The Decellularized Cell-Derived Extracellular Matrix Enhances the Paracrine Function of Human Mesenchymal Stromal/Stem Cells
Abstract
1. Introduction
2. Results
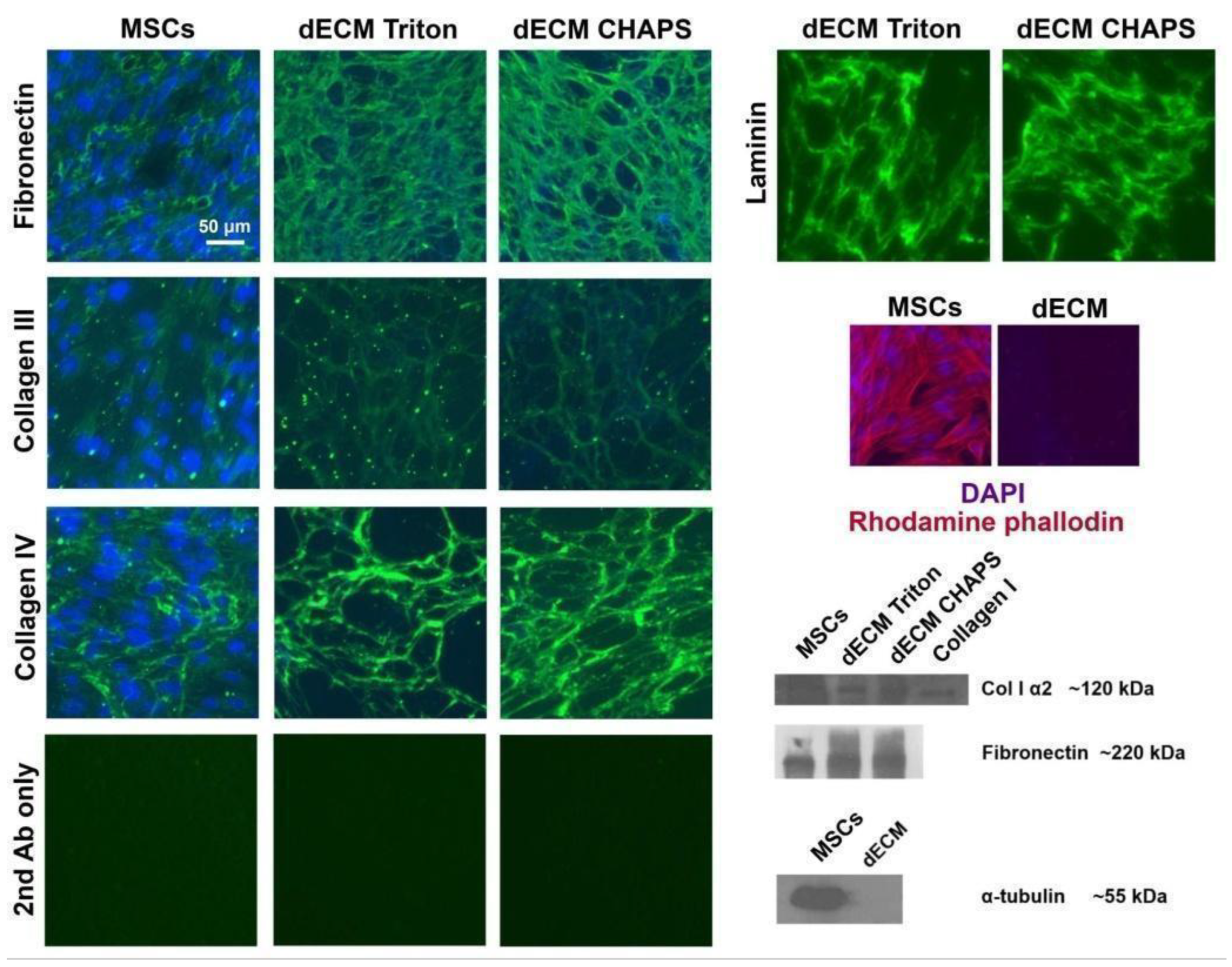
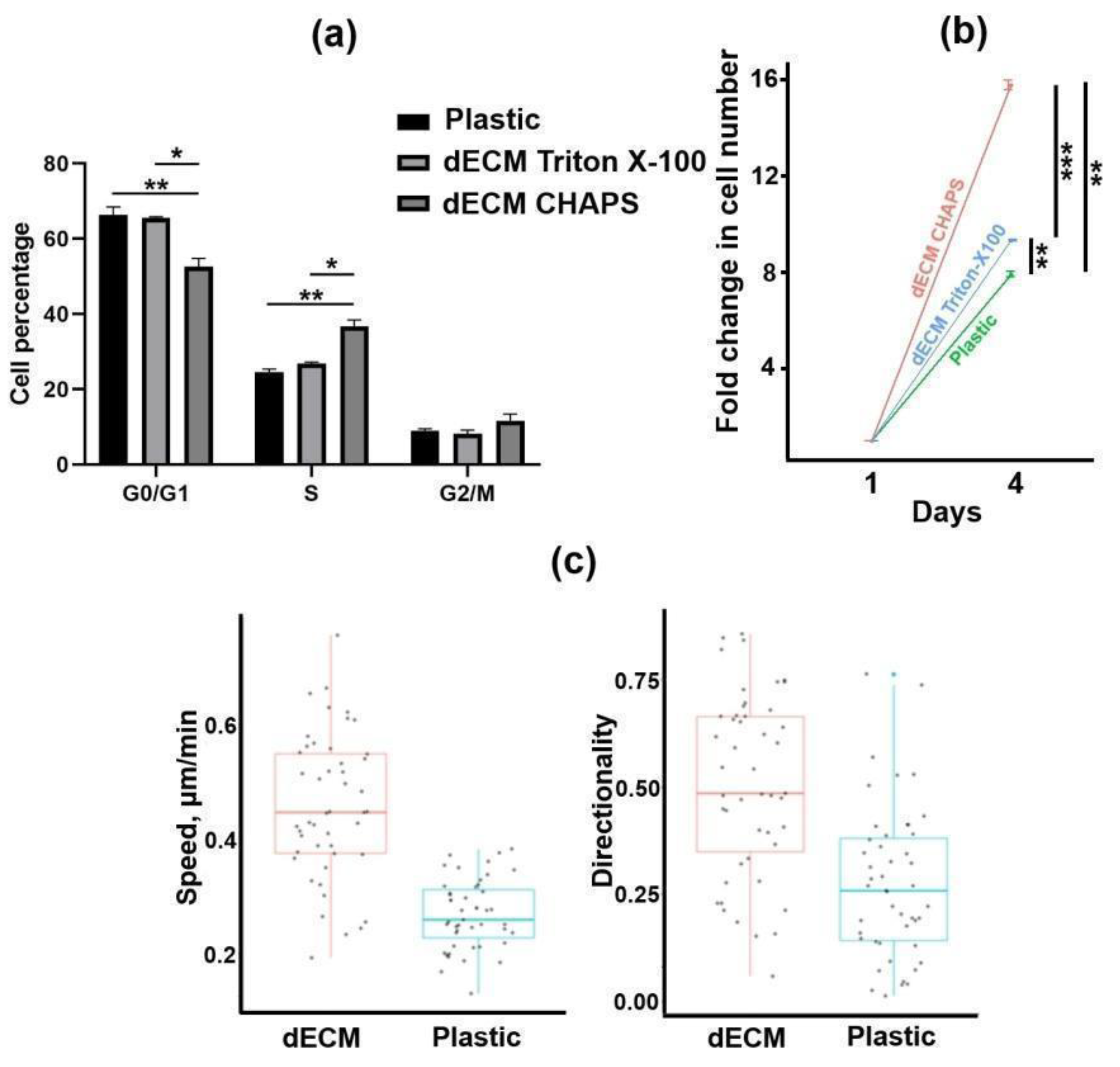
2.1. Production and Characterization of Bioactive dECM
2.2. Expression of Paracrine Factors by MSCs Cultured on dECM
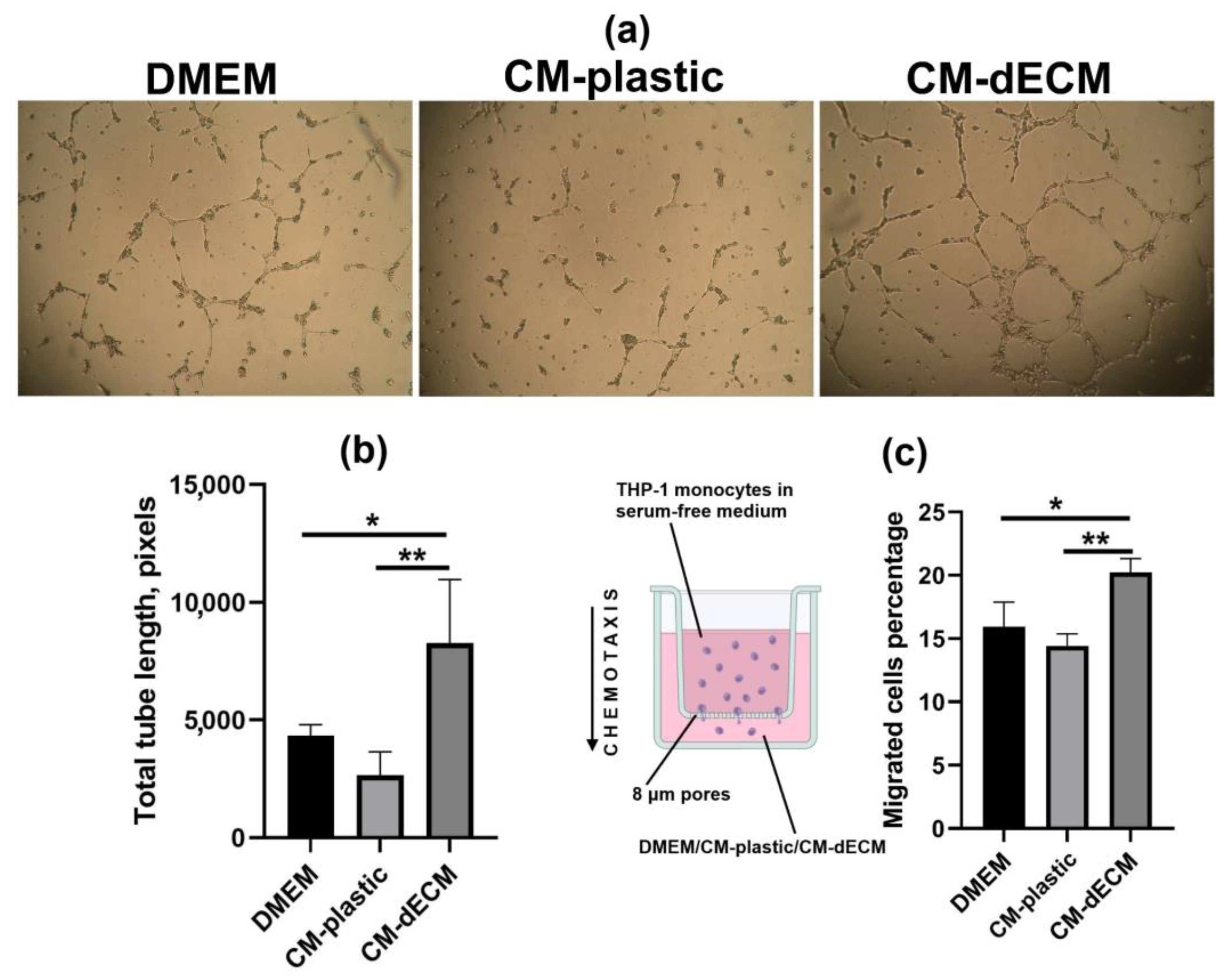
2.3. Conditioned Medium of MESCs Cultured on dECM Acquired Chemotactic and Angiogenic Properties
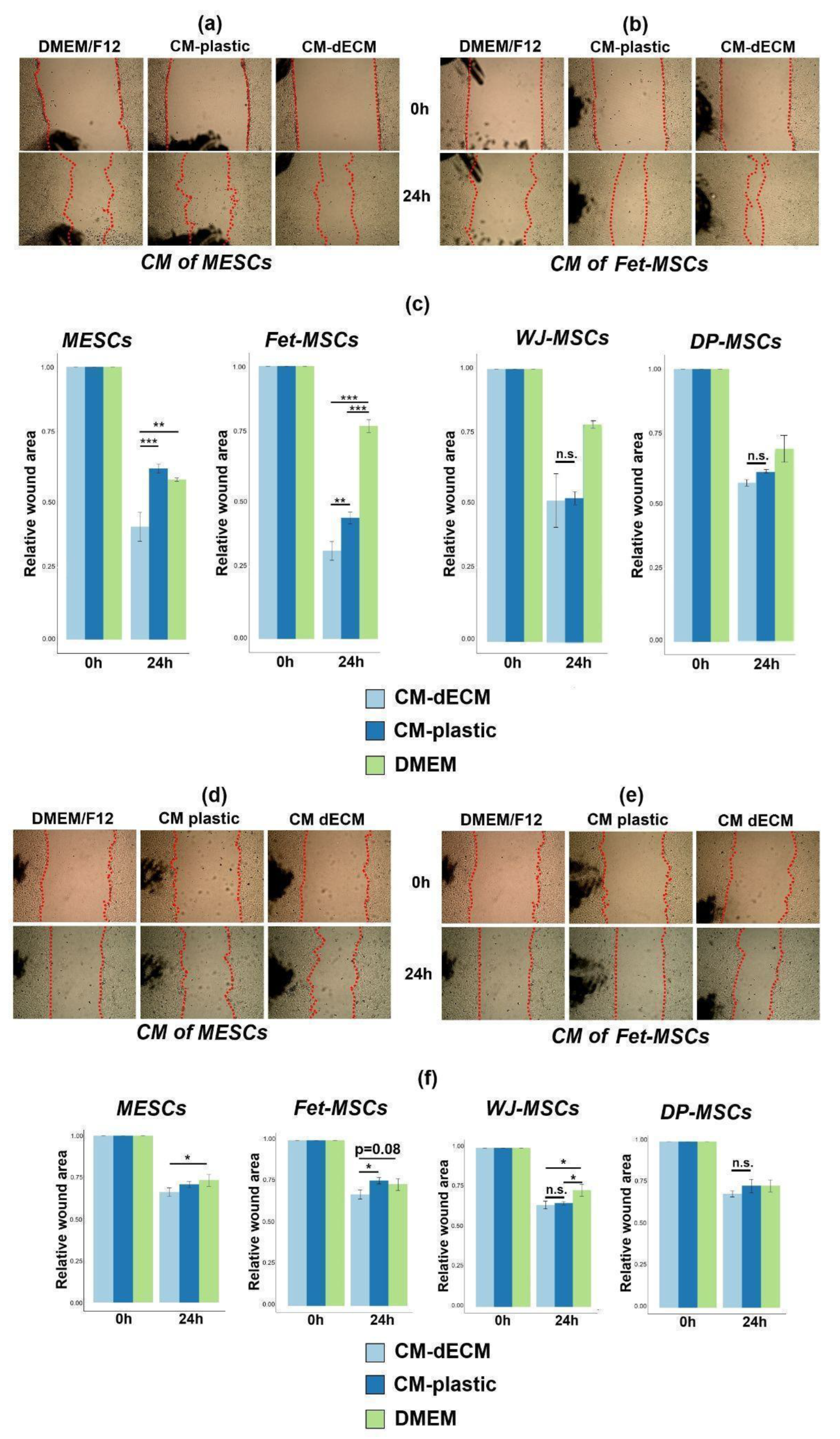
2.4. Conditioned Media of MSCs Cultured on dECM Stimulated 3T3 Fibroblast and HaCaT Keratinocyte Scratch Wound Healing
2.5. FAK Inhibition Promoted dECM-Induced Upregulation of Paracrine Factors
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Preparation of dECM
4.3. Immunofluorescence
4.4. Immunoblotting
4.5. Analysis of MESC Proliferation on dECM
4.6. Analysis of MESC Migration on dECM
4.7. RT-qPCR
4.8. Conditioned Medium Collection
4.9. Multiplex Immunoassay
4.10. Collagen Zymography
4.11. Scratch Wound Healing Assay
4.12. Transwell Migration Assay
4.13. Tube Formation Assay
4.14. IL-8 ELISA
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-Art Review. Sultan Qaboos Univ. Med. J. 2018, 18, e264. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Francos, S.; Eiro, N.; Costa, L.A.; Escudero-Cernuda, S.; Fernández-Sánchez, M.L.; Vizoso, F.J. Mesenchymal Stem Cells as a Cornerstone in a Galaxy of Intercellular Signals: Basis for a New Era of Medicine. Int. J. Mol. Sci. 2021, 22, 3576. [Google Scholar] [CrossRef]
- Galderisi, U.; Peluso, G.; Di Bernardo, G. Clinical Trials Based on Mesenchymal Stromal Cells Are Exponentially Increasing: Where Are We in Recent Years? Stem Cell Rev. Rep. 2022, 18, 23. [Google Scholar] [CrossRef]
- Sandonà, M.; Di Pietro, L.; Esposito, F.; Ventura, A.; Silini, A.R.; Parolini, O.; Saccone, V. Mesenchymal Stromal Cells and Their Secretome: New Therapeutic Perspectives for Skeletal Muscle Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 652970. [Google Scholar] [CrossRef]
- Yin, J.Q.; Zhu, J.; Ankrum, J.A. Manufacturing of Primed Mesenchymal Stromal Cells for Therapy. Nat. Biomed. Eng. 2019, 3, 90–104. [Google Scholar] [CrossRef]
- Noronha Nc, N.D.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming Approaches to Improve the Efficacy of Mesenchymal Stromal Cell-Based Therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef]
- Hynes, R.O. The Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Weng, W.; Zanetti, F.; Bovard, D.; Braun, B.; Ehnert, S.; Uynuk-Ool, T.; Histing, T.; Hoeng, J.; Nussler, A.K.; Aspera-Werz, R.H. A Simple Method for Decellularizing a Cell-Derived Matrix for Bone Cell Cultivation and Differentiation. J. Mater. Sci. Mater. Med. 2021, 32, 124. [Google Scholar] [CrossRef]
- Hoshiba, T.; Chen, G.; Endo, C.; Maruyama, H.; Wakui, M.; Nemoto, E.; Kawazoe, N.; Tanaka, M. Decellularized Extracellular Matrix as an In Vitro Model to Study the Comprehensive Roles of the ECM in Stem Cell Differentiation. Stem Cells Int. 2016, 2016, 6397820. [Google Scholar] [CrossRef]
- Novoseletskaya, E.; Grigorieva, O.; Nimiritsky, P.; Basalova, N.; Eremichev, R.; Milovskaya, I.; Kulebyakin, K.; Kulebyakina, M.; Rodionov, S.; Omelyanenko, N.; et al. Mesenchymal Stromal Cell-Produced Components of Extracellular Matrix Potentiate Multipotent Stem Cell Response to Differentiation Stimuli. Front. Cell Dev. Biol. 2020, 8, 555378. [Google Scholar] [CrossRef]
- Fan, G.; Wen, L.; Li, M.; Li, C.; Luo, B.; Wang, F.; Zhou, L.; Liu, L. Isolation of Mouse Mesenchymal Stem Cells with Normal Ploidy from Bone Marrows by Reducing Oxidative Stress in Combination with Extracellular Matrix. BMC Cell Biol. 2011, 12, 30. [Google Scholar] [CrossRef]
- Garcia-Velasco, J.A.; Arici, A. Interleukin-8 Expression in Endometrial Stromal Cells Is Regulated by Integrin-Dependent Cell Adhesion. Mol. Hum. Reprod. 1999, 5, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.Y.; Liu, Y.H.; Li, Y.W.; Yen, B.L.; Yen, M.L. Extracellular Matrix Protein Laminin Enhances Mesenchymal Stem Cell (MSC) Paracrine Function through Avβ3/CD61 Integrin to Reduce Cardiomyocyte Apoptosis. J. Cell. Mol. Med. 2017, 21, 1572–1583. [Google Scholar] [CrossRef]
- Clark, A.Y.; Martin, K.E.; García, J.R.; Johnson, C.T.; Theriault, H.S.; Han, W.M.; Zhou, D.W.; Botchwey, E.A.; García, A.J. Integrin-Specific Hydrogels Modulate Transplanted Human Bone Marrow-Derived Mesenchymal Stem Cell Survival, Engraftment, and Reparative Activities. Nat. Commun. 2020, 11, 114. [Google Scholar] [CrossRef]
- Choi, J.; Choi, W.; Joo, Y.; Chung, H.; Kim, D.; Oh, S.J.; Kim, S.H. FGF2-Primed 3D Spheroids Producing IL-8 Promote Therapeutic Angiogenesis in Murine Hindlimb Ischemia. NPJ Regen. Med. 2021, 6, 48. [Google Scholar] [CrossRef]
- Yoon, D.S.; Lee, K.M.; Kim, S.H.; Kim, S.H.; Jung, Y.; Kim, S.H.; Park, K.H.; Choi, Y.; Ryu, H.A.; Choi, W.J.; et al. Synergistic Action of IL-8 and Bone Marrow Concentrate on Cartilage Regeneration Through Upregulation of Chondrogenic Transcription Factors. Tissue Eng. Part A 2016, 22, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Bellayr, I.; Pan, H.; Choi, Y.; Li, Y. Regeneration of Soft Tissues Is Promoted by MMP1 Treatment after Digit Amputation in Mice. PLoS ONE 2013, 8, e59105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, S.; Qin, X.; Sun, D.; Wang, Y.; Xie, X.; Fan, W.; Wang, Y.; Liang, D.; Pei, X.; Cao, F. Effects of Hepatocyte Growth Factor Overexpressed Bone Marrow-Derived Mesenchymal Stem Cells on Prevention from Left Ventricular Remodelling and Functional Improvement in Infarcted Rat Hearts. Cell Biochem. Funct. 2012, 30, 574–581. [Google Scholar] [CrossRef]
- Chen, H.; Xia, R.; Li, Z.; Zhang, L.; Xia, C.; Ai, H.; Yang, Z.; Guo, Y. Mesenchymal Stem Cells Combined with Hepatocyte Growth Factor Therapy for Attenuating Ischaemic Myocardial Fibrosis: Assessment Using Multimodal Molecular Imaging. Sci. Rep. 2016, 6, 33700. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, R.T.; Li, Y.; Yang, Y.F.; Xiao, F.J.; Zhang, Y.K.; Wang, S.X.; Sun, H.Y.; Zhang, Q.W.; Wu, C.T.; et al. HGF Gene Modification in Mesenchymal Stem Cells Reduces Radiation-Induced Intestinal Injury by Modulating Immunity. PLoS ONE 2015, 10, e0124420. [Google Scholar] [CrossRef]
- Seo, K.W.; Sohn, S.Y.; Bhang, D.H.; Nam, M.J.; Lee, H.W.; Youn, H.Y. Therapeutic Effects of Hepatocyte Growth Factor-Overexpressing Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells on Liver Fibrosis in Rats. Cell Biol. Int. 2014, 38, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Roseren, F.; Pithioux, M.; Robert, S.; Balasse, L.; Guillet, B.; Lamy, E.; Roffino, S. Systemic Administration of G-CSF Accelerates Bone Regeneration and Modulates Mobilization of Progenitor Cells in a Rat Model of Distraction Osteogenesis. Int. J. Mol. Sci. 2021, 22, 3505. [Google Scholar] [CrossRef]
- Wright, C.R.; Ward, A.C.; Russell, A.P. Granulocyte Colony-Stimulating Factor and Its Potential Application for Skeletal Muscle Repair and Regeneration. Mediat. Inflamm. 2017, 2017, 7517350. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef]
- Ragelle, H.; Naba, A.; Larson, B.L.; Zhou, F.; Prijić, M.; Whittaker, C.A.; Del Rosario, A.; Langer, R.; Hynes, R.O.; Anderson, D.G. Comprehensive Proteomic Characterization of Stem Cell-Derived Extracellular Matrices. Biomaterials 2017, 128, 147–159. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, M.W.; Yoo, K.H.; Lee, T.H.; Kim, H.J.; Jang, I.K.; Chun, Y.H.; Kim, H.J.; Park, S.J.; Lee, S.H.; et al. Gene Expression Profiles of Human Adipose Tissue-Derived Mesenchymal Stem Cells Are Modified by Cell Culture Density. PLoS ONE 2014, 9, e83363. [Google Scholar] [CrossRef]
- Alexandrushkina, N.; Nimiritsky, P.; Eremichev, R.; Popov, V.; Arbatskiy, M.; Danilova, N.; Malkov, P.; Akopyan, Z.; Tkachuk, V.; Makarevich, P. Cell Sheets from Adipose Tissue MSC Induce Healing of Pressure Ulcer and Prevent Fibrosis via Trigger Effects on Granulation Tissue Growth and Vascularization. Int. J. Mol. Sci. 2020, 21, 5567. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Jiao, S.; Han, G.; Zhu, J.; Liu, T. Functional and Clinical Characteristics of Focal Adhesion Kinases in Cancer Progression. Front. Cell Dev. Biol. 2022, 10, 1040311. [Google Scholar] [CrossRef]
- He, X.; Chen, X.; Li, B.; Ji, J.; Chen, S. FAK Inhibitors Induce Cell Multinucleation and Dramatically Increase Pro-Tumoral Cytokine Expression in RAW 264.7 Macrophages. FEBS Lett. 2017, 591, 3861–3871. [Google Scholar] [CrossRef]
- Wu, F. Focal Adhesion Kinase Phosphorylation Increases Stemness, Proliferation, Inflammation and EMT of Breast Cancer Cells on Extracellular Matrix Scaffold. Master’s Thesis, Johns Hopkins University, Baltimore, MD, USA, 2022. [Google Scholar]
- Zemelko, V.I.; Grinchuk, T.M.; Domnina, A.P.; Artzibasheva, I.V.; Zenin, V.V.; Kirsanov, A.A.; Bichevaia, N.K.; Korsak, V.S.; Nikolsky, N.N. Multipotent Mesenchymal Stem Cells of Desquamated Endometrium: Isolation, Characterization, and Application as a Feeder Layer for Maintenance of Human Embryonic Stem Cells. Cell Tissue Biol. 2012, 6, 1–11. [Google Scholar] [CrossRef]
- Krylova, T.A.; Koltsova, A.M.; Zenin, V.V.; Musorina, A.S.; Yakovleva, T.K.; Poljanskaya, G.G. Comparative Characteristics of New Lines of Mesenchymal Stem Cells Derived from Human Embryonic Stem Cells, Bone Marrow, and Foreskin. Cell Tissue Biol. 2012, 6, 95–107. [Google Scholar] [CrossRef]
- Koltsova, A.M.; Krylova, T.A.; Musorina, A.S.; Zenin, V.V.; Turilova, V.I.; Yakovleva, T.K.; Poljanskaya, G.G. The Dynamics of Cell Properties during Long-Term Cultivation of Two Lines of Mesenchymal Stem Cells Derived from Wharton’s Jelly of Human Umbilical Cord. Cell Tissue Biol. 2018, 12, 7–19. [Google Scholar] [CrossRef]
- Koltsova, A.M.; Zenin, V.V.; Turilova, V.I.; Yakovleva, T.K.; Poljanskaya, G.G. The Derivation and Characterization of Mesenchymal Stem Cell Line, Isolated from Human Pulp of a Deciduous Tooth. Tsitologiya 2018, 60, 955–968. [Google Scholar] [CrossRef]
- Burova, E.; Borodkina, A.; Shatrova, A.; Nikolsky, N. Sublethal Oxidative Stress Induces the Premature Senescence of Human Mesenchymal Stem Cells Derived from Endometrium. Oxid. Med. Cell. Longev. 2013, 2013, 474931. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Ratushnyy, A.; Ezdakova, M.; Buravkova, L. Secretome of Senescent Adipose-Derived Mesenchymal Stem Cells Negatively Regulates Angiogenesis. Int. J. Mol. Sci. 2020, 21, 1802. [Google Scholar] [CrossRef]
- Inanc, S.; Keles, D.; Oktay, G. An Improved Collagen Zymography Approach for Evaluating the Collagenases MMP-1, MMP-8, and MMP-13. Biotechniques 2017, 63, 174–180. [Google Scholar] [CrossRef]


| Analyte | pg/mL | |||||||
|---|---|---|---|---|---|---|---|---|
| MESCs | Fet-MSCs | WJ-MSCs | DP-MSCs | |||||
| Plastic | dECM | Plastic | dECM | Plastic | dECM | Plastic | dECM | |
| sCD40L | 13.53 ± 0 | 21.84 ± 2.45 | 5.17 ± 0 | 17.58 ± 1.18 | 25.355 ± 2.5 | 39.94 ± 2.6 | 10.49 ± 0 | 18.43 ± 2.37 |
| EGF | 5.97 ± 0 | 7.195 ± 0.41 | 5.95 ± 0.92 | 5.97 ± 0 | 5.72 ± 0.11 | 8.91 ± 1.01 | 5.81 ± 1.96 | 5.885 ± 0.34 |
| Eotaxin | 4.11 ± 0 | 3.56 ± 0 | 2.99 ± 0 | 4.37 ± 0.36 | 44.785 ± 1.57 | 9.31 ± 0 | 5.61 ± 0 | 4.63 ± 0 |
| FGF-2 | <13.82 | 157.425 ± 10.81 | <13.82 | 131.49 ± 2.79 | 23.72 ± 0 | 672.86 ± 1.2 | 31.92 ± 5.06 | 32.71 ± 0 |
| FLT-3L | 2.34 ± 0 | 2.5 ± 0 | <0.91 | 1.14 ± 0.03 | <0.91 | 1.73 ± 0.02 | <0.91 | <0.91 |
| Fractalkine | <19.38 | < 19.38 | <19.38 | <19.38 | <19.38 | <19.38 | <19.38 | <19.38 |
| G-CSF | <3.39 | 374.21 ± 33.68 | 5.21 ± 0 | 843.07 ± 712.65 | 350.37 ± 23.83 | 2055.5 ± 543.76 | 35.58 ± 2.02 | 37.62 ± 21.99 |
| GM-CSF | 143.03 ± 0 | 917.01 ± 134.32 | 10.03 ± 9.82 | 4.915 ± 1.87 | 21.31 ± 2.88 | 236.49 ± 45.39 | 11.105 ± 9.11 | 9.525 ± 2.25 |
| GRO-α | 0.82 ± 0 | 179.2 ± 20.05 | 2.86 ± 1.46 | 2719.5 ± 392.44 | 2517 ± 214.96 | 9181.5 ± 651.24 | 8.04 ± 2.17 | 94.685 ± 3.95 |
| HGF | <9.26 | 406.13 ± 0 | <9.26 | 131.98 ± 0 | 43.18 ± 0 | 76.64 ± 0 | 13.15 ± 0 | 18.15 ± 0 |
| IFNα2 | <4.86 | <4.86 | <4.86 | <4.86 | <4.86 | <4.86 | <4.86 | <4.86 |
| IFNγ | <1.21 | <1.21 | <1.21 | <1.21 | <1.21 | 1.51 ± 0 | <1.21 | <1.21 |
| IL-1a | 5.89 ± 0 | 13.295 ± 3.93 | <3.83 | 5.23 ± 0 | 47.355 ± 0.27 | 110.53 ± 5.19 | <3.83 | <3.83 |
| IL-1b | <1.13 | 1.49 ± 0.12 | <1.13 | 1.32 ± 0.12 | 3.88 ± 0.07 | 15.45 ± 1.64 | <1.13 | 1.495 ± 0.12 |
| IL-1RA | <1.46 | <1.46 | <1.46 | <1.46 | <1.46 | <1.46 | 6.39 ± 0 | <1.46 |
| IL-2 | <0.46 | 1.12 ± 0.25 | <0.46 | 1.06 ± 0 | 0.73 ± 0.08 | 1.415 ± 0.16 | <0.46 | 0.59 ± 0.05 |
| IL-3 | <0.79 | 0.95 ± 0 | 0.9 ± 0 | <0.79 | <0.79 | <0.79 | 1.74 ± 0 | <0.79 |
| IL-4 | <0.58 | <0.58 | <0.58 | <0.58 | <0.58 | 0.74 ± 0.07 | 1.04 ± 0.24 | 2.565 ± 0.79 |
| IL-5 | <0.24 | <0.24 | <0.24 | <0.24 | <0.24 | <0.24 | <0.24 | <0.24 |
| IL-6 | 40.52 ± 0 | 386.6 ± 39.31 | 12.87 ± 6.02 | 200.57 ± 10.67 | 4962 ± 217.78 | 5913.5 ± 64.34 | 683.64 ± 179.97 | 1131.5 ± 51.61 |
| IL-7 | 1.39 ± 0 | 2.95 ± 0.74 | 1.67 ± 0.96 | 0.87 ± 0.16 | < 0.60 | 1.19 ± 0.32 | 1.68 ± 0.82 | 0.96 ± 0.03 |
| IL-8 | 26.47 ± 0 | 8112 ± 108.89 | 20.01 ± 9.78 | 6800.5 ± 509.82 | 3520 ± 28.28 | 6862 ± 657.60 | 117.41 ± 26.65 | 3008 ± 166.87 |
| IL-9 | 0.92 ± 0 | 1.84 ± 0 | 0.805 ± 0.16 | 1.61 ± 0.32 | 2.005 ± 0.07 | 3.11 ± 0.28 | 1.495 ± 0.16 | 1.61 ± 0 |
| IL-10 | <0.89 | <0.89 | <0.89 | <0.89 | <0.89 | 0.99 ± 0.12 | <0.89 | <0.89 |
| IL-12(p40) | 4.46 ± 0 | 8.25 ± 1.16 | 4.46 ± 0 | 9.08 ± 0 | 19.54 ± 1.45 | 29.18 ± 0 | 6.65 ± 1.09 | 9.95 ± 1.23 |
| IL-12(p70) | <2.24 | <2.24 | <2.24 | <2.24 | <2.24 | <2.24 | <2.24 | <2.24 |
| IL-13 | <4.23 | <4.23 | <4.23 | <4.23 | <4.23 | 5.15 ± 0 | <4.23 | 6.53 ± 0.28 |
| IL-15 | 50.59 ± 0 | 43.88 ± 1.99 | <2.45 | 4.68 ± 0.43 | 4.575 ± 0.28 | 10.035 ± 0.60 | 3.57 ± 0 | 2.97 ± 0.27 |
| IL-17A | <1.11 | 1.6 ± 0 | <1.11 | 1.32 ± 0.12 | 1.74 ± 0.19 | 4.02 ± 0.29 | 1.41 ± 0 | 1.23 ± 0 |
| IL-17/IL-25 | <19.59 | <19.59 | <19.59 | <19.59 | <19.59 | <19.59 | <19.59 | <19.59 |
| IL-17F | <16.95 | <16.95 | <16.95 | <16.95 | <16.95 | <16.95 | <16.95 | <16.95 |
| IL-18 | <0.53 | <0.53 | <0.53 | <0.53 | <0.53 | <0.53 | <0.53 | <0.53 |
| IL-22 | 28.25 ± 0 | 32.725 ± 1.23 | 35.39 ± 9.23 | 33.55 ± 4.09 | 31.25 ± 0.84 | 55.05 ± 5.46 | 48.32 ± 13.65 | 57.48 ± 3.36 |
| IL-27 | <10.65 | 17.65 ± 4.19 | 11.98 ± 0 | 15.94 ± 1.76 | 15.94 ± 1.76 | 24.72 ± 1.37 | 14.58 ± 3.68 | 19.44 ± 3.18 |
| IP-10 | <2.36 | <2.36 | <2.36 | <2.36 | <2.36 | <2.36 | <2.36 | <2.36 |
| MCP-1 | 4547 ± 0 | 7390.5 ± 212.83 | 320.645 ± 59.30 | 5872 ± 548.71 | 8974 ± 87.68 | 12.274.5 ± 596.09 | 2336.5 ± 581.94 | 2218.5 ± 314.66 |
| MCP-3 | <6.94 | 37.47 ± 1.4 | <6.94 | 62.67 ± 29.89 | 89.55 ± 3.84 | 4208.5 ± 3365.12 | 13.11 ± 0.39 | 17.78 ± 3.73 |
| M-CSF | 168.42 ± 0 | 163.5 ± 1.73 | 130.105 ± 12.29 | 150.24 ± 4.80 | 93.89 ± 0 | 104.55 ± 2.65 | 275.18 ± 23.17 | 214.97 ± 0 |
| MDC | <0.62 | <0.62 | <0.62 | <0.62 | <0.62 | <0.62 | <0.62 | <0.62 |
| MIG | <5.47 | <5.47 | <5.47 | <5.47 | <5.47 | <5.47 | <5.47 | <5.47 |
| MIP-1a | 10.71 ± 0 | 14.76 ± 0.25 | 8.27 ± 0.98 | 11.125 ± 0.58 | 13.66 ± 0.79 | 27.16 ± 0.50 | 11.31 ± 1.44 | 11.12 ± 0.58 |
| MIP-1b | <0.42 | <0.42 | <0.42 | <0.42 | <0.42 | 0.76 ± 0.28 | <0.42 | <0.42 |
| PDGF-AA | 10.4 ± 0 | <7.74 | <7.74 | <7.74 | 25.07 ± 0.12 | 121.03 ± 5.58 | <7.74 | <7.74 |
| PDGF-AB/BB | 15.23 ± 0 | 24.78 ± 1.89 | 12.46 ± 0 | 16.61 ± 1.95 | 17.99 ± 0 | 26.10 ± 3.76 | 24.06 ± 6.65 | 28.75 ± 3.72 |
| RANTES | <1.15 | 27.31 ± 0.28 | <1.15 | 9.4 ± 0.16 | <1.15 | 1.62 ± 0 | 1.51 ± 0 | 9.47 ± 0.38 |
| TGFα | <1.04 | 1.45 ± 0 | <1.04 | 1.33 ± 0 | 1.21 ± 0 | 2.715 ± 0.36 | 1.21 ± 0 | 1.21 ± 0 |
| TNFα | <4.02 | 4.28 ± 90 | <4.02 | <4.02 | 20.92 ± 1.34 | 28.405 ± 0.96 | 6.95 ± 1.92 | 12.125 ± 0.98 |
| TNFβ | 1.64 ± 0 | 1.47 ± 0 | <1.38 | 1.98 ± 0 | 6.835 ± 0.16 | 9.455 ± 0.92 | 4.535 ± 1.19 | 11.81 ± 0.16 |
| VEGF-A | 82.38 ± 0 | <2.36 | 39.08 ± 11.32 | 68.985 ± 1.54 | <2.36 | <2.36 | 262.215 ± 52.37 | 1040 ± 11.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ushakov, R.; Ratushnyy, A.; Buravkova, L.; Tolkunova, E.; Burova, E. The Decellularized Cell-Derived Extracellular Matrix Enhances the Paracrine Function of Human Mesenchymal Stromal/Stem Cells. Int. J. Mol. Sci. 2024, 25, 2419. https://doi.org/10.3390/ijms25042419
Ushakov R, Ratushnyy A, Buravkova L, Tolkunova E, Burova E. The Decellularized Cell-Derived Extracellular Matrix Enhances the Paracrine Function of Human Mesenchymal Stromal/Stem Cells. International Journal of Molecular Sciences. 2024; 25(4):2419. https://doi.org/10.3390/ijms25042419
Chicago/Turabian StyleUshakov, Roman, Andrey Ratushnyy, Ludmila Buravkova, Elena Tolkunova, and Elena Burova. 2024. "The Decellularized Cell-Derived Extracellular Matrix Enhances the Paracrine Function of Human Mesenchymal Stromal/Stem Cells" International Journal of Molecular Sciences 25, no. 4: 2419. https://doi.org/10.3390/ijms25042419
APA StyleUshakov, R., Ratushnyy, A., Buravkova, L., Tolkunova, E., & Burova, E. (2024). The Decellularized Cell-Derived Extracellular Matrix Enhances the Paracrine Function of Human Mesenchymal Stromal/Stem Cells. International Journal of Molecular Sciences, 25(4), 2419. https://doi.org/10.3390/ijms25042419







