Abstract
Histones are nuclear proteins essential for packaging genomic DNA and epigenetic gene regulation. Paralogs that can substitute core histones (H2A, H2B, H3, and H4), named histone variants, are constitutively expressed in a replication-independent manner throughout the cell cycle. With specific chaperones, they can be incorporated to chromatin to modify nucleosome stability by modulating interactions with nucleosomal DNA. This allows the regulation of essential fundamental cellular processes for instance, DNA damage repair, chromosomal segregation, and transcriptional regulation. Among all the histone families, histone H2A family has the largest number of histone variants reported to date. Each H2A variant has multiple functions apart from their primary role and some, even be further specialized to perform additional tasks in distinct lineages, such as testis specific shortH2A (sH2A). In the past decades, the discoveries of genetic alterations and mutations in genes encoding H2A variants in cancer had revealed variants’ potentiality in driving carcinogenesis. In addition, there is growing evidence that H2A variants may act as novel prognostic indicators or biomarkers for both early cancer detection and therapeutic treatments. Nevertheless, no studies have ever concluded all identified variants in a single report. Here, in this review, we summarize the respective functions for all the 19 mammalian H2A variants and their roles in cancer biology whilst potentiality being used in clinical setting.
1. Introduction
Genetic material in eukaryotic cells are organized into chromatin. The chromatin is often described as a polymer with nucleosome as the fundamental repeating unit, as described by the classic “beads on a string” model [1]. Each nucleosome consists of 147 base pair (bp) of DNA wrapping around a protein octamer, which is assembled from equal parts of the core histones H2A, H2B, H3, and H4 [2,3]. On both ends of the nucleosome at the DNA entry/exit site, linker histone H1 binds to the nucleosome and play a crucial role in the maintenance of higher-order chromatin structure. Alternations in chromatin structure are associated with change in gene expression modulated by mechanisms such as histone post-translational modifications [4], ATP-dependent nucleosome remodeling and replacement of core histones by variant subspecies by specific chaperones [5].
The expression and deposition of core histones are strictly coupled with DNA replication to fill the gaps behind the progressing replication fork. In the human genome, genes encoding for replication-dependent histones are organized into three gene clusters. The largest cluster, HIST1, consists of 55 genes that reside in chromosome 6 (6p21-6p22). HIST2 and HIST3 which contain six genes and three genes, located at chromosome 1q21 and 1q42 respectively [6].
Histone variants are paralogs of canonical histones. The difference between a variant histone and its canonical counterpart could range from several amino acids to large non-histone domains. Similar to canonical histones, histone variants are also subjected to post-translational modifications (PTMs) such as methylation and ubiquitination [7,8,9,10]. As a result, when canonical histones are replaced, the stability of nucleosomes where variants reside will be altered due to its different structural properties. Variants can further be grouped under two classes, homomorphous and heteromorphous, according to their extent of amino acid sequence changes [7,11]. A group of genes that have no homologs and are not shared among closely related species, known as ORPHAN genes, are quickly evolved and only known to be related to species-specific traits, have characteristics to constitutively expressed throughout the cell cycle and transcripts deposited before and after the S phase, are responsible for the expression of histone variants [12].
Contrary to canonical histone-encoding genes, histone variant-encoding genes contain introns and their mRNAs are polyadenylated [13]. Histone variant genes are expressed in a replication-independent manner and are deposited onto the chromatin by specific histone chaperones and remodelers [14]. Owing to the distinct structural properties of histone variants compared with their canonical counterparts, incorporation of histone variants to the nucleosome will alter the DNA-octamer interaction and hence the overall nucleosome stability. Consequently, histone variants are important players in fundamental cellular processes like DNA damage repair, chromosome condensation, transcription initiation and termination [10,15]. Interestingly, histone variants are mainly expressed in somatic tissues, but some have evolved and exhibit a high degree of variation across species to perform lineage-specific tasks, these variants are predominantly expressed in male germline for sperm packaging [16].
Among all the four classes of core histones, the histone H2A family has the largest number of histone variants with 19 in mammalian species reported to date. In the past decades, reports have revealed the potentiality and capability of H2A variants being the driving cause of cancer. Recently, an increasing number of studies demonstrated the presence and some newly identified, whilst overexpression, down regulation, and mutation of H2A variants in a variety of cancers. Indeed, these variants participate in different stages of cancer progression including migration, invasion, and metastasis. Additionally, the disbalance of variants also induced changes in epigenetic plasticity and sustained oncogene expression programme [16,17]. More importantly, growing evidence suggested that H2A variants may act as novel prognostic indicators and/or biomarkers for both early cancer detection and therapeutic treatments. However, future research is needed to fully explore the role or other unprecedented H2A variants that may play in cancer, as it is thought to be substantially more than what is now understood. Nevertheless, no studies have ever compiled all the found H2A variants in a single published report. Here, in this review, we take the initiative in providing an overview, summarizing the respective functions for all the 19 mammalian H2A variants, with focus on their role in cancers and their potentiality being used in the clinical setting as a therapeutic target.
2. Classification of H2A Variants
H2A variants are categorized as homomorphous or heteromorphous based on the degree of dissimilarity from canonical H2A. Variants like H2A.1 and H2A.2, only differ from the canonical H2A by a few amino acids, are categorized as homomorphous. On the other end of the spectrum, heteromorphous variants carry extra domains on either the N- or C–terminal which result in distinct structure from the canonical histone [7]. Examples of heteromorphous variants are macroH2A (mH2A), H2A.Z and H2A.Bbd (Bar-body deficient) [18].
To visualize the subtle differences in amino acid sequences in homomorphous variants, the classic acetic acid/urea (AU) polyacrylamide gel electrophoresis (PAGE) methodology has been improved with the inclusion of nonionic detergents, Triton X-100, forming TAU PAGE. On the other hand, heteromorphous variants will either be separated by acetic acid-urea or sodium dodecyl-sulfate (NaDodSO4) polyacrylamide gel electrophoresis (SDS-PAGE) [11,18,19,20].
3. The 19 Histone H2A Variants
The H2A family, with 19 mammalian variants reported to date, is the most diverse histone family among the other core histones (Table 1). The amino acid sequences of the 19 histone H2A variants vary greatly from one another but remain highly conserved during evolution, indicating that they all carried out indispensable functions [17,21,22]. In contrast to the other three classes of canonical histones, H2A proteins have both N– and C–terminal tails. Typically, the C–terminal sequences of the H2A variants are the most diverse while the core area is conserved (Figure 1). In addition, the L1 loop on some of the histone H2A variants (H2A.Z.1 and H2A.Z.2) or acidic patch (H2A.Bbd) were found to be clearly different from, and some even vanished within their isoforms [23].

Table 1.
Table showing H2A canonical histone and all the 19 mammalian H2A variants described to date in mammalian species.

Figure 1.
Proteins sequences alignment comparison of all the “Homo sapiens” found histone H2A variants. (GeneBank accession numbers: H2A.X “KAI4074518”; H2A.Z “NP_002097”; H2A.Z.1 “KAI2535323”; H2A.Z.2 “Q71UI9”; MacroH2A.2 “AF151534”; MacroH2A1.1 “AF044286”; MacroH2A1.2 “AF041483”; H2A.Bbd/H2A.B “AAL01652”; H2A.P “KAI3999235”; H2A.1 “P0C0S8”; H2A.2 “NP_003507”. Proteins sequences of H2A.Z.2.2 please refer to (Bönisch et al., 2012) published paper.
3.1. H2A.X—Good Helper in DNA Damage Repair
H2A.X was first identified in the 1980s. It belongs to the heteromorphous class and is found in all eukaryotes [10,24]. It is highly conserved from yeast to human, with 143 amino acids in length and has a molecular weight of −15 kDa [24]. H2A.X is a well-studied variant that plays a critical role in preserving the genome integrity, through taking part in DNA double strand break (DSB) repairing processes to repair DNA damage induced by either chemical, ionizing radiation, or homologous recombination during meiosis [25]. One unique feature of H2A.X is the presence of the SQ motif in the C–terminal region.
Mammalian H2A.X has two SQ motifs (human: TQ, SQ, mice: SQ, SQ) [26]. Upon DNA damage, Serine 139 (S139), which is within the SQE motif, is phosphorylated by DNA damage signaling protein kinases, for instance, Ataxia Telangiectasia Mutated (ATM), ATM-Rad3-related (ATR) and DNA-dependent Protein Kinase (DNA-PK) in the Phosphoinositide 3-kinase (PI3K) pathway, yielding Gamma-H2A.X (γ-H2A.X) [26,27]. Of note, S139 phosphorylation is not necessary for the deposition of H2A.X at DNA repair sites [28].
When DNA double strand break (DSB) is detected, H2A.X is recruited to the DNA damage site by the histone chaperone Facilitate Chromatin Transcription (FACT) [29]. Recent studies demonstrated that H2A.X associated factor contains FACT, DNA-PK, PARP1 and other proteins that are required for DNA damage repair. Phosphorylation of H2A.X by DNA-PK causes conformational changes in the nucleosome, FACT complex thereby primarily catalyzes the exchange activities for H2A and H2A.X. Alternatively, ADP-ribosylation of SPT16 (one of the subunits of FACT), which is driven by Poly (ADP-ribose) Polymerase 1 (PARP1), greatly hindered exchange activities [30]. Importantly, accumulation of H2A.X at repair sites will markedly reduce upon downregulation of SPT16. This illustrated that SPT16 is required to facilitate new H2A.X deposition at repair sites through binding towards Proliferating Cell Nuclear Antigen (PCNA) to the PCNA-interacting protein (PIP) box that it harbors [28,31].
With the deposition of new H2A.X to DSB sites, phosphorylation occurs and the formation of γ-H2A.X can be quickly distributed along the chromosome from DSB sites, spread either bidirectionally or asymmetrically depending on whether DSB is within the topologically associating domains (TADs) or at the TADs border respectively [32]. The spreading creates a DNA damage repair (DDR) platform by initiating a cascade and enlisting many DNA repair components, such as Mediator of DNA damage Checkpoint 1 protein (MDC1), Mre11-Rad50-Nbs1 (MRN) complex, p53 Binding Protein 1 (53BP1), and Breast Cancer Type 1 Protein (BRCA1) [33,34]. Upon completion of this platform, γ-H2A.X will be dephosphorylated by Wild type p53 Induced Phosphatase 1 (Wip1) or displaced by histone exchange [35].
Phosphorylation of H2A.X can also occur on tyrosine 142 (Y142) driven by William Beuren Syndrome Transcription Factor (WSTF) kinase in unstressed cells [36]. EYA1 and EYA3-mediated dephosphorylation of Y142 occurs at DSB to facilitate precise localization of DNA repair factors, which would otherwise result in the recruitment of apoptosis-promoting components [36,37,38].
Interestingly, H2A.X is not only recruited to the chromatin during DNA damage repair (DDR), it also replaces canonical histones in a DSB-independent manner. A recent chromatin immunoprecipitation sequencing (ChIP-seq) on mammalian cells revealed that H2A.X, prior to phosphorylation, already forms uniformly distributed clusters within the nuclear volume, and are especially enriched in sub-telomere regions and active transcription start sites (TSS) in replicating cells [39,40]. While in mouse pluripotent stem cells (PSCs), H2A.X aggregates around extraembryonic lineage genes [40]. Future research is needed to address this issue because the underlying mechanisms governing the distribution of clusters are yet elucidated.
3.1.1. Non-DDR Function of H2A.X
The functions of H2A.X are not confined to DDR. H2A.X is also involved in the regulation of mouse Embryonic Stem Cells (mESCs) proliferation independent of DDR (Table 2). Deposition of H2A.X to rDNA (ribosomal DNA) promoters of mESCs recruits NoRC (Nucleolar Remodeling Complex) to the chromatin to limit mESC proliferation by silencing rRNA transcription [41]. Furthermore, H2A.X knockout (KO) mice, albeit, shows genomic instability, impairment of DNA repair, growth retardation, immune deficiency and even infertility in male mice [42]. The incapacity of sex body formation by condensation of H2A.X-deficient spermatocytes from X and Y chromosome is proposed as the cause of infertility, leading to failure of initiating Meiotic Sex Chromosome Inactivation (MSCI) [43]. In addition, H2A.X can regulate the proper expression of Zygotic Genome Activation (ZGA) genes and Transposable Elements (TE) to allow the establishment of totipotency of mESCs [44]. In human, γ-H2A.X was determined to regulate the fate of human Pluripotent Stem Cells (hPSCs) and progenitor cells. If the level of γ-H2A.X is sustained, hPSCs will have an enhanced fate towards neural development while leukemic progenitors’ hematopoiesis will be suppressed and vice versa [45].

Table 2.
Histone variants’ functions, alterations, and role in cancers. This table summarized all the 19 histone H2A variants’ primary and additional functions, alterations consequences and their role play in different cancer types.
In non-mammalian eukaryotes, H2A.X also manifests its role in regulating zygotic cell division and development of early embryos. H2A.X-F, a H2A.X isoform found only in aquatic species such as Danio and Xenopus, is expressed exclusively in late-stage oocyte, eggs, and early embryos [46]. As our review only focuses on mammalian H2A variants, more detailed information on full eukaryotic histone variants should refer to other published research articles.
3.1.2. H2A.X Role in Cancers–Tumor Suppressor
The crucial role of H2A.X in DDR qualifies it as a tumor suppressor. Indeed, H2A.X has a postulated role in cancer progression and metastasis (Table 2, Table 3 and Table 4). In a p53-null background, heterozygous loss of H2A.X can ameliorate genome instability and incidence of cancer. Furthermore, homozygous loss of H2A.X (11q23) will cause cancer to arise earlier than its heterozygous counterpart. The suggested mechanism for this result is the failure of damage-induced foci formation and co-localize to pre-existing γ-H2A.X platform, hence lowering the DSB signaling efficiency. With that, H2A.X-deficient cells are more sensitive to DNA damaging agents and such H2A.X mutations are being observed in a number of cancers, such as sarcomas, brain tumor, neck squamous cell carcinoma and are predominantly prone to B and T cell lymphomas [47,48].

Table 3.
Histone variants’ tissues expression location, histone chaperones and remodelers and clinical/pathological importance as biomarker/prognostic indicator. This table summarized all the 19 H2A variant’s’ tissues expression location, their corresponding histone chaperones, or remodelers responsible for deposition or exchange in or out the chromatin, and their clinicopathological importance as a prognostic indicator or biomarker for different types of cancers at vary expression levels.

Table 4.
Significance of the altered expression of histone variants in various cancer types. This table summarized the function of all 19 H2A variants according to the difference in their expression levels in cancer.
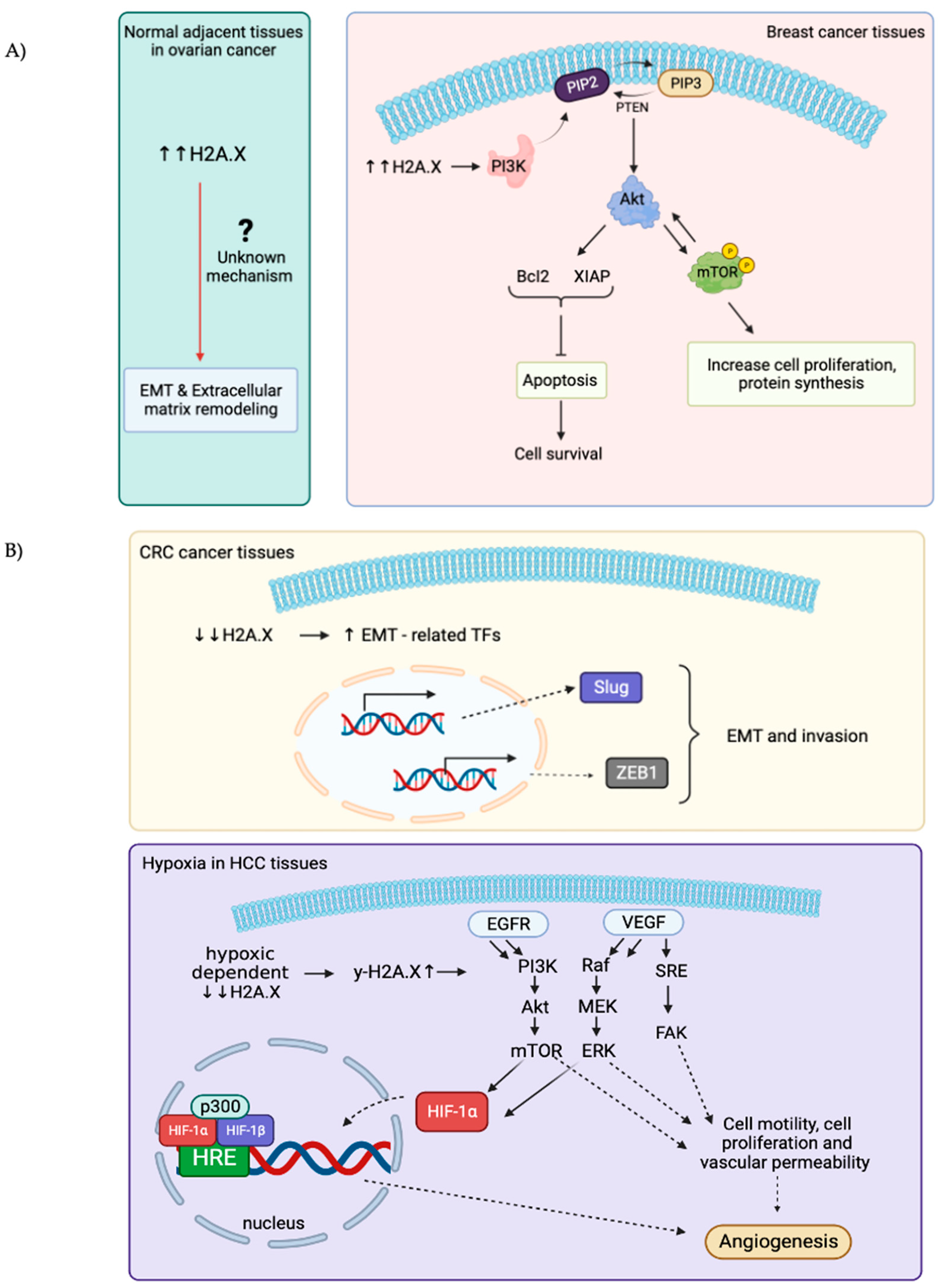
Overexpression of H2A.X is associated with several diseases, the most well-studied are ovarian cancer (OC) and breast cancer [49,50]. High levels of H2A.X are usually associated with upregulation of the PI3K/Akt (Protein kinase B)/Mammalian Target of Rapamycin (mTOR) pathway. This signaling pathway is used to maintain a balance for regular cellular behavior, while activated AKT is used to correlate with mTOR-phosphorylated positivity and is likely to contribute to tumorigenesis by promoting cell proliferation and protein synthesis [51]. With evidence, increased AKT pathways by overexpressing H2A.X assist cancer cells to escape from apoptosis and continuously obtain nutrients to sustain their proliferation [51]. Surprisingly, H2A.X is found selectively overexpressed in normal adjacent tissue (NAT) rather than its origin of malignancy in OC. Extracellular matrix remodeling and epithelial-to-mesenchymal transition (EMT) are observed in these NAT, suggesting that H2A.X may exert a role in helping NAT to obtain well-prepared via cross-talking with cancer microenvironment (Figure 2A) [52]. However, the underlying principles of how a high level of H2A.X in NAT can lead to EMT stays unclear.
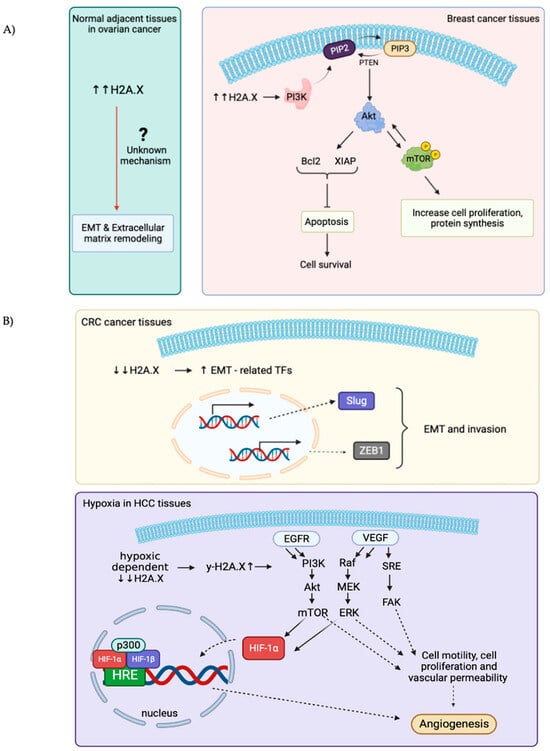
Figure 2.
Schematic illustrations showing the role of overexpressed and down-regulated expressions of H2A.X in different types of cancers. (A): H2A.X is overexpressed in breast cancer and the normal adjacent tissues (NAT) in ovarian cancers. High levels of H2A.X used to activate downstream PI3K/Akt/mTOR signaling pathways to gain advantage for cell growth and cell proliferation in breast cancer. The underlying mechanisms for how the high H2A.X levels in NAT in ovarian cancer lead to EMT and extracellular matrix remodeling remains obscure. (B): H2A.X is downregulated in both CRC and HCC. Decreases in H2A.X in CRC can increase Slug and ZEB1 transcription factors expressions, which further enhance EMT and invasiveness of cancer cells. In HCC, H2A.X levels decrease under hypoxia condition, γ-H2A.X levels thereby increase and regulate multiples signaling pathways (EGFR/HIF-1α/VEGF) to sustain angiogenesis and nutrients uptake for cell growth and proliferation.
EMT in colon cancer has been associated with silencing or downregulation of H2A.X. This is consistent with the suggestion that H2A.X is a regulator of EMT. Low expression of H2A.X upregulated the expression of several key EMT-related Transcription Factors (TFs), Slug and ZEB1 significantly, these two TFs turn out to cooperate to induce EMT programme and invasion [53]. Moreover, hypoxic-dependent reduction in H2A.X abundance in hepatocellular carcinoma (HCC) will increase γ-H2A.X level, consequently promoting angiogenic activity and Epidermal Growth Factor Receptor/Hypoxia-Inducible Factor 1 alpha/Vascular Endothelial Growth Factor (EGFR/HIF-1α/VEGF) signaling pathway to gain nutrients (Figure 2B) [54].
Apart from the amount of H2A.X, the site of phosphorylation also influences cancer progression. Of note, S139 phosphorylation is vital for normal genome stability. However, newly identified phosphorylation in tyrosine 39 (Tyr39) is seen in a variety of cancers as well. Atypically elevated Tyr39 phosphorylation level positively correlates with the proliferation rate of cancer cells, poorer differentiation, higher histological grade, size of tumor, higher risk of metastasis, and lower survival rate [55]. As a result, these studies remarkedly illustrated that a proper expression and regulation of H2A.X securely safeguard our genome stability and function as an essential component in tumor suppressive mechanisms.
3.1.3. H2A.X–A Potential Prognostic/Biomarker for Cancers
The major role of H2A.X in DNA damage repair positions it as attractive candidate as a biomarker. In the past decades, after identifying γ-H2A.X as a universal marker for DSB, its mRNA and protein phosphorylation levels are highly valued and to be applied in the diagnosis of cancers and survival outcome prediction, which indicated a defect in DDR (Table 3). γ-H2A.X is continuously being identified in multiple cancers, including cervical cancer, colorectal cancer (CRC), melanoma and OC, hence its level is credible enough to assess the effectiveness of cancer therapies, like chemotherapy and radiotherapy nowadays [56]. γ-H2A.X therefore serves as an indicator to monitor the radiation and chemo-drug treatment-induced DSB in cancer patients, so as to evaluate patients’ radiosensitivity, together with how patients respond to the drug treatment and try to minimize the side effects caused by chemotherapy [57,58,59].
A widely used approach in vitro is to investigate the amount of γ-H2A.X induced in peripheral blood lymphocytes after ionizing radiation to predict the high risk or early detection of cancers, such as CRC and bladder cancer [60,61]. Usually, high or constitutive accelerated levels of γ-H2A.X point toward the severeness of cancer stage and tumor sizes. Not only limited to in vitro sensing, tissue-specific genotoxicity can also be measured upon the γ-H2A.X signals by immunofluorescence in vivo. Plappert-Helbig et al. revealed that staining of γ-H2A.X can surpass standard comet assay in identifying lesions caused by genotoxicants [62,63]. This may also provide a new platform for future DNA damage quantification in cancer types.
3.2. H2A.Z–Regulator of Gene Transcription
H2A.Z is another heteromorphous class of H2A variants that was identified within the same period as H2A.X. It is 128 amino acids in length and shares only 60% similarity with the canonical H2A. Heterozygous deletion of H2A.Z is embryonically lethal, underlining its importance in early embryo development [64]. H2A.Z plays a pivotal role in establishing an intricate pattern of gene expression necessary for proper early development in mammals [64,65]. H2A.Z is highly abundant and enriched in the pericentric heterochromatin of undifferentiated cells like mESCs, but not in differentiated mouse or human cells. H2A.Z has diverse functions ranging from transcriptional regulation to DNA replication, DNA repair, cell lineage differentiation, chromosome aggregation and neuronal development (Table 2) [66].
In mammals, H2A.Z localizes around transcription start site (TSS), centromere and enhancer region. Accumulating evidence suggested that distinct PTMs of H2A.Z give rise to a variety of functions. Acetylated H2A.Z enriched in promoter regions are known to participate in gene regulation [67,68,69,70]. TIP60-mediated acetylation of H2A.Z destabilizes the nucleosome to facilitate transcription and recruitment of regulatory factors [68,71]. H2A.Z found in the centromere and H3K27me3-marked facultative heterochromatin of the inactive X chromosome (Xi) are mono-ubiquitinated at the C–terminal (K119, K120, K121 and K125) and participate in transcriptional repression [72].
On the structural level, H2A.Z-containing nucleosome is less stable and has higher DNA accessibility compared with canonical nucleosome [73]. It is suggested to be the consequence of an extended acidic patch and L1 loop changes arose when it formed dimer with H2B and interacted with the whole nucleosome complex [74,75,76]. Recent reports revealed that the C–terminal tail of H2A.Z is required for mediating the DNA unwrapping up to 40 base pairs, while N–terminal tail modulates nucleosome gapping [77]. On the other hand, FRET analysis revealed a higher stability of H2A.Z-nucleosome, whilst formation of chromatin fiber [67,74,76,78]. The functional role of H2A.Z remains controversial with multiple contradictory results, as it can have both activate and suppressive functions in transcriptional regulation. A possible reason for such a dynamic functional role in H2A.Z mainly depends on first, its interaction with different variants partners within a nucleosome context and second, PTMs [79,80].
Two H2A.Z isoforms are being identified and are well-conserved in chordates, they are H2A.Z.1 (H2AFZ), and H2A.Z.2 (H2AFV), which differ from H2A.Z by 3 amino acids (14th amino acid: Threonine in H2A.Z.1, Alanine in H2A.Z.2; 38th amino acid: Serine in H2A.Z.1, Threonine in H2A.Z.2; 127th amino acid: Valine in H2A.Z.1, Alanine in H2A.Z.2.) (Figure 1). Although no significant structural differences have been reported between the two isoforms, their L1 loop exhibit structural variation. This is suggested with an evolutionary substitution in the 38th amino acid that specially located within the histone-fold domain (HFD) and the flexibility of the L1 loop in preventing to clash with canonical H2A in heterotypic nucleosomes [23,70]. Although the two isoforms share high similarity structurally, the functions in mammals are non-redundant. While H2A.Z.1 knockout is embryonically lethal; H2A.Z.2 knockout reduces cell proliferation promote apoptosis [67,70,80]. H2A.Z.1 and H2A.Z.2 have recently been reported they play independent, isoforms-specific and tissue-specific role in gene regulation and neuronal development. H2A.Z.1 even has a dual role in interacting with RNA polymerase 2 for transcription initiation and elongation [81].
H2A.Z.2.2 is a recently identified primate-specific variant of H2A.Z.2 [82]. H2A.Z.2.2 is expressed in all human tissues but is predominately found in human brain [82]. The features that set H2A.Z.2.2 apart from H2A.Z is a shortened C–terminal and 6 amino acids differences (Figure 1).
The spliced variants caused an up to date least stable nucleosome complex. A suggested reason for H2A.Z.2.2-nucleosome severely destabilize is due to a unique docking domain (lack part of H3/H4 binding domain) of this spliced variant due to its much shorter C–terminal. Of note, only when the 6 amino acids are present on the docking domain can weaken the chromatin association [83]. Majority of H2A.Z.2.2 is freely diffused in nucleus, only a limited amount is being incorporated into nucleosome via TIP60 and SRCAP chaperone in a regulatory manner. The already known function of H2A.Z.2.2 is limited to its ability in destabilizing nucleosomes, further details are yet to be explored.
3.2.1. H2A.Z Functions Other Than Gene Regulation
Other than gene regulation, recent studies also pointed out that the detection of H2A.Z at centromere guided the localization of SUMOylated Heterochromatin Protein 1-Alpha (HP1α), which contributes to chromosome segregation and chromatic compaction [72,84,85]. H2A.Z also takes part in DNA repair process (Table 2). The deposition of H2A.Z is mediated by the Snf2 Related CREBBP Activator Protein (SRCAP) complex in mammals. Nucleosome Assembly Protein 1 (NAP1) and FACT also recognize H2A.Z-H2B dimer but do not show a high preference over canonical dimer. Recently, a human chaperone Acidic Nuclear Phosphoprotein 32 Family Member E (ANP32E) is reported and found preferentially bind to H2A.Z C–terminal docking domain to facilitate the deposition and removal of H2A.Z [86,87]. ANP32E and human Inositol auxotrophy 80 (INO80) are recruited to the site of DSB at early stages of DDR and evict H2A.Z upon completion of DNA repair [88,89,90,91]. In addition, H2A.Z has been shown in relation to cognitive function, memory processing and neuronal development as aforementioned by inhibiting its incorporation or depletion [92,93,94]. This coincides with the tissue-specific enrichment of H2A.Z in the brain tissues. Furthermore, H2A.Z is shown to be a key regulator in coordinating EMT by having a dual function in both activating and repressing epithelial or mesenchymal related genes in a depletion manner [95].
H2A.Z is involved in cell cycle regulation by indirect regulation of related genes [96]. ChIP-seq in mESCs revealed H2A.Z localization at replication origin in mammals [97]. H2A.Z also plays indispensable role in ESCs and cell lineage differentiation [98]. Depletion of H2A.Z in mESCs can lead to loss of pluripotency and premature differentiation. H2A.Z regulates gene expression in ESCs by facilitating the binding of pluripotency factors to lineage-specific genes promoters, and downstream recruitment of Polycomb Repressive Complex 2 (PRC2). For cell lineage differentiation, H2A.Z has been implicated in variety of lineages including, but not limited to, cardiomyocytes [99], melanocytes (by H2A.Z.2) [100], myoblast [101] and hematopoietic stem cells [102].
3.2.2. H2A.Z Role in Cancers–Oncogenic Variants
H2A.Z is highly expressed in multiple cancers for instance, breast, colorectal, liver, lung, prostate, metastatic melanoma, pancreatic ductal adenocarcinoma (PDACs) and bladder cancer [103,104,105,106,107,108,109,110]. Overexpression of H2A.Z promotes cell proliferation in both estrogen receptor (ER) positive breast cancer and prostate cancer [111]. Other than that, microarray assays and immunostaining in breast tumor revealed high expression of the H2A.Z.1 isoforms, abundance of which correlated with lymph node metastasis and poorer survival prediction (Table 4).
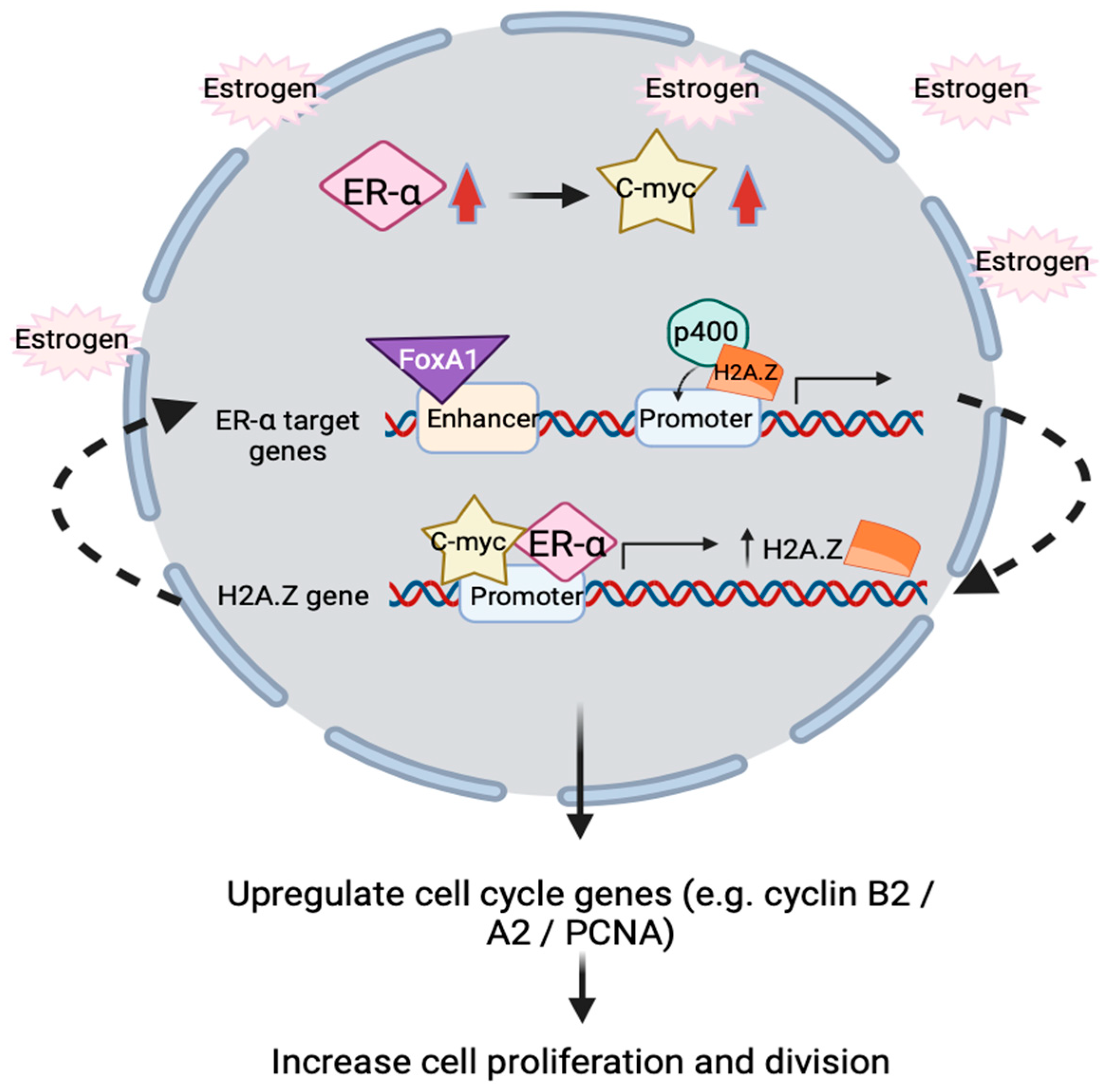
The proposed action of H2A.Z in ER positive breast cancer involves upregulation of estrogen receptor alpha (ERα) and c-Myc to promote proliferation [112,113]. In short, the overall underlying molecular mechanism is that H2A.Z, ERα and c-Myc form a positive feedback loop in regulating estrogen receptor signaling. p400 complex assists H2A.Z incorporation into ERα target promoter in the presence of estrogen, followed by the recruitment of FoxA1 to target enhancers to facilitate target genes’ expression. Activated ERα will bind to H2A.Z gene promoter to increase H2A.Z variants expression. Interestingly, proto-oncogene c-myc will simultaneously be upregulated by ERα in response to estrogen treatment. In line with this, c-Myc is demonstrated to directly regulate and enhance H2A.Z expression level by binding to H2A.Z promoter, turns out subsequently upregulate the transcription of cell cycle genes such as cyclin B2, cyclinA2 and PCNA, which correlates with increasing cell proliferation and division (Figure 3) [111,114].
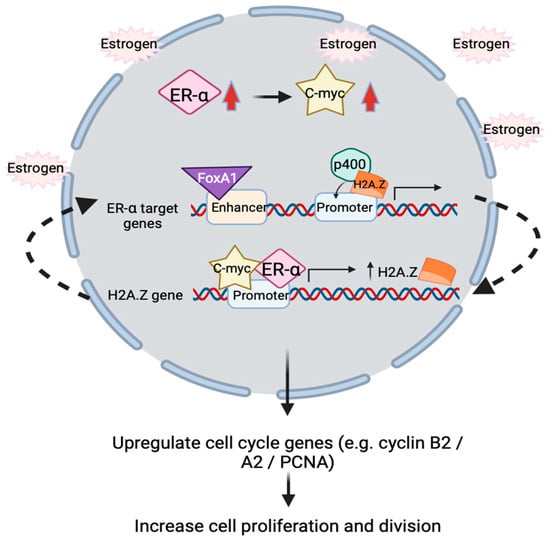
Figure 3.
Schematic illustration showing positive feedback loop relationship between ERα, c-Myc and H2A.Z inside nucleus. In the presence of estrogen, ERα and c-Myc transcription factors (TFs) will simultaneously be upregulated. These two TFs will bind to H2A.Z gene’s promoter to enhance H2A.Z expressions. H2A.Z is incorporated into promoter of ERα target genes by the p400 complex, FoxA1 is then recruited to the enhancer of ERα target genes, resulting in upregulation of gene expression. This turns out upregulate the cell cycle genes and increase breast cancer cells proliferation and cell division.
H2A.Z.1 is implicated in liver tumorigenesis and metastasis. H2A.Z.1 modulates the expression of key genes in cell cycle and EMT in HCC and intrahepatic cholangiocarcinoma (ICC) [115,116]. H2A.Z.1 overexpression in liver cancer cells has been deciphered to suppress cell apoptosis and the expression of negative cell cycle regulators such as p21 and p27. On the other hand, genes involved in cell cycle progression, such as CDK2, CDK4, CDK6 and Cyclin/CDK complex (cyclin D1) are upregulated by H2A.Z.1 overexpression, resulting in pRb hyperphosphorylation and the subsequent loss of its tumor suppressive activity. Furthermore, when H2A.Z.1 is depleted, its metastatic potential was observed with a decrease in chemoattractant-stimulated migratory response and fibronectin whilst an increase in E-cadherin, thereby promote EMT through Transforming Growth Factor–beta (TGF-β) signaling pathway [106,117].
H2A.Z.2 is implicated in the tumorigenesis of aggressive metastatic melanoma. It was postulated that H2A.Z.2 promote proliferation of melanoma cells by recruitment of Bromodomain-containing protein 2 (BRD2) and E2F1 to the promoter region of E2F target genes, where H2A.Z is known to occupy [118]. It works by controlling E2F target genes’ transcription activities in melanoma cells. Recent studies have revealed that H2A.Z.2 interacts with BRD2, hence H2A.Z.2 levels also raised concurrently [118]. BRD2 together with E2F1 bind to E2F target genes in a H2A.Z.2-deposition dependent manner, in turn promote proliferation of melanoma cells. Depletion of H2A.Z.2 resulted in cell cycle arrest in G1/S phase, accompanied by downregulation of cyclin E and cyclin A and Rb hypo-phosphorylation. In addition, PTMs of histone H4 and H2A.Z.2 are also partly involved in driving aggressive melanoma. Hyperacetylation of these two histones enhance the interaction with BRD2, leading to downstream activation of E2F target genes to promote cell proliferation [70,118].
It is important to note that all three isoforms of H2A.Z (H2A.Z.1, H2A.Z.2, H2A.Z.2.2) are highly expressed in PDAC cell lines. Depletion of H2A.Z isoforms resulted in similar cell cycle arrest in G2/M phase [108]. Although all three isoforms have been implicated in the tumorigenesis of PDAC, overexpression of H2A.Z.1 and H2A.Z.2 are more potent activators of oncogenic phenotype, which is consistent with the role of H2A.Z.1 and H2A.Z.2 in cell cycle progression.
Besides melanoma and PDAC, acetylation of H2A.Z at TSS of active genes can lead to oncogene activation in prostate cancer. On the contrary, deacetylation of H2A.Z at TSS will cause tumor suppressor genes silencing [119]. Furthermore, a recent report has revealed that acetylated H2A.Z can generate new ectopic enhancers, resulting in aberrant gene transcriptional activation in androgen receptor (AR) dependent prostate cancer [120].
Collectively, these findings strongly support the oncogenic properties bared by H2A.Z and its isoforms (Table 2). Given that it takes part in multiple essential biological pathways, not only in gene transcriptional regulation but also in orchestrating DNA repair, chromatin compression, memory processing and even cell lineage commitment, which if their regulations either being disturbed or altered, tumorigenesis can be progressed.
3.2.3. H2A.Z–Potential Diagnostic Biomarker and Therapeutic Target for Cancers
H2A.Z overexpression has been reported in other cancers and are associated with poor prognosis (Table 3). The observation that H2A.Z depletion can induce cell cycle arrest and apoptosis positions it as a potential therapeutic target. Proof-of-concept experiments have been carried out in ICC and melanoma cell lines. In ICC where high H2A.Z expression predicts for poor survival, H2A.Z depletion sensitized ICC cell lines to cisplatin treatment. Cisplastin treatment followed by H2A.Z depletion resulted in apoptosis, inhibition of cell proliferation, and attenuated EMT [115]. Similar findings were reported in melanoma cells, where H2A.Z.2 depletion sensitized the cells to targeted therapies and improved drug efficacy [109]. Nevertheless, PDAC cell lines depleted of H2A.Z remain resistant to gemcitabine, this suggests that the action of drugs varies and highly depends on the type of cancers [108].
H2A.Z is also a potential prognostic indicator in cancer in addition to a novel therapeutic target. A fascinating viewpoint suggested that H2A.Z epigenetic signals together with other markers, such as 5MC, H3K9Ac and more were being detected in serum levels of circulating cell-fee nucleosomes (ccfn). By measuring levels of H2A.Z will indeed be crucial for early detection of cancer like CRC and PDAC [104]. Moreover, in HCC patients, H2A.Z.1 is further capable in regulating immune infiltration and T cell differentiation. Therefore, the measuring of both H2A.Z.1 expression and TP53 status may provide a more concrete prediction of HCC survival and a comparatively precise treatment to corresponding patients: high H2A.Z.1 are more likely to be sensitive towards immune checkpoint blockers (ICBs), while low H2A.Z.1 will have a better outcome with radiotherapy and chemotherapy [121]. Altogether, this fully manifests that H2A.Z is a promising diagnostic biomarker and a therapeutic target for cancers.
3.3. MacroH2A–Maintainer of Nuclear Organization and Heterochromatin Architecture
MacroH2A was first discovered in 1992 from rat liver nucleosomes [122]. It is an idiosyncratic histone variant with a tripartite structure—an N–terminus region sharing 64% amino acid sequence identity with H2A (1–122a.a.), an unstructured but lysine rich H1-like linker region (123–160a.a.), and lastly, a globular macro domain which size is two times that of the histone fold region (HFR) (161–371a.a.) [122,123,124,125] (Figure 1).
To date, macroH2A1 and macroH2A2, two mammalian macroH2A proteins are reported and encoded respectively by H2AFY and H2AFY2 genes. Notably, two more alternative spliced variants of macroH2A1 exist, they are macroH2A1.1 and macroH2A1.2. MacroH2A1.2 solely different from macroH2A1.1 in the macrodomain binding pocket arises from alternative splicing of a mutually exclusive exon 5, allowing macroH2A1.1, but not macroH2A1.2 the capacity and implications for NAD+ metabolism and ADP-ribose signaling [126]. Despite a high homology between the two spliced variants, they have distinct function, which adds further complexity to the roles of macroH2A in different cellular processes.
In addition to playing a crucial role in maintaining nuclear organization and heterochromatin development, macroH2A also takes part in other molecular processes such as DNA damage repair and transcriptional repression (Table 2). It acts similarly to a plant-specific variant, H2A.W, in shielding approximately 10bp of extra-nucleosomal DNA from being digested by exonuclease 3. This protecting action is mediated by the interaction of its linker region with the DNA sitting close to the entry or exit site [75,127]. MacroH2A plays vital role in the structural maintenance of heterochromatin and nucleolar and is therefore concentrated at constitutive heterochromatin and repetitive DNA sequences. MacroH2A maintains heterochromatin architecture by promoting contacts between heterochromatic repeat and Lamin B1. Depletion of macroH2A resulted in heterochromatin de-condensation and irregularly shaped and expanded nucleolar. It is believed that the change in nucleolar morphology is a result of a surge in transcriptional activity of ribosomal DNA [128,129].
It is suggested that macroH2A contributes to genome-wide silencing of the X chromosome, as macroH2A (including isoforms) was commonly found to be concentrated on the inactive X chromosome (Xi) of female mammalian cells referred to as a macro chromatin body (MCB). Localization of macroH2A to Xi is dependent on X inactive specific transcript (XIST) lncRNA and two distinct macro-chromatin domains on macroH2A itself [130,131]. X chromosome inactivation (XCI) requires the maintenance of a repressive status, which was completed through several repressive mechanisms, including the deposition of macroH2A-H2B dimers into nucleosomes by Acidic Nuclear Phosphoprotein 32B (ANP32B) histone chaperone [132,133]. Several studies have revealed that heterotypic macroH2A-H2B dimer contribute to a more stable octamer formation, this tends to impede transcriptional activity by propagating into a higher-order chromatin structures, turn out assists in XCI [134,135]. However, macroH2A is non-essential for the initiation of XCI, as the process remains intact in macroH2A knockout mice [136,137].
All macroH2A isoforms are likely engaged in heterochromatin maintenance, each with its distinct role. MacroH2A1 is found predominantly at methylated CpG island within facultative heterochromatin, such as that on the Xi [138]. MacroH2A1 localization to the Xi is dependent on the ubiquitin E3 ligase Cullin3SPOP (Speckle-type POZ (pox virus and zinc finger protein) protein) complex, demonstrated by the loss of Xi localization upon Cullin3 knockdown. Prominent X chromosome reactivation was observed in macroH2A1-depleted cells in the presence of DNA methylase and/or histone deacetylase inhibitor, suggesting that macroH2A1 is part of the epigenetic mechanism required for stable silencing of Xi [139,140,141]. For macroH2A2, evidence also revealed its presence on Xi and its potential role in shaping the chromatin organization in both compacted and accessible chromatin in regional contexts, through modulating as its cognate enhancer element [142,143]. On the other hand, the spliced variants macroH2A1.1 and macroH2A1.2, are present in a diffuse manner within the nucleus, and the formation of MCBs on the Xi is in a XIST-dependent manner [144]. Interestingly, macroH2A1.2 has been shown to inhibit Poly(ADP-ribose) Polymerase 1 (PARP-1 ) enzymatic activity, turn out perturbs the silencing effect of PARP-1 on chromatin [145]. Despite multiple reports on macroH2A1.1 enrichment on Xi, its contribution to the stabilization and maintenance of facultative heterochromatin structure remains obscure.
3.3.1. Other Roles of MacroH2A and Its Isoform
Beside heterochromatin maintenance, macroH2A and its isoforms are engaged in DDR, transcriptional regulation, cell fate differentiation, mitochondrial function, and energy metabolism. MacroH2A1.1 is recruited to DSB sites by its interaction with the PARP1, which then facilitates the recruitment of 53BP1 together with Ku heterodimer: one of the DNA repair factors (KU70/80) for Non-Homologous End Joining (NHEJ) and assists in the activation of Checkpoint Kinase 2 (CHEK2) [146,147]. Unlike macroH2A1.1, macroH2A1.2 that lacks a macrodomain binding pocket, is known to mediate Homologous Repair (HR) through recruitment of BRCA1 [75,148].
MacroH2A not only has a well-known function in gene repression, it also has a dual function in signal-induced gene activation. One of the best examples is macroH2A1, but not macroH2A2, regulates memory processing and formation, which its occupancy in hippocampus is dynamically modified in order to promote learning-induced transcriptional activation, especially in upregulated genes following training [149].
MacroH2A1.1 can also sustain mitochondrial function and energy metabolism by optimizing NAD+ level [150]. Furthermore, macroH2A2 acts as a reprogramming barrier in fully differentiated cells, as it is enriched in early pluripotency genes marked by H3K27me3, which prevent reactivation of these genes [151].
Collectively, macroH2A and its isoforms displace a wide range of functions in a variety of cellular processes and give rise to isoforms-specific functions (Table 2).
3.3.2. MacroH2A Roles in Cancer–Tumor Suppressor
Recently, there has been a surge in interest regarding the role of macroH2A and its isoforms in cancer tumorigenesis. There are strong evidence supporting the role of macroH2A and its isoforms as a tumor suppressor. Low expression of macroH2A has been reported in multiple cancers, such as melanoma, breast, colorectal, liver, lung, bladder, cervical and ovarian cancers [152,153,154]. When the level of macroH2A is restored, cancer cell proliferation, migration and metastasis are alleviated, reducing the malignant phenotypes induced by depletion of macroH2A (Table 4).
MacroH2A1.1 is implicated in EMT suppression as recent reports demonstrated dramatic decrease in macroH2A1.1 expression after the EMT induction in immortalized Human Mammary Epithelial Cells (HMLE). Ectopic expression of macroH2A1.1, but not macroH2A1.2, effectively inhibited EMT induction and HMLE exhibits less mesenchymal morphology. The underlying mechanism for the suppression of EMT is related to the presence of Poly (ADP-Ribose) (PAR) binding domain unique to macroH2A1.1. However, overexpressing macroH2A1 and macroH2A1.2 alone cannot reverse a fully mesenchymal cell to undergo Mesenchymal-Epithelial Transition (MET). Whether the reversible action of cells convert back to epithelial status is mediated through cooperating or interacting with other MET inducing signaling factors requires future investigation [155]. The depletion of macroH2A and its isoforms can also induce malignant melanoma progression through enhancing cell proliferation, migration and metastasis. Melanoma tumorigenesis can be suppressed through a direct transcriptional upregulation of a colorectal cancer oncogene, CDK8, with macroH2A and isoforms [154].
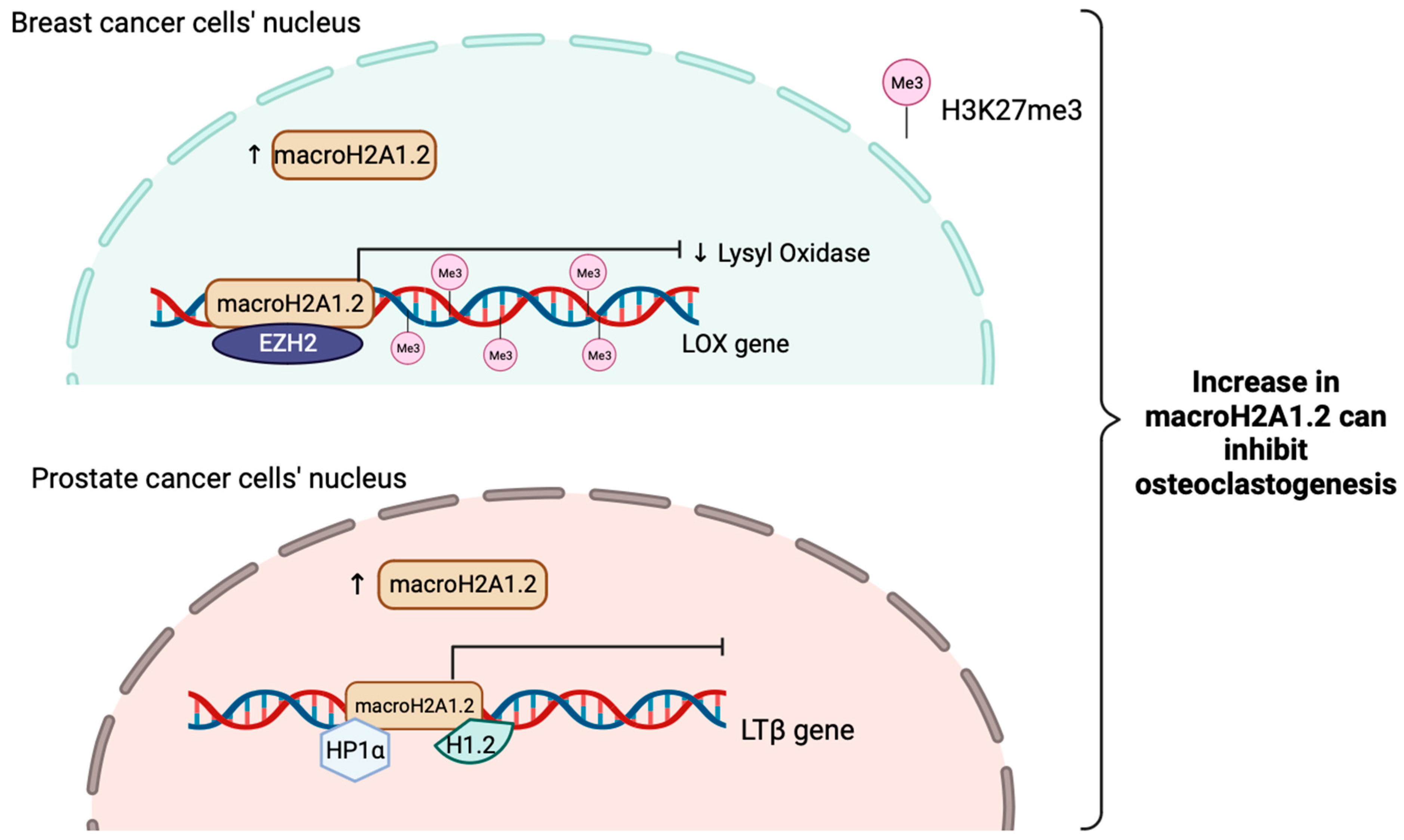
MacroH2A1.2 have been shown to inhibit osteoclastogenesis in bone metastases of breast cancer. This action can be inhibited by macroH2A1.2. It works by attenuating the expression and secretion of Lysyl Oxidase (LOX) by repressing the LOX gene through recruitment of EZH2, resulting in H3K27me3 deposition and ultimately gene silencing [156].
Furthermore, macroH2A1.2 can also inhibit prostate cancer induced osteoclastogenesis via direct interactions with HP1α and H1.2, turn out inactivating a major stimulator of osteoclastogenesis, Lymphotoxin Beta (LTβ) gene in prostate cancer cells. Altogether, this shows that even macroH2A1.2 does not play a role in suppressing EMT just as macroH2A1 and macroH2A1.1 do, it has a novel role in mediating bone metastases (Figure 4) [157].
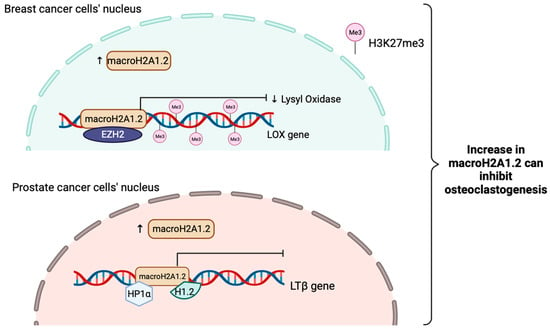
Figure 4.
Schematic illustration showing the tumor suppressive role of macroH2A1.2 when is increased in breast cancer and prostate cancer in inhibiting osteoclastogenesis. MacroH2A1.2 will attenuate the expression of lysyl oxidase (LOX) and interact with EZH2 to raise the H3K27me3 levels to sustain gene silencing in order to inhibit tumor-induced osteoclastogenesis in breast cancer. In prostate cancer, macroH2A1.2 will directly interact with HP1α and H1.2 to suppress LTβ gene expressions, a key stimulator of osteoclastogenesis.
In glioblastoma, the tumor-suppressive function of macroH2A2 is mediated through repression of genes associated with stemness and self-renewal. Depletion of macroH2A2 significantly promoted the self-renewal properties in cancer cells, while overexpression antagonized self-renewal [143,158]. In anal neoplasm, high macroH2A2 expression is associated with low-grade neoplasm and delayed recurrence. Interestingly, macroH2A2 loss in Human Papillomavirus (HPV) positive anal Squamous Cell Carcinoma (SCC) drives tumorigenesis. Of note, oncogene E7 from HPV is vital for inactivating RB to activate E2F for cell proliferation. However, even in HPV-positive SCC samples, where macroH2A2 also functions as an inhibitor to reduce the expressions of E2F-regulate genes, E2F can be activated. Therefore, future work has to be performed to further elucidate the relationships between macroH2A2 and HPV status [159]. Last but not least, high expression of macroH2A2 can impede metastasis in Disseminated Cancer Cells (DCCs) by inhibiting cell cycle and oncogenic signaling programmes [160].
3.3.3. MacroH2A and Isoforms–Novel Prognostic Factor and Diagnostic Markers
MacroH2A and isoforms expression levels highly correlate with patient survival prediction and are proposed to be a novel diagnostic marker in different types of cancers.
It is known that macroH2A1 and isoforms’ expression levels are usually high in different breast cancer types with worst prognosis (Table 3). In Human Epidermal Growth Factor Receptor 2 (HER2) positive breast cancer, macroH2A1.2 expression level is extremely high. MacroH2A1.2 has a trinucleotide insertion (-EIS-) sequences that is absent in macroH2A1.1, is responsible for the interaction and binding between HER2 receptors and its promoter [153]. MacroH2A1.1 contributes to the tumorigenesis of Triple Negative Breast Cancer (TNBC) primarily through modulating the EMT process. The high abundance of macroH2A1.1 has been linked to worse clinical outcome survival. Unlike other prognostic markers, macroH2A1.1 is suggested to be an independent factor that does not rely much on the proliferative status of cells [161,162].
Besides breast cancer, macroH2A1 also showed diagnostic and prognostic value in HCC and lung cancer recurrence. MacroH2A1 is upregulated in HCC and its expression has been associated with poor disease outcome. Gene Set Enrichment Analysis (GSEA) has revealed that high macroH2A1 expression is associated with upregulation of fatty acid metabolism and Mitogen-Activated Protein Kinase (MAPK) signaling pathways, which facilitate HCC tumor cells to better adapt to their microenvironment [163,164]. In lung cancer, macroH2A1.1 can serve as a biomarker for senescence in tumors, which is an anti-tumor mechanism. Patients with low macroH2A1.1 expression are more likely to experience recurrence, a correlation specific to macroH2A1.1 [152].
In anal neoplasm, low macroH2A2 expression is associated with high-grade anal cancer and early recurrence [159]. In contrast, patients with recurrence free survival used to express high level of macroH2A2. These findings suggest that macroH2A2 may serve as a biomarker for accessing the malignancy of anal cancer progression in order to have a more concrete outcome prediction and treatments.
To summarize, all isoforms of macroH2A generally act as a tumor suppressor, with evidence supporting their potential as both diagnostic and prognostic marker in multiple cancers.
3.4. Short H2A (sH2A)–H2A.R
Short H2A histone (sH2A) is a group of H2A variant which displayed a testis-restricted transcription pattern and are expressed exclusively during mammalian spermatogenesis [21]. sH2A is one of the most rapidly evolving group of histone variants and was originated from placental mammals. Features shared among sH2A variants include a truncated docking domain in the C–terminal, almost a complete loss of acidic patch and loss of lysine residues in the N–terminal. Due to the structure differences from canonical H2A, nucleosomes containing sH2A wrap less DNA, which results in loosely packed chromatin.
Recently, an undescribed variant, H2A.R, has been identified and suggested to be a common ancestor for all the known sH2A to date [165,166]. Compared with the previously known sH2A, H2A.R variants are more similar to canonical H2A due to its conserved docking domain in its long C–terminal [167]. To date, there is only one report verified the expression of H2A.R in opossum’s testis (one of the mammals), but not other tissues or organs [167]. Due to a lack of research on this ancestral H2A.R variant, little is known about their role in spermatogenesis and cancer progression. This may be a subject for future investigation.
3.5. Short H2A (sH2A)–H2A.Bbd
sH2A is further classified into 4 different types of variants. Among the four types of variants, Barr body deficient (H2A.Bbd), or H2A.B, is the most researched type of sH2A. H2A.Bbd only shares 48% similarity with canonical H2A. H2A.Bbd differs from H2A in the following ways: (1) The C–terminal tail and the last segment of the docking domain is missing in H2A.Bbd; (2) the N–terminal tail contains 6 consecutive arginine; (3) a more basic L2 loop (Figure 1). H2A.Bbd-nucleosomes contains only 118 bp of DNA, less than the 147 bp in a canonical nucleosome [168]. Studies have demonstrated that H2A.Bbd is found throughout the genome but, is usually associated with transcriptionally active genes on autosomes and the active X chromosome. Little to no H2A.Bbd is incorporated onto the Xi [131,169].
As a result of the missing docking domain in H2A.Bbd, the interaction between H2A.Bbd and H3 is weaker compared with that of H3 and canonical H2A. Therefore, H2A.Bbd incorporation into nucleosome often results in relaxed chromatin [170]. Moreover, H2A.Bbd alters the chromatin remodeling activity mediated by SWItch/Sucrose Non-Fermentable (SW1/SNF) complex [75,171]. It is also shown to enrich at the site of active genes involved in mRNA processing, DNA synthesis and DNA repair [172]. Deposition pattern of H2A.Bbd mostly overlaps with regions marked by H4 acetylation, indicating an active gene transcription region in the genome [173,174]. In addition to that, H2A.Bbd-H2B dimer is spontaneously exchanged within nucleosome with the assistance of a NAP1 histone chaperone [175,176]. Functionally, recent reports have shown that knockdown of H2A.Bbd resulted in an altered chromatin structure in male mouse’s post-meiotic germ cells, yet without affecting spermatogenesis [174,177,178]. It is believed that H2A.Bbd might assist in the transition from histone-based chromatin to protamine-based chromatin during the late stages of spermatogenesis.
H2A.Bbd Role in Cancers
Previous work showed that cells expressing H2A.B will have a shorter S phase and an accelerated sensitivity towards DNA damage, which these are associated with oncogenesis [165]. As sH2A comprised most of the commonly seen canonical H2A mutations in their wildtype sequences, including R29Q or R29F substitutions and a removal of E121 amino acid in the truncated C–terminal [179]. Therefore, this implicated that all sH2As have evolved an onco-histone features and are highly conserved even been through rapid evolution (Table 2).
H2A.B was found to be involved in a broad array of cancer types, where H2A.Bbd’s role in Hodgkin’s lymphoma (HL) was reported. Overexpression of H2A.Bbd positively correlates with cancer cell proliferation (Table 3). It is postulated that H2A.Bbd promote proliferation by enhancing ribosome production. H2A.Bbd containing nucleosomes are commonly found at the promoter of ribosomal genes, at the same time, in a close proximity with RNA polymerase 1, providing a loosen chromatin with highly accessible DNA to facilitate rDNA transcription. The role of H2A.Bbd in promoting ribosomal gene expression is evident from the downregulation of ribosomal gene upon H2A.Bbd depletion.
In addition to promoting rDNA transcription to enhance cell proliferation, H2A.Bbd also engages in the regulation of pre-mRNA splicing of ribosomal proteins, together with PTMs and HIF-1 pathways to establish HL phenotype [180]. Based on data set from Cancer Genome Atlas Program (TCGA), aberrant expression of H2A.Bbd has been reported in the following cancers: urothelial bladder carcinomas (BLCA), cervical squamous cell carcinomas, Uterine Corpus Endometrial Carcinomas (UCEC), endocervical carcinomas and Diffuse Large B-cell Lymphomas (DLBCLs). Among all cancer types, expression of the H2A.Bbd is the highest in DLBCLs (Table 4) [181].
The significance of H2A.Bbd overexpression in the aforementioned cancers is currently unknown. Given its nucleosome-destabilizing effect, it is hypothesized that overexpression of H2A.Bbd might open up the otherwise masked regulatory sequence for oncogenic transcription factors, thereby promoting tumorigenesis.
H2A.Bbd is proposed as a disease biomarker for HL and other cancers since it is commonly seen in a wide range of malignancies; however, its specificity and sensitivity have not been examined and require further research.
3.6. Short H2A (sH2A)–H2A.P, H2A.Q, H2A.L
H2A.P, H2A.Q, and H2A.L are the three remaining histone variants grouped under sH2A according to the most updated research [165,182]. H2A.Q is a newly discovered sH2A present in eutherian mammals. H2A.Q is encoded by a single pseudogene and has a deletion of 7 amino acids in the loop 1 and α-2 helix region. H2A.Q is the shortest eukaryotic histone variants described to date [167]. Interestingly, H2A.Q is only transcribed in the testis of dogs and pigs, but not in other tissues. However, neither human nor any primates’ tissues exhibit H2A.Q expression, suggesting that H2A.Q may not be playing any critical or has lost its role in human or primates.
H2A.P is encoded by a single gene reported so far. Not much detailed information on H2A.P is available until now, by only knowing all sH2As including H2A.P are originated on a portion of X chromosome since the common ancestor of eutherian mammals. Two essential conserved arginine residues that interact with the minor groove of DNA are deleted in H2A.P. Hence, H2A.P containing nucleosomes are expected to be less stable. Furthermore, the last 14 constrained amino acids are a distinctive property that sets H2A.P apart from other sH2A (Figure 1) [167]. Currently, little is known about the actual role that H2A.P plays in mammals, therefore further research is needed to fully uncover that.
H2A.L is the fifth sH2A identified to date. H2A.L is lost in human while conserved in mice. In mice, H2A.L is also known as H2AL1, and has two more isoforms, H2AL2 (H2A.L.2) and H2AL3 (H2A.L.3). The two isoforms differ from each other by no more than 12 amino acids [182,183]. The function of H2A.L.3 has not been documented in any studies so far, necessitating further research to uncover the mystery of this variant.
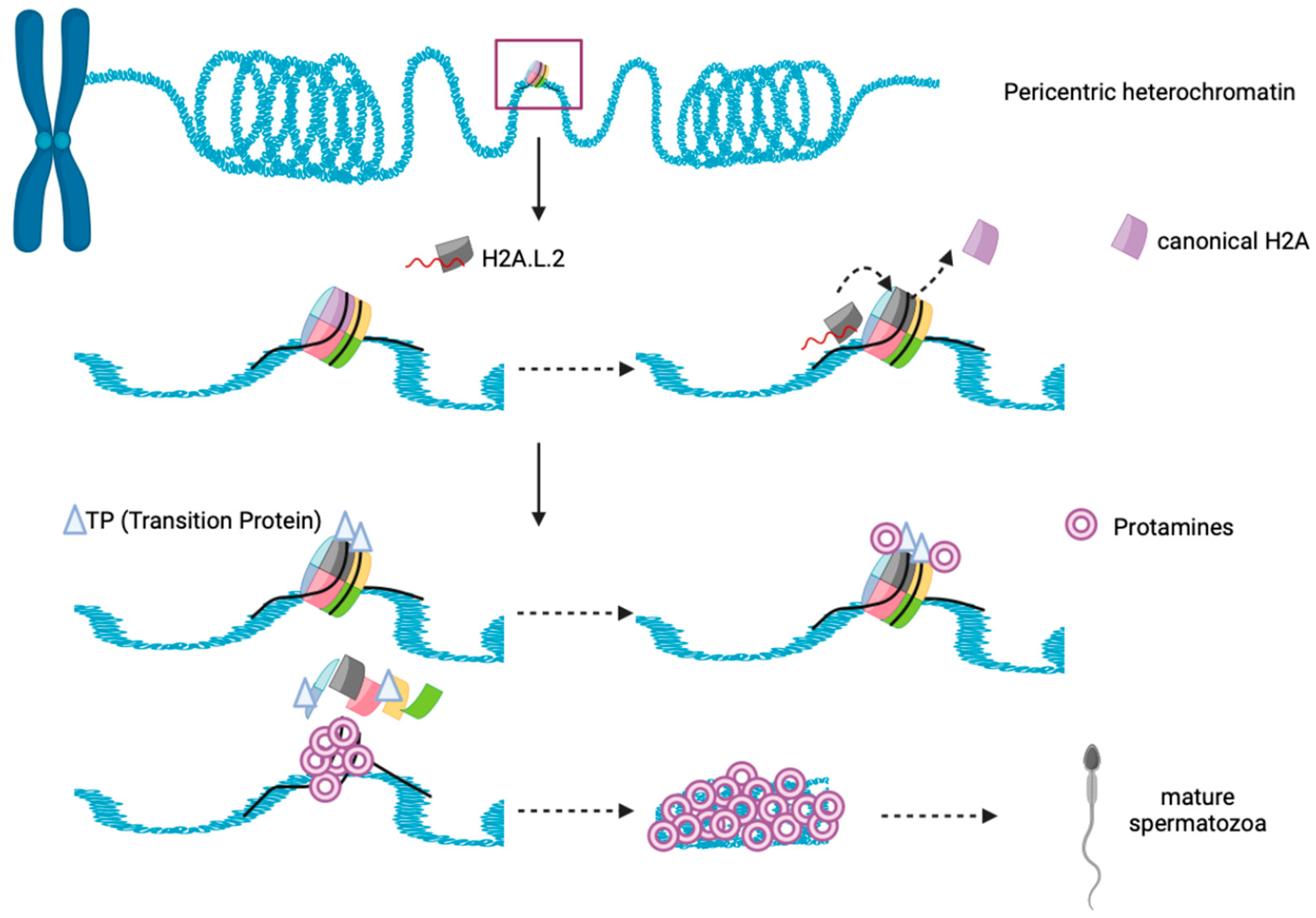
H2A.L.2-containing nucleosome interacts with only 130bp DNA as it shares the same features with other sH2A. Interestingly, H2A.L.2-nucleosome behaves similarly to H2A.Bbd nucleosome, inhibiting both Remodeling the Structure of Chromatin (RSC) and SWI/SNF nucleosome remodeling and mobilization [165,184]. H2A.L.2 favors histone retention on pericentric heterochromatin for spermatogenesis that occur in mice, likely due to the presence of its N–terminal arginine rich motif, that is capable for RNA binding and guide its localization to heterochromatin [185]. After that, the incorporation of H2A.L.2 recruits Transition Protein (TP) loading into nucleosome, and further drives TP-dependent protamine assembly for final sperm genome compaction (Figure 5) [186,187]. The importance of H2A.L.2 has been demonstrated through knock-out experiments, where the mice became sterile. Altogether, H2A.L.2 is important for male mouse fertility but is lost in human, suggesting that H2A.L.2 function may be replaced by other sH2A such as H2A.B or H2A.P, but more research is necessary to determine how credible this suggestion is.
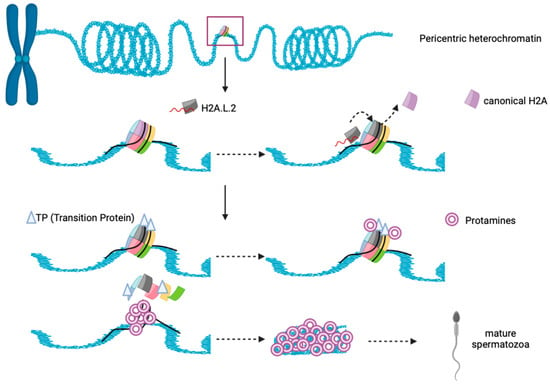
Figure 5.
Schematic illustration showing functional interplay between histone, H2A.L.2, Transition proteins and Protamine during spermatogenesis in mice. N–terminal of H2A.L.2 allow the binding of RNA and it guides the histone variant to the heterochromatin’s nucleosome. Replacement of canonical H2A occurs and H2A.L.2 subsequently recruit transition proteins (TP) loading on nucleosome, and further drives TP-dependent protamine assembly and histone eviction to form mature spermatozoa.
3.7. H2A.22 (H2A.J)
H2A.J is a mammal-specific histone variant encoded by a H2AFJ gene located on human chromosome 12 [166,188]. This variant is specially concentrated in the chromatin of senescent cells, where it stimulates expression of genes in the inflammatory signaling cascade in reaction to DNA damage (Table 2). H2A.J differs from canonical H2A at amino acid 11 (A11V) and contains a potential SQ phosphorylation motif at its C–terminal at around the last 7 amino acids (Figure 1) [189]. With these alterations, H2A.J nucleosome is quite stable through enhancing DNA and histone interaction [190].
To be noted, the C–terminal tail of H2A.J is functionally critical for the expression of inflammatory cytokines/chemokines that belong to the Senescent-Associated Secretory Proteins (SASP) family, as inflammatory related genes are severely downregulated when H2A.J is mutated [191]. Genes that heavily depend on H2A.J for their expression typically have it deposited at the promoter and coding regions. Curiously, H2A.J was not commonly found at TSS regions. It is hypothesized that there may be an internal competition between H2A.J and H2A.Z.
As formerly described, H2A.J shows high abundance in senescent cells having proliferation arrest and associated with persistent DNA damage. It also appears to be accumulated in aging mice in a tissue-specific manner. Reports tested with Hair Follicle Stem Cell (HFSC) and Interfollicular Epidermal Cells (IEC) obtained from both young and old mice, after inducing DNA damage through irradiation, only old mice’s IEC and HFSC show significantly higher H2A.J accumulation [192]. Remarkably, H2A.J’s expression level is very low in proliferating cells, but in young mice, it is seen to be enriched, albeit to varying degrees, in specific organs such as kidney, brain and liver. This suggests that H2A.J may in some way displaying tissues or organs specific functions regardless of the state of cell senescence. To a certain point, H2A.J’s actual role in these organs at young age requires further investigation.
3.7.1. H2A.22/H2A.J Role in Cancer
Inflammatory cytokines produced by senescent cells such as interleukin 1, 6, 8 (IL-1, IL-6, IL-8) are contributor of chronic inflammation. As chronic inflammation is known to promote pro-tumorigenic processes such as angiogenesis, proliferation, invasion, and metastasis, H2A.J might promote tumorigenesis via the upregulation of SASP [193].
H2A.J is highly expressed in a variety of carcinomas, especially in ER-positive breast and prostate cancers [189,194]. The role of H2A.J in breast cancer is by modulating the expression of estrogen and metastasis-associated genes, while H2AFJ tends to be hypo-methylated and overexpressed, suggesting that it might be an oncogene for the luminal B type breast cancer (Table 4). Yet, further functional studies are necessary to validate the role of this new candidate oncogenes in breast tumorigenesis. Other types of cancers that H2A.J are also involved in include Kidney Renal Cell Carcinoma (KIRC), aggressive melanoma, bladder and brain cancer. In contrast to luminal B breast cancer where H2A.J might act as an oncogene, high level of H2A.J is associated with better outcome in prostate cancer, bladder cancer and all other subtypes of breast cancer, yet withdrawing luminal A. On the other hand, high H2A.J levels in KIRC, brain cancer and aggressive melanoma is associated with poor survival [195]. These observations suggested that role of H2A.J in cancer is highly dependent on the cancer types.
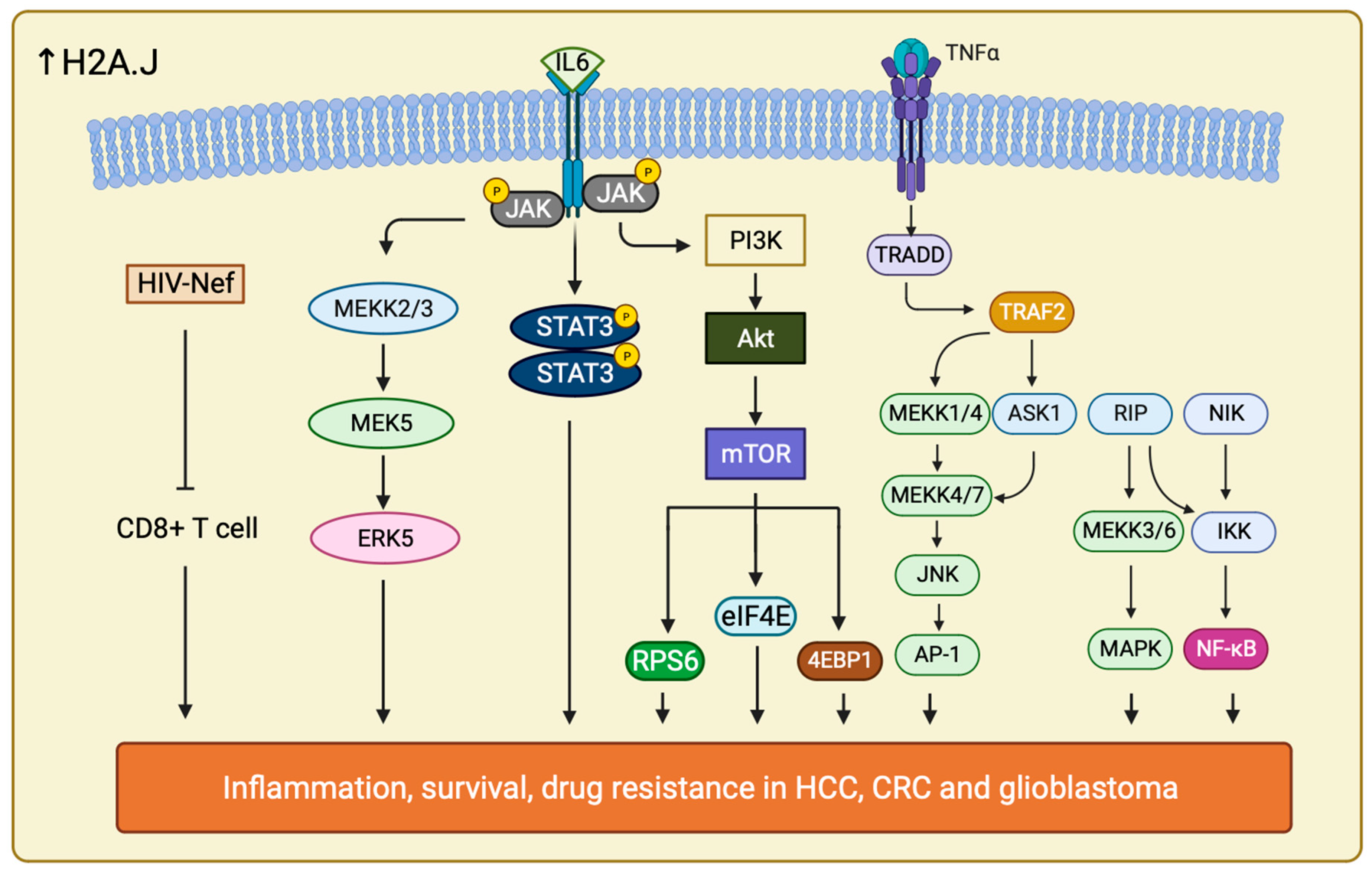
It is interesting to uncover that H2A.J may also play a role in chemoradiotherapy (CRT) resistance in CRC. H2A.J expression is much higher in CRT-resistant CRC compared with CRT-sensitive CRC. It has been confirmed that H2A.J is involved in regulating two important pathways, Mitogen-activated Protein Kinase 7/Extracellular signal Regulated Kinase 5 (MAPK7/ERK5) and Human Immunodeficiency Virus (HIV), Negative Factor (Nef) pathway in CRC, together with the enrichment of several inflammatory pathways to increase CRT resistance in CRC patients (Figure 6) [196].
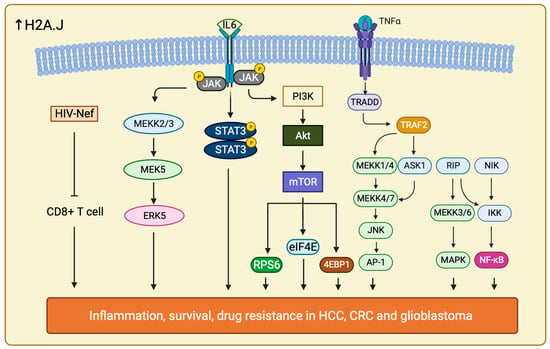
Figure 6.
Schematic illustration showing all the signaling pathways that are involved in drug resistance in cancer therapy when H2A.J is overexpressed. Increase in H2A.J regulates multiple significant signaling pathways including ERK5, TNF-α/NF-κB, PI3K/Akt, JAK /STAT, HIV Nef, IL-6/STAT and more (not listed all here). These pathways play different role and allow cancer cells to survive against different drug treatments through accelerating large amount of inflammatory responses, escaping CD8+ T cell activity and enhance their cell proliferation rate and cell growth.
Furthermore, H2A.J drives radiotherapy and temozolomide (TMZ) resistance in glioblastoma as well. H2A.J is upregulated in mesenchymal type of glioblastoma, the most aggressive subtypes of glioblastoma. H2A.J expression in mesenchymal type of glioblastoma is associated with Proneural-Mesenchymal Transition (PMT) and activation of Tumor Necrosis Factor—Alpha (TNF-α)/Nuclear Factor Kappa-light-chain-enhancer of activated B cells (NF-κB) pathways. These interacting signal networks allow contact also between Signal Transducers and Activators of Transcription 3 (IL-6/STAT3) and Histone Deacetylase 3 (HDAC3) (Figure 6) [197]. The role of H2A.J in promoting TMZ resistance is affirmed by the downregulation of the aforementioned pathways upon H2A.J depletion. Moreover, upregulation of H2A.J in combination with HDAC3 have been associated to poor overall survival in glioblastoma [198]. A more comprehensive and thorough mechanisms by which H2A.J regulate these pathways to modify the PMT progression in glioblastoma should be further explored.
H2A.J has also been implicated in the development of resistance towards sorafenib in HCC [199]. H2AFJ and other hub genes are significantly upregulated in HCC. However, it is unclear how and if H2A.J expression correlate with disease outcome and overall survival prediction. In addition to knowing sorafenib resistance of HCC is due to gene upregulation, it also takes part in accelerating signaling pathways including PI3K/Akt and Janus Kinase (JAK) /STAT pathways. The other hub genes, such as Dynein Light Chain LC8-Type 2 (DYNLL2), SH3 and multiple Ankyrin Repeat Domains Protein 2 (SHANK2), and Metastasis-Associated Protein 3 (MTA3) all contributed to the resistance by regulating metastasis and cell cycle checkpoint [200,201,202]. It is suggested that H2A.J may act similarly as in glioblastoma through upregulating IL-6/STAT signaling, EMT signaling and TNF-α/NF-κB pathways to control inflammatory response, cell proliferation, migration, anti-apoptosis, and survival response (Figure 6) [203].
Collectively, H2A.J is overexpressed in a variety of cancers, but its mode of action is highly context-dependent, which is evident from the contradicting correlation with overall survival/disease outcome in different types of cancer.
3.7.2. H2A.22/H2A.J–Potential Biomarker and Indicator for Therapeutic Treatment
Although H2A.J alone has proven to be an ineffective prognostic marker in glioblastoma [198]. It is possible that the combination of H2A.J expression levels and other established markers such as O6-methylguanine-DNA-Methyltransferase (MGMT), could lead to a more accurate and sensitive prediction for therapeutic treatment outcomes, particularly for those patients who has developed resistance to chemotherapeutic agent.
H2A.J may also serve as a biomarker for senescent stem and aging skin cells. Antigen Kiel 67 (Ki-67) is a well-known marker for proliferating cells, this surface marker will decrease upon aging, while H2A.J expressing keratinocytes will be accelerated [204]. 53BP1 foci used to escalate in aging skin cell but it is difficult to identify discrete 53BP1 foci using immunostaining; in contrast, H2A.J accumulation is much easier to be detected [192]. As a result, H2A.J may become a potential biomarker for senescent cells with increased efficacy and sensitivity.
3.8. H2A.1 and H2A.2
H2A.1 and H2A.2 are the homomorphous group of histone variants with a slight difference in amino acid sequence compared with canonical H2A (Figure 1). H2A.1, also known as TH2A. Genes encoding for H2A.1 are located on chromosome 6p21-22 in human, and chromosome 17p11 in mouse. H2A.1 is a mammalian specific histone variant expressed in testis, oocytes and zygote. H2A.1 differs from the canonical H2A by 10 amino acids and has a preference for forming a dimer with H2B.1.
Co-expression of H2A.1 with the four Yamanaka factors (OKSM: OCT4, SOX2, KLF4 and MYC), induce an open chromatin that enhanced the generation of iPSC. Domain swapping experiment demonstrated that the L1 loop region of H2A.1 is essential for the reprogramming [205]. A similar phenomenon was seen in human as well, co-expression of H2A.1 histone chaperone, Nucleophosmin/Nucleoplasmin 2 (NPM2), with OSKM promoted the generation of iPSCs from naïve stem cell [206]. Remarkably, in zygote, depletion of maternal H2A.1 leads to a perturbation in paternal genome activation, indicating a significant role in genome reprogramming [207].
H2A.1 is uniformly distributed and commonly enriched on X chromosome and autosomes. It was found that H2A.1 can exert both transcriptional upregulation and suppression to genes on the X chromosome. In addition, H2A.1 is also found to be highly expressed in testis and is involved in spermiogenesis and early embryonic mitosis. The 127th amino acid of H2A.1 in mouse is found to be phosphorylated (pH2A.1) during early embryo mitosis and spermatogenesis [208,209]. This data suggested that pH2A.1 is involved in chromatin condensation, which occurs during the last stage of spermiogenesis. Interestingly, paternal pH2A.1 that is rapidly removed upon fertilization will reappear in the pericentromeric heterochromatin of the first mitotic zygote.
Compared with other H2A variants, reports on H2A.2 are limited. Despite the contrasting function of H2A.1 and H2A.2, they are often discussed together in literature. H2A.2 differs from H2A.1 by a few amino acids, two of them are being well-documented; 16th amino acid (H2A.1: Threonine, H2A.2: Serine) and the 51st amino acid (H2A.1: Leucine, H2A.2: Methionine) [210]. H2A.2 coding genes are resided on chromosome 1q21 in human and chromosome 2q34 in mouse [211].
The role of H2A.2 has been investigated in the neuron differentiation, embryogenesis, and aging [212]. Deposition of H2A.2 and H2A.1 is observed during postnatal development for the maturation of rat brain cortical neurons. These two histone variants undergo a dynamic change right after birth and during the postnatal period. At birth, H2A.1 shows a high abundance, whereas H2A.2 represents only 27% of the total H2A. However, H2A.2 expression surged during postnatal development and gradually replaced H2A.1 to become one of the major replication dependent variants (42% of total H2A) [213]. One study pointed out these 2 variants are ubiquitinated in the terminally differentiated cortex neurons [214]. Nevertheless, the precise function of the two histone variants in the differentiation of the neurons remain to be elucidated.
In addition to neuronal development, their expression levels are also being examined during liver cell differentiation, from the embryonic to the adult stage. Notably, H2A.2 will gradually increases, while H2A.1 level decreases in adult hepatocytes [215]. This suggested that H2A.1 functions in epigenetic reprogramming in embryonic stem cells and undifferentiated cells, but the exact role of H2A.2 is unclear.
H2A.1, H2A.2 Role in Cancer
H2A.1 and H2A.2 have been associated with hepatocarcinogenesis. Their expression levels vary at different stages of cancer progression. Interestingly, while H2A.2 is the predominant variant in normal and preneoplastic tissue, H2A.1 is overexpressed in HCC. The high abundance of H2A.1 coincides with the other studies that suggested H2A.1 opens up the chromatin structure to facilitate gene transcription, hence cell proliferation for hepatocarcinogenesis (Table 4).
These two histone variants share almost 98% identity. They are synthesized and deposited during the G1 and S phases when examining hepatoma cells [216]. However, a single amino acid variation between the two variants leads to subtle differences in histone-DNA interaction. This could potentially explain the functional heterogeneity between H2A.1 and H2A.2 in regulating malignancy-associated genes [211]. The H2A.1/H2A.2 ratio is important, for instance, if H2A.1 is overexpressed, reprogramming of hepatocytes could take place through hyperproliferating normal liver cells to return back to preneoplastic and neoplastic phases, which would further promote the development of hepatocarcinogenesis. These results are consistent with earlier studies showing the role of H2A.1 in epigenetic reprogramming.
Recent discovery of CpG island at the promoter region of H2A.1 and TSS-proximal region of H2A.2 suggests that the expression of the two variants might be regulated by different epigenetic factors during tumorigenesis [217]. H2A.1 and H2A.2 are also expressed in colon cancer, and they somehow appeared to have varying degrees of acetylation and methylation [218]. H2A.1 is hypo-methylated in embryonic cells while H2A.2 is hyper-methylated in HCC. This observation explained the phenomenon why H2A.2 is not overexpressed in HCC despite its high expression during preneoplastic stages. Moreover, these data also indicate hypo-methylation of H2A.1, which may facilitate the development and spread of tumors, as the cause of HCC [219,220].
Collectively, it should be highly appreciated that the dynamic expression patterns of these two histone variants contribute to distinct time points of development have different effects (Table 2). More studies are required to solve all the confusion including the unknown mechanisms and roles of both H2A.1 and H2A.2.
4. Conclusions and Perspectives
The histone H2A family is a group of nuclear proteins containing 19 variants. The role of these variants are mainly reported in mammalian species. Remarkably, all H2A variants are involved in safeguarding our genome integrity. Recently, new insights have additionally pointed out some unprecedented variants, including H2A.R and H2A.Q, which are further being grouped together. Furthermore, new alternative spliced forms of variants, such as H2A.Z.2.2 are just being identified and its function is only limited to destabilizing nucleosome complexes. Therefore, the detailed functional role of all the 19 mammalian H2A variants cannot be thoroughly discussed here due to a lack of supporting evidence, which serves a subject for later elucidation.
On the other side of the coin, many H2A variants have been implicated in cancer development. Majority of H2A variants contribute to tumorigenesis and cancer progression. Aberrant expression of histone variants leads to the upregulation of regulatory genes involved in cell proliferation, migration, EMT, invasiveness and angiogenesis; whilst downregulating tumor suppressor genes and inflammatory responses [221]. It is worth noting that the expression of some particular variants is associated with development of drug resistance in certain cancers. Whether other variants may also guide chemotherapy resistance in clinical settings requires further investigation. Last but not least, it is believed that many of the H2A variants have the potential to serve as prognostic indicators and/or effective biomarkers for cancers. However, the specificity and sensitivity of a variant to be used individually for cancer detection remain questionable. With that, we suggested in combination with other well-established markers, more credible diagnostics outcomes may be identified, hence more precise and improved predictions and therapeutics treatments can be proposed. The validity of this suggestion necessitates further research, pointing toward a new direction for future studies.
Author Contributions
Conceptualization, P.M.L. and K.M.C.; writing—original draft preparation, P.M.L. and K.M.C.; writing—review and editing, P.M.L. and K.M.C.; supervision, K.M.C.; funding acquisition, K.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Research Grants Council Hong Kong (Project No. 11105623 and 11104321), National Science Foundation China (Project 8217111397) to K.M. Chan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data availability in a publicly accessible repository that does not issue DOIs: https://www.uniprot.org, https://www.ncbi.nlm.nih.gov (all accessed on 8 February 2024).
Acknowledgments
We are grateful to Tiantian QIN, Shiman HU and Bing HAN for their insightful comments and suggestions. We want to thank Yi Ching Esther WAN for editing and commenting on the revised manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baldi, S.; Korber, P.; Becker, P.B. Beads on a String—Nucleosome Array Arrangements and Folding of the Chromatin Fiber. Nat. Struct. Mol. Biol. 2020, 27, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Luger, K.; Hansen, J.C. Nucleosome and Chromatin Fiber Dynamics. Curr. Opin. Struct. Biol. 2005, 15, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Cutter, A.R.; Hayes, J.J. A Brief Review of Nucleosome Structure. FEBS Lett. 2015, 589, 2914–2922. [Google Scholar] [CrossRef] [PubMed]
- Tessarz, P.; Kouzarides, T. Histone core modifications regulating nucleosome structure and dynamics. Nat. Rev. Mol. Cell Biol. 2014, 15, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.; Hake, S.B. The Nucleosome: A Little Variation Goes a Long way. Biochem. Cell Biol. 2006, 84, 505–507. [Google Scholar] [CrossRef]
- Marzluff, W.F.; Gongidi, P.; Woods, K.R.; Jin, J.; Maltais, L.J. The Human and Mouse Replication-Dependent Histone Genes. Genomics 2002, 80, 487–498. [Google Scholar] [CrossRef]
- Ausió, J. Histone Variants—The Structure behind the Function. Brief. Funct. Genom. 2006, 5, 228–243. [Google Scholar] [CrossRef]
- Chew, Y.C.; Camporeale, G.; Kothapalli, N.; Sarath, G.; Zempleni, J. Lysine Residues in N- and C-Terminal Regions of Human Histone H2A Are Targets for Biotinylation by Biotinidase. J. Nutr. Biochem. 2006, 17, 225–233. [Google Scholar] [CrossRef]
- Franklin, S.G.; Zweidler, A. Non-allelic variants of histones 2a, 2b and 3 in mammals. Nature 1977, 266, 273–275. [Google Scholar] [CrossRef]
- Talbert, P.B.; Henikoff, S. Histone variants–Ancient wrap artists of the epigenome. Nat. Rev. Mol. Cell Biol. 2010, 11, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Ausió, J.; Abbott, D.W.; Wang, X.; Moore, S.C. Histone Variants and Histone Modifications: A Structural Perspective. Biochem. Cell Biol. Biochim. Biol. Cell. 2001, 79, 693–708. [Google Scholar] [CrossRef]
- Sarma, K.; Reinberg, D. Histone variants meet their match. Nat. Rev. Mol. Cell Biol. 2005, 6, 139–149. [Google Scholar] [CrossRef]
- Dominski, Z.; Marzluff, W.F. Formation of the 3′ End of Histone mRNA. Gene 1999, 239, 1–14. [Google Scholar] [CrossRef]
- Albig, W.; Doenecke, D. The Human Histone Gene Cluster at the D6S105 Locus. Hum. Genet. 1997, 101, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Bönisch, C.; Hake, S.B. Histone H2A Variants in Nucleosomes and Chromatin: More or Less Stable? Nucleic Acids Res. 2012, 40, 10719–10741. [Google Scholar] [CrossRef] [PubMed]
- Buschbeck, M.; Hake, S.B. Variants of core histones and their roles in cell fate decisions, development and cancer. Nat. Rev. Mol. Cell Biol. 2017, 18, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Vardabasso, C.; Hasson, D.; Ratnakumar, K.; Chung, C.Y.; Duarte, L.F.; Bernstein, E. Histone variants: Emerging players in cancer biology. Cell. Mol. Life Sci. 2014, 71, 379–404. [Google Scholar] [CrossRef] [PubMed]
- West, M.H.; Bonner, W.M. Histone 2A, a Heteromorphous Family of Eight Protein Species. Biochemistry 1980, 19, 3238–3245. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.B.; Wobig, W.J.; Petering, D.H. Native SDS-PAGE: High Resolution Electrophoretic Separation of Proteins With Retention of Native Properties Including Bound Metal Ions. Metallomics 2014, 6, 1068–1078. [Google Scholar] [CrossRef]
- Ryan, C.A.; Annunziato, A.T. Separation of Histone Variants and Post-Translationally Modified Isoforms by Triton/Acetic Acid/Urea Polyacrylamide Gel Electrophoresis. Curr. Protoc. Mol. Biol. 1999, 45, 21.2.1–21.2.10. [Google Scholar] [CrossRef]
- Karam, G.; Molaro, A. Casting Histone Variants during Mammalian Reproduction. Chromosoma 2023, 132, 153–165. [Google Scholar] [CrossRef]
- Martire, S.; Banaszynski, L.A. The Roles of Histone Variants in Fine-Tuning Chromatin Organization and Function. Nat. Rev. Mol. Cell Biol. 2020, 21, 522–541. [Google Scholar] [CrossRef]
- Horikoshi, N.; Sato, K.; Shimada, K.; Arimura, Y.; Osakabe, A.; Tachiwana, H.; Hayashi-Takanaka, Y.; Iwasaki, W.; Kagawa, W.; Harata, M.; et al. Structural Polymorphism in the L1 Loop Regions of Human H2A.Z.1 and H2A.Z.2. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 2431–2439. [Google Scholar] [CrossRef]
- Redon, C.; Pilch, D.; Rogakou, E.; Sedelnikova, O.; Newrock, K.; Bonner, W. Histone H2A Variants H2AX and H2AZ. Curr. Opin. Genet. Dev. 2002, 12, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Eirín-López, J.M.; Ausió, J. H2AX: Tailoring Histone H2A for Chromatin-Dependent Genomic Integrity. Biochem. Cell Biol. Biochim. Biol. Cell. 2005, 83, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.M.S.; Flaus, A. Structure and Function of Histone H2AX. In Genome Stability and Human Diseases; Nasheuer, H.-P., Ed.; Subcellular Biochemistry; Springer Netherlands: Dordrecht, The Netherlands, 2010; pp. 55–78. [Google Scholar] [CrossRef]
- Mukherjee, B.; Kessinger, C.; Kobayashi, J.; Chen, B.P.C.; Chen, D.J.; Chatterjee, A.; Burma, S. DNA-PK Phosphorylates Histone H2AX during Apoptotic DNA Fragmentation in Mammalian Cells. DNA Repair 2006, 5, 575–590. [Google Scholar] [CrossRef]
- Piquet, S.; Parc, F.L.; Bai, S.-K.; Chevallier, O.; Adam, S.; Polo, S.E. The Histone Chaperone FACT Coordinates H2A.X-Dependent Signaling and Repair of DNA Damage. Mol. Cell 2018, 72, 888–901.e7. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Dong, L.; Hu, M.; Wang, Y.-Z.; Xiao, X.; Zhao, Z.; Yan, J.; Wang, P.-Y.; Reinberg, D.; Li, M.; et al. Functions of FACT in Breaking the Nucleosome and Maintaining Its Integrity at the Single-Nucleosome Level. Mol. Cell 2018, 71, 284–293.e4. [Google Scholar] [CrossRef] [PubMed]
- Heo, K.; Kim, H.; Choi, S.H.; Choi, J.; Kim, K.; Gu, J.; Lieber, M.R.; Yang, A.S.; An, W. FACT-Mediated Exchange of Histone Variant H2AX Regulated by Phosphorylation of H2AX and ADP-Ribosylation of Spt16. Mol. Cell 2008, 30, 86–97. [Google Scholar] [CrossRef]
- Mailand, N.; Gibbs-Seymour, I.; Bekker-Jensen, S. Regulation of PCNA–Protein Interactions for Genome Stability. Nat. Rev. Mol. Cell Biol. 2013, 14, 269–282. [Google Scholar] [CrossRef]
- Collins, P.L.; Purman, C.; Porter, S.I.; Nganga, V.; Saini, A.; Hayer, K.E.; Gurewitz, G.L.; Sleckman, B.P.; Bednarski, J.J.; Bassing, C.H.; et al. DNA Double-Strand Breaks Induce H2Ax Phosphorylation Domains in a Contact-Dependent Manner. Nat. Commun. 2020, 11, 3158. [Google Scholar] [CrossRef]
- Stucki, M.; Clapperton, J.A.; Mohammad, D.; Yaffe, M.B.; Smerdon, S.J.; Jackson, S.P. MDC1 Directly Binds Phosphorylated Histone H2AX to Regulate Cellular Responses to DNA Double-Strand Breaks. Cell 2005, 123, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Lukas, C.; Melander, F.; Stucki, M.; Falck, J.; Bekker-Jensen, S.; Goldberg, M.; Lerenthal, Y.; Jackson, S.P.; Bartek, J.; Lukas, J. Mdc1 couples DNA double-strand break recognition by Nbs1 with its H2AX-dependent chromatin retention. EMBO J. 2004, 23, 2674–2683. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.; Lowe, J.M.; Li, H.; Lee, J.-S.; Belova, G.I.; Bulavin, D.V.; Fornace, A.J. Wip1 Directly Dephosphorylates Gamma-H2AX and Attenuates the DNA Damage Response. Cancer Res. 2010, 70, 4112–4122. [Google Scholar] [CrossRef] [PubMed]
- Hatimy, A.A.; Browne, M.J.G.; Flaus, A.; Sweet, S.M.M. Histone H2AX Y142 Phosphorylation Is a Low Abundance Modification. Int. J. Mass Spectrom. 2015, 391, 139–145. [Google Scholar] [CrossRef]
- Biterge, B.; Schneider, R. Histone Variants: Key Players of Chromatin. Cell Tissue Res. 2014, 356, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Cook, P.J.; Ju, B.G.; Telese, F.; Wang, X.; Glass, C.K.; Rosenfeld, M.G. Tyrosine Dephosphorylation of H2AX Modulates Apoptosis and Survival Decisions. Nature 2009, 458, 591–596. [Google Scholar] [CrossRef]
- Seo, J.; Kim, K.; Chang, D.-Y.; Kang, H.-B.; Shin, E.-C.; Kwon, J.; Choi, J.K. Genome-Wide Reorganization of Histone H2AX toward Particular Fragile Sites on Cell Activation. Nucleic Acids Res. 2014, 42, 1016–1025. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.; Wen, D.; Tseng, Z.; Tahmasian, M.; Zhong, M.; Rafii, S.; Stadtfeld, M.; Hochedlinger, K.; Xiao, A. Histone Variant H2A.X Deposition Pattern Serves as a Functional Epigenetic Mark for Distinguishing the Developmental Potentials of iPSCs. Cell Stem Cell 2014, 15, 281–294. [Google Scholar] [CrossRef]
- Eleuteri, B.; Aranda, S.; Ernfors, P. NoRC Recruitment by H2A.X Deposition at rRNA Gene Promoter Limits Embryonic Stem Cell Proliferation. Cell Rep. 2018, 23, 1853–1866. [Google Scholar] [CrossRef]
- Celeste, A.; Petersen, S.; Romanienko, P.J.; Fernandez-Capetillo, O.; Chen, H.T.; Sedelnikova, O.A.; Reina-San-Martin, N.; Coppola, V.; Meffre, E.; Difilippantonio, M.J. Genomic Instability in Mice Lacking Histone H2AX. Science 2002, 296, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Capetillo, O.; Mahadevaiah, S.K.; Celeste, A.; Romanienko, P.J.; Camerini-Otero, R.D.; Bonner, W.M.; Manova, K.; Burgoyne, P.; Nussenzweig, A. H2AX Is Required for Chromatin Remodeling and Inactivation of Sex Chromosomes in Male Mouse Meiosis. Dev. Cell 2003, 4, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.-Y.; Guo, S.-M.; Cheng, G.-P.; Yin, Y.; He, X.; Zhou, L.-Q. Cleavage-Embryo Genes and Transposable Elements Are Regulated by Histone Variant H2A.X. J. Reprod. Dev. 2021, 67, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Orlando, L.; Tanasijevic, B.; Nakanishi, M.; Reid, J.C.; García-Rodríguez, J.L.; Chauhan, K.D.; Porras, D.P.; Aslostovar, L.; Lu, J.D.; Shapovalova, Z.; et al. Phosphorylation State of the Histone Variant H2A.X Controls Human Stem and Progenitor Cell Fate Decisions. Cell Rep. 2021, 34, 108818. [Google Scholar] [CrossRef]
- Shechter, D.; Chitta, R.K.; Xiao, A.; Shabanowitz, J.; Hunt, D.F.; Allis, C.D. A distinct H2A.X isoform is enriched in Xenopus laevis eggs and early embryos and is phosphorylated in the absence of a checkpoint. Proc. Natl. Acad. Sci. USA 2009, 106, 749–754. [Google Scholar] [CrossRef]
- Celeste, A.; Difilippantonio, S.; Difilippantonio, M.J.; Fernandez-Capetillo, O.; Pilch, D.R.; Sedelnikova, O.A.; Eckhaus, M.; Ried, T.; Bonner, W.M.; Nussenzweig, A. H2AX Haploinsufficiency Modifies Genomic Stability and Tumor Susceptibility. Cell 2003, 114, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.A.; White, J.S.; Huang, X.; Schoppy, D.W.; Baysal, B.E.; Baskaran, R.; Bakkenist, C.J.; Saunders, W.S.; Hsu, L.-C.; Romkes, M.; et al. Loss of Distal 11q Is Associated with DNA Repair Deficiency and Reduced Sensitivity to Ionizing Radiation in Head and Neck Squamous Cell Carcinoma. Genes Chromosomes Cancer 2007, 46, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-F.; Kong, F.-L. Akt Regulates RSK2 to Alter Phosphorylation Level of H2A.X in Breast Cancer. Oncol. Lett. 2021, 21, 187. [Google Scholar] [CrossRef]
- Gao, X.; Bao, H.; Liu, L.; Zhu, W.; Zhang, L.; Yue, L. Systematic Analysis of Lysine Acetylome and Succinylome Reveals the Correlation between Modification of H2A.X Complexes and DNA Damage Response in Breast Cancer. Oncol. Rep. 2020, 43, 1819–1830. [Google Scholar] [CrossRef]
- Altomare, D.A.; Wang, H.Q.; Skele, K.L.; De Rienzo, A.; Klein-Szanto, A.J.; Godwin, A.K.; Testa, J.R. AKT and mTOR Phosphorylation Is Frequently Detected in Ovarian Cancer and Can Be Targeted to Disrupt Ovarian Tumor Cell Growth. Oncogene 2004, 23, 5853–5857. [Google Scholar] [CrossRef]
- Saravi, S.; Katsuta, E.; Jeyaneethi, J.; Amin, H.A.; Kaspar, M.; Takabe, K.; Pados, G.; Drenos, F.; Hall, M.; Karteris, E. H2A Histone Family Member X (H2AX) Is Upregulated in Ovarian Cancer and Demonstrates Utility as a Prognostic Biomarker in Terms of Overall Survival. J. Clin. Med. 2020, 9, 2844. [Google Scholar] [CrossRef]
- Weyemi, U.; Redon, C.E.; Choudhuri, R.; Aziz, T.; Maeda, D.; Boufraqech, M.; Parekh, P.R.; Sethi, T.K.; Kasoji, M.; Abrams, N.; et al. The Histone Variant H2A.X Is a Regulator of the Epithelial–Mesenchymal Transition. Nat. Commun. 2016, 7, 10711. [Google Scholar] [CrossRef]
- Xiao, H.; Tong, R.; Ding, C.; Lv, Z.; Du, C.; Peng, C.; Cheng, S.; Xie, H.; Zhou, L.; Wu, J.; et al. γ-H2AX Promotes Hepatocellular Carcinoma Angiogenesis via EGFR/HIF-1α/VEGF Pathways under Hypoxic Condition. Oncotarget 2015, 6, 2180–2192. [Google Scholar] [CrossRef]
- Liu, Y.; Long, Y.-H.; Wang, S.-Q.; Li, Y.-F.; Zhang, J.-H. Phosphorylation of H2A.XTyr39 Positively Regulates DNA Damage Response and Is Linked to Cancer Progression. FEBS J. 2016, 283, 4462–4473. [Google Scholar] [CrossRef]
- Kuo, L.J.; Yang, L.-X. Gamma-H2AX—A Novel Biomarker for DNA Double-Strand Breaks. In Vivo 2008, 22, 305–309. [Google Scholar]
- Pouliliou, S.; Koukourakis, M.I. Gamma Histone 2AX (γ-H2AX)as a Predictive Tool in Radiation Oncology. Biomarkers 2014, 19, 167–180. [Google Scholar] [CrossRef]
- Bonner, W.M.; Redon, C.E.; Dickey, J.S.; Nakamura, A.J.; Sedelnikova, O.A.; Solier, S.; Pommier, Y. γH2AX and Cancer. Nat. Rev. Cancer 2008, 8, 957–967. [Google Scholar] [CrossRef]
- Palla, V.-V.; Karaolanis, G.; Katafigiotis, I.; Anastasiou, I.; Patapis, P.; Dimitroulis, D.; Perrea, D. Gamma-H2AX: Can It Be Established as a Classical Cancer Prognostic Factor? Tumor Biol. 2017, 39, 1010428317695931. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.I.; Gong, Y.; Ye, Y.; Lin, J.; Chang, D.W.; Kamat, A.M.; Wu, X. γ-H2AX Level in Peripheral Blood Lymphocytes as a Risk Predictor for Bladder Cancer. Carcinogenesis 2013, 34, 2543–2547. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chang, D.W.; Gong, Y.; Eng, C.; Wu, X. Measurement of DNA damage in peripheral blood by the γ-H2AX assay as predictor of colorectal cancer risk. DNA Repair 2017, 53, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ivashkevich, A.; Redon, C.E.; Nakamura, A.J.; Martin, R.F.; Martin, O.A. Use of the γ-H2AX Assay to Monitor DNA Damage and Repair in Translational Cancer Research. Cancer Lett. 2012, 327, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Plappert-Helbig, U.; Libertini, S.; Frieauff, W.; Theil, D.; Martus, H.-J. Gamma-H2AX Immunofluorescence for the Detection of Tissue-Specific Genotoxicity in Vivo. Environ. Mol. Mutagen. 2019, 60, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Faast, R.; Thonglairoam, V.; Schulz, T.C.; Beall, J.; Wells, J.R.; Taylor, H.; Matthaei, K.; Rathjen, P.D.; Tremethick, D.J.; Lyons, I. Histone Variant H2A.Z Is Required for Early Mammalian Development. Curr. Biol. 2001, 11, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Zhou, J.; Bu, G.; Zhu, W.; He, H.; Sun, Q.; Yu, Z.; Xiong, W.; Wang, L.; et al. Hierarchical Accumulation of Histone Variant H2A.Z Regulates Transcriptional States and Histone Modifications in Early Mammalian Embryos. Adv. Sci. 2022, 9, e2200057. [Google Scholar] [CrossRef] [PubMed]
- Colino-Sanguino, Y.; Clark, S.J.; Valdes-Mora, F. The H2A.Z-Nucleosome Code in Mammals: Emerging Functions. Trends Genet. 2022, 38, 273–289. [Google Scholar] [CrossRef]
- Eirín-López, J.M.; González-Romero, R.; Dryhurst, D.; Ishibashi, T.; Ausió, J. The Evolutionary Differentiation of Two Histone H2A.Z Variants in Chordates (H2A.Z-1 and H2A.Z-2) Is Mediated by a Stepwise Mutation Process That Affects Three Amino Acid Residues. BMC Evol. Biol. 2009, 9, 31. [Google Scholar] [CrossRef]
- Ishibashi, T.; Dryhurst, D.; Rose, K.L.; Shabanowitz, J.; Hunt, D.F.; Ausió, J. Acetylation of Vertebrate H2A.Z and Its Effect on the Structure of the Nucleosome. Biochemistry 2009, 48, 5007–5017. [Google Scholar] [CrossRef]
- Thambirajah, A.A.; Dryhurst, D.; Ishibashi, T.; Li, A.; Maffey, A.H.; Ausió, J. H2A.Z Stabilizes Chromatin in a Way That Is Dependent on Core Histone Acetylation. J. Biol. Chem. 2006, 281, 20036–20044. [Google Scholar] [CrossRef]
- Giaimo, B.D.; Ferrante, F.; Herchenröther, A.; Hake, S.B.; Borggrefe, T. The Histone Variant H2A.Z in Gene Regulation. Epigenetics Chromatin 2019, 12, 37. [Google Scholar] [CrossRef]
- Doyon, Y.; Côté, J. The Highly Conserved and Multifunctional NuA4 HAT Complex. Curr. Opin. Genet. Dev. 2004, 14, 147–154. [Google Scholar] [CrossRef]
- Sarcinella, E.; Zuzarte, P.C.; Lau, P.N.I.; Draker, R.; Cheung, P. Monoubiquitylation of H2A.Z Distinguishes Its Association with Euchromatin or Facultative Heterochromatin. Mol. Cell. Biol. 2007, 27, 6457–6468. [Google Scholar] [CrossRef]
- Placek, B.J.; Harrison, L.N.; Villers, B.M.; Gloss, L.M. The H2A.Z/H2B dimer is unstable compared to the dimer containing the major H2A isoform. Protein Sci. 2005, 14, 514–522. [Google Scholar] [CrossRef]
- Park, Y.-J.; Dyer, P.N.; Tremethick, D.J.; Luger, K. A New Fluorescence Resonance Energy Transfer Approach Demonstrates That the Histone Variant H2AZ Stabilizes the Histone Octamer within the Nucleosome. J. Biol. Chem. 2004, 279, 24274–24282. [Google Scholar] [CrossRef]
- Phillips, E.O.N.; Gunjan, A. Histone Variants: The Unsung Guardians of the Genome. DNA Repair 2022, 112, 103301. [Google Scholar] [CrossRef]
- Abbott, D.W.; Ivanova, V.S.; Wang, X.; Bonner, W.M.; Ausió, J. Characterization of the Stability and Folding of H2A.Z Chromatin Particles: IMPLICATIONS FOR TRANSCRIPTIONAL ACTIVATION. J. Biol. Chem. 2001, 276, 41945–41949. [Google Scholar] [CrossRef]
- Li, S.; Wei, T.; Panchenko, A.R. Histone Variant H2A.Z Modulates Nucleosome Dynamics to Promote DNA Accessibility. Nat. Commun. 2023, 14, 769. [Google Scholar] [CrossRef]
- Fan, J.Y.; Gordon, F.; Luger, K.; Hansen, J.C.; Tremethick, D.J. The Essential Histone Variant H2A.Z Regulates the Equilibrium between Different Chromatin Conformational States. Nat. Struct. Biol. 2002, 9, 172–176. [Google Scholar] [CrossRef]
- Jin, C.; Felsenfeld, G. Nucleosome Stability Mediated by Histone Variants H3.3 and H2A.Z. Genes Dev. 2007, 21, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Dryhurst, D.; Ishibashi, T.; Rose, K.L.; Eirín-López, J.M.; McDonald, D.; Silva-Moreno, B.; Veldhoen, N.; Helbing, C.C.; Hendzel, M.J.; Shabanowitz, J.; et al. Characterization of the Histone H2A.Z-1 and H2A.Z-2 Isoforms in Vertebrates. BMC Biol. 2009, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, C.; Lee, C.; Auld, A.L.; Cisse, I.I.; Boyer, L.A. A Dual Role for H2A.Z.1 in Modulating the Dynamics of RNA Polymerase II Initiation and Elongation. Nat. Struct. Mol. Biol. 2021, 28, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Bönisch, C.; Schneider, K.; Pünzeler, S.; Wiedemann, S.M.; Bielmeier, C.; Bocola, M.; Eberl, H.C.; Kuegel, W.; Neumann, J.; Kremmer, E.; et al. H2A.Z.2.2 is an alternatively spliced histone H2A.Z variant that causes severe nucleosome destabilization. Nucleic Acids Res. 2012, 40, 5951–5964. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Dai, L.; Li, C.; Shi, L.; Huang, Y.; Guo, Z.; Wu, F.; Zhu, P.; Zhou, Z. Structural Basis of Nucleosome Dynamics Modulation by Histone Variants H2A.B and H2A.Z.2.2. EMBO J. 2021, 40, e105907. [Google Scholar] [CrossRef]
- Rangasamy, D.; Greaves, I.; Tremethick, D.J. RNA Interference Demonstrates a Novel Role for H2A.Z in Chromosome Segregation. Nat. Struct. Mol. Biol. 2004, 11, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Greaves, I.K.; Rangasamy, D.; Ridgway, P.; Tremethick, D.J. H2A.Z Contributes to the Unique 3D Structure of the Centromere. Proc. Natl. Acad. Sci. USA 2007, 104, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Pan, L.; Wang, W.; Sun, J.; Shan, S.; Dong, Q.; Liang, X.; Dai, L.; Ding, X.; Chen, S.; et al. Anp32e, a Higher Eukaryotic Histone Chaperone Directs Preferential Recognition for H2A.Z. Cell Res. 2014, 24, 389–399. [Google Scholar] [CrossRef]
- Obri, A.; Ouararhni, K.; Papin, C.; Diebold, M.-L.; Padmanabhan, K.; Marek, M.; Stoll, I.; Roy, L.; Reilly, P.T.; Mak, T.W.; et al. ANP32E Is a Histone Chaperone That Removes H2A.Z from Chromatin. Nature 2014, 505, 648–653. [Google Scholar] [CrossRef]
- Gursoy-Yuzugullu, O.; Ayrapetov, M.K.; Price, B.D. Histone Chaperone Anp32e Removes H2A.Z from DNA Double-Strand Breaks and Promotes Nucleosome Reorganization and DNA Repair. Proc. Natl. Acad. Sci. USA 2015, 112, 7507–7512. [Google Scholar] [CrossRef]
- Alatwi, H.E.; Downs, J.A. Removal of H2A.Z by INO80 Promotes Homologous Recombination. EMBO Rep. 2015, 16, 986–994. [Google Scholar] [CrossRef]
- Corujo, D.; Buschbeck, M. Post-Translational Modifications of H2A Histone Variants and Their Role in Cancer. Cancers 2018, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Nishibuchi, I.; Suzuki, H.; Kinomura, A.; Sun, J.; Liu, N.-A.; Horikoshi, Y.; Shima, H.; Kusakabe, M.; Harata, M.; Fukagawa, T.; et al. Reorganization of Damaged Chromatin by the Exchange of Histone Variant H2A.Z-2. Int. J. Radiat. Oncol. 2014, 89, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Narkaj, K.; Stefanelli, G.; Wahdan, M.; Azam, A.B.; Ramzan, F.; Steininger, C.F.D.; Walters, B.J.; Zovkic, I.B. Blocking H2A.Z Incorporation via Tip60 Inhibition Promotes Systems Consolidation of Fear Memory in Mice. eNeuro 2018, 5, ENEURO.0378-18.2018. [Google Scholar] [CrossRef]
- Shen, T.; Ji, F.; Wang, Y.; Lei, X.; Zhang, D.; Jiao, J. Brain-Specific Deletion of Histone Variant H2A.z Results in Cortical Neurogenesis Defects and Neurodevelopmental Disorder. Nucleic Acids Res. 2018, 46, 2290–2307. [Google Scholar] [CrossRef]
- Zovkic, I.B.; Paulukaitis, B.S.; Day, J.J.; Etikala, D.M.; Sweatt, J.D. Histone H2A.Z Subunit Exchange Controls Consolidation of Recent and Remote Memory. Nature 2014, 515, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Domaschenz, R.; Kurscheid, S.; Nekrasov, M.; Han, S.; Tremethick, D.J. The Histone Variant H2A.Z Is a Master Regulator of the Epithelial-Mesenchymal Transition. Cell Rep. 2017, 21, 943–952. [Google Scholar] [CrossRef]
- Dhillon, N.; Oki, M.; Szyjka, S.J.; Aparicio, O.M.; Kamakaka, R.T. H2A.Z Functions To Regulate Progression through the Cell Cycle. Mol. Cell. Biol. 2006, 26, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Cayrou, C.; Ballester, B.; Peiffer, I.; Fenouil, R.; Coulombe, P.; Andrau, J.C.; van Helden, J.; Méchali, M. The chromatin environment shapes DNA replication origin organization and defines origin classes. Genome Res. 2015, 25, 1873–1885. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Cui, K.; Northrup, D.; Liu, C.; Wang, C.; Tang, Q.; Ge, K.; Levens, D.; Crane-Robinson, C.; Zhao, K. H2A.Z Facilitates Access of Active and Repressive Complexes to Chromatin in Embryonic Stem Cell Self-Renewal and Differentiation. Cell Stem Cell 2013, 12, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yao, J.; Shi, Y.; Yi, H.; Zhao, W.; Lin, X.; Yang, Z. The SRCAP Chromatin Remodeling Complex Promotes Oxidative Metabolism during Prenatal Heart Development. Development 2021, 148, dev199026. [Google Scholar] [CrossRef] [PubMed]
- Raja, D.A.; Subramaniam, Y.; Aggarwal, A.; Gotherwal, V.; Babu, A.; Tanwar, J.; Motiani, R.K.; Sivasubbu, S.; Gokhale, R.S.; Natarajan, V.T. Histone Variant Dictates Fate Biasing of Neural Crest Cells to Melanocyte Lineage. Development 2020, 147, dev182576. [Google Scholar] [CrossRef] [PubMed]
- Law, C.; Cheung, P. Expression of Non-Acetylatable H2A.Z in Myoblast Cells Blocks Myoblast Differentiation through Disruption of MyoD Expression. J. Biol. Chem. 2015, 290, 13234–13249. [Google Scholar] [CrossRef]
- Numata, A.; Kwok, H.S.; Zhou, Q.-L.; Li, J.; Tirado-Magallanes, R.; Angarica, V.E.; Hannah, R.; Park, J.; Wang, C.Q.; Krishnan, V.; et al. Lysine Acetyltransferase Tip60 Is Required for Hematopoietic Stem Cell Maintenance. Blood 2020, 136, 1735–1747. [Google Scholar] [CrossRef]
- Rangasamy, D. Histone Variant H2A.Z Can Serve as a New Target for Breast Cancer Therapy. Curr. Med. Chem. 2010, 17, 3155–3161. [Google Scholar] [CrossRef]
- Rasmussen, L.; Christensen, I.J.; Herzog, M.; Micallef, J.; Nielsen, H.J.; Danish Collaborative Group on Early Detection of Colorectal Cancer. Circulating Cell-Free Nucleosomes as Biomarkers for Early Detection of Colorectal Cancer. Oncotarget 2018, 9, 10247–10258. [Google Scholar] [CrossRef]
- Zheng, Y.; Han, X.; Wang, T. Role of H2A.Z.1 in Epithelial-Mesenchymal Transition and Radiation Resistance of Lung Adenocarcinoma in Vitro. Biochem. Biophys. Res. Commun. 2022, 611, 118–125. [Google Scholar] [CrossRef]
- Yang, H.D.; Kim, P.-J.; Eun, J.W.; Shen, Q.; Kim, H.S.; Shin, W.C.; Ahn, Y.M.; Park, W.S.; Lee, J.Y.; Nam, S.W. Oncogenic Potential of Histone-Variant H2A.Z.1 and Its Regulatory Role in Cell Cycle and Epithelial-Mesenchymal Transition in Liver Cancer. Oncotarget 2016, 7, 11412–11423. [Google Scholar] [CrossRef]
- Dryhurst, D.; Ausió, J. Histone H2A.Z Deregulation in Prostate Cancer. Cause or Effect? Cancer Metastasis Rev. 2014, 33, 429–439. [Google Scholar] [CrossRef]
- Ávila-López, P.A.; Guerrero, G.; Nuñez-Martínez, H.N.; Peralta-Alvarez, C.A.; Hernández-Montes, G.; Álvarez-Hilario, L.G.; Herrera-Goepfert, R.; Albores-Saavedra, J.; Villegas-Sepúlveda, N.; Cedillo-Barrón, L.; et al. H2A.Z Overexpression Suppresses Senescence and Chemosensitivity in Pancreatic Ductal Adenocarcinoma. Oncogene 2021, 40, 2065–2080. [Google Scholar] [CrossRef] [PubMed]
- Vardabasso, C.; Gaspar-Maia, A.; Hasson, D.; Pünzeler, S.; Valle-Garcia, D.; Straub, T.; Keilhauer, E.C.; Strub, T.; Dong, J.; Panda, T.; et al. Histone Variant H2A.Z.2 Mediates Proliferation and Drug Sensitivity of Malignant Melanoma. Mol. Cell 2015, 59, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Punj, V.; Choi, J.; Heo, K.; Kim, J.-M.; Laird, P.W.; An, W. Gene Dysregulation by Histone Variant H2A.Z in Bladder Cancer. Epigenetics Chromatin 2013, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Svotelis, A.; Gévry, N.; Grondin, G.; Gaudreau, L. H2A.Z overexpression promotes cellular proliferation of breast cancer cells. Cell Cycle 2010, 9, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Thomas, S.D.; Islam, A.; Muench, D.; Sedoris, K. C-Myc and Cancer Metabolism. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 5546–5553. [Google Scholar] [CrossRef]
- Hua, S.; Kallen, C.B.; Dhar, R.; Baquero, M.T.; Mason, C.E.; A Russell, B.; Shah, P.K.; Liu, J.; Khramtsov, A.; Tretiakova, M.S.; et al. Genomic analysis of estrogen cascade reveals histone variant H2A.Z associated with breast cancer progression. Mol. Syst. Biol. 2008, 4, 188. [Google Scholar] [CrossRef]
- Gévry, N.; Hardy, S.; Jacques P, É.; Laflamme, L.; Svotelis, A.; Robert, F.; Gaudreau, L. Histone H2A.Z is essential for estrogen receptor signaling. Genes Dev. 2009, 23, 1522–1533. [Google Scholar] [CrossRef]
- Yang, B.; Tong, R.; Liu, H.; Wu, J.; Chen, D.; Xue, Z.; Ding, C.; Zhou, L.; Xie, H.; Wu, J.; et al. H2A.Z Regulates Tumorigenesis, Metastasis and Sensitivity to Cisplatin in Intrahepatic Cholangiocarcinoma. Int. J. Oncol. 2018, 52, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Huang, X.; Wang, X.; Zhou, X.; Huang, H.; Qin, L.; Tao, H.; Wang, Q.; Tao, Y. Vital and Distinct Roles of H2A.Z Isoforms in Hepatocellular Carcinoma. OncoTargets Ther. 2020, 13, 4319–4337. [Google Scholar] [CrossRef] [PubMed]
- Sales-Gil, R.; Kommer, D.C.; de Castro, I.J.; Amin, H.A.; Vinciotti, V.; Sisu, C.; Vagnarelli, P. Non-Redundant Functions of H2A.Z.1 and H2A.Z.2 in Chromosome Segregation and Cell Cycle Progression. EMBO Rep. 2021, 22, e52061. [Google Scholar] [CrossRef] [PubMed]
- Vardabasso, C.; Hake, S.B.; Bernstein, E. Histone Variant H2A.Z.2: A Novel Driver of Melanoma Progression. Mol. Cell. Oncol. 2016, 3, e1073417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Valdés-Mora, F.; Song, J.Z.; Statham, A.L.; Strbenac, D.; Robinson, M.D.; Nair, S.S.; Patterson, K.I.; Tremethick, D.J.; Stirzaker, C.; Clark, S.J. Acetylation of H2A.Z Is a Key Epigenetic Modification Associated with Gene Deregulation and Epigenetic Remodeling in Cancer. Genome Res. 2012, 22, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Mora, F.; Gould, C.M.; Colino-Sanguino, Y.; Qu, W.; Song, J.Z.; Taylor, K.M.; Buske, F.A.; Statham, A.L.; Nair, S.S.; Armstrong, N.J.; et al. Acetylated histone variant H2A.Z is involved in the activation of neo-enhancers in prostate cancer. Nat. Commun. 2017, 8, 1346. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Chen, J.; Deng, Y.; Zhang, D.; Dong, L.; Sun, D. H2AFZ Is a Prognostic Biomarker Correlated to TP53 Mutation and Immune Infiltration in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 701736. [Google Scholar] [CrossRef] [PubMed]
- Pehrson, J.R.; Fried, V.A. MacroH2A, a Core Histone Containing a Large Nonhistone Region. Science 1992, 257, 1398–1400. [Google Scholar] [CrossRef]
- Monteiro, F.L.; Baptista, T.; Amado, F.; Vitorino, R.; Jerónimo, C.; Helguero, L.A. Expression and Functionality of Histone H2A Variants in Cancer. Oncotarget 2014, 5, 3428–3443. [Google Scholar] [CrossRef]
- Pehrson, J.R.; Fuji, R.N. Evolutionary Conservation of Histone macroH2A Subtypes and Domains. Nucleic Acids Res. 1998, 26, 2837–2842. [Google Scholar] [CrossRef]
- Sun, Z.; Bernstein, E. Histone Variant macroH2A: From Chromatin Deposition to Molecular Function. Essays Biochem. 2019, 63, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Guberovic, I.; Farkas, M.; Corujo, D.; Buschbeck, M. Evolution, Structure and Function of Divergent macroH2A1 Splice Isoforms. Semin. Cell Dev. Biol. 2023, 135, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, S.; Patel, A.; Bowman, G.D. The basic linker of macroH2A stabilizes DNA at the entry/exit site of the nucleosome. Nucleic Acids Res. 2012, 40, 8285–8295. [Google Scholar] [CrossRef] [PubMed]
- Douet, J.; Corujo, D.; Malinverni, R.; Renauld, J.; Sansoni, V.; Posavec Marjanović, M.; Cantariño, N.; Valero, V.; Mongelard, F.; Bouvet, P.; et al. MacroH2A histone variants maintain nuclear organization and heterochromatin architecture. J. Cell Sci. 2017, 130, 1570–1582. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lv, P.; Yan, G.; Fan, H.; Cheng, L.; Zhang, F.; Dang, Y.; Wu, H.; Wen, B. MacroH2A1 Associates with Nuclear Lamina and Maintains Chromatin Architecture in Mouse Liver Cells. Sci. Rep. 2015, 5, 17186. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, B.P.; Valley, C.M.; Willard, H.F. Histone Variant macroH2A Contains Two Distinct Macrochromatin Domains Capable of Directing macroH2A to the Inactive X Chromosome. Nucleic Acids Res. 2001, 29, 2699–2705. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, B.P.; Willard, H.F. Histone H2A Variants and the Inactive X Chromosome: Identification of a Second macroH2A Variant. Hum. Mol. Genet. 2001, 10, 1101–1113. [Google Scholar] [CrossRef]
- Mandemaker, I.K.; Fessler, E.; Corujo, D.; Kotthoff, C.; Wegerer, A.; Rouillon, C.; Buschbeck, M.; Jae, L.T.; Mattiroli, F.; Ladurner, A.G. The Histone Chaperone ANP32B Regulates Chromatin Incorporation of the Atypical Human Histone Variant macroH2A. Cell Rep. 2023, 42, 113300. [Google Scholar] [CrossRef]
- Pasque, V.; Gillich, A.; Garrett, N.; Gurdon, J.B. Histone Variant macroH2A Confers Resistance to Nuclear Reprogramming. EMBO J. 2011, 30, 2373–2387. [Google Scholar] [CrossRef]
- Bowerman, S.; Hickok, R.J.; Wereszczynski, J. Unique Dynamics in Asymmetric macroH2A–H2A Hybrid Nucleosomes Result in Increased Complex Stability. J. Phys. Chem. B 2019, 123, 419–427. [Google Scholar] [CrossRef]
- Chakravarthy, S.; Luger, K. The Histone Variant Macro-H2A Preferentially Forms “Hybrid Nucleosomes”*♦. J. Biol. Chem. 2006, 281, 25522–25531. [Google Scholar] [CrossRef] [PubMed]
- Boulard, M.; Storck, S.; Cong, R.; Pinto, R.; Delage, H.; Bouvet, P. Histone Variant macroH2A1 Deletion in Mice Causes Female-Specific Steatosis. Epigenetics Chromatin 2010, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Sarma, K.; Lee, J.T. New and Xisting Regulatory Mechanisms of X Chromosome Inactivation. Curr. Opin. Genet. Dev. 2012, 22, 62–71. [Google Scholar] [CrossRef]
- Ma, Y.; Jacobs, S.B.; Jackson-Grusby, L.; Mastrangelo, M.-A.; Torres-Betancourt, J.A.; Jaenisch, R.; Rasmussen, T.P. DNA CpG Hypomethylation Induces Heterochromatin Reorganization Involving the Histone Variant macroH2A. J. Cell Sci. 2005, 118 Pt 8, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Muñoz, I.; Lund, A.H.; van der Stoop, P.; Boutsma, E.; Muijrers, I.; Verhoeven, E.; Nusinow, D.A.; Panning, B.; Marahrens, Y.; van Lohuizen, M. Stable X Chromosome Inactivation Involves the PRC1 Polycomb Complex and Requires Histone MACROH2A1 and the CULLIN3/SPOP Ubiquitin E3 Ligase. Proc. Natl. Acad. Sci. USA 2005, 102, 7635–7640. [Google Scholar] [CrossRef] [PubMed]
- Choo, J.H.; Kim, J.D.; Chung, J.H.; Stubbs, L.; Kim, J. Allele-Specific Deposition of macroH2A1 in Imprinting Control Regions. Hum. Mol. Genet. 2006, 15, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Changolkar, L.N.; Costanzi, C.; Leu, N.A.; Chen, D.; McLaughlin, K.J.; Pehrson, J.R. Developmental Changes in Histone macroH2A1-Mediated Gene Regulation. Mol. Cell. Biol. 2007, 27, 2758–2764. [Google Scholar] [CrossRef] [PubMed]
- Costanzi, C.; Pehrson, J.R. MACROH2A2, a New Member of the MARCOH2A Core Histone Family. J. Biol. Chem. 2001, 276, 21776–21784. [Google Scholar] [CrossRef]
- Nikolic, A.; Maule, F.; Bobyn, A.; Ellestad, K.; Paik, S.; Marhon, S.A.; Mehdipour, P.; Lun, X.; Chen, H.-M.; Mallard, C.; et al. macroH2A2 Antagonizes Epigenetic Programs of Stemness in Glioblastoma. Nat. Commun. 2023, 14, 3062. [Google Scholar] [CrossRef]
- Mermoud, J.E.; Costanzi, C.; Pehrson, J.R.; Brockdorff, N. Histone macroH2A1.2 Relocates to the Inactive X Chromosome after Initiation and Propagation of X-Inactivation. J. Cell Biol. 1999, 147, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Nusinow, D.A.; Hernandez-Munoz, I.; Fazzio, T.G.; Shah, G.M.; Kraus, W.L.; Panning, B. Poly(ADP-ribose) polymerase 1 is inhibited by a histone H2A variant, MacroH2A, and contributes to silencing of the inactive X chromosome. J. Biol. Chem. 2007, 282, 12851–12859. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, Y.; Gursoy-Yuzugullu, O.; Price, B.D. The Histone Variant macroH2A1.1 Is Recruited to DSBs through a Mechanism Involving PARP1. FEBS Lett. 2012, 586, 3920–3925. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.D.; Hamilton, G.A.; Park, J.W.; Gamble, M.J. MacroH2A1 Regulation of Poly(ADP-Ribose) Synthesis and Stability Prevents Necrosis and Promotes DNA Repair. Mol. Cell. Biol. 2019, 40, e00230-19. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, R.; Hosogane, E.K.; Sun, E.G.; Tran, A.D.; Reinhold, W.C.; Burkett, S.; Sturgill, D.M.; Gudla, P.R.; Pommier, Y.; Aladjem, M.I.; et al. Epigenetic Regulation of DNA Repair Pathway Choice by MacroH2A1 Splice Variants Ensures Genome Stability. Mol. Cell 2020, 79, 836–845.e7. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Stefanelli, G.; Narkaj, K.; Brimble, M.A.; Creighton, S.D.; McLean, T.A.B.; Hall, M.; Mitchnick, K.A.; Zakaria, J.; Phung, T.; et al. Histone macroH2A1 Is a Stronger Regulator of Hippocampal Transcription and Memory than macroH2A2 in Mice. Commun. Biol. 2022, 5, 482. [Google Scholar] [CrossRef]
- Hurtado-Bagès, S.; Guberovic, I.; Buschbeck, M. The MacroH2A1.1–PARP1 Axis at the Intersection Between Stress Response and Metabolism. Front. Genet. 2018, 9, 417. [Google Scholar] [CrossRef]
- Gaspar-Maia, A.; Qadeer, Z.A.; Hasson, D.; Ratnakumar, K.; Adrian Leu, N.; Leroy, G.; Liu, S.; Costanzi, C.; Valle-Garcia, D.; Schaniel, C.; et al. MacroH2A Histone Variants Act as a Barrier upon Reprogramming towards Pluripotency. Nat. Commun. 2013, 4, 1565. [Google Scholar] [CrossRef]
- Sporn, J.C.; Kustatscher, G.; Hothorn, T.; Collado, M.; Serrano, M.; Muley, T.; Schnabel, P.; Ladurner, A.G. Histone macroH2A Isoforms Predict the Risk of Lung Cancer Recurrence. Oncogene 2009, 28, 3423–3428. [Google Scholar] [CrossRef]
- Li, X.; Kuang, J.; Shen, Y.; Majer, M.M.; Nelson, C.C.; Parsawar, K.; Heichman, K.A.; Kuwada, S.K. The Atypical Histone MacroH2A1.2 Interacts with HER-2 Protein in Cancer Cells. J. Biol. Chem. 2012, 287, 23171–23183. [Google Scholar] [CrossRef]
- Kapoor, A.; Goldberg, M.S.; Cumberland, L.K.; Ratnakumar, K.; Segura, M.F.; Emanuel, P.O.; Menendez, S.; Vardabasso, C.; LeRoy, G.; Vidal, C.I.; et al. The Histone Variant macroH2A Suppresses Melanoma Progression through Regulation of CDK8. Nature 2010, 468, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Hodge, D.Q.; Cui, J.; Gamble, M.J.; Guo, W. Histone Variant MacroH2A1 Plays an Isoform-Specific Role in Suppressing Epithelial-Mesenchymal Transition. Sci. Rep. 2018, 8, 841. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, Y.; Lee, S.; Kim, M.; Punj, V.; Lu, J.F.; Shin, H.; Kim, K.; Ulmer, T.S.; Koh, J.; et al. Regulation of Breast Cancer-Induced Osteoclastogenesis by MacroH2A1.2 Involving EZH2-Mediated H3K27me3. Cell Rep. 2018, 24, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Shin, Y.; Lee, S.; Kim, M.Y.; Punj, V.; Shin, H.-I.; Kim, K.; Koh, J.-M.; Jeong, D.; An, W. MacroH2A1.2 Inhibits Prostate Cancer-Induced Osteoclastogenesis through Cooperation with HP1α and H1.2. Oncogene 2018, 37, 5749–5765. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Ismail, W.; Mazzone, A.; Ghiraldini, F.G.; Kaur, J.; Bains, M.; Munankarmy, A.; Bagwell, M.S.; Safgren, S.L.; Moore-Weiss, J.; Buciuc, M.; et al. MacroH2A Histone Variants Modulate Enhancer Activity to Repress Oncogenic Programs and Cellular Reprogramming. Commun. Biol. 2023, 6, 215. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-H.; Miyai, K.; Sporn, J.C.; Luo, L.; Wang, J.Y.J.; Cosman, B.; Ramamoorthy, S. Loss of Histone Variant macroH2A2 Expression Associates with Progression of Anal Neoplasm. J. Clin. Pathol. 2016, 69, 627–631. [Google Scholar] [CrossRef]
- Sun, D.; Singh, D.K.; Carcamo, S.; Filipescu, D.; Khalil, B.; Huang, X.; Miles, B.A.; Westra, W.; Sproll, K.C.; Hasson, D.; et al. MacroH2A Impedes Metastatic Growth by Enforcing a Discrete Dormancy Program in Disseminated Cancer Cells. Sci. Adv. 2022, 8, eabo0876. [Google Scholar] [CrossRef]
- Lavigne, A.-C.; Castells, M.; Mermet, J.; Kocanova, S.; Dalvai, M.; Bystricky, K. Increased macroH2A1.1 Expression Correlates with Poor Survival of Triple-Negative Breast Cancer Patients. PLoS ONE 2014, 9, e98930. [Google Scholar] [CrossRef]
- Broggi, G.; Filetti, V.; Ieni, A.; Rapisarda, V.; Ledda, C.; Vitale, E.; Varricchio, S.; Russo, D.; Lombardo, C.; Tuccari, G.; et al. MacroH2A1 Immunoexpression in Breast Cancer. Front. Oncol. 2020, 10, 1519. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ding, Y.; Zeng, L. The diagnostic and prognostic value of H2AFY in hepatocellular carcinoma. BMC Cancer 2021, 21, 418. [Google Scholar] [CrossRef] [PubMed]
- Rappa, F.; Greco, A.; Podrini, C.; Cappello, F.; Foti, M.; Bourgoin, L.; Peyrou, M.; Marino, A.; Scibetta, N.; Williams, R.; et al. Immunopositivity for Histone MacroH2A1 Isoforms Marks Steatosis-Associated Hepatocellular Carcinoma. PLoS ONE 2013, 8, e54458. [Google Scholar] [CrossRef]
- Jiang, X.; Soboleva, T.A.; Tremethick, D.J. Short Histone H2A Variants: Small in Stature but Not in Function. Cells 2020, 9, 867. [Google Scholar] [CrossRef]
- Osakabe, A.; Molaro, A. Histone Renegades: Unusual H2A Histone Variants in Plants and Animals. Semin. Cell Dev. Biol. 2023, 135, 35–42. [Google Scholar] [CrossRef]
- Molaro, A.; Young, J.M.; Malik, H.S. Evolutionary origins and diversification of testis-specific short histone H2A variants in mammals. Genome Res. 2018, 28, 460–473. [Google Scholar] [CrossRef]
- Bao, Y.; Konesky, K.; Park, Y.-J.; Rosu, S.; Dyer, P.N.; Rangasamy, D.; Tremethick, D.J.; Laybourn, P.J.; Luger, K. Nucleosomes containing the histone variant H2A.Bbd organize only 118 base pairs of DNA. EMBO J. 2004, 23, 3314–3324. [Google Scholar] [CrossRef]
- Chadwick, B.P.; Willard, H.F. A Novel Chromatin Protein, Distantly Related to Histone H2a, Is Largely Excluded from the Inactive X Chromosome. J. Cell Biol. 2001, 152, 375–384. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, C.; Hua, X.; Zhang, Z. Molecular Mechanism of Histone Variant H2A.B on Stability and Assembly of Nucleosome and Chromatin Structures. Epigenetics Chromatin 2020, 13, 28. [Google Scholar] [CrossRef]
- González-Romero, R.; Méndez, J.; Ausió, J.; Eirín-López, J.M. Quickly Evolving Histones, Nucleosome Stability and Chromatin Folding: All about Histone H2A.Bbd. Gene 2008, 413, 1–7. [Google Scholar] [CrossRef]
- Sansoni, V.; Casas-Delucchi, C.S.; Rajan, M.; Schmidt, A.; Bönisch, C.; Thomae, A.W.; Staege, M.S.; Hake, S.B.; Cardoso, M.C.; Imhof, A. The histone variant H2A.Bbd is enriched at sites of DNA synthesis. Nucleic Acids Res. 2014, 42, 6405–6420. [Google Scholar] [CrossRef]
- Tolstorukov, M.Y.; Goldman, J.A.; Gilbert, C.; Ogryzko, V.; Kingston, R.E.; Park, P.J. Histone variant H2A.Bbd is associated with active transcription and mRNA processing in human cells. Mol. Cell 2012, 47, 596–607. [Google Scholar] [CrossRef]
- Ishibashi, T.; Li, A.; Eirín-López, J.M.; Zhao, M.; Missiaen, K.; Abbott, D.W.; Meistrich, M.; Hendzel, M.J.; Ausió, J. H2A.Bbd: An X-Chromosome-Encoded Histone Involved in Mammalian Spermiogenesis. Nucleic Acids Res. 2010, 38, 1780–1789. [Google Scholar] [CrossRef]
- Hirano, R.; Arimura, Y.; Kujirai, T.; Shibata, M.; Okuda, A.; Morishima, K.; Inoue, R.; Sugiyama, M.; Kurumizaka, H. Histone variant H2A.B-H2B dimers are spontaneously exchanged with canonical H2A-H2B in the nucleosome. Commun. Biol. 2021, 4, 191. [Google Scholar] [CrossRef] [PubMed]
- Okuwaki, M.; Kato, K.; Shimahara, H.; Tate, S.I.; Nagata, K. Assembly and disassembly of nucleosome core particles containing histone variants by human nucleosome assembly protein I. Mol. Cell. Biol. 2005, 25, 10639–10651. [Google Scholar] [CrossRef]
- Molaro, A.; Wood, A.J.; Janssens, D.; Kindelay, S.M.; Eickbush, M.T.; Wu, S.; Singh, P.; Muller, C.H.; Henikoff, S.; Malik, H.S. Biparental Contributions of the H2A.B Histone Variant Control Embryonic Development in Mice. PLoS Biol. 2020, 18, e3001001. [Google Scholar] [CrossRef]
- Wu, B.J.; Dong, F.L.; Ma, X.S.; Wang, X.G.; Lin, F.; Liu, H.L. Localization and Expression of Histone H2A Variants during Mouse Oogenesis and Preimplantation Embryo Development. Genet. Mol. Res. GMR 2014, 13, 5929–5939. [Google Scholar] [CrossRef]
- Nacev, B.A.; Feng, L.; Bagert, J.D.; Lemiesz, A.E.; Gao, J.; Soshnev, A.A.; Kundra, R.; Schultz, N.; Muir, T.W.; Allis, C.D. The Expanding Landscape of ‘Oncohistone’ Mutations in Human Cancers. Nature 2019, 567, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wen, J.; Paver, E.; Wu, Y.H.; Sun, G.; Bullman, A.; Dahlstrom, J.E.; Tremethick, D.J.; Soboleva, T.A. H2A.B is a cancer/testis factor involved in the activation of ribosome biogenesis in Hodgkin lymphoma. EMBO Rep. 2021, 22, e52462. [Google Scholar] [CrossRef] [PubMed]
- Chew, G.-L.; Bleakley, M.; Bradley, R.K.; Malik, H.S.; Henikoff, S.; Molaro, A.; Sarthy, J. Short H2A Histone Variants Are Expressed in Cancer. Nat. Commun. 2021, 12, 490. [Google Scholar] [CrossRef]
- Shaytan, A.K.; Landsman, D.; Panchenko, A.R. Nucleosome Adaptability Conferred by Sequence and Structural Variations in Histone H2A–H2B Dimers. Curr. Opin. Struct. Biol. 2015, 32, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Govin, J.; Escoffier, E.; Rousseaux, S.; Kuhn, L.; Ferro, M.; Thévenon, J.; Catena, R.; Davidson, I.; Garin, J.; Khochbin, S.; et al. Pericentric Heterochromatin Reprogramming by New Histone Variants during Mouse Spermiogenesis. J. Cell Biol. 2007, 176, 283–294. [Google Scholar] [CrossRef]
- Syed, S.H.; Boulard, M.; Shukla, M.S.; Gautier, T.; Travers, A.; Bednar, J.; Faivre-Moskalenko, C.; Dimitrov, S.; Angelov, D. The incorporation of the novel histone variant H2AL2 confers unusual structural and functional properties of the nucleosome. Nucleic Acids Res. 2009, 37, 4684–4695. [Google Scholar] [CrossRef]
- Hoghoughi, N.; Barral, S.; Curtet, S.; Chuffart, F.; Charbonnier, G.; Puthier, D.; Buchou, T.; Rousseaux, S.; Khochbin, S. RNA-Guided Genomic Localization of H2A.L.2 Histone Variant. Cells 2020, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Barral, S.; Morozumi, Y.; Tanaka, H.; Montellier, E.; Govin, J.; de Dieuleveult, M.; Charbonnier, G.; Couté, Y.; Puthier, D.; Buchou, T.; et al. Histone Variant H2A.L.2 Guides Transition Protein-Dependent Protamine Assembly in Male Germ Cells. Mol. Cell 2017, 66, 89–101.e8. [Google Scholar] [CrossRef] [PubMed]
- Oliva, R.; Dixon, G.H. Vertebrate Protamine Genes and the Histone-to-Protamine Replacement Reaction. In Progress in Nucleic Acid Research and Molecular Biology; Cohn, W.E., Moldave, K., Eds.; Academic Press: Cambridge, MA, USA, 1991; Volume 40, pp. 25–94. [Google Scholar] [CrossRef]
- H2AJ H2A.J Histone [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/55766 (accessed on 18 November 2023).
- Redon, C.E.; Schmal, Z.; Tewary, G.; Mangelinck, A.; Courbeyrette, R.; Thuret, J.-Y.; Aladjem, M.I.; Bonner, W.M.; Rübe, C.E.; Mann, C. Histone Variant H2A.J Is Enriched in Luminal Epithelial Gland Cells. Genes 2021, 12, 1665. [Google Scholar] [CrossRef]
- Tanaka, H.; Sato, S.; Koyama, M.; Kujirai, T.; Kurumizaka, H. Biochemical and Structural Analyses of the Nucleosome Containing Human Histone H2A.J. J. Biochem. 2020, 167, 419–427. [Google Scholar] [CrossRef]
- Isermann, A.; Mann, C.; Rübe, C.E. Histone Variant H2A.J Marks Persistent DNA Damage and Triggers the Secretory Phenotype in Radiation-Induced Senescence. Int. J. Mol. Sci. 2020, 21, 9130. [Google Scholar] [CrossRef]
- Contrepois, K.; Coudereau, C.; Benayoun, B.A.; Schuler, N.; Roux, P.F.; Bischof, O.; Courbeyrette, R.; Carvalho, C.; Thuret, J.-Y.; Ma, Z.; et al. Histone variant H2A.J accumulates in senescent cells and promotes inflammatory gene expression. Nat. Commun. 2017, 8, 14995. [Google Scholar] [CrossRef]
- Ren, J.-L.; Pan, J.-S.; Lu, Y.-P.; Sun, P.; Han, J. Inflammatory Signaling and Cellular Senescence. Cell. Signal. 2009, 21, 378–383. [Google Scholar] [CrossRef]
- Yao, J.; Weremowicz, S.; Feng, B.; Gentleman, R.C.; Marks, J.R.; Gelman, R.; Brennan, C.; Polyak, K. Combined cDNA Array Comparative Genomic Hybridization and Serial Analysis of Gene Expression Analysis of Breast Tumor Progression. Cancer Res. 2006, 66, 4065–4078. [Google Scholar] [CrossRef]
- De Wit, N.J.W.; Rijntjes, J.; Diepstra, J.H.S.; Van Kuppevelt, T.H.; Weidle, U.H.; Ruiter, D.J.; Van Muijen, G.N.P. Analysis of differential gene expression in human melanocytic tumour lesions by custom made oligonucleotide arrays. Br. J. Cancer 2005, 92, 2249–2261. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ghareeb, W.M.; Lu, X.; Huang, Y.; Huang, S.; Chi, P. Coexpression Network Analysis Linked H2AFJ to Chemoradiation Resistance in Colorectal Cancer. J. Cell. Biochem. 2019, 120, 10351–10362. [Google Scholar] [CrossRef] [PubMed]
- Segerman, A.; Niklasson, M.; Haglund, C.; Bergström, T.; Jarvius, M.; Xie, Y.; Westermark, A.; Sönmez, D.; Hermansson, A.; Kastemar, M.; et al. Clonal Variation in Drug and Radiation Response among Glioma-Initiating Cells Is Linked to Proneural-Mesenchymal Transition. Cell Rep. 2016, 17, 2994–3009. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-H.; Lin, C.-H.; Lin, H.-Y.; Kuei, C.-H.; Zheng, J.-Q.; Wang, Y.-H.; Lu, L.-S.; Lee, F.-P.; Hu, C.-J.; Wu, D.; et al. Histone 2A Family Member J Drives Mesenchymal Transition and Temozolomide Resistance in Glioblastoma Multiforme. Cancers 2020, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiu, W.; Shen, X.; Sun, G. Bioinformatics Analysis Revealed Hub Genes and Pathways Involved in Sorafenib Resistance in Hepatocellular Carcinoma. Math. Biosci. Eng. 2019, 16, 6319–6334. [Google Scholar] [CrossRef]
- Yu, Y.; Kovacevic, Z.; Richardson, D.R. Tuning Cell Cycle Regulation with an Iron Key. Cell Cycle 2007, 6, 1982–1994. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Chiba, M.; Murata, S.; Pak, S.; Nagai, K.; Yamamoto, M.; Yanagisawa, K.; Kobayashi, A.; Yasue, H.; Ohkohchi, N. Identification of Natural Antisense Transcripts Involved in Human Colorectal Cancer Development. Int. J. Oncol. 2010, 37, 1425–1432. [Google Scholar] [CrossRef][Green Version]
- Dong, H.; Guo, H.; Xie, L.; Wang, G.; Zhong, X.; Khoury, T.; Tan, D.; Zhang, H. The Metastasis-Associated Gene MTA3, a Component of the Mi-2/NuRD Transcriptional Repression Complex, Predicts Prognosis of Gastroesophageal Junction Adenocarcinoma. PLoS ONE 2013, 8, e62986. [Google Scholar] [CrossRef]
- WANG, X.; LIN, Y. Tumor Necrosis Factor and Cancer, Buddies or Foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef]
- Rübe, C.E.; Bäumert, C.; Schuler, N.; Isermann, A.; Schmal, Z.; Glanemann, M.; Mann, C.; Scherthan, H. Human Skin Aging Is Associated with Increased Expression of the Histone Variant H2A.J in the Epidermis. npj Aging Mech. Dis. 2021, 7, 7. [Google Scholar] [CrossRef]
- Padavattan, S.; Shinagawa, T.; Hasegawa, K.; Kumasaka, T.; Ishii, S.; Kumarevel, T. Structural and Functional Analyses of Nucleosome Complexes with Mouse Histone Variants TH2a and TH2b, Involved in Reprogramming. Biochem. Biophys. Res. Commun. 2015, 464, 929–935. [Google Scholar] [CrossRef]
- Huynh, L.M.; Shinagawa, T.; Ishii, S. Two Histone Variants TH2A and TH2B Enhance Human Induced Pluripotent Stem Cell Generation. Stem Cells Dev. 2016, 25, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Shinagawa, T.; Takagi, T.; Tsukamoto, D.; Tomaru, C.; Huynh, L.M.; Sivaraman, P.; Kumarevel, T.; Inoue, K.; Nakato, R.; Katou, Y.; et al. Histone Variants Enriched in Oocytes Enhance Reprogramming to Induced Pluripotent Stem Cells. Cell Stem Cell 2014, 14, 217–227. [Google Scholar] [CrossRef]
- Hada, M.; Masuda, K.; Yamaguchi, K.; Shirahige, K.; Okada, Y. Identification of a Variant-Specific Phosphorylation of TH2A during Spermiogenesis. Sci. Rep. 2017, 7, 46228. [Google Scholar] [CrossRef] [PubMed]
- Hada, M.; Kim, J.; Inoue, E.; Fukuda, Y.; Tanaka, H.; Watanabe, Y.; Okada, Y. TH2A Is Phosphorylated at Meiotic Centromere by Haspin. Chromosoma 2017, 126, 769–780. [Google Scholar] [CrossRef]
- West, M.H.P.; Bonner, W.M. Structural Comparisons of Mouse Histones 2A.X and 2A.Z with 2A.1 and 2A.2. Comp. Biochem. Physiol. Part B Comp. Biochem. 1983, 76, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.P.; Sharma, A.; Deodhar, K.K.; Gupta, S. Overexpression of Histone Variant H2A.1 and Cellular Transformation Are Related in N-Nitrosodiethylamine-Induced Sequential Hepatocarcinogenesis. Exp. Biol. Med. 2011, 236, 30–35. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Sekeri–Pataryas, K.E. Histone Variants of H2A and H3 Families Are Regulated during in Vitro Aging in the Same Manner as during Differentiation. Exp. Gerontol. 1999, 34, 741–754. [Google Scholar] [CrossRef]
- Piña, B.; Suau, P. Changes in Histones H2A and H3 Variant Composition in Differentiating and Mature Rat Brain Cortical Neurons. Dev. Biol. 1987, 123, 51–58. [Google Scholar] [CrossRef]
- Piña, B.; Suau, P. Core Histone Variants and Ubiquitinated Histones 2A and 2B of Rat Cerebral Cortex Neurons. Biochem. Biophys. Res. Commun. 1985, 133, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, M.; Khade, B.; Khan, S.A.; Ingle, A.; Gupta, S. Expression of Histone Variant, H2A.1 Is Associated with the Undifferentiated State of Hepatocyte. Exp. Biol. Med. 2014, 239, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Jackson, V.; Chalkley, R. Histone Synthesis and Deposition in the G1 and S Phases of Hepatoma Tissue Culture Cells. Biochemistry 1985, 24, 6921–6930. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, M.; Reddy, D.; Gupta, S. Genomic Characterization and Dynamic Methylation of Promoter Facilitates Transcriptional Regulation of H2A Variants, H2A.1 and H2A.2 in Various Pathophysiological States of Hepatocyte. Int. J. Biochem. Cell Biol. 2017, 85, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Naldi, M.; Andrisano, V.; Fiori, J.; Calonghi, N.; Pagnotta, E.; Parolin, C.; Pieraccini, G.; Masotti, L. Histone Proteins Determined in a Human Colon Cancer by High-Performance Liquid Chromatography and Mass Spectrometry. J. Chromatogr. A 2006, 1129, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M. DNA Hypomethylation in Cancer Cells. Epigenomics 2009, 1, 239–259. [Google Scholar] [CrossRef]
- Counts, J.L.; Goodman, J.I. Hypomethylation of DNA: A Possible Epigenetic Mechanism Involved in Tumor Promotion. Prog. Clin. Biol. Res. 1995, 391, 81–101. [Google Scholar] [CrossRef]
- Danielsson, F.; Skogs, M.; Huss, M.; Rexhepaj, E.; O’Hurley, G.; Klevebring, D.; Pontén, F.; Gad, A.K.B.; Uhlén, M.; Lundberg, E. Majority of Differentially Expressed Genes Are Down-Regulated during Malignant Transformation in a Four-Stage Model. Proc. Natl. Acad. Sci. USA 2013, 110, 6853–6858. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).