Assessment of Common Factors Associated with Droplet Digital PCR (ddPCR) Quantification of Paratrichodorus allius in Soil
Abstract
1. Introduction
2. Results
2.1. Effect of Nematode Body Sizes on Quantitative Detection of Paratrichodorus allius
2.2. Soil Pre-Treatment
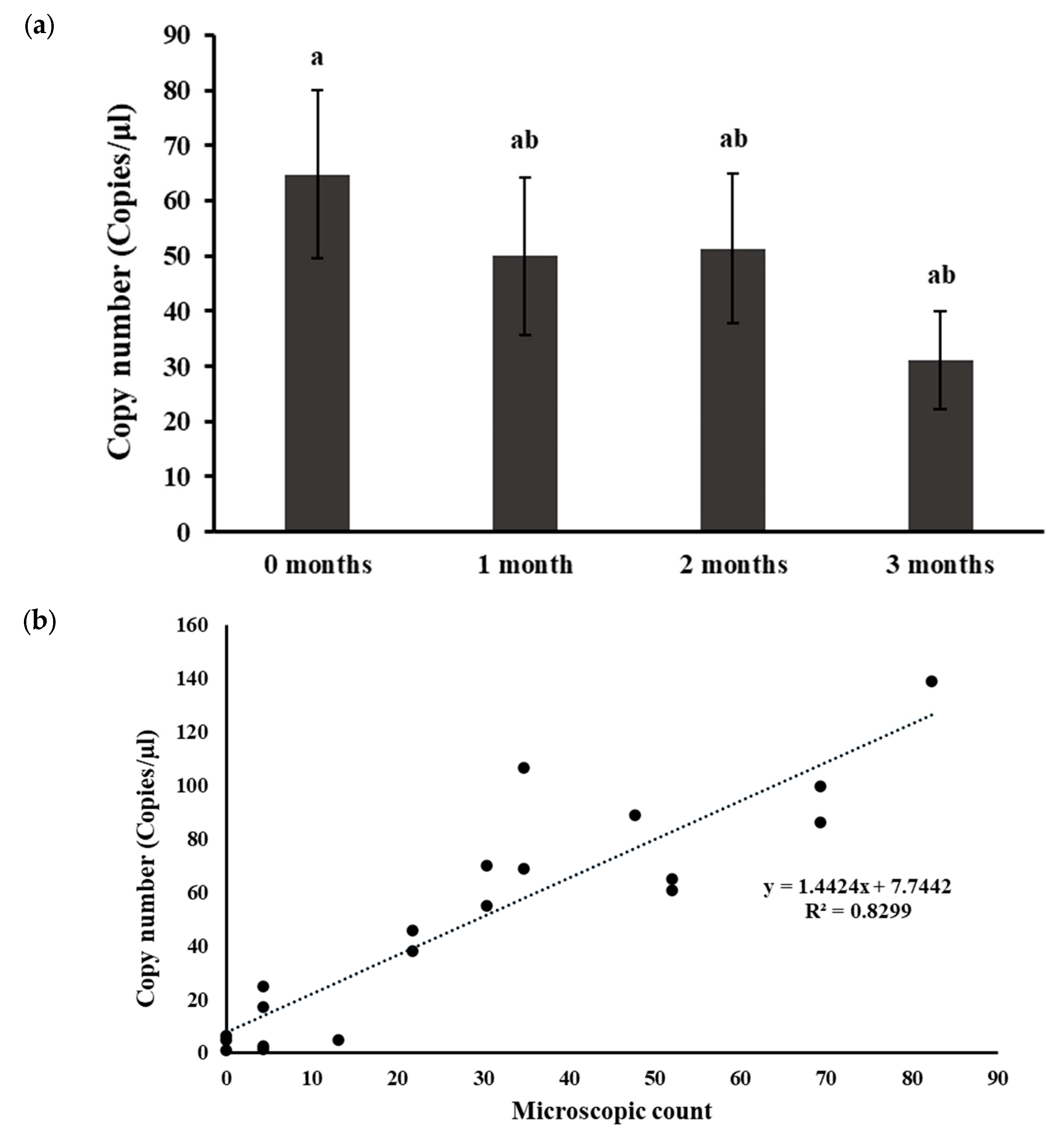
2.3. Effect of Soil Storage Time
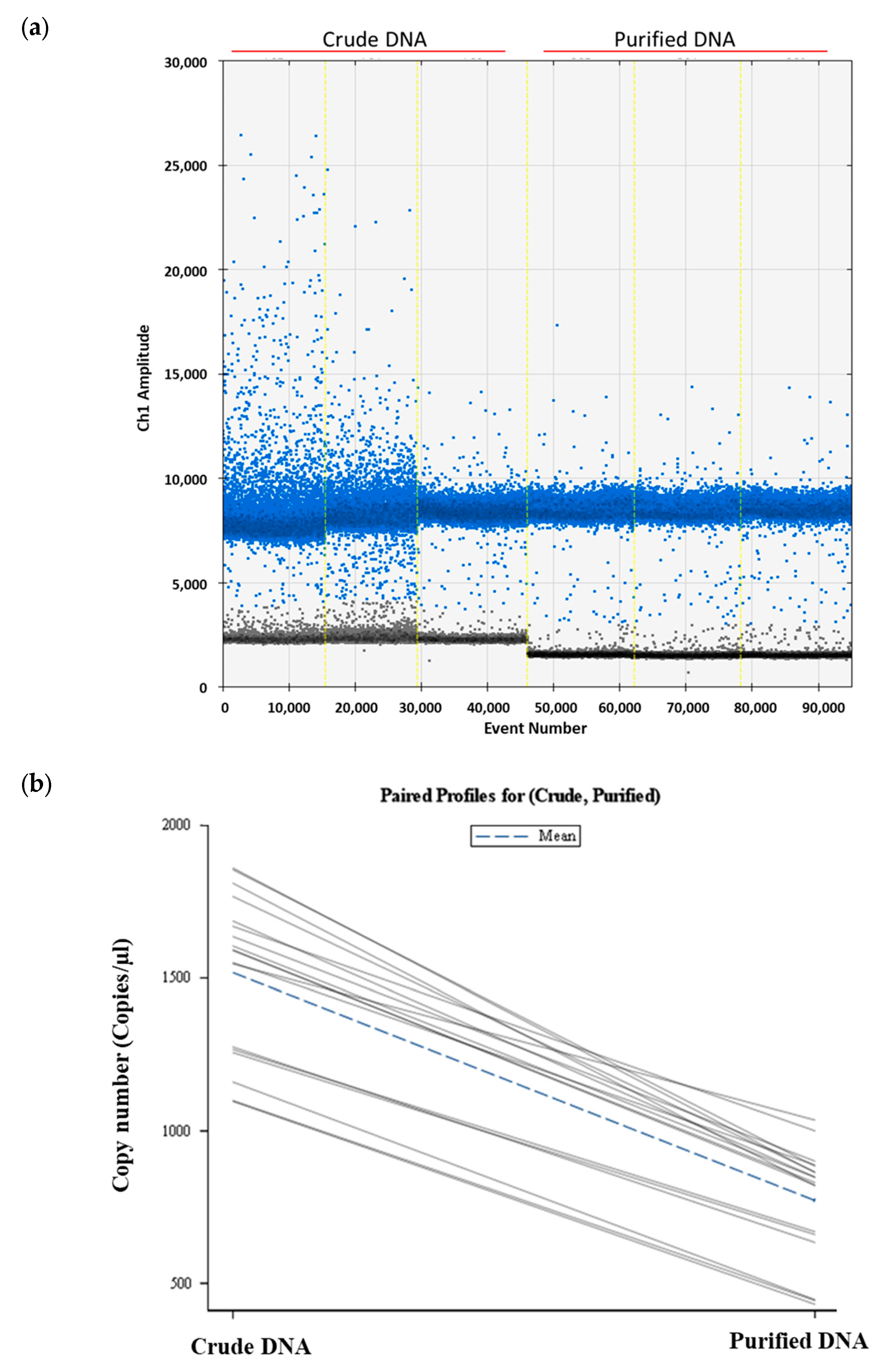
2.4. Effect of Soil DNA Purification
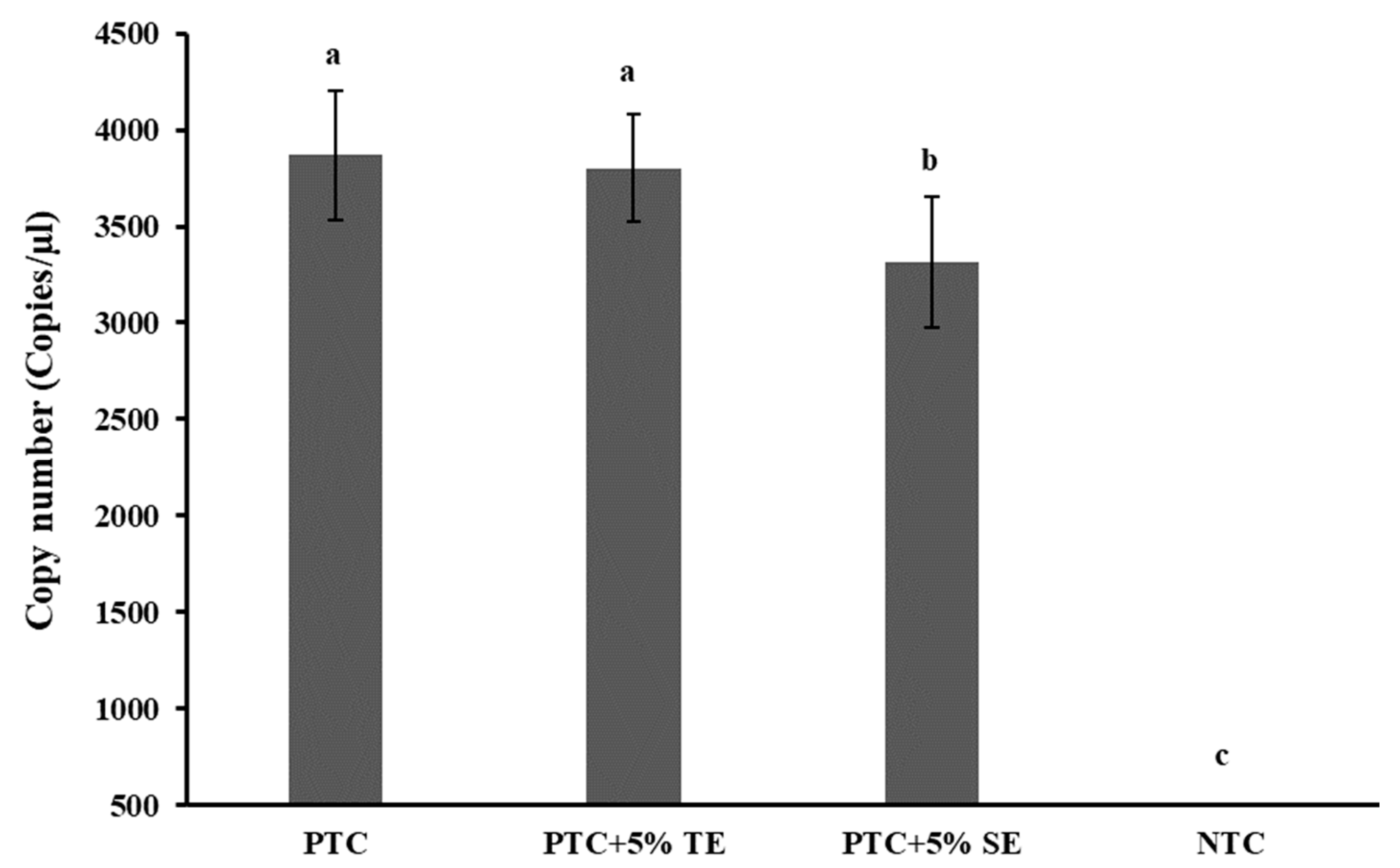
2.5. Effect of PCR Inhibitors Co-Extracted along with Soil DNA
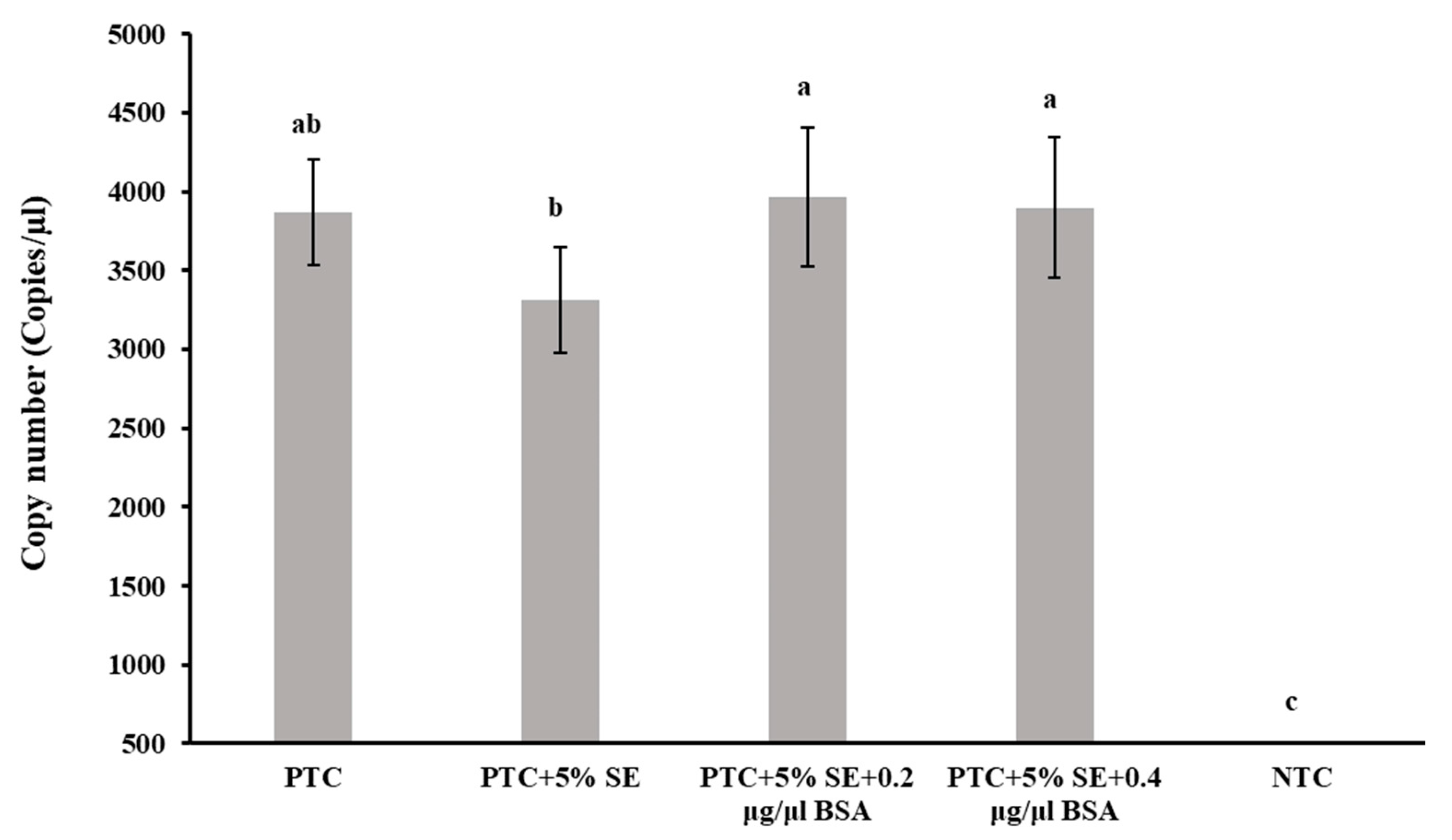
2.6. Effect of Bovine Serum Albumin (BSA)
3. Discussion
4. Materials and Methods
4.1. Soil Sample
4.2. Nematode Extraction, Identification, and Microscopic Count
4.3. Soil DNA Extraction for Nematode Quantification by ddPCR
4.4. ddPCR Assay Condition and Process
4.5. Evaluation of Various Factors on ddPCR
4.5.1. Nematode Body Size
4.5.2. Soil Pre-Treatment
4.5.3. Soil Storage Durations
4.5.4. Soil DNA Purification
4.5.5. PCR Inhibitors from Soil Extract
4.5.6. Bovine Serum Albumin (BSA), a PCR Enhancer
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, K.R.; Olthof, T.H. Relationships between nematode population densities and crop responses. Annu. Rev. Phytopathol. 1976, 14, 327–353. [Google Scholar] [CrossRef]
- Kawanobe, M.; Miyamaru, N.; Yoshida, K.; Kawanaka, T.; Toyota, K. Quantification of lesion nematode (Pratylenchus zeae), stunt nematode (Tylenchorhynchus leviterminalis), spiral nematode (Helicotylenchus dihystera), and lance nematode (Hoplolaimus columbus), parasites of sugarcane in Kitadaito, Okinawa, Japan, using real-time PCR. Nematol. Res. (Jpn. J. Nematol.) 2015, 45, 35–44. [Google Scholar]
- Yan, G.; Smiley, R.W.; Okubara, P.A.; Skantar, A.M.; Reardon, C.L. Developing a real-time PCR assay for detection and quantification of Pratylenchus neglectus in soil. Plant Dis. 2013, 97, 757–764. [Google Scholar] [CrossRef]
- López, M.M.; Llop, P.; Olmos, A.; Marco-Noales, E.; Cambra, M.; Bertolini, E. Are molecular tools solving the challenges posed by detection of plant pathogenic bacteria and viruses? Curr. Issues Mol. Biol. 2009, 11, 13–46. [Google Scholar] [PubMed]
- Huang, D.; Yan, G.; Skantar, A.M. Development of real-time and conventional PCR assays for identifying stubby root nematode Paratrichodorus allius. Plant Dis. 2017, 101, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gleason, S. Loop-mediated isothermal amplification for the diagnostic detection of Meloidogyne chitwoodi and M. fallax. Plant Dis. 2019, 103, 12–18. [Google Scholar] [CrossRef]
- Omer, A.S.; Wallenhammar, A.C.; Viketoft, M. Development of loop-mediated isothermal amplification assay for rapid detection and analysis of the root-knot nematode Meloidogyne hapla in soil. Horticulturae 2022, 8, 87. [Google Scholar] [CrossRef]
- Subbotin, S.A.; Burbridge, J. Recombinase polymerase amplification assays for detection of the major tropical root-knot nematodes. J. Nematol. 2022, 53, e2021-109. [Google Scholar] [CrossRef]
- Goraya, M.; Yan, G.; Whitworth, J.; Grimm, K.S. Advancing nematode identification on potato: An isothermal recombinase polymerase amplification assay for stubby root nematode, Paratrichodorus allius. Am. J. Potato Res. 2023. [Google Scholar] [CrossRef]
- Chen, Y.; Long, H.; Feng, T.; Pei, Y.; Sun, Y.; Zhang, X. Development of a Novel Primer–TaqMan Probe Set for Diagnosis and Quantification of Meloidogyne enterolobii in Soil Using qPCR and Droplet Digital PCR Assays. Int. J. Mol. Sci. 2022, 23, 11185. [Google Scholar] [CrossRef]
- Lawaju, B.; Yan, G.; Whitworth, J. Development of a droplet digital PCR assay for detection and quantification of stubby root nematode, Paratrichodorus allius in soil. Plant Dis. 2023, 107, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Huang, Q.; Zhang, X.; Chen, H. Adsorption of DNA on clay minerals and various colloidal particles from an Alfisol. Soil Biol. Biochem. 2006, 38, 471–476. [Google Scholar] [CrossRef]
- Young, J.M.; Rawlence, N.J.; Weyrich, L.S.; Cooper, A. Limitations and recommendations for successful DNA extraction from forensic soil samples: A review. Sci. Justice 2014, 54, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.; Ferris, J.P.; Gallori, E. Cations as mediators of the adsorption of nucleic acids on clay surfaces in prebiotic environments. Ori. Life Evol. Biosph. 2003, 33, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bogas, V.; Carvalho, M.; Anjos, M.J.; Pinheiro, M.F.; Corte-Real, F. Genetic identification of degraded DNA samples buried in different types of soil. Forensic Sci. Int. Genet. Suppl. Ser. 2009, 2, 169–171. [Google Scholar] [CrossRef]
- Pietramellara, G.; Ascher, J.; Borgogni, F.; Ceccherini, M.T.; Guerri, G.; Nannipieri, P. Extracellular DNA in soil and sediment: Fate and ecological relevance. Biol. Fertil. Soils 2009, 45, 219–235. [Google Scholar] [CrossRef]
- Ivarson, K.C.; Schnitzer, M.; Cortez, J. The biodegradability of nucleic acid bases adsorbed on inorganic and organic soil components. Plant Soil 1982, 64, 343–353. [Google Scholar] [CrossRef]
- Opel, K.L.; Chung, D.; McCord, B.R. A study of PCR inhibition mechanisms using real time PCR. J. Forensic Sci. 2010, 55, 25–33. [Google Scholar] [CrossRef]
- Bessetti, B.J. An introduction to PCR inhibitors. J. Microbiol. Methods 2007, 28, 159–167. [Google Scholar]
- Hedman, J.; Rådström, P. Overcoming inhibition in real-time diagnostic PCR. In PCR Detection of Microbial Pathogens, 2nd ed.; Wilks, M., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 943, pp. 17–48. [Google Scholar]
- Karunanathie, H.; Kee, P.S.; Ng, S.F.; Kennedy, M.A.; Chua, E.W. PCR enhancers: Types, mechanisms, and applications in long-range PCR. Biochimie 2022, 197, 130–143. [Google Scholar] [CrossRef]
- Farell, E.M.; Alexandre, G. Bovine serum albumin further enhances the effects of organic solvents on increased yield of polymerase chain reaction of GC-rich templates. BMC Res. Notes 2012, 5, 257. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Yoshida, A.; Sato, N. Additive effects of bovine serum albumin, dithiothreitol and glycerol on PCR. IUBMB Life 1998, 44, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Olson, B.H.; Chang, J.S. Improving PCR and qPCR detection of hydrogenase A (hyd A) associated with Clostridia in pure cultures and environmental sludges using bovine serum albumin. Appl. Microbiol. Biotechnol. 2007, 77, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.; Kammann, L.; Helfrich, M.; Tebbe, C.C.; Poeplau, C. Impact of common sample pre-treatments on key soil microbial properties. Soil Biol. Biochem. 2021, 160, 108321. [Google Scholar] [CrossRef]
- Aalders, L.T.; McNeill, M.R.; Bell, N.L.; Cameron, C. Plant parasitic nematode survival and detection to inform biosecurity risk assessment. NeoBiota 2017, 36, 1–16. [Google Scholar] [CrossRef]
- Barker, K.R.; Nusbaum, C.J.; Nelson, L. Effects of storage temperature and extraction procedure on recovery of plant-parasitic nematodes from field soils. J. Nematol. 1969, 1, 240–247. [Google Scholar] [PubMed]
- Golden, J.K.; Powell, W.M.; Hendrix, F.F., Jr. The influence of storage temperature on recovery of Pythium spp. and Meloidogyne incognita from field soils. Phytopathology 1972, 62, 819–823. [Google Scholar] [CrossRef]
- Kung, S.P.; Gaugler, R.; Kaya, H.K. Effects of soil temperature, moisture, and relative humidity on entomopathogenic nematode persistence. J. Invertebr. Pathol. 1991, 57, 242–249. [Google Scholar] [CrossRef]
- Tsai, B.Y. Effect of temperature on the survival of Meloidogyne incognita. Plant Pathol. Bull. 2008, 17, 203–208. [Google Scholar]
- Takemoto, S.; Niwa, S.; Okada, H. Effect of storage temperature on soil nematode community structures as revealed by PCR-DGGE. J. Nematol. 2010, 42, 324–331. [Google Scholar]
- Huang, D.; Yan, G.; Gudmestad, N.C.; Whitworth, J. Assessment of factors associated with molecular quantification of stubby root nematode Paratrichodorus allius from field soil DNA. Plant Dis. 2019, 103, 3265–3273. [Google Scholar] [CrossRef] [PubMed]
- Klammer, S.; Mondini, C.; Insam, H. Microbial community fingerprints of composts stored under different conditions. Ann. Microbiol. 2005, 55, 299–305. [Google Scholar]
- Dolfing, J.; Vos, A.; Bloem, J.; Ehlert, P.A.I.; Naumova, N.B.; Kuikman, P.J. Microbial diversity in archived soils. Science 2004, 306, 813. [Google Scholar] [CrossRef] [PubMed]
- Baker, M. Digital PCR hits its stride. Nat. Methods 2012, 9, 541–544. [Google Scholar] [CrossRef]
- Hindson, C.M.; Chevillet, J.R.; Briggs, H.A.; Gallichotte, E.N.; Ruf, I.K.; Hindson, B.J.; Vessella, R.L.; Tewari, M. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat. Methods 2013, 10, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Demeke, T.; Dobnik, D. Critical assessment of digital PCR for the detection and quantification of genetically modified organisms. Anal. Bioanal. Chem. 2018, 410, 4039–4050. [Google Scholar] [CrossRef] [PubMed]
- Kokkoris, V.; Vukicevich, E.; Richards, A.; Thomsen, C.; Hart, M.M. Challenges using droplet digital PCR for environmental samples. Appl. Microbiol. 2021, 1, 74–88. [Google Scholar] [CrossRef]
- Li, H.; Bai, R.; Zhao, Z.; Tao, L.; Ma, M.; Ji, Z.; Jian, M.; Ding, Z.; Dai, X.; Bao, F.; et al. Application of droplet digital PCR to detect the pathogens of infectious diseases. Biosci. Rep. 2018, 38, BSR20181170. [Google Scholar] [CrossRef]
- Morcia, C.; Ghizzoni, R.; Delogu, C.; Andreani, L.; Carnevali, P.; Terzi, V. Digital PCR: What relevance to plant studies? Biology 2020, 9, 433. [Google Scholar] [CrossRef]
- So, S.; Garan, Y.; Miyahara, K.; Ohshima, Y. Body size change in various nematodes depending on bacterial food, sex and growth temperature. Worm 2012, 1, 93–97. [Google Scholar] [CrossRef]
- Sato, E.; Goto, K.; Min, Y.Y.; Toyota, K.; Suzuki, C. Quantitative detection of Pratylenchus penetrans from soil using soil compaction and real-time PCR. Jpn. J. Nematol. 2010, 40, 1–6. [Google Scholar] [CrossRef]
- Oliveira, C.M.D.; Souza Júnior, I.T.D.; Almeida, N.O.; Freitas, M.A.D.; Rocha, M.R.D.; Petrofeza, S. Development of quantitative detection method for Meloidogyne incognita by qPCR. Biosci. J. 2020, 36, 78–86. [Google Scholar] [CrossRef]
- Sulston, J.E.; Brenner, S. The DNA of Caenorhabditis elegans. Genetics 1974, 77, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Tzeneva, V.A.; Salles, J.F.; Naumova, N.; de Vos, W.M.; Kuikman, P.J.; Dolfing, J.; Smidt, H. Effect of soil sample preservation, compared to the effect of other environmental variables, on bacterial and eukaryotic diversity. Res. Microbiol. 2009, 160, 89–98. [Google Scholar] [CrossRef]
- Lillis, P.E.; Griffin, C.T.; Carolan, J.C. The effect of temperature conditioning (9 °C and 20 °C) on the proteome of entomopathogenic nematode infective juveniles. PLoS ONE 2022, 17, e0266164. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Choi, J.H.; Son, A. Necessity of purification during bacterial DNA extraction with environmental soils. Environ. Health Toxicol. 2017, 32, e2017013. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Hou, L.; Woeste, K.; Shang, Z.; Peng, X.; Zhao, P.; Zhang, S. Soil pretreatment and fast cell lysis for direct polymerase chain reaction from forest soils for terminal restriction fragment length polymorphism analysis of fungal communities. Braz. J. Microbiol. 2016, 47, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Kreader, C.A. Relief of amplification inhibition in PCR with bovine serum albumin or T4 gene 32 protein. Appl. Environ. Microbiol. 1992, 62, 1102–1106. [Google Scholar] [CrossRef]
- Albers, C.N.; Banta, G.T.; Jacobsen, O.S.; Hansen, P.E. Characterization and structural modelling of humic substances in field soil displaying significant differences from previously proposed structures. Eur. J. Soil Sci. 2008, 59, 693–705. [Google Scholar] [CrossRef]
- Sidstedt, M.; Jansson, L.; Nilsson, E.; Noppa, L.; Forsman, M.; Rådström, P.; Hedman, J. Humic substances cause fluorescence inhibition in real-time polymerase chain reaction. Anal. Biochem. 2015, 487, 30–37. [Google Scholar] [CrossRef]
- Aoyama, M. Separation of acid-soluble constituents of soil humic acids by dissolution in alkaline urea solution and precipitation with acid. Chem. Biol. Technol. Agric. 2015, 2, 16. [Google Scholar] [CrossRef]
- Dingle, T.C.; Sedlak, R.H.; Cook, L.; Jerome, K.R. Tolerance of droplet-digital PCR vs real-time quantitative PCR to inhibitory substances. Clin. Chem. 2013, 59, 1670–1672. [Google Scholar] [CrossRef] [PubMed]
- Rossen, L.; Nørskov, P.; Holmstrøm, K.; Rasmussen, O.F. Inhibition of PCR by components of food samples, microbial diagnostic assays and DNA-extraction solutions. Int. J. Food Microbiol. 1992, 17, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.P.; Plaisance, A.; Huang, D.; Upadhaya, A.; Gudmestad, N.C.; Handoo, Z.A. First report of the stubby root nematode Paratrichodorus allius on potato in North Dakota. Plant Dis. 2016, 100, 1247. [Google Scholar] [CrossRef]
- Jenkins, W.R.B. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Rep. 1964, 48, 692. [Google Scholar]
- Kumari, S.; Subbotin, S.A. Molecular characterization and diagnostics of stubby root and virus vector nematodes of the family Trichodoridae (Nematoda: Triplonchida) using ribosomal RNA genes. Plant Pathol. 2012, 61, 1021–1031. [Google Scholar] [CrossRef]
- Olsen, R.A.; Bakken, L.R. Viability of soil bacteria: Optimization of plate-counting technique and comparison between total counts and plate counts within different size groups. Microb. Ecol. 1987, 13, 59–74. [Google Scholar] [CrossRef]
| Category Z | Length (µm) y | Width (µm) y | Mean Copy (DNA Copies/µL ± SD) x |
|---|---|---|---|
| Large | 658.02–827.45 | 31.35–43.51 | 38.76 ± 22.53 a |
| Medium | 438.72–623.01 | 26.67–38.21 | 39.21 ± 20.68 a |
| Small | 324.22–397.09 | 19.63–34.92 | 8.54 ± 6.01 b |
| Trt Z | Pre-Trt wt (g) y | After Trt wt (g) y | Wt. Loss (%) | Mean Copy (DNA Copies/µL ± SD) X |
|---|---|---|---|---|
| NT | - | - | - | 275.42 ± 375.18 a |
| RT | 25 | 24.06 | 3.76 | 122.99 ± 195.46 ab |
| OD | 25 | 24.39 | 2.43 | 208.14 ± 208.10 ab |
| AC | 25 | 24.13 | 3.48 | 1.89 ± 1.09 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawaju, B.R.; Yan, G. Assessment of Common Factors Associated with Droplet Digital PCR (ddPCR) Quantification of Paratrichodorus allius in Soil. Int. J. Mol. Sci. 2024, 25, 3104. https://doi.org/10.3390/ijms25063104
Lawaju BR, Yan G. Assessment of Common Factors Associated with Droplet Digital PCR (ddPCR) Quantification of Paratrichodorus allius in Soil. International Journal of Molecular Sciences. 2024; 25(6):3104. https://doi.org/10.3390/ijms25063104
Chicago/Turabian StyleLawaju, Bisho Ram, and Guiping Yan. 2024. "Assessment of Common Factors Associated with Droplet Digital PCR (ddPCR) Quantification of Paratrichodorus allius in Soil" International Journal of Molecular Sciences 25, no. 6: 3104. https://doi.org/10.3390/ijms25063104
APA StyleLawaju, B. R., & Yan, G. (2024). Assessment of Common Factors Associated with Droplet Digital PCR (ddPCR) Quantification of Paratrichodorus allius in Soil. International Journal of Molecular Sciences, 25(6), 3104. https://doi.org/10.3390/ijms25063104






