The Pathophysiology and Treatment of Pyoderma Gangrenosum—Current Options and New Perspectives
Abstract
1. Introduction
2. Pathophysiology
3. Treatment
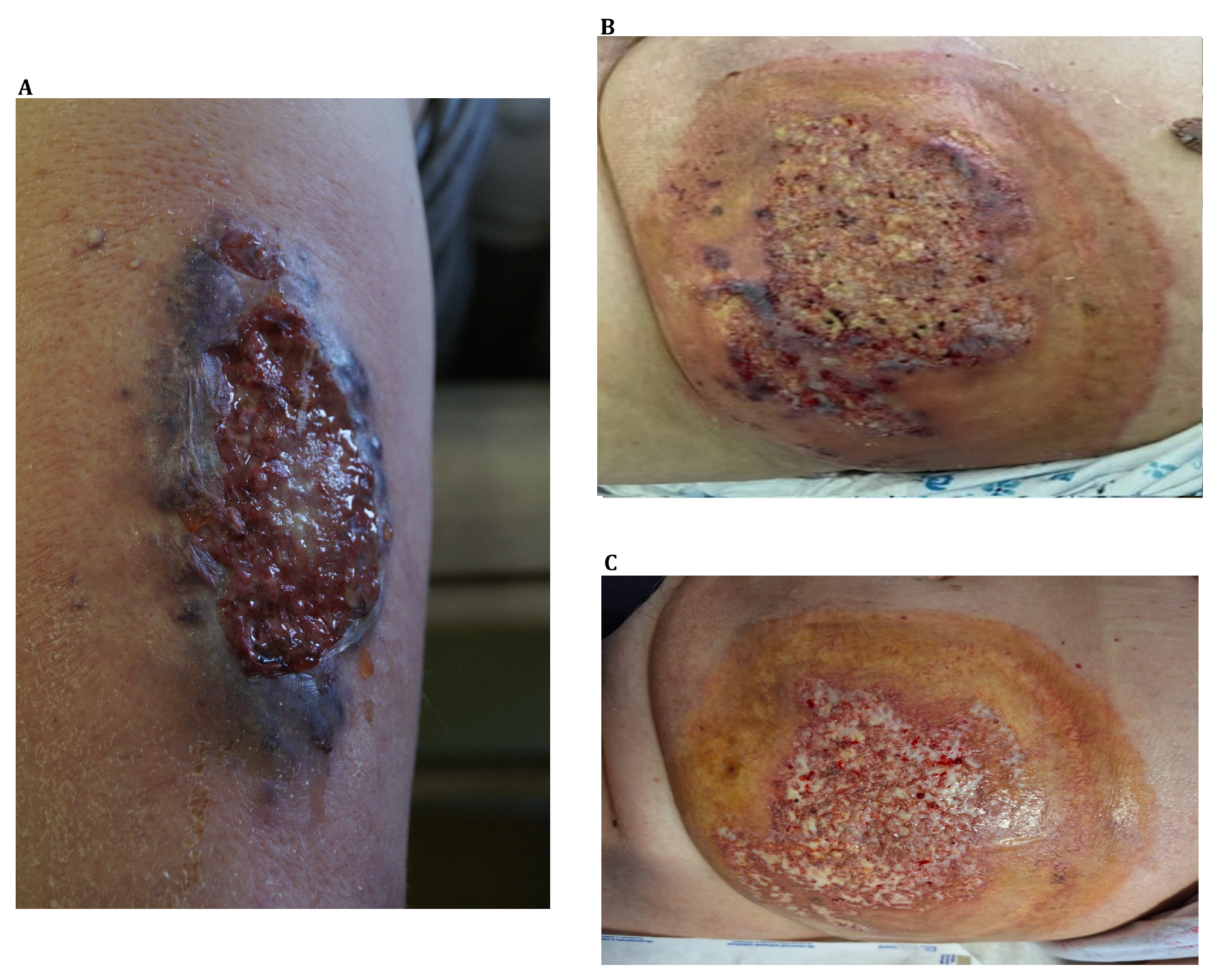
3.1. Wound Management
3.2. Topical Therapies
3.3. Intralesional Therapies
3.4. Systemic Therapies
3.4.1. Corticosteroids
3.4.2. Cyclosporine A (CsA)
3.4.3. Other Immunosuppressants
Mycophenolate Mofetil (MMF)
Methotrexate (MTX)
Azathioprine
3.4.4. Immunosuppressive Antibiotics
Dapsone
Minocycline
3.4.5. Intravenous Immunoglobulin (IVIG)
3.4.6. Tumor Necrosis Factor-α (TNF-α) Inhibitors
Infliximab
| Authors (Year) | Biologic Drug | Dosage Regimen | Study Type | Comorbidities | Efficacy |
|---|---|---|---|---|---|
| T.N. Brooklyn et al. (2006) [64] | Infliximab | 5 mg/kg i.v. at weeks 0, 2, and 6, followed by infusions every 6 to 8 weeks or placebo at week 0 with possible switch at week 2 | Randomized placebo- controlled trial | CD (n = 12) UC (n = 6) no IBD (n = 11) | Out of 29 patients in infliximab group, 20 (67%) demonstrated adequate response |
| M. Regueiro et al. (2003) [106] | Infliximab | 5 mg/kg i.v. | Multicenter retrospective study | IBD in all cases | Complete healing in all 13 cases |
| F. Argüelles-Arias et al. (2013) [107] | Infliximab | 5 mg/kg i.v. | Retrospective observational study | IBD in all cases | Out of 24 patients, 22 (92%) demonstrated complete healing |
| T. Ljung et al. (2002) [109] | Infliximab | 5 mg/kg i.v. | Case series (n = 8) | CD | Complete healing in 3 (37%) cases, partial healing in 3 (37%) |
| F. Salehzadeh et al. (2019) [110] | Infliximab | 100 mg i.v. | Case report | none known | Full recovery in 2-year period |
| M. R. Kaur et al. (2005) [111] | Infliximab | 3 mg/kg i.v. | Case report | none known | Full recovery in 4-month period |
| L. Đ. Betetto et al. (2022) [112] | Infliximab | 10 mg/kg i.v. | Case report | UC | Satisfactory result |
Adalimumab
Etanercept
3.4.7. Ustekinumab
3.4.8. IL-1 Antagonists
3.4.9. IL-17 Inhibitors
IL-23 Inhibitors
4. Future Directions
4.1. Janus Kinase Inhibitors (JAKi)
| Authors (Year) | JAKi | Dosage Regimen | Age and Gender | Comorbidities | Efficacy |
|---|---|---|---|---|---|
| B. Kochar et al. (2019) [173] | Tofacitinib |
|
| all patients with Crohn’s disease and concomitant arthritis previously resistant to various biologics |
|
| P.S. Olavarria et al. (2021) [174] | Tofacitinib | 10 mg p.o. twice daily | 69-year old female | ulcerative colitis and arthralgias | Complete healing after 4 weeks |
| L. G. M. Castro (2023) [168] | Baricitinib Tofacitinib | 2 mg p.o. twice daily for 39 days 5 mg p.o. twice daily for 120 days | 73-year old male 79-year old female | familial Mediterranean fever none known | Complete healing with no relapse |
| M. R. dos Santos et al. (2023) [169] | Upadacitinib | 15 mg p.o. daily | 45-year old female | rheumatoid arthritis | Complete regression after 6 weeks |
| M. Scheinberg et al. (2021) [170] | Baricitinib | 4 mg p.o. daily | 71-year old female | IgA multiple myeloma in remission | Complete regression after 5 weeks |
| S. Nasifoglu et al. (2018) [171] | Ruxolitinib | NA | 64-year old female | polycythemia vera | Complete healing |
4.2. Spesolimab
4.3. Vilobelimab
| Study Number | Medication | Study Phase/Type | Estimated Enrollment | Estimated Study Completion |
|---|---|---|---|---|
| NCT05964413 [179] | Vilobelimab | 3 | 90 | 13 February 2026 |
| NCT04750213 [120] | Adalimumab | observational | 60 | 31 August 2025 |
| NCT06092216 [177] | Spesolimab | 4 | 20 | September 2025 |
| NCT04901325 [172] | Baricitinib | 2 | 10 | 5 December 2024 |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maverakis, E.; Ma, C.; Shinkai, K.; Fiorentino, D.; Callen, J.P.; Wollina, U.; Marzano, A.V.; Wallach, D.; Kim, K.; Schadt, C.; et al. Diagnostic Criteria of Ulcerative Pyoderma Gangrenosum: A Delphi Consensus of International Experts. JAMA Dermatol. 2018, 154, 461–466. [Google Scholar] [CrossRef]
- Jockenhöfer, F.; Wollina, U.; Salva, K.A.; Benson, S.; Dissemond, J. The PARACELSUS score: A novel diagnostic tool for pyoderma gangrenosum. Br. J. Dermatol. 2019, 180, 615–620. [Google Scholar] [CrossRef]
- Haag, C.; Hansen, T.; Hajar, T.; Latour, E.; Keller, J.; Shinkai, K.; Ortega-Loayza, A.G. Comparison of Three Diagnostic Frameworks for Pyoderma Gangrenosum. J. Investig. Dermatol. 2021, 141, 59–63. [Google Scholar] [CrossRef]
- Hughes, A.P.; Jackson, J.M.; Callen, J.P. Clinical features and treatment of peristomal pyoderma gangrenosum. JAMA 2000, 284, 1546–1548. [Google Scholar] [CrossRef] [PubMed]
- Tolkachjov, S.N.; Fahy, A.S.; Cerci, F.B.; Wetter, D.A.; Cha, S.S.; Camilleri, M.J. Postoperative Pyoderma Gangrenosum: A Clinical Review of Published Cases. Mayo Clin. Proc. 2016, 91, 1267–1279. [Google Scholar] [CrossRef]
- Borda, L.J.; Wong, L.L.; Marzano, A.V.; Ortega-Loayza, A.G. Extracutaneous involvement of pyoderma gangrenosum. Arch. Dermatol. Res. 2019, 311, 425–434. [Google Scholar] [CrossRef] [PubMed]
- George, C.; Deroide, F.; Rustin, M. Pyoderma gangrenosum—A guide to diagnosis and management. Clin. Med. 2019, 19, 224–228. [Google Scholar] [CrossRef]
- Honma, M.; Sugawara, M.; Ueno, N.; Honma, M.; Hinooka, R.; Tani, C. Clinical Characteristics of Peristomal Pyoderma Gangrenosum: A Single Center Retrospective Observational Study. J. Dermatol. 2022, 49, 1178–1182. [Google Scholar] [CrossRef]
- Xu, A.; Balgobind, A.; Strunk, A.; Garg, A.; Alloo, A. Prevalence estimates for pyoderma gangrenosum in the United States: An age- and sex-adjusted population analysis. J. Am. Acad. Dermatol. 2020, 83, 425–429. [Google Scholar] [CrossRef]
- Langan, S.M.; Groves, R.W.; Card, T.R.; Gulliford, M.C. Incidence, mortality, and disease associations of pyoderma gangrenosum in the United Kingdom: A retrospective cohort study. J. Investig. Dermatol. 2012, 132, 2166–2170. [Google Scholar] [CrossRef] [PubMed]
- Monari, P.; Moro, R.; Motolese, A.; Misciali, C.; Baraldi, C.; Fanti, P.A.; Caccavale, S.; Puviani, M.; Olezzi, D.; Zampieri, P.; et al. Epidemiology of pyoderma gangrenosum: Results from an Italian prospective multicentre study. Int. Wound J. 2018, 15, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Kolios, A.G.; Gübeli, A.; Meier, B.; Maul, J.-T.; Kündig, T.; Nilsson, J.; Hafner, J.; Guenova, E.; Kerl, K.; Anliker, M.; et al. Clinical Disease Patterns in a Regional Swiss Cohort of 34 Pyoderma Gangrenosum Patients. Dermatology 2017, 233, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Ighani, A.; Al-Mutairi, D.; Rahmani, A.; Weizman, A.V.; Piguet, V.; Alavi, A. Pyoderma gangrenosum and its impact on quality of life: A multicentre, prospective study. Br. J. Dermatol. 2019, 180, 672–673. [Google Scholar] [CrossRef]
- Marzano, A.V.; Borghi, A.; Wallach, D.; Cugno, M. A Comprehensive Review of Neutrophilic Diseases. Clin. Rev. Allergy Immunol. 2018, 54, 114–130. [Google Scholar] [CrossRef]
- Wang, E.A.; Steel, A.; Luxardi, G.; Mitra, A.; Patel, F.; Cheng, M.Y.; Wilken, R.; Kao, J.; de Ga, K.; Sultani, H.; et al. Classic Ulcerative Pyoderma Gangrenosum Is a T Cell-Mediated Disease Targeting Follicular Adnexal Structures: A Hypothesis Based on Molecular and Clinicopathologic Studies. Front. Immunol. 2018, 8, 1980. [Google Scholar] [CrossRef]
- Weiss, D.I.; Ma, F.; Merleev, A.A.; Maverakis, E.; Gilliet, M.; Balin, S.J.; Bryson, B.D.; Ochoa, M.T.; Pellegrini, M.; Bloom, B.R.; et al. IL-1β Induces the Rapid Secretion of the Antimicrobial Protein IL-26 from Th17 Cells. J. Immunol. 2019, 203, 911–921. [Google Scholar] [CrossRef]
- Satoh, T.K.; Mellett, M.; Contassot, E.; French, L.E. Are neutrophilic dermatoses autoinflammatory disorders? Br. J. Dermatol. 2018, 178, 603–613. [Google Scholar] [CrossRef]
- Senra, L.; Mylonas, A.; Kavanagh, R.D.; Fallon, P.G.; Conrad, C.; Borowczyk-Michalowska, J.; Wrobel, L.J.; Kaya, G.; Yawalkar, N.; Boehncke, W.-H.; et al. IL-17E (IL-25) Enhances Innate Immune Responses during Skin Inflammation. J. Investig. Dermatol. 2019, 139, 1732–1742. [Google Scholar] [CrossRef]
- Smith, S.; Wu, P.W.; Seo, J.J.; Fernando, T.; Jin, M.; Contreras, J.; Montano, E.N.; Gabhann, J.N.; Cunningham, K.; Widaa, A.; et al. IL-16/miR-125a axis controls neutrophil recruitment in pristane-induced lung inflammation. JCI Insight 2018, 3, e120798. [Google Scholar] [CrossRef]
- Ergun, T. Pathergy Phenomenon. Front. Med. 2021, 8, 639404. [Google Scholar] [CrossRef]
- Le, S.T.; Wang, J.Z.; Alexanian, C.C.; Johng, S.Y.; Patel, F.B.; Wang, E.A.; Ma, C.; Wilken, R.; Cheng, M.Y.; Maverakis, E. End stage scurvy in the developed world: A diagnostic conundrum but not to be mistaken for pyoderma gangrenosum. Int. Wound J. 2019, 16, 1024. [Google Scholar] [CrossRef]
- Haag, C.K.; Nutan, F.; Cyrus, J.W.; Satpathy, J.; Shinkai, K.; Loayza, A.G.O. Pyoderma gangrenosum misdiagnosis resulting in amputation: A review. J. Trauma Acute Care Surg. 2019, 86, 307–313. [Google Scholar] [CrossRef]
- Bradsher, R.W. The Endemic Mimic: Blastomycosis An Illness Often Misdiagnosed. Trans. Am. Clin. Climatol. Assoc. 2014, 125, 188. [Google Scholar]
- Wallach, D.; Vignon-Pennamen, M.D. From acute febrile neutrophilic dermatosis to neutrophilic disease: Forty years of clinical research. J. Am. Acad. Dermatol. 2006, 55, 1066–1071. [Google Scholar] [CrossRef]
- Lorenz, U. SHP-1 and SHP-2 in T cells: Two phosphatases functioning at many levels. Immunol. Rev. 2009, 228, 342–359. [Google Scholar] [CrossRef]
- Zhang, J.; Somani, A.K.; Siminovitch, K.A. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin. Immunol. 2000, 12, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Nesterovitch, A.B.; Szanto, S.; Gonda, A.; Bardos, T.; Kis-Toth, K.; Adarichev, V.A.; Olasz, K.; Ghassemi-Najad, S.; Hoffman, M.D.; Tharp, M.D.; et al. Spontaneous insertion of a b2 element in the ptpn6 gene drives a systemic autoinflammatory disease in mice resembling neutrophilic dermatosis in humans. Am. J. Pathol. 2011, 178, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Nesterovitch, A.B.; Gyorfy, Z.; Hoffman, M.D.; Moore, E.C.; Elbuluk, N.; Tryniszewska, B.; Rauch, T.A.; Simon, M.; Kang, S.; Fisher, G.J.; et al. Alteration in the gene encoding protein tyrosine phosphatase nonreceptor type 6 (PTPN6/SHP1) may contribute to neutrophilic dermatoses. Am. J. Pathol. 2011, 178, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Nesterovitch, A.B.; Hoffman, M.D.; Simon, M.; Petukhov, P.A.; Tharp, M.D.; Glant, T.T. Mutations in the PSTPIP1 gene and aberrant splicing variants in patients with pyoderma gangrenosum. Clin. Exp. Dermatol. 2011, 36, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.J.; Allantaz, F.; Bennett, L.; Zhang, D.; Gao, X.; Wood, G.; Kastner, D.L.; Punaro, M.; Aksentijevich, I.; Pascual, V.; et al. Clinical, Molecular, and Genetic Characteristics of PAPA Syndrome: A Review. Curr. Genom. 2010, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Kastner, D.L.; Aksentijevich, I.; Goldbach-Mansky, R. Autoinflammatory disease reloaded: A clinical perspective. Cell 2010, 140, 784–790. [Google Scholar] [CrossRef]
- Marzano, A.V.; Borghi, A.; Meroni, P.L.; Cugno, M. Pyoderma gangrenosum and its syndromic forms: Evidence for a link with autoinflammation. Br. J. Dermatol. 2016, 175, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef]
- Wise, C.A.; Gillum, J.D.; Seidman, C.E.; Lindor, N.M.; Veile, R.; Bashiardes, S.; Lovett, M. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum. Mol. Genet. 2002, 11, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Genovese, G.; Moltrasio, C.; Garcovich, S.; Marzano, A.V. PAPA spectrum disorders. G. Ital. Dermatol. Venereol. 2020, 155, 542–550. [Google Scholar] [CrossRef]
- Moura, R.R.; Brandão, L.; Moltrasio, C.; Agrelli, A.; Tricarico, P.M.; Maronese, C.A.; Crovella, S.; Marzano, A.V. Different molecular pathways are disrupted in Pyoderma gangrenosum patients and are associated with the severity of the disease. Sci. Rep. 2023, 13, 4919. [Google Scholar] [CrossRef]
- Marzano, A.V.; Genovese, G.; Moltrasio, C.; Tricarico, P.M.; Gratton, R.; Piaserico, S.; Garcovich, S.; Boniotto, M.; Brandão, L.; Moura, R.; et al. Whole-Exome Sequencing in 10 Unrelated Patients with Syndromic Hidradenitis Suppurativa: A Preliminary Step for a Genotype-Phenotype Correlation. Dermatology 2022, 238, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.M.; Sullivan, G.P.; Clancy, D.M.; Afonina, I.S.; Kulms, D.; Martin, S.J. Neutrophil-Derived Proteases Escalate Inflammation through Activation of IL-36 Family Cytokines. Cell Rep. 2016, 14, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, F.; Streilein, R.D.; Hall, R.P. Increased E-selectin, IL-8 and IL-10 gene expression in human skin after minimal trauma. Exp. Dermatol. 2003, 12, 777–783. [Google Scholar] [CrossRef]
- Maverakis, E.; Van Den Elzen, P.; Sercarz, E.E. Self-reactive T cells and degeneracy of T cell recognition: Evolving concepts-from sequence homology to shape mimicry and TCR flexibility. J. Autoimmun. 2001, 16, 201–209. [Google Scholar] [CrossRef]
- Marzano, A.V.; Damiani, G.; Ceccherini, I.; Berti, E.; Gattorno, M.; Cugno, M. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br. J. Dermatol. 2017, 176, 1588–1598. [Google Scholar] [CrossRef]
- Braun-Falco, M.; Kovnerystyy, O.; Lohse, P.; Ruzicka, T. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)--a new autoinflammatory syndrome distinct from PAPA syndrome. J. Am. Acad. Dermatol. 2012, 66, 409–415. [Google Scholar] [CrossRef]
- Marzano, A.V.; Ishak, R.S.; Colombo, A.; Caroli, F.; Crosti, C. Pyoderma gangrenosum, acne and suppurative hidradenitis syndrome following bowel bypass surgery. Dermatology 2012, 225, 215–219. [Google Scholar] [CrossRef]
- Marzano, A.V.; Ceccherini, I.; Gattorno, M.; Fanoni, D.; Caroli, F.; Rusmini, M.; Grossi, A.; De Simone, C.; Borghi, O.M.; Meroni, P.L.; et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine 2014, 93, e187. [Google Scholar] [CrossRef]
- Lukens, J.R.; Kanneganti, T.-D. SHP-1 and IL-1α conspire to provoke neutrophilic dermatoses. Rare Dis. 2014, 2, e27742. [Google Scholar] [CrossRef]
- Lukens, J.R.; Vogel, P.; Johnson, G.R.; Kelliher, M.A.; Iwakura, Y.; Lamkanfi, M.; Kanneganti, T.-D. RIP1-driven autoinflammation targets IL-1α independently of inflammasomes and RIP3. Nature 2013, 498, 224. [Google Scholar] [CrossRef]
- Tartey, S.; Gurung, P.; Dasari, T.K.; Burton, A.; Kanneganti, T.D. ASK1/2 signaling promotes inflammation in a mouse model of neutrophilic dermatosis. J. Clin. Investig. 2018, 128, 2042–2047. [Google Scholar] [CrossRef]
- Tartey, S.; Gurung, P.; Samir, P.; Burton, A.; Kanneganti, T.-D. Cutting Edge: Dysregulated CARD9 Signaling in Neutrophils Drives Inflammation in a Mouse Model of Neutrophilic Dermatoses. J. Immunol. 2018, 201, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.; Xing, X.; Wolterink, L.; Barnes, D.H.; Yin, Z.; Reingold, L.; Kahlenberg, J.M.; Harms, P.W.; Gudjonsson, J.E. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J. Allergy Clin. Immunol. 2017, 140, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Carrier, Y.; Ma, H.-L.; Ramon, H.E.; Napierata, L.; Small, C.; O’Toole, M.; Young, D.A.; Fouser, L.A.; Nickerson-Nutter, C.; Collins, M.; et al. Inter-regulation of Th17 cytokines and the IL-36 cytokines in vitro and in vivo: Implications in psoriasis pathogenesis. J. Investig. Dermatol. 2011, 131, 2428–2437. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Horan, R.; Stefanska, A.; Carey, A.; Leon, G.; Aguilera, M.; Statovci, D.; Moran, T.; Fallon, P.; Shanahan, F.; et al. IL-36α expression is elevated in ulcerative colitis and promotes colonic inflammation. Mucosal Immunol. 2016, 9, 1193–1204. [Google Scholar] [CrossRef]
- Boutet, M.-A.; Bart, G.; Penhoat, M.; Amiaud, J.; Brulin, B.; Charrier, C.; Morel, F.; Lecron, J.-C.; Rolli-Derkinderen, M.; Bourreille, A.; et al. Distinct expression of interleukin (IL)-36α, β and γ, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and Crohn’s disease. Clin. Exp. Immunol. 2016, 184, 159. [Google Scholar] [CrossRef]
- Hessam, S.; Sand, M.; Gambichler, T.; Skrygan, M.; Rüddel, I.; Bechara, F.G. Interleukin-36 in hidradenitis suppurativa: Evidence for a distinctive proinflammatory role and a key factor in the development of an inflammatory loop. Br. J. Dermatol. 2018, 178, 761–767. [Google Scholar] [CrossRef]
- Pappu, R.; Ramirez-Carrozzi, V.; Sambandam, A. The interleukin-17 cytokine family: Critical players in host defence and inflammatory diseases. Immunology 2011, 134, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Senra, L.; Stalder, R.; Martinez, D.A.; Chizzolini, C.; Boehncke, W.H.; Brembilla, N.C. Keratinocyte-Derived IL-17E Contributes to Inflammation in Psoriasis. J. Investig. Dermatol. 2016, 136, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Marzano, A.V.; Fanoni, D.; Antiga, E.; Quaglino, P.; Caproni, M.; Crosti, C.; Meroni, P.L.; Cugno, M. Expression of cytokines, chemokines and other effector molecules in two prototypic autoinflammatory skin diseases, pyoderma gangrenosum and Sweet’s syndrome. Clin. Exp. Immunol. 2014, 178, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Yeon, H.B.; Lindor, N.M.; Seidman, J.G.; Seidman, C.E. Pyogenic arthritis, pyoderma gangrenosum, and acne syndrome maps to chromosome 15q. Am. J. Hum. Genet. 2000, 66, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Berking, C.; Nesbit, M.; Satyamoorthy, K.; Schaider, H.; Murphy, G.; Ichihashi, M.; Sauter, E.; Herlyn, M. Interleukin-8 overexpression is present in pyoderma gangrenosum ulcers and leads to ulcer formation in human skin xenografts. Lab. Investig. 2000, 80, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Rubas, K.; Reich, A.; Nowicka-Suszko, D.; Maj, J. The role of interleukins 6, 8, 17 and 23 in the pathogenesis of pyoderma gangrenosum. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e660–e662. [Google Scholar] [CrossRef] [PubMed]
- Su, W.P.D.; Sctiroeter, A.L.; Perry, H.O.; Powell, F.C. Histopathologic and immunopathologic study of pyoderma gangrenosum. J. Cutan. Pathol. 1986, 13, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Brooklyn, T.N.; Williams, A.M.; Dunnill, M.G.S.; Probert, C.S. T-cell receptor repertoire in pyoderma gangrenosum: Evidence for clonal expansions and trafficking. Br. J. Dermatol. 2007, 157, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; Mattson, J.; Basham, B.; Sedgwick, J.D.; McClanahan, T.; Kastelein, R.A.; Cua, D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef]
- Brooklyn, T.N.; Dunnill, M.G.S.; Shetty, A.; Bowden, J.J.; Williams, J.D.L.; Griffiths, C.E.M.; Forbes, A.; Greenwood, R.; Probert, C.S. Infliximab for the treatment of pyoderma gangrenosum: A randomised, double blind, placebo controlled trial. Gut 2006, 55, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Craig, F.F.; Thomas, K.S.; Mitchell, E.J.; Williams, H.C.; Norrie, J.; Mason, J.M.; Ormerod, A.D. UK Dermatology Clinical Trials Network’s STOP GAP trial (a multicentre trial of prednisolone versus ciclosporin for pyoderma gangrenosum): Protocol for a randomised controlled trial. Trials 2012, 13, 51. [Google Scholar] [CrossRef]
- Alavi, A.; French, L.E.; Davis, M.D.; Brassard, A.; Kirsner, R.S. Pyoderma Gangrenosum: An Update on Pathophysiology, Diagnosis and Treatment. Am. J. Clin. Dermatol. 2017, 18, 355–372. [Google Scholar] [CrossRef]
- Miller, J.; Yentzer, B.A.; Clark, A.; Jorizzo, J.L.; Feldman, S.R. Pyoderma gangrenosum: A review and update on new therapies. J. Am. Acad. Dermatol. 2010, 62, 646–654. [Google Scholar] [CrossRef]
- Croitoru, D.; Naderi-Azad, S.; Sachdeva, M.; Piguet, V.; Alavi, A. A Wound Care Specialist’s Approach to Pyoderma Gangrenosum. Adv. Wound Care 2020, 9, 686–694. [Google Scholar] [CrossRef]
- Almeida, I.R.; Coltro, P.S.; Gonçalves, H.O.C.; Westin, A.T.; Almeida, J.B.; Lima, R.V.K.S.; Silva, M.F.; Junior, J.A.F. The role of negative pressure wound therapy (NPWT) on the treatment of pyoderma gangrenosum: A systematic review and personal experience. Wound Repair Regen. 2021, 29, 486–494. [Google Scholar] [CrossRef]
- Bazaliński, D.; Krawiec, A.; Kucharzewski, M.; Więch, P. Negative Pressure Wound Therapy in Pyoderma Gangrenosum Treatment. Am. J. Case Rep. 2020, 21, 1–6. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Yanagi, T.; Sato, K.; Yoshimoto, N.; Hirata, Y.; Ujiie, I.; Nishimura, M.; Natsuga, K.; Shiiya, C.; Tsukinaga, I.; et al. Portable negative-pressure wound therapy for pyoderma gangrenosum: Report of two cases. J. Dermatol. 2018, 45, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, O.; Middleton, D.; Hunter, H. Negative-pressure wound therapy as an adjunct to treating pyoderma gangrenosum. J. R. Coll. Physicians Edinb. 2022, 52, 260–262. [Google Scholar] [CrossRef]
- Eisendle, K.; Thuile, T.; Deluca, J.; Pichler, M. Surgical Treatment of Pyoderma Gangrenosum with Negative Pressure Wound Therapy and Skin Grafting, Including Xenografts: Personal Experience and Comprehensive Review on 161 Cases. Adv. Wound Care 2020, 9, 405–425. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.S.; Ormerod, A.D.; Craig, F.E.; Greenlaw, N.; Norrie, J.; Mitchell, E.; Mason, J.M.; Johnston, G.A.; Wahie, S.; Williams, H.C.; et al. Clinical outcomes and response of patients applying topical therapy for pyoderma gangrenosum: A prospective cohort study. J. Am. Acad. Dermatol. 2016, 75, 940–949. [Google Scholar] [CrossRef]
- Lyon, C.C.; Stapleton, M.; Smith, A.J.; Mendelsohn, S.; Beck, M.H.; Griffiths, C.E.M. Topical tacrolimus in the management of peristomal pyoderma gangrenosum. J. Dermatol. Treat. 2001, 12, 13–17. [Google Scholar] [CrossRef]
- Chow, R.K.P.; Ho, V.C. Treatment of pyoderma gangrenosum. J. Am. Acad. Dermatol. 1996, 34, 1047–1060. [Google Scholar] [CrossRef]
- Mrowieiz, U.; Christophers, E. Clearing of pyoderma gangrenosum by intralesional cyclosporin A. Br. J. Dermatol. 1991, 125, 499. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, K.; Aflaki, E.; Akbarzade Jahromi, M.; Dastgheib, L. Successful Treatment of Classic Pyoderma Gangrenosum with Intralesional Infliximab Injection: A Case Report. Australas. J. Dermatol. 2023, 64, e252–e255. [Google Scholar] [CrossRef]
- Cozzani, E.; Gasparini, G.; Parodi, A. Pyoderma gangrenosum: A systematic review. G. Ital. Dermatol. Venereol. 2014, 149, 587–600. Available online: https://pubmed.ncbi.nlm.nih.gov/25213386/ (accessed on 20 April 2023).
- Pyoderma Gangrenosum: Treatment and Prognosis—UpToDate. Available online: https://www.uptodate.com/contents/pyoderma-gangrenosum-treatment-and-prognosis (accessed on 20 April 2023).
- Holt, P.J.; Davies, M.G.; Saunders, K.C.; Nuki, G. Pyoderma gangrenosum: Clinical and laboratory findings in 15 patients with special reference to polyarthritis. Medicine 1980, 59, 114–133. Available online: https://pubmed.ncbi.nlm.nih.gov/7360040/ (accessed on 20 April 2023). [CrossRef]
- Yamauchi, T.; Ishida, K.; Iwashima, Y.; Ikegaya, S.; Kawai, Y.; Ueda, T.; Wakahara, M.; Kumakiri, M. Successful treatment of pyoderma gangrenosum that developed in a patient with myelodysplastic syndrome. J. Infect. Chemother. 2003, 9, 268–271. [Google Scholar] [CrossRef]
- Ambooken, B.; Khader, A.; Muhammed, K.; Rajan, U.; Snigdha, O. Malignant pyoderma gangrenosum eroding the parotid gland successfully treated with dexamethasone pulse therapy. Int. J. Dermatol. 2014, 53, 1536–1538. [Google Scholar] [CrossRef]
- Ormerod, A.D.; Thomas, K.S.; Craig, F.E.; Mitchell, E.; Greenlaw, N.; Norrie, J.; Mason, J.M.; Walton, S.; Johnston, G.A.; Williams, H.C.; et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: Results of the STOP GAP randomised controlled trial. BMJ 2015, 350, h2958. [Google Scholar] [CrossRef] [PubMed]
- Perry, H.O. Pyoderma gangrenosum. South. Med. J. 1969, 62, 899–907. [Google Scholar] [CrossRef]
- Callen, J.P.; Jackson, J.M. Pyoderma gangrenosum: An update. Rheum. Dis. Clin. N. Am. 2007, 33, 787–802. [Google Scholar] [CrossRef]
- Graziano, F.; Macaluso, F.S.; Cassata, N.; Citrano, M.; Orlando, A. Pyoderma Gangrenosum in An Ulcerative Colitis Pediatric Patient During Vedolizumab Therapy Successfully Treated with Oral Cyclosporine. Inflamm. Bowel. Dis. 2021, 27, E110–E111. [Google Scholar] [CrossRef] [PubMed]
- Vidal, D.; Puig, L.; Gilaberte, M.; Alomar, A. Review of 26 cases of classical pyoderma gangrenosum: Clinical and therapeutic features. J. Dermatol. Treat. 2004, 15, 146–152. [Google Scholar] [CrossRef]
- Hasselmann, D.O.; Bens, G.; Tilgen, W.; Reichrath, J. Pyoderma gangrenosum: Clinical presentation and outcome in 18 cases and review of the literature. JDDG J. Der Dtsch. Dermatol. Ges. 2007, 5, 560–564. [Google Scholar] [CrossRef]
- Eaton, P.A.; Callen, J.P. Mycophenolate mofetil as therapy for pyoderma gangrenosum. Arch. Dermatol. 2009, 145, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kelly, R. Treatment of pyoderma gangrenosum with mycophenolate mofetil as a steroid-sparing agent. J. Am. Acad. Dermatol. 2013, 69, 565–569. [Google Scholar] [CrossRef]
- Hrin, M.L.; Bashyam, A.M.; Huang, W.W.; Feldman, S.R. Mycophenolate mofetil as adjunctive therapy to corticosteroids for the treatment of pyoderma gangrenosum: A case series and literature review. Int. J. Dermatol. 2021, 60, e486–e492. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Hrin, M.L.; Bowers, N.L.; Jorizzo, J.L.; Feldman, S.R.; Huang, W.W. Methotrexate for pyoderma gangrenosum: A retrospective case series of 33 patients. J. Am. Acad. Dermatol. 2023, 90, 642–644. [Google Scholar] [CrossRef]
- Sardar, P.; Guha, P.; Das, N.K.; Gharami, R.C.; Majumdar, S.; Banerjee, D.; Banerjee, R. Ulcerative pyoderma gangrenosum in mixed connective tissue disorder: A rare association and role of azathioprine in the management. Indian J. Dermatol. 2011, 56, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Nazir, A.; Zafar, A. Management of Idiopathic Pyoderma Gangrenosum with Azathioprine As the Primary Adjunct in an Asian Man: A Case Report. Cureus 2022, 14, e25177. [Google Scholar] [CrossRef]
- Wozel, G.; Blasum, C. Dapsone in dermatology and beyond. Arch. Dermatol. Res. 2014, 306, 103–124. [Google Scholar] [CrossRef]
- Galun, E.; Flugelman, M.Y.; Rachmilewitz, D. Pyoderma gangrenosum complicating ulcerative colitis: Successful treatment with methylprednisolone pulse therapy and dapsone. Am. J. Gastroenterol. 1986, 81, 988–989. Available online: https://pubmed.ncbi.nlm.nih.gov/3766502/ (accessed on 20 April 2023).
- Brown, R.E.; Lay, L.; Graham, D. Bilateral pyoderma gangrenosum of the hand: Treatment with dapsone. J. Hand Surg. Br. 1993, 18, 119–121. [Google Scholar] [CrossRef]
- Teasley, L.A.; Foster, C.S.; Baltatzis, S. Sclerokeratitis and facial skin lesions: A case report of pyoderma gangrenosum and its response to dapsone therapy. Cornea 2007, 26, 215–219. [Google Scholar] [CrossRef]
- Din, R.S.; Tsiaras, W.G.; Li, D.G.; Mostaghimi, A. Efficacy of Systemic Dapsone Treatment for Pyoderma Gangrenosum: A Retrospective Review. J. Drugs Dermatol. 2018, 17, 1058–1060. Available online: https://pubmed.ncbi.nlm.nih.gov/30365585/ (accessed on 20 April 2023). [PubMed]
- Shenefelt, P.D. Pyoderma gangrenosum associated with cystic acne and hidradenitis suppurativa controlled by adding minocycline and sulfasalazine to the treatment regimen. Cutis 1996, 57, 315–319. Available online: https://pubmed.ncbi.nlm.nih.gov/8726710/ (accessed on 20 April 2023).
- Reynolds, N.J.; Peachey, R.D. Response of atypical bullous pyoderma gangrenosum to oral minocycline hydrochloride and topical steroids. Acta. Derm. Venereol. 1990, 138, 538–539. Available online: https://pubmed.ncbi.nlm.nih.gov/1981437/ (accessed on 20 April 2023). [CrossRef]
- Miralles, E.S.; Nunez, M.; Perez, B.; Ledo, A. Minocycline hydrochloride hyperpigmentation complicating treatment of pyoderma gangrenosum. J. Dermatol. 1994, 21, 965–967. [Google Scholar] [CrossRef]
- Song, H.; Lahood, N.; Mostaghimi, A. Intravenous immunoglobulin as adjunct therapy for refractory pyoderma gangrenosum: Systematic review of cases and case series. Br. J. Dermatol. 2018, 178, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, U.; Romano, P.; Mulcahy, L.D.; Dooley, L.T.; Baker, D.G.; Gottlieb, A.B. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: A randomised trial. Lancet 2001, 357, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, M.; Valentine, J.; Plevy, S.; Fleisher, M.R.; Lichtenstein, G.R. Infliximab for treatment of pyoderma gangrenosum associated with inflammatory bowel disease. Am. J. Gastroenterol. 2003, 98, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Argüelles-Arias, F.; Castro-Laria, L.; Lobatón, T.; Aguas-Peris, M.; Rojas-Feria, M.; Acosta, M.B.-D.; Soto-Escribano, P.; Calvo-Moya, M.; Ginard-Vicens, D.; Chaparro-Sánchez, M.; et al. Characteristics and treatment of pyoderma gangrenosum in inflammatory bowel disease. Dig. Dis. Sci. 2013, 58, 2949–2954. [Google Scholar] [CrossRef]
- Tumor Necrosis Factor-Alpha Inhibitors: An Overview of Adverse Effects—UpToDate. Available online: https://www.uptodate.com/contents/tumor-necrosis-factor-alpha-inhibitors-an-overview-of-adverse-effects (accessed on 20 April 2023).
- Ljung, T.; Staun, M.; Grove, O.; Fausa, O.; Vatn, M.H.; Hellström, P.M. Pyoderma gangrenosum associated with crohn disease: Effect of TNF-alpha blockade with infliximab. Scand. J. Gastroenterol. 2002, 37, 1108–1110. [Google Scholar] [CrossRef] [PubMed]
- Salehzadeh, F.; Mohammadikebar, Y.; Enteshary, A.; Ghanbarpour, O.; Mirzarahimi, M. Infliximab in treatment of idiopathic refractory childhood pyoderma gangrenosum (PG). Biologics 2019, 13, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.R.; Lewis, H.M. Severe recalcitrant pyoderma gangrenosum treated with infliximab. Br. J. Dermatol. 2005, 153, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Betetto, L.Đ.; Točkova, O.; Suhodolčan, A.B. Mucocutaneous pyoderma gangrenosum: A case report and literature review. Acta Dermatovenerol. Alp. Pannonica Adriat. 2022, 31, S10–S13. [Google Scholar] [CrossRef]
- Yamasaki, K.; Yamanaka, K.; Zhao, Y.; Iwano, S.; Takei, K.; Suzuki, K.; Yamamoto, T. Adalimumab in Japanese patients with active ulcers of pyoderma gangrenosum: Final analysis of a 52-week phase 3 open-label study. J. Dermatol. 2022, 49, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Seishima, M.; Sasaki, M.; Sugie, S. Successful treatment of pyoderma gangrenosum using adalimumab in a patient undergoing hemodialysis. J. Dermatol. 2022, 49, e435–e436. [Google Scholar] [CrossRef] [PubMed]
- Ohmura, S.; Homma, Y.; Hanai, S.; Otsuki, Y.; Miyamoto, T. Successful switching treatment of adalimumab for refractory pyoderma gangrenosum in a patient with rheumatoid arthritis with prior use of tumour necrosis factor inhibitors: A case report and review of the literature. Mod. Rheumatol. Case Rep. 2023, 7, 9–13. [Google Scholar] [CrossRef]
- Campanati, A.; Brisigotti, V.; Ganzetti, G.; Molinelli, E.; Giuliodori, K.; Consales, V.; Racchini, S.; Bendia, E.; Offidani, A. Finally, recurrent pyoderma gangrenosum treated with Adalimumab: Case report and review of the literature. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1245–1247. [Google Scholar] [CrossRef]
- Kikuchi, N.; Hiraiwa, T.; Ohashi, T.; Hanami, Y.; Satoh, M.; Takenoshita, H.; Yamamoto, T. Pyoderma gangrenosum possibly triggered by adalimumab. Eur. J. Dermatol. 2012, 22, 804–805. [Google Scholar] [CrossRef]
- Benzaquen, M.; Monnier, J.; Beaussault, Y.; Rouby, F.; Berbis, P. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: Effectiveness of ustekinumab. Australas J. Dermatol. 2017, 58, e270–e271. [Google Scholar] [CrossRef]
- Tan, Y.; Kavaklieva, S.; Wood, F. Pyoderma gangrenosum induced by adalimumab in a seropositive rheumatoid arthritis patient: A paradoxical effect of adalimumab? Rheumatology 2021, 60, e288–e289. [Google Scholar] [CrossRef]
- A Study to Assess Adverse Events and Change in Disease State in Adult Participants Being Treated with Humira in Participants Diagnosed with Pyoderma Gangrenosum (PG). Available online: https://clinicaltrials.gov/study/NCT04750213?cond=Pyoderma%20Gangrenosum&rank=4&limit=10&aggFilters=status:rec%20not&page=1 (accessed on 2 January 2024).
- Ariane, M.; Bouaziz, J.D.; de Masson, A.; Jachiet, M.; Bagot, M.; Lepelletier, C. Efficacy and safety of etanercept for postoperative pyoderma gangrenosum after infliximab serum sickness. Dermatol. Ther. 2019, 32, e12774. [Google Scholar] [CrossRef]
- Kim, F.S.; Pandya, A.G. The use of etanercept in the treatment of peristomal pyoderma gangrenosum. Clin. Exp. Dermatol. 2012, 37, 442–443. [Google Scholar] [CrossRef]
- Roy, D.B.; Conte, E.T.; Cohen, D.J. The treatment of pyoderma gangrenosum using etanercept. J. Am. Acad. Dermatol. 2006, 54, S128–S134. [Google Scholar] [CrossRef]
- Rogge, F.J.; Pacifico, M.; Kang, N. Treatment of pyoderma gangrenosum with the anti-TNFalpha drug—Etanercept. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 431–433. [Google Scholar] [CrossRef]
- Haridas, V.; Shetty, P.; Dsouza, L.C.; Dinesh, U.S.; Haridas, K.; Bargale, A. Pyoderma gangrenosum in Sjögren’s syndrome and its successful treatment with topical application of etanercept. Int. J. Rheum. Dis. 2017, 20, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, G.; Jorizzo, J.L. Use of etanercept in treatment of pyoderma gangrenosum in a patient with autoimmune hepatitis. J. Dermatol. Treat. 2005, 16, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Pastor, N.; Betlloch, I.; Pascual, J.C.; Blanes, M.; Bañuls, J.; Silvestre, J.F. Pyoderma gangrenosum treated with anti-TNF alpha therapy (etanercept). Clin. Exp. Dermatol. 2006, 31, 152–153. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.W., 4th; Johnson, C.A.; Lynn, A. Treatment of pyoderma gangrenosum with etanercept. J. Drugs Dermatol. 2004, 3, 441–444. Available online: https://pubmed.ncbi.nlm.nih.gov/15303791/ (accessed on 20 April 2023). [PubMed]
- Guedes, R.; Moreira, A.; Menezes, N.; Baptista, A.; Varela, P. Treatment of thalidomide resistant pyoderma gangrenosum with etanercept. Acta Dermatovenerol. Croat. 2012, 20, 175–180. Available online: https://pubmed.ncbi.nlm.nih.gov/23069303/ (accessed on 20 April 2023). [PubMed]
- Abdallah, H.B.; Fogh, K.; Bech, R. Pyoderma gangrenosum and tumour necrosis factor alpha inhibitors: A semi-systematic review. Int. Wound J. 2019, 16, 511–521. [Google Scholar] [CrossRef]
- Kowalzick, L.; Bertolini, J.; Baumann, C.; Walther, B.; Truhm, B.; Eickenscheidt, L. Paradoxical Reaction to Etanercept: Development of Pyoderma Gangraenosum during Therapy of Psoriasis Arthritis. J. Dtsch. Dermatol. Ges. 2013, 11, 447–449. [Google Scholar] [CrossRef]
- Kleinpenning, M.M.; Langewouters, A.M.G.; Van De Kerkhof, P.C.M.; Greebe, R.J. Severe pyoderma gangrenosum unresponsive to etanercept and adalimumab. J. Dermatol. Treat. 2011, 22, 261–265. [Google Scholar] [CrossRef]
- Vallerand, I.A.; Hardin, J. Ustekinumab for the treatment of recalcitrant pyoderma gangrenosum: A case report. SAGE Open Med. Case Rep. 2019, 7, 2050313X1984520. [Google Scholar] [CrossRef]
- González, J.L.; Sáez, M.L.; Moraleda, I.M.; Martínez, Á.H. Pyoderma gangrenosum solved by ustekinumab therapy. Gastroenterol. Hepatol. 2021, 44, 299–300. [Google Scholar] [CrossRef]
- Fahmy, M.; Ramamoorthy, S.; Hata, T.; Sandborn, W.J. Ustekinumab for peristomal pyoderma gangrenosum. Am. J. Gastroenterol. 2012, 107, 794–795. [Google Scholar] [CrossRef] [PubMed]
- Low, Z.M.; Mar, A. Treatment of severe recalcitrant pyoderma gangrenosum with ustekinumab. Australas J. Dermatol. 2018, 59, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Cámara, P.G.; Ara, M.L.Z.; López, S.G. Ustekinumab in a patient with pyoderma gangrenosum and refractory Crohn’s disease. Med. Clin. 2019, 153, e35–e36. [Google Scholar] [CrossRef]
- Piqueras-García, J.; Sahuquillo-Torralba, A.J.; Torres-Navarro, I.; Botella-Estrada, R. Pyoderma Gangrenosum with Ulcerative Colitis Successfully Treated with Ustekinumab. Actas Dermosifiliogr. 2019, 110, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Nieto, D.; Sendagorta, E.; Rueda, J.M.; Herranz, P. Successful treatment with ustekinumab and vacuum-assisted closure therapy in recalcitrant myelodysplastic syndrome-associated pyoderma gangrenosum: Case report and literature review. Clin. Exp. Dermatol. 2019, 44, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Goldminz, A.M.; Botto, N.C.; Gottlieb, A.B. Severely recalcitrant pyoderma gangrenosum successfully treated with ustekinumab. J. Am. Acad. Dermatol. 2012, 67, e237–e238. [Google Scholar] [CrossRef] [PubMed]
- Petty, A.J.; Whitley, M.J.; Balaban, A.; Ellington, K.; Marano, A.L. Pyoderma gangrenosum induced by secukinumab in a patient with psoriasis successfully treated with ustekinumab. JAAD Case Rep. 2020, 6, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Cosgarea, I.; Lovric, Z.; Körber, A.; Dissemond, J. Successful treatment of refractory pyoderma gangrenosum with ustekinumab only after excision of renal cell carcinoma. Int. Wound J. 2016, 13, 1041–1042. [Google Scholar] [CrossRef]
- Guenova, E.; Teske, A.; Fehrenbacher, B.; Hoerber, S.; Adamczyk, A.; Schaller, M.; Hoetzenecker, W.; Biedermann, T. Interleukin 23 expression in pyoderma gangrenosum and targeted therapy with ustekinumab. Arch. Dermatol. 2011, 147, 1203–1205. [Google Scholar] [CrossRef]
- Nunes, G.; Patita, M.; Fernandes, V. Refractory Pyoderma Gangrenosum in a Patient with Crohn’s Disease: Complete Response to Ustekinumab. J. Crohns Colitis 2019, 13, 812–813. [Google Scholar] [CrossRef] [PubMed]
- Kolios, A.; Maul, J.; Meier, B.; Kerl, K.; Traidl-Hoffmann, C.; Hertl, M.; Zillikens, D.; Röcken, M.; Ring, J.; Facchiano, A.; et al. Canakinumab in adults with steroid-refractory pyoderma gangrenosum. Br. J. Dermatol. 2015, 173, 1216–1223. [Google Scholar] [CrossRef]
- Acierno, S.; Angrisani, F.; Marino, A.; Caporali, R.F.; Cimaz, R.; Giani, T. Canakinumab treatment in a young girl with refractory chronic recurrent multifocal osteomyelitis associated with pyoderma gangrenosum. Int. J. Rheum. Dis. 2022, 25, 1333–1338. [Google Scholar] [CrossRef]
- Jaeger, T.; Andres, C.; Grosber, M.; Zirbs, M.; Hein, R.; Ring, J.; Traidl-Hoffmann, C. Pyoderma gangrenosum and concomitant hidradenitis suppurativa--rapid response to canakinumab (anti-IL-1β). Eur. J. Dermatol. 2013, 23, 408–410. [Google Scholar] [CrossRef]
- O’Connor, C.; Gallagher, C.; Hollywood, A.; Paul, L.; O’Connell, M. Anakinra for recalcitrant pyoderma gangrenosum. Clin. Exp. Dermatol. 2021, 46, 1558–1560. [Google Scholar] [CrossRef] [PubMed]
- Coe, J.; Kudva, S.; Shams, K. Matching the dose to the disease: Successful treatment of recalcitrant pyoderma gangrenosum using high-dose secukinumab. Dermatol. Ther. 2022, 35, e15669. [Google Scholar] [CrossRef] [PubMed]
- Kao, A.S.; King, A.D.; Bardhi, R.; Daveluy, S. Targeted therapy with ixekizumab in pyoderma gangrenosum: A case series and a literature overview. JAAD Case Rep. 2023, 37, 49–53. [Google Scholar] [CrossRef]
- Tee, M.W.; Avarbock, A.B.; Ungar, J.; Frew, J.W. Rapid resolution of pyoderma gangrenosum with brodalumab therapy. JAAD Case Rep. 2020, 6, 1167–1169. [Google Scholar] [CrossRef]
- McPhie, M.L.; Kirchhof, M.G. Pyoderma gangrenosum treated with secukinumab: A case report. SAGE Open Med. Case Rep. 2020, 8, 2050313X2094043. [Google Scholar] [CrossRef]
- García, M.M.; González, M.M.; Lobato, J.M.P. Secukinumab for pyoderma gangrenosum: A case report. Med. Clin. 2019, 152, 246. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhou, S.; Xiong, H.; Yang, J.; Yang, Z.; Zhou, N.; Mao, J.; Li, M. A Case of Paradoxical Reactions to Biologic Therapy for Psoriasis. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Orita, A.; Hoshina, D.; Hirosaki, K. Pyoderma gangrenosum caused by secukinumab successfully treated with risankizumab: A case report and literature review. Clin. Exp. Dermatol. 2022, 47, 1372–1374. [Google Scholar] [CrossRef]
- Sadik, C.D.; Thieme, M.; Zillikens, D.; Terheyden, P. First emergence of pyoderma gangraenosum, palmoplantar pustulosis and sacroiliitis in a psoriasis patient associated with switching from secukinumab to brodalumab. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e406–e407. [Google Scholar] [CrossRef]
- Kao, A.S.; King, A.D.; Daveluy, S. Successful treatment of cabozantinib-induced pyoderma gangrenosum with ixekizumab therapy: A case report. Dermatol. Ther. 2022, 35, e15716. [Google Scholar] [CrossRef]
- Pollack, I.R.; Wolner, Z.J.; Hammett, J.; Swerlick, R.A. Pyoderma gangrenosum in a patient on ixekizumab. JAAD Case Rep. 2021, 16, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Oak, A.S.W.; Elewski, B.E. Use of IL-23 Inhibitors for the Treatment of Plaque Psoriasis and Psoriatic Arthritis: A Comprehensive Review. Am. J. Clin. Dermatol. 2021, 22, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K. New treatment of pyoderma gangrenosum and hidradenitis suppurativa: A review. J. Dermatol. 2023, 51, 172–179. [Google Scholar] [CrossRef]
- Leow, L.J.; Zubrzycki, N. Recalcitrant Ulcerative Pyoderma Gangrenosum of the Leg Responsive to Tildrakizumab: A Case Report. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1729–1736. [Google Scholar] [CrossRef]
- John, J.M.; Sinclair, R.D. Tildrakizumab for treatment of refractory pyoderma gangrenosum of the penis and polymyalgia rheumatica: Killing two birds with one stone. Australas. J. Dermatol. 2020, 61, 170–171. [Google Scholar] [CrossRef]
- Çalışkan, E.; Edek, Y.C.; Adışen, E.; İlter, N. Peristomal pyoderma gangrenosum treated with interleukin 23 inhibitor treatment: A case report. J. Eur. Acad. Dermatol. Venereol. 2023. [Google Scholar] [CrossRef]
- Baier, C.; Barak, O. Guselkumab as a treatment option for recalcitrant pyoderma gangrenosum. JAAD Case Rep. 2020, 8, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Reese, A.M.; Erickson, K.; Reed, K.B.; Ortega-Loayza, A.G. Modified dose of guselkumab for treatment of pyoderma gangrenosum. JAAD Case Rep. 2022, 21, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, B.; Schlott, S.; Ivanov, I.H.; Dissemond, J. Successful treatment of a refractory pyoderma gangrenosum with Risankizumab. Int. Wound J. 2020, 17, 1086–1088. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, L.V.; Martínez, M.L.; Pin, A.I.C. Off-label use of guselkumab for pyoderma gangrenosum. Med. Clin. 2023, 161, 226–227. [Google Scholar] [CrossRef]
- Castro, L.G.M. JAK inhibitors: A novel, safe, and efficacious therapy for pyoderma gangrenosum. Int. J. Dermatol. 2023, 62, 1088–1093. [Google Scholar] [CrossRef]
- Dos Santos, M.R.D.; Ianhez, M.; Ribeiro, B.N.; de Queiroz, B.B.; Miot, H.A. Refractory pyoderma gangrenosum associated with rheumatoid arthritis successfully treated with upadacitinib. Comments on: ‘JAK inhibitors: A novel, safe, and efficacious therapy for pyoderma gangrenosum. Int. J. Dermatol. 2023, 62, e595–e598. [Google Scholar] [CrossRef] [PubMed]
- Scheinberg, M.; Machado, L.A.; Castro, L.G.M.; Ferreira, S.B.; Michalany, N. Successful treatment of ulcerated pyoderma gangrenosum with baricitinib, a novel JAK inhibitor. J. Transl. Autoimmun. 2021, 4, 100099. [Google Scholar] [CrossRef]
- Nasifoglu, S.; Heinrich, B.; Welzel, J. Successful therapy for pyoderma gangrenosum with a Janus kinase 2 inhibitor. Br. J. Dermatol. 2018, 179, 504–505. [Google Scholar] [CrossRef]
- Baricitinib in the Treatment of Adults with Pyoderma Gangrenosum (PG). Available online: https://clinicaltrials.gov/study/NCT04901325?cond=Pyoderma%20Gangrenosum&rank=8&limit=10&aggFilters=status:rec%20not&page=1 (accessed on 2 January 2024).
- Kochar, B.; Herfarth, N.; Mamie, C.; Navarini, A.A.; Scharl, M.; Herfarth, H.H. Tofacitinib for the Treatment of Pyoderma Gangrenosum. Clin. Gastroenterol. Hepatol. 2019, 17, 991–993. [Google Scholar] [CrossRef]
- Olavarría, P.S.; Iturria, S.R.; Castillejo, Ó.N. Tofacitinib, a useful option for the treatment of pyoderma gangrenosum in an ulcerative colitis patient. Rev. Esp. De Enfermedades Dig. 2021, 113, 733–734. [Google Scholar] [CrossRef]
- Guénin, S.H.; Khattri, S.; Lebwohl, M.G. Spesolimab use in treatment of pyoderma gangrenosum. JAAD Case Rep. 2023, 34, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, X.; Guo, Q.; Qiao, Z.; Wang, N.; Pai, P.; Liu, X. Rapid Response to Spesolimab in a Patient with severe refractory Pyoderma Gangrenosum. Clin. Exp. Dermatol. 2023, 49, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Spesolimab in Pyoderma Gangrenosum. Available online: https://clinicaltrials.gov/study/NCT06092216?cond=Pyoderma%20Gangrenosum&rank=2&limit=10&aggFilters=status:rec%20not&page=1 (accessed on 2 January 2024).
- Exploratory Study of IFX-1 in Patients with Pyoderma Gangrenosum. Available online: https://clinicaltrials.gov/study/NCT03971643?cond=Pyoderma%20Gangrenosum&rank=12&limit=10&page=2 (accessed on 2 January 2024).
- Phase III Trial to Investigate Efficacy and Safety of Vilobelimab in Ulcerative Pyoderma Gangrenos. Available online: https://clinicaltrials.gov/study/NCT05964413?cond=Pyoderma%20Gangrenosum&rank=5&limit=10&aggFilters=status:rec%20not&page=1 (accessed on 2 January 2024).

| Authors (Year) | Systemic Drug | Dosage Regimen | Method of Administration | Others |
|---|---|---|---|---|
| T. Yamauchi et al. (2003) [82] | Methylprednisolone | 1 g for 3 days | i.v. | Dosage reduced within 2 weeks—therapy maintained with 30 mg prednisolone daily for 6 months |
| B. Ambooken et al. (2014) [83] | Dexamethasone | 100 mg in 500 mL 5% dextrose infused over 3–4 h on 3 consecutive days | i.v. | 9 pulses at 28 days intervals |
| A. D. Ormerod et al. (2015) [84] | Prednisolone | 0.75 mg/kg/day; maximum dose 75 mg/day | p.o. | - |
| A. D. Ormerod et al. (2015) [84] | Cyclosporine A | 4 mg/kg/day; maximum dose 400 mg/day | p.o. | - |
| P. A. Eaton et al. (2009). [90] | Mycophenolate mofetil | Initial dose 0.5/day or 1g/day; maximal dosages from 0.5 g 4 times daily to 2 g twice daily | p.o. | - |
| J. Li et al. (2013). [91] | Mycophenolate mofetil | 1 g or 2 g total daily | p.o. | The maintenance dose was 2 g or 3 g total daily; the average duration of treatment was 12.1 months |
| M. L. Hrin et al. (2021) [92] | Mycophenolate mofetil | 1g to 2.5 g daily | p.o. | - |
| J. A.Williams et al. (2023) [93] | Methotrexate | 5–25 mg | ND | 97% received concominant prednisone |
| P. Sardar et al. (2011) [94] | Azathioprine | 1 mg/kg daily | p.o. | Patient was unresponsive to systemic steroid and dapsone |
| E. Galun (1986) [97]; R.E. Brown (1993) [98] L. A. Teasley et al. (2007) [99]; R. S. Din et al. (2018) [100] | Dapsone | 50–200 mg daily | p.o. | Screening for glucose-6-phosphate dehydrogenase (G6PD) before and during treatment due to hematologic toxicity |
| P. D. Shenefelt et al. (1990) [101]; N. J. Reynolds et al. (1996) [102] | Minocycline | 100 mg twice daily | p.o. | Combination with other therapeutics |
| H. Song et al. (2018) [104] | Intravenous immunoglobulin | 2 g/kg | i.v. | The mean time to initial response of 3–5 weeks |
| Authors (Year) | Biologic Drug | Dosage Regimen | Study Type | Comorbidities | Efficacy |
|---|---|---|---|---|---|
| F. Argüelles-Arias et al. (2013) [107] | Adalimumab | 160/80 mg given s.c. at 0 and 2 weeks, and then every 2 weeks | Retrospective observational study | IBD in all cases | 7 patients, complete response |
| K. Yamasaki et al. (2022) [113] | Adalimumab | 160 mg s.c. at week 0, 80 mg at week 2, and then 40 mg every week from week 4 | Open-label study | UC; RA; hypertension; hyperlipidemia; hyperuricemia; osteoporosis | 12 (54.5%) of 22 patients reached a satisfactory outcome |
| M. Seishima et al. (2022) [114] | Adalimumab | 160 and 80 mg given s.c., biweekly, and then 40 mg weekly | Case report | History of systemic sarcoidosis; renal failure | Satisfactory result |
| S. Ohmura et al. (2023) [115] | Adalimumab | NA | Case report | RA | Satisfactory result |
| A. Campanati et al. (2015) [116] | Adalimumab | 160 mg s.c. at week 0, 80 mg at week 1, and then 40 mg every 2 weeks | Case report | CD | Complete healing after 12 weeks |
| Authors (Year) | Biologic Drug | Dosage Regimen | Study Type | Comorbidities | Efficacy |
|---|---|---|---|---|---|
| M. Ariane et al. (2019) [121] | Etanercept | 50 mg per week s.c. | Case report | None known; breast plastic surgery | Complete remission |
| F. S. Kim et al. (2012) [122] | Etanercept | 50 mg twice weekly; at 9 months, 50 mg per week s.c. | Case report | CD | Satisfactory result |
| D. B. Roy et al. (2006) [123] | Etanercept | 25 mg twice weekly s.c. | Case reports (n = 3) |
| Complete healing after 2 months in patients 1 and 3; satisfactory result in patient 2 |
| F. J. Rogge et al. (2008) [124] | Etanercept | 50 mg per week s.c. | Case report | None known | Complete healing after 7 months |
| V. Haridas et al. (2017) [125] | Etanercept | 1 mg/2 × 2 cm area—topical | Case report | Sjogren’s syndrome | Satisfactory result |
| G. Goldenberg et al. (2005) [126] | Etanercept | 25 mg twice weekly s.c. | Case report | Autoimmune hepatitis | Complete healing after 5 months |
| N. Pastor et al. (2005) [127] | Etanercept | 25 mg twice weekly s.c. | Case report | NA | Complete healing after 8 weeks |
| JW 4th McGowan et al. (2004) [128] | Etanercept | NA | Case report | NA | Satisfactory result |
| R. Guedes et al. (2012) [129] | Etanercept | NA | Case report | NA | Satisfactory result |
| M. M. Kleinpenning et al. (2011) [132] | Etanercept | 50 mg twice weekly s.c. | Case report | Hypogammaglobulinemia | Insufficient clinical improvement |
| Authors (Year) | Biologic Drug | Dosage Regimen | Study Type | Comorbidities | Efficacy |
|---|---|---|---|---|---|
| M. Benzaquen et al. (2017) [118] | Ustekinumab | 45 mg s.c. | Case report | psoriasis | Satisfactory result |
| I. A. Vallerand et al. (2019) [133] | Ustekinumab | 520 mg iv. Infusion at week 0, 90 mg s.c. at week 8 and then every 8 weeks | Case report | MG, DM, hypertension, dyslipidemia, CKD, gout, and obstructive sleep apnea | Complete healing after 6 months |
| J. López González et al. (2021) [134] | Ustekinumab | 260 mg iv. Infusion, then 90 mg s.c. every 8 weeks | Case report | CD | Satisfactory result |
| M. Fahmy et al. (2012) [135] | Ustekinumab | 90 mg s.c. at weeks 0 and 2, then every 8 weeks beginning at week 10 | Case report | UC | Complete healing by week 10 |
| Z. M. Low et al. (2018) [136] | Ustekinumab | 90 mg s.c. at weeks 0 and 4, then every 6 weeks, and later 45 mg every 3 weeks | Case report | NA | Significant improvement at 3 months |
| P. García Cámara et al. (2019) [137] | Ustekinumab | 520 mg iv. Infusion at week 0, then 90 mg s.c. every 8 weeks | Case report | CD | Complete healing after 12 months |
| J. Piqueras-García et al. (2019) [138] | Ustekinumab | 90 mg s.c. at weeks 0, 4, 10, and every 8 weeks thereafter | Case report | UC | Satisfactory result |
| D. Nieto et al. (2019) [139] | Ustekinumab | 90 mg s.c. every 8 weeks | Case report | Myelodysplastic syndrome | Complete healing after 20 weeks |
| A. M. Goldminz et al. (2012) [140] | Ustekinumab | 90 mg s.c. at weeks 0 and 4, and then every 8 weeks | Case report | None known | Satisfactory results after 22 weeks |
| A. J. Petty et al. (2020) [141] | Ustekinumab | 90 mg s.c. at weeks 0 and 4, and then every 8 weeks | Case report | Psoriasis and palmoplantar pustulosis | Satisfactory results after 2 doses |
| I. Cosgarea et al. (2016) [142] | Ustekinumab | NA | Case report | Renal cell carcinoma, chronic venous insufficiency, diabetes, hypertension | Complete healing after 3 months |
| E. Guenova et al. (2011) [143] | Ustekinumab | 45 mg s.c. at week 0 and week 4 | Case report | None known | Complete healing after 14 weeks |
| G. Nunes et al. (2019) [144] | Ustekinumab | 520 mg iv. Infusion, then 90 mg s.c. every 8 weeks | Case report | CD | Satisfactory result |
| Authors (Year) | Biologic Drug | Dosage Regimen | Study Type | Comorbidities | Efficacy |
|---|---|---|---|---|---|
| A. G. A. Kolios et al. (2015) [145] | Canakinumab | 150 mg s.c. at weeks 0 and 2, then 150–300 mg at week 4 if needed | Prospective, open-label study | none known | Complete healing in 4 out of 5 patients |
| S. Acierno et al. (2022) [146] | Canakinumab | 4 mg/kg s.c. every 4 weeks, after a year, 4 mg/kg every 8 weeks, because of exacerbation of the disease after a year of remission, return to the dosage of 4 mg/kg every 4 weeks | Case report | refractory chronic recurrent multifocal osteomyelitis | Satisfactory response |
| T. Jaeger et al. (2013) [147] | Canakinumab | 150 mg s.c. every 3–6 weeks, a total of 8 injections | Case report | HS | Complete remission in 1 year |
| C. O’Connor et al. (2021) [148] | Anakinra | 2 mg/kg s.c. daily in 4 weeks, then 100 mg daily | Case report |
| Complete healing in 4 months |
| Authors (Year) | Biologic Drug | Dosage Regimen | Study Type | Comorbidities | Efficacy |
|---|---|---|---|---|---|
| J. Coe et al. (2022) [149] | Secukinumab | 300 mg s.c. four weekly; after 2 months, 300 mg two weekly | Case report | Depression, osteoarthritis, hiatus hernia, Gilbert’s syndrome, and previous hepatitis A infection | Complete healing after a year of high-dose therapy |
| A.S. Kao et al. (2023) [150] | Ixekizumab | 160 mg s.c. at week 0, then 80 mg every 2 weeks until week 12, then 80 mg every 4 weeks | Case series |
|
|
| M. W. Tee et al. [151] | Brodalumab | 210 s.c. every week | Case series |
| Complete healing in both cases |
| M.L. McPhie et al. (2020) [152] | Secukinumab | 300 mg s.c. at weeks 0, 1, 2, 3, and 4, followed by monthly maintenance dosing | Case report | NA | Complete healing |
| M.M. Garcia et al. (2018) [153] | Secukinumab | 300 mg s.c. at weeks 0, 1, 2, 3, and 4, then every 4 weeks; beginning week 16, 300 mg every other week | Case report | RA, post-surgery for Quervain’s tenosynovitis | Partial response after 20 months of treatment |
| Authors (Year) | Biologic Drug | Dosage Regimen | Study Type | Comorbidities | Efficacy |
|---|---|---|---|---|---|
| L. J. Leow et al. (2022) [161] | Tildrakizumab | 100 mg s.c. on week 0 and 4, then every 8 weeks | Case report | NA | Constant improvement after 82 weeks |
| E. Çalışkan et al. (2023) [163] | Risankizumab | NA | Case report | ankylosing spondylitis, ileostomy due to megacolon toxicum | Refractory to treatment; closed primary ostomy—regression of lesions; no new lesions at the side of new ostomy |
| C. Baier et al. (2020) [164] | Guselkumab | 100 mg s.c. monthly | Case report | monoclonal gammopathy of undetermined significance and type 2 diabetes | Complete healing within 3 months |
| A. M. Reese et al. (2022) [165] | Guselkumab | 200 mg s.c. at week 0, 100 mg at week 4, then every 6 weeks | Case report | type 2 diabetes mellitus | Complete healing after 4 doses |
| J. M. John et al. (2020) [162] | Tildrakizumab | 100 mg s.c. on week 0, 4, then every 12 weeks | Case report | gout, polymyalgia rheumatica, renal impairment | Almost complete healing |
| B. Burgdorf et al. (2020) [166] | Risankizumab | 150 mg s.c. on weeks 0, 4, then every 12 weeks | Case report | none | Significant improvement |
| L.V. Piñeiro et al. (2023) [167] | Guselkumab | 100 mg s.c. at week 0, 4, then every 8 weeks | Case report | NA | Complete healing with residual post-inflammatory lesions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łyko, M.; Ryguła, A.; Kowalski, M.; Karska, J.; Jankowska-Konsur, A. The Pathophysiology and Treatment of Pyoderma Gangrenosum—Current Options and New Perspectives. Int. J. Mol. Sci. 2024, 25, 2440. https://doi.org/10.3390/ijms25042440
Łyko M, Ryguła A, Kowalski M, Karska J, Jankowska-Konsur A. The Pathophysiology and Treatment of Pyoderma Gangrenosum—Current Options and New Perspectives. International Journal of Molecular Sciences. 2024; 25(4):2440. https://doi.org/10.3390/ijms25042440
Chicago/Turabian StyleŁyko, Magdalena, Anna Ryguła, Michał Kowalski, Julia Karska, and Alina Jankowska-Konsur. 2024. "The Pathophysiology and Treatment of Pyoderma Gangrenosum—Current Options and New Perspectives" International Journal of Molecular Sciences 25, no. 4: 2440. https://doi.org/10.3390/ijms25042440
APA StyleŁyko, M., Ryguła, A., Kowalski, M., Karska, J., & Jankowska-Konsur, A. (2024). The Pathophysiology and Treatment of Pyoderma Gangrenosum—Current Options and New Perspectives. International Journal of Molecular Sciences, 25(4), 2440. https://doi.org/10.3390/ijms25042440







