Abstract
The effect of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on the coagulation system is not fully understood. SARS-CoV-2 penetrates cells through angiotensin-converting enzyme 2 (ACE2) receptors, leading to its downregulation. Des-arginine9-bradykinin (DA9B) is degraded by ACE2 and causes vasodilation and increased vascular permeability. Furthermore, DA9B is associated with impaired platelet function. Therefore, the aim of this study was to evaluate the effects of DA9B on platelet function and coagulopathy in critically ill coronavirus disease 2019 (COVID-19) patients. In total, 29 polymerase-positive SARS-CoV-2 patients admitted to the intensive care unit of the University Hospital of Giessen and 29 healthy controls were included. Blood samples were taken, and platelet impedance aggregometry and rotational thromboelastometry were performed. Enzyme-linked immunosorbent assays measured the concentrations of DA9B, bradykinin, and angiotensin 2. Significantly increased concentrations of DA9B and angiotensin 2 were found in the COVID-19 patients. A negative effect of DA9B on platelet function and intrinsic coagulation was also found. A sub-analysis of moderate and severe acute respiratory distress syndrome patients revealed a negative association between DA9B and platelet counts and fibrinogen levels. DA9B provokes inhibitory effects on the intrinsic coagulation system in COVID-19 patients. This negative feedback seems reasonable as bradykinin, which is transformed to DA9B, is released after contact activation. Nevertheless, further studies are needed to confirm our findings.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was declared a pandemic in March 2020 by the World Health Organization. To date, more than 770 million cases and 6.9 million deaths associated with the coronavirus disease 2019 (COVID-19) have been reported [1]. However, with the development of novel RNA vaccines, the lethality of COVID-19 has decreased significantly [2]. Nevertheless, morbidity remains a relevant healthcare problem and is associated with coagulopathy [3]. Despite tremendous research efforts, COVID-19-associated alterations to the human coagulatory system are not yet fully understood. It has been demonstrated that SARS-CoV-2 proteins contain a large number of antigens that mimic human blood proteins [4]. The virus spike and replicase 1a protein show similarities to rhesus blood antigens, prothrombin, and von Willebrand factor. Following this, SARS-CoV-2 might not only directly interact with blood coagulation, either as an agonist or antagonist, but also autoantibodies could be synthesized [4]. Moreover, bacterial co-infection, which is common in critically ill COVID-19 patients, is also known to mimic human blood antigens, like cardiolipin, prothrombin, albumin, and platelet factor 4 [4]. It has been shown that cardiolipin antibodies are found in nearly half of patients with COVID-19, and a higher prevalence was found during a severe course of the disease [5]. Furthermore, cardiolipin antibodies are known to induce neutrophilia extracellular traps (NETs), which are elevated during COVID-19 infection and are associated with increased coagulation of the blood [6,7].
Furthermore, activation of the complement system is also involved in COVID-19-associated coagulopathy. It has been shown, that the nucleocapsid protein of SARS-CoV-2 binds to the mannan-binding lectin serin protease 2 (MASP2), which activates the lectin pathway of the complement system and cleaves prothrombin into thrombin [8]. Furthermore, the levels of the complement proteins C3a and C5-b9 were found to be higher in COVID-19 patients with thromboembolic events [8].
COVID-19 has been recognized as a systemic inflammatory disease with vascular endotheliitis [9,10,11,12]. The excessive host inflammatory response, also known as cytokine storm, has been associated with thrombotic complications during COVID-19 [7,13]. Increased levels of IL-6, IL-8, IL-1β, and TNF-α, which correlate with disease severity, have been reported in COVID-19 patients [7]. Furthermore, TNF-α leads to the release of IL-6 from endothelial cells [14]. It must be noted that IL-6 is associated with several pro-coagulatory effects like increased concentrations of fibrinogen, tissue factor, platelets, and von Willebrand factor [14].
Furthermore, platelets are activated by cytokines and complement products [7]. Variable levels of tissue factor mRNA were detected in the platelets of patients with COVID-19, indicating another mechanism for thrombotic events [7].
Another factor involved in COVID-19-associated coagulopathy is the hematopoietic system. Progenitor cells of the erythroid and myeloid lineage express angiotensin-converting enzyme type 2 (ACE2) and transmembrane protease serine subtype 2 on the cell surface. Infection of these cells leads to infected platelets with hyperactivity [15].
It has been shown that the binding of SARS-CoV-2 to the angiotensin-converting enzyme type 2 (ACE2) leads to the downregulation of ACE2 and the upregulation of ACE1 [16]. ACE1 catalyses the synthesis of angiotensin 2 (ANG2), and it has been shown that increased concentrations of ANG2 can be measured during the course of COVID-19 infection [17,18,19]. Both ACE1 and ACE2 enzymes are connected to the kinin system. While short-acting bradykinin (BK) is rapidly degraded by ACE1, des-argine9-bradykinin (DA9B) is depleted more slowly by ACE2, resulting in a longer half-life [16,20]. Beyond the ACE pathway, bradykinin is also converted to DA9B by carboxypeptidase N, which is upregulated during COVID-19 infection [21]. Thus, increased concentrations of DA9B can be expected during COVID-19 infection. Mendes et al. measured the plasma concentration of DA9B and BK in patients with COVID-19. Interestingly, compared to health controls, elevated concentrations of DA9B and decreased levels of BK were found [22]. Furthermore, the expression of the BK receptor 1 (B1R) was assessed in the postmortem liver specimens of 27 COVID-19 individuals and compared to patients with colon cancer, with the results showing increased B1R expression levels in the hepatic tissues of patients with COVID-19 [22].
Several physiological functions of DA9B have been identified. First, it binds to the inducible B1R, which is expressed on the vascular endothelium and leads to the release of pro-inflammatory chemokines and an increase in endothelial permeability [23]. Second, the DA9B-induced activation of inducible nitric oxide synthetase leads to vasodilation, which was observed in critically ill COVID-19 patients. Lastly, the release of prostacyclin (PGI2) is caused by DA9B. Besides its vasodilatory effects, PGI2 is known to inhibit platelet function [23,24].
Currently, it is unknown as to whether DA9B-induced dysfunction can be detected during the clinical course of severe COVID-19 infection. Since platelet dysfunction has been well described in COVID-19 patients, this study hypothesized that SARS-CoV-2-induced changes in DA9B are associated with relevant platelet dysfunction [25,26,27]. Therefore, data from a prospective, observational study were used in a secondary analysis evaluating the concentration and time course of DA9B, BK, and ANG2 and their effects on the coagulatory system [28].
2. Results
The characteristics of the study cohorts are summarized in Table 1.

Table 1.
Description of the study cohorts.
2.1. Bradykinin, Des-Arginine9-Bradykinin, and Angiotensin 2 Concentrations
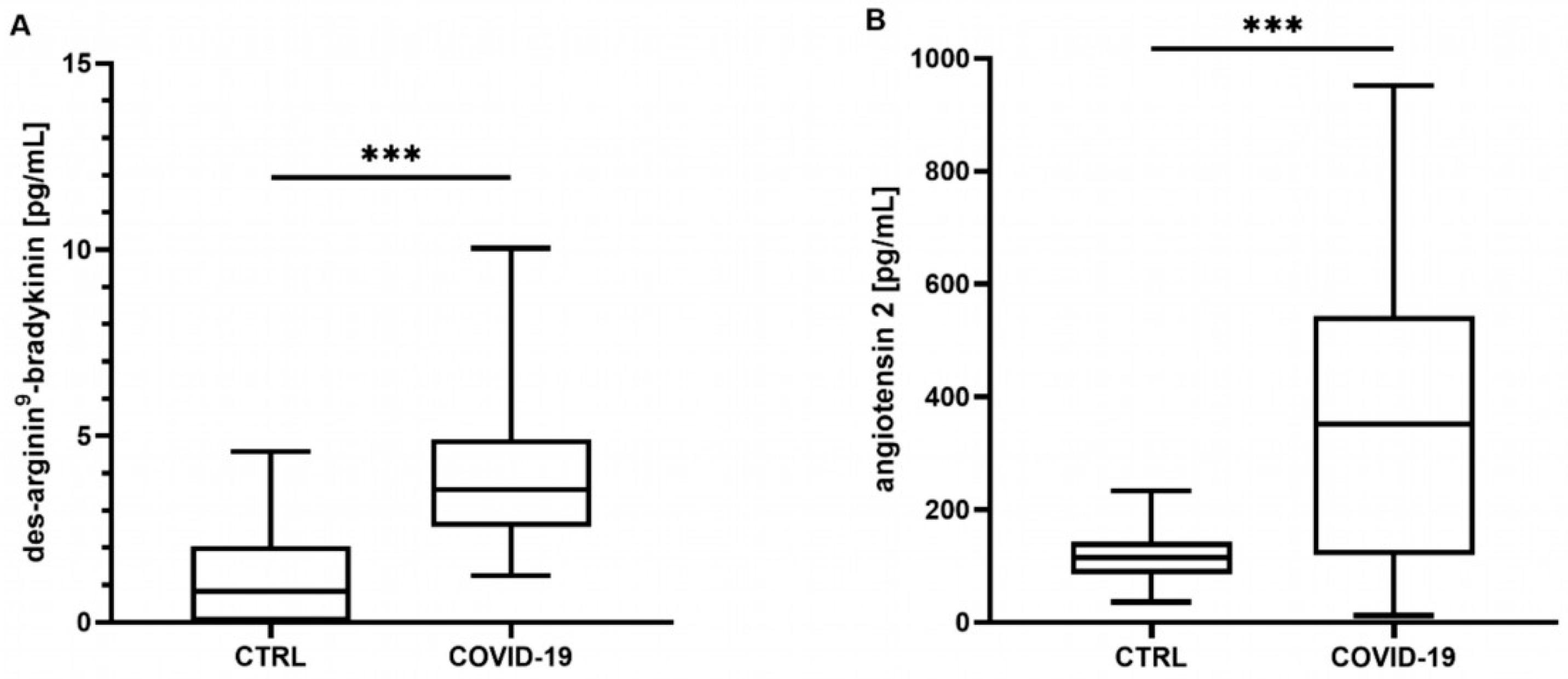
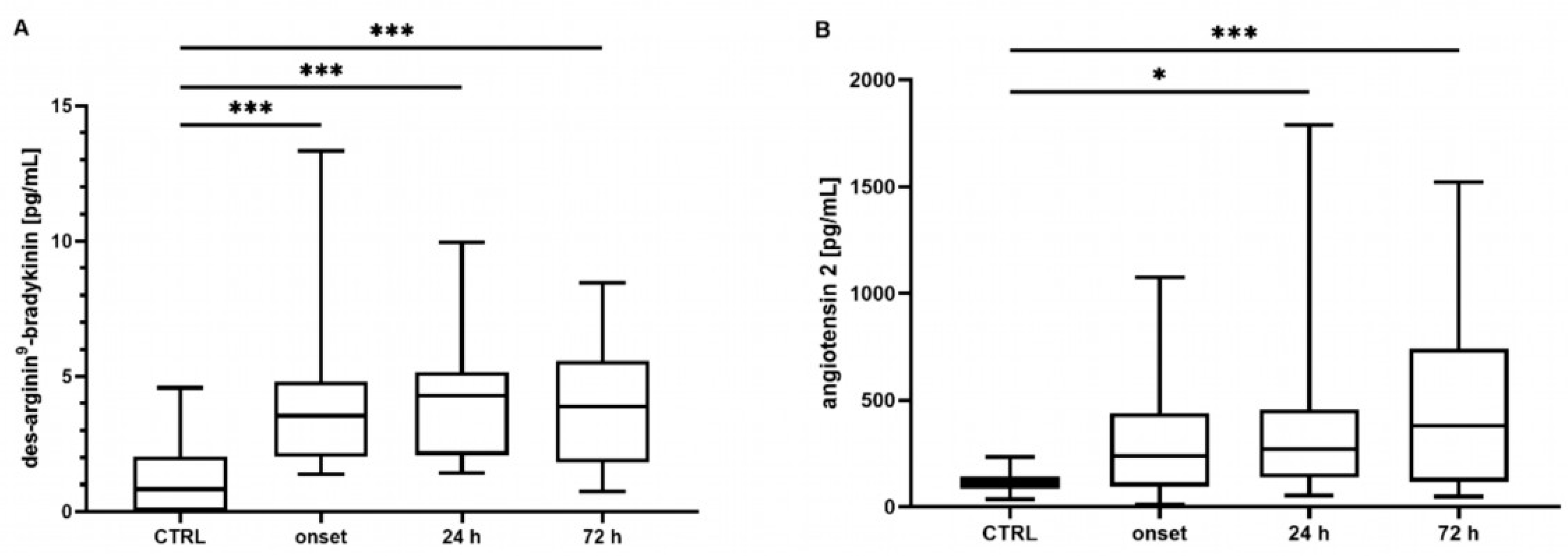
Neither the mean concentrations nor the time point analysis revealed differences in the BK concentrations between the controls and the COVID-19 patients (mean: control: 340 [301–487] pg/mL, COVID-19: 390 [211–586] pg/mL, p = 0.807; t0 348 [219–649] pg/mL, t24 324 [201–772] pg/mL, t72 272 [186–437] pg/mL, all p > 0.05, data not shown). In contrast, the COVID-19 patients showed significantly elevated concentrations of DA9B (4.16 [2.55–5.35] pg/mL) compared to in the controls (0.82 [0.0–2.22] pg/mL, p < 0.001, Figure 1A), which persisted over the entire study period (COVID-19: t0 4.12 [2.25–5.95] pg/mL, t24 4.32 [2.00–5.28] pg/mL, t72 3.98 [1.7–5.69] pg/mL, all p < 0.001, Figure 2A).
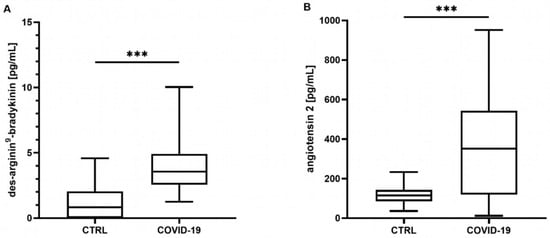
Figure 1.
(A) Des-arginine9-bradykinin (DA9B) and (B) angiotensin 2 (ANG2) at all time points. In patients with COVID-19, significantly higher concentrations of DA9B and ANG2 were found. The asterisks denote the degree of statistical significance (*** p < 0.001). Box and whisker plots indicate the median, interquartile range (box), and minimum and maximum (whiskers). Abbreviations: COVID-19 = coronavirus disease 2019; CTRL = control group.
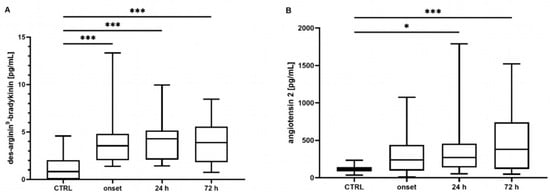
Figure 2.
Time course of (A) des-Arginine9-Bradykinin (DA9B) and (B) angiotensin 2 (ANG2) concentrations. Increased concentrations of DA9B were measured in patients with COVID-19 at all time points. Elevated concentrations of ANG2 were measured in COVID-19 patients at t24 and t72. The asterisks denote the degree of statistical significance (* p ≤ 0.05; *** p < 0.001). Box and whisker plots indicate the median, interquartile range (box), and minimum and maximum (whiskers). Abbreviations: CTRL = control group.
ANG2 was significantly increased in the COVID-19 patients compared to the matched controls (360 [161–577] vs. 113 [78–139] pg/mL, p < 0.001, Figure 1B). An analysis of single time points indicated elevated concentrations at t24 and t72 (COVID-19: t0 246 [123–439] pg/mL, t24 202 [129–457] pg/mL, t72 378 [117–756] pg/mL, t0 p = 0.134, t24 p = 0.023, t72 p < 0.001, Figure 2B).
2.2. Coagulatory Function in Patients with COVID-19
2.2.1. Clot Formation and Stability
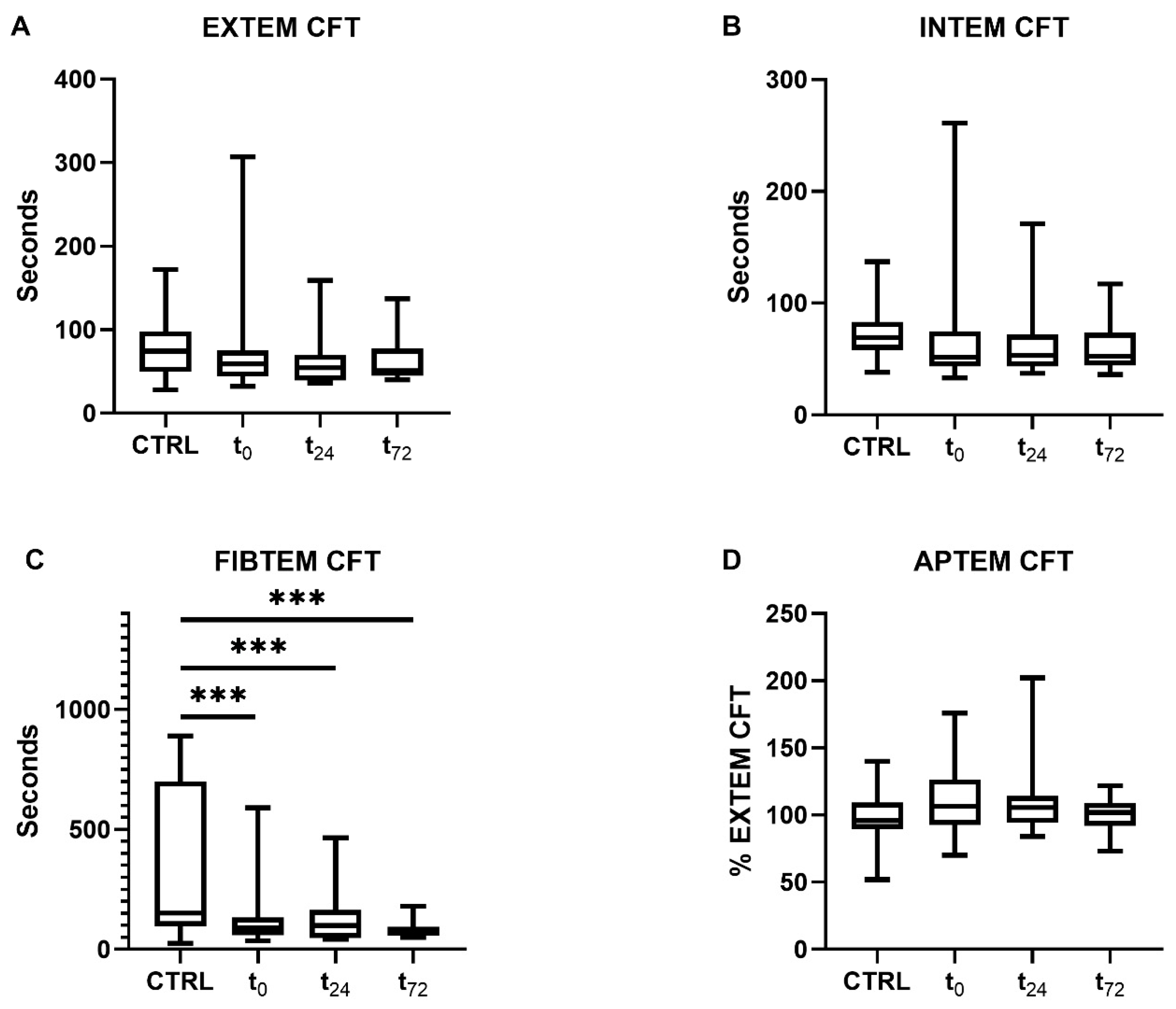
Patients with COVID-19 showed a decrease in their clot formation time (CFT) in the extrinsically and intrinsically activated thromboelastometry (EXTEM and INTEM) assays, which did not reach statistical significance, however (Figure 3A,B). Overall, data variance was high, particularly at t0.
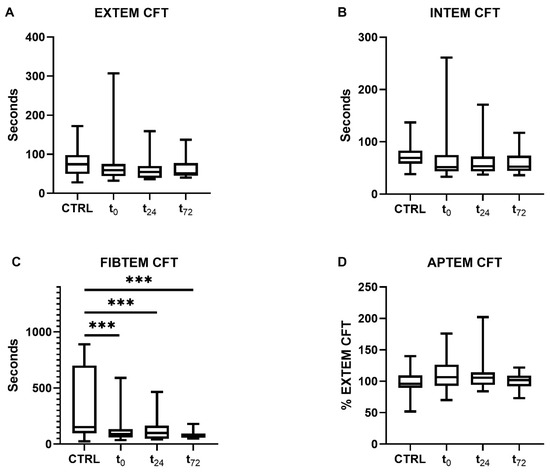
Figure 3.
Clot formation time (CFT) determined using rotational thromboelastometry. CFT was measured through (A) extrinsically and (B) intrinsically activated thromboelastometry and (C) fibrinogen- and (D) aprotinin-based thromboelastometry. Patients with COVID-19 demonstrated significantly reduced CFTs compared with the controls in the FIBTEM assay at all time points (p < 0.001). The asterisks denote the degree of statistical significance (*** p < 0.001). Box and whisker plots indicate the median, interquartile range (box), and minimum and maximum (whiskers). Abbreviations: APTEM = aprotinin-based thromboelastometry; CTRL = control group; EXTEM = extrinsically activated thromboelastometry; FIBTEM = fibrinogen-based thromboelastometry; INTEM = intrinsically activated thromboelastometry; CFT = clot formation time.
Furthermore, a significant reduction in CFT in the fibrinogen-based thromboelastometry (FIBTEM) assay was measured compared to the controls (controls: 171 [90–760] s, COVID-19: t0 90 [59–134] s, t24 99 [47–165] s, t72 67 [56–94] s, all p < 0.001, Figure 3C). As previously shown, the maximum clot firmness (MCF) in the FIBTEM test was increased in patients with COVID-19 [28]. The concentrations of d-dimers were only measured in COVID-19 patients (t0 1.8 [0.8–4.8] µg/mL, t24 1.7 [0.9–3.5] µg/mL, t72 2.5 [0.8–5.3] µg/mL).
2.2.2. Platelet Function
As previously shown in the primary study, COVID-19 patients demonstrated significantly impaired platelet functioning in the arachidonic acid (ASPI, t0) and adenosine diphosphate (ADP, t24) tests [28]. No differences between the controls and the COVID-19 patients were found for platelet counts (control: 250 [208–291] giga/L, COVID-19: t0 210 [150–286] giga/L, t24 215 [159–268] giga/L, t72 245 [148–335] giga/L).
2.3. Correlation Analysis of Des-Arginine9-Bradykinin in All COVID-19 Patients
A positive correlation between DA9B and d-dimers was shown at all time points (Table 2). In addition, a positive relationship was found between DA9B and lactate dehydrogenase (LDH) at t0 and t72 (Table 2). Ferritin and glutamic oxaloacetic transaminase (GOT) also exhibited a positive correlation (Table 2). The thromboelastographic data demonstrated positive dependency with CFT in the INTEM (t0 and t24) and EXTEM (t24) assays (Table 2). Furthermore, all COVID-19 patients were separated regarding prophylactic or therapeutic anticoagulation and the relationship between DA9B and INTEM CFT was analyzed (prophylactic: t0 r = 0.69, p = 0.013 t24 r = 0.80, p = 0.017 t72 r = 0.86, p = 0.014; therapeutic: t0 r = 0.36, p = 0.244 t24 r = 0.41, p = 0.12 t72 r = 0.22, p = 0.491).

Table 2.
Correlations between laboratory results and ROTEM data with des-arginine9-bradykinin.
2.4. Correlation Analysis of Des-Arginine9-Bradykinin in ARDS COVID-19 Patients
The following calculations were conducted for patients with moderate or severe forms of acute respiratory distress syndrome (ARDS) according to the Berlin definition [29]. An analysis of coagulation parameters revealed a positive correlation with D-dimers (t0, t72) and an inverse correlation with platelets (t24, t72) and fibrinogen (t24, Table 3). Inflammatory markers such as ferritin (t0, t24) and pro-calcitonin (PCT, t0, t24) demonstrated a positive association with DA9B (Table 3). A positive correlation was found for GOT (t24) and LDH (t0, Table 3).

Table 3.
Correlation between laboratory results and ROTEM data with des-arginine9-bradykinin in acute respiratory distress patients.
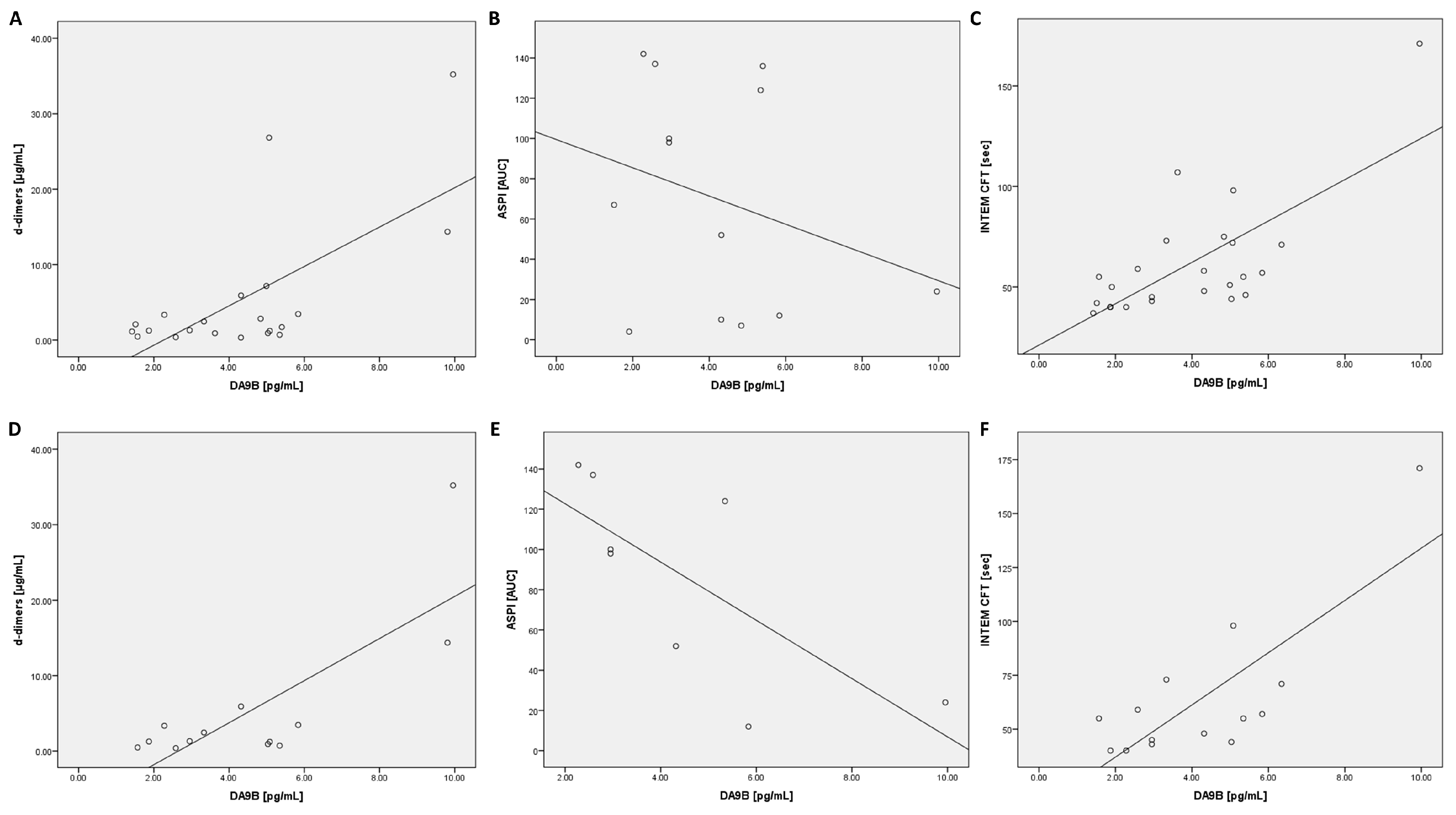
An analysis of the ROTEM data revealed a positive correlation for CFT in the INTEM assay (t0, t24, Table 3 and Figure 4). In contrast, an inverse correlation was found for clot firmness after 20 min of clot formation (A20) (at all time points) and MCF (t72) in the INTEM test (Table 3). In addition, a positive correlation was found for A20 (t24) and MCF (t24) in the aprotinin-based thromboelastometry (APTEM) assay (Table 3).
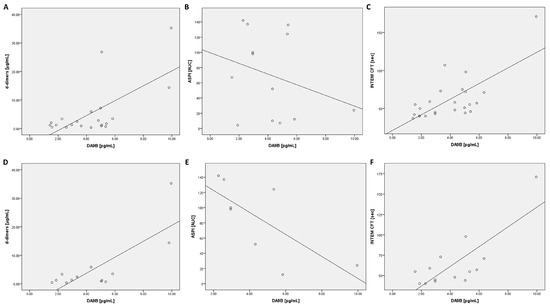
Figure 4.
Scatter plots for DA9B with d-dimers, ASPI, and INTEM CFT at t24: scatter plots are shown for all COVID-19 patients (A–C) and COVID-19 patients with moderate or severe acute respiratory distress syndrome (D–F). Abbreviations: ASPI = arachidonic acid; INTEM = intrinsically activated thromboelastometry; CFT = clot formation time.
2.5. Correlation Analysis of Angiotensin 2 in All COVID-19 Patients
An analysis of the laboratory results revealed a positive correlation with C-reactive protein (CRP) at t72 (r = 0.64, p = 0.001). Furthermore, CRP (t24 r = 0.51, p = 0.009), PCT (t0 r = 0.46, p = 0.013), IL-6 (t24 r = 0.53, p = 0.020), ferritin (t72 r = 0.55, p = 0.013), GOT (t0 r = 0.60, p = 0.001), glutamic pyruvic transaminase (GPT, t0 r = 0.52, p = 0.004), and LDH (t72 r = 0.53, p = 0.018) showed significant correlations, although there was a high degree of data scattering.
Furthermore, there was a positive dependency in the INTEM test for CFT (t0 r = 0.41, p = 0.047) and an inverse correlation with A20 (t0 r = −0.62, p = 0.002) and MCF (t0 r = −0.45, p = 0.028).
2.6. Correlation Analysis of Angiotensin 2 in ARDS COVID-19 Patients
The laboratory results showed a positive connection with IL-6 (t24 r = 0.69, p = 0.013). All other significant correlations showed a low determination coefficient (ferritin t24 r = 0.59, p = 0.035 and GOT t0 r = 0.63, p = 0.022). ANG2 was positively correlated with A20 (t24 r = 0.64, p = 0.018) and MCF (t24 r = 0.63, p = 0.023) in the APTEM assay, while an inverse correlation was found for A20 (t0 r = −0.92, p < 0.001) in the INTEM test.
2.7. Correlation Analysis of Impedance Aggregometry with Des-Arginine9-Bradykinin and Angiotensin 2
No significant correlations were found between impedance aggregometry and DA9B or ANG2. However, an analysis of patients with moderate or severe ARDS revealed an inverse correlation between DA9B and the ASPI test (t24: r = −0.83, p = 0.011).
3. Discussion
To the best of our knowledge, this is the first study investigating the effects of DA9B on in vivo blood coagulation in critically ill COVID-19 patients. Elevated concentrations of DA9B were measured for all time points, while correlation analyses revealed positive dependencies with d-dimers, LDH, and the CFT in the EXTEM and INTEM assays. Furthermore, a sub-analysis of patients with moderate or severe ARDS revealed a positive correlation with markers of inflammation such as PCT and an inverse dependency with platelets, fibrinogen, A20, and MCF in the INTEM assay. Moreover, an analysis of ANG2 demonstrated elevated concentrations at t24 and t72. A positive correlation was also found for CRP and IL-6 in ARDS patients, while an inverse correlation was identified for A20 in the INTEM assay. However, no differences in BK concentrations were found, and no correlation analyses were conducted.
As DA9B is depleted by ACE2, which is downregulated during COVID-19 infection, many authors have linked the kinin system to COVID-19 [13,30,31]. DA9B is of specific interest because, contrary to BK, it has a longer half-life and binds to B1R, which is induced by systemic inflammation through TNF-α [23]. Effects following the activation of B1R include vasodilation and increased vascular permeability [23]. Therefore, investigation into DA9B during COVID-19 infection is of particular interest.
Mendes et al. found increased concentrations of DA9B in patients with COVID-19 [22]. While these findings align with our results, some important differences exist between their study and ours. The Mendes et al. study included patients hospitalized 17 to 30 days after a positive PCR test for SARS-CoV-2 [22], while only critically ill ICU patients were included in our study. Furthermore, it should be noted that rather than an ELISA, Mendes et al. used liquid chromatography–tandem mass spectrometry to measure DA9B concentrations [22]. Therefore, the absolute amount of DA9B cannot be compared between the studies.
Interestingly, our correlation analysis revealed a positive correlation between DA9B and d-dimers at all time points. On the one hand, the number of d-dimers could have increased during inflammation due to immunothrombosis [32]. However, no correlations between DA9B and markers of inflammation were found in our analysis in any of the COVID-19 patients. However, there are several reasons for elevated d-dimer levels in critically ill COVID-19 patients. COVID-19 pathology is known for close interaction between the inflammatory and coagulatory systems, leading to an increase in fibrinolysis and d-dimers [33,34]. Furthermore, d-dimers can accumulate during renal failure, which was indicated by an impaired glomerular filtration rate in the study collective (t0 49 [31–90]; t24 52 [30–90]; t72 41 [24–79] mL/min; Supplementary Table S1) [28]. Lastly, the variance in the number of d-dimers might reflect the heterogeneity of the study group. Since adrenomedullin has been described as an indicator of sepsis and organ dysfunction in bacterial and viral infections, further studies should focus on the association between DA9B and adrenomedullin [35]. On the other hand, d-dimers could also reflect signs of increased fibrinolysis. Moreover, a positive dependence between DA9B and signs of cell damage (LDH) was found, which could reflect endotheliitis during COVID-19. Therefore, our further analysis focused on the impact of DA9B on coagulation.
The thromboelastographic analysis revealed a positive correlation between DA9B and the CFT in the EXTEM and INTEM assays. This suggests that a prolonged time was needed to reach clot formation. Thus, DA9B might have an inhibitory effect on the coagulation system’s extrinsic and intrinsic pathways. Since INTEM CFT could be affected by therapeutic anticoagulation, all COVID-19 patients were divided into receiving prophylactic or therapeutic anticoagulation. Interestingly, no dependency between DA9B and INTEM CFT were seen in patients with therapeutic anticoagulation, whereas a strong connection was found in patients with prophylactic anticoagulation. Since BK is released after contact activation, the kinin system is connected to the intrinsic coagulation system. As BK is converted to DA9B, a negative feedback loop between DA9B and the intrinsic pathway of the coagulation system appears possible. It should be emphasized that these inhibitory effects on the extrinsic and intrinsic coagulation systems were also seen in the ARDS sub-analysis. Furthermore, an inverse correlation between DA9B with A20 and MCF in the INTEM assay was found in this sub-group, which highlights the inhibitory effect on the intrinsic coagulation system.
Since TNF-α leads to the release of IL-6 and the induction of B1R, an association between IL-6 and DA9B could be hypothesized [14,23]. Interestingly, no significant correlations were found between IL-6 and DA9B. However, it must be highlighted that only slightly elevated concentrations of IL-6 were measured in the underlying primary study (t0 49.5 [35.1–149.8]; t24 17.1 [13.6–37.4]; t72 20.9 [0.0–54.8] pg/mL; Supplementary Table S1) [28]. Since IL-6 is known to be an early marker of an inflammatory response and the disease severity varied, the slight elevation in IL-6 might be explainable. Nevertheless, the heterogeneity of the study group could also give an explanation. Nonetheless, all patients had to be treated in the intensive care unit.
It has been shown that the activation of B1R results in the release of tissue plasminogen activator [36]. This could explain the positive connection between DA9B and d-dimers. Data from endothelial cells of the pulmonary artery of the calf have shown that the activation of B1R with DA9B leads to the release of PGI2 [36,37]. Since PGI2 is known to reverse the activation of platelets, this could account for the impaired platelet function found in the primary study [28,38]. Data from animal studies suggest that this inhibitory effect is dose dependent, and a two to threefold increase in PGI2 is needed to inhibit platelets [36]. Although no association between DA9B and the results of impedance aggregometry were found in our study population, a sub-analysis of the moderate and severe ARDS sub-groups demonstrated an inverse correlation between DA9B and the AUC in the ASPI test. In addition, an inverse correlation with DA9B was also found between platelets and fibrinogen in ARDS patients, which could be interpreted as an increase in the degree of consumption. Furthermore, SNPs in the gens for MTHFR, fibrinogen or IL-6 receptor could also offer an explanation for the inverse connection between fibrinogen and DA9B. Furthermore, additional studies should include the expression of glycoprotein IIb/IIIa and P-selection on the platelets. Since SNPs have also been found in platelet disorders like macrothrombocytopenia, SNPs could also be associated with lower platelet counts [39]. Moreover, additional platelet function tests like those for collagen, adrenaline, and ristocetin could improve our understanding of COVID-19-associated platelet dysfunction.
Since ANG2 is depleted by ACE2, which is decreased during COVID-19 infection, it was an early research target. Many authors have demonstrated elevated concentrations of ANG2 during COVID-19 infection, which aligns with our results [17,18,19]. Furthermore, a positive correlation between ANG2 and markers of inflammation was found, which could be explained by the fact that SARS-CoV-2 enters cells after binding to ACE2. Therefore, increased virus concentrations are associated with increased concentrations of ANG2.
Interestingly, the ROTEM analysis revealed an inhibitory effect on the intrinsic coagulation system, which could only be seen in the ARDS sub-analysis for A20. However, contrary to our results, data from human monocytes have demonstrated tissue factor release due to ANG2 [40]. It has also been shown that ANG2 enhances thrombosis development in hypertensive rats [41]. However, this is the first study investigating the connection between ANG2 and ROTEM in COVID-19 patients. Since these results were found only at one time point for one marker, these findings should be interpreted with caution.
No differences in BK were found in our study. From a pathophysiologic perspective, BK may be impaired due to its increased degradation by ACE1. Mendes et al. measured the concentration of BK in 20 hospitalized patients with COVID-19 and compared them with healthy volunteers [22]. Contrary to our findings, they found decreased concentrations of BK in COVID-19 patients. Unfortunately, no information about the severity of the disease was given in their study. Moreover, liquid chromatography–tandem mass spectrometry was used for the measurements. Therefore, their results cannot be directly compared to ours. As proteases degrade BK, it is important to add protease inhibitors to the blood collection system prior to its use. Since the primary study was designed to measure mtDNA and blood coagulation, no protease inhibitors were added to the blood-collecting tubes. Hence, the results of our BK analysis should be interpreted with caution. Cyr et al. measured the half-life of bradykinin (27 s) and DA9B (643 s) in 116 healthy individuals [42]. For this reason, all blood samples were centrifugated immediately after withdrawal at 4 °C. Therefore, the impact of the spontaneous degradation of DA9B on the study results is negligible.
This study has some limitations. First, due to the exploratory nature of the primary study, the sample size was small. Nevertheless, in vivo effects were already detectable with our sample size. Second, as mentioned above, no protease inhibitors were added to the blood collection tube, as the primary study was designed to measure mtDNA. Therefore, the results of the BK analysis must be interpreted with caution. Third, since no analyses of possible mutations of SARS-CoV-2 were performed, the effect of possible mutations remains unclear. Fourth, the presence of single nucleotide polymorphisms (SNPs) was not analysed. It has been shown that SNPs in the gene of methylenetetrahydrofolate reductase (MTHFR) are associated with increased concentrations of homocysteine and consecutive venous thrombosis [43]. Furthermore, SNPs have also been identified in the genes of the IL-6 receptor, fibrinogen beta chain, and fibrinogen gamma chain and have been found to be associated with increased concentrations of fibrinogen [44]. Therefore, the presence of SNPs should be considered during further studies with the analysis of blood coagulation.
4. Materials and Methods
4.1. Study Design
As previously described, the primary study was designed as a single-center, prospective, observational, proof-of-concept study [28]. In total, 29 critically ill COVID-19 patients treated in the intensive care unit of the University Hospital of Giessen and 29 healthy controls were included in the primary study. The secondary analysis was approved by the local ethics committee (Justus-Liebig University of Giessen, trial code: 65/20).
The primary study was conducted in accordance with the Declaration of Helsinki and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The inclusion criteria consisted of having been admitted to the surgical intensive care unit (ICU) of the University Hospital of Giessen within 24 h, having a positive SARS-CoV-2 PCR test, being of legal age, and giving informed consent, which was obtained through a legal representative when applicable. The exclusion criteria comprised having a negative SARS-CoV-2 PCR test or being under 18 years of age.
4.2. Sample Processing
Blood from the controls was drawn through the cubital vein, whereas that of the COVID-19 patients was collected through arterial or central lines. The blood was collected in hirudin and citrate tubes for platelet impedance and thromboelastometry, respectively. Ethylenediaminetetraacetic acid tubes were used for enzyme-linked immunosorbent assays (ELISAs). The blood samples from the controls were collected only once, while those of the patients were collected at study inclusion (t0), after 24 h (t24), and after 72h (t72) thereafter. Platelet impedance aggregometric and thromboelastometric measurements were carried out immediately after blood sampling. Following centrifugation, plasma samples were stored at −80 °C for further analysis. Clinical data were extracted from the local patient data management system (IMESO GmbH, Giessen, Germany).
4.3. Coagulation Analysis
Coagulatory function was assessed using thromboelastography (ROTEM Delta analyser; Tem Innovations GmbH, Munich, Germany). EXTEM and INTEM reagents were used to evaluate the coagulatory function of the intrinsic and extrinsic pathways, respectively. FIBTEM and APTEM reagents were used to investigate fibrinogen-dependent coagulation and the extent of fibrinolysis, respectively [28].
The clotting time in seconds, CFT in seconds, clot strength based on MCF in millimeters, A20 in millimeters, and fibrinolysis based on maximum lysis as the percentage of the MCF were recorded.
Impedance aggregometry (Multiplate Analyzer, Roche Diagnostics, Mannheim, Germany) was performed according to the manufacturer’s instructions to describe platelet function. In brief, thrombin receptor-activating peptide (TRAPtest, Verum Diagnostica GmbH, Munich, Germany), ADP (ADPtest, Verum Diagnostica GmbH) or ASPI (ASPItest, Verum Diagnostica GmbH) were used. The area under the impedance aggregometric curve (AUC) was used to describe aggregation capacity.
4.4. Laboratory Analysis
The laboratory parameters assessed included leukocyte, thrombocyte, neutrophilic granulocyte, and lymphocyte counts, glomerular filtration rate, international normalized ratio, and the levels of fibrinogen, D-dimer, CRP, PCT, IL-6, ferritin, creatinine, urea, GOT, GPT, and LDH. All parameters were measured during routine clinical tests at the local laboratory of the University Hospital of Giessen [28].
4.5. ELISA
The concentration of BK, DA9B, and ANG2 were quantified using ELISA (ELISA Kits MBS766033, MBS109439, and MBS 703599; MyBioSource.com, San Diego, CA, USA) according to the manufacturer’s instructions. The assays were carried out in duplicate readings. The probes were unfrozen only once.
4.6. Statistical Analysis
All data are expressed as medians and interquartile ranges (25–75th percentiles). An analysis of variance followed by a post hoc Bonferroni test was used to compare the groups. The Shapiro–Wilk test was performed and revealed that DA9B is not normally distributed at t0 and t24. Furthermore, a non-normal distribution was found for ANG2 regarding any timepoint. Therefore, Spearman’s correlation coefficients were used for the correlation between DA9B and ANG2 and the results of the laboratory, aggregometric, and thromboelastometric analyses. A p-value of ≤0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 20 (IBM Corp., Armonk, NY, USA).
5. Conclusions
In summary, our study demonstrates an inhibitory effect of DA9B on the intrinsic coagulation system in COVID-19 patients. This potential negative feedback might offer an explanation as bradykinin, which is transformed to DA9B, is released after contact activation. Nevertheless, further larger scale studies should be performed to confirm our findings.
Author Contributions
Conceptualization, F.E., C.K., M.S. and E.S.; methodology, F.E., S.E., C.K., G.S. and E.S.; software, G.S.; validation, G.S., M.S. and C.K.; formal analysis, G.S., F.E. and E.S.; investigation, F.E., C.K. and E.S.; resources, M.S.; data curation, G.S.; writing—original draft preparation, F.E., S.E. and E.S.; writing—review and editing, C.K., G.S. and M.S.; visualization, F.E. and E.S.; supervision, M.S. and C.K.; project administration, F.E., C.K. and E.S.; funding acquisition, F.E. All authors have read and agreed to the published version of the manuscript.
Funding
F.E. is a participant of the JLU CAREER program, which is funded by the German Research Council.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Justus-Liebig University of Giessen (protocol code 65/20; 6 November 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank Ilona Magel for her expert technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- World Health Organisation. Coronavirus Disease (COVID-19) Weekly Epidemiological Update. Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update (accessed on 29 September 2023).
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021, 20, 172. [Google Scholar] [CrossRef]
- Root-Bernstein, R. COVID-19 coagulopathies: Human blood proteins mimic SARS-CoV-2 virus, vaccine proteins and bacterial co-infections inducing autoimmunity: Combinations of bacteria and SARS-CoV-2 synergize to induce autoantibodies targeting cardiolipin, cardiolipin-binding proteins, platelet factor 4, prothrombin, and coagulation factors. Bioessays 2021, 43, e2100158. [Google Scholar] [CrossRef]
- Taha, M.; Samavati, L. Antiphospholipid antibodies in COVID-19: A meta-analysis and systematic review. RMD Open 2021, 7, e001580. [Google Scholar] [CrossRef]
- Szturmowicz, M.; Demkow, U. Neutrophil Extracellular Traps (NETs) in Severe SARS-CoV-2 Lung Disease. Int. J. Mol. Sci. 2021, 22, 8854. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, N.; Colucci, M. The Prothrombotic State Associated with SARS-CoV-2 Infection: Pathophysiological Aspects. Mediterr. J. Hematol. Infect. Dis. 2021, 13, e2021045. [Google Scholar] [CrossRef] [PubMed]
- Bekassy, Z.; Lopatko Fagerström, I.; Bader, M.; Karpman, D. Crosstalk between the renin-angiotensin, complement and kallikrein-kinin systems in inflammation. Nat. Rev. Immunol. 2022, 22, 411–428. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef]
- Nägele, M.P.; Haubner, B.; Tanner, F.C.; Ruschitzka, F.; Flammer, A.J. Endothelial dysfunction in COVID-19: Current findings and therapeutic implications. Atherosclerosis 2020, 314, 58–62. [Google Scholar] [CrossRef]
- Zhang, J.; Tecson, K.M.; McCullough, P.A. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev. Cardiovasc. Med. 2020, 21, 315–319. [Google Scholar] [CrossRef]
- Mahmudpour, M.; Roozbeh, J.; Keshavarz, M.; Farrokhi, S.; Nabipour, I. COVID-19 cytokine storm: The anger of inflammation. Cytokine 2020, 133, 155151. [Google Scholar] [CrossRef]
- Kerr, R.; Stirling, D.; Ludlam, C.A. Interleukin 6 and haemostasis. Br. J. Haematol. 2001, 115, 3–12. [Google Scholar] [CrossRef]
- Balzanelli, M.G.; Distratis, P.; Dipalma, G.; Vimercati, L.; Inchingolo, A.D.; Lazzaro, R.; Aityan, S.K.; Maggiore, M.E.; Mancini, A.; Laforgia, R.; et al. SARS-CoV-2 Virus Infection May Interfere CD34+ Hematopoietic Stem Cells and Megakaryocyte-Erythroid Progenitors Differentiation Contributing to Platelet Defection towards Insurgence of Thrombocytopenia and Thrombophilia. Microorganisms 2021, 9, 1632. [Google Scholar] [CrossRef]
- Datta, P.K.; Liu, F.; Fischer, T.; Rappaport, J.; Qin, X. SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics 2020, 10, 7448–7464. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Li, J.; Feng, C.; et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hu, R.; Zhang, C.; Ren, W.; Yu, A.; Zhou, X. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients. Crit. Care 2020, 24, 290. [Google Scholar] [CrossRef] [PubMed]
- El-Arif, G.; Farhat, A.; Khazaal, S.; Annweiler, C.; Kovacic, H.; Wu, Y.; Cao, Z.; Fajloun, Z.; Khattar, Z.A.; Sabatier, J.M. The Renin-Angiotensin System: A Key Role in SARS-CoV-2-Induced COVID-19. Molecules 2021, 26, 6945. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, I.A.; Kaplan, A.P. Studies of the digestion of bradykin, lys-bradykinin, and des-Arg9-bradykinin by angiotensin converting enzyme. Biochem. Pharmacol. 1986, 35, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, E.; Díaz-García, E.; García-Tovar, S.; Zamarrón, E.; Mangas, A.; Galera, R.; Nanwani-Nanwani, K.; Pérez-de-Diego, R.; López-Collazo, E.; García-Río, F.; et al. Impaired Kallikrein-Kinin System in COVID-19 Patients’ Severity. Front. Immunol. 2022, 13, 909342. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.M.d.M.; Do Nascimento, I.J.B.; Marazzi-Diniz, P.H.; Da Silveira, I.B.; Itaborahy, M.F.; Viana, L.E.; Silva, F.A.; Santana, M.F.; Pinto, R.A.; Dutra, B.G.; et al. The des-Arg9-bradykinin/B1R axis: Hepatic damage in COVID-19. Front. Physiol. 2022, 13, 1080837. [Google Scholar] [CrossRef] [PubMed]
- Marceau, F.; Bachelard, H.; Bouthillier, J.; Fortin, J.-P.; Morissette, G.; Bawolak, M.-T.; Charest-Morin, X.; Gera, L. Bradykinin receptors: Agonists, antagonists, expression, signaling, and adaptation to sustained stimulation. Int. Immunopharmacol. 2020, 82, 106305. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.C.; Voetsch, B.; Loscalzo, J. Endogenous mechanisms of inhibition of platelet function. Microcirculation 2005, 12, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Bertolin, A.J.; Dalçóquio, T.F.; Salsoso, R.; de M Furtado, R.H.; Kalil-Filho, R.; Hajjar, L.A.; Siciliano, R.F.; Kallás, E.G.; Baracioli, L.M.; Lima, F.G.; et al. Platelet Reactivity and Coagulation Markers in Patients with COVID-19. Adv. Ther. 2021, 38, 3911–3923. [Google Scholar] [CrossRef] [PubMed]
- Heinz, C.; Miesbach, W.; Herrmann, E.; Sonntagbauer, M.; Raimann, F.J.; Zacharowski, K.; Weber, C.F.; Adam, E.H. Greater Fibrinolysis Resistance but No Greater Platelet Aggregation in Critically Ill COVID-19 Patients. Anesthesiology 2021, 134, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Notz, Q.; Schlesinger, T.; Stumpner, J.; Kredel, M.; Sitter, M.; Schmid, B.; Kranke, P.; Schulze, H.; Meybohm, P.; et al. Point of care diagnostic of hypercoagulability and platelet function in COVID-19 induced acute respiratory distress syndrome: A retrospective observational study. Thromb. J. 2021, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Edinger, F.; Edinger, S.; Koch, C.; Markmann, M.; Hecker, M.; Sander, M.; Schneck, E. Peak Plasma Levels of mtDNA Serve as a Predictive Biomarker for COVID-19 in-Hospital Mortality. J. Clin. Med. 2022, 11, 7161. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Meini, S.; Zanichelli, A.; Sbrojavacca, R.; Iuri, F.; Roberts, A.T.; Suffritti, C.; Tascini, C. Understanding the Pathophysiology of COVID-19: Could the Contact System Be the Key? Front. Immunol. 2020, 11, 2014. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, S.; Zhang, Y.; Zhi, Y. Does hereditary angioedema make COVID-19 worse? World Allergy Organ. J. 2020, 13, 100454. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- De Azambuja Pias Weber, A.; Viero, F.T.; Pillat, M.M.; de Lima Gonçalves, T. Changes in markers of inflammation and their correlation with death in patients with COVID-19 in the intensive care unit. Cytokine 2024, 175, 156509. [Google Scholar] [CrossRef] [PubMed]
- De Maistre, E.; Savard, P.; Guinot, P.-G. COVID-19 and the Concept of Thrombo-Inflammation: Review of the Relationship between Immune Response, Endothelium and Coagulation. J. Clin. Med. 2023, 12, 7245. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, O.H.M.; Lengquist, M.; Spångfors, M.; Annborn, M.; Bergmann, D.; Schulte, J.; Levin, H.; Melander, O.; Frigyesi, A.; Friberg, H. Circulating bioactive adrenomedullin as a marker of sepsis, septic shock and critical illness. Crit. Care 2020, 24, 636. [Google Scholar] [CrossRef] [PubMed]
- Schmaier, A.H. The contact activation and kallikrein/kinin systems: Pathophysiologic and physiologic activities. J. Thromb. Haemost. 2016, 14, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Cahill, M.; Fishman, J.B.; Polgar, P. Effect of des arginine9-bradykinin and other bradykinin fragments on the synthesis of prostacyclin and the binding of bradykinin by vascular cells in culture. Agents Actions 1988, 24, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.Z.; Raslan, Z.; Atkinson, L.; Aburima, A.; Thomas, S.G.; Naseem, K.M.; Calaminus, S.D.J. Prostacyclin reverses platelet stress fibre formation causing platelet aggregate instability. Sci. Rep. 2017, 7, 5582. [Google Scholar] [CrossRef]
- Ghevaert, C.; Salsmann, A.; Watkins, N.A.; Schaffner-Reckinger, E.; Rankin, A.; Garner, S.F.; Stephens, J.; Smith, G.A.; Debili, N.; Vainchenker, W.; et al. A nonsynonymous SNP in the ITGB3 gene disrupts the conserved membrane-proximal cytoplasmic salt bridge in the alphaIIbbeta3 integrin and cosegregates dominantly with abnormal proplatelet formation and macrothrombocytopenia. Blood 2008, 111, 3407–3414. [Google Scholar] [CrossRef]
- He, M.; He, X.; Xie, Q.; Chen, F.; He, S. Angiotensin II induces the expression of tissue factor and its mechanism in human monocytes. Thromb. Res. 2006, 117, 579–590. [Google Scholar] [CrossRef]
- Mogielnicki, A.; Chabielska, E.; Pawlak, R.; Szemraj, J.; Buczko, W. Angiotensin II enhances thrombosis development in renovascular hypertensive rats. Thromb. Haemost. 2005, 93, 1069–1076. [Google Scholar] [CrossRef]
- Cyr, M.; Lepage, Y.; Blais, C.; Gervais, N.; Cugno, M.; Rouleau, J.L.; Adam, A. Bradykinin and des-Arg(9)-bradykinin metabolic pathways and kinetics of activation of human plasma. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H275–H283. [Google Scholar] [CrossRef] [PubMed]
- Den Heijer, M.; Lewington, S.; Clarke, R. Homocysteine, MTHFR and risk of venous thrombosis: A meta-analysis of published epidemiological studies. J. Thromb. Haemost. 2005, 3, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Wassel, C.L.; Lange, L.A.; Keating, B.J.; Taylor, K.C.; Johnson, A.D.; Palmer, C.; Ho, L.A.; Smith, N.L.; Lange, E.M.; Li, Y.; et al. Association of genomic loci from a cardiovascular gene SNP array with fibrinogen levels in European Americans and African-Americans from six cohort studies: The Candidate Gene Association Resource (CARe). Blood 2011, 117, 268–275. [Google Scholar] [CrossRef] [PubMed][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).