The Role of Extracellular Vesicles in Atopic Dermatitis
Abstract
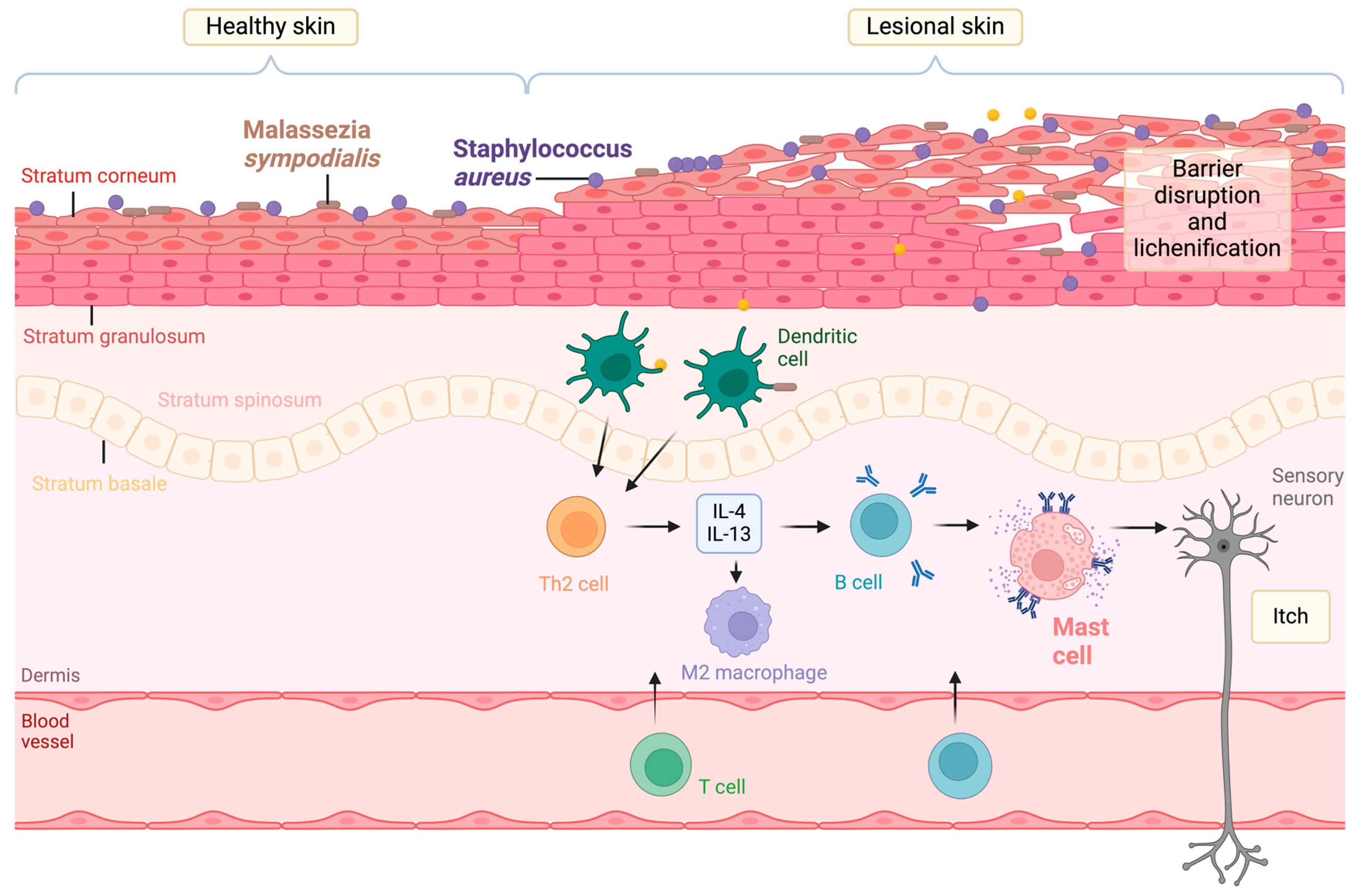
:1. Introduction
2. Staphylococcus aureus-Derived EVs in Atopic Dermatitis
2.1. The Secretion of EVs by Staphylococcus aureus
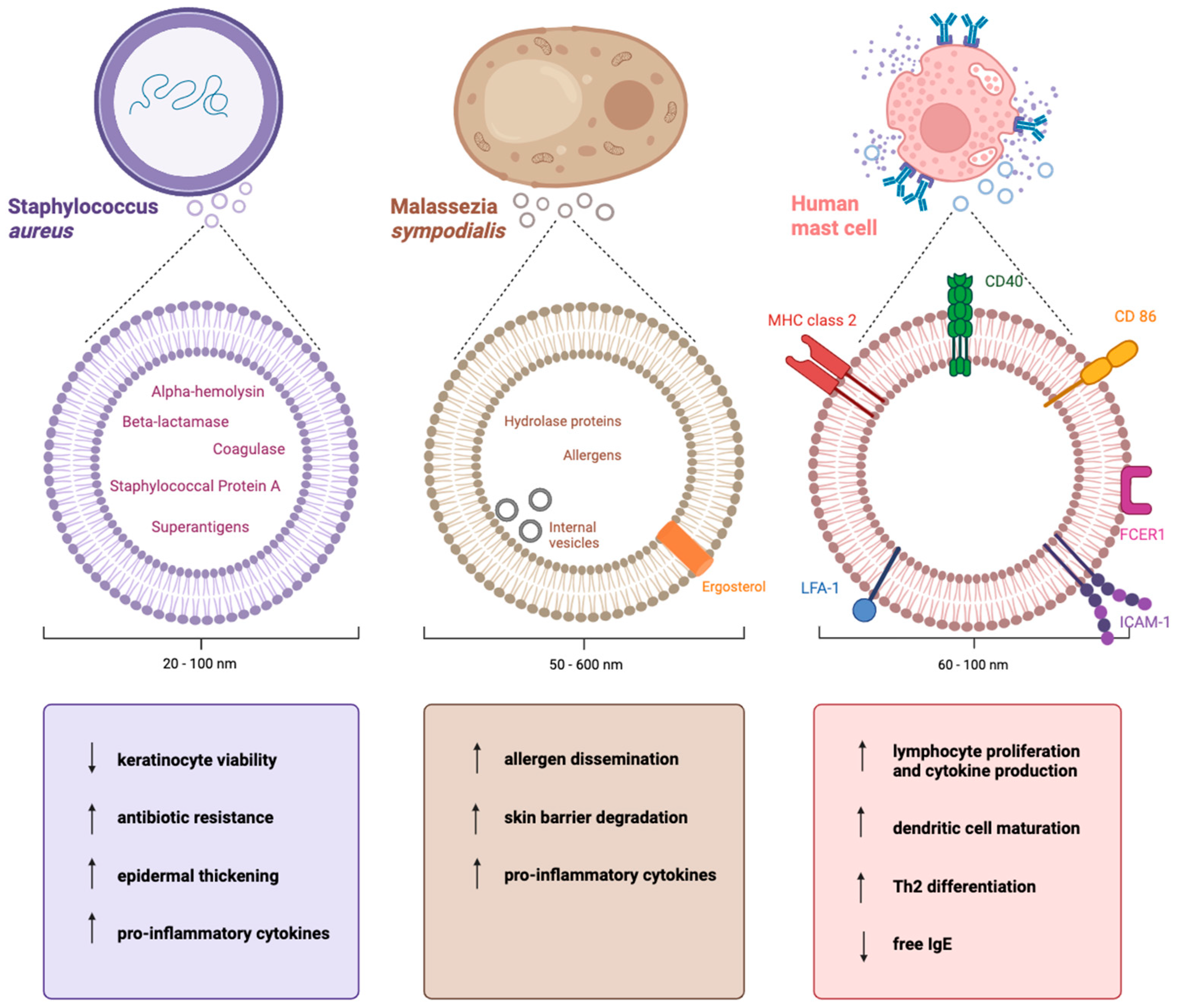
2.2. The Cargo of Staphylococcus aureus-Derived EVs
2.3. The Functions of Staphylococcus aureus EVs in Atopic Dermatitis
2.3.1. Antibiotic Resistance
2.3.2. Epidermal Disruption
2.3.3. Immune Response Modulation
2.3.4. Biofilm Formation
3. Malassezia sympodialis-Derived EVs in Atopic Dermatitis
3.1. The Secretion of Malassezia sympodialis-Derived EVs
3.2. The Cargo of Malassezia sympodialis-Derived EVs
3.3. The Functions of Malassezia sympodialis-Derived EVs in Atopic Dermatitis
3.3.1. Dissemination of Allergens
3.3.2. Skin Barrier Degradation
3.3.3. Immunomodulation
4. Host Mast Cell-Derived EVs in Atopic Dermatitis
4.1. The Secretion of Mast Cell-Derived EVs
4.2. The Cargo of Mast Cell-Derived EVs
4.3. The Functions of Mast Cell-Derived EVs
4.3.1. Lymphocyte Activation
4.3.2. Dendritic Cell Maturation and Activation
4.3.3. Reducing Free IgE
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology, 10th ed.; Elsevier: Philadelphia, PA, USA, 2021. [Google Scholar]
- Hanifin, J.M.; Rajka, G. Diagnostic Features of Atopic Dermatitis. Acta Derm. Venereol. 1980, 60, 44–47. [Google Scholar] [CrossRef]
- Silverberg, J.; Margolis, D.; Boguniewicz, M.; Fonacier, L.; Grayson, M.; Ong, P.; Fuxench, Z.C.; Simpson, E.; Gelfand, J. Distribution of atopic dermatitis lesions in United States adults. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Panther, D.J.; Jacob, S.E. The Importance of Acidification in Atopic Eczema: An Underexplored Avenue for Treatment. J. Clin. Med. 2015, 4, 970–978. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.M. Significance of skin barrier dysfunction in atopic dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 207–215. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Ahn, K.; Leung, D.Y.M. Interactions Between Atopic Dermatitis and Staphylococcus aureus Infection: Clinical Implications. Allergy Asthma Immunol. Res. 2019, 11, 593–603. [Google Scholar] [CrossRef]
- Rojahn, T.B.; Vorstandlechner, V.; Krausgruber, T.; Bauer, W.M.; Alkon, N.; Bangert, C.; Thaler, F.M.; Sadeghyar, F.; Fortelny, N.; Gernedl, V.; et al. Single-cell transcriptomics combined with interstitial fluid proteomics defines cell type-specific immune regulation in atopic dermatitis. J. Allergy Clin. Immunol. 2020, 146, 1056–1069. [Google Scholar] [CrossRef]
- Reynolds, G.; Vegh, P.; Fletcher, J.; Poyner, E.F.M.; Stephenson, E.; Goh, I.; Botting, R.A.; Huang, N.; Olabi, B.; Dubois, A.; et al. Developmental cell programs are co-opted in inflammatory skin disease. Science 2021, 371, eaba6500. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Andriantsitohaina, R.; Baharvand, H.; Bauer, N.N.; Baxter, A.A.; Beckham, C.; Bielska, E.; Boireau, W.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, 640. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhao, W.-L.; Ye, Y.-Y.; Bai, X.-C.; Liu, R.-Q.; Chang, L.-F.; Zhou, Q.; Sui, S.-F. Cellular Internalization of Exosomes Occurs Through Phagocytosis. Traffic 2010, 11, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Fitzner, D.; Schnaars, M.; van Rossum, D.; Krishnamoorthy, G.; Dibaj, P.; Bakhti, M.; Regen, T.; Hanisch, U.-K.; Simons, M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 2011, 124, 447–458. [Google Scholar] [CrossRef]
- Shao, S.; Fang, H.; Li, Q.; Wang, G. Extracellular vesicles in Inflammatory Skin Disorders: From Pathophysiology to Treatment. Theranostics 2020, 10, 9937–9955. [Google Scholar] [CrossRef]
- Chung, E.J.; Luo, C.-H.; Thio, C.L.-P.; Chang, Y.-J. Immunomodulatory Role of Staphylococcus aureus in Atopic Dermatitis. Pathogens 2022, 11, 422. [Google Scholar] [CrossRef]
- Rustad, A.M.; Nickles, M.A.; Lio, P.A. Atopic Dermatitis and Staphylococcus aureus: A Complex Relationship With Therapeutic Implications. J. Dermatol. Nurses’ Assoc. 2021, 13, 162–167. [Google Scholar] [CrossRef]
- Tauber, M.M.D.; Balica, S.M.D.; Hsu, C.-Y.P.; Jean-Decoster, C.P.; Lauze, C.M.; Redoules, D.P.; Viodé, C.P.; Schmitt, A.-M.M.D.P.; Serre, G.M.D.P.; Simon, M.P.; et al. Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 1272–1274.e1273. [Google Scholar] [CrossRef]
- Morishita, Y.; Tada, J.; Sato, A.; Toi, Y.; Kanzaki, H.; Akiyama, H.; Arata, J. Possible influences of Staphylococcus aureus on atopic dermatitis the colonizing features and the effects of staphylococcal enterotoxins. Clin. Exp. Allergy 1999, 29, 1110–1117. [Google Scholar] [CrossRef]
- Macias, E.S.M.D.; Pereira, F.A.M.D.; Rietkerk, W.M.D.M.B.A.; Safai, B.M.D. Superantigens in dermatology. J. Am. Acad. Dermatol. 2011, 64, 455–472. [Google Scholar] [CrossRef]
- Lee, E.-Y.; Choi, D.-Y.; Kim, D.-K.; Kim, J.-W.; Park, J.O.; Kim, S.; Kim, S.-H.; Desiderio, D.M.; Kim, Y.-K.; Kim, K.-P.; et al. Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 2009, 9, 5425–5436. [Google Scholar] [CrossRef]
- Wang, X.; Thompson, C.D.; Weidenmaier, C.; Lee, J.C. Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat. Commun. 2018, 9, 1379. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lin, H. Characterization and function of membrane vesicles in Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2021, 105, 1795–1801. [Google Scholar] [CrossRef]
- Schlatterer, K.; Beck, C.; Hanzelmann, D.; Lebtig, M.; Fehrenbacher, B.; Schaller, M.; Ebner, P.; Nega, M.; Otto, M.; Kretschmer, D.; et al. The mechanism behind bacterial lipoprotein release: Phenol-soluble modulins mediate toll-like receptor 2 activation via extracellular vesicle release from staphylococcus aureus. mBio 2018, 9, e01851-18. [Google Scholar] [CrossRef] [PubMed]
- Andreoni, F.; Toyofuku, M.; Menzi, C.; Kalawong, R.; Shambat, S.M.; François, P.; Zinkernagel, A.S.; Eberl, L. Antibiotics stimulate formation of vesicles in staphylococcus aureus in both phage-dependent and -independent fashions and via different routes. Antimicrob. Agents Chemother. 2019, 63, e01439-18. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, E.-Y.; Kim, S.-H.; Kim, D.-K.; Park, K.-S.; Kim, K.P.; Kim, Y.-K.; Roh, T.-Y.; Gho, Y.S. Staphylococcus aureus Extracellular Vesicles Carry Biologically Active β-Lactamase. Antimicrob. Agents Chemother. 2013, 57, 2589–2595. [Google Scholar] [CrossRef]
- Jun, S.H.; Lee, J.H.; Kim, S.I.; Choi, C.W.; Park, T.I.; Jung, H.R.; Cho, J.W.; Kim, S.H.; Lee, J.C. Staphylococcus aureus-derived membrane vesicles exacerbate skin inflammation in atopic dermatitis. Clin. Exp. Allergy 2017, 47, 85–96. [Google Scholar] [CrossRef]
- Hong, S.-W.; Choi, E.-B.; Min, T.-K.; Kim, J.-H.; Kim, M.-H.; Jeon, S.G.; Lee, B.-J.; Gho, Y.S.; Jee, Y.-K.; Pyun, B.-Y.; et al. An important role of α-hemolysin in extracellular vesicles on the development of atopic dermatitis induced by Staphylococcus aureus. PLoS ONE 2014, 9, e100499. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Kim, M.R.; Lee, E.Y.; Kim, J.H.; Kim, Y.S.; Jeon, S.G.; Yang, J.M.; Lee, B.J.; Pyun, B.Y.; Gho, Y.S.; et al. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy 2011, 66, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Staudenmaier, L.; Focken, J.; Schlatterer, K.; Kretschmer, D.; Schittek, B. Bacterial membrane vesicles shape Staphylococcus aureus skin colonization and induction of innate immune responses. Exp. Dermatol. 2022, 31, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bin, B.H.; Choi, E.J.; Lee, H.G.; Lee, T.R.; Cho, E.G. Staphylococcus aureus-derived extracellular vesicles induce monocyte recruitment by activating human dermal microvascular endothelial cells in vitro. Clin. Exp. Allergy 2019, 49, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Lee, S.; Soper, S.A.; Mitchell, R.J. Staphylococcus aureus extracellular vesicles (EVs): Surface-binding antagonists of biofilm formation. Mol. Biosyst. 2017, 13, 2704–2714. [Google Scholar] [CrossRef] [PubMed]
- Homey, B.; Steinhoff, M.; Ruzicka, T.; Leung, D.Y.M. Cytokines and chemokines orchestrate atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 118, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Masuda, K.; Tamagawa-Mineoka, R.; Matsunaka, H.; Murakami, Y.; Yamashita, R.; Morita, E.; Katoh, N. Thymic stromal lymphopoietin expression is increased in the horny layer of patients with atopic dermatitis. Clin. Exp. Immunol. 2013, 171, 330–337. [Google Scholar] [CrossRef]
- Yawalkar, N.; Uguccioni, M.; Schärer, J.; Braunwalder, J.; Karlen, S.; Dewald, B.; Braathen, L.R.; Baggiolini, M. Enhanced Expression of Eotaxin and CCR3 in Atopic Dermatitis. J. Investig. Dermatol. 1999, 113, 43–48. [Google Scholar] [CrossRef]
- Corren, J.; Ziegler, S.F. TSLP: From allergy to cancer. Nat. Immunol. 2019, 20, 1603–1609. [Google Scholar] [CrossRef]
- Son, E.D.; Kim, H.-J.; Park, T.; Shin, K.; Bae, I.-H.; Lim, K.-M.; Cho, E.-G.; Lee, T.R. Staphylococcus aureus inhibits terminal differentiation of normal human keratinocytes by stimulating interleukin-6 secretion. J. Dermatol. Sci. 2014, 74, 64–71. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Hanke, M.L.; Fritz, T.; Angle, A.; Aldrich, A.; Williams, S.H.; Engebretsen, I.L.; Bayles, K.W.; Horswill, A.R.; Kielian, T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 2011, 186, 6585–6596. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tan, X.; Chen, G.; Liu, X.; Feng, A.; Liu, Z.; Liu, W. Extracellular Vesicles of Commensal Skin Microbiota Alleviate Cutaneous Inflammation in Atopic Dermatitis Mouse Model by Re-Establishing Skin Homeostasis. J. Investig. Dermatol. 2023. [Google Scholar] [CrossRef]
- Gonzalez, T.; Biagini Myers, J.M.; Herr, A.B.; Khurana Hershey, G.K. Staphylococcal Biofilms in Atopic Dermatitis. Curr. Allergy Asthma Rep. 2017, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Ianiri, G.; LeibundGut-Landmann, S.; Dawson, T.L. Malassezia: A Commensal, Pathogen, and Mutualist of Human and Animal Skin. Annu. Rev. Microbiol. 2022, 76, 757–782. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, U.; Qazi, K.R.; Johansson, C.; Hultenby, K.; Karlsson, M.; Lundeberg, L.; Gabrielsson, S.; Scheynius, A. Nanovesicles from malassezia sympodialis and host exosomes induce cytokine responses—Novel mechanisms for host-microbe interactions in atopic eczema. PLoS ONE 2011, 6, e21480. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-D.; Chen, S.H.; Camacho, E.; Casadevall, A.; Williamson, P.R. Role of the ESCRT Pathway in Laccase Trafficking and Virulence of Cryptococcus neoformans. Infect. Immun. 2020, 88. [Google Scholar] [CrossRef]
- de Souza Pereira, R.; Geibel, J. Direct observation of oxidative stress on the cell wall of Saccharomyces cerevisiae strains with atomic force microscopy. Mol. Cell. Biochem. 1999, 201, 17–24. [Google Scholar] [CrossRef]
- Johansson, H.J.; Vallhov, H.; Holm, T.; Gehrmann, U.; Andersson, A.; Johansson, C.; Blom, H.; Carroni, M.; Lehtiö, J.; Scheynius, A. Extracellular nanovesicles released from the commensal yeast Malassezia sympodialis are enriched in allergens and interact with cells in human skin. Sci. Rep. 2018, 8, 9182. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Godinho, R.M.C.; Zamith-Miranda, D.; Nimrichter, L. Traveling into Outer Space: Unanswered Questions about Fungal Extracellular Vesicles. PLoS Pathog. 2015, 11, e1005240. [Google Scholar] [CrossRef]
- Liebana-Jordan, M.; Brotons, B.; Falcon-Perez, J.M.; Gonzalez, E. Extracellular Vesicles in the Fungi Kingdom. Int. J. Mol. Sci. 2021, 22, 7221. [Google Scholar] [CrossRef]
- Rayner, S.; Bruhn, S.; Vallhov, H.; Andersson, A.; Billmyre, R.B.; Scheynius, A. Identification of small RNAs in extracellular vesicles from the commensal yeast Malassezia sympodialis. Sci. Rep. 2017, 7, 39742. [Google Scholar] [CrossRef] [PubMed]
- Bleackley, M.R.; Dawson, C.S.; Anderson, M.A. Fungal Extracellular Vesicles with a Focus on Proteomic Analysis. Proteomics 2019, 19, e1800232. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Nimrichter, L.; Oliveira, D.L.; Frases, S.; Miranda, K.; Zaragoza, O.; Alvarez, M.; Nakouzi, A.; Feldmesser, M.; Casadevall, A. Vesicular Polysaccharide Export in Cryptococcus neoformans Is a Eukaryotic Solution to the Problem of Fungal Trans-Cell Wall Transport. Eukaryot. Cell 2007, 6, 48–59. [Google Scholar] [CrossRef]
- Vallhov, H.; Johansson, H.; Holm, T.; Gehrmann, U.; Johansson, C.; Andersson, A.; Lehtio, J.; Scheynius, A. The role of extracellular vesicles (MalaEx) from the commensal yeast Malassezia sympodialis in atopic eczema. Scand. J. Immunol. 2017, 86, 253. [Google Scholar]
- Glatz, M.; Bosshard, P.P.; Hoetzenecker, W.; Schmid-Grendelmeier, P. The role of malassezia spp in atopic dermatitis. J. Clin. Med. 2015, 4, 1217–1228. [Google Scholar] [CrossRef]
- Tanojo, H.; Boelsma, E.; Junginger, H.E.; Ponec, M.; Boddé, H.E. In vivo Human Skin Barrier Modulation by Topical Application of Fatty Acids. Ski. Pharmacol. Appl. Ski. Physiol. 1998, 11, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef]
- Lecce, M.; Molfetta, R.; Milito, N.D.; Santoni, A.; Paolini, R. Fcεri signaling in the modulation of allergic response: Role of mast cell-derived exosomes. Int. J. Mol. Sci. 2020, 21, 5464. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef]
- Skokos, D.; Le Panse, S.; Villa, I.; Rousselle, J.-C.; Peronet, R.; David, B.; Namane, A.; Mecheri, S. Mast Cell-Dependent B and T Lymphocyte Activation Is Mediated by the Secretion of Immunologically Active Exosomes. J. Immunol. 2001, 166, 868–876. [Google Scholar] [CrossRef]
- Raposo, G.; Tenza, D.; Mecheri, S.; Peronet, R.; Bonnerot, C.; Desaymard, C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol. Biol. Cell 1997, 8, 2631–2645. [Google Scholar] [CrossRef]
- Groot Kormelink, T.; Arkesteijn, G.J.A.; van de Lest, C.H.A.; Geerts, W.J.C.; Goerdayal, S.S.; Altelaar, M.A.F.; Redegeld, F.A.; Nolte-’t Hoen, E.N.M.; Wauben, M.H.M. Mast Cell Degranulation Is Accompanied by the Release of a Selective Subset of Extracellular Vesicles That Contain Mast Cell-Specific Proteases. J. Immunol. 2016, 197, 3382–3392. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Y.; Lin, L.; Wang, J.; Xiao, H.; Li, J.; Peng, X.; Dai, H.; Li, L. Mast Cell-Derived Exosomes Promote Th2 Cell Differentiation via OX40L-OX40 Ligation. J. Immunol. Res. 2016, 2016, 3623898. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, S.; Sakamoto-Sasaki, T.; Kurosawa, Y.; Hayama, K.; Matsuda, A.; Watanabe, Y.; Terui, T.; Gon, Y.; Matsumoto, K.; Okayama, Y. miR103a-3p in extracellular vesicles from FcεRI-aggregated human mast cells enhances IL-5 production by group 2 innate lymphoid cells. J. Allergy Clin. Immunol. 2021, 147, 1878–1891. [Google Scholar] [CrossRef] [PubMed]
- Skokos, D.; Botros, H.G.; Demeure, C.; Morin, J.; Peronet, R.; Birkenmeier, G.; Boudaly, S.; Mecheri, S. Mast Cell-Derived Exosomes Induce Phenotypic and Functional Maturation of Dendritic Cells and Elicit Specific Immune Responses In Vivo. J. Immunol. 2003, 170, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Yang, H.; Peng, X.; Lin, L.; Wang, J.; Lin, K.; Cui, Z.; Li, J.; Xiao, H.; Liang, Y.; et al. Mast cell exosomes can suppress allergic reactions by binding to IgE. J. Allergy Clin. Immunol. 2018, 141, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, C.; Villa, I.; Peroneta, R.; David, B.; Chouaib, S.; Mécheri, S. In vitro and in vivo immunostimulatory potential of bone marrow–derived mast cells on B- and T-lymphocyte activation. J. Allergy Clin. Immunol. 2000, 105, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Guttman-Yassky, E. Deciphering the complexities of atopic dermatitis: Shifting paradigms in treatment approaches. J. Allergy Clin. Immunol. 2014, 134, 769–779. [Google Scholar] [CrossRef]
- Cho, B.S.; Kim, J.O.; Ha, D.H.; Yi, Y.W. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res. Ther. 2018, 9, 187. [Google Scholar] [CrossRef]
- Garima; Sharma, D.; Kumar, A.; Mostafavi, E. Extracellular vesicle-based biovectors in chronic wound healing: Biogenesis and delivery approaches. Mol. Ther. Nucleic Acids 2023, 32, 822–840. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; McDowell, A.; Seo, H.; Kim, S.; Min, T.K.; Jee, Y.K.; Choi, Y.; Park, H.S.; Pyun, B.Y.; Kim, Y.K. Diagnostic Models for Atopic Dermatitis Based on Serum Microbial Extracellular Vesicle Metagenomic Analysis: A Pilot Study. Allergy Asthma Immunol. Res. 2020, 12, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Go, G.; Yun, C.W.; Yea, J.H.; Yoon, S.; Han, S.Y.; Lee, G.; Lee, M.Y.; Lee, S.H. Topical Administration of Melatonin-Loaded Extracellular Vesicle-Mimetic Nanovesicles Improves 2,4-Dinitrofluorobenzene-Induced Atopic Dermatitis. Biomolecules 2021, 11, 1450. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Zhu, H.; Zhang, X. Extracellular vesicle-mediated regulation of macrophage polarization in bacterial infections. Front. Microbiol. 2022, 13, 1039040. [Google Scholar] [CrossRef]
- Lai, Y.; Jiang, B.; Hou, F.; Huang, X.; Ling, B.; Lu, H.; Zhong, T.; Huang, J. The emerging role of extracellular vesicles in fungi: A double-edged sword. Front. Microbiol. 2023, 14, 1216895. [Google Scholar] [CrossRef]
| Author, Year [Ref] | Model System | Conclusion |
|---|---|---|
| Lee et al., 2013 [30] | Bacterial, in vitro | SEVs mediated the survival of BlaZ |
| Jun et al., 2017 [31] | Human, in vitro | SEVs detected on surface and in cytoplasm of keratinocytes as well as in intercellular space of epidermis. HaCaT cells increased expression of IL-1beta, IL-6, IL-8 and MIP-1alpha when treated with SEVs |
| Hong et al., 2014 [32] | Human, in vitro | HaCaT viability significantly decreased upon treatment with SEVs from AD patients |
| Hong et al., 2011 [33] | Murine, in vitro Murine, in vivo | Dermal fibroblasts increased production of IL-6, TSLP, MIP-1alpha, and eotaxin when treated with SEVs. SEV application in vivo caused epidermal thickening and infiltration of dermis by mast cells and eosinophils |
| Staudenmaier et al., 2022 [34] | Human, in vitro | SEVs induced CXCL8 and TNF-alpha expression in keratinocytes. |
| Kim et al., 2019 [35] | Human, in vitro | SEVs increased expression of E-selectin, VCAM1, ICAM-1, and IL-6 in HDMECs. |
| Im et al., 2017 [36] | Bacterial, in vitro | SEVs dose-dependently inhibited A. baumannii biofilm development |
| Author, Year [Ref] | Methods | Conclusion |
|---|---|---|
| Gehrmann et al., 2011 [46] | Human, in vitro | MalaEx significantly enhanced IL-4 and TNF-alpha production in CD14- and CD34-depleted PBMCs |
| Johansson et al., 2018 [49] | Human, in vitro | MalaEx contain Mala s1 and s5–13 allergens. MalaEx are internalized by keratinocytes and monocytes. |
| Valhov et al., 2020 [55] | Human, in vitro | MalaEx induce a dose-dependent increase in ICAM-1 expression in keratinocytes. |
| Author, Year [Ref] | Methods | Conclusion |
|---|---|---|
| Skokos et al., 2001 [62] | Murine, in vivo | Proliferation of spleen and lymph node cells as well as IL-2 and IFN-gamma production. Bone-derived mast cells require pretreatment with IL-4 to secrete MCEVs. |
| Li et al., 2016 [65] | Murine, in vitro | MCEVs induced a higher proliferation rate of T cells than control and a higher proportion of Th2 differentiated cells. |
| Toyoshima et al., 2021 [66] | Human, in vitro. | MCEVs enhanced IL-5 production in ILC2s in the presence of IL-33. They did not enhance IL-13 production. |
| Skokos et al., 2003 [67] | Murine, in vivo | MCEVs induced the upregulation of DC maturation markers MHC II, CD80, CD86, and CD40. |
| Xie et al., 2018 [68] | Murine, in vitro | Each MCEV contained 25 to 40 FcERI molecules. MCEVs bind to free IgE via FcERI, reducing mast cell activation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harvey-Seutcheu, C.; Hopkins, G.; Fairclough, L.C. The Role of Extracellular Vesicles in Atopic Dermatitis. Int. J. Mol. Sci. 2024, 25, 3255. https://doi.org/10.3390/ijms25063255
Harvey-Seutcheu C, Hopkins G, Fairclough LC. The Role of Extracellular Vesicles in Atopic Dermatitis. International Journal of Molecular Sciences. 2024; 25(6):3255. https://doi.org/10.3390/ijms25063255
Chicago/Turabian StyleHarvey-Seutcheu, Catherine, Georgina Hopkins, and Lucy C. Fairclough. 2024. "The Role of Extracellular Vesicles in Atopic Dermatitis" International Journal of Molecular Sciences 25, no. 6: 3255. https://doi.org/10.3390/ijms25063255
APA StyleHarvey-Seutcheu, C., Hopkins, G., & Fairclough, L. C. (2024). The Role of Extracellular Vesicles in Atopic Dermatitis. International Journal of Molecular Sciences, 25(6), 3255. https://doi.org/10.3390/ijms25063255






