The Differences in the Developmental Stages of the Cardiomyocytes and Endothelial Cells in Human and Mouse Embryos at the Single-Cell Level
Abstract
:1. Introduction
2. Results
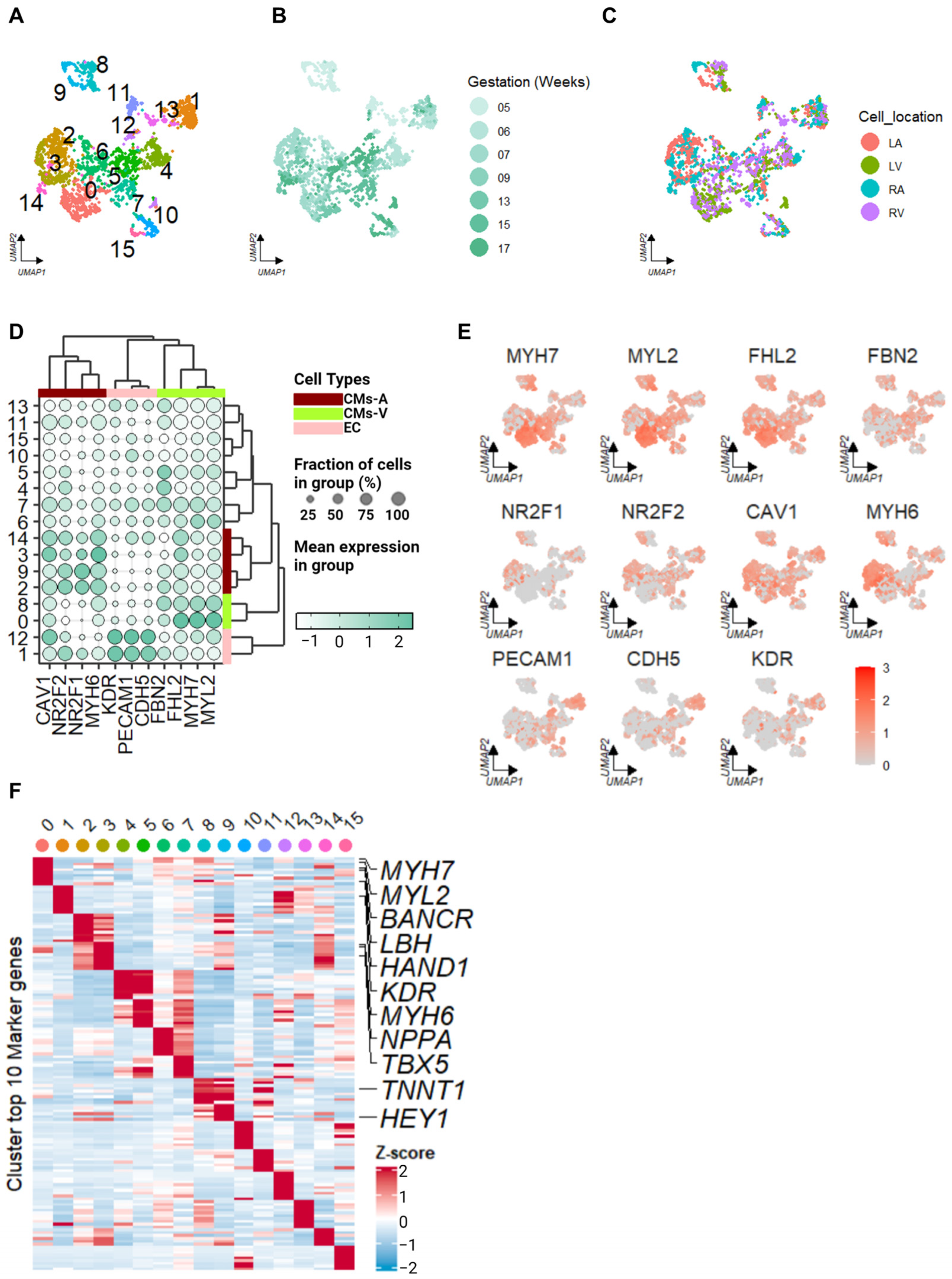
2.1. Identification of Cell Types in Human Embryonic Hearts
2.2. Identification of Cell Types in the Mouse Embryonic Cardiac Muscle Lineages and Mouse Fetal Heart
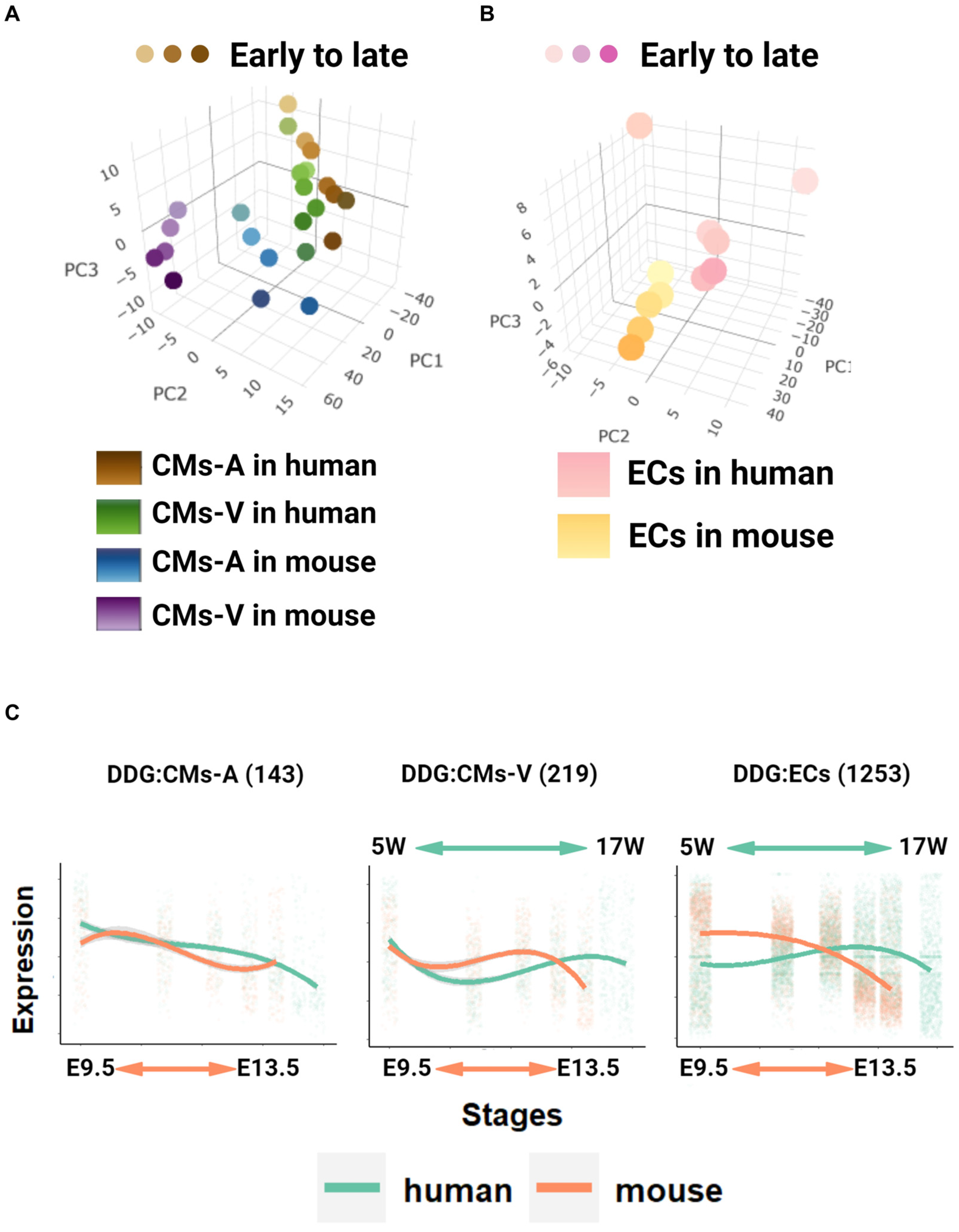
2.3. Global Transcriptome Analysis in Pseudo-Bulk and Identification of Developmentally Dynamic Genes (DDGs)
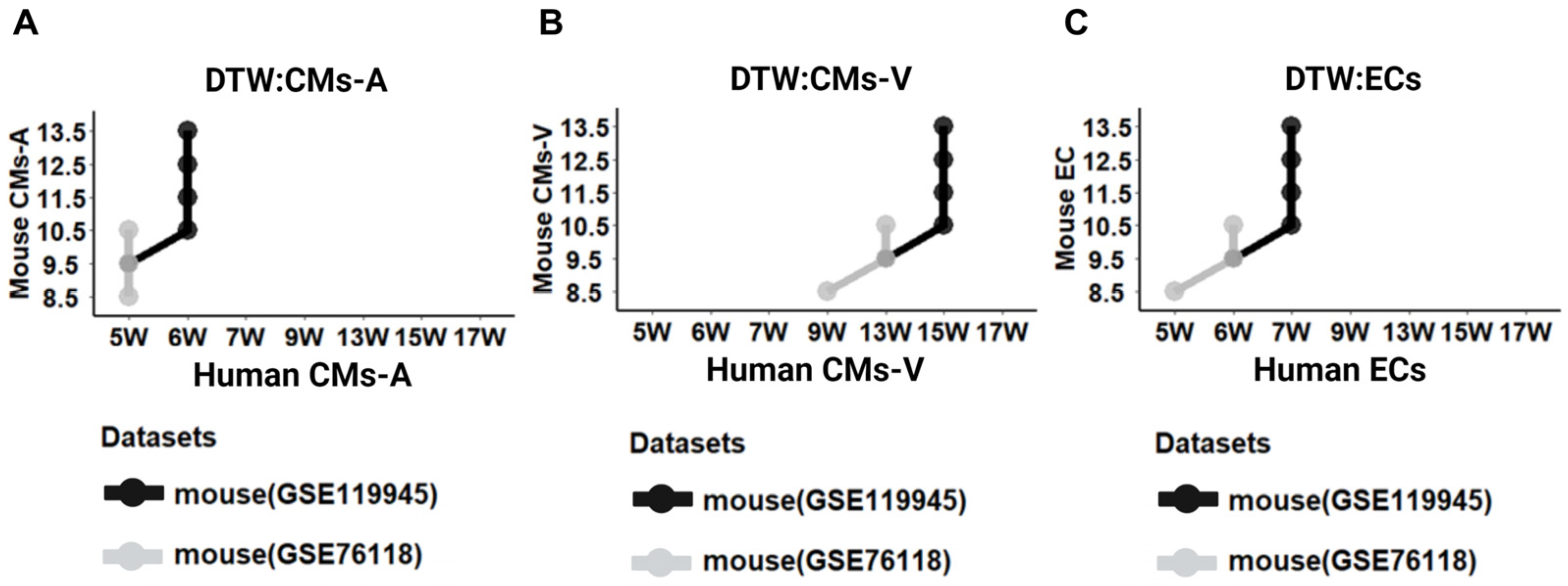
2.4. Stage Correspondence across Species with Dynamic Time Warping (DTW)
2.5. Differences in Transcription Factors of Different Species of Cardiomyocytes in Atria and Ventricles
2.6. Joint Analysis with the Chamber-like Cardiomyocytes Derived from Human Pluripotent Stem Cells (hPSCs)
3. Discussion
4. Materials and Methods
4.1. Identification of Cell Types
4.2. Construction of Pseudo-Bulk Matrix and Execution of PCA
4.3. Developmentally Dynamic Genes (DDGs)
4.4. Stage Correspondence across Species in Dynamic Time Warping (DTW)
4.5. The Transcription Factor (TF) in Differentially Expressed Genes (DEGs)
4.6. Combining Analysis between Prdm16cKO Mouse Heart and hPSC-Derived Cardiomyocytes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savoji, H.; Mohammadi, M.H.; Rafatian, N.; Toroghi, M.K.; Wang, E.Y.; Zhao, Y.; Korolj, A.; Ahadian, S.; Radisic, M. Cardiovascular Disease Models: A Game Changing Paradigm in Drug Discovery and Screening. Biomaterials 2019, 198, 3–26. [Google Scholar] [CrossRef]
- Dickinson, M.E.; Flenniken, A.M.; Ji, X.; Teboul, L.; Wong, M.D.; White, J.K.; Meehan, T.F.; Weninger, W.J.; Westerberg, H.; Adissu, H.; et al. High-Throughput Discovery of Novel Developmental Phenotypes. Nature 2016, 537, 508–514. [Google Scholar] [CrossRef]
- Brawand, D.; Soumillon, M.; Necsulea, A.; Julien, P.; Csárdi, G.; Harrigan, P.; Weier, M.; Liechti, A.; Aximu-Petri, A.; Kircher, M.; et al. The Evolution of Gene Expression Levels in Mammalian Organs. Nature 2011, 478, 343–348. [Google Scholar] [CrossRef]
- Cardoso-Moreira, M.; Halbert, J.; Valloton, D.; Velten, B.; Chen, C.; Shao, Y.; Liechti, A.; Ascenção, K.; Rummel, C.; Ovchinnikova, S.; et al. Gene Expression across Mammalian Organ Development. Nature 2019, 571, 505–509. [Google Scholar] [CrossRef]
- Krishnan, A.; Samtani, R.; Dhanantwari, P.; Lee, E.; Yamada, S.; Shiota, K.; Donofrio, M.T.; Leatherbury, L.; Lo, C.W. A Detailed Comparison of Mouse and Human Cardiac Development. Pediatr. Res. 2014, 76, 500–507. [Google Scholar] [CrossRef]
- Wessels, A.; Sedmera, D. Developmental Anatomy of the Heart: A Tale of Mice and Man. Physiol. Genom. 2003, 15, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Anzai, T.; Yamagata, T.; Uosaki, H. Comparative Transcriptome Landscape of Mouse and Human Hearts. Front. Cell Dev. Biol. 2020, 8, 268. [Google Scholar] [CrossRef]
- Gao, J.; Zheng, Y.; Li, L.; Lu, M.; Chen, X.; Wang, Y.; Li, Y.; Liu, X.; Gao, Y.; Mao, Y.; et al. Integrated transcriptomics and epigenomics reveal chamber-specific and species-specific characteristics of human and mouse hearts. PLoS Biol. 2021, 19, e3001229. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Hu, Y.; Cao, H.; Lin, Q.; Yi, N.; Zhang, J.; Gu, Y.; Yang, Y.; He, S.; Lu, M.; et al. Concordant and Heterogeneity of Single-Cell Transcriptome in Cardiac Development of Human and Mouse. Front. Genet. 2022, 13, 892766. [Google Scholar] [CrossRef]
- Basu, M.; Garg, V. Maternal Hyperglycemia and Fetal Cardiac Development: Clinical Impact and Underlying Mechanisms. Birth Defects Res. 2018, 110, 1504–1516. [Google Scholar] [CrossRef] [PubMed]
- Brade, T.; Pane, L.S.; Moretti, A.; Chien, K.R.; Laugwitz, K.-L. Embryonic Heart Progenitors and Cardiogenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a013847. [Google Scholar] [CrossRef]
- de Boer, B.A.; van den Berg, G.; de Boer, P.A.J.; Moorman, A.F.M.; Ruijter, J.M. Growth of the Developing Mouse Heart: An Interactive Qualitative and Quantitative 3D Atlas. Dev. Biol. 2012, 368, 203–213. [Google Scholar] [CrossRef]
- Savolainen, S.M.; Foley, J.F.; Elmore, S.A. Histology Atlas of the Developing Mouse Heart with Emphasis on E11.5 to E18.5. Toxicol. Pathol. 2009, 37, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the Adult Human Heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef]
- Zhou, P.; Pu, W.T. Recounting Cardiac Cellular Composition. Circ. Res. 2016, 118, 368–370. [Google Scholar] [CrossRef]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef]
- Liu, X.; Somel, M.; Tang, L.; Yan, Z.; Jiang, X.; Guo, S.; Yuan, Y.; He, L.; Oleksiak, A.; Zhang, Y.; et al. Extension of Cortical Synaptic Development Distinguishes Humans from Chimpanzees and Macaques. Genome Res. 2012, 22, 611–622. [Google Scholar] [CrossRef]
- Cui, Y.; Zheng, Y.; Liu, X.; Yan, L.; Fan, X.; Yong, J.; Hu, Y.; Dong, J.; Li, Q.; Wu, X.; et al. Single-Cell Transcriptome Analysis Maps the Developmental Track of the Human Heart. Cell Rep. 2019, 26, 1934–1950.e5. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, F.; Wang, L.; Li, Z.; Ren, Z.; Li, D.; Zhang, M.; Han, L.; Wang, S.; Zhou, B.; et al. Single-Cell Analysis of Murine Fibroblasts Identifies Neonatal to Adult Switching That Regulates Cardiomyocyte Maturation. Nat. Commun. 2020, 11, 2585. [Google Scholar] [CrossRef] [PubMed]
- Gawronski, K.A.B.; Kim, J. Single Cell Transcriptomics of Noncoding RNAs and Their Cell-Specificity. Wiley Interdiscip. Rev. RNA 2017, 8, e1433. [Google Scholar] [CrossRef] [PubMed]
- Paik, D.T.; Tian, L.; Williams, I.M.; Rhee, S.; Zhang, H.; Liu, C.; Mishra, R.; Wu, S.M.; Red-Horse, K.; Wu, J.C. Single-Cell RNA Sequencing Unveils Unique Transcriptomic Signatures of Organ-Specific Endothelial Cells. Circulation 2020, 142, 1848–1862. [Google Scholar] [CrossRef] [PubMed]
- Kanton, S.; Boyle, M.J.; He, Z.; Santel, M.; Weigert, A.; Sanchís-Calleja, F.; Guijarro, P.; Sidow, L.; Fleck, J.S.; Han, D.; et al. Organoid Single-Cell Genomic Atlas Uncovers Human-Specific Features of Brain Development. Nature 2019, 574, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Spielmann, M.; Qiu, X.; Huang, X.; Ibrahim, D.M.; Hill, A.J.; Zhang, F.; Mundlos, S.; Christiansen, L.; Steemers, F.J.; et al. The Single-Cell Transcriptional Landscape of Mammalian Organogenesis. Nature 2019, 566, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pu, W.T. Cardiomyocyte Maturation. Circ. Res. 2020, 126, 1086–1106. [Google Scholar] [CrossRef]
- Padula, S.L.; Velayutham, N.; Yutzey, K.E. Transcriptional Regulation of Postnatal Cardiomyocyte Maturation and Regeneration. Int. J. Mol. Sci. 2021, 22, 3288. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xu, A.; Sim, S.; Priest, J.R.; Tian, X.; Khan, T.; Quertermous, T.; Zhou, B.; Tsao, P.S.; Quake, S.R.; et al. Transcriptomic Profiling Maps Anatomically Patterned Subpopulations among Single Embryonic Cardiac Cells. Dev. Cell 2016, 39, 491–507. [Google Scholar] [CrossRef]
- Wilson, K.D.; Ameen, M.; Guo, H.; Abilez, O.J.; Tian, L.; Mumbach, M.R.; Diecke, S.; Qin, X.; Liu, Y.; Yang, H.; et al. Endogenous Retrovirus-Derived LncRNA BANCR Promotes Cardiomyocyte Migration in Humans and Non-Human Primates. Dev. Cell 2020, 54, 694–709.e9. [Google Scholar] [CrossRef]
- Cibi, D.M.; Bi-Lin, K.W.; Shekeran, S.G.; Sandireddy, R.; Tee, N.; Singh, A.; Wu, Y.; Srinivasan, D.K.; Kovalik, J.-P.; Ghosh, S.; et al. Prdm16 Deficiency Leads to Age-Dependent Cardiac Hypertrophy, Adverse Remodeling, Mitochondrial Dysfunction, and Heart Failure. Cell Rep. 2020, 33, 108288. [Google Scholar] [CrossRef]
- Wu, T.; Liang, Z.; Zhang, Z.; Liu, C.; Zhang, L.; Gu, Y.; Peterson, K.L.; Evans, S.M.; Fu, X.-D.; Chen, J. PRDM16 Is a Compact Myocardium-Enriched Transcription Factor Required to Maintain Compact Myocardial Cardiomyocyte Identity in Left Ventricle. Circulation 2022, 145, 586–602. [Google Scholar] [CrossRef]
- Kong, Y.; Shelton, J.M.; Rothermel, B.; Li, X.; Richardson, J.A.; Bassel-Duby, R.; Williams, R.S. Cardiac-Specific LIM Protein FHL2 Modifies the Hypertrophic Response to β-Adrenergic Stimulation. Circulation 2001, 103, 2731–2738. [Google Scholar] [CrossRef]
- Pantalacci, S.; Sémon, M. Transcriptomics of Developing Embryos and Organs: A Raising Tool for Evo–Devo. J. Exp. Zool. Part B Mol. Dev. Evol. 2015, 324, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.-M.; Jing, H. Cardiac Pathologies in Relation to Smad-Dependent Pathways. Interact. CardioVascular Thorac. Surg. 2010, 11, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Barak, Y.; Hemberger, M.; Sucov, H.M. Phases and Mechanisms of Embryonic Cardiomyocyte Proliferation and Ventricular Wall Morphogenesis. Pediatr. Cardiol. 2019, 40, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Manukjan, N.; Ahmed, Z.; Fulton, D.; Blankesteijn, W.M.; Foulquier, S. A Systematic Review of WNT Signaling in Endothelial Cell Oligodendrocyte Interactions: Potential Relevance to Cerebral Small Vessel Disease. Cells 2020, 9, 1545. [Google Scholar] [CrossRef] [PubMed]
- Arndt, A.-K.; Schafer, S.; Drenckhahn, J.-D.; Sabeh, M.K.; Plovie, E.R.; Caliebe, A.; Klopocki, E.; Musso, G.; Werdich, A.A.; Kalwa, H.; et al. Fine Mapping of the 1p36 Deletion Syndrome Identifies Mutation of PRDM16 as a Cause of Cardiomyopathy. Am. J. Hum. Genet. 2013, 93, 67–77. [Google Scholar] [CrossRef]
- Myasnikov, R.P.; Bukaeva, A.A.; Kulikova, O.V.; Ershova, A.I.; Petukhova, A.V.; Zotova, E.D.; Meshkov, A.N.; Mershina, E.A.; Kiseleva, A.V.; Divashuk, M.G.; et al. New variant of PRDM16 gene nucleotide sequence in a family with various phenotypic manifestations of the non-compacted myocardium. Russ. J. Cardiol. 2021, 26, 4315. [Google Scholar] [CrossRef]
- Yang, D.; Gomez-Garcia, J.; Funakoshi, S.; Tran, T.; Fernandes, I.; Bader, G.D.; Laflamme, M.A.; Keller, G.M. Modeling Human Multi-Lineage Heart Field Development with Pluripotent Stem Cells. Cell Stem Cell 2022, 29, 1382–1401.e8. [Google Scholar] [CrossRef]
- de Soysa, T.Y.; Ranade, S.S.; Okawa, S.; Ravichandran, S.; Huang, Y.; Salunga, H.T.; Schricker, A.; del Sol, A.; Gifford, C.A.; Srivastava, D. Single-Cell Analysis of Cardiogenesis Reveals Basis for Organ-Level Developmental Defects. Nature 2019, 572, 120–124. [Google Scholar] [CrossRef]
- Xu, X.Q.; Soo, S.Y.; Sun, W.; Zweigerdt, R. Global Expression Profile of Highly Enriched Cardiomyocytes Derived from Human Embryonic Stem Cells. Stem Cells 2009, 27, 2163–2174. [Google Scholar] [CrossRef]
- Jensen, B.; Agger, P.; de Boer, B.A.; Oostra, R.-J.; Pedersen, M.; van der Wal, A.C.; Nils Planken, R.; Moorman, A.F.M. The Hypertrabeculated (Noncompacted) Left Ventricle Is Different from the Ventricle of Embryos and Ectothermic Vertebrates. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 1696–1706. [Google Scholar] [CrossRef]
- Samsa, L.A.; Yang, B.; Liu, J. Embryonic Cardiac Chamber Maturation: Trabeculation, Conduction, and Cardiomyocyte Proliferation. Am. J. Med. Genet. Part C Semin. Med. Genet. 2013, 163, 157–168. [Google Scholar] [CrossRef]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M.; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M.; et al. Integrated Analysis of Multimodal Single-Cell Data. Cell 2021, 184, 3573–3587.e29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. scRNAtoolVis: Useful Functions to Make Your scRNA-seq Plot More Cool! 2022. Available online: https://github.com/junjunlab/scRNAtoolVis (accessed on 4 March 2023).
- Durinck, S.; Spellman, P.T.; Birney, E.; Huber, W. Mapping Identifiers for the Integration of Genomic Datasets with the R/Bioconductor Package BiomaRt. Nat. Protoc. 2009, 4, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Sievert, C. Interactive Web-Based Data Visualization with R, Plotly, and Shiny; Chapman and Hall/CRC: Boca Raton, FL, USA, 2020; ISBN 978-1-138-33145-7. [Google Scholar]
- Nueda, M.J.; Tarazona, S.; Conesa, A. Next MaSigPro: Updating MaSigPro Bioconductor Package for RNA-Seq Time Series. Bioinformatics 2014, 30, 2598–2602. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Shen, L.; Icahn School of Medicine at Mount Sinai. GeneOverlap: Test and Visualize Gene Overlaps. R Package Version 1.30.0. 2021. Available online: http://shenlab-sinai.github.io/shenlab-sinai/ (accessed on 4 March 2023).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Zhang, H.-M.; Liu, T.; Liu, C.-J.; Song, S.; Zhang, X.; Liu, W.; Jia, H.; Xue, Y.; Guo, A.-Y. AnimalTFDB 2.0: A Resource for Expression, Prediction and Functional Study of Animal Transcription Factors. Nucleic Acids Res. 2015, 43, D76–D81. [Google Scholar] [CrossRef]
- Jesse, H. Krijthe Rtsne: T-Distributed Stochastic Neighbor Embedding using a Barnes-Hut Implementation. 2015. Available online: https://github.com/jkrijthe/Rtsne (accessed on 4 March 2023).



| GEO Accessibility | First Author Name | Heart Region/Cell Type | Time Points |
|---|---|---|---|
| GSE106118 (human) | Yueli Cui | Whole heart | 5 weeks (5 W) to 25 W |
| GSE119945 (mouse) | Junyue Cao | Whole heart and other organs | E9.5 to E13.5 days |
| GSE76118 (mouse) | Guang Li | Whole heart | E8.5 to E10.5 days |
| GSE179393 (mouse) | Tongbin Wu | Whole heart (Prdm16cKO) | E13.5 days |
| GSE173486 (hPSCs) | Donghe Yang | hPSC-induced cardiomyocytes | Day 6, day 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Shao, N.-Y. The Differences in the Developmental Stages of the Cardiomyocytes and Endothelial Cells in Human and Mouse Embryos at the Single-Cell Level. Int. J. Mol. Sci. 2024, 25, 3240. https://doi.org/10.3390/ijms25063240
Liu C, Shao N-Y. The Differences in the Developmental Stages of the Cardiomyocytes and Endothelial Cells in Human and Mouse Embryos at the Single-Cell Level. International Journal of Molecular Sciences. 2024; 25(6):3240. https://doi.org/10.3390/ijms25063240
Chicago/Turabian StyleLiu, Chuyu, and Ning-Yi Shao. 2024. "The Differences in the Developmental Stages of the Cardiomyocytes and Endothelial Cells in Human and Mouse Embryos at the Single-Cell Level" International Journal of Molecular Sciences 25, no. 6: 3240. https://doi.org/10.3390/ijms25063240





