Pathogenetic Contributions and Therapeutic Implications of Transglutaminase 2 in Neurodegenerative Diseases
Abstract
1. Introduction
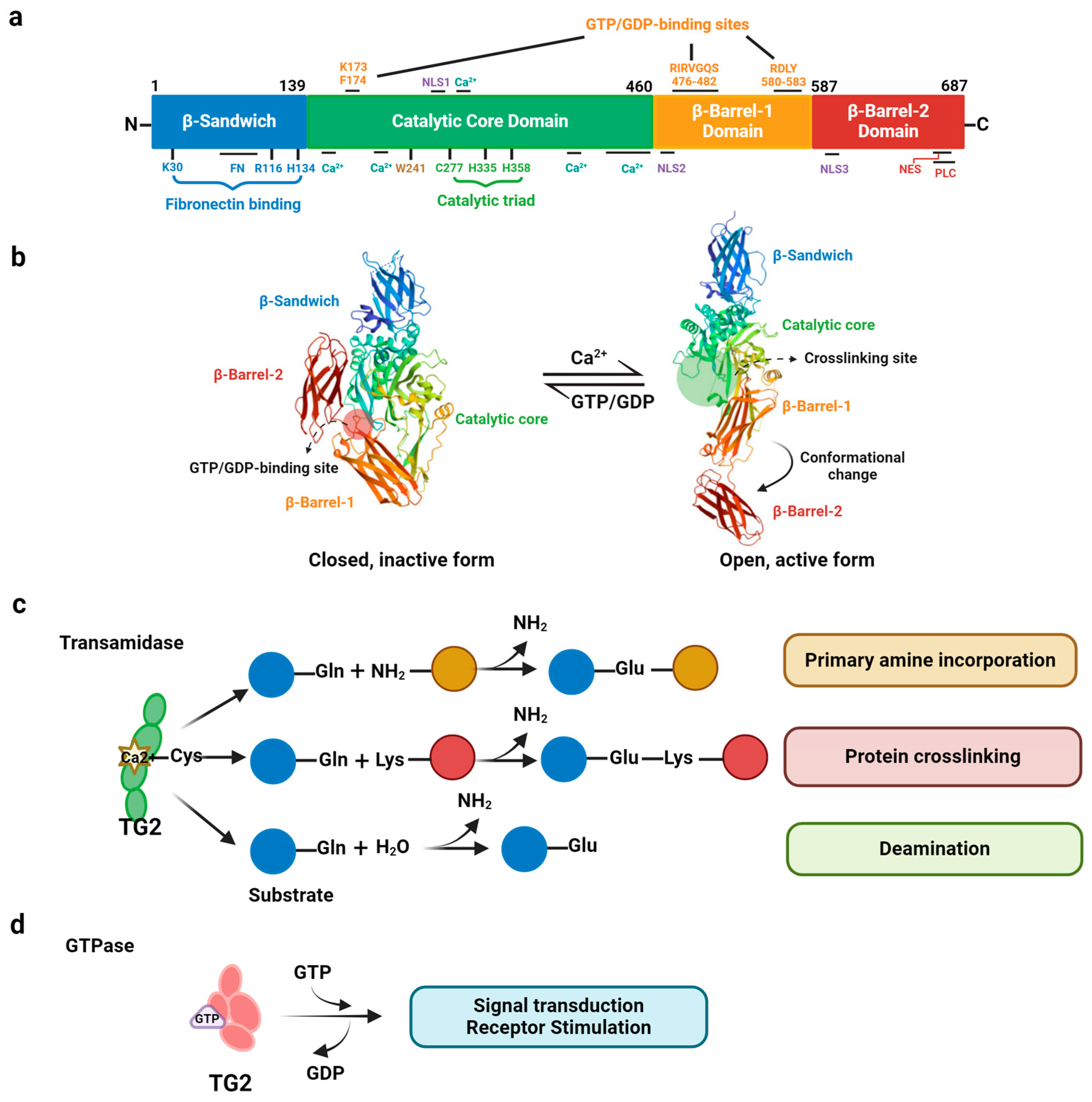
2. Structure and Multifunctional Activities of TG2
3. Regulation of TG2 Expression and Its Activation
3.1. Regulation of TG2 Transcription
3.2. Regulation of TG2 Activation
4. Distribution of TG2
4.1. Cytosolic TG2
4.2. Mitochondrial TG2
4.3. Nuclear TG2
4.4. Extracellular TG2
4.5. TG2 Expression in Various Cell Types
5. Pathological Role of TG2 in Neurodegenerative Diseases
5.1. TG2 in Alzheimer’s Disease
5.2. TG2 in Parkinson’s Disease and Dementia with Lewy Bodies
5.3. TG2 in Huntington’s Disease
5.4. TG2 in Other Neurodegenerative Disorders
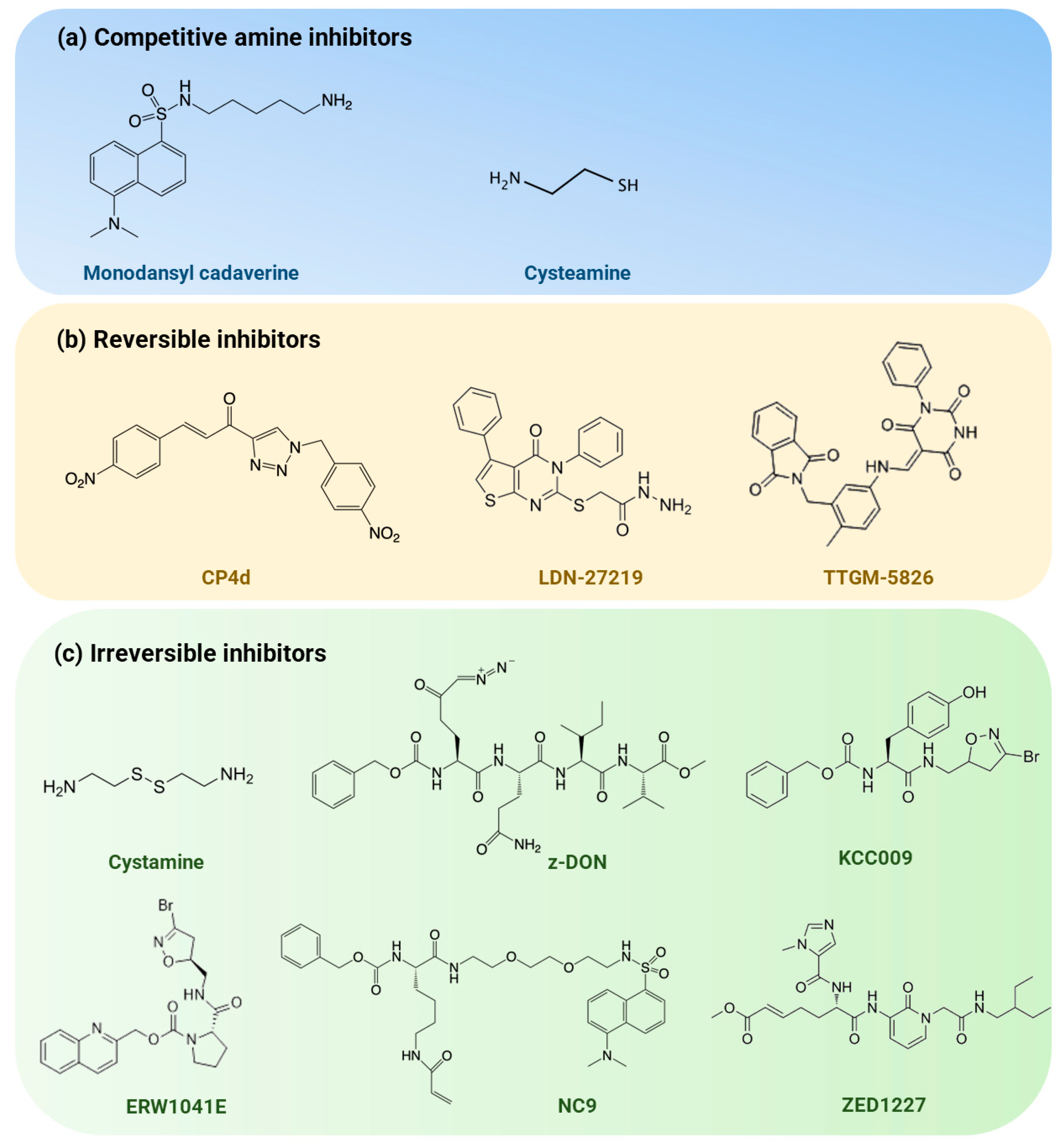
6. Current Progress in Targeting TG2 for Therapeutic Purposes
6.1. Competitive Amine Inhibitors
6.2. Reversible Inhibitors
6.3. Irreversible Inhibitors
7. Conclusions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Folk, J.E.; Finlayson, J.S. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv. Protein Chem. 1977, 31, 1–133. [Google Scholar]
- Iismaa, S.E.; Holman, S.; Wouters, M.A.; Lorand, L.; Graham, R.M.; Husain, A. Evolutionary specialization of a tryptophan indole group for transition-state stabilization by eukaryotic transglutaminases. Proc. Natl. Acad. Sci. USA 2003, 100, 12636–12641. [Google Scholar] [CrossRef] [PubMed]
- Tatsukawa, H.; Hitomi, K. Role of Transglutaminase 2 in Cell Death, Survival, and Fibrosis. Cells 2021, 10, 1842. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, G.; Grun, D.; Alexander, H.R.; Friedberg, J.S.; Xu, W.; Keillor, J.W.; Kandasamy, S.; Eckert, R.L. Transglutaminase is a mesothelioma cancer stem cell survival protein that is required for tumor formation. Oncotarget 2018, 9, 34495–34505. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Chung, K.C. New insight into transglutaminase 2 and link to neurodegenerative diseases. BMB Rep. 2018, 51, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Bhedi, C.D.; Nasirova, S.; Toksoz, D.; Warburton, R.R.; Morine, K.J.; Kapur, N.K.; Galper, J.B.; Preston, I.R.; Hill, N.S.; Fanburg, B.L.; et al. Glycolysis regulated transglutaminase 2 activation in cardiopulmonary fibrogenic remodeling. FASEB J. 2020, 34, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; Choi, Y.; Kim, K.H.; Seo, A.; Kwon, S.; Kim, Y.C.; Kim, D.K.; Kim, Y.S.; Yang, S.H. Inhibiting Transglutaminase 2 Mediates Kidney Fibrosis via Anti-Apoptosis. Biomedicines 2022, 10, 1345. [Google Scholar] [CrossRef] [PubMed]
- Paolella, G.; Sposito, S.; Romanelli, A.M.; Caputo, I. Type 2 Transglutaminase in Coeliac Disease: A Key Player in Pathogenesis, Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7513. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Fan, Y.; Lin, L.; Kellems, R.E.; Xia, Y. Tissue transglutaminase: A multifunctional and multisite regulator in health and disease. Physiol. Rev. 2024, 104, 281–325. [Google Scholar] [CrossRef]
- Kim, S.Y.; Grant, P.; Lee, J.H.; Pant, H.C.; Steinert, P.M. Differential expression of multiple transglutaminases in human brain. Increased expression and cross-linking by transglutaminases 1 and 2 in Alzheimer’s disease. J. Biol. Chem. 1999, 274, 30715–30721. [Google Scholar] [CrossRef]
- Mastroberardino, P.G.; Piacentini, M. Type 2 transglutaminase in Huntington’s disease: A double-edged sword with clinical potential. J. Intern. Med. 2010, 268, 419–431. [Google Scholar] [CrossRef]
- Verhaar, R.; Drukarch, B.; Bol, J.G.; Jongenelen, C.A.; Musters, R.J.; Wilhelmus, M.M. Increase in endoplasmic reticulum-associated tissue transglutaminase and enzymatic activation in a cellular model of Parkinson’s disease. Neurobiol. Dis. 2012, 45, 839–850. [Google Scholar] [CrossRef]
- Andre, W.; Nondier, I.; Valensi, M.; Guillonneau, F.; Federici, C.; Hoffner, G.; Djian, P. Identification of brain substrates of transglutaminase by functional proteomics supports its role in neurodegenerative diseases. Neurobiol. Dis. 2017, 101, 40–58. [Google Scholar] [CrossRef]
- Schuppan, D.; Maki, M.; Lundin, K.E.A.; Isola, J.; Friesing-Sosnik, T.; Taavela, J.; Popp, A.; Koskenpato, J.; Langhorst, J.; Hovde, O.; et al. A Randomized Trial of a Transglutaminase 2 Inhibitor for Celiac Disease. N. Engl. J. Med. 2021, 385, 35–45. [Google Scholar] [CrossRef]
- Buchold, C.; Hils, M.; Gerlach, U.; Weber, J.; Pelzer, C.; Heil, A.; Aeschlimann, D.; Pasternack, R. Features of ZED1227: The First-In-Class Tissue Transglutaminase Inhibitor Undergoing Clinical Evaluation for the Treatment of Celiac Disease. Cells 2022, 11, 1667. [Google Scholar] [CrossRef] [PubMed]
- Phatak, V.M.; Croft, S.M.; Rameshaiah Setty, S.G.; Scarpellini, A.; Hughes, D.C.; Rees, R.; McArdle, S.; Verderio, E.A. Expression of transglutaminase-2 isoforms in normal human tissues and cancer cell lines: Dysregulation of alternative splicing in cancer. Amino Acids 2013, 44, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Begg, G.E.; Holman, S.R.; Stokes, P.H.; Matthews, J.M.; Graham, R.M.; Iismaa, S.E. Mutation of a critical arginine in the GTP-binding site of transglutaminase 2 disinhibits intracellular cross-linking activity. J. Biol. Chem. 2006, 281, 12603–12609. [Google Scholar] [CrossRef]
- Nurminskaya, M.V.; Belkin, A.M. Cellular functions of tissue transglutaminase. Int. Rev. Cell Mol. Biol. 2012, 294, 1–97. [Google Scholar] [PubMed]
- Sestito, C.; Breve, J.J.P.; Killestein, J.; Teunissen, C.E.; Wilhelmus, M.M.M.; Drukarch, B.; van Dam, A.M. Differential Expression of Tissue Transglutaminase Splice Variants in Peripheral Blood Mononuclear Cells of Primary Progressive Multiple Sclerosis Patients. Med. Sci. 2018, 6, 108. [Google Scholar] [CrossRef]
- Sima, L.E.; Matei, D.; Condello, S. The Outside-In Journey of Tissue Transglutaminase in Cancer. Cells 2022, 11, 1779. [Google Scholar] [CrossRef] [PubMed]
- Hang, J.; Zemskov, E.A.; Lorand, L.; Belkin, A.M. Identification of a novel recognition sequence for fibronectin within the NH2-terminal beta-sandwich domain of tissue transglutaminase. J. Biol. Chem. 2005, 280, 23675–23683. [Google Scholar] [CrossRef]
- Cardoso, I.; Osterlund, E.C.; Stamnaes, J.; Iversen, R.; Andersen, J.T.; Jorgensen, T.J.; Sollid, L.M. Dissecting the interaction between transglutaminase 2 and fibronectin. Amino Acids 2017, 49, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, R.; Csosz, E.; Kurtan, T.; Antus, S.; Szigeti, K.; Simon-Vecsei, Z.; Korponay-Szabo, I.R.; Keresztessy, Z.; Fesus, L. Functional significance of five noncanonical Ca2+-binding sites of human transglutaminase 2 characterized by site-directed mutagenesis. FEBS J. 2009, 276, 7083–7096. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cerione, R.A.; Clardy, J. Structural basis for the guanine nucleotide-binding activity of tissue transglutaminase and its regulation of transamidation activity. Proc. Natl. Acad. Sci. USA 2002, 99, 2743–2747. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.N.; Lomasney, J.W.; Mak, E.C.; Lorand, L. Interactions of G(h)/transglutaminase with phospholipase Cdelta1 and with GTP. Proc. Natl. Acad. Sci. USA 1999, 96, 11815–11819. [Google Scholar] [CrossRef]
- Lee, K.N.; Arnold, S.A.; Birckbichler, P.J.; Patterson, M.K., Jr.; Fraij, B.M.; Takeuchi, Y.; Carter, H.A. Site-directed mutagenesis of human tissue transglutaminase: Cys-277 is essential for transglutaminase activity but not for GTPase activity. Biochim. Biophys. Acta 1993, 1202, 1–6. [Google Scholar] [CrossRef]
- Ulukan, B.; Bihorac, A.; Sipahioglu, T.; Kiraly, R.; Fesus, L.; Telci, D. Role of Tissue Transglutaminase Catalytic and Guanosine Triphosphate-Binding Domains in Renal Cell Carcinoma Progression. ACS Omega 2020, 5, 28273–28284. [Google Scholar] [CrossRef] [PubMed]
- Begg, G.E.; Carrington, L.; Stokes, P.H.; Matthews, J.M.; Wouters, M.A.; Husain, A.; Lorand, L.; Iismaa, S.E.; Graham, R.M. Mechanism of allosteric regulation of transglutaminase 2 by GTP. Proc. Natl. Acad. Sci. USA 2006, 103, 19683–19688. [Google Scholar] [CrossRef]
- Murthy, S.N.; Iismaa, S.; Begg, G.; Freymann, D.M.; Graham, R.M.; Lorand, L. Conserved tryptophan in the core domain of transglutaminase is essential for catalytic activity. Proc. Natl. Acad. Sci. USA 2002, 99, 2738–2742. [Google Scholar] [CrossRef]
- Zhang, J.; Jasutkar, H.G.; Mouradian, M.M. Targeting transglutaminase 2 as a potential disease modifying therapeutic strategy for synucleinopathies. Neural Regen. Res. 2021, 16, 1560–1561. [Google Scholar]
- Kiraly, R.; Demeny, M.; Fesus, L. Protein transamidation by transglutaminase 2 in cells: A disputed Ca2+-dependent action of a multifunctional protein. FEBS J. 2011, 278, 4717–4739. [Google Scholar] [CrossRef]
- Keillor, J.W.; Clouthier, C.M.; Apperley, K.Y.P.; Akbar, A.; Mulani, A. Acyl transfer mechanisms of tissue transglutaminase. Bioorg. Chem. 2014, 57, 186–197. [Google Scholar] [CrossRef]
- Maddock, R.M.A.; Pollard, G.J.; Moreau, N.G.; Perry, J.J.; Race, P.R. Enzyme-catalysed polymer cross-linking: Biocatalytic tools for chemical biology, materials science and beyond. Biopolymers 2020, 111, e23390. [Google Scholar] [CrossRef]
- Savoca, M.P.; Tonoli, E.; Atobatele, A.G.; Verderio, E.A.M. Biocatalysis by Transglutaminases: A Review of Biotechnological Applications. Micromachines 2018, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Tabolacci, C.; De Martino, A.; Mischiati, C.; Feriotto, G.; Beninati, S. The Role of Tissue Transglutaminase in Cancer Cell Initiation, Survival and Progression. Med. Sci. 2019, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Occhigrossi, L.; D’Eletto, M.; Barlev, N.; Rossin, F. The Multifaceted Role of HSF1 in Pathophysiology: Focus on Its Interplay with TG2. Int. J. Mol. Sci. 2021, 22, 6366. [Google Scholar] [CrossRef] [PubMed]
- Occhigrossi, L.; Rossin, F.; D’Eletto, M.; Farrace, M.G.; Ciccosanti, F.; Petrone, L.; Sacchi, A.; Nardacci, R.; Falasca, L.; Del Nonno, F.; et al. Transglutaminase 2 Regulates Innate Immunity by Modulating the STING/TBK1/IRF3 Axis. J. Immunol. 2021, 206, 2420–2429. [Google Scholar] [CrossRef]
- Rossin, F.; Ciccosanti, F.; D’Eletto, M.; Occhigrossi, L.; Fimia, G.M.; Piacentini, M. Type 2 transglutaminase in the nucleus: The new epigenetic face of a cytoplasmic enzyme. Cell Mol. Life Sci. 2023, 80, 52. [Google Scholar] [CrossRef] [PubMed]
- Gluck, S.; Guey, B.; Gulen, M.F.; Wolter, K.; Kang, T.W.; Schmacke, N.A.; Bridgeman, A.; Rehwinkel, J.; Zender, L.; Ablasser, A. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat. Cell Biol. 2017, 19, 1061–1070. [Google Scholar] [CrossRef]
- Gulen, M.F.; Samson, N.; Keller, A.; Schwabenland, M.; Liu, C.; Gluck, S.; Thacker, V.V.; Favre, L.; Mangeat, B.; Kroese, L.J.; et al. cGAS-STING drives ageing-related inflammation and neurodegeneration. Nature 2023, 620, 374–380. [Google Scholar] [CrossRef]
- Chen, J.S.; Mehta, K. Tissue transglutaminase: An enzyme with a split personality. Int. J. Biochem. Cell Biol. 1999, 31, 817–836. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.S.; Lin, C.J.; Wu, Y.T.; Wu, C.J. Tissue transglutaminase (TG2) and mitochondrial function and dysfunction. Front. Biosci. 2017, 22, 1114–1137. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.J.; Murtaugh, M.P.; Moore, W.T., Jr.; Johnson, G.S.; Lucas, D. Retinoic acid-induced expression of tissue transglutaminase in human promyelocytic leukemia (HL-60) cells. J. Biol. Chem. 1985, 260, 5166–5174. [Google Scholar] [CrossRef]
- Lesort, M.; Tucholski, J.; Miller, M.L.; Johnson, G.V. Tissue transglutaminase: A possible role in neurodegenerative diseases. Prog. Neurobiol. 2000, 61, 439–463. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, D.; Curro, M.; Ferlazzo, N.; Condello, S.; Ientile, R. Monitoring of transglutaminase 2 under different oxidative stress conditions. Amino Acids 2012, 42, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.Y.; Jeon, J.H.; Cho, S.Y.; Shin, D.M.; Kim, C.W.; Jeong, E.M.; Bae, H.C.; Kim, T.W.; Lee, S.H.; Choi, Y.; et al. Transglutaminase 2 suppresses apoptosis by modulating caspase 3 and NF-kappaB activity in hypoxic tumor cells. Oncogene 2010, 29, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Qin, X.Y.; Furutani, Y. Transglutaminase 2 as a Marker for Inflammation and Therapeutic Target in Sepsis. Int. J. Mol. Sci. 2021, 22, 1897. [Google Scholar] [CrossRef]
- Philp, C.J.; Siebeke, I.; Clements, D.; Miller, S.; Habgood, A.; John, A.E.; Navaratnam, V.; Hubbard, R.B.; Jenkins, G.; Johnson, S.R. Extracellular Matrix Cross-Linking Enhances Fibroblast Growth and Protects against Matrix Proteolysis in Lung Fibrosis. Am. J. Respir. Cell Mol. Biol. 2018, 58, 594–603. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.S.; Choi, D.H.; Bang, M.S.; Han, T.R.; Joh, T.H.; Kim, S.Y. Transglutaminase 2 induces nuclear factor-kappaB activation via a novel pathway in BV-2 microglia. J. Biol. Chem. 2004, 279, 53725–53735. [Google Scholar] [CrossRef]
- Maiuri, L.; Luciani, A.; Giardino, I.; Raia, V.; Villella, V.R.; D’Apolito, M.; Pettoello-Mantovani, M.; Guido, S.; Ciacci, C.; Cimmino, M.; et al. Tissue transglutaminase activation modulates inflammation in cystic fibrosis via PPARgamma down-regulation. J. Immunol. 2008, 180, 7697–7705. [Google Scholar] [CrossRef]
- Takano, K.; Shiraiwa, K.; Moriyama, M.; Nakamura, Y. Transglutaminase 2 expression induced by lipopolysaccharide stimulation together with NO synthase induction in cultured astrocytes. Neurochem. Int. 2010, 57, 812–818. [Google Scholar] [CrossRef]
- Ritter, S.J.; Davies, P.J. Identification of a transforming growth factor-beta1/bone morphogenetic protein 4 (TGF-beta1/BMP4) response element within the mouse tissue transglutaminase gene promoter. J. Biol. Chem. 1998, 273, 12798–12806. [Google Scholar] [CrossRef]
- Quan, G.; Choi, J.Y.; Lee, D.S.; Lee, S.C. TGF-beta1 up-regulates transglutaminase two and fibronectin in dermal fibroblasts: A possible mechanism for the stabilization of tissue inflammation. Arch. Dermatol. Res. 2005, 297, 84–90. [Google Scholar] [CrossRef]
- Prud’homme, G.J.; Piccirillo, C.A. The inhibitory effects of transforming growth factor-beta-1 (TGF-beta1) in autoimmune diseases. J. Autoimmun. 2000, 14, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Wakabayashi, Y.; Mori, T. Cellular and molecular basis for the regulation of inflammation by TGF-beta. J. Biochem. 2010, 147, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Grosso, H.; Mouradian, M.M. Transglutaminase 2: Biology, relevance to neurodegenerative diseases and therapeutic implications. Pharmacol. Ther. 2012, 133, 392–410. [Google Scholar] [CrossRef] [PubMed]
- Pinkas, D.M.; Strop, P.; Brunger, A.T.; Khosla, C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007, 5, e327. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.L.; Kaartinen, M.T.; Nurminskaya, M.; Belkin, A.M.; Colak, G.; Johnson, G.V.; Mehta, K. Transglutaminase regulation of cell function. Physiol. Rev. 2014, 94, 383–417. [Google Scholar] [CrossRef]
- Di Venere, A.; Rossi, A.; De Matteis, F.; Rosato, N.; Agro, A.F.; Mei, G. Opposite effects of Ca(2+) and GTP binding on tissue transglutaminase tertiary structure. J. Biol. Chem. 2000, 275, 3915–3921. [Google Scholar] [CrossRef] [PubMed]
- Nakaoka, H.; Perez, D.M.; Baek, K.J.; Das, T.; Husain, A.; Misono, K.; Im, M.J.; Graham, R.M. Gh: A GTP-binding protein with transglutaminase activity and receptor signaling function. Science 1994, 264, 1593–1596. [Google Scholar] [CrossRef]
- Achyuthan, K.E.; Greenberg, C.S. Identification of a guanosine triphosphate-binding site on guinea pig liver transglutaminase. Role of GTP and calcium ions in modulating activity. J. Biol. Chem. 1987, 262, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Casadio, R.; Polverini, E.; Mariani, P.; Spinozzi, F.; Carsughi, F.; Fontana, A.; Polverino de Laureto, P.; Matteucci, G.; Bergamini, C.M. The structural basis for the regulation of tissue transglutaminase by calcium ions. Eur. J. Biochem. 1999, 262, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Tempest, R.; Guarnerio, S.; Maani, R.; Cooper, J.; Peake, N. The Biological and Biomechanical Role of Transglutaminase-2 in the Tumour Microenvironment. Cancers 2021, 13, 2788. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.; Strnad, P.; Watts, R.E.; Choi, K.; Jabri, B.; Omary, M.B.; Khosla, C. Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS ONE 2008, 3, e1861. [Google Scholar] [CrossRef] [PubMed]
- Stamnaes, J.; Pinkas, D.M.; Fleckenstein, B.; Khosla, C.; Sollid, L.M. Redox regulation of transglutaminase 2 activity. J. Biol. Chem. 2010, 285, 25402–25409. [Google Scholar] [CrossRef]
- Jin, X.; Stamnaes, J.; Klock, C.; DiRaimondo, T.R.; Sollid, L.M.; Khosla, C. Activation of extracellular transglutaminase 2 by thioredoxin. J. Biol. Chem. 2011, 286, 37866–37873. [Google Scholar] [CrossRef]
- Luciani, A.; Villella, V.R.; Vasaturo, A.; Giardino, I.; Raia, V.; Pettoello-Mantovani, M.; D’Apolito, M.; Guido, S.; Leal, T.; Quaratino, S.; et al. SUMOylation of tissue transglutaminase as link between oxidative stress and inflammation. J. Immunol. 2009, 183, 2775–2784. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.M.; Kim, C.W.; Cho, S.Y.; Jang, G.Y.; Shin, D.M.; Jeon, J.H.; Kim, I.G. Degradation of transglutaminase 2 by calcium-mediated ubiquitination responding to high oxidative stress. FEBS Lett. 2009, 583, 648–654. [Google Scholar] [CrossRef]
- Shin, D.M.; Jeon, J.H.; Kim, C.W.; Cho, S.Y.; Lee, H.J.; Jang, G.Y.; Jeong, E.M.; Lee, D.S.; Kang, J.H.; Melino, G.; et al. TGFbeta mediates activation of transglutaminase 2 in response to oxidative stress that leads to protein aggregation. FASEB J. 2008, 22, 2498–2507. [Google Scholar] [CrossRef]
- Gorlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef]
- Baev, A.Y.; Vinokurov, A.Y.; Novikova, I.N.; Dremin, V.V.; Potapova, E.V.; Abramov, A.Y. Interaction of Mitochondrial Calcium and ROS in Neurodegeneration. Cells 2022, 11, 706. [Google Scholar] [CrossRef]
- Lee, Z.W.; Kwon, S.M.; Kim, S.W.; Yi, S.J.; Kim, Y.M.; Ha, K.S. Activation of in situ tissue transglutaminase by intracellular reactive oxygen species. Biochem. Biophys. Res. Commun. 2003, 305, 633–640. [Google Scholar] [CrossRef]
- Wongmekiat, O.; Leelarungrayub, D.; Thamprasert, K. Alpha-lipoic acid attenuates renal injury in rats with obstructive nephropathy. Biomed. Res. Int. 2013, 2013, 138719. [Google Scholar] [CrossRef]
- Houldsworth, A. Role of oxidative stress in neurodegenerative disorders: A review of reactive oxygen species and prevention by antioxidants. Brain Commun. 2024, 6, fcad356. [Google Scholar] [CrossRef]
- Attarwala, H.Z.; Suri, K.; Amiji, M.M. Co-Silencing of Tissue Transglutaminase-2 and Interleukin-15 Genes in a Celiac Disease Mimetic Mouse Model Using a Nanoparticle-in-Microsphere Oral System. Mol. Pharm. 2021, 18, 3099–3107. [Google Scholar] [CrossRef]
- Nouri Nojadeh, J.; Bildiren Eryilmaz, N.S.; Erguder, B.I. CRISPR/Cas9 genome editing for neurodegenerative diseases. EXCLI J. 2023, 22, 567–582. [Google Scholar] [PubMed]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.F.; Yu, T. CRISPR/Cas9 therapeutics: Progress and prospects. Signal Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.J.; Stahlberg, S.; Vowinckel, J. Novel roles for biogenic monoamines: From monoamines in transglutaminase-mediated post-translational protein modification to monoaminylation deregulation diseases. FEBS J. 2011, 278, 4740–4755. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Antonyak, M.A.; Cerione, R.A. GTP-binding-defective forms of tissue transglutaminase trigger cell death. Biochemistry 2007, 46, 14819–14829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piacentini, M.; Farrace, M.G.; Piredda, L.; Matarrese, P.; Ciccosanti, F.; Falasca, L.; Rodolfo, C.; Giammarioli, A.M.; Verderio, E.; Griffin, M.; et al. Transglutaminase overexpression sensitizes neuronal cell lines to apoptosis by increasing mitochondrial membrane potential and cellular oxidative stress. J. Neurochem. 2002, 81, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Choi, S.S.; Ha, K.S. Transglutaminase 2: A multi-functional protein in multiple subcellular compartments. Amino Acids 2010, 39, 619–631. [Google Scholar] [CrossRef]
- D’Eletto, M.; Rossin, F.; Occhigrossi, L.; Farrace, M.G.; Faccenda, D.; Desai, R.; Marchi, S.; Refolo, G.; Falasca, L.; Antonioli, M.; et al. Transglutaminase Type 2 Regulates ER-Mitochondria Contact Sites by Interacting with GRP75. Cell Rep. 2018, 25, 3573–3581 e4. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.O.; Lim, Y.C.; Kim, Y.M.; Ha, K.S. Transglutaminase 2 promotes both caspase-dependent and caspase-independent apoptotic cell death via the calpain/Bax protein signaling pathway. J. Biol. Chem. 2012, 287, 14377–14388. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, Y.; Zhang, H.; Graner, S.; Williams, J.F.; Levitt, M.L.; Lokshin, A. Interaction of tissue transglutaminase with nuclear transport protein importin-alpha3. FEBS Lett. 1999, 446, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Tatsukawa, H.; Shrestha, R.; Ishibashi, N.; Matsuura, T.; Kagechika, H.; Kose, S.; Hitomi, K.; Imamoto, N.; Kojima, S. Molecular mechanism by which acyclic retinoid induces nuclear localization of transglutaminase 2 in human hepatocellular carcinoma cells. Cell Death Dis. 2015, 6, e2002. [Google Scholar] [CrossRef] [PubMed]
- Dardik, R.; Inbal, A. Complex formation between tissue transglutaminase II (tTG) and vascular endothelial growth factor receptor 2 (VEGFR-2): Proposed mechanism for modulation of endothelial cell response to VEGF. Exp. Cell Res. 2006, 312, 2973–2982. [Google Scholar] [CrossRef] [PubMed]
- Filiano, A.J.; Bailey, C.D.; Tucholski, J.; Gundemir, S.; Johnson, G.V. Transglutaminase 2 protects against ischemic insult, interacts with HIF1beta, and attenuates HIF1 signaling. FASEB J. 2008, 22, 2662–2675. [Google Scholar] [CrossRef]
- Yunes-Medina, L.; Feola, J.; Johnson, G.V.W. Subcellular localization patterns of transglutaminase 2 in astrocytes and neurons are differentially altered by hypoxia. Neuroreport 2017, 28, 1208–1214. [Google Scholar] [CrossRef]
- Tatsukawa, H.; Fukaya, Y.; Frampton, G.; Martinez-Fuentes, A.; Suzuki, K.; Kuo, T.F.; Nagatsuma, K.; Shimokado, K.; Okuno, M.; Wu, J.; et al. Role of transglutaminase 2 in liver injury via cross-linking and silencing of transcription factor Sp1. Gastroenterology 2009, 136, 1783–1795.e10. [Google Scholar] [CrossRef]
- Farrelly, L.A.; Thompson, R.E.; Zhao, S.; Lepack, A.E.; Lyu, Y.; Bhanu, N.V.; Zhang, B.; Loh, Y.E.; Ramakrishnan, A.; Vadodaria, K.C.; et al. Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature 2019, 567, 535–539. [Google Scholar] [CrossRef]
- Ballestar, E.; Abad, C.; Franco, L. Core histones are glutaminyl substrates for tissue transglutaminase. J. Biol. Chem. 1996, 271, 18817–18824. [Google Scholar] [CrossRef]
- Ballestar, E.; Boix-Chornet, M.; Franco, L. Conformational changes in the nucleosome followed by the selective accessibility of histone glutamines in the transglutaminase reaction: Effects of ionic strength. Biochemistry 2001, 40, 1922–1929. [Google Scholar] [CrossRef]
- Piacentini, M.; D’Eletto, M.; Farrace, M.G.; Rodolfo, C.; Del Nonno, F.; Ippolito, G.; Falasca, L. Characterization of distinct sub-cellular location of transglutaminase type II: Changes in intracellular distribution in physiological and pathological states. Cell Tissue Res. 2014, 358, 793–805. [Google Scholar] [CrossRef]
- Lukasak, B.J.; Mitchener, M.M.; Kong, L.; Dul, B.E.; Lazarus, C.D.; Ramakrishnan, A.; Ni, J.; Shen, L.; Maze, I.; Muir, T.W. TGM2-mediated histone transglutamination is dictated by steric accessibility. Proc. Natl. Acad. Sci. USA 2022, 119, e2208672119. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Esfarjani, P.; La Spada, A.R. Deja vu with a twist: Transglutaminases in bioenergetics and transcriptional dysfunction in Huntington’s disease. EMBO Mol. Med. 2010, 2, 335–337. [Google Scholar] [CrossRef] [PubMed]
- McConoughey, S.J.; Basso, M.; Niatsetskaya, Z.V.; Sleiman, S.F.; Smirnova, N.A.; Langley, B.C.; Mahishi, L.; Cooper, A.J.; Antonyak, M.A.; Cerione, R.A.; et al. Inhibition of transglutaminase 2 mitigates transcriptional dysregulation in models of Huntington disease. EMBO Mol. Med. 2010, 2, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Nelea, V.; Nakano, Y.; Kaartinen, M.T. Size distribution and molecular associations of plasma fibronectin and fibronectin crosslinked by transglutaminase 2. Protein J. 2008, 27, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Spurlin, T.A.; Bhadriraju, K.; Chung, K.H.; Tona, A.; Plant, A.L. The treatment of collagen fibrils by tissue transglutaminase to promote vascular smooth muscle cell contractile signaling. Biomaterials 2009, 30, 5486–5496. [Google Scholar] [CrossRef] [PubMed]
- Chau, D.Y.; Collighan, R.J.; Verderio, E.A.; Addy, V.L.; Griffin, M. The cellular response to transglutaminase-cross-linked collagen. Biomaterials 2005, 26, 6518–6529. [Google Scholar] [CrossRef] [PubMed]
- Forsprecher, J.; Wang, Z.; Nelea, V.; Kaartinen, M.T. Enhanced osteoblast adhesion on transglutaminase 2-crosslinked fibronectin. Amino Acids 2009, 36, 747–753. [Google Scholar] [CrossRef]
- Lorand, L.; Graham, R.M. Transglutaminases: Crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 2003, 4, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Belkin, A.M. Extracellular TG2: Emerging functions and regulation. FEBS J. 2011, 278, 4704–4716. [Google Scholar] [CrossRef] [PubMed]
- Lortat-Jacob, H.; Burhan, I.; Scarpellini, A.; Thomas, A.; Imberty, A.; Vives, R.R.; Johnson, T.; Gutierrez, A.; Verderio, E.A. Transglutaminase-2 interaction with heparin: Identification of a heparin binding site that regulates cell adhesion to fibronectin-transglutaminase-2 matrix. J. Biol. Chem. 2012, 287, 18005–18017. [Google Scholar] [CrossRef] [PubMed]
- Espitia Pinzon, N.; Sanz-Morello, B.; Breve, J.J.; Bol, J.G.; Drukarch, B.; Bauer, J.; Baron, W.; van Dam, A.M. Astrocyte-derived tissue Transglutaminase affects fibronectin deposition, but not aggregation, during cuprizone-induced demyelination. Sci. Rep. 2017, 7, 40995. [Google Scholar] [CrossRef]
- Condello, S.; Prasad, M.; Atwani, R.; Matei, D. Tissue transglutaminase activates integrin-linked kinase and beta-catenin in ovarian cancer. J. Biol. Chem. 2022, 298, 102242. [Google Scholar] [CrossRef] [PubMed]
- Tatsukawa, H.; Tani, Y.; Otsu, R.; Nakagawa, H.; Hitomi, K. Global identification and analysis of isozyme-specific possible substrates crosslinked by transglutaminases using substrate peptides in mouse liver fibrosis. Sci. Rep. 2017, 7, 45049. [Google Scholar] [CrossRef]
- Shinde, A.V.; Dobaczewski, M.; de Haan, J.J.; Saxena, A.; Lee, K.K.; Xia, Y.; Chen, W.; Su, Y.; Hanif, W.; Kaur Madahar, I.; et al. Tissue transglutaminase induction in the pressure-overloaded myocardium regulates matrix remodelling. Cardiovasc. Res. 2017, 113, 892–905. [Google Scholar] [CrossRef]
- Wang, Z.; Stuckey, D.J.; Murdoch, C.E.; Camelliti, P.; Lip, G.Y.H.; Griffin, M. Cardiac fibrosis can be attenuated by blocking the activity of transglutaminase 2 using a selective small-molecule inhibitor. Cell Death Dis. 2018, 9, 613. [Google Scholar] [CrossRef]
- Rudlong, J.; Cheng, A.; Johnson, G.V.W. The role of transglutaminase 2 in mediating glial cell function and pathophysiology in the central nervous system. Anal. Biochem. 2020, 591, 113556. [Google Scholar] [CrossRef]
- Elahi, A.; Emerson, J.; Rudlong, J.; Keillor, J.W.; Salois, G.; Visca, A.; Girardi, P.; Johnson, G.V.W.; Proschel, C. Deletion or Inhibition of Astrocytic Transglutaminase 2 Promotes Functional Recovery after Spinal Cord Injury. Cells 2021, 10, 2942. [Google Scholar] [CrossRef]
- Giera, S.; Luo, R.; Ying, Y.; Ackerman, S.D.; Jeong, S.J.; Stoveken, H.M.; Folts, C.J.; Welsh, C.A.; Tall, G.G.; Stevens, B.; et al. Microglial transglutaminase-2 drives myelination and myelin repair via GPR56/ADGRG1 in oligodendrocyte precursor cells. Elife 2018, 7, e33385. [Google Scholar] [CrossRef]
- Szondy, Z.; Korponay-Szabo, I.; Kiraly, R.; Sarang, Z.; Tsay, G.J. Transglutaminase 2 in human diseases. Biomedicine 2017, 7, 15. [Google Scholar] [CrossRef]
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-beta and tau in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chetelat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef]
- Tong, B.C.; Wu, A.J.; Li, M.; Cheung, K.H. Calcium signaling in Alzheimer’s disease & therapies. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1745–1760. [Google Scholar] [PubMed]
- Supnet, C.; Bezprozvanny, I. The dysregulation of intracellular calcium in Alzheimer disease. Cell Calcium 2010, 47, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Jeitner, T.M.; Muma, N.A.; Battaile, K.P.; Cooper, A.J. Transglutaminase activation in neurodegenerative diseases. Future Neurol. 2009, 4, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Schrank, S.; Barrington, N.; Stutzmann, G.E. Calcium-Handling Defects and Neurodegenerative Disease. Cold Spring Harb. Perspect. Biol. 2020, 12, a035212. [Google Scholar] [CrossRef]
- Panes, J.D.; Godoy, P.A.; Silva-Grecchi, T.; Celis, M.T.; Ramirez-Molina, O.; Gavilan, J.; Munoz-Montecino, C.; Castro, P.A.; Moraga-Cid, G.; Yevenes, G.E.; et al. Changes in PGC-1alpha/SIRT1 Signaling Impact on Mitochondrial Homeostasis in Amyloid-Beta Peptide Toxicity Model. Front. Pharmacol. 2020, 11, 709. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Rodriguez, M.; Bacskai, B.J. Mitochondria and Calcium in Alzheimer’s Disease: From Cell Signaling to Neuronal Cell Death. Trends Neurosci. 2021, 44, 136–151. [Google Scholar] [CrossRef]
- Yu, W.; Jin, H.; Huang, Y. Mitochondria-associated membranes (MAMs): A potential therapeutic target for treating Alzheimer’s disease. Clin. Sci. 2021, 135, 109–126. [Google Scholar] [CrossRef]
- Benilova, I.; Karran, E.; De Strooper, B. The toxic Abeta oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat. Neurosci. 2012, 15, 349–357. [Google Scholar] [CrossRef]
- Panes-Fernandez, J.; Godoy, P.A.; Gavilan, J.; Ramirez-Molina, O.; Burgos, C.F.; Marileo, A.; Flores-Nunez, O.; Castro, P.A.; Moraga-Cid, G.; Yevenes, G.E.; et al. TG2 promotes amyloid beta aggregates: Impact on ER-mitochondria crosstalk, calcium homeostasis and synaptic function in Alzheimer’s disease. Biomed. Pharmacother. 2023, 162, 114596. [Google Scholar] [CrossRef]
- Wilhelmus, M.M.; de Jager, M.; Smit, A.B.; van der Loo, R.J.; Drukarch, B. Catalytically active tissue transglutaminase colocalises with Abeta pathology in Alzheimer’s disease mouse models. Sci. Rep. 2016, 6, 20569. [Google Scholar] [CrossRef]
- Wilhelmus, M.M.M.; Chouchane, O.; Loos, M.; Jongenelen, C.A.M.; Breve, J.J.P.; Jonker, A.; Bol, J.; Smit, A.B.; Drukarch, B. Absence of tissue transglutaminase reduces amyloid-beta pathology in APP23 mice. Neuropathol. Appl. Neurobiol. 2022, 48, e12796. [Google Scholar] [CrossRef]
- Wilhelmus, M.M.M.; Tonoli, E.; Coveney, C.; Boocock, D.J.; Jongenelen, C.A.M.; Breve, J.J.P.; Verderio, E.A.M.; Drukarch, B. The Transglutaminase-2 Interactome in the APP23 Mouse Model of Alzheimer’s Disease. Cells 2022, 11, 389. [Google Scholar] [CrossRef]
- Johnson, G.V.; Cox, T.M.; Lockhart, J.P.; Zinnerman, M.D.; Miller, M.L.; Powers, R.E. Transglutaminase activity is increased in Alzheimer’s disease brain. Brain Res. 1997, 751, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, R.M.; Aschoff, A.; Niederwieser, G.; Heuberger, C.; Jirikowski, G. Cerebrospinal fluid tissue transglutaminase as a biochemical marker for Alzheimer’s disease. Neurobiol. Dis. 2002, 11, 106–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilhelmus, M.M.; Grunberg, S.C.; Bol, J.G.; van Dam, A.M.; Hoozemans, J.J.; Rozemuller, A.J.; Drukarch, B. Transglutaminases and transglutaminase-catalyzed cross-links colocalize with the pathological lesions in Alzheimer’s disease brain. Brain Pathol. 2009, 19, 612–622. [Google Scholar] [CrossRef] [PubMed]
- de Jager, M.; van der Wildt, B.; Schul, E.; Bol, J.G.; van Duinen, S.G.; Drukarch, B.; Wilhelmus, M.M. Tissue transglutaminase colocalizes with extracellular matrix proteins in cerebral amyloid angiopathy. Neurobiol. Aging 2013, 34, 1159–1169. [Google Scholar] [CrossRef][Green Version]
- Wilhelmus, M.M.M.; Jongenelen, C.A.; Bol, J.; Drukarch, B. Interaction between tissue transglutaminase and amyloid-beta: Protein-protein binding versus enzymatic crosslinking. Anal. Biochem. 2020, 592, 113578. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.N.; Wilson, J.H.; Lukas, T.J.; Kuret, J.; Lorand, L. Cross-linking sites of the human tau protein, probed by reactions with human transglutaminase. J. Neurochem. 1998, 71, 2607–2614. [Google Scholar] [CrossRef]
- Tucholski, J.; Kuret, J.; Johnson, G.V. Tau is modified by tissue transglutaminase in situ: Possible functional and metabolic effects of polyamination. J. Neurochem. 1999, 73, 1871–1880. [Google Scholar]
- Wenger, K.; Viode, A.; Schlaffner, C.N.; van Zalm, P.; Cheng, L.; Dellovade, T.; Langlois, X.; Bannon, A.; Chang, R.; Connors, T.R.; et al. Common mouse models of tauopathy reflect early but not late human disease. Mol. Neurodegener. 2023, 18, 10. [Google Scholar] [CrossRef]
- Halverson, R.A.; Lewis, J.; Frausto, S.; Hutton, M.; Muma, N.A. Tau protein is cross-linked by transglutaminase in P301L tau transgenic mice. J. Neurosci. 2005, 25, 1226–1233. [Google Scholar] [CrossRef]
- Khan, K.M.; Balasubramanian, N.; Gaudencio, G.; Wang, R.; Selvakumar, G.P.; Kolling, L.; Pierson, S.; Tadinada, S.M.; Abel, T.; Hefti, M.; et al. Human tau-overexpressing mice recapitulate brainstem involvement and neuropsychiatric features of early Alzheimer’s disease. Acta Neuropathol. Commun. 2023, 11, 57. [Google Scholar] [CrossRef]
- Citron, B.A.; SantaCruz, K.S.; Davies, P.J.; Festoff, B.W. Intron-exon swapping of transglutaminase mRNA and neuronal Tau aggregation in Alzheimer’s disease. J. Biol. Chem. 2001, 276, 3295–3301. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.M.; Zainelli, G.M.; Norlund, M.A.; Lee, J.M.; Muma, N.A. Transglutaminase bonds in neurofibrillary tangles and paired helical filament tau early in Alzheimer’s disease. Neurochem. Int. 2002, 40, 17–30. [Google Scholar] [CrossRef] [PubMed]
- McFarthing, K.; Buff, S.; Rafaloff, G.; Fiske, B.; Mursaleen, L.; Fuest, R.; Wyse, R.K.; Stott, S.R.W. Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2023 Update. J. Park. Dis. 2023, 13, 427–439. [Google Scholar] [CrossRef]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 101–113. [Google Scholar] [CrossRef]
- Junn, E.; Ronchetti, R.D.; Quezado, M.M.; Kim, S.Y.; Mouradian, M.M. Tissue transglutaminase-induced aggregation of alpha-synuclein: Implications for Lewy body formation in Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. USA 2003, 100, 2047–2052. [Google Scholar] [CrossRef]
- Andringa, G.; Lam, K.Y.; Chegary, M.; Wang, X.; Chase, T.N.; Bennett, M.C. Tissue transglutaminase catalyzes the formation of alpha-synuclein crosslinks in Parkinson’s disease. FASEB J. 2004, 18, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Grosso, H.; Woo, J.M.; Lee, K.W.; Im, J.Y.; Masliah, E.; Junn, E.; Mouradian, M.M. Transglutaminase 2 exacerbates alpha-synuclein toxicity in mice and yeast. FASEB J. 2014, 28, 4280–4291. [Google Scholar] [CrossRef]
- Zhang, J.; Grosso Jasutkar, H.; Yan, R.; Woo, J.M.; Lee, K.W.; Im, J.Y.; Junn, E.; Iismaa, S.E.; Mouradian, M.M. Transglutaminase 2 Depletion Attenuates alpha-Synuclein Mediated Toxicity in Mice. Neuroscience 2020, 441, 58–64. [Google Scholar] [CrossRef]
- Verhaar, R.; Jongenelen, C.A.; Gerard, M.; Baekelandt, V.; Van Dam, A.M.; Wilhelmus, M.M.; Drukarch, B. Blockade of enzyme activity inhibits tissue transglutaminase-mediated transamidation of alpha-synuclein in a cellular model of Parkinson’s disease. Neurochem. Int. 2011, 58, 785–793. [Google Scholar] [CrossRef]
- Citron, B.A.; Suo, Z.; SantaCruz, K.; Davies, P.J.; Qin, F.; Festoff, B.W. Protein crosslinking, tissue transglutaminase, alternative splicing and neurodegeneration. Neurochem. Int. 2002, 40, 69–78. [Google Scholar] [CrossRef]
- Schmid, A.W.; Chiappe, D.; Pignat, V.; Grimminger, V.; Hang, I.; Moniatte, M.; Lashuel, H.A. Dissecting the mechanisms of tissue transglutaminase-induced cross-linking of alpha-synuclein: Implications for the pathogenesis of Parkinson disease. J. Biol. Chem. 2009, 284, 13128–13142. [Google Scholar] [CrossRef]
- Min, B.; Kwon, Y.C.; Choe, K.M.; Chung, K.C. PINK1 phosphorylates transglutaminase 2 and blocks its proteasomal degradation. J. Neurosci. Res. 2015, 93, 722–735. [Google Scholar] [CrossRef]
- Leitao, A.D.G.; Rudolffi-Soto, P.; Chappard, A.; Bhumkar, A.; Lau, D.; Hunter, D.J.B.; Gambin, Y.; Sierecki, E. Selectivity of Lewy body protein interactions along the aggregation pathway of alpha-synuclein. Commun. Biol. 2021, 4, 1124. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Flower, M.D.; Ross, C.A.; Wild, E.J. Huntington disease: New insights into molecular pathogenesis and therapeutic opportunities. Nat. Rev. Neurol. 2020, 16, 529–546. [Google Scholar] [CrossRef]
- Thompson, L.M.; Orr, H.T. HD and SCA1: Tales from two 30-year journeys since gene discovery. Neuron 2023, 111, 3517–3530. [Google Scholar] [CrossRef]
- Arrasate, M.; Finkbeiner, S. Protein aggregates in Huntington’s disease. Exp. Neurol. 2012, 238, 1–11. [Google Scholar] [CrossRef]
- DiFiglia, M.; Sapp, E.; Chase, K.O.; Davies, S.W.; Bates, G.P.; Vonsattel, J.P.; Aronin, N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 1997, 277, 1990–1993. [Google Scholar] [CrossRef]
- Riguet, N.; Mahul-Mellier, A.L.; Maharjan, N.; Burtscher, J.; Croisier, M.; Knott, G.; Hastings, J.; Patin, A.; Reiterer, V.; Farhan, H.; et al. Nuclear and cytoplasmic huntingtin inclusions exhibit distinct biochemical composition, interactome and ultrastructural properties. Nat. Commun. 2021, 12, 6579. [Google Scholar] [CrossRef]
- Czeredys, M.; Gruszczynska-Biegala, J.; Schacht, T.; Methner, A.; Kuznicki, J. Expression of genes encoding the calcium signalosome in cellular and transgenic models of Huntington’s disease. Front. Mol. Neurosci. 2013, 6, 42. [Google Scholar] [CrossRef]
- Tang, T.S.; Tu, H.; Chan, E.Y.; Maximov, A.; Wang, Z.; Wellington, C.L.; Hayden, M.R.; Bezprozvanny, I. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron 2003, 39, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, M.; Oliveros, J.C.; Naranjo, J.R.; Carafoli, E. Neuronal Ca(2+) dyshomeostasis in Huntington disease. Prion 2013, 7, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Zainelli, G.M.; Ross, C.A.; Troncoso, J.C.; Muma, N.A. Transglutaminase cross-links in intranuclear inclusions in Huntington disease. J. Neuropathol. Exp. Neurol. 2003, 62, 14–24. [Google Scholar] [CrossRef]
- Zainelli, G.M.; Ross, C.A.; Troncoso, J.C.; Fitzgerald, J.K.; Muma, N.A. Calmodulin regulates transglutaminase 2 cross-linking of huntingtin. J. Neurosci. 2004, 24, 1954–1961. [Google Scholar] [CrossRef]
- Karpuj, M.V.; Garren, H.; Slunt, H.; Price, D.L.; Gusella, J.; Becher, M.W.; Steinman, L. Transglutaminase aggregates huntingtin into nonamyloidogenic polymers, and its enzymatic activity increases in Huntington’s disease brain nuclei. Proc. Natl. Acad. Sci. USA 1999, 96, 7388–7393. [Google Scholar] [CrossRef]
- Jeitner, T.M.; Matson, W.R.; Folk, J.E.; Blass, J.P.; Cooper, A.J. Increased levels of gamma-glutamylamines in Huntington disease CSF. J. Neurochem. 2008, 106, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Munsie, L.; Caron, N.; Atwal, R.S.; Marsden, I.; Wild, E.J.; Bamburg, J.R.; Tabrizi, S.J.; Truant, R. Mutant huntingtin causes defective actin remodeling during stress: Defining a new role for transglutaminase 2 in neurodegenerative disease. Hum. Mol. Genet. 2011, 20, 1937–1951. [Google Scholar] [CrossRef]
- Wurz, A.I.; Schulz, A.M.; O’Bryant, C.T.; Sharp, J.F.; Hughes, R.M. Cytoskeletal dysregulation and neurodegenerative disease: Formation, monitoring, and inhibition of cofilin-actin rods. Front. Cell Neurosci. 2022, 16, 982074. [Google Scholar] [CrossRef]
- Obrdlik, A.; Percipalle, P. The F-actin severing protein cofilin-1 is required for RNA polymerase II transcription elongation. Nucleus 2011, 2, 72–79. [Google Scholar] [CrossRef]
- Kelpsch, D.J.; Tootle, T.L. Nuclear Actin: From Discovery to Function. Anat. Rec. 2018, 301, 1999–2013. [Google Scholar] [CrossRef]
- Schulze-Krebs, A.; Canneva, F.; Stemick, J.; Plank, A.C.; Harrer, J.; Bates, G.P.; Aeschlimann, D.; Steffan, J.S.; von Horsten, S. Transglutaminase 6 Is Colocalized and Interacts with Mutant Huntingtin in Huntington Disease Rodent Animal Models. Int. J. Mol. Sci. 2021, 22, 8914. [Google Scholar] [CrossRef]
- Azam, S.; Haque, M.E.; Balakrishnan, R.; Kim, I.S.; Choi, D.K. The Ageing Brain: Molecular and Cellular Basis of Neurodegeneration. Front. Cell Dev. Biol. 2021, 9, 683459. [Google Scholar] [CrossRef]
- Jankovska, N.; Matej, R. Molecular Pathology of ALS: What We Currently Know and What Important Information Is Still Missing. Diagnostics 2021, 11, 1365. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.B.; Kittel, T.E.; Kim, R.B.; Bach, T.N.; Zhang, T.; Mitchell, C.S. Comparing therapeutic modulators of the SOD1 G93A Amyotrophic Lateral Sclerosis mouse pathophysiology. Front. Neurosci. 2022, 16, 1111763. [Google Scholar] [CrossRef]
- Fujita, K.; Honda, M.; Hayashi, R.; Ogawa, K.; Ando, M.; Yamauchi, M.; Nagata, Y. Transglutaminase activity in serum and cerebrospinal fluid in sporadic amyotrophic lateral sclerosis: A possible use as an indicator of extent of the motor neuron loss. J. Neurol. Sci. 1998, 158, 53–57. [Google Scholar] [CrossRef]
- Oono, M.; Okado-Matsumoto, A.; Shodai, A.; Ido, A.; Ohta, Y.; Abe, K.; Ayaki, T.; Ito, H.; Takahashi, R.; Taniguchi, N.; et al. Transglutaminase 2 accelerates neuroinflammation in amyotrophic lateral sclerosis through interaction with misfolded superoxide dismutase 1. J. Neurochem. 2014, 128, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Van Strien, M.E.; Baron, W.; Bakker, E.N.; Bauer, J.; Bol, J.G.; Breve, J.J.; Binnekade, R.; Van Der Laarse, W.J.; Drukarch, B.; Van Dam, A.M. Tissue transglutaminase activity is involved in the differentiation of oligodendrocyte precursor cells into myelin-forming oligodendrocytes during CNS remyelination. Glia 2011, 59, 1622–1634. [Google Scholar] [CrossRef]
- Chrobok, N.L.; Bol, J.; Jongenelen, C.A.; Breve, J.J.P.; El Alaoui, S.; Wilhelmus, M.M.M.; Drukarch, B.; van Dam, A.M. Characterization of Transglutaminase 2 activity inhibitors in monocytes in vitro and their effect in a mouse model for multiple sclerosis. PLoS ONE 2018, 13, e0196433. [Google Scholar]
- Chrobok, N.L.; Bol, J.; Wilhelmus, M.M.M.; Drukarch, B.; van Dam, A.M. Tissue Transglutaminase Appears in Monocytes and Macrophages but Not in Lymphocytes in White Matter Multiple Sclerosis Lesions. J. Neuropathol. Exp. Neurol. 2019, 78, 492–500. [Google Scholar] [CrossRef]
- Sestito, C.; Leurs, C.E.; Steenwijk, M.D.; Breve, J.J.P.; Twisk, J.W.R.; Wilhelmus, M.M.M.; Drukarch, B.; Teunissen, C.E.; van Dam, A.M.; Killestein, J. Tissue Transglutaminase Expression Associates With Progression of Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e998. [Google Scholar] [CrossRef]
- Pearse, D.D.; Hefley, A.B.; Morales, A.A.; Ghosh, M. Comparative Profiling of TG2 and Its Effectors in Human Relapsing Remitting and Progressive Multiple Sclerosis. Biomedicines 2022, 10, 1241. [Google Scholar] [CrossRef]
- Oh, K.; Park, H.B.; Seo, M.W.; Byoun, O.J.; Lee, D.S. Transglutaminase 2 exacerbates experimental autoimmune encephalomyelitis through positive regulation of encephalitogenic T cell differentiation and inflammation. Clin. Immunol. 2012, 145, 122–132. [Google Scholar] [CrossRef]
- van Strien, M.E.; de Vries, H.E.; Chrobok, N.L.; Bol, J.; Breve, J.J.P.; van der Pol, S.M.P.; Kooij, G.; van Buul, J.D.; Karpuj, M.; Steinman, L.; et al. Tissue Transglutaminase contributes to experimental multiple sclerosis pathogenesis and clinical outcome by promoting macrophage migration. Brain Behav. Immun. 2015, 50, 141–154. [Google Scholar] [CrossRef]
- Keillor, J.W.; Johnson, G.V.W. Transglutaminase 2 as a therapeutic target for neurological conditions. Expert. Opin. Ther. Targets 2021, 25, 721–731. [Google Scholar] [CrossRef]
- Keillor, J.W.; Apperley, K.Y.; Akbar, A. Inhibitors of tissue transglutaminase. Trends Pharmacol. Sci. 2015, 36, 32–40. [Google Scholar] [CrossRef]
- Kargbo, R.B. Development and Utilization of Novel Transglutaminase 2 Inhibitors for Potential Treatment of Autoimmune Disease and Gastrointestinal Disorder. ACS Med. Chem. Lett. 2023, 14, 1496–1497. [Google Scholar] [CrossRef]
- Siegel, M.; Khosla, C. Transglutaminase 2 inhibitors and their therapeutic role in disease states. Pharmacol. Ther. 2007, 115, 232–245. [Google Scholar] [CrossRef]
- Dickson, R.B.; Willingham, M.C.; Pastan, I. Binding and internalization of 125I-alpha 2-macroglobulin by cultured fibroblasts. J. Biol. Chem. 1981, 256, 3454–3459. [Google Scholar] [CrossRef]
- Igarashi, S.; Koide, R.; Shimohata, T.; Yamada, M.; Hayashi, Y.; Takano, H.; Date, H.; Oyake, M.; Sato, T.; Sato, A.; et al. Suppression of aggregate formation and apoptosis by transglutaminase inhibitors in cells expressing truncated DRPLA protein with an expanded polyglutamine stretch. Nat. Genet. 1998, 18, 111–117. [Google Scholar] [CrossRef]
- Jeitner, T.M.; Pinto, J.T.; Cooper, A.J.L. Cystamine and cysteamine as inhibitors of transglutaminase activity in vivo. Biosci. Rep. 2018, 38, BSR20180691. [Google Scholar] [CrossRef]
- Palanski, B.A.; Khosla, C. Cystamine and Disulfiram Inhibit Human Transglutaminase 2 via an Oxidative Mechanism. Biochemistry 2018, 57, 3359–3363. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. Therapeutic Applications of Cysteamine and Cystamine in Neurodegenerative and Neuropsychiatric Diseases. Front. Neurol. 2019, 10, 1315. [Google Scholar] [CrossRef]
- Arbez, N.; Roby, E.; Akimov, S.; Eddings, C.; Ren, M.; Wang, X.; Ross, C.A. Cysteamine Protects Neurons from Mutant Huntingtin Toxicity. J. Huntingt. Dis. 2019, 8, 129–143. [Google Scholar] [CrossRef]
- Basso, M.; Ratan, R.R. Transglutaminase is a therapeutic target for oxidative stress, excitotoxicity and stroke: A new epigenetic kid on the CNS block. J. Cereb. Blood Flow. Metab. 2013, 33, 809–818. [Google Scholar] [CrossRef]
- Dubinsky, R.; Gray, C. CYTE-I-HD: Phase I dose finding and tolerability study of cysteamine (Cystagon) in Huntington’s disease. Mov. Disord. 2006, 21, 530–533. [Google Scholar] [CrossRef]
- Prundean, A.; Youssov, K.; Humbert, S.; Bonneau, D.; Verny, C. A phase II, open-label evaluation of cysteamine tolerability in patients with Huntington’s disease. Mov. Disord. 2015, 30, 288–289. [Google Scholar] [CrossRef]
- Pardin, C.; Roy, I.; Lubell, W.D.; Keillor, J.W. Reversible and competitive cinnamoyl triazole inhibitors of tissue transglutaminase. Chem. Biol. Drug Des. 2008, 72, 189–196. [Google Scholar] [CrossRef]
- Caron, N.S.; Munsie, L.N.; Keillor, J.W.; Truant, R. Using FLIM-FRET to measure conformational changes of transglutaminase type 2 in live cells. PLoS ONE 2012, 7, e44159. [Google Scholar] [CrossRef]
- Kerr, C.; Szmacinski, H.; Fisher, M.L.; Nance, B.; Lakowicz, J.R.; Akbar, A.; Keillor, J.W.; Lok Wong, T.; Godoy-Ruiz, R.; Toth, E.A.; et al. Transamidase site-targeted agents alter the conformation of the transglutaminase cancer stem cell survival protein to reduce GTP binding activity and cancer stem cell survival. Oncogene 2017, 36, 2981–2990. [Google Scholar] [CrossRef]
- Eckert, R.L.; Fisher, M.L.; Grun, D.; Adhikary, G.; Xu, W.; Kerr, C. Transglutaminase is a tumor cell and cancer stem cell survival factor. Mol. Carcinog. 2015, 54, 947–958. [Google Scholar] [CrossRef]
- Case, A.; Stein, R.L. Kinetic analysis of the interaction of tissue transglutaminase with a nonpeptidic slow-binding inhibitor. Biochemistry 2007, 46, 1106–1115. [Google Scholar] [CrossRef]
- Pinilla, E.; Comerma-Steffensen, S.; Prat-Duran, J.; Rivera, L.; Matchkov, V.V.; Buus, N.H.; Simonsen, U. Transglutaminase 2 Inhibitor LDN 27219 Age-Dependently Lowers Blood Pressure and Improves Endothelium-Dependent Vasodilation in Resistance Arteries. Hypertension 2021, 77, 216–227. [Google Scholar] [CrossRef]
- Malkomes, P.; Lunger, I.; Oppermann, E.; Lorenz, J.; Faqar-Uz-Zaman, S.F.; Han, J.; Bothur, S.; Ziegler, P.; Bankov, K.; Wild, P.; et al. Transglutaminase 2 is associated with adverse colorectal cancer survival and represents a therapeutic target. Cancer Gene Ther. 2023, 30, 1346–1354. [Google Scholar] [CrossRef]
- Katt, W.P.; Blobel, N.J.; Komarova, S.; Antonyak, M.A.; Nakano, I.; Cerione, R.A. A small molecule regulator of tissue transglutaminase conformation inhibits the malignant phenotype of cancer cells. Oncotarget 2018, 9, 34379–34397. [Google Scholar] [CrossRef][Green Version]
- Cundy, N.J.; Arciszewski, J.; Gates, E.W.J.; Acton, S.L.; Passley, K.D.; Awoonor-Williams, E.; Boyd, E.K.; Xu, N.; Pierson, E.; Fernandez-Ansieta, C.; et al. Novel irreversible peptidic inhibitors of transglutaminase 2. RSC Med. Chem. 2023, 14, 378–385. [Google Scholar] [CrossRef]
- Mader, L.; Watt, S.K.I.; Iyer, H.R.; Nguyen, L.; Kaur, H.; Keillor, J.W. The war on hTG2: Warhead optimization in small molecule human tissue transglutaminase inhibitors. RSC Med. Chem. 2023, 14, 277–298. [Google Scholar] [CrossRef]
- Almami, I.S.; Aldubayan, M.A.; Felemban, S.G.; Alyamani, N.; Howden, R.; Robinson, A.J.; Pearson, T.D.Z.; Boocock, D.; Algarni, A.S.; Garner, A.C.; et al. Neurite outgrowth inhibitory levels of organophosphates induce tissue transglutaminase activity in differentiating N2a cells: Evidence for covalent adduct formation. Arch. Toxicol. 2020, 94, 3861–3875. [Google Scholar] [CrossRef]
- Choi, K.; Siegel, M.; Piper, J.L.; Yuan, L.; Cho, E.; Strnad, P.; Omary, B.; Rich, K.M.; Khosla, C. Chemistry and biology of dihydroisoxazole derivatives: Selective inhibitors of human transglutaminase 2. Chem. Biol. 2005, 12, 469–475. [Google Scholar] [CrossRef]
- Yuan, L.; Siegel, M.; Choi, K.; Khosla, C.; Miller, C.R.; Jackson, E.N.; Piwnica-Worms, D.; Rich, K.M. Transglutaminase 2 inhibitor, KCC009, disrupts fibronectin assembly in the extracellular matrix and sensitizes orthotopic glioblastomas to chemotherapy. Oncogene 2007, 26, 2563–2573. [Google Scholar] [CrossRef]
- Dafik, L.; Albertelli, M.; Stamnaes, J.; Sollid, L.M.; Khosla, C. Activation and inhibition of transglutaminase 2 in mice. PLoS ONE 2012, 7, e30642. [Google Scholar] [CrossRef][Green Version]
- Watts, R.E.; Siegel, M.; Khosla, C. Structure-activity relationship analysis of the selective inhibition of transglutaminase 2 by dihydroisoxazoles. J. Med. Chem. 2006, 49, 7493–7501. [Google Scholar] [CrossRef]
- Fisher, M.L.; Adhikary, G.; Xu, W.; Kerr, C.; Keillor, J.W.; Eckert, R.L. Type II transglutaminase stimulates epidermal cancer stem cell epithelial-mesenchymal transition. Oncotarget 2015, 6, 20525–20539. [Google Scholar] [CrossRef]
- Gundemir, S.; Monteagudo, A.; Akbar, A.; Keillor, J.W.; Johnson, G.V.W. The complex role of transglutaminase 2 in glioblastoma proliferation. Neuro Oncol. 2017, 19, 208–218. [Google Scholar] [CrossRef]
- Keillor, J.W.; Apperley, K.Y. Transglutaminase inhibitors: A patent review. Expert. Opin. Ther. Pat. 2016, 26, 49–63. [Google Scholar] [CrossRef]
- Akbar, A.; McNeil, N.M.R.; Albert, M.R.; Ta, V.; Adhikary, G.; Bourgeois, K.; Eckert, R.L.; Keillor, J.W. Structure-Activity Relationships of Potent, Targeted Covalent Inhibitors That Abolish Both the Transamidation and GTP Binding Activities of Human Tissue Transglutaminase. J. Med. Chem. 2017, 60, 7910–7927. [Google Scholar] [CrossRef]
- McNeil, N.M.R.; Gates, E.W.J.; Firoozi, N.; Cundy, N.J.; Leccese, J.; Eisinga, S.; Tyndall, J.D.A.; Adhikary, G.; Eckert, R.L.; Keillor, J.W. Structure-activity relationships of N-terminal variants of peptidomimetic tissue transglutaminase inhibitors. Eur. J. Med. Chem. 2022, 232, 114172. [Google Scholar] [CrossRef]
- Cano, A.; Sanchez-Lopez, E.; Ettcheto, M.; Lopez-Machado, A.; Espina, M.; Souto, E.B.; Galindo, R.; Camins, A.; Garcia, M.L.; Turowski, P. Current advances in the development of novel polymeric nanoparticles for the treatment of neurodegenerative diseases. Nanomedicine 2020, 15, 1239–1261. [Google Scholar] [CrossRef]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- Mistretta, M.; Farini, A.; Torrente, Y.; Villa, C. Multifaceted nanoparticles: Emerging mechanisms and therapies in neurodegenerative diseases. Brain 2023, 146, 2227–2240. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Mouradian, M.M. Pathogenetic Contributions and Therapeutic Implications of Transglutaminase 2 in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 2364. https://doi.org/10.3390/ijms25042364
Liu J, Mouradian MM. Pathogenetic Contributions and Therapeutic Implications of Transglutaminase 2 in Neurodegenerative Diseases. International Journal of Molecular Sciences. 2024; 25(4):2364. https://doi.org/10.3390/ijms25042364
Chicago/Turabian StyleLiu, Jun, and M. Maral Mouradian. 2024. "Pathogenetic Contributions and Therapeutic Implications of Transglutaminase 2 in Neurodegenerative Diseases" International Journal of Molecular Sciences 25, no. 4: 2364. https://doi.org/10.3390/ijms25042364
APA StyleLiu, J., & Mouradian, M. M. (2024). Pathogenetic Contributions and Therapeutic Implications of Transglutaminase 2 in Neurodegenerative Diseases. International Journal of Molecular Sciences, 25(4), 2364. https://doi.org/10.3390/ijms25042364






