PI3K/Akt/mTOR Signaling Pathway as a Target for Colorectal Cancer Treatment
Abstract
1. Introduction
2. Overview of PI3K/Akt/mTOR Signaling
3. PI3K/Akt/mTOR Pathway Alteration in CRC
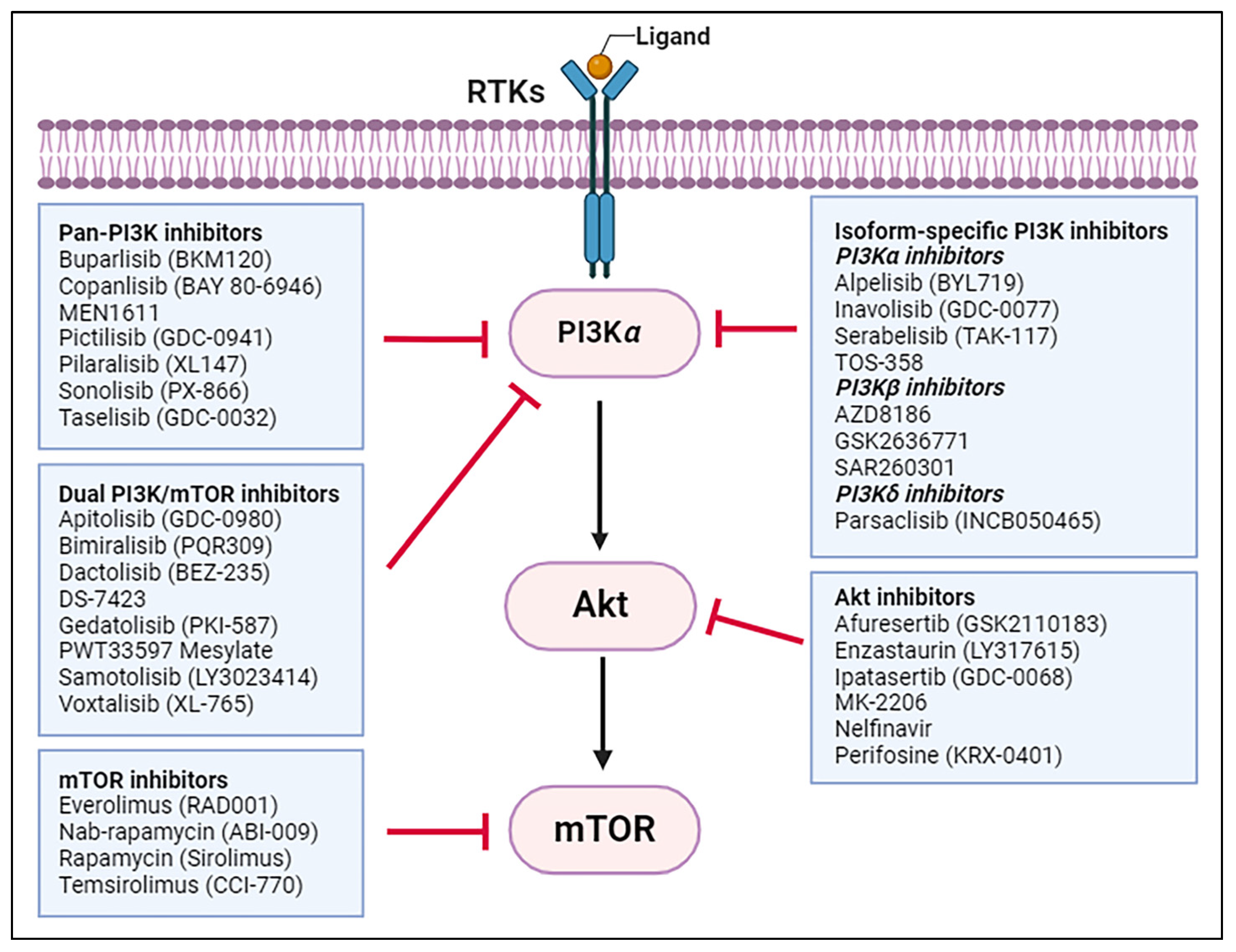
4. Recent Advances in PI3K/Akt/mTOR Inhibitor Research for CRC Treatment
4.1. Pan-PI3K Inhibitors
4.2. Isoform-Specific PI3K Inhibitors
4.3. Dual PI3K/mTOR Inhibitors
4.4. Akt Inhibitors
4.5. mTOR Inhibitors
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Leiphrakpam, P.D.; Rajappa, S.J.; Krishnan, M.; Batra, R.; Murthy, S.S.; Are, C. Colorectal cancer: Review of signaling pathways and associated therapeutic strategies. J. Surg. Oncol. 2023, 127, 1277–1295. [Google Scholar] [CrossRef]
- Shen, C.; Tannenbaum, D.; Horn, R.; Rogers, J.; Eng, C.; Zhou, S.; Johnson, B.; Kopetz, S.; Morris, V.; Overman, M.; et al. Overall Survival in Phase 3 Clinical Trials and the Surveillance, Epidemiology, and End Results Database in Patients With Metastatic Colorectal Cancer, 1986-2016: A Systematic Review. JAMA Netw Open 2022, 5, e2213588. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT Pathway for Cancer Drug Discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Mayer, I.A.; Arteaga, C.L. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annu. Rev. Med. 2016, 67, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Courtney, K.D.; Corcoran, R.B.; Engelman, J.A. The PI3K pathway as drug target in human cancer. J. Clin. Oncol. 2010, 28, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat. Rev. Cancer 2009, 9, 550–562. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Burke, J.E. Structural Basis for Regulation of Phosphoinositide Kinases and Their Involvement in Human Disease. Mol. Cell 2018, 71, 653–673. [Google Scholar] [CrossRef]
- Alessi, D.R.; James, S.R.; Downes, C.P.; Holmes, A.B.; Gaffney, P.R.; Reese, C.B.; Cohen, P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 1997, 7, 261–269. [Google Scholar] [CrossRef]
- Vander Haar, E.; Lee, S.I.; Bandhakavi, S.; Griffin, T.J.; Kim, D.H. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell Biol. 2007, 9, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, B.A.; Restuccia, D.F. PI3K-PKB/Akt pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011189. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Katso, R.; Okkenhaug, K.; Ahmadi, K.; White, S.; Timms, J.; Waterfield, M.D. Cellular function of phosphoinositide 3-kinases: Implications for development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 2001, 17, 615–675. [Google Scholar] [CrossRef]
- Samuels, Y.; Wang, Z.; Bardelli, A.; Silliman, N.; Ptak, J.; Szabo, S.; Yan, H.; Gazdar, A.; Powell, S.M.; Riggins, G.J.; et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 2004, 304, 554. [Google Scholar] [CrossRef]
- Parsons, D.W.; Wang, T.L.; Samuels, Y.; Bardelli, A.; Cummins, J.M.; DeLong, L.; Silliman, N.; Ptak, J.; Szabo, S.; Willson, J.K.; et al. Colorectal cancer: Mutations in a signalling pathway. Nature 2005, 436, 792. [Google Scholar] [CrossRef]
- COSMIC. The Catalogue of Somatic Mutations. In Cancer; Sanger Institute: Hinxton, UK; Available online: https://cancer.sanger.ac.uk/cosmic (accessed on 20 November 2023).
- Goel, A.; Arnold, C.N.; Niedzwiecki, D.; Carethers, J.M.; Dowell, J.M.; Wasserman, L.; Compton, C.; Mayer, R.J.; Bertagnolli, M.M.; Boland, C.R. Frequent inactivation of PTEN by promoter hypermethylation in microsatellite instability-high sporadic colorectal cancers. Cancer Res. 2004, 64, 3014–3021. [Google Scholar] [CrossRef] [PubMed]
- Carpten, J.D.; Faber, A.L.; Horn, C.; Donoho, G.P.; Briggs, S.L.; Robbins, C.M.; Hostetter, G.; Boguslawski, S.; Moses, T.Y.; Savage, S.; et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 2007, 448, 439–444. [Google Scholar] [CrossRef]
- Philp, A.J.; Campbell, I.G.; Leet, C.; Vincan, E.; Rockman, S.P.; Whitehead, R.H.; Thomas, R.J.; Phillips, W.A. The phosphatidylinositol 3’-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001, 61, 7426–7429. [Google Scholar] [PubMed]
- Janku, F.; Yap, T.A.; Meric-Bernstam, F. Targeting the PI3K pathway in cancer: Are we making headway? Nat. Rev. Clin. Oncol. 2018, 15, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, R.; Zhao, J.; Zhu, L.; Xu, J.; Ma, F.; Guo, R.; Xu, C.; Wang, W.-x.; Yi, X.; et al. Mutation profiling of TSC1 and TSC2 genes in solid tumors using comprehensive NGS panel. J. Clin. Oncol. 2018, 36, e24244. [Google Scholar] [CrossRef]
- Leiphrakpam, P.D.; Chowdhury, S.; Wang, J.; Black, J.D.; Are, C. The role and therapeutic implications of PI3K signaling pathway in cancer. J. Surg. Oncol. 2021, 123, 39–41. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, Y.L.; Zhou, K.; Wang, L.L.; Yan, Z.X.; Liu, Y.L.; Xu, L.L.; Zhao, S.W.; Chu, H.L.; Shi, T.T.; et al. PIK3CA mutations confer resistance to first-line chemotherapy in colorectal cancer. Cell Death Dis. 2018, 9, 739. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Mehrotra, S.; Languino, L.R.; Raskett, C.M.; Mercurio, A.M.; Dohi, T.; Altieri, D.C. IAP regulation of metastasis. Cancer Cell 2010, 17, 53–64. [Google Scholar] [CrossRef]
- Agarwal, E.; Chaudhuri, A.; Leiphrakpam, P.D.; Haferbier, K.L.; Brattain, M.G.; Chowdhury, S. Akt inhibitor MK-2206 promotes anti-tumor activity and cell death by modulation of AIF and Ezrin in colorectal cancer. BMC Cancer 2014, 14, 145. [Google Scholar] [CrossRef]
- Cully, M.; You, H.; Levine, A.J.; Mak, T.W. Beyond PTEN mutations: The PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat. Rev. Cancer 2006, 6, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-F.; Yu, B.-H.; Li, D.-L.; Ke, H.-L.; Guo, X.-Z.; Xiao, X.-Y. PI3K expression and PIK3CA mutations are related to colorectal cancer metastases. World J. Gastroenterol. 2012, 18, 3745–3751. [Google Scholar] [CrossRef] [PubMed]
- Leiphrakpam, P.D.; Lazenby, A.J.; Smith, L.M.; Brattain, M.G.; Black, J.D.; Wang, J.; Are, C. Correlation of PRL3 expression with colorectal cancer progression. J. Surg. Oncol. 2021, 123, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Quah, S.Y.; Dong, J.M.; Manser, E.; Tang, J.P.; Zeng, Q. PRL-3 down-regulates PTEN expression and signals through PI3K to promote epithelial-mesenchymal transition. Cancer Res. 2007, 67, 2922–2926. [Google Scholar] [CrossRef] [PubMed]
- Rios, P.; Li, X.; Köhn, M. Molecular mechanisms of the PRL phosphatases. FEBS J. 2013, 280, 505–524. [Google Scholar] [CrossRef]
- Leiphrakpam, P.D.; Rajput, A.; Mathiesen, M.; Agarwal, E.; Lazenby, A.J.; Are, C.; Brattain, M.G.; Chowdhury, S. Ezrin expression and cell survival regulation in colorectal cancer. Cell Signal 2014, 26, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Leiphrakpam, P.D.; Lazenby, A.J.; Chowdhury, S.; Smith, L.M.; Mathiesen, M.; Brattain, M.G.; Wang, J.; Black, J.D.; Are, C. Prognostic and therapeutic implications of NHERF1 expression and regulation in colorectal cancer. J. Surg. Oncol. 2020, 121, 547–560. [Google Scholar] [CrossRef]
- Leiphrakpam, P.D.; Brattain, M.G.; Black, J.D.; Wang, J.J. TGFβ and IGF1R signaling activate protein kinase A through differential regulation of ezrin phosphorylation in colon cancer cells. J. Biol. Chem. 2018, 293, 8242–8254. [Google Scholar] [CrossRef]
- Martini, M.; Ciraolo, E.; Gulluni, F.; Hirsch, E. Targeting PI3K in Cancer: Any Good News? Front. Oncol. 2013, 3, 108. [Google Scholar] [CrossRef]
- Bendell, J.C.; Rodon, J.; Burris, H.A.; de Jonge, M.; Verweij, J.; Birle, D.; Demanse, D.; De Buck, S.S.; Ru, Q.C.; Peters, M.; et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2012, 30, 282–290. [Google Scholar] [CrossRef]
- Rodon, J.; Braña, I.; Siu, L.L.; De Jonge, M.J.; Homji, N.; Mills, D.; Di Tomaso, E.; Sarr, C.; Trandafir, L.; Massacesi, C.; et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Invest. New Drugs 2014, 32, 670–681. [Google Scholar] [CrossRef]
- Bedard, P.L.; Tabernero, J.; Janku, F.; Wainberg, Z.A.; Paz-Ares, L.; Vansteenkiste, J.; Van Cutsem, E.; Pérez-García, J.; Stathis, A.; Britten, C.D.; et al. A phase Ib dose-escalation study of the oral pan-PI3K inhibitor buparlisib (BKM120) in combination with the oral MEK1/2 inhibitor trametinib (GSK1120212) in patients with selected advanced solid tumors. Clin. Cancer Res. 2015, 21, 730–738. [Google Scholar] [CrossRef]
- Zambrano, C.C.; Schuler, M.H.; Machiels, J.-P.H.; Hess, D.; Paz-Ares, L.; Awada, A.; Moos, R.v.; Steeghs, N.; Ahnert, J.R.; Mesmaeker, P.D.; et al. Phase lb study of buparlisib (BKM120) plus either paclitaxel (PTX) in advanced solid tumors (aST) or PTX plus trastuzumab (TZ) in HER2+ breast cancer (BC). J. Clin. Oncol. 2014, 32, 627. [Google Scholar] [CrossRef]
- Baranda, J.C.; Reed, G.; Williamson, S.K.; Perez, R.P.; Stoltz, M.L.; Mackay, C.; Madan, R.; Pessetto, Z.Y.; Godwin, A.K. Irinotecan (Iri) and buparlisib (B) in previously treated patients (pts) with metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2015, 33, 655. [Google Scholar] [CrossRef]
- Bardia, A.; Gounder, M.; Rodon, J.; Janku, F.; Lolkema, M.P.; Stephenson, J.J.; Bedard, P.L.; Schuler, M.; Sessa, C.; LoRusso, P.; et al. Phase Ib Study of Combination Therapy with MEK Inhibitor Binimetinib and Phosphatidylinositol 3-Kinase Inhibitor Buparlisib in Patients with Advanced Solid Tumors with RAS/RAF Alterations. Oncologist 2020, 25, e160–e169. [Google Scholar] [CrossRef] [PubMed]
- McRee, A.J.; Sanoff, H.K.; Carlson, C.; Ivanova, A.; O’Neil, B.H. A phase I trial of mFOLFOX6 combined with the oral PI3K inhibitor BKM120 in patients with advanced refractory solid tumors. Invest. New Drugs 2015, 33, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.S.; Mahipal, A.; Schuler, M.; De Braud, F.G.M.; Dirix, L.; Rampersad, A.; Zhou, J.; Wu, Y.; Kalambakas, S.; Wen, P.Y. Dose-Escalation Study of Sonidegib (Lde225) Plus Buparlisib (Bkm120) in Patients (Pts) with Advanced Solid Tumors. Ann. Oncol. 2014, 25, iv147. [Google Scholar] [CrossRef]
- Goodwin, R.; Jonker, D.; Chen, E.; Kennecke, H.; Cabanero, M.; Tsao, M.S.; Vickers, M.; Bohemier, C.; Lim, H.; Ritter, H.; et al. A phase Ib study of a PI3Kinase inhibitor BKM120 in combination with panitumumab in patients with KRAS wild-type advanced colorectal cancer. Invest. New Drugs 2020, 38, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Zhang, L.I.; Trandafir, L.; Dong, T.; Duval, V.; Hazell, K.; Xu, B. Phase I Study of the Pan-PI3K Inhibitor Buparlisib in Adult Chinese Patients with Advanced Solid Tumors. Anticancer. Res. 2016, 36, 6185–6194. [Google Scholar] [CrossRef][Green Version]
- Patnaik, A.; Appleman, L.J.; Tolcher, A.W.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Weiss, G.J.; Sachdev, J.C.; Chadha, M.; Fulk, M.; et al. First-in-human phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin’s lymphomas. Ann. Oncol. 2016, 27, 1928–1940. [Google Scholar] [CrossRef]
- Doi, T.; Fuse, N.; Yoshino, T.; Kojima, T.; Bando, H.; Miyamoto, H.; Kaneko, M.; Osada, M.; Ohtsu, A. A Phase I study of intravenous PI3K inhibitor copanlisib in Japanese patients with advanced or refractory solid tumors. Cancer Chemother. Pharmacol. 2017, 79, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, S.; Zhao, F.; Deming, D.A.; Mitchell, E.P.; Wright, J.J.; Gray, R.J.; Wang, V.; McShane, L.M.; Rubinstein, L.V.; Patton, D.R.; et al. Phase II Study of Copanlisib in Patients With Tumors With PIK3CA Mutations: Results From the NCI-MATCH ECOG-ACRIN Trial (EAY131) Subprotocol Z1F. J. Clin. Oncol. 2022, 40, 1552–1561. [Google Scholar] [CrossRef]
- Jakubowski, C.; Collins, N.B.; Sugar, E.A.; Berg, M.; Cao, H.; Giannakis, M.; Jaffee, E.M.; Azad, N.S. A phase I/II study of PI3Kinase inhibition with copanlisib combined with the anti-PD-1 antibody nivolumab in relapsed/refractory solid tumors with expansions in MSS colorectal cancer. J. Clin. Oncol. 2020, 38, TPS4114. [Google Scholar] [CrossRef]
- Capano, S.; Merlino, G.; Bigioni, M.; Tunici, P.; Cottino, F.; Zanella, E.; Vurchio, V.; Bertotti, A.; Trusolino, L.; Laurent, D.O.; et al. 489P MEN1611 in combination with cetuximab: Targeting PIK3CA mutations in RAS-wild-type patient-derived colorectal cancer xenografts. Ann. Oncol. 2021, 32, S573. [Google Scholar] [CrossRef]
- Sarker, D.; Ang, J.E.; Baird, R.; Kristeleit, R.; Shah, K.; Moreno, V.; Clarke, P.A.; Raynaud, F.I.; Levy, G.; Ware, J.A.; et al. First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin. Cancer Res. 2015, 21, 77–86. [Google Scholar] [CrossRef]
- Leong, S.; Moss, R.A.; Bowles, D.W.; Ware, J.A.; Zhou, J.; Spoerke, J.M.; Lackner, M.R.; Shankar, G.; Schutzman, J.L.; van der Noll, R.; et al. A Phase I Dose-Escalation Study of the Safety and Pharmacokinetics of Pictilisib in Combination with Erlotinib in Patients with Advanced Solid Tumors. Oncologist 2017, 22, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.I.; LoRusso, P.; Kwak, E.; Pandya, S.; Rudin, C.M.; Kurkjian, C.; Cleary, J.M.; Pilat, M.J.; Jones, S.; de Crespigny, A.; et al. Phase Ib study of the MEK inhibitor cobimetinib (GDC-0973) in combination with the PI3K inhibitor pictilisib (GDC-0941) in patients with advanced solid tumors. Invest. New Drugs 2020, 38, 419–432. [Google Scholar] [CrossRef]
- Shapiro, G.I.; Rodon, J.; Bedell, C.; Kwak, E.L.; Baselga, J.; Braña, I.; Pandya, S.S.; Scheffold, C.; Laird, A.D.; Nguyen, L.T.; et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245408 (XL147), an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. Clin. Cancer Res. 2014, 20, 233–245. [Google Scholar] [CrossRef]
- Edelman, G.; Rodon, J.; Lager, J.; Castell, C.; Jiang, J.; Van Allen, E.M.; Wagle, N.; Lindeman, N.I.; Sholl, L.M.; Shapiro, G.I. Phase I Trial of a Tablet Formulation of Pilaralisib, a Pan-Class I PI3K Inhibitor, in Patients with Advanced Solid Tumors. Oncologist 2018, 23, 401-e438. [Google Scholar] [CrossRef]
- Wheler, J.; Mutch, D.; Lager, J.; Castell, C.; Liu, L.; Jiang, J.; Traynor, A.M. Phase I Dose-Escalation Study of Pilaralisib (SAR245408, XL147) in Combination with Paclitaxel and Carboplatin in Patients with Solid Tumors. Oncologist 2017, 22, 377-e337. [Google Scholar] [CrossRef]
- Bowles, D.W.; Kochenderfer, M.; Cohn, A.; Sideris, L.; Nguyen, N.; Cline-Burkhardt, V.; Schnadig, I.; Choi, M.; Nabell, L.; Chaudhry, A.; et al. A Randomized, Phase II Trial of Cetuximab With or Without PX-866, an Irreversible Oral Phosphatidylinositol 3-Kinase Inhibitor, in Patients With Metastatic Colorectal Carcinoma. Clin. Colorectal Cancer 2016, 15, 337–344.e332. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; Jegede, O.A.; Grilley-Olson, J.E.; Lauring, J.D.; Mitchell, E.P.; Zwiebel, J.A.; Gray, R.J.; Wang, V.; McShane, L.M.; Rubinstein, L.V.; et al. Phase II Study of Taselisib in PIK3CA-Mutated Solid Tumors Other Than Breast and Squamous Lung Cancer: Results From the NCI-MATCH ECOG-ACRIN Trial (EAY131) Subprotocol I. JCO Precis. Oncol. 2022, 6, e2100424. [Google Scholar] [CrossRef] [PubMed]
- Maira, S.M.; Pecchi, S.; Huang, A.; Burger, M.; Knapp, M.; Sterker, D.; Schnell, C.; Guthy, D.; Nagel, T.; Wiesmann, M.; et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol. Cancer Ther. 2012, 11, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Rowley, B.R.; Bull, C.O.; Schneider, C.; Haegebarth, A.; Schatz, C.A.; Fracasso, P.R.; Wilkie, D.P.; Hentemann, M.; Wilhelm, S.M.; et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110α and p110δ activities in tumor cell lines and xenograft models. Mol. Cancer Ther. 2013, 12, 2319–2330. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Khazaei, M.; Hasanzadeh, M.; ShahidSales, S.; Joudi Mashhad, M.; Farazestanian, M.; Sadeghnia, H.R.; Rezayi, M.; Maftouh, M.; Hassanian, S.M.; et al. Therapeutic Potential of Targeting PI3K/AKT Pathway in Treatment of Colorectal Cancer: Rational and Progress. J. Cell Biochem. 2018, 119, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Juric, D.; Rodon, J.; Tabernero, J.; Janku, F.; Burris, H.A.; Schellens, J.H.M.; Middleton, M.R.; Berlin, J.; Schuler, M.; Gil-Martin, M.; et al. Phosphatidylinositol 3-Kinase α-Selective Inhibition With Alpelisib (BYL719) in PIK3CA-Altered Solid Tumors: Results From the First-in-Human Study. J. Clin. Oncol. 2018, 36, 1291–1299. [Google Scholar] [CrossRef]
- Van Geel, R.M.J.M.; Tabernero, J.; Elez, E.; Bendell, J.C.; Spreafico, A.; Schuler, M.; Yoshino, T.; Delord, J.P.; Yamada, Y.; Lolkema, M.P.; et al. A Phase Ib Dose-Escalation Study of Encorafenib and Cetuximab with or without Alpelisib in Metastatic BRAF-Mutant Colorectal Cancer. Cancer Discov. 2017, 7, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.R.; Kim, B.; Kim, J.H.; Park, K.H.; Kim, Y.H.; Lee, S. Phase Ib and pharmacokinetics study of alpelisib, a PIK3CA inhibitor, and capecitabine in patients with advanced solid tumors. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Juric, D.; de Bono, J.S.; LoRusso, P.M.; Nemunaitis, J.; Heath, E.I.; Kwak, E.L.; Macarulla Mercadé, T.; Geuna, E.; Jose de Miguel-Luken, M.; Patel, C.; et al. A First-in-Human, Phase I, Dose-Escalation Study of TAK-117, a Selective PI3Kα Isoform Inhibitor, in Patients with Advanced Solid Malignancies. Clin. Cancer Res. 2017, 23, 5015–5023. [Google Scholar] [CrossRef]
- Choudhury, A.D.; Higano, C.S.; de Bono, J.S.; Cook, N.; Rathkopf, D.E.; Wisinski, K.B.; Martin-Liberal, J.; Linch, M.; Heath, E.I.; Baird, R.D.; et al. A Phase I Study Investigating AZD8186, a Potent and Selective Inhibitor of PI3Kβ/δ, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2022, 28, 2257–2269. [Google Scholar] [CrossRef]
- Arkenau, H.-T.; Mateo, J.; Lemech, C.R.; Infante, J.R.; Burris, H.A.; Bang, Y.-J.; Eder, J.P.; Herbst, R.S.; Sharma, S.; Chung, H.C.; et al. A phase I/II, first-in-human dose-escalation study of GSK2636771 in patients (pts) with PTEN-deficient advanced tumors. J. Clin. Oncol. 2014, 32, 2514. [Google Scholar] [CrossRef]
- Dumbrava, E.E.; Burton, E.M.; Subudhi, S.K.; Milton, D.R.; Aparicio, A.; Yap, T.A.; Naing, A.; Corn, P.G.; Pilié, P.G.; Zurita, A.J.; et al. Phase I/II study of the selective PI3Kβ inhibitor GSK2636771 in combination with pembrolizumab in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and PTEN loss. J. Clin. Oncol. 2022, 40, 5052. [Google Scholar] [CrossRef]
- Bédard, P.L.; Davies, M.A.; Kopetz, S.; Juric, D.; Shapiro, G.I.; Luke, J.J.; Spreafico, A.; Wu, B.; Castell, C.; Gomez, C.; et al. First-in-human trial of the PI3Kβ-selective inhibitor SAR260301 in patients with advanced solid tumors. Cancer 2018, 124, 315–324. [Google Scholar] [CrossRef]
- Fritsch, C.; Huang, A.; Chatenay-Rivauday, C.; Schnell, C.; Reddy, A.; Liu, M.; Kauffmann, A.; Guthy, D.; Erdmann, D.; De Pover, A.; et al. Characterization of the novel and specific PI3Kα inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol. Cancer Ther. 2014, 13, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Alpelisib: First Global Approval. Drugs 2019, 79, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Geel, R.V.; Guren, T.K.; Yaeger, R.D.; Spreafico, A.; Faris, J.E.; Yoshino, T.; Yamada, Y.; Kim, T.W.; Bendell, J.C.; et al. Phase 2 results: Encorafenib (ENCO) and cetuximab (CETUX) with or without alpelisib (ALP) in patients with advanced BRAF-mutant colorectal cancer (BRAFm CRC). J. Clin. Oncol. 2016, 34, 3544. [Google Scholar] [CrossRef]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Dolly, S.O.; Wagner, A.J.; Bendell, J.C.; Kindler, H.L.; Krug, L.M.; Seiwert, T.Y.; Zauderer, M.G.; Lolkema, M.P.; Apt, D.; Yeh, R.F.; et al. Phase I Study of Apitolisib (GDC-0980), Dual Phosphatidylinositol-3-Kinase and Mammalian Target of Rapamycin Kinase Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 2874–2884. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Brown, N.; Xyrafas, A.; Bize, V.; Hawle, H.; Berardi, S.; Cmiljanović, N.; Cmiljanović, V.; Stumm, M.; Dimitrijević, S.; et al. First-in human, phase 1, dose-escalation pharmacokinetic and pharmacodynamic study of the oral dual PI3K and mTORC1/2 inhibitor PQR309 in patients with advanced solid tumors (SAKK 67/13). Eur. J. Cancer 2018, 96, 6–16. [Google Scholar] [CrossRef]
- Rodon, J.; Pérez-Fidalgo, A.; Krop, I.E.; Burris, H.; Guerrero-Zotano, A.; Britten, C.D.; Becerra, C.; Schellens, J.; Richards, D.A.; Schuler, M.; et al. Phase 1/1b dose escalation and expansion study of BEZ235, a dual PI3K/mTOR inhibitor, in patients with advanced solid tumors including patients with advanced breast cancer. Cancer Chemother. Pharmacol. 2018, 82, 285–298. [Google Scholar] [CrossRef]
- Toyoda, M.; Watanabe, K.; Amagasaki, T.; Natsume, K.; Takeuchi, H.; Quadt, C.; Shirao, K.; Minami, H. A phase I study of single-agent BEZ235 special delivery system sachet in Japanese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2019, 83, 289–299. [Google Scholar] [CrossRef]
- Ahnert, J.R.; Schuler, M.H.; Machiels, J.-P.H.; Hess, D.; Paz-Ares, L.; Awada, A.; Moos, R.v.; Steeghs, N.; Zambrano, C.C.; Mesmaeker, P.D.; et al. Phase lb study of BEZ235 plus either paclitaxel (PTX) in advanced solid tumors (aST) or PTX plus trastuzumab (TZ) in HER2+ breast cancer (BC). J. Clin. Oncol. 2014, 32, 621. [Google Scholar] [CrossRef]
- Bendell, J.C.; Kurkjian, C.; Infante, J.R.; Bauer, T.M.; Burris, H.A., 3rd; Greco, F.A.; Shih, K.C.; Thompson, D.S.; Lane, C.M.; Finney, L.H.; et al. A phase 1 study of the sachet formulation of the oral dual PI3K/mTOR inhibitor BEZ235 given twice daily (BID) in patients with advanced solid tumors. Invest. New Drugs 2015, 33, 463–471. [Google Scholar] [CrossRef]
- Wise-Draper, T.M.; Moorthy, G.; Salkeni, M.A.; Karim, N.A.; Thomas, H.E.; Mercer, C.A.; Beg, M.S.; O’Gara, S.; Olowokure, O.; Fathallah, H.; et al. A Phase Ib Study of the Dual PI3K/mTOR Inhibitor Dactolisib (BEZ235) Combined with Everolimus in Patients with Advanced Solid Malignancies. Target. Oncol. 2017, 12, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Bendell, J.C.; LoRusso, P.; Tsushima, T.; Desai, V.; Kenmotsu, H.; Watanabe, J.; Ono, A.; Murugesan, B.G.; Silva, J.; et al. A call for global harmonization of phase I oncology trials: Results from two parallel, first-in-human phase I studies of DS-7423, an oral PI3K/mTOR dual inhibitor in advanced solid tumors conducted in the United States and Japan. J. Clin. Oncol. 2017, 35, 2536. [Google Scholar] [CrossRef]
- Shapiro, G.I.; Bell-McGuinn, K.M.; Molina, J.R.; Bendell, J.; Spicer, J.; Kwak, E.L.; Pandya, S.S.; Millham, R.; Borzillo, G.; Pierce, K.J.; et al. First-in-Human Study of PF-05212384 (PKI-587), a Small-Molecule, Intravenous, Dual Inhibitor of PI3K and mTOR in Patients with Advanced Cancer. Clin. Cancer Res. 2015, 21, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, Z.A.; Alsina, M.; Soares, H.P.; Braña, I.; Britten, C.D.; Del Conte, G.; Ezeh, P.; Houk, B.; Kern, K.A.; Leong, S.; et al. A Multi-Arm Phase I Study of the PI3K/mTOR Inhibitors PF-04691502 and Gedatolisib (PF-05212384) plus Irinotecan or the MEK Inhibitor PD-0325901 in Advanced Cancer. Target. Oncol. 2017, 12, 775–785. [Google Scholar] [CrossRef]
- Bendell, J.C.; Varghese, A.M.; Hyman, D.M.; Bauer, T.M.; Pant, S.; Callies, S.; Lin, J.; Martinez, R.; Wickremsinhe, E.; Fink, A.; et al. A First-in-Human Phase 1 Study of LY3023414, an Oral PI3K/mTOR Dual Inhibitor, in Patients with Advanced Cancer. Clin. Cancer Res. 2018, 24, 3253–3262. [Google Scholar] [CrossRef] [PubMed]
- Azaro, A.; Massard, C.; Tap, W.D.; Cassier, P.A.; Merchan, J.; Italiano, A.; Anderson, B.; Yuen, E.; Yu, D.; Oakley, G., 3rd; et al. A phase 1b study of the Notch inhibitor crenigacestat (LY3039478) in combination with other anticancer target agents (taladegib, LY3023414, or abemaciclib) in patients with advanced or metastatic solid tumors. Invest. New Drugs 2021, 39, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.P.; Tabernero, J.; Markman, B.; Patnaik, A.; Tolcher, A.W.; Baselga, J.; Shi, W.; Egile, C.; Ruiz-Soto, R.; Laird, A.D.; et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245409 (XL765), a novel, orally administered PI3K/mTOR inhibitor in patients with advanced solid tumors. Clin. Cancer Res. 2014, 20, 2445–2456. [Google Scholar] [CrossRef] [PubMed]
- Jänne, P.A.; Cohen, R.B.; Laird, A.D.; Macé, S.; Engelman, J.A.; Ruiz-Soto, R.; Rockich, K.; Xu, J.; Shapiro, G.I.; Martinez, P.; et al. Phase I safety and pharmacokinetic study of the PI3K/mTOR inhibitor SAR245409 (XL765) in combination with erlotinib in patients with advanced solid tumors. J. Thorac. Oncol. 2014, 9, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Schram, A.M.; Gandhi, L.; Mita, M.M.; Damstrup, L.; Campana, F.; Hidalgo, M.; Grande, E.; Hyman, D.M.; Heist, R.S. A phase Ib dose-escalation and expansion study of the oral MEK inhibitor pimasertib and PI3K/MTOR inhibitor voxtalisib in patients with advanced solid tumours. Br. J. Cancer 2018, 119, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Maira, S.M.; Stauffer, F.; Brueggen, J.; Furet, P.; Schnell, C.; Fritsch, C.; Brachmann, S.; Chène, P.; De Pover, A.; Schoemaker, K.; et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 2008, 7, 1851–1863. [Google Scholar] [CrossRef]
- Vasudevan, K.M.; Barbie, D.A.; Davies, M.A.; Rabinovsky, R.; McNear, C.J.; Kim, J.J.; Hennessy, B.T.; Tseng, H.; Pochanard, P.; Kim, S.Y.; et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell 2009, 16, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Tolcher, A.W.; Patnaik, A.; Papadopoulos, K.P.; Rasco, D.W.; Becerra, C.R.; Allred, A.J.; Orford, K.; Aktan, G.; Ferron-Brady, G.; Ibrahim, N.; et al. Phase I study of the MEK inhibitor trametinib in combination with the AKT inhibitor afuresertib in patients with solid tumors and multiple myeloma. Cancer Chemother. Pharmacol. 2015, 75, 183–189. [Google Scholar] [CrossRef]
- Wolff, R.A.; Fuchs, M.; Di Bartolomeo, M.; Hossain, A.M.; Stoffregen, C.; Nicol, S.; Heinemann, V. A double-blind, randomized, placebo-controlled, phase 2 study of maintenance enzastaurin with 5-fluorouracil/leucovorin plus bevacizumab after first-line therapy for metastatic colorectal cancer. Cancer 2012, 118, 4132–4138. [Google Scholar] [CrossRef]
- Shapiro, G.I.; LoRusso, P.; Cho, D.C.; Musib, L.; Yan, Y.; Wongchenko, M.; Chang, I.; Patel, P.; Chan, I.T.; Sanabria-Bohorquez, S.; et al. A phase Ib open-label dose escalation study of the safety, pharmacokinetics, and pharmacodynamics of cobimetinib (GDC-0973) and ipatasertib (GDC-0068) in patients with locally advanced or metastatic solid tumors. Invest. New Drugs 2021, 39, 163–174. [Google Scholar] [CrossRef]
- Yap, T.A.; Yan, L.; Patnaik, A.; Fearen, I.; Olmos, D.; Papadopoulos, K.; Baird, R.D.; Delgado, L.; Taylor, A.; Lupinacci, L.; et al. First-in-Man Clinical Trial of the Oral Pan-AKT Inhibitor MK-2206 in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2011, 29, 4688–4695. [Google Scholar] [CrossRef]
- Do, K.; Speranza, G.; Bishop, R.; Khin, S.; Rubinstein, L.; Kinders, R.J.; Datiles, M.; Eugeni, M.; Lam, M.H.; Doyle, L.A.; et al. Biomarker-driven phase 2 study of MK-2206 and selumetinib (AZD6244, ARRY-142886) in patients with colorectal cancer. Invest. New Drugs 2015, 33, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Overman, M.J.; Fogelman, D.R.; Kee, B.K.; Menter, D.; Raghav, K.P.S.; Morris, V.K.; Oh, J.; Wu, J.; Jiang, Z.; et al. A phase II and co-clinical study of an AKT inhibitor in patients (pts) with biomarker-enriched, previously treated metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2016, 34, 3563. [Google Scholar] [CrossRef]
- Bendell, J.C.; Nemunaitis, J.; Vukelja, S.J.; Hagenstad, C.; Campos, L.T.; Hermann, R.C.; Sportelli, P.; Gardner, L.; Richards, D.A. Randomized placebo-controlled phase II trial of perifosine plus capecitabine as second- or third-line therapy in patients with metastatic colorectal cancer. J. Clin. Oncol. 2011, 29, 4394–4400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bendell, J.C.; Ervin, T.J.; Senzer, N.N.; Richards, D.A.; Firdaus, I.; Lockhart, A.C.; Cohn, A.L.; Saleh, M.N.; Gardner, L.R.; Sportelli, P.; et al. Results of the X-PECT study: A phase III randomized double-blind placebo-controlled study of perifosine plus capecitabine (P-CAP) versus placebo plus capecitabine (CAP) in patients (pts) with refractory metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2012, 30, LBA3501. [Google Scholar] [CrossRef]
- Ng, K.; Tabernero, J.; Hwang, J.; Bajetta, E.; Sharma, S.; Del Prete, S.A.; Arrowsmith, E.R.; Ryan, D.P.; Sedova, M.; Jin, J.; et al. Phase II study of everolimus in patients with metastatic colorectal adenocarcinoma previously treated with bevacizumab-, fluoropyrimidine-, oxaliplatin-, and irinotecan-based regimens. Clin. Cancer Res. 2013, 19, 3987–3995. [Google Scholar] [CrossRef]
- Hecht, J.R.; Reid, T.R.; Garrett, C.R.; Beck, J.T.; Davidson, S.J.; Mackenzie, M.J.; Brandt, U.; Rizvi, S.; Sharma, S. Phase I study of everolimus, cetuximab and irinotecan as second-line therapy in metastatic colorectal cancer. Anticancer. Res. 2015, 35, 1567–1573. [Google Scholar]
- Chiorean, E.G.; Picus, J.; Breen, T.; Ansari, R.H.; Harb, W.A.; Burns, M.; Spittler, A.J.; Loehrer, P.J. Phase I/II study of everolimus (E) with irinotecan (Iri) and cetuximab (C) in 2nd line metastatic colorectal cancer (mCRC): Hoosier Cancer Research Network GI05-102. J. Clin. Oncol. 2015, 33, 3618. [Google Scholar] [CrossRef]
- Altomare, I.; Bendell, J.C.; Bullock, K.E.; Uronis, H.E.; Morse, M.A.; Hsu, S.D.; Zafar, S.Y.; Blobe, G.C.; Pang, H.; Honeycutt, W.; et al. A phase II trial of bevacizumab plus everolimus for patients with refractory metastatic colorectal cancer. Oncologist 2011, 16, 1131–1137. [Google Scholar] [CrossRef]
- Weldon Gilcrease, G.; Stenehjem, D.D.; Wade, M.L.; Weis, J.; McGregor, K.; Whisenant, J.; Boucher, K.M.; Thorne, K.; Orgain, N.; Garrido-Laguna, I.; et al. Phase I/II study of everolimus combined with mFOLFOX-6 and bevacizumab for first-line treatment of metastatic colorectal cancer. Invest. New Drugs 2019, 37, 482–489. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Ng, K.; Zhu, A.X.; Abrams, T.; Enzinger, P.C.; McCleary, N.J.; Schrag, D.; Kwak, E.L.; Allen, J.N.; Bhargava, P.; et al. Multicenter phase II study of tivozanib (AV-951) and everolimus (RAD001) for patients with refractory, metastatic colorectal cancer. Oncologist 2013, 18, 377–378. [Google Scholar] [CrossRef] [PubMed]
- Townsend, A.; Tebbutt, N.; Karapetis, C.; Cooper, P.; Singhal, N.; Yeend, S.; Pirc, L.; Joshi, R.; Hardingham, J.; Price, T. Phase IB/II Study of Second-Line Therapy with Panitumumab, Irinotecan, and Everolimus (PIE) in KRAS Wild-Type Metastatic Colorectal Cancer. Clin. Cancer Res. 2018, 24, 3838–3844. [Google Scholar] [CrossRef]
- Bendell, J.C.; Jones, S.F.; Hart, L.; Spigel, D.R.; Lane, C.M.; Earwood, C.; Infante, J.R.; Barton, J.; Burris, H.A. A phase Ib study of linsitinib (OSI-906), a dual inhibitor of IGF-1R and IR tyrosine kinase, in combination with everolimus as treatment for patients with refractory metastatic colorectal cancer. Invest. New Drugs 2015, 33, 187–193. [Google Scholar] [CrossRef]
- Spindler, K.G.; Demuth, C.; Sorensen, B.S.; Johansen, J.S.; Nielsen, D.; Pallisgaard, N.; Hoegdall, E.; Pfeiffer, P.; Vittrup Jensen, B. Total cell-free DNA, carcinoembryonic antigen, and C-reactive protein for assessment of prognosis in patients with metastatic colorectal cancer. Tumour Biol. 2018, 40, 1010428318811207. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.M.; Meric-Bernstam, F.; Chawla, S.; Falchook, G.; Hong, D.; Akcakanat, A.; Chen, H.; Naing, A.; Fu, S.; Wheler, J.; et al. Weekly nab-Rapamycin in patients with advanced nonhematologic malignancies: Final results of a phase I trial. Clin. Cancer Res. 2013, 19, 5474–5484. [Google Scholar] [CrossRef]
- Sharma, S.; Chiorean, E.G.; Alistar, A.T.; Braiteh, F.S.; Matrana, M.R.; Becerra, C.R.; Grigorian, B.; Hou, S.; Schmid, A.N.; Desai, N. Phase I results from a phase 1/2 multi-center study of nab-sirolimus combined with mFOLFOX6+bevacizumab (FB) as first-line (1L) therapy in patients (pts) with metastatic colorectal cancer (mCRC) with or without PTEN loss. J. Clin. Oncol. 2020, 38, e16050. [Google Scholar] [CrossRef]
- Cohen, E.E.; Wu, K.; Hartford, C.; Kocherginsky, M.; Eaton, K.N.; Zha, Y.; Nallari, A.; Maitland, M.L.; Fox-Kay, K.; Moshier, K.; et al. Phase I studies of sirolimus alone or in combination with pharmacokinetic modulators in advanced cancer patients. Clin. Cancer Res. 2012, 18, 4785–4793. [Google Scholar] [CrossRef]
- Buijsen, J.; van den Bogaard, J.; Jutten, B.; Belgers, E.; Sosef, M.; Leijtens, J.W.; Beets, G.L.; Jansen, R.L.; Riedl, R.G.; Clarijs, R.; et al. A phase I-II study on the combination of rapamycin and short course radiotherapy in rectal cancer. Radiother. Oncol. 2015, 116, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Spindler, K.L.; Sorensen, M.M.; Pallisgaard, N.; Andersen, R.F.; Havelund, B.M.; Ploen, J.; Lassen, U.; Jakobsen, A.K. Phase II trial of temsirolimus alone and in combination with irinotecan for KRAS mutant metastatic colorectal cancer: Outcome and results of KRAS mutational analysis in plasma. Acta Oncol. 2013, 52, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Piha-Paul, S.A.; Naing, A.; Falchook, G.S.; Wheler, J.J.; Janku, F.; Zinner, R.; Laday, S.; Kies, M.S.; Tsimberidou, A.M. Phase I clinical trial of lenalidomide with temsirolimus in patients with advanced cancer. J. Clin. Oncol. 2013, 31, 2604. [Google Scholar] [CrossRef]
- Kondapaka, S.B.; Singh, S.S.; Dasmahapatra, G.P.; Sausville, E.A.; Roy, K.K. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol. Cancer Ther. 2003, 2, 1093–1103. [Google Scholar] [PubMed]
- Sabatini, D.M. mTOR and cancer: Insights into a complex relationship. Nat. Rev. Cancer 2006, 6, 729–734. [Google Scholar] [CrossRef]
- Teng, Q.-X.; Ashar, Y.V.; Gupta, P.; Gadee, E.; Fan, Y.-F.; Reznik, S.E.; Wurpel, J.N.D.; Chen, Z.-S. Revisiting mTOR inhibitors as anticancer agents. Drug Discov. Today 2019, 24, 2086–2095. [Google Scholar] [CrossRef]
- Sparks, C.A.; Guertin, D.A. Targeting mTOR: Prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene 2010, 29, 3733–3744. [Google Scholar] [CrossRef]
- Francipane, M.G.; Lagasse, E. mTOR pathway in colorectal cancer: An update. Oncotarget 2014, 5, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cui, J.F.; Chen, M.B.; Liu, C.Y.; Liu, F.; Zhang, Q.D.; Zou, J.; Lu, P.H. The preclinical evaluation of the dual mTORC1/2 inhibitor INK-128 as a potential anti-colorectal cancer agent. Cancer Biol. Ther. 2015, 16, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Fricke, S.L.; Payne, S.N.; Favreau, P.F.; Kratz, J.D.; Pasch, C.A.; Foley, T.M.; Yueh, A.E.; Van De Hey, D.R.; Depke, M.G.; Korkos, D.P.; et al. MTORC1/2 Inhibition as a Therapeutic Strategy for PIK3CA Mutant Cancers. Mol. Cancer Ther. 2019, 18, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Ding, J.; Huang, Y.; Liu, J.; Liu, N.; Ao, Y.; Hong, Y.; Wang, L.; Zhang, L.; et al. Targeting mTOR suppressed colon cancer growth through 4EBP1/eIF4E/PUMA pathway. Cancer Gene Ther. 2020, 27, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; De Santis, M.C.; Braccini, L.; Gulluni, F.; Hirsch, E. PI3K/AKT signaling pathway and cancer: An updated review. Ann. Med. 2014, 46, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.L.; Cantley, L.C. PI3K pathway alterations in cancer: Variations on a theme. Oncogene 2008, 27, 5497–5510. [Google Scholar] [CrossRef]
- Foley, T.M.; Payne, S.N.; Pasch, C.A.; Yueh, A.E.; Van De Hey, D.R.; Korkos, D.P.; Clipson, L.; Maher, M.E.; Matkowskyj, K.A.; Newton, M.A.; et al. Dual PI3K/mTOR Inhibition in Colorectal Cancers with APC and PIK3CA Mutations. Mol. Cancer Res. 2017, 15, 317–327. [Google Scholar] [CrossRef]
- Jun, T.; Gjoerup, O.; Roberts, T.M. Tangled Webs: Evidence of Cross-Talk Between c-Raf-1 and Akt. Sci. STKE 1999, 1999, pe1. [Google Scholar] [CrossRef] [PubMed]
- Dienstmann, R.; Salazar, R.; Tabernero, J. Overcoming Resistance to Anti-EGFR Therapy in Colorectal Cancer. Am. Soc. Clin. Oncol. Educ. Book. 2015, 35, e149–e156. [Google Scholar] [CrossRef]
- Prossomariti, A.; Piazzi, G.; Alquati, C.; Ricciardiello, L. Are Wnt/β-Catenin and PI3K/AKT/mTORC1 Distinct Pathways in Colorectal Cancer? Cell Mol. Gastroenterol. Hepatol. 2020, 10, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Gu, T.; Hu, X.; Zhang, J.; Luo, P. Comprehensive Analysis Identifies PI3K/Akt Pathway Alternations as an Immune-Related Prognostic Biomarker in Colon Adenocarcinoma Patients Receiving Immune Checkpoint Inhibitor Treatment. J. Immunol. Res. 2022, 2022, 8179799. [Google Scholar] [CrossRef]
- Nusrat, M.; Syed, M.A.; Katkhuda, R.; Parra, E.R.; Wistuba, I.I.; Kong, P.; Koehne, A.; Dasari, A.; Overman, M.J.; Menter, D.; et al. The immune impact of PI3K-AKT pathway inhibition in colorectal cancer. J. Clin. Oncol. 2022, 40, 154. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, F.; ten Dijke, P. Signaling interplay between transforming growth factor–β receptor and PI3K/AKT pathways in cancer. Trends Biochem. Sci. 2013, 38, 612–620. [Google Scholar] [CrossRef]
- Muellner, M.K.; Uras, I.Z.; Gapp, B.V.; Kerzendorfer, C.; Smida, M.; Lechtermann, H.; Craig-Mueller, N.; Colinge, J.; Duernberger, G.; Nijman, S.M. A chemical-genetic screen reveals a mechanism of resistance to PI3K inhibitors in cancer. Nat. Chem. Biol. 2011, 7, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, L.; Qiu, H.; Zhang, M.; Sun, L.; Peng, P.; Yu, Q.; Yuan, X. Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget 2017, 8, 3980–4000. [Google Scholar] [CrossRef]
- Esposito, A.; Viale, G.; Curigliano, G. Safety, Tolerability, and Management of Toxic Effects of Phosphatidylinositol 3-Kinase Inhibitor Treatment in Patients With Cancer: A Review. JAMA Oncol. 2019, 5, 1347–1354. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Pauli, C.; Du, X.; Wang, D.G.; Li, X.; Wu, D.; Amadiume, S.C.; Goncalves, M.D.; Hodakoski, C.; Lundquist, M.R.; et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 2018, 560, 499–503. [Google Scholar] [CrossRef] [PubMed]

| Gene | Alteration | Expected Outcome | References |
|---|---|---|---|
| PIK3CA | Mutation | Gain of function | [19,20,21] |
| Amplification | |||
| PTEN | Mutation | Loss of function | [20,21,22] |
| Deletion | |||
| Epigenetic silencing | |||
| IRS2 | Amplification | Gain of function | [20] |
| PIK3R1 | Mutation | Gain of function | [24] |
| PDK1 | Mutation | Gain of function | [20] |
| AKT1 | Mutation | Gain of function | [23] |
| AKT2 | Mutation | Gain of function | [20] |
| Co-amplification | |||
| PAK4 | Mutation | Gain of function | [20] |
| Co-amplification | |||
| mTOR | Mutation | Gain of function | [25] |
| TSC1/2 | Mutation | Loss of function | [25,26] |
| Drug | Condition | Phase | Status | NCT Number, Reference |
|---|---|---|---|---|
| Buparlisib (BKM120) | Advanced solid tumors | I | Completed | NCT01068483 [41,42] |
| Advanced solid tumors | I | Completed | NCT01155453 [43] | |
| Advanced solid tumors | I | Completed | NCT01285466 [44] | |
| Advanced CRC | I | Completed | NCT01304602 [45] | |
| Advanced solid tumors | I | Completed | NCT01363232 [46] | |
| PIK3CA mutated cancers | I | Withdrawn | NCT01501604 | |
| Advanced solid tumors | I | Completed | NCT01571024 [47] | |
| Advanced solid tumors | I | Completed | NCT01576666 [48] | |
| RAS-WT mCRC | I/II | Completed | NCT01591421 [49] | |
| Advanced solid tumors | I | Completed | NCT01626209 [50] | |
| Copanlisib (BAY 80-6946) | Advanced tumors | I | Completed | NCT00962611 [51] |
| Advanced or refractory solid tumors | I | Completed | NCT01404390 [52] | |
| Advanced or refractory solid tumors | II | Active, not recruiting | NCT02465060 [53] | |
| Relapsed or refractory solid tumors with expansions in MSS CRC | I/II | Active, not recruiting | NCT03711058 [54] | |
| PIK3CA and PTEN mutated advanced solid tumors | I/II | Active, not recruiting | NCT04317105 | |
| MEN1611 | mCRC | I/II | Active, not recruiting | NCT04495621 [55] |
| Pictilisib (GDC-0941) | Locally advanced or metastatic solid tumors | I | Completed | NCT00876122 [56] |
| Advanced solid tumors | I | Completed | NCT00975182 [57] | |
| Locally advanced or metastatic solid tumors | I | Terminated | NCT00996892 [58] | |
| Pilaralisib (XL147) | Advanced solid tumors | I | Completed | NCT00486135 [59,60] |
| Solid tumors | I | Completed | NCT00756847 [61] | |
| Locally advanced or metastatic solid tumors | I | Completed | NCT01357330 | |
| Sonolisib (PX-866) | mCRC | I/II | Completed | NCT01252628 [62] |
| Taselisib (GDC-0032) | Advanced refractory solid tumors | II | Active, not recruiting | NCT02465060 [63] |
| Drug | Condition | Phase | Status | NCT, Reference |
|---|---|---|---|---|
| PI3Kα inhibitors | ||||
| Alpelisib (BYL719) | PIK3CA-mutated advanced solid tumors | I | Completed | NCT01219699 [67] |
| Advanced solid tumors | I | Completed | NCT01449058 | |
| BRAF-mutated mCRC | I/II | Completed | NCT01719380 [68] | |
| PIK3CA-mutated mCRC | I/II | Active, not recruiting | NCT04753203 [69] | |
| Inavolisib (GDC-0077) | mCRC | I | Recruiting | NCT04929223 |
| Serabelisib (TAK-117) | Advanced solid tumors | I | Completed | NCT01449370 [70] |
| Advanced solid tumors | I/II | Unknown | NCT04073680 | |
| PIK3CA-mutated advanced solid tumors | I | Active, not recruiting | NCT05300048 | |
| TOS-358 | Solid tumors | I | Recruiting | NCT05683418 |
| PI3Kβ inhibitors | ||||
| AZD8186 | Advanced solid tumors | I | Completed | NCT01884285 [71] |
| PTEN/PIK3CB-altered advanced solid tumors | I | Active, not recruiting | NCT03218826 | |
| GSK2636771 | PTEN-deficient advanced solid tumors | I/II | Completed | NCT01458067 [72,73] |
| Advanced refractory Solid Tumors | I | Active, not recruiting | NCT02465060 | |
| SAR260301 | Advanced cancers | I | Completed | NCT01673737 [74] |
| PI3Kδ inhibitors | ||||
| Parsaclisib (INCB050465) | Advanced solid tumors | I | Completed | NCT02646748 |
| Drug | Condition | Phase | Status | NCT, Reference |
|---|---|---|---|---|
| Apitolisib (GDC-0980) | Refractory solid tumors | I | Completed | NCT00854152 [81] |
| Advanced solid tumors | I | Completed | NCT01332604 | |
| Bimiralisib (PQR309) | Advanced solid tumors | I | Completed | NCT01940133 [82] |
| Dactolisib (BEZ-235) | Advanced solid tumors | I/II | Completed | NCT00620594 [83] |
| Advanced solid tumors | I | Completed | NCT01195376 [84] | |
| Metastatic or locally advanced solid tumors | I | Completed | NCT01285466 [85] | |
| Advanced solid tumors | I | Completed | NCT01337765 | |
| Advanced solid tumors | I | Completed | NCT01343498 [86] | |
| Advanced solid tumors | I | Terminated | NCT01508104 [87] | |
| DS-7423 | Advanced solid tumors | I | Completed | NCT01364844 [88] |
| Gedatolisib (PKI-587) | Solid tumors | I | Completed | NCT00940498 [89] |
| Advanced cancer | I | Terminated | NCT01347866 [90] | |
| mCRC | II | Terminated | NCT01925274 | |
| PWT33597 Mesylate | Advanced malignancies | I | Completed | NCT01407380 |
| Samotolisib (LY3023414) | Advanced cancer | I | Completed | NCT01655225 [91] |
| Advanced or metastatic solid tumors | I | Completed | NCT02784795 [92] | |
| Voxtalisib (XL-765) | Solid tumors | I | Completed | NCT00485719 [93] |
| Solid tumors | I | Completed | NCT00777699 [94] | |
| Locally advanced or metastatic solid tumors | I | Completed | NCT01390818 [95] |
| Drug | Condition | Phase | Status | NCT, Reference |
|---|---|---|---|---|
| Akt inhibitors | ||||
| Afuresertib (GSK2110183) | Solid tumors | I | Completed | NCT01476137 [98] |
| Enzastaurin (LY317615) | Recurrent CRC | II | Completed | NCT00437268 |
| mCRC | II | Completed | NCT00612586 [99] | |
| mCRC | II | Completed | NCT00192114 | |
| Ipatasertib (GDC-0068) | Locally advanced or metastatic solid tumors | I | Completed | NCT01562275 [100] |
| MK-2206 | Advanced solid tumors | I | Completed | NCT00670488 [101] |
| Advanced CRC | II | Completed | NCT01333475 [102] | |
| PTEN loss and PIK3CA mutated mCRC | II | Completed | NCT01802320 [103] | |
| Nelfinavir | Locally advanced CRC | I/II | Completed | NCT00704600 |
| Perifosine (KRX-0401) | mCRC | II | Completed | NCT00398879 [104] |
| Refractory advanced CRC | III | Completed | NCT01097018 [105] | |
| mTOR inhibitors | ||||
| Everolimus (RAD001) | mCRC | II | Completed | NCT00337545 |
| Refractory mCRC | II | Completed | NCT00419159 [106] | |
| mCRC | I | Completed | NCT00478634 [107] | |
| mCRC | I/II | Completed | NCT00522665 [108] | |
| Refractory mCRC | II | Completed | NCT00597506 [109] | |
| CRC | I/II | Completed | NCT01047293 [110] | |
| mCRC | I/II | Completed | NCT01058655 [111] | |
| KRAS WT mCRC | I/II | Completed | NCT01139138 [112] | |
| Refractory mCRC | I | Completed | NCT01154335 [113] | |
| mCRC | II | Completed | NCT01387880 [114] | |
| Solid tumors | I | Completed | NCT02890069 | |
| mCRC | II | Recruiting | NCT05725200 | |
| Nab-rapamycin (ABI-009) | Advanced carcinoma | I/II | Completed | NCT03190174 [115] |
| Advanced or mCRC | I/II | Completed | NCT03439462 [116] | |
| Rapamycin (Sirolimus) | Advanced malignancies | I | Completed | NCT00375245 [117] |
| Rectum cancer | I/II | Completed | NCT00409994 [118] | |
| Advanced malignancies | I | Completed | NCT00707135 [117] | |
| Solid tumors | I | Completed | NCT01522820 | |
| Temsirolimus (CCI-770) | Refractory CRC | I | Completed | NCT00593060 |
| KRAS mutated mCRC | II | Completed | NCT00827684 [119] | |
| Advanced cancers | I | Completed | NCT01183663 [120] | |
| Hepatic metastatic cancer | I | Recruiting | NCT03203525 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leiphrakpam, P.D.; Are, C. PI3K/Akt/mTOR Signaling Pathway as a Target for Colorectal Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 3178. https://doi.org/10.3390/ijms25063178
Leiphrakpam PD, Are C. PI3K/Akt/mTOR Signaling Pathway as a Target for Colorectal Cancer Treatment. International Journal of Molecular Sciences. 2024; 25(6):3178. https://doi.org/10.3390/ijms25063178
Chicago/Turabian StyleLeiphrakpam, Premila D., and Chandrakanth Are. 2024. "PI3K/Akt/mTOR Signaling Pathway as a Target for Colorectal Cancer Treatment" International Journal of Molecular Sciences 25, no. 6: 3178. https://doi.org/10.3390/ijms25063178
APA StyleLeiphrakpam, P. D., & Are, C. (2024). PI3K/Akt/mTOR Signaling Pathway as a Target for Colorectal Cancer Treatment. International Journal of Molecular Sciences, 25(6), 3178. https://doi.org/10.3390/ijms25063178






