GC-MS with Headspace Extraction for Non-Invasive Diagnostics of IBD Dynamics in a Model of DSS-Induced Colitis in Rats
Abstract
:1. Introduction
2. Results
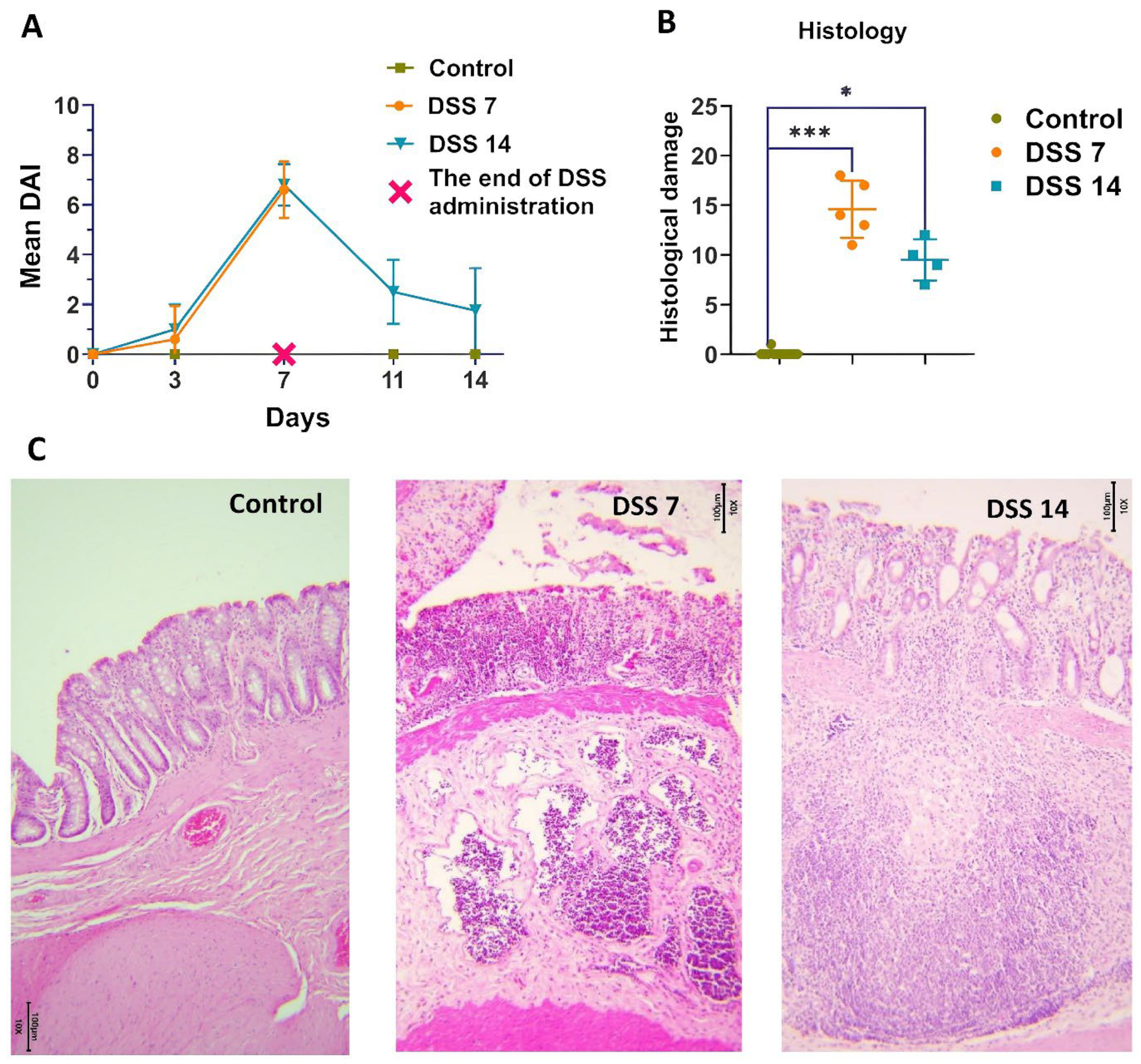
2.1. Histopathological Changes in Acute Phase and in Remission
2.2. Metabolomic Data from Acute Phase to Remission Stage
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ethics Statement
4.3. Reagents
4.4. Clinical Disease Score
4.5. Sample Preparation
4.6. HS-GC/MS
4.7. Histology
4.8. Data Processing
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adamkova, P.; Hradicka, P.; Skalnikova, H.K.; Cizkova, V.; Vodicka, P.; Iannaccone, S.F.; Kassayova, M.; Gancarcikova, S.; Demeckova, V. Dextran Sulphate Sodium Acute Colitis Rat Model: A Suitable Tool for Advancing Our Understanding of Immune and Microbial Mechanisms in the Pathogenesis of Inflammatory Bowel Disease. Vet. Sci. 2022, 9, 238. [Google Scholar] [CrossRef]
- Mezoff, E.A.; Williams, K.C.; Erdman, S.H. Gastrointestinal Endoscopy in the Neonate. Clin. Perinatol. 2020, 47, 413–422. [Google Scholar] [CrossRef]
- Valdés, L.; Cuervo, A.; Salazar, N.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. The relationship between phenolic compounds from diet and microbiota: Impact on human health. Food Funct. 2015, 6, 2424–2439. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Niccolai, E.; Baldi, S.; Ricci, F.; Russo, E.; Nannini, G.; Menicatti, M.; Poli, G.; Taddei, A.; Bartolucci, G.; Calabrò, A.S.; et al. Evaluation and comparison of short chain fatty acids composition in gut diseases. World J. Gastroenterol. 2019, 25, 5543–5558. [Google Scholar] [CrossRef]
- Zhang, V.R.; Ramachandran, G.K.; Loo, E.X.L.; Soh, A.Y.S.; Yong, W.P.; Siah, K.T.H. Volatile organic compounds as potential biomarkers of irritable bowel syndrome: A systematic review. Neurogastroenterol. Motil. 2023, 35, e14536. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, W.; Lan, P.; Mou, X. The microbiome in in fl ammatory bowel diseases: From pathogenesis to therapy. Protein Cell 2021, 12, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Greenwood, R.; Costello, B.; Ratcliffe, N.; Probert, C.S. Investigation of faecal volatile organic metabolites as novel diagnostic biomarkers in in fl ammatory bowel disease. Aliment. Pharmacol. Ther. 2016, 43, 596–611. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; Fowler, D.P.; Turner, C.; Jia, W.; Whitehead, R.N.; Griffiths, L.; Dawson, C.; Waring, R.H.; Ramsden, D.B.; Cole, J.A.; et al. Analysis of Volatile Organic Compounds of Bacterial Origin in Chronic Gastrointestinal Diseases. Inflamm. Bowel Dis. 2013, 19, 2069–2078. [Google Scholar] [CrossRef]
- Filipiak, W.; Żuchowska, K.; Marszałek, M.; Depka, D.; Bogiel, T.; Warmuzi, N. GC-MS profiling of volatile metabolites produced by Klebsiella pneumoniae. Front. Mol. Biosci. 2022, 9, 1019290. [Google Scholar] [CrossRef]
- Shagaleeva, O.Y.; Kashatnikova, D.A.; Kardonsky, D.A.; Konanov, D.N.; Efimov, B.A.; Bagrov, D.V.; Evtushenko, E.G.; Chaplin, A.V.; Silantiev, A.S.; Filatova, J.V.; et al. Investigating volatile compounds in the Bacteroides secretome. Front. Microbiol. 2023, 14, 1164877. [Google Scholar] [CrossRef]
- Karu, N.; Deng, L.; Slae, M.; Guo, A.C.; Sajed, T.; Huynh, H.; Wine, E.; Wishart, D.S. A review on human fecal metabolomics: Methods, applications and the human fecal metabolome database. Anal. Chim. Acta 2018, 1030, 1–24. [Google Scholar] [CrossRef]
- Antoniou, E.; Margonis, G.A.; Angelou, A.; Pikouli, A.; Argiri, P.; Karavokyros, I.; Papalois, A.; Pikoulis, E. The TNBS-induced colitis animal model: An overview. Ann. Med. Surg. 2016, 11, 9–15. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental models of inflammatory bowel diseases. Med. Hyg. 2001, 59, 241–248. [Google Scholar] [CrossRef]
- Silva, I.; Solas, J.; Pinto, R.; Mateus, V. Chronic Experimental Model of TNBS-Induced Colitis to Study Inflammatory Bowel Disease. Int. J. Mol. Sci. 2022, 23, 4739. [Google Scholar] [CrossRef]
- Weigmann, B.; Neurath, M.F. Oxazolone-induced colitis as a model of Th2 immune responses in the intestinal mucosa. Methods Mol. Biol. 2016, 1422, 253–261. [Google Scholar]
- Martin, J.C.; Bériou, G.; Josien, R. Dextran sulfate sodium (DSS)-induced acute colitis in the rat. Methods Mol. Biol. 2016, 1371, 197–203. [Google Scholar]
- Johansson, M.E.V.; Gustafsson, J.K.; Holmén-Larsson, J.; Jabbar, K.S.; Xia, L.; Xu, H.; Ghishan, F.K.; Carvalho, F.A.; Gewirtz, A.T.; Sjövall, H.; et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014, 63, 281–291. [Google Scholar] [CrossRef]
- Xu, H.-M.; Huang, H.-L.; Liu, Y.-D.; Zhu, J.-Q.; Zhou, Y.-L.; Chen, H.-T.; Xu, J.; Zhao, H.-L.; Guo, X.; Shi, W.; et al. Selection strategy of dextran sulfate sodium-induced acute or chronic colitis mouse models based on gut microbial profile. BMC Microbiol. 2021, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Han, X.; Li, J.-X.; Shi, R.; Liu, L.-L.; Wang, K.; Liao, Y.-T.; Jiang, H.; Zhang, Y.; Hu, J.-C.; et al. Differential analysis of intestinal microbiota and metabolites in mice with dextran sulfate sodium-induced colitis. World J. Gastroenterol. 2022, 28, 6109–6130. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.E.; Zheng, B.; Koelink, P.J.; van de Kant, H.J.G.; Haazen, L.C.J.M.; van Roest, M.; Garssen, J.; Folkerts, G.; Kraneveld, A.D. New Perspective on Dextran Sodium Sulfate Colitis: Antigen-Specific T Cell Development during Intestinal Inflammation. PLoS ONE 2013, 8, e69936. [Google Scholar] [CrossRef]
- Ma, T.; Shen, X.; Shi, X.; Arbab, H.; Quan, K. Targeting gut microbiota and metabolism as the major probiotic mechanism—An evidence-based review. Trends Food Sci. Technol. 2023, 138, 178–198. [Google Scholar] [CrossRef]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993, 69, 238–249. [Google Scholar] [PubMed]
- Chen, L.; Zhou, Z.; Yang, Y.; Chen, N.; Xiang, H. Therapeutic effect of imiquimod on dextran sulfate sodium-induced ulcerative colitis in mice. PLOS ONE 2017, 12, e0186138. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence ? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Venegas, D.P.; De la Fuente, M.K.; Landskron, G.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 424615. [Google Scholar]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Dehkhodaie, E.; Bouzari, B.; Rahimi, M. Dual role of microbiota-derived short-chain fatty acids on host and pathogen. Biomed. Pharmacother. 2022, 145, 112352. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]
- Moolenbeek, C.; Ruitenberg, E.J. The ‘Swiss roll’: A simple technique for histological studies of the rodent intestine. Lab. Anim. 1981, 15, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Mascaraque, C.; González, R.; Suárez, M.D.; Zarzuelo, A.; de Medina, F.S.; Martínez-Augustin, O. Intestinal anti-inflammatory activity of apigenin K in two rat colitis models induced by trinitrobenzenesulfonic acid and dextran sulphate sodium. Dig. Dis. Sci. 2016, 113, 1458–1475. [Google Scholar] [CrossRef] [PubMed]


| Metabolite | Percent of Samples in Which the Marker Is Detected Out of a Total of n = 44 | Control Group, Amount% | Group DSS, Day 0, Amount% | Group DSS, Day 3, Amount% | Group DSS, Day 7, Amount% | Group DSS, Day 11, Amount% | Group DSS, Day 14, Amount% |
|---|---|---|---|---|---|---|---|
| Acetic acid | 65 | 26.42 ± 7.24 | 34.83 ± 7.97 | 72.21 ± 10.5 | 34.71 ± 20.66 | 70.72 ± 12.6 | 48.55 ± 13.3 |
| Propanoic acid | 70 | 18.26 ± 7.48 | 19.99 ± 5.14 | 12.73 ± 7.36 | 30.4 ± 16.08 | 10.69 ± 4.19 | 16.58 ± 3 |
| Propanoic acid, 2-methyl- | 91 | 7.52 ± 2.36 | 5.2 ± 2.61 | 10.93 ± 9.18 | 7.61 ± 4.31 | 2.61 ± 1.39 | 5.63 ± 0.98 |
| Butanoic acid | 98 | 33.96 ± 6.73 | 37.3 ± 8.6 | 9.66 ± 5.81 | 33.01 ± 4.61 | 13.3 ± 5.31 | 25.51 ± 12.93 |
| Pentanoic acid | 100 | 14.47 ± 3.68 | 12.65 ± 3.85 | 11.39 ± 7.4 | 8.00 ± 5.26 | 2.44 ± 2.51 | 15.30 ± 14.02 |
| Hexanoic acid | 77 | 10.23 ± 4.63 | 10.36 ± 3.07 | 21.71 ± 22.61 | 2.20 ± 2.22 | 0.21 ± 0.07 | 6.35 ± 5.92 |
| Heptanoic acid | 73 | 1.81 ± 1.19 | 1.08 ± 0.63 | 6.44 ± 7.53 | 0.31 ± 0.40 | 0.14 ± 0.00 | 1.13 ± 0.75 |
| Phenol 4-methyl- | 93 | 2.20 ± 1.30 | 1.21 ± 1.81 | 5.49 ± 6.11 | 5.24 ± 5.37 | 0.32 ± 0 | 1.44 ± 1.33 |
| Benzenepropanoic acid | 84 | 0.39 ± 0.17 | 0.97 ± 1.12 | 0.27 ± 0.27 | 0.09 ± 0.03 | 0.04 ± 0.00 | 0.36 ± 0.23 |
| Indole | 75 | 0.11 ± 0.12 | 0.10 ± 0.1 | 0.01 ± 0.02 | 0.42 ± 0.18 | 0.004 ± 0.000 | 0.01 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shagaleeva, O.Y.; Kashatnikova, D.A.; Kardonsky, D.A.; Danilova, E.Y.; Ivanov, V.A.; Evsiev, S.S.; Zubkov, E.A.; Abramova, O.V.; Zorkina, Y.A.; Morozova, A.Y.; et al. GC-MS with Headspace Extraction for Non-Invasive Diagnostics of IBD Dynamics in a Model of DSS-Induced Colitis in Rats. Int. J. Mol. Sci. 2024, 25, 3295. https://doi.org/10.3390/ijms25063295
Shagaleeva OY, Kashatnikova DA, Kardonsky DA, Danilova EY, Ivanov VA, Evsiev SS, Zubkov EA, Abramova OV, Zorkina YA, Morozova AY, et al. GC-MS with Headspace Extraction for Non-Invasive Diagnostics of IBD Dynamics in a Model of DSS-Induced Colitis in Rats. International Journal of Molecular Sciences. 2024; 25(6):3295. https://doi.org/10.3390/ijms25063295
Chicago/Turabian StyleShagaleeva, Olga Yu., Daria A. Kashatnikova, Dmitry A. Kardonsky, Elena Yu. Danilova, Viktor A. Ivanov, Suleiman S. Evsiev, Eugene A. Zubkov, Olga V. Abramova, Yana A. Zorkina, Anna Y. Morozova, and et al. 2024. "GC-MS with Headspace Extraction for Non-Invasive Diagnostics of IBD Dynamics in a Model of DSS-Induced Colitis in Rats" International Journal of Molecular Sciences 25, no. 6: 3295. https://doi.org/10.3390/ijms25063295
APA StyleShagaleeva, O. Y., Kashatnikova, D. A., Kardonsky, D. A., Danilova, E. Y., Ivanov, V. A., Evsiev, S. S., Zubkov, E. A., Abramova, O. V., Zorkina, Y. A., Morozova, A. Y., Konanov, D. N., Silantiev, A. S., Efimov, B. A., Kolesnikova, I. V., Bespyatykh, J. A., Stimpson, J., & Zakharzhevskaya, N. B. (2024). GC-MS with Headspace Extraction for Non-Invasive Diagnostics of IBD Dynamics in a Model of DSS-Induced Colitis in Rats. International Journal of Molecular Sciences, 25(6), 3295. https://doi.org/10.3390/ijms25063295







