Timeframe Analysis of Novel Synthetic Cannabinoids Effects: A Study on Behavioral Response and Endogenous Cannabinoids Disruption
Abstract
:1. Introduction
2. Results
2.1. Behavioral Response Studies
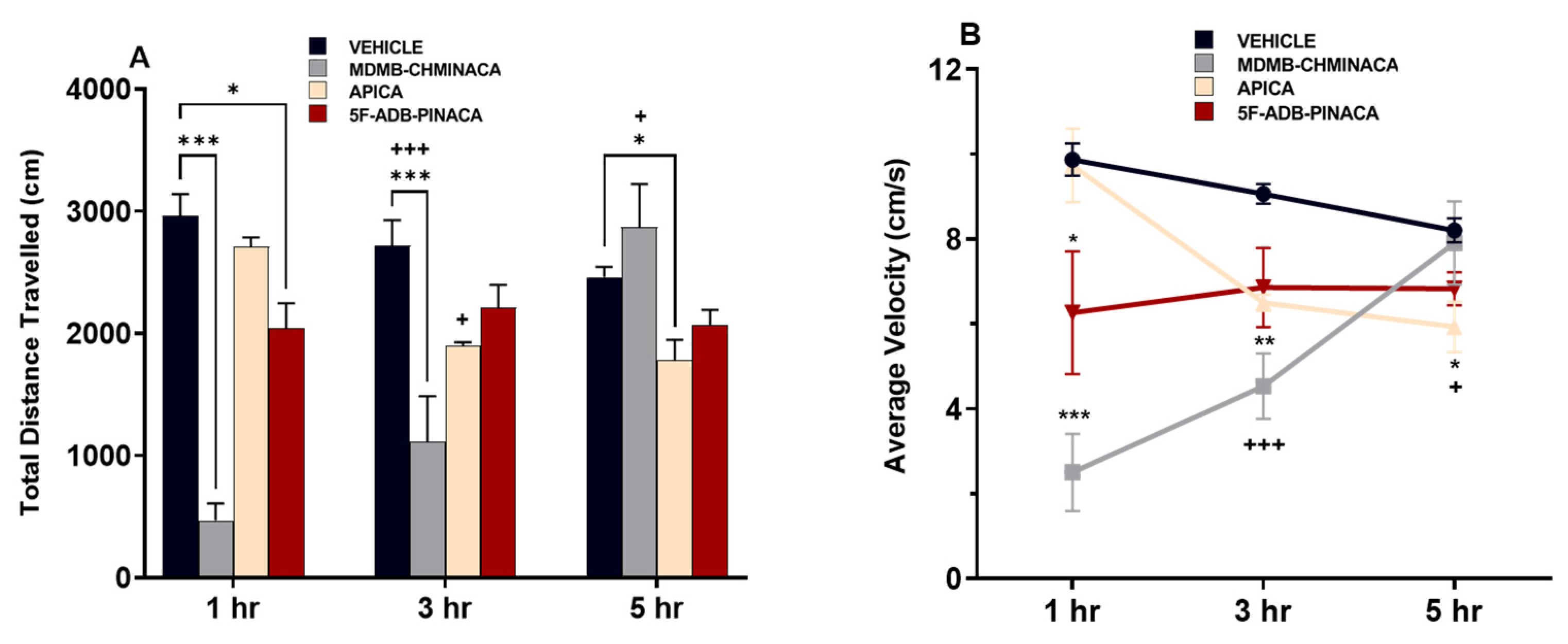
2.1.1. Distance Travelled and Velocity
2.1.2. Anxiety-like Behavior
2.1.3. Memory and Learning
2.2. Alteration of Brain Derived Neurotrophic Factor (BDNF) in the Hippocampus
2.3. Effects on Endocannabinoid Concentrations in the Hippocampus
2.4. Alteration of Endocannabinoids Metabolic Enzymes, FAAH and MAGL
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Treatment
4.3. Overview of the Experimental Design
Behavioral Response Assessment
Exploration
Anxiety
Recognition Index
4.4. Determination of Endocannabinoid AEA & 2-AG Contents in Hippocampus
4.5. Real Time-Reverse Transcription (RT)-Polymerase Chain Reaction (PCR)
4.6. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Herkenham, M.; Lynn, A.; Johnson, M.R.; Melvin, L.; de Costa, B.; Rice, K. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J. Neurosci. 1991, 11, 563–583. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylgylcerol: A Possible Endogenous Cannabinoid Receptor Ligand in Brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef]
- Rumińska, A.; Dobrzyn, A. The endocannabinoid system and its role in regulation of metabolism in peripheral tissues. Postep. Biochem. 2012, 58, 127–134. [Google Scholar]
- Gurney, S.M.; Scott, K.S.; Kacinko, S.L.; Presley, B.C.; Logan, B.K. Pharmacology, toxicology, and adverse effects of syn-thetic cannabinoid drugs. Forensic Sci. Rev. 2014, 26, 53–78. [Google Scholar]
- Maurya, N.; Velmurugan, B.K. Therapeutic applications of cannabinoids. Chem. Interact. 2018, 293, 77–88. [Google Scholar] [CrossRef]
- Di Marzo, V.; Fontana, A.; Cadas, H.; Schinelli, S.; Cimino, G.; Schwartz, J.-C.; Piomelli, D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 1994, 372, 686–691. [Google Scholar] [CrossRef]
- Cadas, H.; Gaillet, S.; Beltramo, M.; Venance, L.; Piomelli, D. Biosynthesis of an Endogenous Cannabinoid Precursor in Neurons and its Control by Calcium and cAMP. J. Neurosci. 1996, 16, 3934–3942. [Google Scholar] [CrossRef]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef]
- Beltramo, M.; Stella, N.; Calignano, A.; Lin, S.Y.; Makriyannis, A.; Piomelli, D. Functional Role of High-Affinity Anandamide Transport, as Revealed by Selective Inhibition. Science 1997, 277, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2019, 16, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Braile, M.; Marcella, S.; Marone, G.; Galdiero, M.R.; Varricchi, G.; Loffredo, S. The Interplay between the Immune and the Endocannabinoid Systems in Cancer. Cells 2021, 10, 1282. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Bidaut-Russell, M.; A Devane, W.; Melvin, L.S.; Johnson, M.; Herkenham, M. The cannabinoid receptor: Biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990, 13, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Makriyannis, A.; Deng, H. Cannabimimetic Indole Derivatives. U.S. Patent US20050119234A1, 2 June 2005. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. EMCDDA Perspectives on Drugs—Synthetic Cannabinoids in Europe; Publication Office of the European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2017.
- Kronstrand, R.; Roman, M.; Andersson, M.; Eklund, A. Toxicological Findings of Synthetic Cannabinoids in Recreational Users. J. Anal. Toxicol. 2013, 37, 534–541. [Google Scholar] [CrossRef]
- Baumann, M.H.; Solis, E.; Watterson, L.R.; Marusich, J.A.; Fantegrossi, W.E.; Wiley, J.L. Baths Salts, Spice, and Related Designer Drugs: The Science Behind the Headlines. J. Neurosci. 2014, 34, 15150–15158. [Google Scholar] [CrossRef]
- Soussan, C.; Kjellgren, A. The users of Novel Psychoactive Substances: Online survey about their characteristics, attitudes and motivations. Int. J. Drug Policy 2016, 32, 77–84. [Google Scholar] [CrossRef]
- Cooper, Z.D. Adverse Effects of Synthetic Cannabinoids: Management of Acute Toxicity and Withdrawal. Curr. Psychiatry Rep. 2016, 18, 52. [Google Scholar] [CrossRef]
- Weinstein, A.M.; Rosca, P.; Fattore, L.; London, E.D. Synthetic Cathinone and Cannabinoid Designer Drugs Pose a Major Risk for Public Health. Front. Psychiatry 2017, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, A.; Pascali, J.P.; Fais, P.; Pelletti, G.; Gabbin, A.; Franchetti, G.; Cecchetto, G.; Viel, G. Molecular Mechanisms of Action of Novel Psychoactive Substances (NPS). A New Threat for Young Drug Users with Forensic-Toxicological Implications. Life 2021, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Castaneto, M.S.; Gorelick, D.A.; Desrosiers, N.A.; Hartman, R.L.; Pirard, S.; Huestis, M.A. Synthetic cannabinoids: Epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014, 144, 12–41. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drugs Addiction and Europol. EMCDDA EU Drug Markets Report; Publication Office of the European Union: Luxemburg, 2019.
- Shevyrin, V.A.; Morzherin, Y.Y. Cannabinoids: Structures, effects, and classification. Russ. Chem. Bull. 2015, 64, 1249–1266. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime UNODC Early Warning Advisory on New Psychoactive Substances. NPS Sub-stances Group-Synthetic Cannabinoids. 2022. Available online: https://www.unodc.org/LSS/SubstanceGroup/Details/ae45ce06-6d33-4f5f-916a-e873f07bde02 (accessed on 26 July 2022).
- Banister, S.D.; Wilkinson, S.M.; Longworth, M.; Stuart, J.; Apetz, N.; English, K.; Brooker, L.; Goebel, C.; Hibbs, D.E.; Glass, M.; et al. The Synthesis and Pharmacological Evaluation of Adamantane-Derived Indoles: Cannabimimetic Drugs of Abuse. ACS Chem. Neurosci. 2013, 4, 1081–1092. [Google Scholar] [CrossRef]
- Banister, S.D.; Longworth, M.; Kevin, R.; Sachdev, S.; Santiago, M.; Stuart, J.; Mack, J.B.C.; Glass, M.; McGregor, I.S.; Connor, M.; et al. Pharmacology of Valinate and tert-Leucinate Synthetic Cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and Their Analogues. ACS Chem. Neurosci. 2016, 7, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction. EMCDDA Europol Joint Report on a New Psychoactive Substance: Methyl 2-[[1-(cyclohexylmethyl)indole-3-carbonyl]amino]-3,3-dimethylbutanoate (MDMB-CHMICA); Publication Office of the European Monitoring Centre for Drugs and Drug Addiction: Luxemburg, 2016.
- Banister, S.D.; Moir, M.; Stuart, J.; Kevin, R.C.; Wood, K.E.; Longworth, M.; Wilkinson, S.M.; Beinat, C.; Buchanan, A.S.; Glass, M.; et al. Pharmacology of Indole and Indazole Synthetic Cannabinoid Designer Drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA. ACS Chem. Neurosci. 2015, 6, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Schoeder, C.T.; Hess, C.; Madea, B.; Meiler, J.; Müller, C.E. Pharmacological evaluation of new constituents of “Spice”: Synthetic cannabinoids based on indole, indazole, benzimidazole and carbazole scaffolds. Forensic Toxicol. 2018, 36, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Franz, F.; Jechle, H.; Wilde, M.; Angerer, V.; Huppertz, L.M.; Longworth, M.; Kassiou, M.; Jung, M.; Auwärter, V. Structure-metabolism relationships of valine and tert-leucine-derived synthetic cannabinoid receptor agonists: A systematic comparison of the in vitro phase I metabolism using pooled human liver microsomes and high-resolution mass spectrometry. Forensic Toxicol. 2019, 37, 316–329. [Google Scholar] [CrossRef]
- Brandon, A.M.; Antonides, L.H.; Riley, J.; Epemolu, O.; McKeown, D.A.; Read, K.D.; McKenzie, C. A Systematic Study of the In Vitro Pharmacokinetics and Estimated Human In Vivo Clearance of Indole and Indazole-3-Carboxamide Synthetic Cannabinoid Receptor Agonists Detected on the Illicit Drug Market. Molecules 2021, 26, 1396. [Google Scholar] [CrossRef]
- Presley, B.C.; Logan, B.K.; Jansen-Varnum, S.A. In vitro Phase I metabolism of indazole carboxamide synthetic cannabinoid MDMB-CHMINACA via human liver microsome incubation and high-resolution mass spectrometry. Drug Test. Anal. 2019, 11, 1264–1276. [Google Scholar] [CrossRef]
- Xu, D.-Q.; Dai, Y.; Zhang, W.-F.; Wang, J.-F.; Wang, Y.-Y.; Zhang, Y.; Xin, G.; Zhao, Q.-L.; Li, X. Rapid identification of MDMB-CHMINACA metabolites using Zebrafish and Human Liver microsomes as the Biotransformation system by LC-QE-HF-MS. J. Anal. Toxicol. 2020, 44, 1012–1026. [Google Scholar] [CrossRef]
- Li, R.-S.; Fukumori, R.; Takeda, T.; Song, Y.; Morimoto, S.; Kikura-Hanajiri, R.; Yamaguchi, T.; Watanabe, K.; Aritake, K.; Tanaka, Y.; et al. Elevation of endocannabinoids in the brain by synthetic cannabinoid JWH-018: Mechanism and effect on learning and memory. Sci. Rep. 2019, 9, 9621. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, M.; Egashira, N. New Perspectives in the Studies on Endocannabinoid and Cannabis: Abnormal Behaviors Associate with CB1 Cannabinoid Receptor and Development of Therapeutic Application. J. Pharmacol. Sci. 2004, 96, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Jang, Y.-S.; Jeon, W.K.; Han, J.-S. Assessment of Cognitive Phenotyping in Inbred, Genetically Modified Mice, and Transgenic Mouse Models of Alzheimer’s Disease. Exp. Neurobiol. 2019, 28, 146–157. [Google Scholar] [CrossRef]
- Basavarajappa, B.S.; Subbanna, S. Potential Mechanisms Underlying the Deleterious Effects of Synthetic Cannabinoids Found in Spice/K2 Products. Brain Sci. 2019, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Wiebelhaus, J.M.; Poklis, J.L.; Poklis, A.; Vann, R.E.; Lichtman, A.H.; Wise, L.E. Inhalation exposure to smoke from synthetic “marijuana” produces potent cannabimimetic effects in mice. Drug Alcohol Depend. 2012, 126, 316–323. [Google Scholar] [CrossRef]
- Wilson, C.D.; Tai, S.; Ewing, L.; Crane, J.; Lockhart, T.; Fujiwara, R.; Radominska-Pandya, A.; Fantegrossi, W.E. Convulsant Effects of Abused Synthetic Cannabinoids JWH-018 and 5F-AB-PINACA Are Mediated by Agonist Actions at CB1 Receptors in Mice. J. Pharmacol. Exp. Ther. 2018, 368, 146–156. [Google Scholar] [CrossRef]
- Ito, S.; Deyama, S.; Domoto, M.; Zhang, T.; Sasase, H.; Fukao, A.; Esaki, H.; Hinoi, E.; Kaneko, S.; Kaneda, K. Effects of the synthetic cannabinoid 5F-AMB on anxiety and recognition memory in mice. Psychopharmacology 2019, 236, 2235–2242. [Google Scholar] [CrossRef]
- Kathuria, S.; Gaetani, S.; Fegley, D.; Valiño, F.; Duranti, A.; Tontini, A.; Mor, M.; Tarzia, G.; La Rana, G.; Calignano, A.; et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2002, 9, 76–81. [Google Scholar] [CrossRef]
- Winters, B.D.; Saksida, L.M.; Bussey, T.J. Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci. Biobehav. Rev. 2008, 32, 1055–1070. [Google Scholar] [CrossRef]
- Prickaerts, J.; de Vente, J.; Honig, W.; Steinbusch, H.W.; Blokland, A. cGMP, but not cAMP, in rat hippocampus is involved in early stages of object memory consolidation. Eur. J. Pharmacol. 2002, 436, 83–87. [Google Scholar] [CrossRef]
- D’Isa, R.; Brambilla, R.; Fasano, S. Behavioral methods for the study of the Ras-ERK pathway in memory formation and consolidation: Passive avoidance and novel object recognition tests. Methods Mol. Biol. 2014, 1120, 131–156. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Cammarota, M.; Katche, C.; Slipczuk, L.; Rossato, J.I.; Goldin, A.; Izquierdo, I.; Medina, J.H. BDNF is essential to promote persistence of long-term memory storage. Proc. Natl. Acad. Sci. USA 2008, 105, 2711–2716. [Google Scholar] [CrossRef]
- Yamada, K.; Nabeshima, T. Brain-Derived Neurotrophic Factor/TrkB Signaling in Memory Processes. J. Pharmacol. Sci. 2003, 91, 267–270. [Google Scholar] [CrossRef]
- Cunha, C.; Brambilla, R.; Thomas, K.L. A simple role for BDNF in learning and memory? Front. Mol. Neurosci. 2010, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kobayashi, Y.; Oka, S.; Waku, K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Schlosburg, J.E.; Blankman, J.L.; Long, J.Z.; Nomura, D.K.; Pan, B.; Kinsey, S.G.; Nguyen, P.T.; Ramesh, D.; Booker, L.; Burston, J.J.; et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat. Neurosci. 2010, 13, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Luchicchi, A.; Pistis, M. Anandamide and 2-arachidonoylglycerol: Pharmacological Properties, Functional Features, and Emerging Specificities of the Two Major Endocannabinoids. Mol. Neurobiol. 2012, 46, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, N.; Kawamura, M.; Kikura-Hanajiri, R.; Goda, Y. Identification of two new-type synthetic cannabinoids, N-(1-adamantyl)-1-pentyl-1H-indole-3-carboxamide (APICA) and N-(1-adamantyl)-1-pentyl-1H-indazole-3-carboxamide (APINACA), and detection of five synthetic cannabinoids, AM-1220, AM-2233, AM-1241, CB-13 (CRA-13), and AM-1248, as designer drugs in illegal products. Forensic Toxicol. 2012, 30, 114–125. [Google Scholar] [CrossRef]
- Longworth, M.; Connor, M.; Banister, S.D.; Kassiou, M. Synthesis and Pharmacological Profiling of the Metabolites of Synthetic Cannabinoid Drugs APICA, STS-135, ADB-PINACA, and 5F-ADB-PINACA. ACS Chem. Neurosci. 2017, 8, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Cravatt, B.F.; Demarest, K.; Patricelli, M.P.; Bracey, M.H.; Giang, D.K.; Martin, B.R.; Lichtman, A.H. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. USA 2001, 98, 9371–9376. [Google Scholar] [CrossRef] [PubMed]
- Piomelli, D.; Tarzia, G.; Duranti, A.; Tontini, A.; Mor, M.; Compton, T.R.; Dasse, O.; Monaghan, E.P.; Parrott, J.A.; Putman, D. Pharmacological Profile of the Selective FAAH Inhibitor KDS-4103 (URB597). CNS Drug Rev. 2006, 12, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Fagundo, A.B.; de la Torre, R.; Jiménez-Murcia, S.; Agüera, Z.; Pastor, A.; Casanueva, F.F.; Granero, R.; Baños, R.; Botella, C.; del Pino-Gutierrez, A.; et al. Modulation of the Endocannabinoids N-Arachidonoylethanolamine (AEA) and 2-Arachidonoylglycerol (2-AG) on Executive Functions in Humans. PLoS ONE 2013, 8, e66387. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. UNODC Drug Market Trends: Opioids, Cannabis; World Drug Report (United Nations publication, Sales No. E.21.XI.8); United Nations Office on Drugs and Crime: Vienna, Austria, 2021. [Google Scholar]
- Barbieri, M.; Ossato, A.; Canazza, I.; Trapella, C.; Borelli, A.; Beggiato, S.; Rimondo, C.; Serpelloni, G.; Ferraro, L.; Marti, M. Synthetic cannabinoid JWH-018 and its halogenated derivatives JWH-018-Cl and JWH-018-Br impair Novel Object Recognition in mice: Behavioral, electrophysiological and neurochemical evidence. Neuropharmacology 2016, 109, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.; Hyatt, W.; Gu, C.; Franks, L.; Vasiljevik, T.; Brents, L.; Prather, P.; Fantegrossi, W. Repeated administration of phytocannabinoid Δ9-THC or synthetic cannabinoids JWH-018 and JWH-073 induces tolerance to hypothermia but not locomotor suppression in mice, and reduces CB1 receptor expression and function in a brain region-specific manner. Pharmacol. Res. 2015, 102, 22–32. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J. Vis. Exp. 2017, 2017, e55718. [Google Scholar] [CrossRef]
- Deacon, R.M.J. Housing, husbandry and handling of rodents for behavioral experiments. Nat. Protoc. 2006, 1, 936–946. [Google Scholar] [CrossRef]
- Dere, E.; Huston, J.P.; Silva, M.A.D.S. Integrated memory for objects, places, and temporal order: Evidence for episodic-like memory in mice. Neurobiol. Learn. Mem. 2005, 84, 214–221. [Google Scholar] [CrossRef]
- Mood and Anxiety Related Phenotypes in Mice; Springer Science and Business Media LLC.: Dordrecht, The Netherlands, 2009; ISBN 9780896037939.
- van Goethem, N.P.; Rutten, K.; van der Staay, F.J.; Jans, L.A.; Akkerman, S.; Steinbusch, H.W.; Blokland, A.; Klooster, J.V.; Prickaerts, J. Object recognition testing: Rodent species, strains, housing conditions, and estrous cycle. Behav. Brain Res. 2012, 232, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Festing, M.F.W.; Altman, D.G. Guidelines for the Design and Statistical Analysis of Experiments Using Laboratory Animals. ILAR J. 2002, 43, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, N.J.; Gaskin, S.; Squire, L.R.; Clark, R.E. Object recognition memory and the rodent hippocampus. Learn. Mem. 2010, 17, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.M.; Hawk, J.D.; Abel, T.; Havekes, R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn. Mem. 2010, 17, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Katona, I.; Urbán, G.M.; Wallace, M.; Ledent, C.; Jung, K.-M.; Piomelli, D.; Mackie, K.; Freund, T.F. Molecular Composition of the Endocannabinoid System at Glutamatergic Synapses. J. Neurosci. 2006, 26, 5628–5637. [Google Scholar] [CrossRef]
- Simon, P.; Dupuis, R.; Costentin, J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 1994, 61, 59–64. [Google Scholar] [CrossRef]
- Bailey, K.R.; Crawley, J.N. Anxiety-related behaviors in mice. In Methods of Behavior Analysis in Neuro-Science, 2nd ed.; Buccafusco, J.J., Ed.; Taylor & Francis Publishers: Boca Raton, FL, USA, 2009; Chapter 5. [Google Scholar]
- Lezak, K.R.; Missig, G.; Carlezon, W.A., Jr. Behavioral methods to study anxiety in rodents. Dialogues Clin. Neurosci. 2017, 19, 181–191. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2011, 13, 93–110. [Google Scholar] [CrossRef]
- Qi, M.; Morena, M.; Vecchiarelli, H.A.; Hill, M.N.; Schriemer, D.C. A robust capillary liquid chromatography/tandem mass spectrometry method for quantitation of neuromodulatory endocannabinoids. Rapid Commun. Mass Spectrom. 2015, 29, 1889–1897. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda Garcia, J.C.; Li, R.-S.; Kikura-Hanajiri, R.; Tanaka, Y.; Ishii, Y. Timeframe Analysis of Novel Synthetic Cannabinoids Effects: A Study on Behavioral Response and Endogenous Cannabinoids Disruption. Int. J. Mol. Sci. 2024, 25, 3083. https://doi.org/10.3390/ijms25063083
Pineda Garcia JC, Li R-S, Kikura-Hanajiri R, Tanaka Y, Ishii Y. Timeframe Analysis of Novel Synthetic Cannabinoids Effects: A Study on Behavioral Response and Endogenous Cannabinoids Disruption. International Journal of Molecular Sciences. 2024; 25(6):3083. https://doi.org/10.3390/ijms25063083
Chicago/Turabian StylePineda Garcia, Jorge Carlos, Ren-Shi Li, Ruri Kikura-Hanajiri, Yoshitaka Tanaka, and Yuji Ishii. 2024. "Timeframe Analysis of Novel Synthetic Cannabinoids Effects: A Study on Behavioral Response and Endogenous Cannabinoids Disruption" International Journal of Molecular Sciences 25, no. 6: 3083. https://doi.org/10.3390/ijms25063083
APA StylePineda Garcia, J. C., Li, R.-S., Kikura-Hanajiri, R., Tanaka, Y., & Ishii, Y. (2024). Timeframe Analysis of Novel Synthetic Cannabinoids Effects: A Study on Behavioral Response and Endogenous Cannabinoids Disruption. International Journal of Molecular Sciences, 25(6), 3083. https://doi.org/10.3390/ijms25063083





