Bavachin Rejuvenates Sensitivity of Colistin against Colistin-Resistant Gram-Negative Bacteria
Abstract
:1. Introduction
2. Results
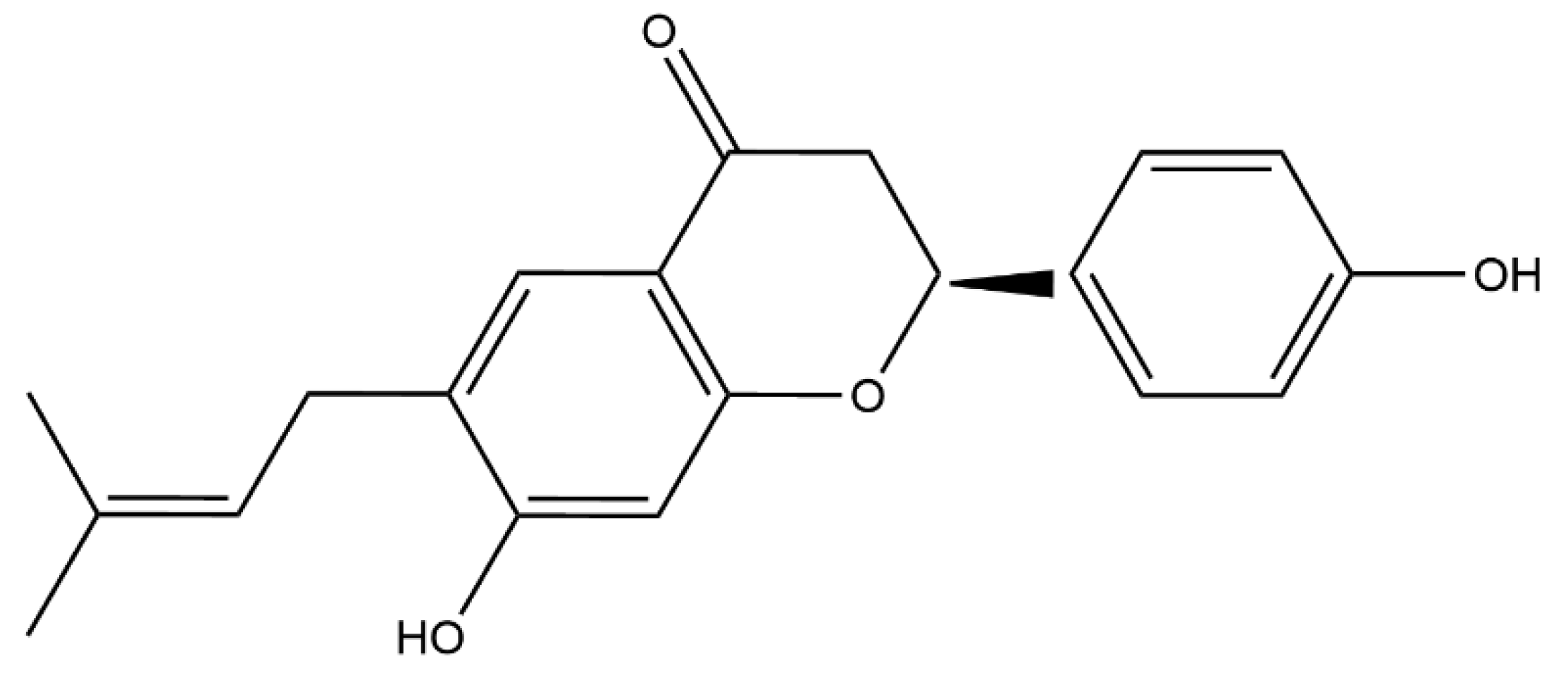
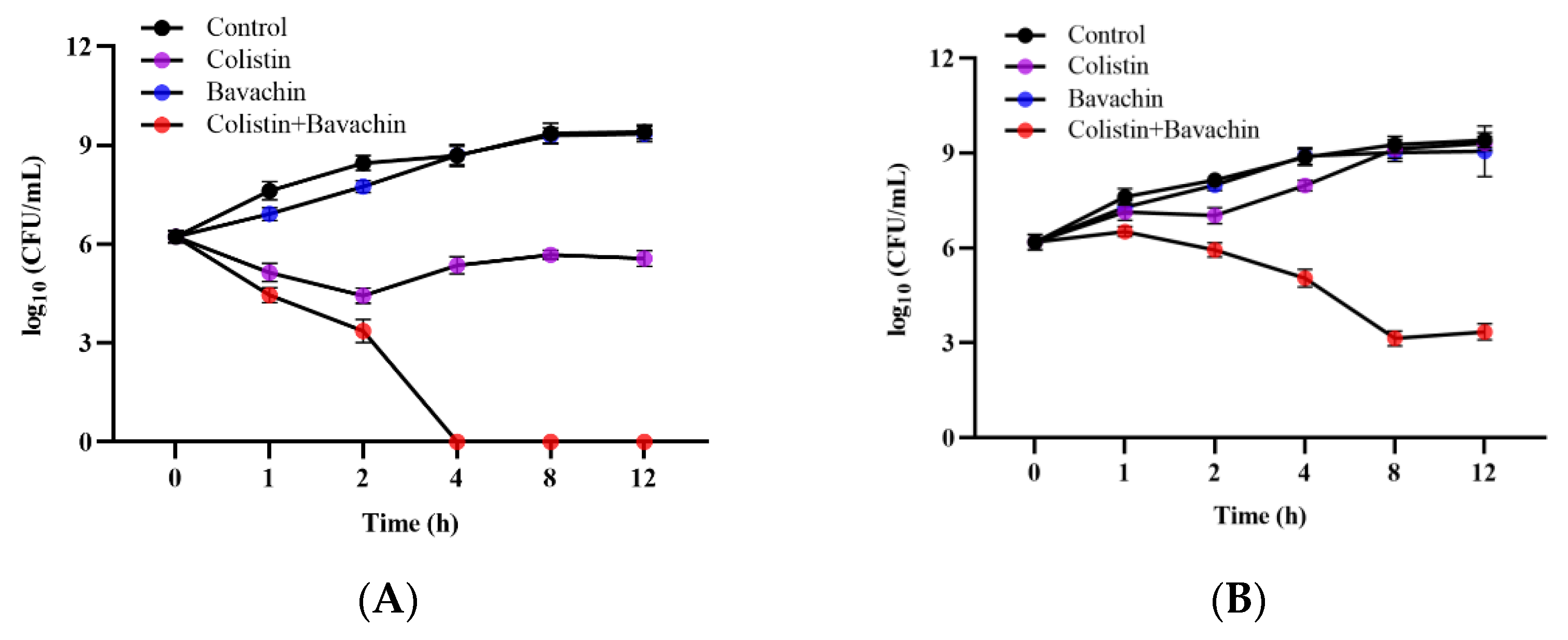
2.1. Bavachin Combined with Colistin Has Strong Synergistic Effects on MDR GNB
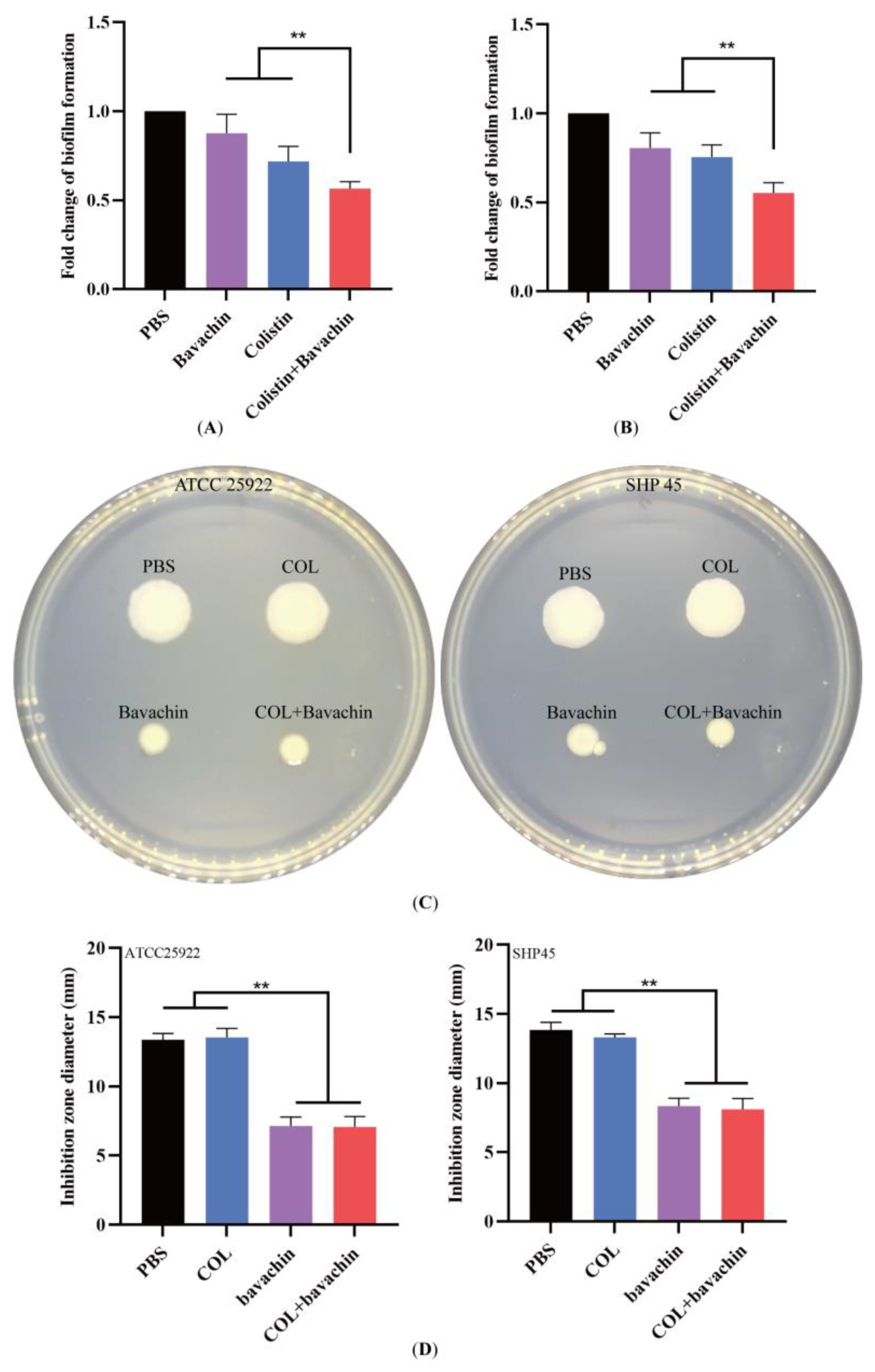
2.2. Effect of the Colistin Combined with Bavachin on Antibacterial Activity and Bacterial Biofilm Formation
2.3. Bavachin Enhances the Efficacy of Colistin In Vivo Infection Models
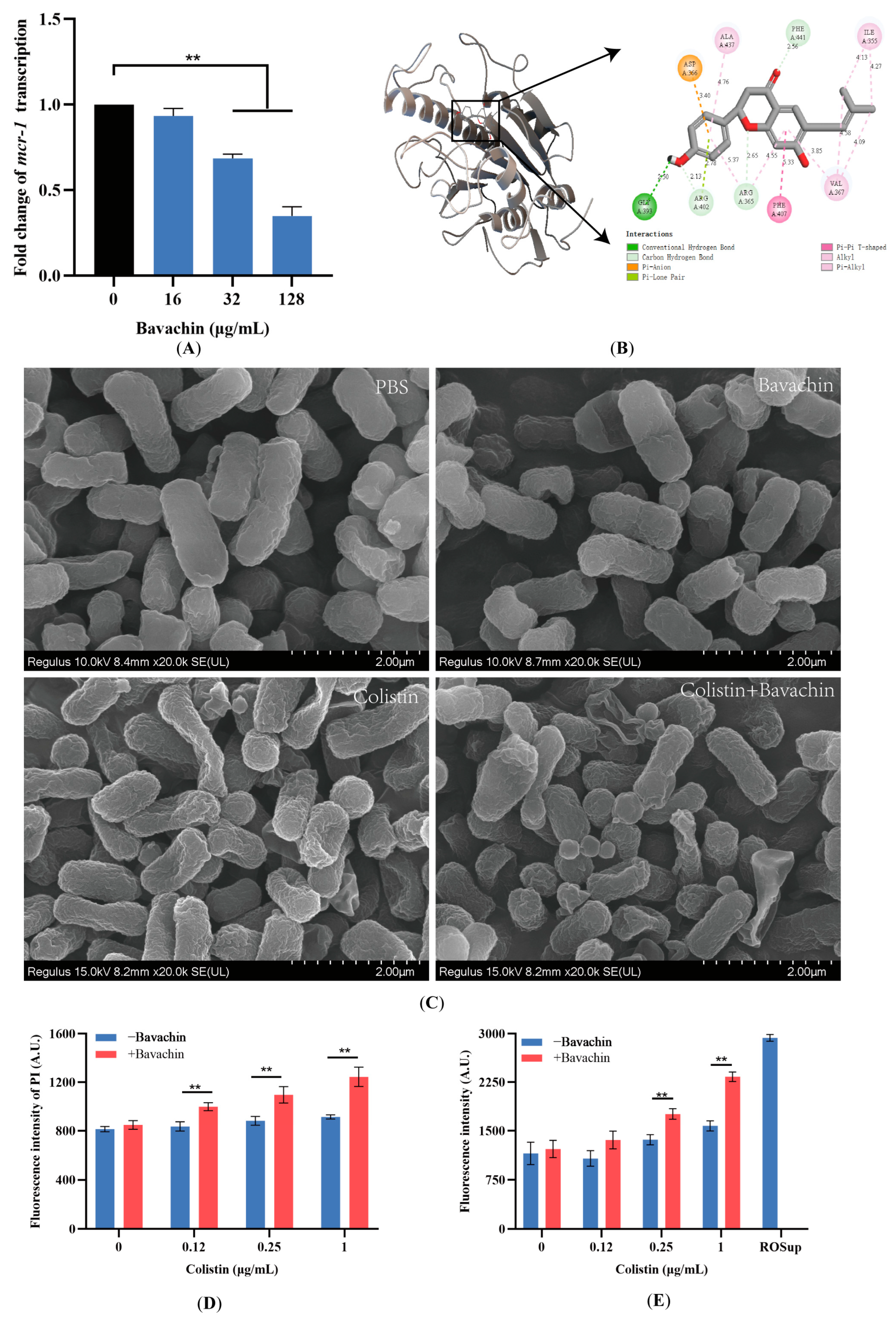
2.4. Bavachin Plays an Important Role in the Function of the MCR Protein
2.5. Bavachin Potentiates the Membrane-Damaging Activity of Colistin
3. Discussion
4. Materials and Methods
4.1. Reagents and Bacterial Strains
4.2. The Antimicrobial Susceptibility Test
4.3. The Hemolytic Activity Assay
4.4. The Time–Kill Assay
4.5. The Biofilm Formation Assay
4.6. Motility Assay
4.7. RNA Isolation and Reverse Transcription (RT)-PCR
4.8. The Molecular Docking Assay
4.9. Scanning Electron Microscopy (SEM)
4.10. The Protein Leakage Assay
4.11. Membrane Permeability Evaluation
4.12. Reactive Oxygen Species (ROS) Measurement
4.13. The Galleria Mellonella Infection Model
4.14. The Mice Infection Model In Vivo
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| E. coli | Escherichia coli |
| GNB | Gram-negative bacteria |
| MDR | Multidrug resistant |
| CM | Cytoplasmic membrane |
| CLSI | Clinical and laboratory standards institute |
| MH | Mueller–Hinton |
| SEM | Scanning electron microscopy |
| FICI | Fractional inhibitory concentration index |
| PBS | Phosphate-buffered saline |
| PI | Propidium iodide |
| SPF | Specified pathogen free |
| MIC | Minimum inhibitory concentration |
| S. typhimurium | Salmonella typhimurium |
| K. pneumoniae | Klebsiella pneumoniae |
References
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Antibiotic Resistance: Moving from Individual Health Norms to Social Norms in One Health and Global Health. Front. Microbiol. 2020, 11, 1914. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L.; Harris, P.N.A. Colistin resistance: A major breach in our last line of defence. Lancet Infect. Dis. 2016, 16, 132–133. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya-Parlakay, A.; Kara, A.; Cengiz, A.B. Increased risk of nephrotoxicity: Side effect of colistin use in paediatric patients. Int. J. Antimicrob. Agents 2015, 45, 327. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Sun, W.; Simeonov, A. Drug repurposing screens and synergistic drug-combinations for infectious diseases. Br. J. Pharmacol. 2018, 175, 181–191. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, Z.; Shi, J.; Li, R.; Upton, M.; Wang, Z. Drug repurposing for next-generation combination therapies against multidrug-resistant bacteria. Theranostics 2021, 11, 4910–4928. [Google Scholar] [CrossRef]
- Wright, G.D. Antibiotic Adjuvants: Rescuing Antibiotics from Resistance. Trends Microbiol. 2016, 24, 862–871. [Google Scholar] [CrossRef]
- Zimmermann, A.; Kainz, K.; Hofer, S.J.; Bauer, M.A.; Schroeder, S.; Dengjel, J.; Pietrocola, F.; Kepp, O.; Ruckenstuhl, C.; Eisenberg, T.; et al. 4,4′Dimethoxychalcone: A natural flavonoid that promotes health through autophagy-dependent and -independent effects. Autophagy 2019, 15, 1662–1664. [Google Scholar] [CrossRef]
- Rathore, D.; McCutchan, T.F.; Sullivan, M.; Kumar, S. Antimalarial drugs: Current status and new developments. Expert Opin. Investig. Drugs 2005, 14, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-S.; Weng, J.-K. Demystifying traditional herbal medicine with modern approach. Nat. Plants 2017, 3, 17109. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.D.; Gupta, A.; Evangelopoulos, D.; Basavannacharya, C.; Pabon, L.C.; Plazas, E.A.; Muñoz, D.R.; Delgado, W.A.; Cuca, L.E.; Ribon, W.; et al. Anti-tubercular screening of natural products from Colombian plants: 3-methoxynordomesticine, an inhibitor of MurE ligase of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2010, 65, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Li, T.; Liu, X.; Hao, Z.; Ding, S.; Panichayupakaranant, P.; Zhu, K.; Shen, J. Plant Natural Flavonoids Against Multidrug Resistant Pathogens. Adv. Sci. 2021, 8, e2100749. [Google Scholar] [CrossRef] [PubMed]
- Wathoni, N.; Rusdin, A.; Motoyama, K.; Joni, I.M.; Lesmana, R.; Muchtaridi, M. Nanoparticle Drug Delivery Systems for α-Mangostin. Nanotechnol. Sci. Appl. 2020, 13, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Q.; Hou, X.; Wang, Y.; Wang, N.; Deng, X.; Wen, Z.; Li, D.; Li, L.; Zhou, Y.; Wang, J. Naringenin Microsphere as a Novel Adjuvant Reverses Colistin Resistance via Various Strategies against Multidrug-Resistant Klebsiella pneumoniae Infection. J. Agric. Food Chem. 2022, 70, 16201–16217. [Google Scholar] [CrossRef]
- Yao, Z.; Feng, L.; Zhao, Y.; Zhang, X.; Chen, L.; Wang, L.; Zhang, Y.; Sun, Y.; Zhou, T.; Cao, J. Thymol Increases Sensitivity of Clinical Col-R Gram-Negative Bacteria to Colistin. Microbiol. Spectr. 2022, 10, e0018422. [Google Scholar] [CrossRef]
- Wei, X.; Lin, L.; Yuan, Q.-Q.; Wang, X.-Y.; Zhang, Q.; Zhang, X.-M.; Tang, K.-C.; Guo, M.-Y.; Dong, T.-Y.; Han, W.; et al. Bavachin protects against diet-induced hepatic steatosis and obesity in mice. Acta Pharmacol. Sin. 2023, 44, 1416–1428. [Google Scholar] [CrossRef]
- Zhang, C.; Qian, D.-D.; Yu, T.; Yang, H.; Li, P.; Li, H.-J. Multi-parametric cellular imaging coupled with multi-component quantitative profiling for screening of hepatotoxic equivalent markers from Psoraleae fructus. Phytomedicine Int. J. Phytother. Phytopharm. 2021, 93, 153518. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Wang, Q.; Ren, Y.; Wang, Q.; Li, Z. Bavachin exerted anti-neuroinflammatory effects by regulation of A20 ubiquitin-editing complex. Int. Immunopharmacol. 2021, 100, 108085. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lim, W.; Song, G. Bavachin suppresses human placental choriocarcinoma cells by targeting electron transport chain complexes and mitochondrial dysfunction. Free Radic. Biol. Med. 2020, 156, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tang, Z.; Wang, M.; Shi, J.; Lin, Y.; Sun, T.; Zou, Z.; Weng, Z. Exploration of Antimicrobial Ingredients in Psoralea corylifolia L. Seed and Related Mechanism against Methicillin-Resistant Staphylococcus aureus. Molecules 2022, 27, 6952. [Google Scholar] [CrossRef]
- Jin, Y.-H.; Kim, D.E.; Jang, M.S.; Min, J.S.; Kwon, S. Bavachin produces immunoadjuvant activity by targeting the NFAT signaling pathway. Phytomedicine Int. J. Phytother. Phytopharm. 2021, 93, 153796. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2021. [Google Scholar]
- Li, J.; Zhang, X.; Han, N.; Wan, P.; Zhao, F.; Xu, T.; Peng, X.; Xiong, W.; Zeng, Z. Mechanism of Action of Isopropoxy Benzene Guanidine against Multidrug-Resistant Pathogens. Microbiol. Spectr. 2023, 11, e0346922. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, M.; Pan, J.; Shen, X.; Liu, W.; Zhang, X.; Li, H.; Deng, X. Quercetin impairs Streptococcus pneumoniae biofilm formation by inhibiting sortase A activity. J. Cell. Mol. Med. 2018, 22, 6228–6237. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Bassler, B.L. Surviving as a Community: Antibiotic Tolerance and Persistence in Bacterial Biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef]
- Harms, A.; Maisonneuve, E.; Gerdes, K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 2016, 354, aaf4268. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Barken, K.B.; Pamp, S.J.; Yang, L.; Gjermansen, M.; Bertrand, J.J.; Klausen, M.; Givskov, M.; Whitchurch, C.B.; Engel, J.N.; Tolker-Nielsen, T. Roles of type IV pili, flagellum-mediated motility and extracellular DNA in the formation of mature multicellular structures in Pseudomonas aeruginosa biofilms. Environ. Microbiol. 2008, 10, 2331–2343. [Google Scholar] [CrossRef] [PubMed]
- Shui, J.; Wang, H.; Tao, X.; Min, C.; Li, J.; Zou, M. Relationship of biofilm-forming ability of with swimming motility, twitching motility and virulence gene distribution. Zhejiang Da Xue Xue Bao Yi Xue Ban J. Zhejiang Univ. Med. Sci. 2021, 50, 345–351. [Google Scholar]
- Yahav, D.; Tau, N.; Shepshelovich, D. Assessment of Data Supporting the Efficacy of New Antibiotics for Treating Infections Caused by Multidrug-resistant Bacteria. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 72, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; van Dorp, L.; Shaw, L.P.; Bradley, P.; Wang, Q.; Wang, X.; Jin, L.; Zhang, Q.; Liu, Y.; Rieux, A.; et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun. 2018, 9, 1179. [Google Scholar] [CrossRef] [PubMed]
- Taglialegna, A. Heat up the antibiotics. Nat. Rev. Microbiol. 2023, 21, 277. [Google Scholar] [CrossRef]
- Veitch, N.C.; Grayer, R.J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 2011, 28, 1626–1695. [Google Scholar] [CrossRef] [PubMed]
- Wedick, N.M.; Pan, A.; Cassidy, A.; Rimm, E.B.; Sampson, L.; Rosner, B.; Willett, W.; Hu, F.B.; Sun, Q.; van Dam, R.M. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012, 95, 925–933. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Kiyama, R. Estrogenic flavonoids and their molecular mechanisms of action. J. Nutr. Biochem. 2023, 114, 109250. [Google Scholar] [CrossRef]
- Vissenaekens, H.; Criel, H.; Grootaert, C.; Raes, K.; Smagghe, G.; Van Camp, J. Flavonoids and cellular stress: A complex interplay affecting human health. Crit. Rev. Food Sci. Nutr. 2022, 62, 8535–8566. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, Q.; Zhou, X.; Wang, X.; Li, H.; Zhang, W.; Yuan, H.; Sun, C. Flavonoids regulate tumor-associated macrophages—From structure-activity relationship to clinical potential (Review). Pharmacol. Res. 2022, 184, 106419. [Google Scholar] [CrossRef]
- Yi, Y.-S. Regulatory Roles of Flavonoids on Inflammasome Activation during Inflammatory Responses. Mol. Nutr. Food Res. 2018, 62, e1800147. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Sun, D.; Ren, X.; Zhao, Y.; Zhang, H.; Jiang, T.; Guan, J.; Tang, Y.; Song, W.; Li, S.; et al. Bavachin Suppresses Alpha-Hemolysin Expression and Protects Mice from Pneumonia Infection by Staphylococcus aureus. J. Microbiol. Biotechnol. 2022, 32, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Venter, H.; Veltman, T.; Williams, R.; O′Donovan, L.A.; Russell, C.C.; McCluskey, A.; Page, S.W.; Ogunniyi, A.D.; Trott, D.J. In vitro synergistic activity of NCL195 in combination with colistin against Gram-negative bacterial pathogens. Int. J. Antimicrob. Agents 2021, 57, 106323. [Google Scholar] [CrossRef]
- Nemeth, A.M.; Basak, A.K.; Weig, A.W.; Marrujo, S.A.; Barker, W.T.; Jania, L.A.; Hendricks, T.A.; Sullivan, A.E.; O′Connor, P.M.; Melander, R.J.; et al. Structure-Function Studies on IMD-0354 Identifies Highly Active Colistin Adjuvants. ChemMedChem 2020, 15, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Si, Z.; Zheng, W.; Prananty, D.; Li, J.; Koh, C.H.; Kang, E.-T.; Pethe, K.; Chan-Park, M.B. Polymers as advanced antibacterial and antibiofilm agents for direct and combination therapies. Chem. Sci. 2022, 13, 345–364. [Google Scholar] [CrossRef]
- Fivenson, E.M.; Bernhardt, T.G. An Essential Membrane Protein Modulates the Proteolysis of LpxC to Control Lipopolysaccharide Synthesis in Escherichia coli. MBio 2020, 11, 10–1128. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Xue, K.; Xiao, M.; Wu, K.; Lv, S.; Hao, B.; Zhu, C. Polarity-Sensitive Fluorescent Probe for Reflecting the Packing Degree of Bacterial Membrane Lipids. Anal. Chem. 2022, 94, 3303–3312. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Zhang, Y.-B.; Chen, Z.-J.; Zhang, Y.-T.; Yang, X.-W. Plasma pharmacokinetics and cerebral nuclei distribution of major constituents of Psoraleae fructus in rats after oral administration. Phytomedicine Int. J. Phytother. Phytopharm. 2018, 38, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, C.; Xu, J.; Qin, Z.; Li, S.; Hu, L.; Yao, Z.; Gonzalez, F.J.; Yao, X. Characterization of metabolic activity, isozyme contribution and species differences of bavachin, and identification of efflux transporters for bavachin-O-glucuronide in HeLa1A1 cells. J. Pharm. Pharmacol. 2020, 72, 1771–1786. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Han, N.; He, Z.; Dai, X.; Zhao, F.; Li, Y.; Xiong, W.; Zeng, Z. Bavachin Rejuvenates Sensitivity of Colistin against Colistin-Resistant Gram-Negative Bacteria. Int. J. Mol. Sci. 2024, 25, 2349. https://doi.org/10.3390/ijms25042349
Li J, Han N, He Z, Dai X, Zhao F, Li Y, Xiong W, Zeng Z. Bavachin Rejuvenates Sensitivity of Colistin against Colistin-Resistant Gram-Negative Bacteria. International Journal of Molecular Sciences. 2024; 25(4):2349. https://doi.org/10.3390/ijms25042349
Chicago/Turabian StyleLi, Jie, Ning Han, Zhengyuan He, Xiaolan Dai, Feifei Zhao, Yangyang Li, Wenguang Xiong, and Zhenling Zeng. 2024. "Bavachin Rejuvenates Sensitivity of Colistin against Colistin-Resistant Gram-Negative Bacteria" International Journal of Molecular Sciences 25, no. 4: 2349. https://doi.org/10.3390/ijms25042349
APA StyleLi, J., Han, N., He, Z., Dai, X., Zhao, F., Li, Y., Xiong, W., & Zeng, Z. (2024). Bavachin Rejuvenates Sensitivity of Colistin against Colistin-Resistant Gram-Negative Bacteria. International Journal of Molecular Sciences, 25(4), 2349. https://doi.org/10.3390/ijms25042349



