Gold Nanoparticles in Neurological Diseases: A Review of Neuroprotection
Abstract
:1. Introduction
1.1. The Role of Neuroinflammation in Neurodegenerative Diseases and Stroke
1.2. Properties and Applications of AuNPs for Biomedical Applications
1.3. Effect of Size and Charge of AuNPs or Surface Modification of AuNPs in Neuroprotection
2. Mechanisms of Neuroprotection and Anti-Neuro-Inflammatory Effects of AuNPs
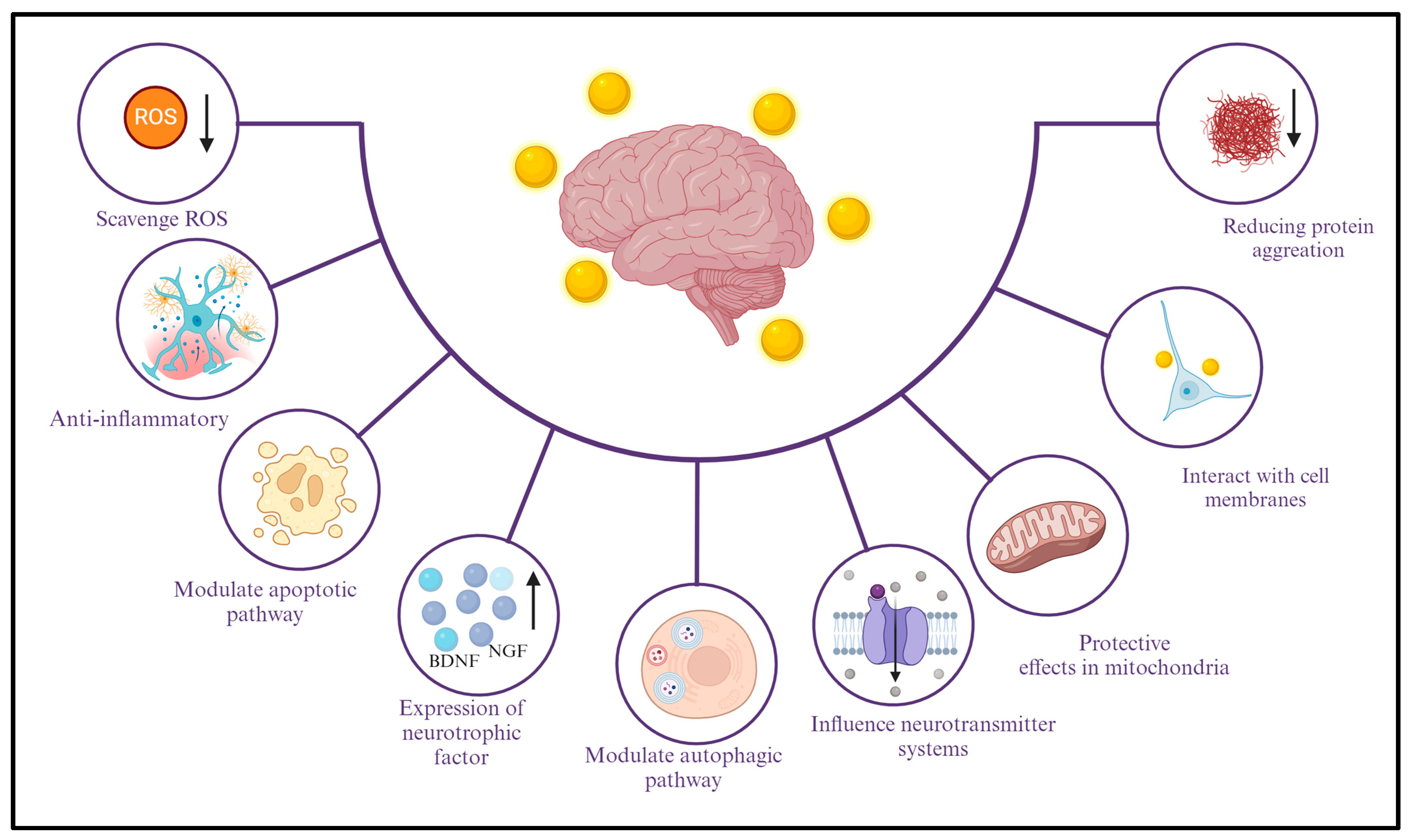
2.1. Explanation of the Different Mechanisms through Which AuNPs Exhibit Neuroprotective Effects
2.2. Anti-Inflammatory Properties of AuNPs
3. Application of AuNPs in AD, PD, and Stroke
3.1. Potential Use of AuNPs in the Treatment of AD
3.2. Prospects for the Application of AuNPs in the Treatment of PD
3.3. Potential of AuNPs for Neuroprotection after Stroke
4. Cellular and Animal Research
4.1. Experimental Results in Cellular Models
4.2. Experimental Results in Animal Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Villani, E.R.; Marzetti, E. Molecular Signals and Genetic Regulations of Neurological Disorders. Int. J. Mol. Sci. 2023, 24, 5902. [Google Scholar] [CrossRef]
- Yen, C.; Lin, C.L.; Chiang, M.C. Exploring the Frontiers of Neuroimaging: A Review of Recent Advances in Understanding Brain Functioning and Disorders. Life 2023, 13, 1472. [Google Scholar] [CrossRef] [PubMed]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar]
- Isik, S.; Yeman Kiyak, B.; Akbayir, R.; Seyhali, R.; Arpaci, T. Microglia Mediated Neuroinflammation in Parkinson’s Disease. Cells 2023, 12, 1012. [Google Scholar] [CrossRef]
- Zeng, J.; Bao, T.; Yang, K.; Zhu, X.; Wang, S.; Xiang, W.; Ge, A.; Zeng, L.; Ge, J. The mechanism of microglia-mediated immune inflammation in ischemic stroke and the role of natural botanical components in regulating microglia: A review. Front. Immunol. 2022, 13, 1047550. [Google Scholar] [CrossRef]
- Tarnawski, L.; Olofsson, P.S. Inflammation neuroscience: Neuro-immune crosstalk and interfaces. Clin. Transl. Immunol. 2021, 10, e1352. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef]
- Megha, K.B.; Joseph, X.; Akhil, V.; Mohanan, P.V. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine 2021, 91, 153712. [Google Scholar] [CrossRef]
- Chiang, M.C.; Tsai, T.Y.; Wang, C.J. The Potential Benefits of Quercetin for Brain Health: A Review of Anti-Inflammatory and Neuroprotective Mechanisms. Int. J. Mol. Sci. 2023, 24, 6328. [Google Scholar] [CrossRef]
- Vashist, A.; Manickam, P.; Raymond, A.D.; Arias, A.Y.; Kolishetti, N.; Vashist, A.; Arias, E.; Nair, M. Recent Advances in Nanotherapeutics for Neurological Disorders. ACS Appl. Bio Mater. 2023, 6, 2614–2621. [Google Scholar] [CrossRef] [PubMed]
- Aili, M.; Zhou, K.; Zhan, J.; Zheng, H.; Luo, F. Anti-inflammatory role of gold nanoparticles in the prevention and treatment of Alzheimer’s disease. J. Mater. Chem. B 2023, 11, 8605–8621. [Google Scholar] [CrossRef]
- Xue, J.; Liu, T.; Liu, Y.; Jiang, Y.; Seshadri, V.D.D.; Mohan, S.K.; Ling, L. Neuroprotective effect of biosynthesised gold nanoparticles synthesised from root extract of Paeonia moutan against Parkinson disease—In vitro &In vivo model. J. Photochem. Photobiol. B 2019, 200, 111635. [Google Scholar]
- Salatin, S.; Farhoudi, M.; Farjami, A.; Maleki Dizaj, S.; Sharifi, S.; Shahi, S. Nanoparticle Formulations of Antioxidants for the Management of Oxidative Stress in Stroke: A Review. Biomedicines 2023, 11, 3010. [Google Scholar] [CrossRef] [PubMed]
- Milan, J.; Niemczyk, K.; Kus-Liskiewicz, M. Treasure on the Earth—Gold Nanoparticles and Their Biomedical Applications. Materials 2022, 15, 3355. [Google Scholar] [CrossRef]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef]
- Nguyen, N.H.A.; Falagan-Lotsch, P. Mechanistic Insights into the Biological Effects of Engineered Nanomaterials: A Focus on Gold Nanoparticles. Int. J. Mol. Sci. 2023, 24, 4109. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, J.Q.; Ashby, C.R., Jr.; Zeng, L.; Fan, Y.F.; Chen, Z.S. Gold nanoparticles: Synthesis, physiochemical properties and therapeutic applications in cancer. Drug Discov. Today 2021, 26, 1284–1292. [Google Scholar] [CrossRef]
- Sarfraz, N.; Khan, I. Plasmonic Gold Nanoparticles (AuNPs): Properties, Synthesis and their Advanced Energy, Environmental and Biomedical Applications. Chem. Asian J. 2021, 16, 720–742. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef]
- Okoampah, E.; Mao, Y.; Yang, S.; Sun, S.; Zhou, C. Gold nanoparticles-biomembrane interactions: From fundamental to simulation. Colloids Surf. B Biointerfaces 2020, 196, 111312. [Google Scholar] [CrossRef]
- Mulens-Arias, V.; Balfourier, A.; Nicolas-Boluda, A.; Carn, F.; Gazeau, F. Endocytosis-driven gold nanoparticle fractal rearrangement in cells and its influence on photothermal conversion. Nanoscale 2020, 12, 21832–21849. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Q. Protein-gold nanoparticle interactions and their possible impact on biomedical applications. Acta Biomater. 2017, 55, 13–27. [Google Scholar] [CrossRef]
- Graczyk, A.; Pawlowska, R.; Jedrzejczyk, D.; Chworos, A. Gold Nanoparticles in Conjunction with Nucleic Acids as a Modern Molecular System for Cellular Delivery. Molecules 2020, 25, 204. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M.; Jomaa, A.A.; Kamoun, M. Gold nanoparticles: Synthesis properties and applications. J. King Saud. Univ. Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Bano, A.; Dawood, A.; Rida Saira, F.; Malik, A.; Alkholief, M.; Ahmad, H.; Khan, M.A.; Ahmad, Z.; Bazighifan, O. Enhancing catalytic activity of gold nanoparticles in a standard redox reaction by investigating the impact of AuNPs size, temperature and reductant concentrations. Sci. Rep. 2023, 13, 12359. [Google Scholar] [CrossRef]
- Sibuyi, N.R.S.; Moabelo, K.L.; Fadaka, A.O.; Meyer, S.; Onani, M.O.; Madiehe, A.M.; Meyer, M. Multifunctional Gold Nanoparticles for Improved Diagnostic and Therapeutic Applications: A Review. Nanoscale Res. Lett. 2021, 16, 174. [Google Scholar] [CrossRef]
- Kus-Liskiewicz, M.; Fickers, P.; Ben Tahar, I. Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations. Int. J. Mol. Sci. 2021, 22, 10952. [Google Scholar] [CrossRef]
- Zhang, J.; Mou, L.; Jiang, X. Surface chemistry of gold nanoparticles for health-related applications. Chem. Sci. 2020, 11, 923–936. [Google Scholar] [CrossRef]
- Huang, H.; Liu, R.; Yang, J.; Dai, J.; Fan, S.; Pi, J.; Wei, Y.; Guo, X. Gold Nanoparticles: Construction for Drug Delivery and Application in Cancer Immunotherapy. Pharmaceutics 2023, 15, 1868. [Google Scholar] [CrossRef]
- Khongkow, M.; Yata, T.; Boonrungsiman, S.; Ruktanonchai, U.R.; Graham, D.; Namdee, K. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood-brain barrier penetration. Sci. Rep. 2019, 9, 8278. [Google Scholar] [CrossRef]
- Zhang, R.; Kiessling, F.; Lammers, T.; Pallares, R.M. Clinical translation of gold nanoparticles. Drug Deliv. Transl. Res. 2023, 13, 378–385. [Google Scholar] [CrossRef]
- Talarska, P.; Boruczkowski, M.; Zurawski, J. Current Knowledge of Silver and Gold Nanoparticles in Laboratory Research-Application, Toxicity, Cellular Uptake. Nanomaterials 2021, 11, 2454. [Google Scholar] [CrossRef]
- Kumar, P.A.-O.; Lim, D.K. Gold-Polymer Nanocomposites for Future Therapeutic and Tissue Engineering Applications. Pharmaceutics 2022, 14, 70. [Google Scholar] [CrossRef]
- Koushki, K.; Keshavarz Shahbaz, S.; Keshavarz, M.; Bezsonov, E.E.; Sathyapalan, T.; Sahebkar, A. Gold Nanoparticles: Multifaceted Roles in the Management of Autoimmune Disorders. Biomolecules 2021, 11, 1289. [Google Scholar] [CrossRef]
- Kumar, P.P.P.; Mahajan, R. Gold Polymer Nanomaterials: A Promising Approach for Enhanced Biomolecular Imaging. Nanotheranostics 2024, 8, 64–89. [Google Scholar] [CrossRef]
- Porras, J.C.; Bernuz, M.; Marfa, J.; Pallares-Rusinol, A.; Marti, M.; Pividori, M.I. Comparative Study of Gold and Carbon Nanoparticles in Nucleic Acid Lateral Flow Assay. Nanomaterials 2021, 11, 741. [Google Scholar] [CrossRef]
- Mahor, A.; Singh, P.P.; Bharadwaj, P.; Sharma, N.; Yadav, S.; Rosenholm, J.M.; Bansal, K.K. Carbon-Based Nanomaterials for Delivery of Biologicals and Therapeutics: A Cutting-Edge Technology. C 2021, 7, 19. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Perez-Aranda, M.; Martinez, G.; Merinero, M.; Arguelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Raman, S.; Mahmood, S.; Hilles, A.R.; Javed, M.N.; Azmana, M.; Al-Japairai, K.A.S. Polymeric Nanoparticles for Brain Drug Delivery—A Review. Curr. Drug Metab. 2020, 21, 649–660. [Google Scholar] [CrossRef]
- Navarro Martínez, N.; Toledo Hernández, J.; Morales, J.O. Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach. Nanotechnol. Rev. 2023, 12, 20220548. [Google Scholar] [CrossRef]
- Ohta, S.; Kikuchi, E.; Ishijima, A.; Azuma, T.; Sakuma, I.; Ito, T. Investigating the optimum size of nanoparticles for their delivery into the brain assisted by focused ultrasound-induced blood-brain barrier opening. Sci. Rep. 2020, 10, 18220. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: A review. J. Nanopart Res. 2023, 25, 43. [Google Scholar] [CrossRef]
- Ferrari, E. Gold Nanoparticle-Based Plasmonic Biosensors. Biosensors 2023, 13, 411. [Google Scholar] [CrossRef]
- Reznickova, A.; Slavikova, N.; Kolska, Z.; Kolarova, K.; Belinova, T.; Hubalek Kalbacova, M.; Cieslar, M.; Svorcik, V. PEGylated gold nanoparticles: Stability, cytotoxicity and antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 26–34. [Google Scholar] [CrossRef]
- Del Amo, L.; Cano, A.; Ettcheto, M.; Souto, E.B.; Espina, M.; Camins, A.; García, M.L.; Sánchez-López, E. Surface Functionalization of PLGA Nanoparticles to Increase Transport across the BBB for Alzheimer’s Disease. Appl. Sci. 2021, 11, 4305. [Google Scholar] [CrossRef]
- Sartaj, A.; Qamar, Z.; Md, S.; Alhakamy, N.A.; Baboota, S.; Ali, J. An Insight to Brain Targeting Utilizing Polymeric Nanoparticles: Effective Treatment Modalities for Neurological Disorders and Brain Tumor. Front. Bioeng. Biotechnol. 2022, 10, 788128. [Google Scholar]
- Papastefanaki, F.; Jakovcevski, I.; Poulia, N.; Djogo, N.; Schulz, F.; Martinovic, T.; Ciric, D.; Loers, G.; Vossmeyer, T.; Weller, H.; et al. Intraspinal Delivery of Polyethylene Glycol-coated Gold Nanoparticles Promotes Functional Recovery After Spinal Cord Injury. Mol. Ther. 2015, 23, 993–1002. [Google Scholar] [CrossRef]
- Chiang, M.C.; Nicol, C.J.B.; Lin, C.H.; Chen, S.J.; Yen, C.; Huang, R.N. Nanogold induces anti-inflammation against oxidative stress induced in human neural stem cells exposed to amyloid-beta peptide. Neurochem. Int. 2021, 145, 104992. [Google Scholar] [CrossRef]
- Mihailovic, V.; Katanic Stankovic, J.S.; Selakovic, D.; Rosic, G. An Overview of the Beneficial Role of Antioxidants in the Treatment of Nanoparticle-Induced Toxicities. Oxid. Med. Cell Longev. 2021, 2021, 7244677. [Google Scholar] [CrossRef]
- Chiang, M.C.; Nicol, C.J.B. GSH-AuNP anti-oxidative stress, ER stress and mitochondrial dysfunction in amyloid-beta peptide-treated human neural stem cells. Free Radic. Biol. Med. 2022, 187, 185–201. [Google Scholar] [CrossRef]
- Alotaibi, B.S.; Buabeid, M.; Ibrahim, N.A.; Kharaba, Z.J.; Ijaz, M.; Noreen, S.; Murtaza, G. Potential of Nanocarrier-Based Drug Delivery Systems for Brain Targeting: A Current Review of Literature. Int. J. Nanomed. 2021, 16, 7517–7533. [Google Scholar] [CrossRef]
- Kumar, P.P.; Lim, D.-K. Photothermal Effect of Gold Nanoparticles as a Nanomedicine for Diagnosis and Therapeutics. Pharmaceutics 2023, 15, 2349. [Google Scholar] [CrossRef]
- Vinod, C.; Jena, S. Nano-Neurotheranostics: Impact of Nanoparticles on Neural Dysfunctions and Strategies to Reduce Toxicity for Improved Efficacy. Front. Pharmacol. 2021, 12, 612692. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, R.; Mishra, J.; Dutta, K.; Ahlawat, P.; Kumar, A.; Dhanasekaran, S.; Gupta, A.K.; Sinha, S.; Bishi, D.K.; et al. Strategies facilitating the permeation of nanoparticles through blood-brain barrier: An insight towards the development of brain-targeted drug delivery system. J. Drug Deliv. Sci. Technol. 2023, 86, 104694. [Google Scholar] [CrossRef]
- Nayak, V.; Patra, S.; Rout, S.; Jena, A.B.; Sharma, R.; Pattanaik, K.P.; Singh, J.; Pandey, S.S.; Singh, R.P.; Majhi, S.; et al. Regulation of neuroinflammation in Alzheimer’s disease via nanoparticle-loaded phytocompounds with anti-inflammatory and autophagy-inducing properties. Phytomedicine 2024, 122, 155150. [Google Scholar] [CrossRef]
- Kumar, M.; Kulkarni, P.; Liu, S.; Chemuturi, N.; Shah, D.K. Nanoparticle biodistribution coefficients: A quantitative approach for understanding the tissue distribution of nanoparticles. Adv. Drug Deliv. Rev. 2023, 194, 114708. [Google Scholar] [CrossRef]
- Khan, M.A.R.; Al Mamun, M.S.; Habib, M.A.; Islam, A.B.M.N.; Mahiuddin, M.; Karim, K.M.R.; Naime, J.; Saha, P.; Dey, S.K.; Ara, M.H. A review on gold nanoparticles: Biological synthesis, characterizations, and analytical applications. Results Chem. 2022, 4, 100478. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J. Funct. Biomater. 2021, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.M.; Bhaskar, S.; Cheerala, V.S.K.; Battampara, P.; Reddy, R.; Neelakantan, S.C.; Reddy, N.; Ramamurthy, S.S. Review of Gold Nanoparticles in Surface Plasmon-Coupled Emission Technology: Effect of Shape, Hollow Nanostructures, Nano-Assembly, Metal-Dielectric and Heterometallic Nanohybrids. Nanomaterials 2024, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Aguirre, C.; Morales, F.; Gallardo-Toledo, E.; Guerrero, S.; Giralt, E.; Araya, E.; Kogan, M.J. Peptides and proteins used to enhance gold nanoparticle delivery to the brain: Preclinical approaches. Int. J. Nanomed. 2015, 10, 4919–4936. [Google Scholar]
- Scarpa, E.; Cascione, M.; Griego, A.; Pellegrino, P.; Moschetti, G.; De Matteis, V. Gold and silver nanoparticles in Alzheimer’s and Parkinson’s diagnostics and treatments. Ibrain 2023, 9, 298–315. [Google Scholar] [CrossRef]
- Abdelkader, N.F.; El-Batal, A.I.; Amin, Y.M.; Hawas, A.M.; Hassan, S.H.M.; Eid, N.I. Neuroprotective Effect of Gold Nanoparticles and Alpha-Lipoic Acid Mixture against Radiation-Induced Brain Damage in Rats. Int. J. Mol. Sci. 2022, 23, 9640. [Google Scholar] [CrossRef]
- Chiang, M.C.; Nicol, C.J.B.; Cheng, Y.C.; Yen, C.; Lin, C.H.; Chen, S.J.; Huang, R.N. Nanogold Neuroprotection in Human Neural Stem Cells Against Amyloid-beta-induced Mitochondrial Dysfunction. Neuroscience 2020, 435, 44–57. [Google Scholar] [CrossRef]
- da Silva, L.E.; Abel, J.S.; Tartari, G.; da Silva, M.R.; de Oliveira, M.P.; Vedova, L.M.D.; Mendes, T.F.; Mendes, R.L.; Soares, H.J.; Vernke, C.N.; et al. Combination of Gold Nanoparticles with Carnitine Attenuates Brain Damage in an Obesity Animal Model. Mol. Neurobiol. 2024. [Google Scholar] [CrossRef]
- Silveira, P.C.L.; Rodrigues, M.S.; Gelain, D.P.; de Oliveira, J. Gold nanoparticles application to the treatment of brain dysfunctions related to metabolic diseases: Evidence from experimental studies. Metab. Brain Dis. 2023, 38, 123–135. [Google Scholar] [CrossRef]
- Jiang, Y.; Kang, Y.; Liu, J.; Yin, S.; Huang, Z.; Shao, L. Nanomaterials alleviating redox stress in neurological diseases: Mechanisms and applications. J. Nanobiotechnol. 2022, 20, 265. [Google Scholar] [CrossRef]
- Ke, S.; Zhou, T.; Yang, P.; Wang, Y.; Zhang, P.; Chen, K.; Ren, L.; Ye, S. Gold nanoparticles enhance TRAIL sensitivity through Drp1-mediated apoptotic and autophagic mitochondrial fission in NSCLC cells. Int. J. Nanomed. 2017, 12, 2531–2551. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.H.; Ryu, N.E.; Lim, D.J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef]
- Kesharwani, P.; Ma, R.; Sang, L.; Fatima, M.; Sheikh, A.; Abourehab, M.A.S.; Gupta, N.; Chen, Z.S.; Zhou, Y. Gold nanoparticles and gold nanorods in the landscape of cancer therapy. Mol. Cancer 2023, 22, 98. [Google Scholar] [CrossRef]
- Razavi, S.; Seyedebrahimi, R.; Jahromi, M. Biodelivery of nerve growth factor and gold nanoparticles encapsulated in chitosan nanoparticles for schwann-like cells differentiation of human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2019, 513, 681–687. [Google Scholar] [CrossRef]
- Paviolo, C.; Stoddart, P.R. Gold Nanoparticles for Modulating Neuronal Behavior. Nanomaterials 2017, 7, 92. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Mukherjee, S.; Das, S.; Bhat, F.A.; Raja Singh, P.; Patra, C.R.; Arunakaran, J. Gold nanoparticles-conjugated quercetin induces apoptosis via inhibition of EGFR/PI3K/Akt-mediated pathway in breast cancer cell lines (MCF-7 and MDA-MB-231). Cell Biochem. Funct. 2017, 35, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Saha, D.; Chadha, T.S.; Raman, B.; Biswas, P. Non-invasive aerosol delivery and transport of gold nanoparticles to the brain. Sci. Rep. 2017, 7, 44718. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, V.; Mekky, G.; van der Meer, S.B.; Seeds, M.C.; Atala, A.J.; Epple, M. Transport of ultrasmall gold nanoparticles (2 nm) across the blood-brain barrier in a six-cell brain spheroid model. Sci. Rep. 2020, 10, 18033. [Google Scholar] [CrossRef] [PubMed]
- Masoudi Asil, S.; Ahlawat, J.; Guillama Barroso, G.; Narayan, M. Nanomaterial based drug delivery systems for the treatment of neurodegenerative diseases. Biomater. Sci. 2020, 8, 4109–4128. [Google Scholar] [CrossRef] [PubMed]
- Morfill, C.; Pankratova, S.; Machado, P.; Fernando, N.K.; Regoutz, A.; Talamona, F.; Pinna, A.; Klosowski, M.; Wilkinson, R.J.; Fleck, R.A.; et al. Nanostars Carrying Multifunctional Neurotrophic Dendrimers Protect Neurons in Preclinical In Vitro Models of Neurodegenerative Disorders. ACS Appl. Mater. Interfaces 2022, 14, 47445–47460. [Google Scholar] [CrossRef]
- Spinelli, A.; Girelli, M.; Arosio, D.; Polito, L.; Podini, P.; Martino, G.; Seneci, P.; Muzio, L.; Menegon, A. Intracisternal delivery of PEG-coated gold nanoparticles results in high brain penetrance and long-lasting stability. J. Nanobiotechnol. 2019, 17, 49. [Google Scholar] [CrossRef]
- Kim, M.J.; Rehman, S.U.; Amin, F.U.; Kim, M.O. Enhanced neuroprotection of anthocyanin-loaded PEG-gold nanoparticles against Abeta(1-42)-induced neuroinflammation and neurodegeneration via the NF-(K)B/JNK/GSK3beta signaling pathway. Nanomedicine 2017, 13, 2533–2544. [Google Scholar] [CrossRef]
- Di Bella, D.; Ferreira, J.P.S.; Silva, R.N.O.; Echem, C.; Milan, A.; Akamine, E.H.; Carvalho, M.H.; Rodrigues, S.F. Gold nanoparticles reduce inflammation in cerebral microvessels of mice with sepsis. J. Nanobiotechnol. 2021, 19, 52. [Google Scholar] [CrossRef]
- Farhana, A.; Alsrhani, A.; Rasheed, N.; Rasheed, Z. Gold nanoparticles attenuate the interferon-gamma induced SOCS1 expression and activation of NF-κB p65/50 activity via modulation of microRNA-155-5p in triple-negative breast cancer cells. Front. Immunol. 2023, 14, 1228458. [Google Scholar] [CrossRef]
- Silveira, G.B.; Muller, A.P.; Machado-de-Avila, R.A.; Silveira, P.C.L. Advance in the use of gold nanoparticles in the treatment of neurodegenerative diseases: New perspectives. Neural Regen. Res. 2021, 16, 2425–2426. [Google Scholar] [CrossRef]
- Puranik, N.; Yadav, D.; Song, M. Advancements in the Application of Nanomedicine in Alzheimer’s Disease: A Therapeutic Perspective. Int. J. Mol. Sci. 2023, 24, 14044. [Google Scholar]
- Zhao, L.; Lan, T.; Jiang, G.; Yan, B. Protective effect of the gold nanoparticles green synthesized by Calendula officinalis L. extract on cerebral ischemia stroke-reperfusion injury in rats: A preclinical trial study. Inorg. Chem. Commun. 2022, 141, 109486. [Google Scholar] [CrossRef]
- Nayab, D.E.; Din, F.U.; Ali, H.; Kausar, W.A.; Urooj, S.; Zafar, M.; Khan, I.; Shabbir, K.; Khan, G.M. Nano biomaterials based strategies for enhanced brain targeting in the treatment of neurodegenerative diseases: An up-to-date perspective. J. Nanobiotechnol. 2023, 21, 477. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.C.; Wang, S.J.; Hsiao, C.Y.; Hung, C.T.; Hsu, Y.J.; Chang, D.C.; Hung, C.F. Pharmacological Role of Functionalized Gold Nanoparticles in Disease Applications. Molecules 2022, 27, 1551. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Shen, X.; Song, X.; Wang, N.; Wo, X.; Gao, Y. Protective mechanism of gold nanoparticles on human neural stem cells injured by beta-amyloid protein through miR-21-5p/SOCS6 pathway. Neurotoxicology 2023, 95, 12–22. [Google Scholar] [CrossRef]
- Hou, K.; Zhao, J.; Wang, H.; Li, B.; Li, K.; Shi, X.; Wan, K.; Ai, J.; Lv, J.; Wang, D.; et al. Chiral gold nanoparticles enantioselectively rescue memory deficits in a mouse model of Alzheimer’s disease. Nat. Commun. 2020, 11, 4790. [Google Scholar] [CrossRef]
- Anand, B.G.; Wu, Q.; Karthivashan, G.; Shejale, K.P.; Amidian, S.; Wille, H.; Kar, S. Mimosine functionalized gold nanoparticles (Mimo-AuNPs) suppress beta-amyloid aggregation and neuronal toxicity. Bioact. Mater. 2021, 6, 4491–4505. [Google Scholar]
- Zhao, J.; Xu, N.; Yang, X.; Ling, G.; Zhang, P. The roles of gold nanoparticles in the detection of amyloid-β peptide for Alzheimer’s disease. Colloid. Interface Sci. Commun. 2022, 46, 100579. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Jia, Z.; Yuan, X.; Liu, J. Electrostatic assembly of gold nanoparticle and metal-organic framework nanoparticles attenuates amyloid beta aggregate-mediated neurotoxicity. J. Mater. Chem. B 2023, 11, 4453–4463. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhou, Y.; Dong, H.; Xu, M.; Zhang, J.; Yan, M. Ultrasensitive electrochemical detection of amyloid-beta oligomers using double amplification strategy by MXene substrate and covalent organic framework-based probe. Talanta 2024, 266, 125134. [Google Scholar] [CrossRef]
- Warerkar, O.D.; Mudliar, N.H.; Momin, M.M.; Singh, P.K. Targeting Amyloids with Coated Nanoparticles: A Review on Potential Combinations of Nanoparticles and Bio-Compatible Coatings. Crit. Rev. Ther. Drug Carr. Syst. 2024, 41, 85–119. [Google Scholar] [CrossRef]
- Chang, Y.J.; Chien, Y.H.; Chang, C.C.; Wang, P.N.; Chen, Y.R.; Chang, Y.C. Detection of Femtomolar Amyloid-beta Peptides for Early-Stage Identification of Alzheimer’s Amyloid-beta Aggregation with Functionalized Gold Nanoparticles. ACS Appl. Mater. Interfaces 2024, 16, 3819–3828. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, Z.; Liu, R.; Liu, L.; Li, H.; Li, X.; Chen, Y.; Lu, C.; Liu, Y. AuNPs as an important inorganic nanoparticle applied in drug carrier systems. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4222–4233. [Google Scholar] [CrossRef] [PubMed]
- Mieszawska, A.J.; Mulder, W.J.; Fayad, Z.A.; Cormode, D.P. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol. Pharm. 2013, 10, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Ghobashy, M.M.; Alkhursani, S.A.; Alqahtani, H.A.; El-damhougy, T.K.; Madani, M. Gold nanoparticles in microelectronics advancements and biomedical applications. Mater. Sci. Eng. B 2024, 301, 117191. [Google Scholar] [CrossRef]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Mojzych, I.; Zawadzka, A.; Kaczynska, K.; Wojciechowski, P.; Zajac, D.; Chotkowski, M.; Wiktorska, K.; Maurin, J.K.; Mazur, M. A tetrahydroacridine derivative and its conjugate with gold nanoparticles: Promising agents for the treatment of Alzheimer’s disease. Phys. Chem. Chem. Phys. 2023, 25, 16796–16806. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Tramontin, N.; da Silva, S.; Arruda, R.; Ugioni, K.S.; Canteiro, P.B.; de Bem Silveira, G.; Mendes, C.; Silveira, P.C.L.; Muller, A.P. Gold Nanoparticles Treatment Reverses Brain Damage in Alzheimer’s Disease Model. Mol. Neurobiol. 2020, 57, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Sheng, X.; Xie, H.; Zhou, S.; Zhong, M.; Liu, A. Inhibition of Alzheimer’s Abeta(1-42) Fibrillogenesis and Removal of Copper Ions by Polypeptides Modified Gold Nanoparticles. Chem. Biodivers. 2022, 19, e202200342. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, X.; Ji, D.; Tian, J.; Peng, Q.; Shen, Y.; Xiao, Y. Functionalised penetrating peptide-chondroitin sulphate-gold nanoparticles: Synthesis, characterization, and applications as an anti-Alzheimer’s disease drug. Int. J. Biol. Macromol. 2023, 230, 123125. [Google Scholar] [CrossRef]
- Sanati, M.; Khodagholi, F.; Aminyavari, S.; Ghasemi, F.; Gholami, M.; Kebriaeezadeh, A.; Sabzevari, O.; Hajipour, M.J.; Imani, M.; Mahmoudi, M.; et al. Impact of Gold Nanoparticles on Amyloid beta-Induced Alzheimer’s Disease in a Rat Animal Model: Involvement of STIM Proteins. ACS Chem. Neurosci. 2019, 10, 2299–2309. [Google Scholar] [CrossRef]
- Ling, L.; Jiang, Y.; Liu, Y.; Li, H.; Bari, A.; Ullah, R.; Xue, J. Role of gold nanoparticle from Cinnamomum verum against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) induced mice model. J. Photochem. Photobiol. B 2019, 201, 111657. [Google Scholar] [CrossRef]
- Gao, G.; Chen, R.; He, M.; Li, J.; Li, J.; Wang, L.; Sun, T. Gold nanoclusters for Parkinson’s disease treatment. Biomaterials 2019, 194, 36–46. [Google Scholar] [CrossRef]
- Maity, A.; Mondal, A.; Kundu, S.; Shome, G.; Misra, R.; Singh, A.; Pal, U.; Mandal, A.K.; Bera, K.; Maiti, N.C. Naringenin-Functionalized Gold Nanoparticles and Their Role in alpha-Synuclein Stabilization. Langmuir 2023, 39, 7231–7248. [Google Scholar] [CrossRef]
- Kalcec, N.; Peranic, N.; Barbir, R.; Hall, C.R.; Smith, T.A.; Sani, M.A.; Frkanec, R.; Separovic, F.; Vinkovic Vrcek, I. Spectroscopic study of L-DOPA and dopamine binding on novel gold nanoparticles towards more efficient drug-delivery system for Parkinson’s disease. Spectrochim Acta A Mol Biomol Spectrosc 2022, 268, 120707. [Google Scholar]
- Hu, K.; Chen, X.; Chen, W.; Zhang, L.; Li, J.; Ye, J.; Zhang, Y.; Zhang, L.; Li, C.H.; Yin, L.; et al. Neuroprotective effect of gold nanoparticles composites in Parkinson’s disease model. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- da Silva Corneo, E.; de Bem Silveira, G.; Scussel, R.; Correa, M.; da Silva Abel, J.; Luiz, G.P.; Feuser, P.E.; Silveira, P.C.L.; Machado-de-Avila, R.A. Effects of gold nanoparticles administration through behavioral and oxidative parameters in animal model of Parkinson’s disease. Colloids Surf. B Biointerfaces 2020, 196, 111302. [Google Scholar] [CrossRef]
- Liu, L.; Li, M.; Xu, M.; Wang, Z.; Zeng, Z.; Li, Y.; Zhang, Y.; You, R.; Li, C.H.; Guan, Y.Q. Actively targeted gold nanoparticle composites improve behavior and cognitive impairment in Parkinson’s disease mice. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111028. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zhao, M.; Chen, H.; Lenahan, C.; Zhou, X.; Ou, Y.; He, Y. The Role of Nanomaterials in Stroke Treatment: Targeting Oxidative Stress. Oxid. Med. Cell Longev. 2021, 2021, 8857486. [Google Scholar] [CrossRef]
- Bonnard, T.; Gauberti, M.; Martinez de Lizarrondo, S.; Campos, F.; Vivien, D. Recent Advances in Nanomedicine for Ischemic and Hemorrhagic Stroke. Stroke 2019, 50, 1318–1324. [Google Scholar] [CrossRef]
- Hunt, R.D.; Sedighi, O.; Clark, W.M.; Doiron, A.L.; Cipolla, M.J. Differential effect of gold nanoparticles on cerebrovascular function and biomechanical properties. Physiol. Rep. 2023, 11, e15789. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, Y.; Liu, Y.; Guo, Z.; Bai, T.; Zhou, P.; Wu, J.; Yang, Q.; Liu, Z.; Lu, X. Intrinsic Effects of Gold Nanoparticles on Oxygen-Glucose Deprivation/Reperfusion Injury in Rat Cortical Neurons. Neurochem. Res. 2019, 44, 1549–1566. [Google Scholar] [CrossRef] [PubMed]
- Nazarian, S.; Abdolmaleki, Z.; Torfeh, A.; Shirazi Beheshtiha, S.H. Mesenchymal stem cells with modafinil (gold nanoparticles) significantly improves neurological deficits in rats after middle cerebral artery occlusion. Exp. Brain Res. 2020, 238, 2589–2601. [Google Scholar] [CrossRef]
- Huang, W.; Wang, L.; Zou, Y.; Ding, X.; Geng, X.; Li, J.; Zhao, H.; Qi, R.; Li, S. Preparation of gastrodin-modified dendrimer-entrapped gold nanoparticles as a drug delivery system for cerebral ischemia-reperfusion injury. Brain Behav. 2022, 12, e2810. [Google Scholar] [CrossRef]
- Rathore, P.; Arora, I.; Rastogi, S.; Akhtar, M.; Singh, S.; Samim, M. Collagen Nanoparticle-Mediated Brain Silymarin Delivery: An Approach for Treating Cerebral Ischemia and Reperfusion-Induced Brain Injury. Front. Neurosci. 2020, 14, 538404. [Google Scholar] [CrossRef]
- Elbassal, E.A.; Morris, C.; Kent, T.W.; Lantz, R.; Ojha, B.; Wojcikiewicz, E.P.; Du, D. Gold Nanoparticles as a Probe for Amyloid-beta Oligomer and Amyloid Formation. J. Phys. Chem. C Nanomater. Interfaces 2017, 121, 20007–20015. [Google Scholar] [CrossRef]
- Correa-Paz, C.; da Silva-Candal, A.; Polo, E.; Parcq, J.; Vivien, D.; Maysinger, D.; Pelaz, B.; Campos, F. New Approaches in Nanomedicine for Ischemic Stroke. Pharmaceutics 2021, 13, 757. [Google Scholar] [CrossRef]
- Naqvi, S.; Panghal, A.; Flora, S.J.S. Nanotechnology: A Promising Approach for Delivery of Neuroprotective Drugs. Front. Neurosci. 2020, 14, 494. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.D.; Hu, Y.J.; Yu, L.; Zhou, X.G.; Wu, J.M.; Tang, Y.; Qin, D.L.; Fan, Q.Z.; Wu, A.G. Nanoparticles: A Hope for the Treatment of Inflammation in CNS. Front. Pharmacol. 2021, 12, 683935. [Google Scholar] [CrossRef]
- Sadhukhan, S.; Moniruzzaman, M.; Maity, S.; Ghosh, S.; Pattanayak, A.K.; Chakraborty, S.B.; Maity, B.; Das, M. Organometallic Folate Gold Nanoparticles Ameliorate Lipopolysaccharide-Induced Oxidative Damage and Inflammation in Zebrafish Brain. ACS Omega 2022, 7, 9917–9928. [Google Scholar] [CrossRef] [PubMed]
- Piersimoni, M.E.; Teng, X.; Cass, A.E.G.; Ying, L. Antioxidant lipoic acid ligand-shell gold nanoconjugates against oxidative stress caused by alpha-synuclein aggregates. Nanoscale Adv. 2020, 2, 5666–5681. [Google Scholar] [CrossRef]
- Khan, F.A.; Almohazey, D.; Alomari, M.; Almofty, S.A. Impact of nanoparticles on neuron biology: Current research trends. Int. J. Nanomed. 2018, 13, 2767–2776. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cho, B.; Lee, E.; Kim, J.; Yoo, J.; Sung, J.S.; Kwon, Y.; Kim, J. Electromagnetized gold nanoparticles improve neurogenesis and cognition in the aged brain. Biomaterials 2021, 278, 121157. [Google Scholar] [CrossRef]
- Xia, Q.; Huang, J.; Feng, Q.; Chen, X.; Liu, X.; Li, X.; Zhang, T.; Xiao, S.; Li, H.; Zhong, Z.; et al. Size- and cell type-dependent cellular uptake, cytotoxicity and in vivo distribution of gold nanoparticles. Int. J. Nanomed. 2019, 14, 6957–6970. [Google Scholar] [CrossRef]
- Liu, Z.; Shen, Y.; Wu, Y.; Yang, Y.; Wu, J.; Zhou, P.; Lu, X.; Guo, Z. An intrinsic therapy of gold nanoparticles in focal cerebral ischemia-reperfusion injury in rats. J. Biomed. Nanotechnol. 2013, 9, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Etame, A.B.; Diaz, R.J.; O’Reilly, M.A.; Smith, C.A.; Mainprize, T.G.; Hynynen, K.; Rutka, J.T. Enhanced delivery of gold nanoparticles with therapeutic potential into the brain using MRI-guided focused ultrasound. Nanomedicine 2012, 8, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Adewale, O.B.; Davids, H.; Cairncross, L.; Roux, S. Toxicological Behavior of Gold Nanoparticles on Various Models: Influence of Physicochemical Properties and Other Factors. Int. J. Toxicol. 2019, 38, 357–384. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]





| Biological Model | Pathways | Targets/Mechanisms | References |
|---|---|---|---|
| AD model induced by 100 μg okadaic acid in male Wistar rats and then treated with 20 nm AuNPs at a dose of 2.5 mg/kg every 48 h for 21 days. | AuNPs prevented neuroinflammation, preserved mitochondrial function, restored antioxidant status, and improved cognitive impairment. | AuNP treatment restored abnormal tau phosphorylation, BDNF, NGF-β, IL-1β, ATP synthase activity, SOD, catalase activities, and glutathione (GSH) levels and maintained them at normal levels. | [104] |
| The effect of AuNPs on Aβ-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. | AuNPs have the potential to serve as effective inhibitors of Aβ fibrillogenesis. | AuNPs showed a significant protective effect, suggesting that inhibiting Aβ fibrillation and copper ion chelation had a beneficial effect on neurons. | [105] |
| AuNP applications as an anti-AD drug in Aβ1–40-induced apoptosis in SH-SY5Y cells. | Inhibition of Aβ1–40 accumulation, reduction in Aβ1–40-induced apoptosis, protection against oxidative stress and cholinergic injury, inhibition of aberrant tau phosphorylation, and suppression of inflammatory response. | AuNPs reduced abnormal tau phosphorylation, inflammatory factors, and oxidative stress damage by regulating GSK3β, NF-κB signaling pathway, malondialdehyde (MDA), and glutathione peroxidase (GSH-Px) levels. | [106] |
| Inhibition effect of AuNPs (110 nM) on Aβ42 (40 μM)-induced neuronal death in SH-SY5Y cells. Effect of AuNPs (25 mg kg−1) on rescuing memory impairments in APP/PS1 mice. | AuNPs demonstrated the ability to protect neuronal cells from Aβ42-induced death in vitro and improve memory impairments in a mouse model of AD. | By inhibiting Aβ42 fibrillation and effectively crossing the BBB, these nanoparticles could offer a way to treat behavioral impairments. | [92] |
| The investigation concerns the potential therapeutic advantages of 10 ppm of AuNPs in a 3D cell culture model, utilizing human neural stem cells exposed to 5 μM of Aβ1–42. | The research shows that AuNPs effectively reduce inflammation and oxidative stress in hNSCs exposed to Aβ, specifically under 3D scaffold conditions. | The AuNPs led to the normalization of the expression of inflammatory cytokines (specifically TNF-α and IL-1β), NF-κB (p65), nuclear factor erythroid 2-related factor 2 (Nrf2), and aggregates in Aβ-treated human neural stem cells. | [53] |
| The possible therapeutic benefit of AuNPs in mitigating cognitive and memory deficits in a rat model induced by the Aβ model. | In the Morris water maze, rats who received treatments of Aβ and AuNPs demonstrated extended presence in the target quadrant, thus indicating memory retention enhancements. | The levels of critical proteins necessary for the survival and adaptability of neurons, including BDNF, CREB, and stromal interaction molecules (STIM1 and STIM2), were increased in rats subjected to Aβ and AuNP treatment. | [107] |
| Biological Model | Pathways | Targets/Mechanisms | References |
|---|---|---|---|
| The therapeutic effectiveness of the composites of AuNPs has been demonstrated in both in vitro (in PC12 cell cultures) and in vivo (in living organisms) models of PD. | In a model of PD, AuNP composites show neuroprotective effects. | The AuNPs are transfected into cells via endocytosis. This process inhibits apoptosis in PC12 cells and dopaminergic neurons, potentially preserving these cells from degeneration. | [112] |
| AuNPs were administered at 2.5 mg/kg (20 nm) for five consecutive days in 0.25 mg/kg reserpine-induced male C57BL/6 mice. | AuNPs have positive effects in reversing behavioral and oxidative stress parameters in a reserpine-induced PD model. | AuNPs reversed the behavioral and oxidative stress parameters observed in the reserpine-induced PD mice. Additionally, AuNPs partially improved neurotrophic factors that are crucial for neuronal survival. | [113] |
| AuNPs exhibit effective neuroprotective properties in cellular PD models and mouse PD models. | A new direction for the application of AuNPs in medicinal contexts, specifically in treating neurodegenerative disorders like PD. | AuNPs can potentially prevent α-synuclein fibrillation, provide neuroprotection in cell models, improve behavioral symptoms, and reverse dopaminergic neuron loss in a mouse model of PD. | [109] |
| AuNPs demonstrated substantial neuroprotective effects on both motor and non-motor aspects of the PD mouse model induced by MPTP (30 mg/kg intraperitoneal twice a week). | AuNPs showed a considerable neuroprotective impact to enhance the behavioral and cognitive deficits of mice with PD. | The treatment with AuNPs led to a reduction in the aggregation of α-synuclein in the substantia nigra. AuNP-treated mice exhibited improved long-term potentiation and exploration ability, positively impacting their cognitive and behavioral function. | [114] |
| In vitro, assays demonstrated the capacity of AuNPs to suppress inflammation in murine microglial BV2 cells. In vivo, studies established their beneficial effects in preventing neuroinflammation and improving motor coordination in PD-induced mice. | These findings suggest the potential therapeutic use of these AuNPs in treating PD, highlighting their neuroprotective and anti-inflammation properties. | The neuroprotective effects were assessed by measuring nitric oxide, prostaglandin E2 assays, and inflammatory cytokines (IL-6 and IL-1β). The results suggest that the AuNPs can mitigate inflammatory conditions induced by LPS in BV2 cells—moreover, gold nanoparticles alleviate neuroinflammation and improve motor coordination in the C57BL/6 mice induced with PD. | [15] |
| Biological Model | Effects | Targets/Mechanisms | References |
|---|---|---|---|
| The impact of 20 nm AuNPs on neuronal injury and survival in primary rat cortical neurons during oxygen–glucose deprivation/reperfusion (OGD/R) injury. | AuNPs might exhibit neuroprotective effects and anti-inflammatory properties in OGD/R injury in rat cortical neurons. | AuNPs might modulate neuronal cell viability, cell survival, antiapoptotic pathways, mitochondrial oxygen consumption, autophagic processes, and neurotransmitter release during OGD/R injury. | [118] |
| Neuroprotective effects of 50 nm AuNPs (100 mg/kg/day) in a rat model of middle cerebral artery occlusion (MCAO). | AuNPs could enhance neuronal survival and neurotrophic factor levels in the MCAO model. | AuNPs significantly reduced brain infarct volume and apoptosis while increasing BDNF, GDNF, and NeuN levels in ischemic brain injuries. | [119] |
| Neuroprotective effects of AuNPs in the MCAO rats. | AuNPs notably reduced MCAO-induced apoptosis. | Pathological alterations in brain tissue and significant organs were identified through staining with hematoxylin and eosin. Apoptotic rat astrocytes and hypothalamic neurons were discovered using TUNEL staining and flow cytometry. AuNPs exerted anti-inflammatory and antiapoptotic effects against cerebral ischemia–reperfusion injury. | [120] |
| Nanoparticles enhanced their effectiveness in treating rat brain injury induced by cerebral ischemia and reperfusion. | The use of nanoparticles has resulted in improved drug bioavailability and targeted drug delivery, leading to enhanced neuroprotective effects. | Nanoparticles showed a significant reduction in the expression of inflammatory proteins NF-kB, iNOS, and apoptotic protein caspase-3 in the MCAO group. | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, M.-C.; Yang, Y.-P.; Nicol, C.J.B.; Wang, C.-J. Gold Nanoparticles in Neurological Diseases: A Review of Neuroprotection. Int. J. Mol. Sci. 2024, 25, 2360. https://doi.org/10.3390/ijms25042360
Chiang M-C, Yang Y-P, Nicol CJB, Wang C-J. Gold Nanoparticles in Neurological Diseases: A Review of Neuroprotection. International Journal of Molecular Sciences. 2024; 25(4):2360. https://doi.org/10.3390/ijms25042360
Chicago/Turabian StyleChiang, Ming-Chang, Yu-Ping Yang, Christopher J. B. Nicol, and Chieh-Ju Wang. 2024. "Gold Nanoparticles in Neurological Diseases: A Review of Neuroprotection" International Journal of Molecular Sciences 25, no. 4: 2360. https://doi.org/10.3390/ijms25042360
APA StyleChiang, M.-C., Yang, Y.-P., Nicol, C. J. B., & Wang, C.-J. (2024). Gold Nanoparticles in Neurological Diseases: A Review of Neuroprotection. International Journal of Molecular Sciences, 25(4), 2360. https://doi.org/10.3390/ijms25042360







