Beta-Caryophyllene, a Cannabinoid Receptor Type 2 Selective Agonist, in Emotional and Cognitive Disorders
Abstract
1. Introduction
2. CB2R as a Potential Modulator of Neuroinflammation and Neuropsychiatric Disorders
2.1. CB2R in Neuroinflammation
2.2. CB2R in Neuropsychiatric Disorders
2.2.1. CB2R in Depression
2.2.2. CB2R in Anxiety and Anxiety-Related Disorders
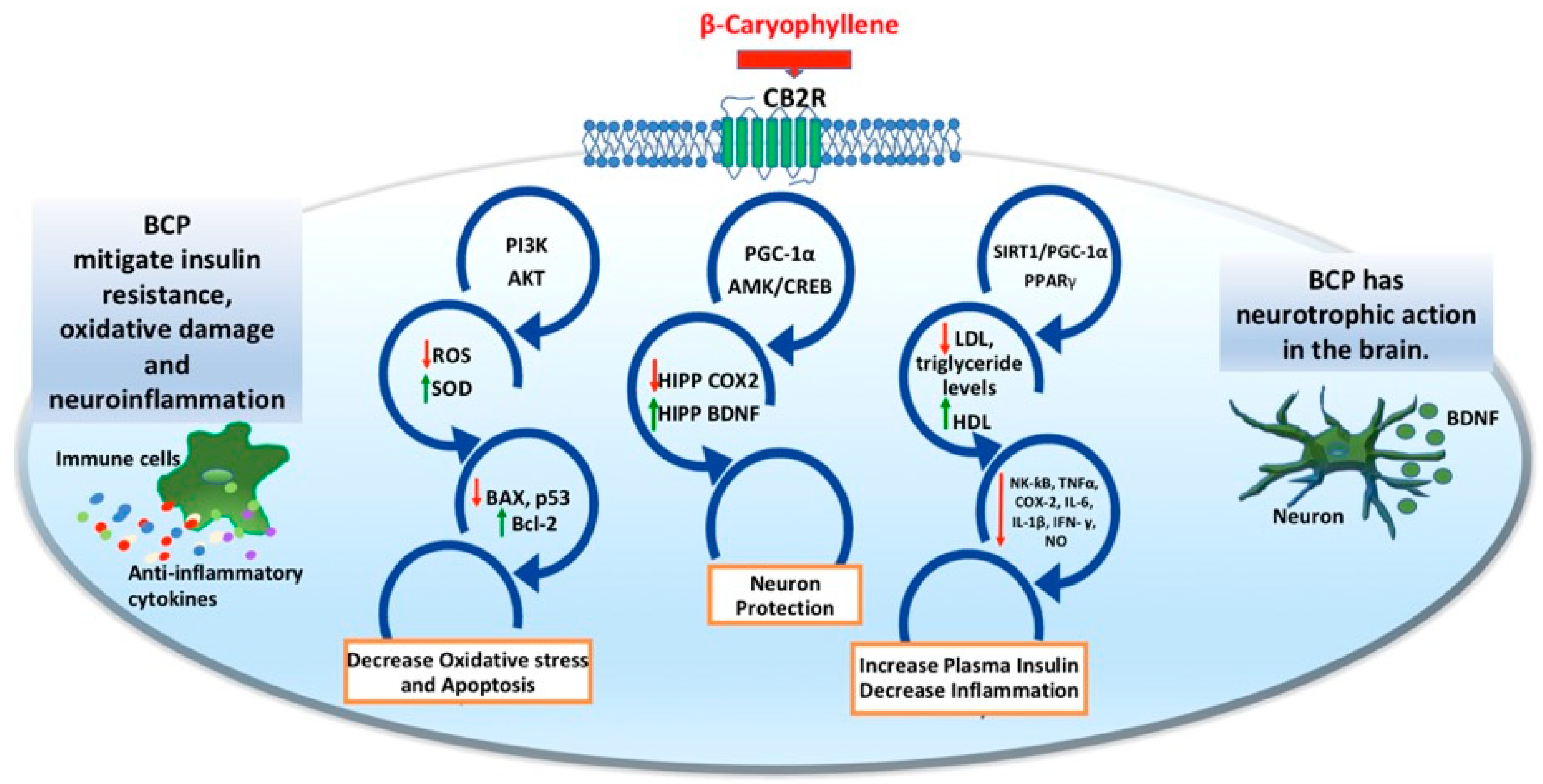
3. BCP as a Functional CB2R Agonist to Target Neuropsychiatric Disorders
3.1. Immunomodulatory Roles of BCP
3.2. Anti-Inflammatory Roles of BCP
3.3. Beta-Caryophyllene May Be Prospective in the Treatment of Anxiety and Depression
| Type | Strain | Paradigm | Behavioral Effects | Neurochemical Effects | References |
|---|---|---|---|---|---|
| BCP (acute; 50 mg/kg; i.p.) | C57BL/6 mice | acute stress | ↑ sociability ↓ anxiety ↓ depressive-like behavior | [60] | |
| BCP (chronic; 25, 50, 100 mg/kg; i.p.) | Sprague Dawley rats | CR+S | ↓ depressive-like behavior | in the HIPP: ↓ COX-2 ↑ CB2R ↑ BDNF | [102] |
| Organotypic hippocampal slices | LPS (1 µg/mL) | ↓ LTD | |||
| BCP (chronic; 30 mg/kg; p.o.) | Wistar rats | HFFD | ↓ Anxiety ↓ Depressive-like behavior ↓ Memory deficits | ↓ Insulin resistance ↓ Oxidative stress ↓ Neuroinflammation | [90] |
| BCP (200 mg/kg) | Swiss mice | Acute stress | ↓ Anxiety | [103] | |
| BCP (acute; 10, 25, 50 mg/kg; i.p.) | Swiss mice | Acute stress | ↓ Anxiety | [104] | |
| BCP (acute; 50, 100, 200 mg/kg; p.o.) | Swiss mice | Acute stress | ↓ Anxiety | [105] | |
| PEO (chronic; 50, 100, 200 mg/kg; p.o.) | ICR mice | Restraint stress | ↓ Anxiety | [107] | |
| ViphyllinTM (chronic; 50, 100 mg/kg, p.o.) | Mice | DSS-induced anxiety | ↓ Anxiety | [108] | |
| BCP (chronic; 0.02, 0.2, 2, 4%; p.o. in dosing beaker) | Zebrafish | Acute stress | ↓ Immobility at the highest dose used (i.e., 4%)
| [109] | |
| BCP (chronic; 10 mg/kg; p.o.) | BALB/c mice | STZ-induced diabetes | ↓ Depressive-like behavior | ↓ IL-1β ↓ TNFα ↓ IL-6 | [110] |
3.4. BCP May Be Prospective in COVID-19-Associated Psychiatric Disorders
3.5. Safety and Toxicity of BCP
3.6. Dosage Forms and Pharmaceutical Development of BCP
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Whiteford, H.A.; Degenhardt, L.; Rehm, J.; Baxter, A.J.; Ferrari, A.J.; Erskine, H.E.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Johns, N.; et al. Global Burden of Disease Attributable to Mental and Substance Use Disorders: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 382, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Marazziti, D.; Avella, M.T.; Mucci, N.; Della Vecchia, A.; Ivaldi, T.; Palermo, S.; Mucci, F. Impact of Economic Crisis on Mental Health: A 10-Year Challenge. CNS Spectr. 2021, 26, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, K.N.; Karakatsoulis, G.; Abraham, S.; Adorjan, K.; Ahmed, H.U.; Alarcón, R.D.; Arai, K.; Auwal, S.S.; Berk, M.; Bjedov, S.; et al. Results of the COVID-19 Mental Health International for the General Population (COMET-G) Study. Eur. Neuropsychopharmacol. 2022, 54, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.A.K.; Mitra, A.K.; Bhuiyan, A.R. Impact of COVID-19 on Mental Health in Adolescents: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 2470. [Google Scholar] [CrossRef] [PubMed]
- Alvi, T.; Kumar, D.; Tabak, B.A. Social Anxiety and Behavioral Assessments of Social Cognition: A Systematic Review. J. Affect. Disord. 2022, 311, 17–30. [Google Scholar] [CrossRef]
- Goldsmith, D.R.; Bekhbat, M.; Mehta, N.D.; Felger, J.C. Inflammation-Related Functional and Structural Dysconnectivity as a Pathway to Psychopathology. Biol. Psychiatry 2023, 93, 405–418. [Google Scholar] [CrossRef]
- Viveros, M.P.; Marco, E.M.; File, S.E. Endocannabinoid System and Stress and Anxiety Responses. Pharmacol. Biochem. Behav. 2005, 81, 331–342. [Google Scholar] [CrossRef]
- Riebe, C.J.; Pamplona, F.A.; Kamprath, K.; Wotjak, C.T. Fear Relief-toward a New Conceptual Frame Work and What Endocannabinoids Gotta Do with It. Neuroscience 2012, 204, 159–185. [Google Scholar] [CrossRef]
- Ruehle, S.; Rey, A.A.; Remmers, F.; Lutz, B. The Endocannabinoid System in Anxiety, Fear Memory and Habituation. J. Psychopharmacol. 2012, 26, 23–39. [Google Scholar] [CrossRef]
- Bluett, R.J.; Gamble-George, J.C.; Hermanson, D.J.; Hartley, N.D.; Marnett, L.J.; Patel, S. Central Anandamide Deficiency Predicts Stress-Induced Anxiety: Behavioral Reversal through Endocannabinoid Augmentation. Transl. Psychiatry 2014, 4, e408. [Google Scholar] [CrossRef]
- Gunduz-Cinar, O.; MacPherson, K.P.; Cinar, R.; Gamble-George, J.; Sugden, K.; Williams, B.; Godlewski, G.; Ramikie, T.S.; Gorka, A.X.; Alapafuja, S.O.; et al. Convergent Translational Evidence of a Role for Anandamide in Amygdala-Mediated Fear Extinction, Threat Processing and Stress-Reactivity. Mol. Psychiatry 2013, 18, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.M.; Vecchiarelli, H.A.; Morena, M.; Lee, T.T.Y.; Hermanson, D.J.; Kim, A.B.; McLaughlin, R.J.; Hassan, K.I.; Kühne, C.; Wotjak, C.T.; et al. Corticotropin-Releasing Hormone Drives Anandamide Hydrolysis in the Amygdala to Promote Anxiety. J. Neurosci. 2015, 35, 3879–3892. [Google Scholar] [CrossRef] [PubMed]
- Akirav, I. The Role of Cannabinoids in Modulating Emotional and Non-Emotional Memory Processes in the Hippocampus. Front. Behav. Neurosci. 2011, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Tadijan, A.; Vlašić, I.; Vlainić, J.; Đikić, D.; Oršolić, N.; Jazvinšćak Jembrek, M. Intracellular Molecular Targets and Signaling Pathways Involved in Antioxidative and Neuroprotective Effects of Cannabinoids in Neurodegenerative Conditions. Antioxidants 2022, 11, 2049. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Gao, M.; Gao, F.; Su, Q.; Wu, J. Brain Cannabinoid Receptor 2: Expression, Function and Modulation. Acta Pharmacol. Sin. 2017, 38, 312–316. [Google Scholar] [CrossRef]
- Joshi, N.; Onaivi, E.S. Endocannabinoid System Components: Overview and Tissue Distribution. Adv. Exp. Med. Biol. 2019, 1162, 1–12. [Google Scholar] [CrossRef]
- Morcuende, A.; García-Gutiérrez, M.S.; Tambaro, S.; Nieto, E.; Manzanares, J.; Femenia, T. Immunomodulatory Role of CB2 Receptors in Emotional and Cognitive Disorders. Front. Psychiatry 2022, 13, 866052. [Google Scholar] [CrossRef]
- Hashiesh, H.M.; Sharma, C.; Goyal, S.N.; Sadek, B.; Jha, N.K.; Kaabi, J.A.; Ojha, S. A Focused Review on CB2 Receptor-Selective Pharmacological Properties and Therapeutic Potential of β-Caryophyllene, a Dietary Cannabinoid. Biomed. Pharmacother. 2021, 140, 111639. [Google Scholar] [CrossRef]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Gallo, M.P.; Maffei, M.E.; Bovolin, P. Protective Effects of (E)-β-Caryophyllene (BCP) in Chronic Inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef]
- Ferber, S.G.; Namdar, D.; Hen-Shoval, D.; Eger, G.; Koltai, H.; Shoval, G.; Shbiro, L.; Weller, A. The “Entourage Effect”: Terpenes Coupled with Cannabinoids for the Treatment of Mood Disorders and Anxiety Disorders. Curr. Neuropharmacol. 2020, 18, 87–96. [Google Scholar] [CrossRef]
- Rathod, S.S.; Agrawal, Y.O.; Nakhate, K.T.; Meeran, M.F.N.; Ojha, S.; Goyal, S.N. Neuroinflammation in the Central Nervous System: Exploring the Evolving Influence of Endocannabinoid System. Biomedicines 2023, 11, 2642. [Google Scholar] [CrossRef] [PubMed]
- Benito, C.; Tolón, R.M.; Pazos, M.R.; Núñez, E.; Castillo, A.I.; Romero, J. Cannabinoid CB2 Receptors in Human Brain Inflammation. Br. J. Pharmacol. 2008, 153, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Hernangómez, M.; Mestre, L.; Correa, F.G.; Loría, F.; Mecha, M.; Iñigo, P.M.; Docagne, F.; Williams, R.O.; Borrell, J.; Guaza, C. CD200-CD200R1 Interaction Contributes to Neuroprotective Effects of Anandamide on Experimentally Induced Inflammation. Glia 2012, 60, 1437–1450. [Google Scholar] [CrossRef]
- Bauer, M.E.; Teixeira, A.L. Inflammation in Psychiatric Disorders: What Comes First? Ann. N. Y. Acad. Sci. 2019, 1437, 57–67. [Google Scholar] [CrossRef]
- Rom, S.; Persidsky, Y. Cannabinoid Receptor 2: Potential Role in Immunomodulation and Neuroinflammation. J. Neuroimmune Pharmacol. 2013, 8, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Mou, X.; Huang, J.; Xiong, N.; Li, H. Trans-Caryophyllene Suppresses Hypoxia-Induced Neuroinflammatory Responses by Inhibiting NF-κB Activation in Microglia. J. Mol. Neurosci. 2014, 54, 41–48. [Google Scholar] [CrossRef]
- Sheng, W.S.; Hu, S.; Min, X.; Cabral, G.A.; Lokensgard, J.R.; Peterson, P.K. Synthetic Cannabinoid WIN55,212-2 Inhibits Generation of Inflammatory Mediators by IL-1beta-Stimulated Human Astrocytes. Glia 2005, 49, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Deng, H.; Qin, Q.; Ma, Z. JWH133 Inhibits MPP+-Induced Inflammatory Response and Iron Influx in Astrocytes. Neurosci. Lett. 2020, 720, 134779. [Google Scholar] [CrossRef]
- Alkaitis, M.S.; Solorzano, C.; Landry, R.P.; Piomelli, D.; DeLeo, J.A.; Romero-Sandoval, E.A. Evidence for a Role of Endocannabinoids, Astrocytes and P38 Phosphorylation in the Resolution of Postoperative Pain. PLoS ONE 2010, 5, e10891. [Google Scholar] [CrossRef]
- Sheng, W.S.; Hu, S.; Ni, H.T.; Rock, R.B.; Peterson, P.K. WIN55,212-2 Inhibits Production of CX3CL1 by Human Astrocytes: Involvement of P38 MAP Kinase. J. Neuroimmune Pharmacol. 2009, 4, 244–248. [Google Scholar] [CrossRef]
- Jing, N.; Fang, B.; Li, Z.; Tian, A. Exogenous Activation of Cannabinoid-2 Receptor Modulates TLR4/MMP9 Expression in a Spinal Cord Ischemia Reperfusion Rat Model. J. Neuroinflamm. 2020, 17, 101. [Google Scholar] [CrossRef]
- Marchalant, Y.; Brothers, H.M.; Norman, G.J.; Karelina, K.; DeVries, A.C.; Wenk, G.L. Cannabinoids Attenuate the Effects of Aging upon Neuroinflammation and Neurogenesis. Neurobiol. Dis. 2009, 34, 300–307. [Google Scholar] [CrossRef]
- Palazuelos, J.; Ortega, Z.; Díaz-Alonso, J.; Guzmán, M.; Galve-Roperh, I. CB2 Cannabinoid Receptors Promote Neural Progenitor Cell Proliferation via mTORC1 Signaling. J. Biol. Chem. 2012, 287, 1198–1209. [Google Scholar] [CrossRef]
- Ishiguro, H.; Kibret, B.G.; Horiuchi, Y.; Onaivi, E.S. Potential Role of Cannabinoid Type 2 Receptors in Neuropsychiatric and Neurodegenerative Disorders. Front. Psychiatry 2022, 13, 828895. [Google Scholar] [CrossRef]
- Marco, E.M.; Echeverry-Alzate, V.; López-Moreno, J.A.; Giné, E.; Peñasco, S.; Viveros, M.P. Consequences of Early Life Stress on the Expression of Endocannabinoid-Related Genes in the Rat Brain. Behav. Pharmacol. 2014, 25, 547–556. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.-P.; Patel, S.; Meozzi, P.A.; Myers, L.; Perchuk, A.; Mora, Z.; Tagliaferro, P.A.; Gardner, E.; et al. Functional Expression of Brain Neuronal CB2 Cannabinoid Receptors Are Involved in the Effects of Drugs of Abuse and in Depression. Ann. N. Y. Acad. Sci. 2008, 1139, 434–449. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; Navarrete, F.; Navarro, G.; Reyes-Resina, I.; Franco, R.; Lanciego, J.L.; Giner, S.; Manzanares, J. Alterations in Gene and Protein Expression of Cannabinoid CB2 and GPR55 Receptors in the Dorsolateral Prefrontal Cortex of Suicide Victims. Neurotherapeutics 2018, 15, 796–806. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.-P.; Patel, S.; Meozzi, P.A.; Myers, L.; Perchuk, A.; Mora, Z.; Tagliaferro, P.A.; Gardner, E.; et al. Brain Neuronal CB2 Cannabinoid Receptors in Drug Abuse and Depression: From Mice to Human Subjects. PLoS ONE 2008, 3, e1640. [Google Scholar] [CrossRef]
- Ortega-Alvaro, A.; Aracil-Fernández, A.; García-Gutiérrez, M.S.; Navarrete, F.; Manzanares, J. Deletion of CB2 Cannabinoid Receptor Induces Schizophrenia-Related Behaviors in Mice. Neuropsychopharmacology 2011, 36, 1489–1504. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The Role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Roohi, E.; Jaafari, N.; Hashemian, F. On Inflammatory Hypothesis of Depression: What Is the Role of IL-6 in the Middle of the Chaos? J. Neuroinflamm. 2021, 18, 45. [Google Scholar] [CrossRef]
- Wu, Z.; Xiao, L.; Wang, H.; Wang, G. Neurogenic Hypothesis of Positive Psychology in Stress-Induced Depression: Adult Hippocampal Neurogenesis, Neuroinflammation, and Stress Resilience. Int. Immunopharmacol. 2021, 97, 107653. [Google Scholar] [CrossRef]
- Ming, Z.; Sawicki, G.; Bekar, L.K. Acute Systemic LPS-Mediated Inflammation Induces Lasting Changes in Mouse Cortical Neuromodulation and Behavior. Neurosci. Lett. 2015, 590, 96–100. [Google Scholar] [CrossRef]
- Casaril, A.M.; Domingues, M.; Fronza, M.; Vieira, B.; Begnini, K.; Lenardão, E.J.; Seixas, F.K.; Collares, T.; Nogueira, C.W.; Savegnago, L. Antidepressant-like Effect of a New Selenium-Containing Compound Is Accompanied by a Reduction of Neuroinflammation and Oxidative Stress in Lipopolysaccharide-Challenged Mice. J. Psychopharmacol. 2017, 31, 1263–1273. [Google Scholar] [CrossRef]
- O’Connor, J.C.; Lawson, M.A.; André, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-Induced Depressive-like Behavior Is Mediated by Indoleamine 2,3-Dioxygenase Activation in Mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef]
- Carlisle, S.J.; Marciano-Cabral, F.; Staab, A.; Ludwick, C.; Cabral, G.A. Differential Expression of the CB2 Cannabinoid Receptor by Rodent Macrophages and Macrophage-like Cells in Relation to Cell Activation. Int. Immunopharmacol. 2002, 2, 69–82. [Google Scholar] [CrossRef]
- Lataliza, A.A.B.; de Assis, P.M.; da Rocha Laurindo, L.; Gonçalves, E.C.D.; Raposo, N.R.B.; Dutra, R.C. Antidepressant-like Effect of Rosmarinic Acid during LPS-Induced Neuroinflammatory Model: The Potential Role of Cannabinoid Receptors/PPAR-γ Signaling Pathway. Phytother. Res. 2021, 35, 6974–6989. [Google Scholar] [CrossRef]
- Sahu, P.; Mudgal, J.; Arora, D.; Kinra, M.; Mallik, S.B.; Rao, C.M.; Pai, K.S.R.; Nampoothiri, M. Cannabinoid Receptor 2 Activation Mitigates Lipopolysaccharide-Induced Neuroinflammation and Sickness Behavior in Mice. Psychopharmacology 2019, 236, 1829–1838. [Google Scholar] [CrossRef]
- Roche, M.; Diamond, M.; Kelly, J.P.; Finn, D.P. In Vivo Modulation of LPS-Induced Alterations in Brain and Peripheral Cytokines and HPA Axis Activity by Cannabinoids. J. Neuroimmunol. 2006, 181, 57–67. [Google Scholar] [CrossRef]
- Zoppi, S.; Madrigal, J.L.; Caso, J.R.; García-Gutiérrez, M.S.; Manzanares, J.; Leza, J.C.; García-Bueno, B. Regulatory Role of the Cannabinoid CB2 Receptor in Stress-Induced Neuroinflammation in Mice. Br. J. Pharmacol. 2014, 171, 2814–2826. [Google Scholar] [CrossRef]
- Chakrapani, S.; Eskander, N.; De Los Santos, L.A.; Omisore, B.A.; Mostafa, J.A. Neuroplasticity and the Biological Role of Brain Derived Neurotrophic Factor in the Pathophysiology and Management of Depression. Cureus 2020, 12, e11396. [Google Scholar] [CrossRef]
- Porter, G.A.; O’Connor, J.C. Brain-Derived Neurotrophic Factor and Inflammation in Depression: Pathogenic Partners in Crime? World J. Psychiatry 2022, 12, 77–97. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; Ortega-Álvaro, A.; Busquets-García, A.; Pérez-Ortiz, J.M.; Caltana, L.; Ricatti, M.J.; Brusco, A.; Maldonado, R.; Manzanares, J. Synaptic Plasticity Alterations Associated with Memory Impairment Induced by Deletion of CB2 Cannabinoid Receptors. Neuropharmacology 2013, 73, 388–396. [Google Scholar] [CrossRef]
- Tang, J.; Miao, H.; Jiang, B.; Chen, Q.; Tan, L.; Tao, Y.; Zhang, J.; Gao, F.; Feng, H.; Zhu, G.; et al. A Selective CB2R Agonist (JWH133) Restores Neuronal Circuit after Germinal Matrix Hemorrhage in the Preterm via CX3CR1+ Microglia. Neuropharmacology 2017, 119, 157–169. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; Pérez-Ortiz, J.M.; Gutiérrez-Adán, A.; Manzanares, J. Depression-Resistant Endophenotype in Mice Overexpressing Cannabinoid CB2 Receptors. Br. J. Pharmacol. 2010, 160, 1773–1784. [Google Scholar] [CrossRef]
- Llorente-Berzal, A.; Assis, M.A.; Rubino, T.; Zamberletti, E.; Marco, E.M.; Parolaro, D.; Ambrosio, E.; Viveros, M.-P. Sex-Dependent Changes in Brain CB1R Expression and Functionality and Immune CB2R Expression as a Consequence of Maternal Deprivation and Adolescent Cocaine Exposure. Pharmacol. Res. 2013, 74, 23–33. [Google Scholar] [CrossRef]
- Robertson, J.M.; Achua, J.K.; Smith, J.P.; Prince, M.A.; Staton, C.D.; Ronan, P.J.; Summers, T.R.; Summers, C.H. Anxious Behavior Induces Elevated Hippocampal Cb2 Receptor Gene Expression. Neuroscience 2017, 352, 273–284. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; Manzanares, J. Overexpression of CB2 Cannabinoid Receptors Decreased Vulnerability to Anxiety and Impaired Anxiolytic Action of Alprazolam in Mice. J. Psychopharmacol. 2011, 25, 111–120. [Google Scholar] [CrossRef]
- Bahi, A.; Al Mansouri, S.; Al Memari, E.; Al Ameri, M.; Nurulain, S.M.; Ojha, S. β-Caryophyllene, a CB2 Receptor Agonist Produces Multiple Behavioral Changes Relevant to Anxiety and Depression in Mice. Physiol. Behav. 2014, 135, 119–124. [Google Scholar] [CrossRef]
- Ivy, D.; Palese, F.; Vozella, V.; Fotio, Y.; Yalcin, A.; Ramirez, G.; Mears, D.; Wynn, G.; Piomelli, D. Cannabinoid CB2 Receptors Mediate the Anxiolytic-like Effects of Monoacylglycerol Lipase Inhibition in a Rat Model of Predator-Induced Fear. Neuropsychopharmacology 2020, 45, 1330–1338. [Google Scholar] [CrossRef]
- Papagianni, E.P.; Stevenson, C.W. Cannabinoid Regulation of Fear and Anxiety: An Update. Curr. Psychiatry Rep. 2019, 21, 38. [Google Scholar] [CrossRef]
- Marazziti, D.; Carmassi, C.; Cappellato, G.; Chiarantini, I.; Massoni, L.; Mucci, F.; Arone, A.; Violi, M.; Palermo, S.; De Iorio, G.; et al. Novel Pharmacological Targets of Post-Traumatic Stress Disorders. Life 2023, 13, 1731. [Google Scholar] [CrossRef]
- Howlett, A.C.; Abood, M.E. CB1 and CB2 Receptor Pharmacology. Adv. Pharmacol. 2017, 80, 169–206. [Google Scholar] [CrossRef]
- Tahamtan, A.; Samieipoor, Y.; Nayeri, F.S.; Rahbarimanesh, A.A.; Izadi, A.; Rashidi-Nezhad, A.; Tavakoli-Yaraki, M.; Farahmand, M.; Bont, L.; Shokri, F.; et al. Effects of Cannabinoid Receptor Type 2 in Respiratory Syncytial Virus Infection in Human Subjects and Mice. Virulence 2018, 9, 217–230. [Google Scholar] [CrossRef]
- Costantino, C.M.; Gupta, A.; Yewdall, A.W.; Dale, B.M.; Devi, L.A.; Chen, B.K. Cannabinoid Receptor 2-Mediated Attenuation of CXCR4-Tropic HIV Infection in Primary CD4+ T Cells. PLoS ONE 2012, 7, e33961. [Google Scholar] [CrossRef]
- Ramirez, S.H.; Reichenbach, N.L.; Fan, S.; Rom, S.; Merkel, S.F.; Wang, X.; Ho, W.-Z.; Persidsky, Y. Attenuation of HIV-1 Replication in Macrophages by Cannabinoid Receptor 2 Agonists. J. Leukoc. Biol. 2013, 93, 801–810. [Google Scholar] [CrossRef]
- Askari, V.R.; Baradaran Rahimi, V.; Tabatabaee, S.A.; Shafiee-Nick, R. Combination of Imipramine, a Sphingomyelinase Inhibitor, and β-Caryophyllene Improve Their Therapeutic Effects on Experimental Autoimmune Encephalomyelitis (EAE). Int. Immunopharmacol. 2019, 77, 105923. [Google Scholar] [CrossRef]
- Alberti, T.B.; Barbosa, W.L.R.; Vieira, J.L.F.; Raposo, N.R.B.; Dutra, R.C. (-)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 691. [Google Scholar] [CrossRef]
- Carvalho, C.E.S.; Sobrinho-Junior, E.P.C.; Brito, L.M.; Nicolau, L.a.D.; Carvalho, T.P.; Moura, A.K.S.; Rodrigues, K.a.F.; Carneiro, S.M.P.; Arcanjo, D.D.R.; Citó, A.M.G.L.; et al. Anti-Leishmania Activity of Essential Oil of Myracrodruon Urundeuva (Engl.) Fr. All.: Composition, Cytotoxity and Possible Mechanisms of Action. Exp. Parasitol. 2017, 175, 59–67. [Google Scholar] [CrossRef]
- Ku, C.-M.; Lin, J.-Y. Anti-Inflammatory Effects of 27 Selected Terpenoid Compounds Tested through Modulating Th1/Th2 Cytokine Secretion Profiles Using Murine Primary Splenocytes. Food Chem. 2013, 141, 1104–1113. [Google Scholar] [CrossRef]
- Brito, L.F.; Oliveira, H.B.M.; das Neves Selis, N.; E Souza, C.L.S.; Júnior, M.N.S.; de Souza, E.P.; da Silva, L.S.C.; de Souza Nascimento, F.; Amorim, A.T.; Campos, G.B.; et al. Anti-Inflammatory Activity of β-Caryophyllene Combined with Docosahexaenoic Acid in a Model of Sepsis Induced by Staphylococcus Aureus in Mice. J. Sci. Food Agric. 2019, 99, 5870–5880. [Google Scholar] [CrossRef]
- Toguri, J.T.; Moxsom, R.; Szczesniak, A.M.; Zhou, J.; Kelly, M.E.M.; Lehmann, C. Cannabinoid 2 Receptor Activation Reduces Leukocyte Adhesion and Improves Capillary Perfusion in the Iridial Microvasculature during Systemic Inflammation. Clin. Hemorheol. Microcirc. 2015, 61, 237–249. [Google Scholar] [CrossRef]
- Sardinha, J.; Kelly, M.E.M.; Zhou, J.; Lehmann, C. Experimental Cannabinoid 2 Receptor-Mediated Immune Modulation in Sepsis. Mediat. Inflamm. 2014, 2014, 978678. [Google Scholar] [CrossRef]
- Zhang, Q.; An, R.; Tian, X.; Yang, M.; Li, M.; Lou, J.; Xu, L.; Dong, Z. β-Caryophyllene Pretreatment Alleviates Focal Cerebral Ischemia-Reperfusion Injury by Activating PI3K/Akt Signaling Pathway. Neurochem. Res. 2017, 42, 1459–1469. [Google Scholar] [CrossRef]
- Tian, X.; Liu, H.; Xiang, F.; Xu, L.; Dong, Z. β-Caryophyllene Protects against Ischemic Stroke by Promoting Polarization of Microglia toward M2 Phenotype via the TLR4 Pathway. Life Sci. 2019, 237, 116915. [Google Scholar] [CrossRef]
- Choi, I.-Y.; Ju, C.; Anthony Jalin, A.M.A.; Lee, D.I.; Prather, P.L.; Kim, W.-K. Activation of Cannabinoid CB2 Receptor-Mediated AMPK/CREB Pathway Reduces Cerebral Ischemic Injury. Am. J. Pathol. 2013, 182, 928–939. [Google Scholar] [CrossRef]
- Lou, J.; Cao, G.; Li, R.; Liu, J.; Dong, Z.; Xu, L. β-Caryophyllene Attenuates Focal Cerebral Ischemia-Reperfusion Injury by Nrf2/HO-1 Pathway in Rats. Neurochem. Res. 2016, 41, 1291–1304. [Google Scholar] [CrossRef]
- Wyganowska-Swiatkowska, M.; Nohawica, M.; Grocholewicz, K.; Nowak, G. Influence of Herbal Medicines on HMGB1 Release, SARS-CoV-2 Viral Attachment, Acute Respiratory Failure, and Sepsis. A Literature Review. Int. J. Mol. Sci. 2020, 21, 4639. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. An Update on PPAR Activation by Cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Levy, R.M. Metaxalone Suppresses Production of Inflammatory Cytokines Associated with Painful Conditions in Mouse Macrophages RAW264.7 Cells in Vitro: Synergistic Effect with β-Caryophyllene. Curr. Mol. Med. 2020, 20, 643–652. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-Caryophyllene Is a Dietary Cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef]
- Oliveira-Tintino, C.D.d.M.; Pessoa, R.T.; Fernandes, M.N.M.; Alcântara, I.S.; da Silva, B.A.F.; de Oliveira, M.R.C.; Martins, A.O.B.P.B.; da Silva, M.d.S.; Tintino, S.R.; Rodrigues, F.F.G.; et al. Anti-Inflammatory and Anti-Edematogenic Action of the Croton Campestris A. St.-Hil (Euphorbiaceae) Essential Oil and the Compound β-Caryophyllene in in Vivo Models. Phytomedicine 2018, 41, 82–95. [Google Scholar] [CrossRef]
- Klauke, A.-L.; Racz, I.; Pradier, B.; Markert, A.; Zimmer, A.M.; Gertsch, J.; Zimmer, A. The Cannabinoid CB2 Receptor-Selective Phytocannabinoid Beta-Caryophyllene Exerts Analgesic Effects in Mouse Models of Inflammatory and Neuropathic Pain. Eur. Neuropsychopharmacol. 2014, 24, 608–620. [Google Scholar] [CrossRef]
- Aly, E.; Khajah, M.A.; Masocha, W. β-Caryophyllene, a CB2-Receptor-Selective Phytocannabinoid, Suppresses Mechanical Allodynia in a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Molecules 2019, 25, 106. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Tang, S.; Lu, L.; Zhang, L.; Bu, X.; Li, H.; Hu, X.; Hu, X.; Jiang, P.; et al. Large-Scale Network Dysfunction in the Acute State Compared to the Remitted State of Bipolar Disorder: A Meta-Analysis of Resting-State Functional Connectivity. EBioMedicine 2020, 54, 102742. [Google Scholar] [CrossRef]
- Askari, V.R.; Baradaran Rahimi, V.; Shafiee-Nick, R. Low Doses of β-Caryophyllene Reduced Clinical and Paraclinical Parameters of an Autoimmune Animal Model of Multiple Sclerosis: Investigating the Role of CB2 Receptors in Inflammation by Lymphocytes and Microglial. Brain Sci. 2023, 13, 1092. [Google Scholar] [CrossRef]
- Lindsey, L.P.; Daphney, C.M.; Oppong-Damoah, A.; Uchakin, P.N.; Abney, S.E.; Uchakina, O.N.; Khusial, R.D.; Akil, A.; Murnane, K.S. The Cannabinoid Receptor 2 Agonist, β-Caryophyllene, Improves Working Memory and Reduces Circulating Levels of Specific Proinflammatory Cytokines in Aged Male Mice. Behav. Brain Res. 2019, 372, 112012. [Google Scholar] [CrossRef]
- Chávez-Hurtado, P.; González-Castañeda, R.E.; Beas-Zarate, C.; Flores-Soto, M.E.; Viveros-Paredes, J.M. β-Caryophyllene Reduces DNA Oxidation and the Overexpression of Glial Fibrillary Acidic Protein in the Prefrontal Cortex and Hippocampus of d-Galactose-Induced Aged BALB/c Mice. J. Med. Food 2020, 23, 515–522. [Google Scholar] [CrossRef]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-Caryophyllene Alleviates Diet-Induced Neurobehavioral Changes in Rats: The Role of CB2 and PPAR-γ Receptors. Biomed. Pharmacother. 2019, 110, 145–154. [Google Scholar] [CrossRef]
- Segat, G.C.; Manjavachi, M.N.; Matias, D.O.; Passos, G.F.; Freitas, C.S.; Costa, R.; Calixto, J.B. Antiallodynic Effect of β-Caryophyllene on Paclitaxel-Induced Peripheral Neuropathy in Mice. Neuropharmacology 2017, 125, 207–219. [Google Scholar] [CrossRef]
- Lou, J.; Teng, Z.; Zhang, L.; Yang, J.; Ma, L.; Wang, F.; Tian, X.; An, R.; Yang, M.; Zhang, Q.; et al. β-Caryophyllene/Hydroxypropyl-β-Cyclodextrin Inclusion Complex Improves Cognitive Deficits in Rats with Vascular Dementia through the Cannabinoid Receptor Type 2 -Mediated Pathway. Front. Pharmacol. 2017, 8, 2. [Google Scholar] [CrossRef]
- Ojha, S.; Javed, H.; Azimullah, S.; Haque, M.E. β-Caryophyllene, a Phytocannabinoid Attenuates Oxidative Stress, Neuroinflammation, Glial Activation, and Salvages Dopaminergic Neurons in a Rat Model of Parkinson Disease. Mol. Cell. Biochem. 2016, 418, 59–70. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, Z.; Liu, S. β-Caryophyllene Ameliorates the Alzheimer-Like Phenotype in APP/PS1 Mice through CB2 Receptor Activation and the PPARγ Pathway. Pharmacology 2014, 94, 1–12. [Google Scholar] [CrossRef]
- Poddighe, L.; Carta, G.; Serra, M.P.; Melis, T.; Boi, M.; Lisai, S.; Murru, E.; Muredda, L.; Collu, M.; Banni, S.; et al. Acute Administration of Beta-Caryophyllene Prevents Endocannabinoid System Activation during Transient Common Carotid Artery Occlusion and Reperfusion. Lipids Health Dis. 2018, 17, 23. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Haque, M.E.; Ojha, S.K. Cannabinoid Type 2 (CB2) Receptors Activation Protects against Oxidative Stress and Neuroinflammation Associated Dopaminergic Neurodegeneration in Rotenone Model of Parkinson’s Disease. Front. Neurosci. 2016, 10, 321. [Google Scholar] [CrossRef]
- Viveros-Paredes, J.M.; González-Castañeda, R.E.; Gertsch, J.; Chaparro-Huerta, V.; López-Roa, R.I.; Vázquez-Valls, E.; Beas-Zarate, C.; Camins-Espuny, A.; Flores-Soto, M.E. Neuroprotective Effects of β-Caryophyllene against Dopaminergic Neuron Injury in a Murine Model of Parkinson’s Disease Induced by MPTP. Pharmaceuticals 2017, 10, 60. [Google Scholar] [CrossRef]
- Wang, G.; Ma, W.; Du, J. β-Caryophyllene (BCP) Ameliorates MPP+ Induced Cytotoxicity. Biomed. Pharmacother. 2018, 103, 1086–1091. [Google Scholar] [CrossRef]
- Solas, M.; Francis, P.T.; Franco, R.; Ramirez, M.J. CB2 Receptor and Amyloid Pathology in Frontal Cortex of Alzheimer’s Disease Patients. Neurobiol. Aging 2013, 34, 805–808. [Google Scholar] [CrossRef]
- Carrier, E.J.; Patel, S.; Hillard, C.J. Endocannabinoids in Neuroimmunology and Stress. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 657–665. [Google Scholar] [CrossRef]
- Hillard, C.J. Role of Cannabinoids and Endocannabinoids in Cerebral Ischemia. Curr. Pharm. Des. 2008, 14, 2347–2361. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Kim, H.-B.; Lee, S.; Kim, M.-J.; Kim, K.-J.; Han, G.; Han, S.-Y.; Lee, E.-A.; Yoon, J.-H.; Kim, D.-O.; et al. Antidepressant-like Effects of β-Caryophyllene on Restraint plus Stress-Induced Depression. Behav. Brain Res. 2020, 380, 112439. [Google Scholar] [CrossRef] [PubMed]
- da Silva Oliveira, G.L.; da Silva, J.C.C.L.; Dos Santos C L da Silva, A.P.; Feitosa, C.M.; de Castro Almeida, F.R. Anticonvulsant, Anxiolytic and Antidepressant Properties of the β-Caryophyllene in Swiss Mice: Involvement of Benzodiazepine-GABAAergic, Serotonergic and Nitrergic Systems. Curr. Mol. Pharmacol. 2021, 14, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.D.C.; Paz, M.F.C.J.; Oliveira Santos, J.V.D.; Da Silva, F.C.C.; Tchekalarova, J.D.; Salehi, B.; Islam, M.T.; Setzer, W.N.; Sharifi-Rad, J.; De Castro E Sousa, J.M.; et al. Anxiety Therapeutic Interventions of β-Caryophyllene: A Laboratory-Based Study. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- Galdino, P.M.; Nascimento, M.V.M.; Florentino, I.F.; Lino, R.C.; Fajemiroye, J.O.; Chaibub, B.A.; de Paula, J.R.; de Lima, T.C.M.; Costa, E.A. The Anxiolytic-like Effect of an Essential Oil Derived from Spiranthera Odoratissima A. St. Hil. Leaves and Its Major Component, β-Caryophyllene, in Male Mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 38, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Mony, T.J.; Elahi, F.; Choi, J.W.; Park, S.J. Neuropharmacological Effects of Terpenoids on Preclinical Animal Models of Psychiatric Disorders: A Review. Antioxidants 2022, 11, 1834. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Xuan, H.-Z.; Shou, Q.-Y.; Zhan, Z.-G.; Lu, X.; Hu, F.-L. Therapeutic Effects of Propolis Essential Oil on Anxiety of Restraint-Stressed Mice. Hum. Exp. Toxicol. 2012, 31, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Sudeep, H.V.; Venkatakrishna, K.; Raj, A.; Reethi, B.; Shyamprasad, K. ViphyllinTM, a Standardized Extract from Black Pepper Seeds, Mitigates Intestinal Inflammation, Oxidative Stress, and Anxiety-like Behavior in DSS-Induced Colitis Mice. J. Food Biochem. 2022, 46, e14306. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Stewart, A.; El-Hakim, I.; Hamilton, T.J. Effects of Super-Class Cannabis Terpenes Beta-Caryophyllene and Alpha-Pinene on Zebrafish Behavioural Biomarkers. Sci. Rep. 2022, 12, 17250. [Google Scholar] [CrossRef]
- Aguilar-Ávila, D.S.; Flores-Soto, M.E.; Tapia-Vázquez, C.; Pastor-Zarandona, O.A.; López-Roa, R.I.; Viveros-Paredes, J.M. β-Caryophyllene, a Natural Sesquiterpene, Attenuates Neuropathic Pain and Depressive-Like Behavior in Experimental Diabetic Mice. J. Med. Food 2019, 22, 460–468. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Inflammation in COVID-19: From Pathogenesis to Treatment. Int. J. Clin. Exp. Pathol. 2021, 14, 831–844. [Google Scholar]
- Jha, N.K.; Sharma, C.; Hashiesh, H.M.; Arunachalam, S.; Meeran, M.N.; Javed, H.; Patil, C.R.; Goyal, S.N.; Ojha, S. β-Caryophyllene, A Natural Dietary CB2 Receptor Selective Cannabinoid Can Be a Candidate to Target the Trinity of Infection, Immunity, and Inflammation in COVID-19. Front. Pharmacol. 2021, 12, 590201. [Google Scholar] [CrossRef]
- Costiniuk, C.T.; Jenabian, M.-A. Acute Inflammation and Pathogenesis of SARS-CoV-2 Infection: Cannabidiol as a Potential Anti-Inflammatory Treatment? Cytokine Growth Factor. Rev. 2020, 53, 63–65. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Miranda, K.; Nagarkatti, M. Use of Cannabinoids to Treat Acute Respiratory Distress Syndrome and Cytokine Storm Associated with Coronavirus Disease-2019. Front. Pharmacol. 2020, 11, 589438. [Google Scholar] [CrossRef]
- Sexton, M. Cannabis in the Time of Coronavirus Disease 2019: The Yin and Yang of the Endocannabinoid System in Immunocompetence. J. Altern. Complement. Med. 2020, 26, 444–448. [Google Scholar] [CrossRef]
- Raj, V.; Park, J.G.; Cho, K.-H.; Choi, P.; Kim, T.; Ham, J.; Lee, J. Assessment of Antiviral Potencies of Cannabinoids against SARS-CoV-2 Using Computational and in Vitro Approaches. Int. J. Biol. Macromol. 2021, 168, 474–485. [Google Scholar] [CrossRef]
- Byrareddy, S.N.; Mohan, M. SARS-CoV2 Induced Respiratory Distress: Can Cannabinoids Be Added to Anti-Viral Therapies to Reduce Lung Inflammation? Brain Behav. Immun. 2020, 87, 120–121. [Google Scholar] [CrossRef]
- Esposito, G.; Pesce, M.; Seguella, L.; Sanseverino, W.; Lu, J.; Corpetti, C.; Sarnelli, G. The Potential of Cannabidiol in the COVID-19 Pandemic. Br. J. Pharmacol. 2020, 177, 4967–4970. [Google Scholar] [CrossRef]
- Iorio, R.; Celenza, G.; Petricca, S. Multi-Target Effects of ß-Caryophyllene and Carnosic Acid at the Crossroads of Mitochondrial Dysfunction and Neurodegeneration: From Oxidative Stress to Microglia-Mediated Neuroinflammation. Antioxidants 2022, 11, 1199. [Google Scholar] [CrossRef]
- Alvarez-González, I.; Madrigal-Bujaidar, E.; Castro-García, S. Antigenotoxic Capacity of Beta-Caryophyllene in Mouse, and Evaluation of Its Antioxidant and GST Induction Activities. J. Toxicol. Sci. 2014, 39, 849–859. [Google Scholar] [CrossRef]
- Di Sotto, A.; Mazzanti, G.; Carbone, F.; Hrelia, P.; Maffei, F. Inhibition by β-Caryophyllene of Ethyl Methanesulfonate-Induced Clastogenicity in Cultured Human Lymphocytes. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2010, 699, 23–28. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Mazzanti, G.; Di Sotto, A. Mutagenicity of Cigarette Butt Waste in the Bacterial Reverse Mutation Assay: The Protective Effects of β-Caryophyllene and β-Caryophyllene Oxide. Environ. Toxicol. 2016, 31, 1319–1328. [Google Scholar] [CrossRef]
- Ambrož, M.; Šmatová, M.; Šadibolová, M.; Pospíšilová, E.; Hadravská, P.; Kašparová, M.; Skarková, V.H.; Králová, V.; Skálová, L. Sesquiterpenes α-Humulene and β-Caryophyllene Oxide Enhance the Efficacy of 5-Fluorouracil and Oxaliplatin in Colon Cancer Cells. Acta Pharm. 2019, 69, 121–128. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.A.; Abd El-Alim, A.E.-A.F.; Galal, A.A.A.; El-Sayed, R.G.; El-Naseery, N.I. Anti-Arthritic Effect of β-Caryophyllene and Its Ameliorative Role on Methotrexate and/or Leflunomide-Induced Side Effects in Arthritic Rats. Life Sci. 2019, 233, 116750. [Google Scholar] [CrossRef]
- Shim, H.I.; Song, D.J.; Shin, C.M.; Yoon, H.; Park, Y.S.; Kim, N.; Lee, D.H. Inhibitory Effects of β-caryophyllene on Helicobacter pylori Infection: A Randomized Double-blind, Placebo-controlled Study. Korean J. Gastroenterol. 2019, 74, 199. [Google Scholar] [CrossRef]
- Semprini, R.; Martorana, A.; Ragonese, M.; Motta, C. Observational Clinical and Nerve Conduction Study on Effects of a Nutraceutical Combination on Painful Diabetic Distal Symmetric Sensory-Motor Neuropathy in Patients with Diabetes Type 1 and Type 2. Minerva Med. 2018, 109, 358–362. [Google Scholar] [CrossRef]
- Bahr, T.; Allred, K.; Martinez, D.; Rodriguez, D.; Winterton, P. Effects of a Massage-like Essential Oil Application Procedure Using Copaiba and Deep Blue Oils in Individuals with Hand Arthritis. Complement. Ther. Clin. Pract. 2018, 33, 170–176. [Google Scholar] [CrossRef]
- Tarumi, W.; Shinohara, K. Olfactory Exposure to β-Caryophyllene Increases Testosterone Levels in Women’s Saliva. Sex. Med. 2020, 8, 525–531. [Google Scholar] [CrossRef]
- Santos, P.S.; Oliveira, T.C.; R Júnior, L.M.; Figueiras, A.; Nunes, L.C.C. β-Caryophyllene Delivery Systems: Enhancing the Oral Pharmacokinetic and Stability. Curr. Pharm. Des. 2018, 24, 3440–3453. [Google Scholar] [CrossRef]
- Alberti, T.B.; Coelho, D.S.; Maraschin, M. β-Caryophyllene Nanoparticles Design and Development: Controlled Drug Delivery of Cannabinoid CB2 Agonist as a Strategic Tool towards Neurodegeneration. Mater. Sci. Eng. C 2021, 121, 111824. [Google Scholar] [CrossRef]
- Mödinger, Y.; Knaub, K.; Dharsono, T.; Wacker, R.; Meyrat, R.; Land, M.H.; Petraglia, A.L.; Schön, C. Enhanced Oral Bioavailability of β-Caryophyllene in Healthy Subjects Using the VESIsorb® Formulation Technology, a Novel Self-Emulsifying Drug Delivery System (SEDDS). Molecules 2022, 27, 2860. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-X.; Artmann, C. Relative Bioavailability Comparison of Different Coenzyme Q10 Formulations with a Novel Delivery System. Altern. Ther. Health Med. 2009, 15, 42–46. [Google Scholar]
- Patra, K.C.; Singh, B.; Pareta, S.; Kumar, K.J. A Validated HPTLC Method for Determination of Trans-Caryophyllene from Polyherbal Formulations. Nat. Prod. Res. 2010, 24, 1933–1938. [Google Scholar] [CrossRef]
- Nieto-Bobadilla, M.S.; Siepmann, F.; Djouina, M.; Dubuquoy, L.; Tesse, N.; Willart, J.-F.; Dubreuil, L.; Siepmann, J.; Neut, C. Controlled Delivery of a New Broad Spectrum Antibacterial Agent against Colitis: In Vitro and in Vivo Performance. Eur. J. Pharm. Biopharm. 2015, 96, 152–161. [Google Scholar] [CrossRef]
- Geddo, F.; Scandiffio, R.; Antoniotti, S.; Cottone, E.; Querio, G.; Maffei, M.E.; Bovolin, P.; Gallo, M.P. PipeNig®-FL, a Fluid Extract of Black Pepper (Piper Nigrum L.) with a High Standardized Content of Trans-β-Caryophyllene, Reduces Lipid Accumulation in 3T3-L1 Preadipocytes and Improves Glucose Uptake in C2C12 Myotubes. Nutrients 2019, 11, 2788. [Google Scholar] [CrossRef]
- Elangovan, P.; Ramasamy, G.; Sundaram, M.; Ramasamy, M. Efficacy of Siddha Therapeutics on Mantha Sanni (Autism Spectrum Disorder) Among Pediatric Patients: An Interventional Non-Randomized Open-Label Clinical Trial. Cureus 2023, 15, e47128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricardi, C.; Barachini, S.; Consoli, G.; Marazziti, D.; Polini, B.; Chiellini, G. Beta-Caryophyllene, a Cannabinoid Receptor Type 2 Selective Agonist, in Emotional and Cognitive Disorders. Int. J. Mol. Sci. 2024, 25, 3203. https://doi.org/10.3390/ijms25063203
Ricardi C, Barachini S, Consoli G, Marazziti D, Polini B, Chiellini G. Beta-Caryophyllene, a Cannabinoid Receptor Type 2 Selective Agonist, in Emotional and Cognitive Disorders. International Journal of Molecular Sciences. 2024; 25(6):3203. https://doi.org/10.3390/ijms25063203
Chicago/Turabian StyleRicardi, Caterina, Serena Barachini, Giorgio Consoli, Donatella Marazziti, Beatrice Polini, and Grazia Chiellini. 2024. "Beta-Caryophyllene, a Cannabinoid Receptor Type 2 Selective Agonist, in Emotional and Cognitive Disorders" International Journal of Molecular Sciences 25, no. 6: 3203. https://doi.org/10.3390/ijms25063203
APA StyleRicardi, C., Barachini, S., Consoli, G., Marazziti, D., Polini, B., & Chiellini, G. (2024). Beta-Caryophyllene, a Cannabinoid Receptor Type 2 Selective Agonist, in Emotional and Cognitive Disorders. International Journal of Molecular Sciences, 25(6), 3203. https://doi.org/10.3390/ijms25063203








