Comparative Characterization of Human Meibomian Glands, Free Sebaceous Glands, and Hair-Associated Sebaceous Glands Based on Biomarkers, Analysis of Secretion Composition, and Gland Morphology
Abstract
1. Introduction
2. Results
2.1. Histological Analyses of the Morphology of MGs, Free SGs, and Hair-Associated SGs
2.2. Immunohistochemical Localization of Biomarkers in MGs, Free SGs, and Hair-Associated SGs
2.2.1. Cytokeratins
2.2.2. Stem Cell Marker
2.2.3. Cell–Cell Contact Marker
2.3. Lipid Accumulation in MGs, Free SGs, and Hair-Associated SGs
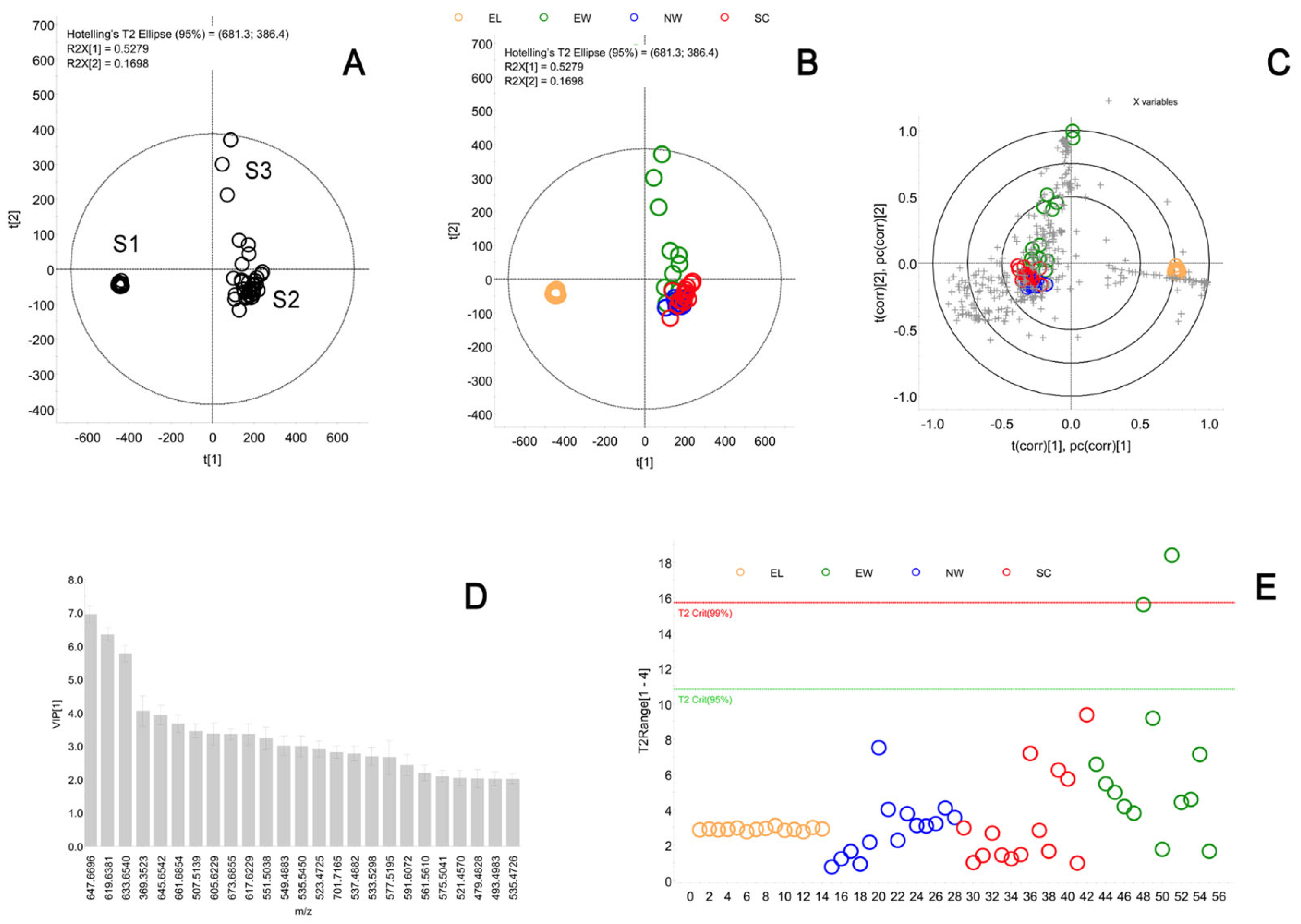
2.4. Meibum and Sebum Component Analysis
2.4.1. Basic Information about the Volunteers
2.4.2. Comparison of the Composition of Meibum and Sebum
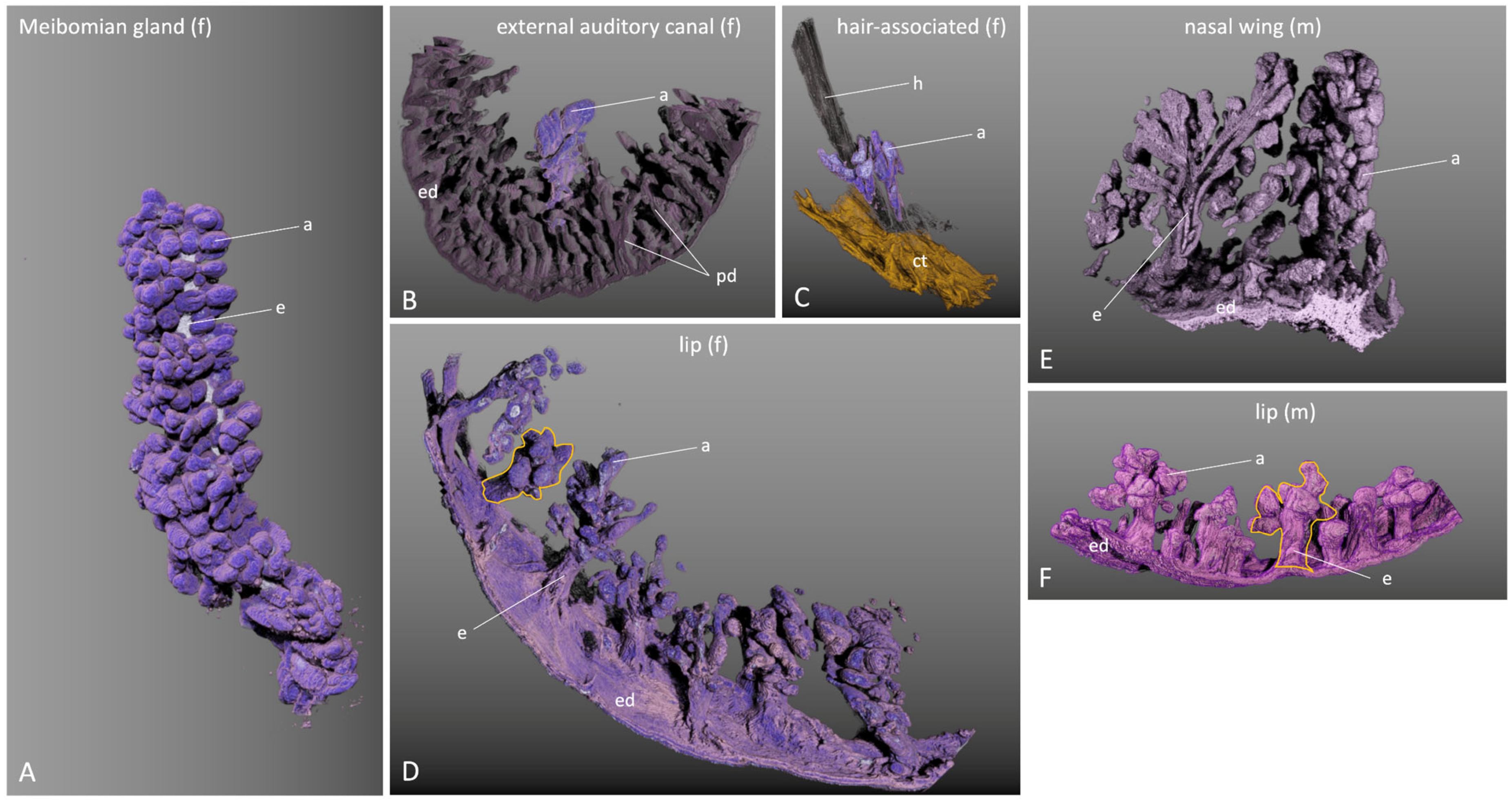
2.5. Three-Dimensional Gland Reconstruction of Different Glands
3. Discussion
4. Materials and Methods
4.1. Collection of Human Samples
4.2. Fixation and Preparation of the Tissue
4.3. Immunohistochemistry
4.4. Oil Red O Staining
4.5. Analysis of Secretion Composition
4.5.1. Collection of Meibum and Sebum
4.5.2. Liquid Chromatography–Mass Spectrometry and Statistical Analysis
4.6. Analysis of the Gland Morphology
4.6.1. Serial Sectioning and Light Microscopy
4.6.2. Scanning of Serial Sections
4.6.3. Processing and 3D Reconstruction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Messmer, E.M. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch. Arztebl. Int. 2015, 112, 71–81, quiz 82. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 75–92. [CrossRef] [PubMed]
- Tsubota, K.; Yokoi, N.; Watanabe, H.; Dogru, M.; Kojima, T.; Yamada, M.; Kinoshita, S.; Kim, H.-M.; Tchah, H.-W.; Hyon, J.Y.; et al. A New Perspective on Dry Eye Classification: Proposal by the Asia Dry Eye Society. Eye Contact Lens. 2020, 46 (Suppl. S1), S2–S13. [Google Scholar] [CrossRef]
- Tong, L.; Chaurasia, S.S.; Mehta, J.S.; Beuerman, R.W. Screening for meibomian gland disease: Its relation to dry eye subtypes and symptoms in a tertiary referral clinic in singapore. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3449–3454. [Google Scholar] [CrossRef]
- Nichols, K.K. The international workshop on meibomian gland dysfunction: Introduction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Knop, E.; Knop, N.; Millar, T.; Obata, H.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978. [Google Scholar] [CrossRef]
- Martel, J.L.; Miao, J.H.; Badri, T. (Eds.) Anatomy, Hair Follicle. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Olami, Y.; Zajicek, G.; Cogan, M.; Gnessin, H.; Pe’er, J. Turnover and migration of meibomian gland cells in rats’ eyelids. Ophthalmic Res. 2001, 33, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Nicolaides, N.; Smith, R.E. Meibomian gland studies: Histologic and ultrastructural investigations. Investig. Ophthalmol. Vis. Sci. 1981, 20, 537–547. [Google Scholar]
- Jester, J.V.; Nicolaides, N.; Kiss-Palvolgyi, I.; Smith, R.E. Meibomian gland dysfunction. II. The role of keratinization in a rabbit model of MGD. Investig. Ophthalmol. Vis. Sci. 1989, 30, 936–945. [Google Scholar]
- Bolognia, J.L.; Schaffer, J.V.; Duncan, K.O.; Ko, C. Dermatology Essentials; Elsevier—Health Sciences Division: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Thody, A.J.; Shuster, S. Control and function of sebaceous glands. Physiol. Rev. 1989, 69, 383–416. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Moreno, I.Y.; Trapp, M.E.; Ramirez, L.; Gesteira, T.F.; Coulson-Thomas, V.J. Meibomian gland development: Where, when and how? Differ. Res. Biol. Divers. 2023, 132, 41–50. [Google Scholar] [CrossRef]
- Lovászi, M.; Szegedi, A.; Zouboulis, C.C.; Törőcsik, D. Sebaceous-immunobiology is orchestrated by sebum lipids. Derm.-Endocrinol. 2017, 9, e1375636. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, H.; Huang, C.; Li, Z.; Li, W.; Zhang, X.; Liu, Z. Impact of Chronic Smoking on Meibomian Gland Dysfunction. PLoS ONE 2016, 11, e0168763. [Google Scholar] [CrossRef] [PubMed]
- Shamloul, G.; Khachemoune, A. An updated review of the sebaceous gland and its role in health and diseases Part 1: Embryology, evolution, structure, and function of sebaceous glands. Dermatol. Ther. 2021, 34, e14695. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Picardo, M.; Ju, Q.; Kurokawa, I.; Törőcsik, D.; Bíró, T.; Schneider, M.R. Beyond acne: Current aspects of sebaceous gland biology and function. Rev. Endocr. Metab. Disord. 2016, 17, 319–334. [Google Scholar] [CrossRef]
- Jeyalatha, M.V.; Qu, Y.; Liu, Z.; Ou, S.; He, X.; Bu, J.; Li, S.; Reinach, P.S.; Liu, Z.; Li, W. Function of meibomian gland: Contribution of proteins. Exp. Eye Res. 2017, 163, 29–36. [Google Scholar] [CrossRef]
- Foulks, G.N.; Bron, A.J. Meibomian gland dysfunction: A clinical scheme for description, diagnosis, classification, and grading. Ocul. Surf. 2003, 1, 107–126. [Google Scholar] [CrossRef]
- Rötzer, V.; Egu, D.; Waschke, J. Meibomian gland cells display a differentiation-dependent composition of desmosomes. Histochem. Cell Biol. 2016, 146, 685–694. [Google Scholar] [CrossRef]
- Tektaş, O.Y.; Yadav, A.; Garreis, F.; Schlötzer-Schrehardt, U.; Schicht, M.; Hampel, U.; Bräuer, L.; Paulsen, F. Characterization of the mucocutaneous junction of the human eyelid margin and meibomian glands with different biomarkers. Ann. Anat. 2012, 194, 436–445. [Google Scholar] [CrossRef]
- Pappas, A.; Johnsen, S.; Liu, J.-C.; Eisinger, M. Sebum analysis of individuals with and without acne. Derm.-Endocrinol. 2009, 1, 157–161. [Google Scholar] [CrossRef]
- Ewurum, A.; Ankem, A.; Georgiev, G.; Borchman, D. A spectroscopic study of the composition and conformation of cholesteryl and wax esters purified from meibum. Chem. Phys. Lipids 2021, 238, 105088. [Google Scholar] [CrossRef]
- Mudgil, P.; Borchman, D.; Gerlach, D.; Yappert, M.C. Sebum/Meibum Surface Film Interactions and Phase Transitional Differences. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2401–2411. [Google Scholar] [CrossRef]
- Butovich, I.A. Meibomian glands, meibum, and meibogenesis. Exp. Eye Res. 2017, 163, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Nien, C.J.; Massei, S.; Lin, G.; Liu, H.; Paugh, J.R.; Liu, C.-Y.; Kao, W.W.-Y.; Brown, D.J.; Jester, J.V. The development of meibomian glands in mice. Mol. Vis. 2010, 16, 1132–1140. [Google Scholar] [PubMed]
- Wolff, E.; Bron, A.J.; Tripathi, R.C.; Tripathi, B.J. Wolff’s Anatomy of the Eye and Orbit; Chapman & Hall: Boca Raton, FL, USA, 1997. [Google Scholar]
- Fenteany, G.; Glogauer, M. Cytoskeletal remodeling in leukocyte function. Curr. Opin. Hematol. 2004, 11, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Tan, D.T.H.; Beuerman, R.W. The eyelid margin: A transitional zone for 2 epithelial phenotypes. Arch. Ophthalmol. 2007, 125, 523–532. [Google Scholar] [CrossRef]
- Riau, A.K.; Barathi, V.A.; Beuerman, R.W. Mucocutaneous junction of eyelid and lip: A study of the transition zone using epithelial cell markers. Curr. Eye Res. 2008, 33, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Knop, E.; Ludescher, M.; Knop, N. Keratinization of the Human Meibomian Gland and Its Contribution to Meibomian Gland Dysfunction (mgd). Investig. Ophthalmol. Vis. Sci. 2010, 51, 2366. [Google Scholar]
- Latham, J.A.; Redfern, C.P.; Thody, A.J.; de Kretser, T.A. Immunohistochemical markers of human sebaceous gland differentiation. J. Histochem. Cytochem. 1989, 37, 729–734. [Google Scholar] [CrossRef]
- Reneker, L.W.; Irlmeier, R.T.; Shui, Y.-B.; Liu, Y.; Huang, A.J.W. Histopathology and selective biomarker expression in human meibomian glands. Br. J. Ophthalmol. 2020, 104, 999–1004. [Google Scholar] [CrossRef]
- Wong, H.H.; Chu, P. Immunohistochemical features of the gastrointestinal tract tumors. J. Gastrointest. Oncol. 2012, 3, 262–284. [Google Scholar] [CrossRef]
- Kurokawa, I.; Nishimura, K.; Hakamada, A.; Isoda, K.-I.; Yamanaka, K.-I.; Mizutani, H.; Tsubura, A. Cutaneous dermoid cyst: Cytokeratin and filaggrin expression suggesting differentiation towards follicular infundibulum and mature sebaceous gland. Oncol. Rep. 2006, 16, 295–299. [Google Scholar] [CrossRef][Green Version]
- Kurzen, H.; Esposito, L.; Langbein, L.; Hartschuh, W. Cytokeratins as markers of follicular differentiation: An immunohistochemical study of trichoblastoma and basal cell carcinoma. Am. J. Dermatopathol. 2001, 23, 501–509. [Google Scholar] [CrossRef]
- Parfitt, G.J.; Geyfman, M.; Xie, Y.; Jester, J.V. Characterization of quiescent epithelial cells in mouse meibomian glands and hair follicle/sebaceous glands by immunofluorescence tomography. J. Investig. Dermatol. 2015, 135, 1175–1177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Abbas, A.K.; Fausto, N.; Robbins, S.L.; Cotran, R.S. (Eds.) Robbins and Cotran Pathologic Basis of Disease, 7th ed.; Elsevier, Saunders: Philadelphia, PA, USA, 2005; ISBN 9780721601878. [Google Scholar]
- Lanza, R.P.; Atala, A. (Eds.) Pluripotent Stem Cells, 2nd ed.; Elsevier, AP: Amsterdam, The Netherlands, 2013; ISBN 9780124017191. [Google Scholar]
- Parfitt, G.J.; Lewis, P.N.; Young, R.D.; Richardson, A.; Lyons, J.G.; Di Girolamo, N.; Jester, J.V. Renewal of the Holocrine Meibomian Glands by Label-Retaining, Unipotent Epithelial Progenitors. Stem Cell Rep. 2016, 7, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.-T.; Sullivan, D.A.; Chen, D.; Hatton, M.P.; Kam, W.R.; Liu, Y. Biomarkers for Progenitor and Differentiated Epithelial Cells in the Human Meibomian Gland. Stem Cells Transl. Med. 2018, 7, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.; Garreis, F.; Paulsen, F. Pathophysiology of Meibomian Glands—An Overview. Ocul. Immunol. Inflamm. 2021, 29, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Teh, M.-T.; Mackenzie, I.C.; Waseem, A. Keratin k15 as a biomarker of epidermal stem cells. Int. J. Mol. Sci. 2013, 14, 19385–19398. [Google Scholar] [CrossRef]
- Walko, G.; Castañón, M.J.; Wiche, G. Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 2015, 360, 529–544. [Google Scholar] [CrossRef]
- Waschke, J. The desmosome and pemphigus. Histochem. Cell Biol. 2008, 130, 21–54. [Google Scholar] [CrossRef]
- Hampel, U.; Schröder, A.; Mitchell, T.; Brown, S.; Snikeris, P.; Garreis, F.; Kunnen, C.; Willcox, M.; Paulsen, F. Serum-induced keratinization processes in an immortalized human meibomian gland epithelial cell line. PLoS ONE 2015, 10, e0128096. [Google Scholar] [CrossRef] [PubMed]
- Butovich, I.A.; Uchiyama, E.; McCulley, J.P. Lipids of human meibum: Mass-spectrometric analysis and structural elucidation. J. Lipid Res. 2007, 48, 2220–2235. [Google Scholar] [CrossRef]
- Thiboutot, D. Regulation of human sebaceous glands. J. Investig. Dermatol. 2004, 123, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Picardo, M.; Ottaviani, M.; Camera, E.; Mastrofrancesco, A. Sebaceous gland lipids. Derm.-Endocrinol. 2009, 1, 68–71. [Google Scholar] [CrossRef]
- Nakahara, T.; Moroi, Y.; Takayama, K.; Nakanishi, Y.; Furue, M. Analysis of sebum lipid composition and the development of acneiform rash before and after administration of egfr inhibitor. Curr. Oncol. 2015, 22, 124–127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Butovich, I.A.; McMahon, A.; Wojtowicz, J.C.; Lin, F.; Mancini, R.; Itani, K. Dissecting lipid metabolism in meibomian glands of humans and mice: An integrative study reveals a network of metabolic reactions not duplicated in other tissues. Biochim. Biophys. Acta 2016, 1861, 538–553. [Google Scholar] [CrossRef]
- Gorgas, K.; Völkl, A. Peroxisomes in sebaceous glands. IV. Aggregates of tubular peroxisomes in the mouse Meibomian gland. Histochem. J. 1984, 16, 1079–1098. [Google Scholar] [CrossRef]
- Sirigu, P.; Shen, R.L.; Da Pinto Silva, P. Human meibomian glands: The ultrastructure of acinar cells as viewed by thin section and freeze-fracture transmission electron microscopies. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2284–2292. [Google Scholar]
- Green-Church, K.B.; Butovich, I.; Willcox, M.; Borchman, D.; Paulsen, F.; Barabino, S.; Glasgow, B.J. The international workshop on meibomian gland dysfunction: Report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1979–1993. [Google Scholar] [CrossRef]
- Pucker, A.D.; Nichols, J.J. Analysis of meibum and tear lipids. Ocul. Surf. 2012, 10, 230–250. [Google Scholar] [CrossRef]
- Nicolaides, N.; Santos, E.C.; Smith, R.E.; Jester, J.V. Meibomian gland dysfunction. III. Meibomian gland lipids. Investig. Ophthalmol. Vis. Sci. 1989, 30, 946–951. [Google Scholar]
- Joffre, C.; Souchier, M.; Grégoire, S.; Viau, S.; Bretillon, L.; Acar, N.; Bron, A.M.; Creuzot-Garcher, C. Differences in meibomian fatty acid composition in patients with meibomian gland dysfunction and aqueous-deficient dry eye. Br. J. Ophthalmol. 2008, 92, 116–119. [Google Scholar] [CrossRef]
- Guest, J.F.; Greener, M.J.; Robinson, A.C.; Smith, A.F. Impacted cerumen: Composition, production, epidemiology and management. QJM 2004, 97, 477–488. [Google Scholar] [CrossRef]
- Yoshida, S.; Shimmura, S.; Kawakita, T.; Miyashita, H.; Den, S.; Shimazaki, J.; Tsubota, K. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4780–4786. [Google Scholar] [CrossRef]
- Rötzer, V.; Melega, F.; Garreis, F.; Paulsen, F.; Waschke, J. E-Cadherin Is Important for Meibomian Gland Function as Revealed by a New Human ex Vivo Slice Culture Model. Am. J. Pathol. 2019, 189, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Tesch, F.; Siegerist, F.; Hay, E.; Artelt, N.; Daniel, C.; Amann, K.; Zimmermann, U.; Kavvadas, P.; Grisk, O.; Chadjichristos, C.; et al. Super-resolved local recruitment of CLDN5 to filtration slits implicates a direct relationship with podocyte foot process effacement. J. Cell. Mol. Med. 2021, 25, 7631–7641. [Google Scholar] [CrossRef]
- Henker, R.; Scholz, M.; Gaffling, S.; Asano, N.; Hampel, U.; Garreis, F.; Hornegger, J.; Paulsen, F. Morphological features of the porcine lacrimal gland and its compatibility for human lacrimal gland xenografting. PLoS ONE 2013, 8, e74046. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.J.; Zetzsche, M.; Scholz, M.; Hahn, D.; Gaffling, S.; Heichel, J.; Hammer, C.M.; Bräuer, L.; Paulsen, F. New insights into the lacrimal pump. Ocul. Surf. 2020, 18, 689–698. [Google Scholar] [CrossRef] [PubMed]
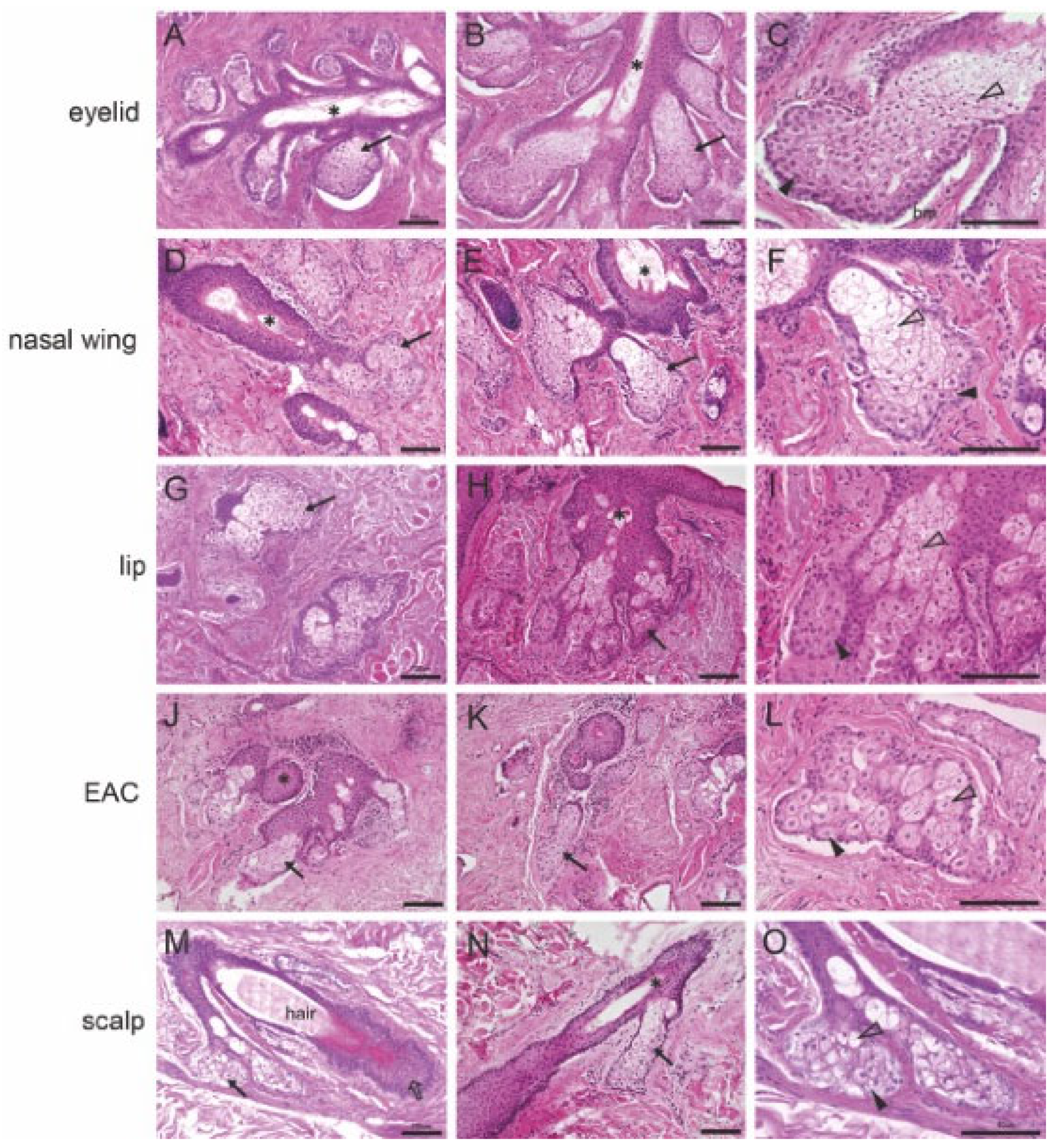
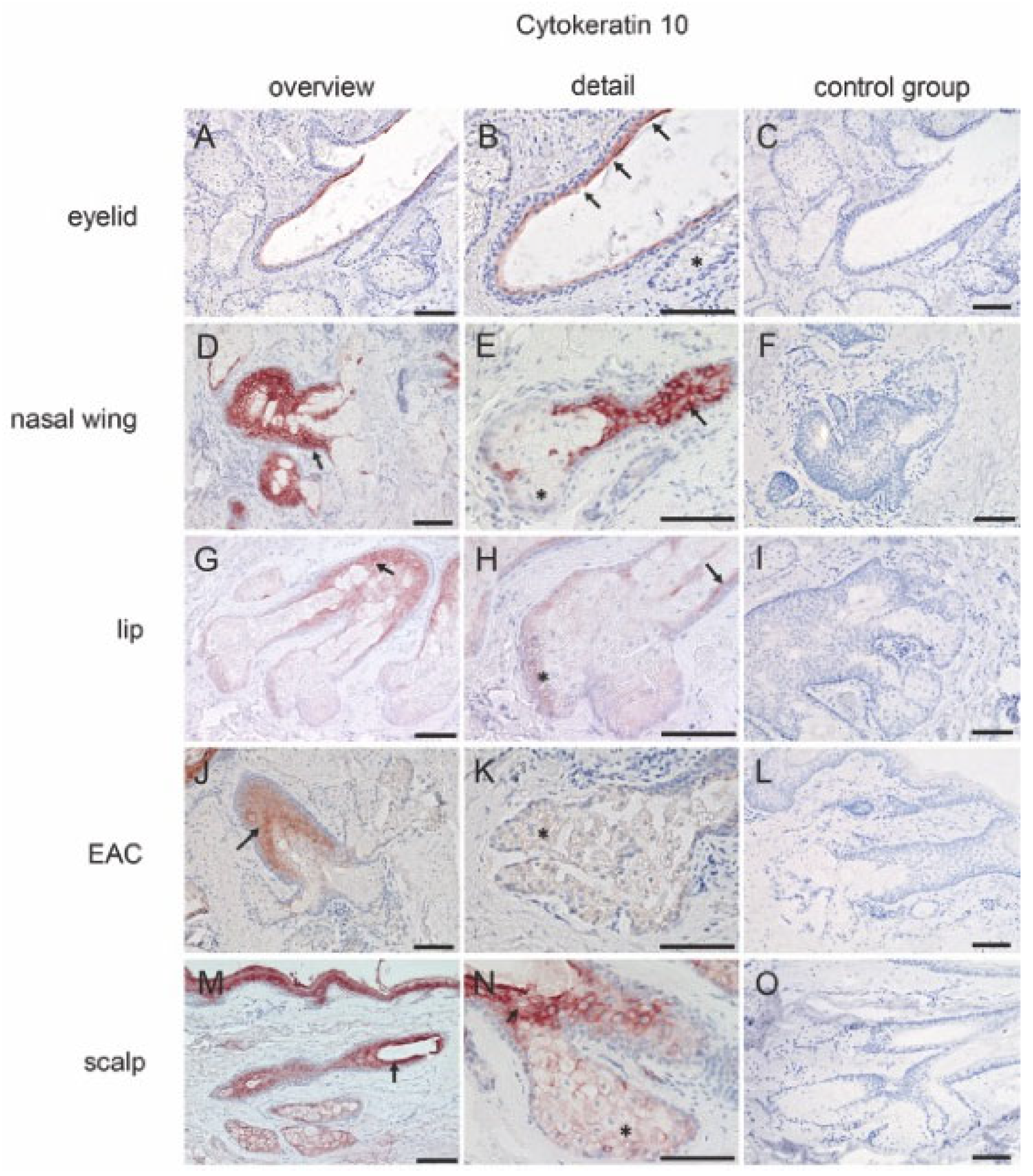
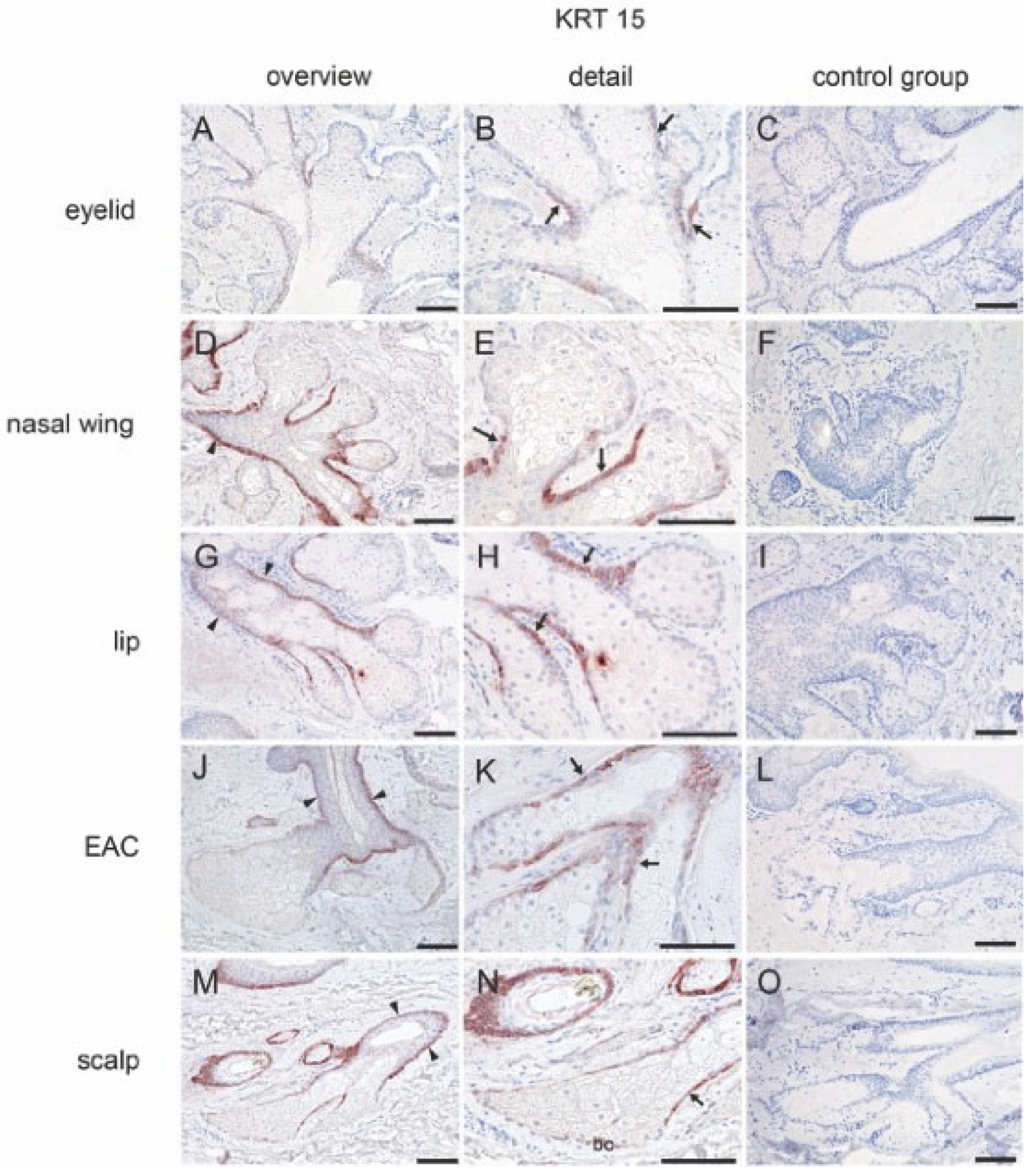

| Markers | MGs | Free SGs | Hair-Associated SGs | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eyelid | Nasal Wing | Lip | EAC | Scalp | ||||||||||||||||
| Duct | bc | mc | Duct | bc | mc | Duct | bc | mc | Duct | bc | mc | Duct | bc | mc | ||||||
| sl | bl | sl | bl | sl | bl | sl | bl | sl | bl | |||||||||||
| cytokeratins | ||||||||||||||||||||
| CK1 | + | − | − | − | + | − | − | − | + | − | − | − | + | − | − | − | + | − | + | + |
| CK8 | − | − | + | − | − | − | + | − | − | − | + | − | − | − | + | − | − | − | + | − |
| CK10 | + | − | − | − | + | − | + | + | + | − | + | + | + | − | + | + | + | − | + | + |
| CK14 | − | + | + | − | − | + | + | + | − | + | + | + | − | + | + | + | − | + | + | + |
| stem cell marker | ductule | cd | bc | mc | ductule | cd | bc | mc | ductule | cd | bc | mc | ductule | cd | bc | mc | ductule | cd | bc | mc |
| KRT15 | + | − | − | − | + | + | − | − | + | + | − | − | + | + | − | − | + | + | + | − |
| N-cadherin | − | − | + | − | − | − | + | − | − | − | + | − | − | − | + | − | − | − | + | − |
| cell–cell contact marker | ||||||||||||||||||||
| Dsg1 | + | + | + | +/− | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Dsc3 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Dp | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Pg | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| E-cadherin | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Claudin5 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Antibody | Species | Clonality | Company | Product No. | Dilution |
|---|---|---|---|---|---|
| Primary antibodies | |||||
| CK1 | Mouse | Monoclonal | Santa Cruz | sc-376224 | 1:25 |
| CK8 | Rabbit | Monoclonal | Abcam | ab53280 | 1:250 |
| CK10 | Mouse | Monoclonal | Santa Cruz | sc-53252 | 1:250 |
| CK14 | Mouse | Monoclonal | Novocastra | NCL-LL002 | 1:20 |
| KRT15 | Mouse | Monoclonal | Abcam | ab80522 | 1:50 |
| N-cadherin | Rabbit | Polyclonal | Abcam | ab225719 | 1:50 |
| E-cadherin | Rabbit | Monoclonal | Abcam | ab40772 | 1:250 |
| Dsg1 | Mouse | Monoclonal | Progen | 651111 | 1:20 |
| Pg | Mouse | Monoclonal | Progen | 61005 | 1:5 |
| Dp | Rabbit | Polyclonal | St. John’s laboratory | ABIN2736659 | 1:100 |
| Dsc3 | Rabbit | Polyclonal | Abcam | ab190118 | 1:100 |
| Claudin 5 | Rabbit | Monoclonal | Thermo Fisher | JM11-22 | 1:50 |
| Secondary antibodies | |||||
| Goat anti-rabbit biotinylated IgG | Polyclonal | Dako | E0432 | 1:200 | |
| Goat anti-rabbit biotinylated IgG | Polyclonal | Invitrogen | 31820 | 1:200 | |
| Goat anti-mouse biotinylated IgG | Polyclonal | Dako | E0433 | 1:200 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Butovich, I.A.; Garreis, F.; Zahn, I.; Scholz, M.; Gaffling, S.; Jabari, S.; Dietrich, J.; Paulsen, F. Comparative Characterization of Human Meibomian Glands, Free Sebaceous Glands, and Hair-Associated Sebaceous Glands Based on Biomarkers, Analysis of Secretion Composition, and Gland Morphology. Int. J. Mol. Sci. 2024, 25, 3109. https://doi.org/10.3390/ijms25063109
Liu Y, Butovich IA, Garreis F, Zahn I, Scholz M, Gaffling S, Jabari S, Dietrich J, Paulsen F. Comparative Characterization of Human Meibomian Glands, Free Sebaceous Glands, and Hair-Associated Sebaceous Glands Based on Biomarkers, Analysis of Secretion Composition, and Gland Morphology. International Journal of Molecular Sciences. 2024; 25(6):3109. https://doi.org/10.3390/ijms25063109
Chicago/Turabian StyleLiu, Yuqiuhe, Igor A. Butovich, Fabian Garreis, Ingrid Zahn, Michael Scholz, Simone Gaffling, Samir Jabari, Jana Dietrich, and Friedrich Paulsen. 2024. "Comparative Characterization of Human Meibomian Glands, Free Sebaceous Glands, and Hair-Associated Sebaceous Glands Based on Biomarkers, Analysis of Secretion Composition, and Gland Morphology" International Journal of Molecular Sciences 25, no. 6: 3109. https://doi.org/10.3390/ijms25063109
APA StyleLiu, Y., Butovich, I. A., Garreis, F., Zahn, I., Scholz, M., Gaffling, S., Jabari, S., Dietrich, J., & Paulsen, F. (2024). Comparative Characterization of Human Meibomian Glands, Free Sebaceous Glands, and Hair-Associated Sebaceous Glands Based on Biomarkers, Analysis of Secretion Composition, and Gland Morphology. International Journal of Molecular Sciences, 25(6), 3109. https://doi.org/10.3390/ijms25063109








