Clinical Insights into Structure, Regulation, and Targeting of ABL Kinases in Human Leukemia
Abstract
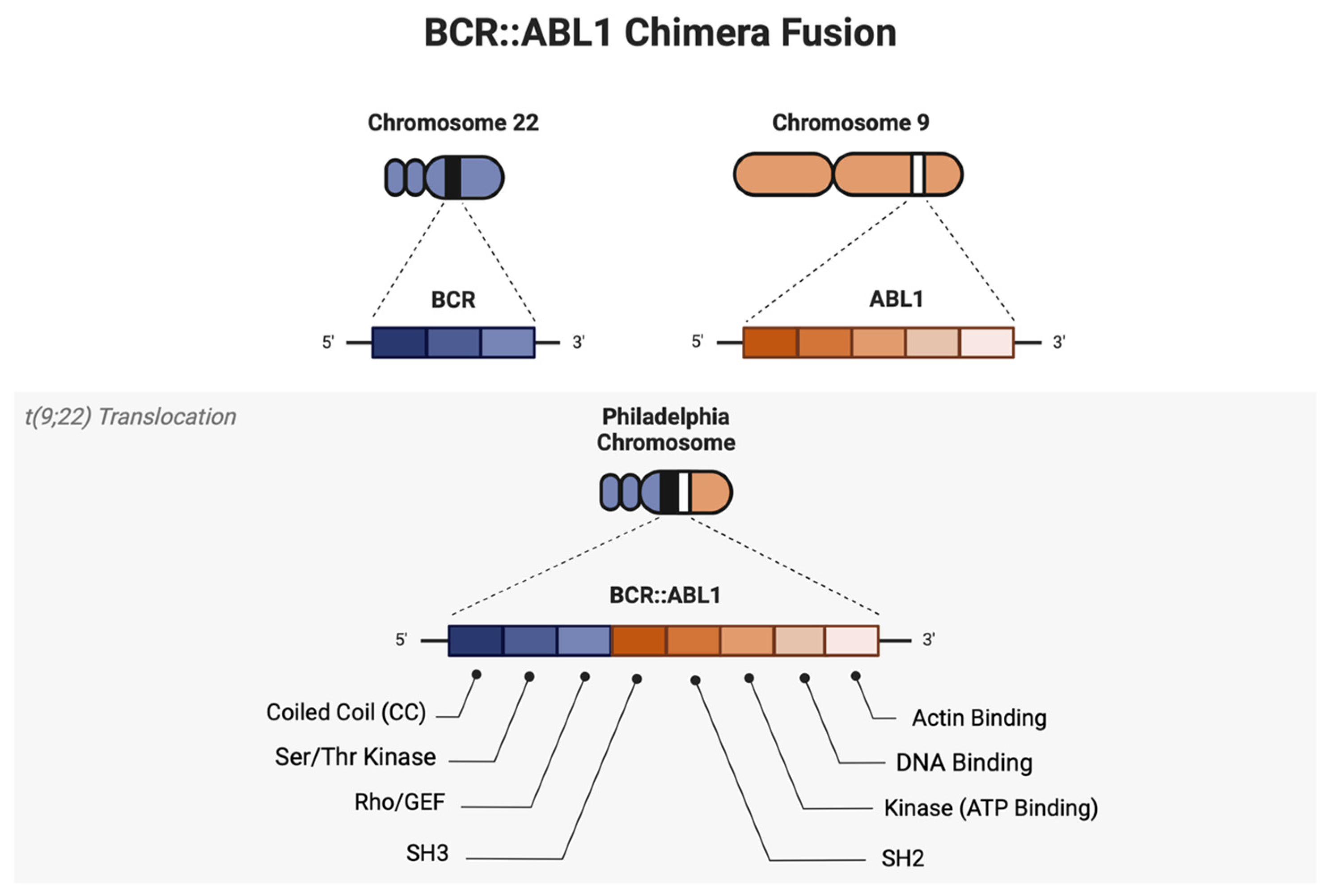
1. Introduction
2. Structural and Molecular Description of ABL1 Regulation
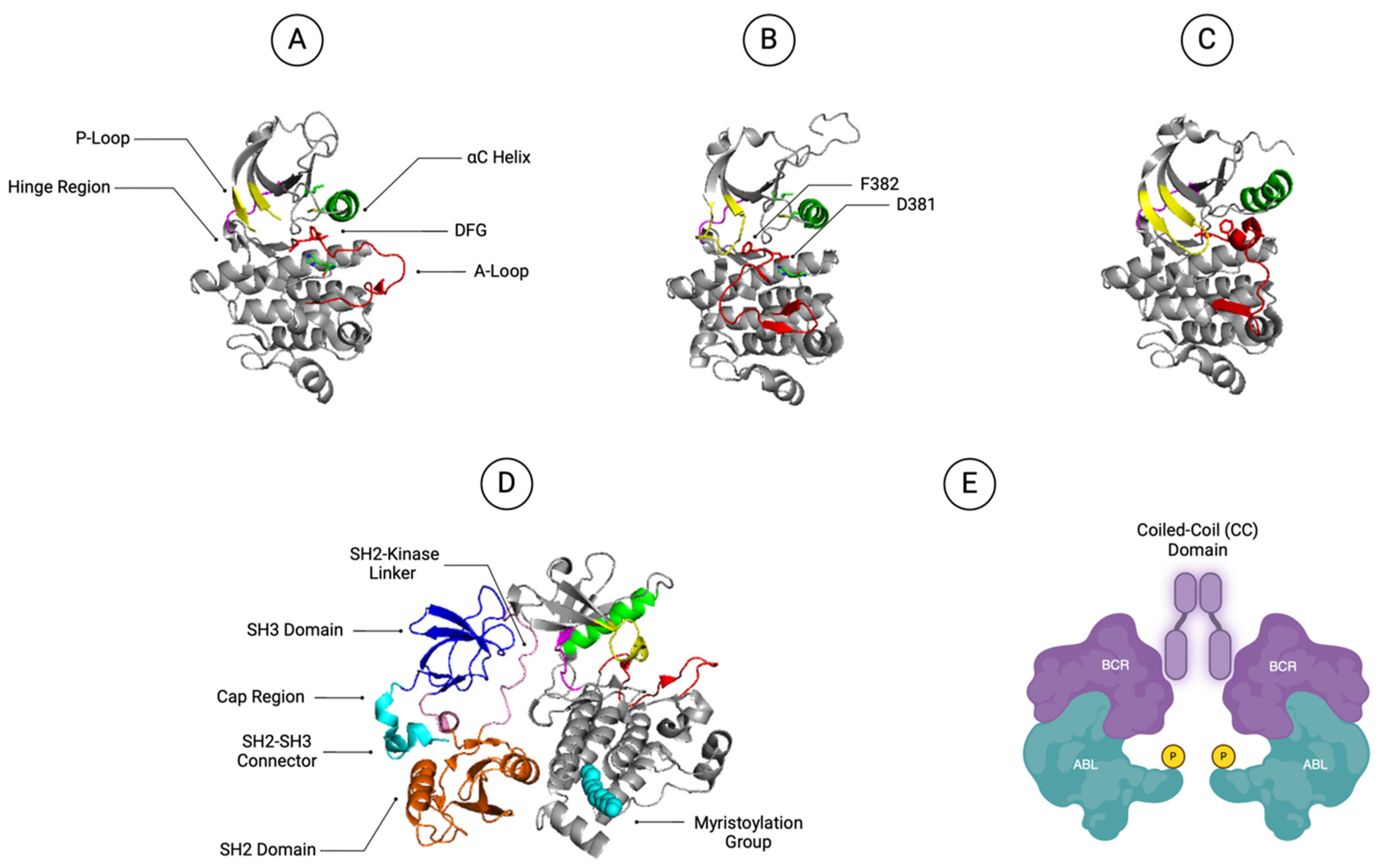
2.1. ABL1 Kinase Domain
2.2. ABL SH2 and SH3 Domains
2.3. ABL1 N-Terminal Myristoylation Group and Cap Region
2.4. BCR::ABL1 Coiled-Coil Domain
3. Structural Characterization of BCR::ABL1 Kinase Inhibitors
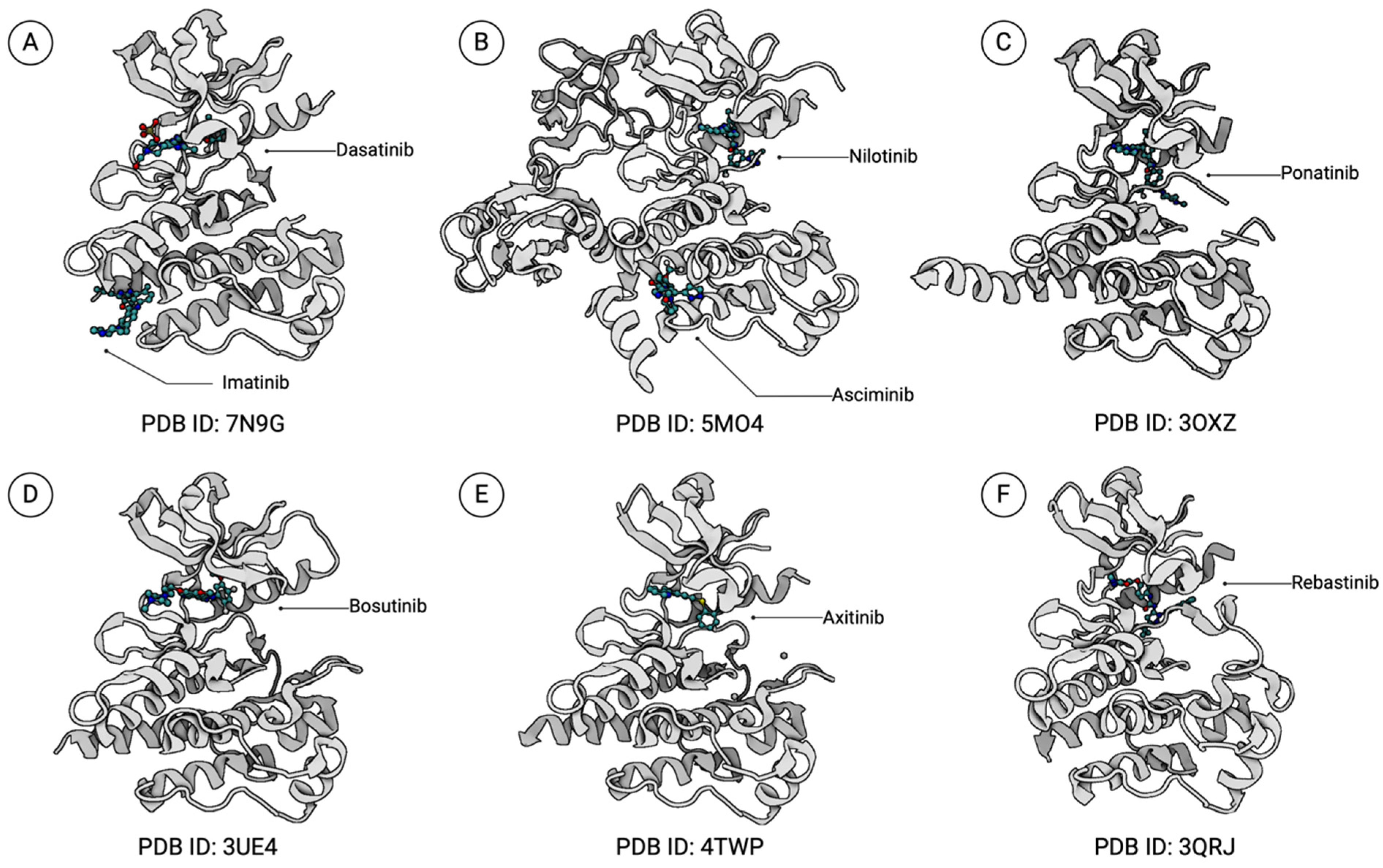
3.1. BCR::ABL1 DFG-Out TKIs
3.1.1. Imatinib
3.1.2. Nilotinib
3.1.3. Ponatinib
3.2. BCR::ABL1 DFG-In TKIs
3.2.1. Dasatinib
3.2.2. Bosutinib
3.3. Allosteric Inhibitors of the ABL1 Kinase Domain
Asciminib
3.4. Other BCR::ABL1 Inhibitors
3.4.1. Axitinib
3.4.2. Rebastinib
4. TKI Treatment Strategies in the Clinic
4.1. Long-Term Clinical Efficacy of TKIs and TKI Tolerance
4.2. Clinical Trials of Developing TKI Treatment Strategies for CML
4.2.1. ASC4FIRST
4.2.2. ASC2ESCALATE
4.2.3. PACE and OPTIC
4.2.4. Decitabine, Venetoclax, and Ponatinib for the Treatment of Philadelphia Chromosome-Positive Acute Myeloid Leukemia or Myeloid Blast Phase or Accelerated Phase CML (M.D. Anderson Cancer Center)
4.2.5. TFR and TKI Discontinuation Trials
4.2.6. Olverembatinib
4.2.7. Preclinical Developments of CML Therapeutic Strategies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jabbour, E.; Kantarjian, H. Chronic Myeloid Leukemia: 2022 Update on Diagnosis, Therapy, and Monitoring. Am. J. Hematol. 2022, 97, 1236–1256. [Google Scholar] [CrossRef]
- Pane, F.; Intrieri, M.; Quintarelli, C.; Izzo, B.; Muccioli, G.C.; Salvatore, F. BCR/ABL Genes and Leukemic Phenotype: From Molecular Mechanisms to Clinical Correlations. Oncogene 2002, 21, 8652–8667. [Google Scholar] [CrossRef]
- Cross, N.C.P.; Ernst, T.; Branford, S.; Cayuela, J.-M.; Deininger, M.; Fabarius, A.; Kim, D.D.H.; Machova Polakova, K.; Radich, J.P.; Hehlmann, R.; et al. European LeukemiaNet Laboratory Recommendations for the Diagnosis and Management of Chronic Myeloid Leukemia. Leukemia 2023, 37, 2150–2167. [Google Scholar] [CrossRef]
- Senapati, J.; Jabbour, E.; Kantarjian, H.; Short, N.J. Pathogenesis and Management of Accelerated and Blast Phases of Chronic Myeloid Leukemia. Leukemia 2023, 37, 5–17. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Molica, M.; Zacheo, I.; Diverio, D.; Alimena, G.; Breccia, M. Long-Term Outcome of Chronic Myeloid Leukaemia Patients with P210 and P190 Co-Expression at Baseline. Br. J. Haematol. 2015, 169, 148–150. [Google Scholar] [CrossRef]
- Adnan-Awad, S.; Kim, D.; Hohtari, H.; Javarappa, K.K.; Brandstoetter, T.; Mayer, I.; Potdar, S.; Heckman, C.A.; Kytölä, S.; Porkka, K.; et al. Characterization of P190-Bcr-Abl Chronic Myeloid Leukemia Reveals Specific Signaling Pathways and Therapeutic Targets. Leukemia 2021, 35, 1964–1975. [Google Scholar] [CrossRef]
- Verstovsek, S.; Lin, H.; Kantarjian, H.; Saglio, G.; De Micheli, D.; Pane, F.; Garcia-Manero, G.; Intrieri, M.; Rotoli, B.; Salvatore, F.; et al. Neutrophilic-Chronic Myeloid Leukemia: Low Levels of P230 BCR/ABL mRNA and Undetectable BCR/ABL Protein May Predict an Indolent Course. Cancer 2002, 94, 2416–2425. [Google Scholar] [CrossRef]
- Senapati, J.; Sasaki, K.; Issa, G.C.; Lipton, J.H.; Radich, J.P.; Jabbour, E.; Kantarjian, H.M. Management of Chronic Myeloid Leukemia in 2023—Common Ground and Common Sense. Blood Cancer J. 2023, 13, 58. [Google Scholar] [CrossRef]
- Graham, S.M.; Jørgensen, H.G.; Allan, E.; Pearson, C.; Alcorn, M.J.; Richmond, L.; Holyoake, T.L. Primitive, Quiescent, Philadelphia-Positive Stem Cells from Patients with Chronic Myeloid Leukemia Are Insensitive to STI571 in Vitro. Blood 2002, 99, 319–325. [Google Scholar] [CrossRef]
- Shah, N.P.; Nicoll, J.M.; Nagar, B.; Gorre, M.E.; Paquette, R.L.; Kuriyan, J.; Sawyers, C.L. Multiple BCR-ABL Kinase Domain Mutations Confer Polyclonal Resistance to the Tyrosine Kinase Inhibitor Imatinib (STI571) in Chronic Phase and Blast Crisis Chronic Myeloid Leukemia. Cancer Cell 2002, 2, 117–125. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, Y.; Smith, C.; Gasparetto, M.; Turhan, A.; Eaves, A.; Eaves, C. Chronic Myeloid Leukemia Stem Cells Possess Multiple Unique Features of Resistance to BCR-ABL Targeted Therapies. Leukemia 2007, 21, 926–935. [Google Scholar] [CrossRef]
- Corbin, A.S.; Agarwal, A.; Loriaux, M.; Cortes, J.; Deininger, M.W.; Druker, B.J. Human Chronic Myeloid Leukemia Stem Cells Are Insensitive to Imatinib despite Inhibition of BCR-ABL Activity. J. Clin. Investig. 2011, 121, 396–409. [Google Scholar] [CrossRef]
- Zabriskie, M.S.; Eide, C.A.; Tantravahi, S.K.; Vellore, N.A.; Estrada, J.; Nicolini, F.E.; Khoury, H.J.; Larson, R.A.; Konopleva, M.; Cortes, J.E.; et al. BCR-ABL1 Compound Mutations Combining Key Kinase Domain Positions Confer Clinical Resistance to Ponatinib in Ph Chromosome-Positive Leukemia. Cancer Cell 2014, 26, 428–442. [Google Scholar] [CrossRef]
- Holyoake, T.L.; Vetrie, D. The Chronic Myeloid Leukemia Stem Cell: Stemming the Tide of Persistence. Blood 2017, 129, 1595–1606. [Google Scholar] [CrossRef]
- Zhai, X.; Jiang, X. Properties of Leukemic Stem Cells in Regulating Drug Resistance in Acute and Chronic Myeloid Leukemias. Biomedicines 2022, 10, 1841. [Google Scholar] [CrossRef]
- Baccarani, M.; Abruzzese, E.; Accurso, V.; Albano, F.; Annunziata, M.; Barulli, S.; Beltrami, G.; Bergamaschi, M.; Binotto, G.; Bocchia, M.; et al. Managing Chronic Myeloid Leukemia for Treatment-Free Remission: A Proposal from the GIMEMA CML WP. Blood Adv. 2019, 3, 4280–4290. [Google Scholar] [CrossRef]
- Hantschel, O.; Superti-Furga, G. Regulation of the C-Abl and Bcr–Abl Tyrosine Kinases. Nat. Rev. Mol. Cell Biol. 2004, 5, 33–44. [Google Scholar] [CrossRef]
- Nagar, B.; Hantschel, O.; Young, M.A.; Scheffzek, K.; Veach, D.; Bornmann, W.; Clarkson, B.; Superti-Furga, G.; Kuriyan, J. Structural Basis for the Autoinhibition of C-Abl Tyrosine Kinase. Cell 2003, 112, 859–871. [Google Scholar] [CrossRef]
- Rossari, F.; Minutolo, F.; Orciuolo, E. Past, Present, and Future of Bcr-Abl Inhibitors: From Chemical Development to Clinical Efficacy. J. Hematol. Oncol. 2018, 11, 84. [Google Scholar] [CrossRef]
- Sonti, R.; Hertel-Hering, I.; Lamontanara, A.J.; Hantschel, O.; Grzesiek, S. ATP Site Ligands Determine the Assembly State of the Abelson Kinase Regulatory Core via the Activation Loop Conformation. J. Am. Chem. Soc. 2018, 140, 1863–1869. [Google Scholar] [CrossRef]
- Knighton, D.R.; Zheng, J.H.; Ten Eyck, L.F.; Ashford, V.A.; Xuong, N.H.; Taylor, S.S.; Sowadski, J.M. Crystal Structure of the Catalytic Subunit of Cyclic Adenosine Monophosphate-Dependent Protein Kinase. Science 1991, 253, 407–414. [Google Scholar] [CrossRef]
- Nagar, B.; Bornmann, W.G.; Pellicena, P.; Schindler, T.; Veach, D.R.; Miller, W.T.; Clarkson, B.; Kuriyan, J. Crystal Structures of the Kinase Domain of C-Abl in Complex with the Small Molecule Inhibitors PD173955 and Imatinib (STI-571). Cancer Res. 2002, 62, 4236–4243. [Google Scholar]
- Tokarski, J.S.; Newitt, J.A.; Chang, C.Y.J.; Cheng, J.D.; Wittekind, M.; Kiefer, S.E.; Kish, K.; Lee, F.Y.F.; Borzillerri, R.; Lombardo, L.J.; et al. The Structure of Dasatinib (BMS-354825) Bound to Activated ABL Kinase Domain Elucidates Its Inhibitory Activity against Imatinib-Resistant ABL Mutants. Cancer Res. 2006, 66, 5790–5797. [Google Scholar] [CrossRef]
- Young, M.A.; Shah, N.P.; Chao, L.H.; Seeliger, M.; Milanov, Z.V.; Biggs, W.H., III; Treiber, D.K.; Patel, H.K.; Zarrinkar, P.P.; Lockhart, D.J.; et al. Structure of the Kinase Domain of an Imatinib-Resistant Abl Mutant in Complex with the Aurora Kinase Inhibitor VX-680. Cancer Res. 2006, 66, 1007–1014. [Google Scholar] [CrossRef]
- Reddy, E.P.; Aggarwal, A.K. The Ins and Outs of Bcr-Abl Inhibition. Genes Cancer 2012, 3, 447–454. [Google Scholar] [CrossRef]
- Schindler, T.; Bornmann, W.; Pellicena, P.; Miller, W.T.; Clarkson, B.; Kuriyan, J. Structural Mechanism for STI-571 Inhibition of Abelson Tyrosine Kinase. Science 2000, 289, 1938–1942. [Google Scholar] [CrossRef]
- Seeliger, M.A.; Ranjitkar, P.; Kasap, C.; Shan, Y.; Shaw, D.E.; Shah, N.P.; Kuriyan, J.; Maly, D.J. Equally Potent Inhibition of C-Src and Abl by Compounds That Recognize Inactive Kinase Conformations. Cancer Res. 2009, 69, 2384–2392. [Google Scholar] [CrossRef]
- Levinson, N.M.; Kuchment, O.; Shen, K.; Young, M.A.; Koldobskiy, M.; Karplus, M.; Cole, P.A.; Kuriyan, J. A Src-Like Inactive Conformation in the Abl Tyrosine Kinase Domain. PLoS Biol. 2006, 4, e144. [Google Scholar] [CrossRef]
- Seeliger, M.A.; Nagar, B.; Frank, F.; Cao, X.; Henderson, M.N.; Kuriyan, J. C-Src Binds to the Cancer Drug Imatinib with an Inactive Abl/c-Kit Conformation and a Distributed Thermodynamic Penalty. Structure 2007, 15, 299–311. [Google Scholar] [CrossRef]
- Nagar, B.; Hantschel, O.; Seeliger, M.; Davies, J.M.; Weis, W.I.; Superti-Furga, G.; Kuriyan, J. Organization of the SH3-SH2 Unit in Active and Inactive Forms of the c-Abl Tyrosine Kinase. Mol. Cell 2006, 21, 787–798. [Google Scholar] [CrossRef]
- Panjarian, S.; Iacob, R.E.; Chen, S.; Engen, J.R.; Smithgall, T.E. Structure and Dynamic Regulation of Abl Kinases*. J. Biol. Chem. 2013, 288, 5443–5450. [Google Scholar] [CrossRef]
- Hantschel, O.; Nagar, B.; Guettler, S.; Kretzschmar, J.; Dorey, K.; Kuriyan, J.; Superti-Furga, G. A Myristoyl/Phosphotyrosine Switch Regulates c-Abl. Cell 2003, 112, 845–857. [Google Scholar] [CrossRef]
- Wylie, A.A.; Schoepfer, J.; Jahnke, W.; Cowan-Jacob, S.W.; Loo, A.; Furet, P.; Marzinzik, A.L.; Pelle, X.; Donovan, J.; Zhu, W.; et al. The Allosteric Inhibitor ABL001 Enables Dual Targeting of BCR-ABL1. Nature 2017, 543, 733–737. [Google Scholar] [CrossRef]
- Hughes, T.P.; Mauro, M.J.; Cortes, J.E.; Minami, H.; Rea, D.; DeAngelo, D.J.; Breccia, M.; Goh, Y.-T.; Talpaz, M.; Hochhaus, A.; et al. Asciminib in Chronic Myeloid Leukemia after ABL Kinase Inhibitor Failure. N. Engl. J. Med. 2019, 381, 2315–2326. [Google Scholar] [CrossRef]
- Braun, T.P.; Eide, C.A.; Druker, B.J. Response and Resistance to BCR-ABL1-Targeted Therapies. Cancer Cell 2020, 37, 530–542. [Google Scholar] [CrossRef]
- McWhirter, J.R.; Galasso, D.L.; Wang, J.Y. A Coiled-Coil Oligomerization Domain of Bcr Is Essential for the Transforming Function of Bcr-Abl Oncoproteins. Mol. Cell. Biol. 1993, 13, 7587–7595. [Google Scholar] [CrossRef]
- Million, R.P.; Van Etten, R.A. The Grb2 Binding Site Is Required for the Induction of Chronic Myeloid Leukemia-like Disease in Mice by the Bcr/Abl Tyrosine Kinase. Blood 2000, 96, 664–670. [Google Scholar] [CrossRef]
- Brehme, M.; Hantschel, O.; Colinge, J.; Kaupe, I.; Planyavsky, M.; Köcher, T.; Mechtler, K.; Bennett, K.L.; Superti-Furga, G. Charting the Molecular Network of the Drug Target Bcr-Abl. Proc. Natl. Acad. Sci. USA 2009, 106, 7414–7419. [Google Scholar] [CrossRef]
- He, Y.; Wertheim, J.A.; Xu, L.; Miller, J.P.; Karnell, F.G.; Choi, J.K.; Ren, R.; Pear, W.S. The Coiled-Coil Domain and Tyr177 of Bcr Are Required to Induce a Murine Chronic Myelogenous Leukemia-like Disease by Bcr/Abl. Blood 2002, 99, 2957–2968. [Google Scholar] [CrossRef]
- Xie, T.; Saleh, T.; Rossi, P.; Miller, D.; Kalodimos, C.G. Imatinib Can Act as an Allosteric Activator of Abl Kinase. J. Mol. Biol. 2022, 434, 167349. [Google Scholar] [CrossRef]
- Cowan-Jacob, S.W.; Fendrich, G.; Floersheimer, A.; Furet, P.; Liebetanz, J.; Rummel, G.; Rheinberger, P.; Centeleghe, M.; Fabbro, D.; Manley, P.W. Structural Biology Contributions to the Discovery of Drugs to Treat Chronic Myelogenous Leukaemia. Acta Crystallogr. D Biol. Crystallogr. 2007, 63, 80–93. [Google Scholar] [CrossRef]
- Azam, M.; Seeliger, M.A.; Gray, N.S.; Kuriyan, J.; Daley, G.Q. Activation of Tyrosine Kinases by Mutation of the Gatekeeper Threonine. Nat. Struct. Mol. Biol. 2008, 15, 1109–1118. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Weisberg, E.; Manley, P.W.; Breitenstein, W.; Brüggen, J.; Cowan-Jacob, S.W.; Ray, A.; Huntly, B.; Fabbro, D.; Fendrich, G.; Hall-Meyers, E.; et al. Characterization of AMN107, a Selective Inhibitor of Native and Mutant Bcr-Abl. Cancer Cell 2005, 7, 129–141. [Google Scholar] [CrossRef]
- Soverini, S.; Branford, S.; Nicolini, F.E.; Talpaz, M.; Deininger, M.W.N.; Martinelli, G.; Müller, M.C.; Radich, J.P.; Shah, N.P. Implications of BCR-ABL1 Kinase Domain-Mediated Resistance in Chronic Myeloid Leukemia. Leuk. Res. 2014, 38, 10–20. [Google Scholar] [CrossRef]
- Patel, A.B.; O’Hare, T.; Deininger, M.W. Mechanisms of Resistance to ABL Kinase Inhibition in CML and the Development of next Generation ABL Kinase Inhibitors. Hematol. Oncol. Clin. N. Am. 2017, 31, 589–612. [Google Scholar] [CrossRef]
- Zhou, T.; Commodore, L.; Huang, W.-S.; Wang, Y.; Thomas, M.; Keats, J.; Xu, Q.; Rivera, V.M.; Shakespeare, W.C.; Clackson, T.; et al. Structural Mechanism of the Pan-BCR-ABL Inhibitor Ponatinib (AP24534): Lessons for Overcoming Kinase Inhibitor Resistance. Chem. Biol. Drug Des. 2011, 77, 1–11. [Google Scholar] [CrossRef]
- O’Hare, T.; Shakespeare, W.C.; Zhu, X.; Eide, C.A.; Rivera, V.M.; Wang, F.; Adrian, L.T.; Zhou, T.; Huang, W.-S.; Xu, Q.; et al. AP24534, a Pan-BCR-ABL Inhibitor for Chronic Myeloid Leukemia, Potently Inhibits the T315I Mutant and Overcomes Mutation-Based Resistance. Cancer Cell 2009, 16, 401–412. [Google Scholar] [CrossRef]
- O’Hare, T.; Walters, D.K.; Stoffregen, E.P.; Jia, T.; Manley, P.W.; Mestan, J.; Cowan-Jacob, S.W.; Lee, F.Y.; Heinrich, M.C.; Deininger, M.W.N.; et al. In Vitro Activity of Bcr-Abl Inhibitors AMN107 and BMS-354825 against Clinically Relevant Imatinib-Resistant Abl Kinase Domain Mutants. Cancer Res. 2005, 65, 4500–4505. [Google Scholar] [CrossRef]
- Hochhaus, A.; Kantarjian, H. The Development of Dasatinib as a Treatment for Chronic Myeloid Leukemia (CML): From Initial Studies to Application in Newly Diagnosed Patients. J. Cancer Res. Clin. Oncol. 2013, 139, 1971–1984. [Google Scholar] [CrossRef]
- Vajpai, N.; Strauss, A.; Fendrich, G.; Cowan-Jacob, S.W.; Manley, P.W.; Grzesiek, S.; Jahnke, W. Solution Conformations and Dynamics of ABL Kinase-Inhibitor Complexes Determined by NMR Substantiate the Different Binding Modes of Imatinib/Nilotinib and Dasatinib. J. Biol. Chem. 2008, 283, 18292–18302. [Google Scholar] [CrossRef]
- Zhou, T.; Commodore, L.; Huang, W.-S.; Wang, Y.; Sawyer, T.K.; Shakespeare, W.C.; Clackson, T.; Zhu, X.; Dalgarno, D.C. Structural Analysis of DFG-in and DFG-out Dual Src-Abl Inhibitors Sharing a Common Vinyl Purine Template. Chem. Biol. Drug Des. 2010, 75, 18–28. [Google Scholar] [CrossRef]
- Levinson, N.M.; Boxer, S.G. Structural and Spectroscopic Analysis of the Kinase Inhibitor Bosutinib and an Isomer of Bosutinib Binding to the Abl Tyrosine Kinase Domain. PLoS ONE 2012, 7, e29828. [Google Scholar] [CrossRef]
- Schoepfer, J.; Jahnke, W.; Berellini, G.; Buonamici, S.; Cotesta, S.; Cowan-Jacob, S.W.; Dodd, S.; Drueckes, P.; Fabbro, D.; Gabriel, T.; et al. Discovery of Asciminib (ABL001), an Allosteric Inhibitor of the Tyrosine Kinase Activity of BCR-ABL1. J. Med. Chem. 2018, 61, 8120–8135. [Google Scholar] [CrossRef]
- Manley, P.W.; Barys, L.; Cowan-Jacob, S.W. The Specificity of Asciminib, a Potential Treatment for Chronic Myeloid Leukemia, as a Myristate-Pocket Binding ABL Inhibitor and Analysis of Its Interactions with Mutant Forms of BCR-ABL1 Kinase. Leuk. Res. 2020, 98, 106458. [Google Scholar] [CrossRef]
- Jones, J.K.; Thompson, E.M. Allosteric Inhibition of ABL Kinases: Therapeutic Potential in Cancer. Mol. Cancer Ther. 2020, 19, 1763–1769. [Google Scholar] [CrossRef]
- Qiang, W.; Antelope, O.; Zabriskie, M.S.; Pomicter, A.D.; Vellore, N.A.; Szankasi, P.; Rea, D.; Cayuela, J.M.; Kelley, T.W.; Deininger, M.W.; et al. Mechanisms of Resistance to the BCR-ABL1 Allosteric Inhibitor Asciminib. Leukemia 2017, 31, 2844–2847. [Google Scholar] [CrossRef]
- Eide, C.A.; Zabriskie, M.S.; Savage Stevens, S.L.; Antelope, O.; Vellore, N.A.; Than, H.; Schultz, A.R.; Clair, P.; Bowler, A.D.; Pomicter, A.D.; et al. Combining the Allosteric Inhibitor Asciminib with Ponatinib Suppresses Emergence of and Restores Efficacy against Highly Resistant BCR-ABL1 Mutants. Cancer Cell 2019, 36, 431–443.e5. [Google Scholar] [CrossRef]
- Han, H.-J.; Kim, J.J.; Pyne, D.; Travas, A.; Ambalavanan, A.; Kimura, S.; Deininger, M.W.; Kim, J.-W.; Kim, D.D.H. Asciminib Enhances Its Treatment Efficacy Synergistically in the Treatment of Chronic Myeloid Leukemia Harboring ABL1 Kinase Domain Mutation When Combined with a Reduced Dose of Ponatinib, Dasatinib, or Bosutinib, but Not with Nilotinib or Imatinib. Blood 2023, 142, 6337. [Google Scholar] [CrossRef]
- Pemovska, T.; Johnson, E.; Kontro, M.; Repasky, G.A.; Chen, J.; Wells, P.; Cronin, C.N.; McTigue, M.; Kallioniemi, O.; Porkka, K.; et al. Axitinib Effectively Inhibits BCR-ABL1(T315I) with a Distinct Binding Conformation. Nature 2015, 519, 102–105. [Google Scholar] [CrossRef]
- Okabe, S.; Tauchi, T.; Tanaka, Y.; Sakuta, J.; Ohyashiki, K. Anti-Leukemic Activity of Axitinib against Cells Harboring the BCR-ABL T315I Point Mutation. J. Hematol. Oncol. 2015, 8, 97. [Google Scholar] [CrossRef][Green Version]
- Lindström, H.J.G.; Friedman, R. The Effects of Combination Treatments on Drug Resistance in Chronic Myeloid Leukaemia: An Evaluation of the Tyrosine Kinase Inhibitors Axitinib and Asciminib. BMC Cancer 2020, 20, 397. [Google Scholar] [CrossRef]
- Chan, W.W.; Wise, S.C.; Kaufman, M.D.; Ahn, Y.M.; Ensinger, C.L.; Haack, T.; Hood, M.M.; Jones, J.; Lord, J.W.; Lu, W.P.; et al. Conformational Control Inhibition of the BCR-ABL1 Tyrosine Kinase, Including the Gatekeeper T315I Mutant, by the Switch-Control Inhibitor DCC-2036. Cancer Cell 2011, 19, 556–568. [Google Scholar] [CrossRef]
- Eide, C.A.; Adrian, L.T.; Tyner, J.W.; Mac Partlin, M.; Anderson, D.J.; Wise, S.C.; Smith, B.; Petillo, P.A.; Flynn, D.L.; Deininger, M.W.N.; et al. The ABL Switch Control Inhibitor DCC-2036 Is Active against the Chronic Myeloid Leukemia Mutant BCR-ABLT315I and Exhibits a Narrow Resistance Profile. Cancer Res. 2011, 71, 3189–3195. [Google Scholar] [CrossRef]
- Cortes, J.; Talpaz, M.; Smith, H.P.; Snyder, D.S.; Khoury, J.; Bhalla, K.N.; Pinilla-Ibarz, J.; Larson, R.; Mitchell, D.; Wise, S.C.; et al. Phase 1 Dose-Finding Study of Rebastinib (DCC-2036) in Patients with Relapsed Chronic Myeloid Leukemia and Acute Myeloid Leukemia. Haematologica 2017, 102, 519–528. [Google Scholar] [CrossRef]
- Molica, M.; Naqvi, K.; Cortes, J.E.; Paul, S.; Kadia, T.M.; Breccia, M.; Kantarjian, H.; Jabbour, E.J. Treatment-Free Remission in Chronic Myeloid Leukemia. Clin. Adv. Hematol. Oncol. 2019, 17, 686–696. [Google Scholar]
- Gorre, M.E.; Mohammed, M.; Ellwood, K.; Hsu, N.; Paquette, R.; Rao, P.N.; Sawyers, C.L. Clinical Resistance to STI-571 Cancer Therapy Caused by BCR-ABL Gene Mutation or Amplification. Science 2001, 293, 876–880. [Google Scholar] [CrossRef]
- Kantarjian, H.; O’Brien, S.; Cortes, J.; Giles, F.; Thomas, D.; Kornblau, S.; Shan, J.; Beth Rios, M.; Keating, M.; Freireich, E.; et al. Sudden Onset of the Blastic Phase of Chronic Myelogenous Leukemia: Patterns and Implications. Cancer 2003, 98, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Tantiworawit, A.; Power, M.M.; Barnett, M.J.; Hogge, D.E.; Nantel, S.H.; Nevill, T.J.; Shepherd, J.D.; Song, K.W.; Sutherland, H.J.; Toze, C.L.; et al. Long-Term Follow-up of Patients with Chronic Myeloid Leukemia in Chronic Phase Developing Sudden Blast Phase on Imatinib Therapy. Leuk. Lymphoma 2012, 53, 1321–1326. [Google Scholar] [CrossRef]
- Hochhaus, A.; Masszi, T.; Giles, F.J.; Radich, J.P.; Ross, D.M.; Gómez Casares, M.T.; Hellmann, A.; Stentoft, J.; Conneally, E.; García-Gutiérrez, V.; et al. Treatment-Free Remission Following Frontline Nilotinib in Patients with Chronic Myeloid Leukemia in Chronic Phase: Results from the ENESTfreedom Study. Leukemia 2017, 31, 1525–1531. [Google Scholar] [CrossRef]
- Radich, J.P.; Hochhaus, A.; Masszi, T.; Hellmann, A.; Stentoft, J.; Casares, M.T.G.; García-Gutiérrez, J.V.; Conneally, E.; le Coutre, P.D.; Gattermann, N.; et al. Treatment-Free Remission Following Frontline Nilotinib in Patients with Chronic Phase Chronic Myeloid Leukemia: 5-Year Update of the ENESTfreedom Trial. Leukemia 2021, 35, 1344–1355. [Google Scholar] [CrossRef]
- Atallah, E.; Schiffer, C.A.; Radich, J.P.; Weinfurt, K.P.; Zhang, M.-J.; Pinilla-Ibarz, J.; Kota, V.; Larson, R.A.; Moore, J.O.; Mauro, M.J.; et al. Assessment of Outcomes After Stopping Tyrosine Kinase Inhibitors Among Patients With Chronic Myeloid Leukemia: A Nonrandomized Clinical Trial. JAMA Oncol. 2021, 7, 42–50. [Google Scholar] [CrossRef]
- Gener-Ricos, G.; Haddad, F.; Sasaki, K.; Issa, G.C.; Skinner, J.; Takahashi, K.; Masarova, L.; Burger, J.A.; Borthakur, G.; Bose, P.; et al. Long-Term Follow-up of Low-Dose Dasatinib (50 mg Daily) As Frontline Therapy in Newly Diagnosed Chronic Myeloid Leukemia. Blood 2022, 140, 1493–1494. [Google Scholar] [CrossRef]
- Jabbour, E.; Sasaki, K.; Haddad, F.G.; Issa, G.C.; Skinner, J.; Dellasala, S.; Yilmaz, M.; Ferrajoli, A.; Bose, P.; Thompson, P.; et al. Low-Dose Dasatinib 50 mg/day versus Standard-Dose Dasatinib 100 mg/day as Frontline Therapy in Chronic Myeloid Leukemia in Chronic Phase: A Propensity Score Analysis. Am. J. Hematol. 2022, 97, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, K.; Jabbour, E.; Skinner, J.; Anderson, K.; Dellasala, S.; Yilmaz, M.; Ferrajoli, A.; Bose, P.; Thompson, P.; Alvarado, Y.; et al. Long-Term Follow-up of Lower Dose Dasatinib (50 mg Daily) as Frontline Therapy in Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia. Cancer 2020, 126, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.-Z.; Jiang, Q.; Jiang, H.; Lai, Y.-Y.; Zhu, H.-H.; Liu, Y.-R.; Jiang, B.; Huang, X.-J. Combination of White Blood Cell Count at Presentation With Molecular Response at 3 Months Better Predicts Deep Molecular Responses to Imatinib in Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia Patients. Medicine 2016, 95, e2486. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Yen, R.; Grasedieck, S.; Lin, H.; Nakamoto, H.; Forrest, D.L.; Eaves, C.J.; Jiang, X. Identification of Multivariable microRNA and Clinical Biomarker Panels to Predict Imatinib Response in Chronic Myeloid Leukemia at Diagnosis. Leukemia 2023, 37, 2426–2435. [Google Scholar] [CrossRef]
- Marin, D.; Ibrahim, A.R.; Lucas, C.; Gerrard, G.; Wang, L.; Szydlo, R.M.; Clark, R.E.; Apperley, J.F.; Milojkovic, D.; Bua, M.; et al. Assessment of BCR-ABL1 Transcript Levels at 3 Months Is the Only Requirement for Predicting Outcome for Patients with Chronic Myeloid Leukemia Treated with Tyrosine Kinase Inhibitors. J. Clin. Oncol. 2012, 30, 232–238. [Google Scholar] [CrossRef]
- Neelakantan, P.; Gerrard, G.; Lucas, C.; Milojkovic, D.; May, P.; Wang, L.; Paliompeis, C.; Bua, M.; Reid, A.; Rezvani, K.; et al. Combining BCR-ABL1 Transcript Levels at 3 and 6 Months in Chronic Myeloid Leukemia: Implications for Early Intervention Strategies. Blood 2013, 121, 2739–2742. [Google Scholar] [CrossRef]
- Kotagama, K.; Chang, Y.; Mangone, M. miRNAs as Biomarkers in Chronic Myelogenous Leukemia. Drug Dev. Res. 2015, 76, 278–285. [Google Scholar] [CrossRef]
- Glauche, I.; Kuhn, M.; Baldow, C.; Schulze, P.; Rothe, T.; Liebscher, H.; Roy, A.; Wang, X.; Roeder, I. Quantitative Prediction of Long-Term Molecular Response in TKI-Treated CML—Lessons from an Imatinib versus Dasatinib Comparison. Sci. Rep. 2018, 8, 12330. [Google Scholar] [CrossRef]
- Alves, R.; Gonçalves, A.C.; Jorge, J.; Marques, G.; Luís, D.; Ribeiro, A.B.; Freitas-Tavares, P.; Oliveiros, B.; Almeida, A.M.; Sarmento-Ribeiro, A.B. MicroRNA Signature Refine Response Prediction in CML. Sci. Rep. 2019, 9, 9666. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.R.B.; de Moraes, L.N.; Cury, S.S.; Dadalto, J.; Capannacci, J.; Carvalho, R.F.; Nogueira, C.R.; Hokama, N.K.; Hokama, P.d.O.M. Comparison of microRNA Expression Profile in Chronic Myeloid Leukemia Patients Newly Diagnosed and Treated by Allogeneic Hematopoietic Stem Cell Transplantation. Front. Oncol. 2020, 10, 1544. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.E.; Apperley, J.F.; Copland, M.; Cicconi, S. Additional Chromosomal Abnormalities at Chronic Myeloid Leukemia Diagnosis Predict an Increased Risk of Progression. Blood Adv. 2021, 5, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Yen, R.; Grasedieck, S.; Wu, A.; Lin, H.; Su, J.; Rothe, K.; Nakamoto, H.; Forrest, D.L.; Eaves, C.J.; Jiang, X. Identification of Key microRNAs as Predictive Biomarkers of Nilotinib Response in Chronic Myeloid Leukemia: A Sub-Analysis of the ENESTxtnd Clinical Trial. Leukemia 2022, 36, 2443–2452. [Google Scholar] [CrossRef]
- Réa, D.; Mauro, M.J.; Boquimpani, C.; Minami, Y.; Lomaia, E.; Voloshin, S.; Turkina, A.; Kim, D.-W.; Apperley, J.F.; Abdo, A.; et al. A Phase 3, Open-Label, Randomized Study of Asciminib, a STAMP Inhibitor, vs. Bosutinib in CML after 2 or More Prior TKIs. Blood 2021, 138, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; Jabbour, E.; Deininger, M.; Abruzzese, E.; Apperley, J.; Cortes, J.; Chuah, C.; DeAngelo, D.J.; DiPersio, J.; Hochhaus, A.; et al. Ponatinib after Failure of Second-Generation Tyrosine Kinase Inhibitor in Resistant Chronic-Phase Chronic Myeloid Leukemia. Am. J. Hematol. 2022, 97, 1419–1426. [Google Scholar] [CrossRef]
- Hughes, T.P.; Shanmuganathan, N. Management of TKI-Resistant Chronic Phase CML. Hematology 2022, 2022, 129–137. [Google Scholar] [CrossRef]
- Özgür Yurttaş, N.; Eşkazan, A.E. Novel Therapeutic Approaches in Chronic Myeloid Leukemia. Leuk. Res. 2020, 91, 106337. [Google Scholar] [CrossRef]
- Loscocco, F.; Visani, G.; Galimberti, S.; Curti, A.; Isidori, A. BCR-ABL Independent Mechanisms of Resistance in Chronic Myeloid Leukemia. Front. Oncol. 2019, 9, 939. [Google Scholar] [CrossRef]
- Choi, E.-J. Asciminib: The First-in-Class Allosteric Inhibitor of BCR::ABL1 Kinase. Blood Res. 2023, 58, S29–S36. [Google Scholar] [CrossRef]
- Hughes, T.; Cortes, J.E.; Takahashi, N.; Larson, R.A.; Issa, G.C.; Bombaci, F.; Ramscar, N.; Kapoor, S.; Ifrah, S.; Hochhaus, A. ASC4FIRST: A Phase III Study of Asciminib vs. Investigator-Selected Tyrosine Kinase Inhibitor in Patients with Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (CML-CP). Blood 2022, 140, 6767–6768. [Google Scholar] [CrossRef]
- Cortes, J.E.; Hochhaus, A.; Takahashi, N.; Larson, R.A.; Issa, G.C.; Bombaci, F.; Ramscar, N.; Ifrah, S.; Hughes, T.P. Asciminib Monotherapy for Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase: The ASC4FIRST Phase III Trial. Future Oncol. 2022, 18, 4161–4170. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 Recommendations from an International Expert Panel on Behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Sasaki, K.; Mauro, M.; Levy, M.Y.; Atallah, E.L.; Koller, P.B.; Maegawa, R.; Damon, A.; Kumar, J.; Khan, M.; Cortes, J.E. ASC2ESCALATE: A Phase 2, Single-Arm, Dose-Escalation Study of Asciminib Monotherapy in Patients (Pts) with Chronic Myeloid Leukemia in Chronic Phase (CML-CP) Previously Treated with 1 Prior Tyrosine Kinase Inhibitor (TKI). Blood 2022, 140, 6784–6786. [Google Scholar] [CrossRef]
- Cortes, J.E.; Kim, D.-W.; Pinilla-Ibarz, J.; le Coutre, P.D.; Paquette, R.; Chuah, C.; Nicolini, F.E.; Apperley, J.F.; Khoury, H.J.; Talpaz, M.; et al. Ponatinib Efficacy and Safety in Philadelphia Chromosome–Positive Leukemia: Final 5-Year Results of the Phase 2 PACE Trial. Blood 2018, 132, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Apperley, J.; Lomaia, E.; Moiraghi, B.; Undurraga Sutton, M.; Pavlovsky, C.; Chuah, C.; Sacha, T.; Lipton, J.H.; Schiffer, C.; et al. OPTIC Primary Analysis: A Dose-Optimization Study of 3 Starting Doses of Ponatinib (PON). J. Clin. Oncol. 2021, 39, 7000. [Google Scholar] [CrossRef]
- Cortes, J.; Apperley, J.; Lomaia, E.; Moiraghi, B.; Undurraga Sutton, M.; Pavlovsky, C.; Chuah, C.; Sacha, T.; Lipton, J.H.; Schiffer, C.A.; et al. Ponatinib Dose-Ranging Study in Chronic-Phase Chronic Myeloid Leukemia: A Randomized, Open-Label Phase 2 Clinical Trial. Blood 2021, 138, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Deininger, M.W.; Lomaia, E.; Moiraghi, B.; Sutton, M.U.; Pavlovsky, C.; Chuah, C.; Sacha, T.; Lipton, J.H.; McCloskey, J.; et al. Three-Year Update from the Optic Trial: A Dose-Optimization Study of 3 Starting Doses of Ponatinib. Blood 2022, 140, 1495–1497. [Google Scholar] [CrossRef]
- Jabbour, E.; Apperley, J.; Cortes, J.; Rea, D.; Deininger, M.; Abruzzese, E.; Chuah, C.; DeAngelo, D.J.; Hochhaus, A.; Lipton, J.H.; et al. Dose Modification Dynamics of Ponatinib in Patients with Chronic-Phase Chronic Myeloid Leukemia (CP-CML) from the PACE and OPTIC Trials. Leukemia 2024, 38, 475–481. [Google Scholar] [CrossRef]
- Abaza, Y.; Kantarjian, H.; Alwash, Y.; Borthakur, G.; Champlin, R.; Kadia, T.; Garcia-Manero, G.; Daver, N.; Ravandi, F.; Verstovsek, S.; et al. Phase I/II Study of Dasatinib in Combination with Decitabine in Patients with Accelerated or Blast Phase Chronic Myeloid Leukemia. Am. J. Hematol. 2020, 95, 1288–1295. [Google Scholar] [CrossRef]
- Leonard, J.T.; Rowley, J.S.J.; Eide, C.A.; Traer, E.; Hayes-Lattin, B.; Loriaux, M.; Spurgeon, S.E.; Druker, B.J.; Tyner, J.W.; Chang, B.H. Targeting BCL-2 and ABL/LYN in Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Sci. Transl. Med. 2016, 8, 354ra114. [Google Scholar] [CrossRef] [PubMed]
- Maiti, A.; Franquiz, M.J.; Ravandi, F.; Cortes, J.E.; Jabbour, E.J.; Sasaki, K.; Marx, K.; Daver, N.G.; Kadia, T.M.; Konopleva, M.Y.; et al. Venetoclax and BCR-ABL Tyrosine Kinase Inhibitor Combinations: Outcome in Patients with Philadelphia Chromosome-Positive Advanced Myeloid Leukemias. Acta Haematol. 2020, 143, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Senapati, J.; Ravandi, F.; DiNardo, C.D.; Issa, G.C.; Sasaki, K.; Konopleva, M.; Pemmaraju, N.; Chien, K.S.; Ohanian, M.; Jabbour, E.; et al. A Phase II Study of the Combination of Decitabine, Venetoclax and Ponatinib in Patients with Chronic Myeloid Leukemia (CML) in Myeloid Blast Phase (MBP) or Philadelphia-Chromosome Positive (Ph+) Acute Myeloid Leukemia (AML). Blood 2022, 140, 3880–3882. [Google Scholar] [CrossRef]
- Etienne, G.; Guilhot, J.; Rea, D.; Rigal-Huguet, F.; Nicolini, F.; Charbonnier, A.; Guerci-Bresler, A.; Legros, L.; Varet, B.; Gardembas, M.; et al. Long-Term Follow-Up of the French Stop Imatinib (STIM1) Study in Patients With Chronic Myeloid Leukemia. J. Clin. Oncol. 2017, 35, 298–305. [Google Scholar] [CrossRef]
- Saussele, S.; Richter, J.; Guilhot, J.; Gruber, F.X.; Hjorth-Hansen, H.; Almeida, A.; Janssen, J.J.W.M.; Mayer, J.; Koskenvesa, P.; Panayiotidis, P.; et al. Discontinuation of Tyrosine Kinase Inhibitor Therapy in Chronic Myeloid Leukaemia (EURO-SKI): A Prespecified Interim Analysis of a Prospective, Multicentre, Non-Randomised, Trial. Lancet Oncol. 2018, 19, 747–757. [Google Scholar] [CrossRef]
- Dulucq, S.; Astrugue, C.; Etienne, G.; Mahon, F.-X.; Benard, A. Risk of Molecular Recurrence after Tyrosine Kinase Inhibitor Discontinuation in Chronic Myeloid Leukaemia Patients: A Systematic Review of Literature with a Meta-Analysis of Studies over the Last Ten Years. Br. J. Haematol. 2020, 189, 452–468. [Google Scholar] [CrossRef]
- Richter, J.; Lübking, A.; Söderlund, S.; Lotfi, K.; Markevärn, B.; Själander, A.; Stenke, L.; Deneberg, S.; Ahlstrand, E.; Myhr-Eriksson, K.; et al. Molecular Status 36 Months after TKI Discontinuation in CML Is Highly Predictive for Subsequent Loss of MMR-Final Report from AFTER-SKI. Leukemia 2021, 35, 2416–2418. [Google Scholar] [CrossRef]
- Rousselot, P.; Loiseau, C.; Delord, M.; Cayuela, J.M.; Spentchian, M. Late Molecular Recurrences in Patients with Chronic Myeloid Leukemia Experiencing Treatment-Free Remission. Blood Adv. 2020, 4, 3034–3040. [Google Scholar] [CrossRef] [PubMed]
- Dulucq, S.; Rigal-Huguet, F.; Nicolini, F.E.; Cony-Makhoul, P.; Escoffre-Barbe, M.; Gardembas, M.; Legros, L.; Rousselot, P.; Liu, J.; Rea, D.; et al. Efficacy and Safety of Nilotinib in Chronic Myeloid Leukaemia Patients Who Failed to Achieve a Treatment-Free Remission Period after Imatinib Discontinuation: Results of the French Nilo Post-STIM Study. Br. J. Haematol. 2023, 201, 1116–1124. [Google Scholar] [CrossRef]
- Flygt, H.; Söderlund, S.; Richter, J.; Saussele, S.; Koskenvesa, P.; Stenke, L.; Mustjoki, S.; Dimitrijevic, A.; Stentoft, J.; Majeed, W.; et al. Treatment-Free Remission after a Second TKI Discontinuation Attempt in Patients with Chronic Myeloid Leukemia Re-Treated with Dasatinib—Interim Results from the DAstop2 Trial. Leukemia 2024, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Pan, X.; Zhang, Z.; Wang, D.; Lu, X.; Li, Y.; Wen, D.; Long, H.; Luo, J.; Feng, Y.; et al. Identification of GZD824 as an Orally Bioavailable Inhibitor That Targets Phosphorylated and Nonphosphorylated Breakpoint Cluster Region-Abelson (Bcr-Abl) Kinase and Overcomes Clinically Acquired Mutation-Induced Resistance against Imatinib. J. Med. Chem. 2013, 56, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, Z.; Zhang, G.; Hu, Y.; Li, W.; Song, Y.; Li, J.; Zhou, L.; Liu, B.; Liu, X.; et al. Olverembatinib (HQP1351) Demonstrates Efficacy Vs. Best Available Therapy (BAT) in Patients (Pts) with Tyrosine Kinase Inhibitor (TKI)-Resistant Chronic Myeloid Leukemia Chronic-Phase (CML-CP) in a Registrational Randomized Phase 2 Study. Blood 2023, 142, 869. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, Z.; Qin, Y.; Li, W.; Xu, N.; Liu, B.; Zhang, Y.; Meng, L.; Zhu, H.; Du, X.; et al. Olverembatinib (HQP1351), a Well-Tolerated and Effective Tyrosine Kinase Inhibitor for Patients with T315I-Mutated Chronic Myeloid Leukemia: Results of an Open-Label, Multicenter Phase 1/2 Trial. J. Hematol. Oncol. 2022, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lei, Z.; Yao, X.; Wang, H.; Zhang, M.; Hou, Z.; Li, Y.; Zhao, Y.; Li, H.; Liu, D.; et al. Potential Drug-Drug Interaction of Olverembatinib (HQP1351) Using Physiologically Based Pharmacokinetic Models. Front. Pharmacol. 2022, 13, 1065130. [Google Scholar] [CrossRef]
- Jabbour, E.; Koller, P.B.; Oehler, V.G.; Jamy, O.H.; Mukherjee, S.; Hunter, A.M.; Baer, M.R.; Beck, J.T.; Chen, Z.; Guo, H.; et al. Olverembatinib (HQP1351) Overcomes Ponatinib Resistance in Patients with Heavily Pretreated/Refractory Chronic Myeloid Leukemia (CML) and Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia (Ph+ ALL). Blood 2022, 140, 200–202. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, Z.; Qin, Y.-Z.; Zhao, T.; Liu, B.; Chen, Z.; Niu, Q.; Men, L.; Wang, H.; Yang, D.; et al. A Five-Year Follow-up on Safety and Efficacy of Olverembatinib (HQP1351), a Novel Third-Generation BCR-ABL Tyrosine Kinase Inhibitor (TKI), in Patients with TKI-Resistant Chronic Myeloid Leukemia (CML) in China. Blood 2022, 140, 198–199. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, Z.; Hou, Y.; Hu, Y.; Li, W.; Liu, X.; Xu, N.; Zhang, Y.; Song, Y.; Meng, L.; et al. Updated Results of Pivotal Phase 2 Trials of Olverembatinib (HQP1351) in Patients (Pts) with Tyrosine Kinase Inhibitor (TKI)-Resistant Chronic- and Accelerated-Phase Chronic Myeloid Leukemia (CML-CP and CML-AP) with T315I Mutation. Blood 2022, 140, 203–204. [Google Scholar] [CrossRef]
- Lu, M.; Deng, C.; Xiong, Y.; Wang, H.; Xu, P.; Men, L.; Xie, T.; Jiang, Q.; Chen, Z.; Niu, Q.; et al. Exposure-Response (E-R) Analysis of Olverembatinib (HQP1351) in Chinese Patients with Chronic Myeloid Leukemia (CML). Blood 2020, 136, 5–6. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H.M.; Koller, P.B.; Jamy, O.; Oehler, V.G.; Lomaia, E.; Hunter, A.M.; Uspenskaya, O.; Samarina, S.; Mukherjee, S.; et al. Update of Olverembatinib (HQP1351) Overcoming Ponatinib and/or Asciminib Resistance in Patients (Pts) with Heavily Pretreated/Refractory Chronic Myeloid Leukemia (CML) and Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia (Ph+ ALL). Blood 2023, 142, 1798. [Google Scholar] [CrossRef]
- Gong, X.; Fang, Q.; Gu, R.; Qiu, S.; Liu, K.; Lin, D.; Zhou, C.; Zhang, G.; Gong, B.; Liu, Y.; et al. Olverembatinib Combined with Venetoclax and Reduced-Intensity Chemotherapy for Patients with Newly Diagnosed Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: Early Results from a Phase II Study. Blood 2023, 142, 827. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Tang, X.; Luo, J.; Tu, Z.; Jiang, K.; Ren, X.; Xu, F.; Chan, S.; Li, Y.; et al. GZD824 as a FLT3, FGFR1 and PDGFRα Inhibitor Against Leukemia In Vitro and In Vivo. Transl. Oncol. 2020, 13, 100766. [Google Scholar] [CrossRef]
- Kumar, V.; Jyotirmayee; Verma, M. Developing Therapeutic Approaches for Chronic Myeloid Leukemia: A Review. Mol. Cell. Biochem. 2023, 478, 1013–1029. [Google Scholar] [CrossRef] [PubMed]
- Sadaf, S.; Awasthi, D.; Singh, A.K.; Nagarkoti, S.; Kumar, S.; Barthwal, M.K.; Dikshit, M. Pyroptotic and Apoptotic Cell Death in iNOS and nNOS Overexpressing K562 Cells: A Mechanistic Insight. Biochem. Pharmacol. 2020, 176, 113779. [Google Scholar] [CrossRef] [PubMed]
- García-Tuñón, I.; Hernández-Sánchez, M.; Ordoñez, J.L.; Alonso-Pérez, V.; Álamo-Quijada, M.; Benito, R.; Guerrero, C.; Hernández-Rivas, J.M.; Sánchez-Martín, M. The CRISPR/Cas9 System Efficiently Reverts the Tumorigenic Ability of BCR/ABL in Vitro and in a Xenograft Model of Chronic Myeloid Leukemia. Oncotarget 2017, 8, 26027–26040. [Google Scholar] [CrossRef] [PubMed]
- Vuelta, E.; Ordoñez, J.L.; Sanz, D.J.; Ballesteros, S.; Hernández-Rivas, J.M.; Méndez-Sánchez, L.; Sánchez-Martín, M.; García-Tuñón, I. CRISPR/Cas9-Directed Gene Trap Constitutes a Selection System for Corrected BCR/ABL Leukemic Cells in CML. Int. J. Mol. Sci. 2022, 23, 6386. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lage, M.; Torres-Ruiz, R.; Puig-Serra, P.; Moreno-Gaona, P.; Martin, M.C.; Moya, F.J.; Quintana-Bustamante, O.; Garcia-Silva, S.; Carcaboso, A.M.; Petazzi, P.; et al. In Vivo CRISPR/Cas9 Targeting of Fusion Oncogenes for Selective Elimination of Cancer Cells. Nat. Commun. 2020, 11, 5060. [Google Scholar] [CrossRef] [PubMed]
- Vuelta, E.; García-Tuñón, I.; Hernández-Carabias, P.; Méndez, L.; Sánchez-Martín, M. Future Approaches for Treating Chronic Myeloid Leukemia: CRISPR Therapy. Biology 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Nisar, S.; Mukherjee, S.; Saha, N.; Yarravarapu, N.; Lone, S.N.; Masoodi, T.; Chauhan, R.; Maacha, S.; Bagga, P.; et al. Integration of CRISPR/Cas9 with Artificial Intelligence for Improved Cancer Therapeutics. J. Transl. Med. 2022, 20, 534. [Google Scholar] [CrossRef]
- Chuai, G.-H.; Wang, Q.-L.; Liu, Q. In Silico Meets In Vivo: Towards Computational CRISPR-Based sgRNA Design. Trends Biotechnol. 2017, 35, 12–21. [Google Scholar] [CrossRef] [PubMed]

| Trial Name (Clinical Trial Number) | Compound(s) | Combination | Rationale | Phase | Number of Patients | Outcome | Status |
|---|---|---|---|---|---|---|---|
| ASC4FIRST (NCT04971226) | Asciminib (80 mg QD) | Monotherapy | Comparison of efficacy of ASC as a first-line therapy against other first-line TKI: | III | TBD | Active Not recruiting | |
| IM 400 mg QD | 404 | ||||||
| NL 300 mg BID | |||||||
| DA 100 mg QD | Newly diagnosed CML-CP | ||||||
| BOS 400 mg QD | |||||||
| ASC2ESCALATE (NCT05384587) | Asciminib | Monotherapy | Safety and efficacy of ASC dose escalation | II | 92 | TBD | Recruiting |
| (80 mg, 200 mg QD, 200 mg BID) | CML-CP with prior TKI failure | ||||||
| PACE (NCT01207440) | Ponatinib (45 mg QD) | Monotherapy | Safety and efficacy of PON to overcome the T315I mutation | II | 449 | CML-CP (267 patients): | Completed |
| 60% MCyR | |||||||
| 54% CCyR | |||||||
| CML-CP, AP, BP | 40% MMR | ||||||
| Ph+ ALL | 24% MR4.5 | ||||||
| Resistant to DA, NL or have the T315I mutation | CML-AP (83 patients): | ||||||
| 61% MHR | |||||||
| 49% MCyR | |||||||
| 31% CCyR | |||||||
| 22% MMR | |||||||
| CML-BP (62 patients): | |||||||
| 31% MHR | |||||||
| 23% MCyR | |||||||
| 38% CCyR | |||||||
| 13% MMR | |||||||
| Ph+ ALL (32): | |||||||
| 47% MCyR | |||||||
| 38% CCyR | |||||||
| OPTIC (NCT02467270) | Ponatinib (45 mg, 30 mg, 15 mg QD) | Monotherapy | Safety and efficacy of 3 different starting doses of PON | II | 283 | Patients with T315I MR2 | Active Not recruiting |
| 64% (45 mg) | |||||||
| 25% (30 mg) | |||||||
| 16% (15 mg) | |||||||
| Patients without T315I MR2 | |||||||
| CML-CP with prior TKI failure or have the T315I mutation | 59% (45 mg) | ||||||
| 44% (30 mg) | |||||||
| 46% (15 mg) | |||||||
| DAC-VEN-PON (NCT04188405) | Ponatinib (45 mg QD) | +Decitabine (20 mg/m2 QD) +Venetoclax (400 mg QD) | Safety and efficacy of DAC-VEN-PON combination Assess BCL-2 dependency in response to treatment regimen | II | 14 | 11 patients responded | Active Not recruiting |
| CML-AP, BP with prior TKI or chemotherapy exposure | 40% CR/CRi | ||||||
| 33% MLFS | |||||||
| Nilo Post-STIM (NCT01774630) | Nilotinib (300 mg BID) | Monotherapy | Safety and efficacy of NL to achieve 2nd TFR after prior IM discontinuation | II | 31 | 7 patients discontinued therapy after experiencing adverse events | Completed |
| CML patients with molecular relapse after IM discontinuation attempt | 22 patients achieved TFR rates of: | ||||||
| |||||||
| DAstop2 (NCT03573596) | Dasatinib (100 mg, 70 mg QD) | Monotherapy | Safety and efficacy of DA to achieve 2nd TFR after prior failed TKI discontinuation attempt | II | 94 | 62 patients attempted 2nd TFR attempt with TFR rates of: | Recruiting |
| CML-CP patients who relapsed after 3 years of TKI therapy and achieved deep molecular response (EURO-SKI) |
| ||||||
| HQP1351 vs. BAT (NCT04126681) | Olverembatinib (40 mg QD) | Monotherapy | Safety and efficacy of olverembatinib compared to best available therapy (BAT) | II | 144 | 97 patients discontinued treatment due to adverse events | Active Not recruiting |
| Olverembatinib arm: | |||||||
| CML-CP patients resistant and/or intolerant to IM, DA, and NL | 85% CHR | ||||||
| 48% MCyR | |||||||
| 36% CCyR | |||||||
| 27% MMR | |||||||
| BAT arm: | |||||||
| 35% CR | |||||||
| 30% MCyR | |||||||
| 16% CCyR | |||||||
| 8% MMR | |||||||
| HQP1351-CP (NCT03883087) | Olverembatinib (40 mg QD) | Monotherapy | Safety and efficacy of olverembatinib against CML-CP | I/II | 127 | 79% MCyR (95% CI: 70–85%) | Active Not recruiting |
| 69% CCyR (95% CI: 60–77%) | |||||||
| CML-CP patients with the T315I mutation | 56% MMR (95% CI: 47–64%) | ||||||
| 44% MR4.0 (95% CI: 35–52%) | |||||||
| 39% MR4.5 (95% CI: 47–64%) | |||||||
| HQP1351-AP (NCT03883100) | Olverembatinib (40 mg QD) | Monotherapy | Safety and efficacy of olverembatinib against CML-AP | II | 38 | 47% MCyR (95% CI: 31–62%) | Active Not recruiting |
| 47% CCyR (95% CI: 31–62%) | |||||||
| CML-AP patients with the T315I mutation | 45% MMR (95% CI: 28–60%) | ||||||
| 39% MR4.0 (95% CI: 22–56%) | |||||||
| 32% MR4.5 (95% CI: 18–48%) | |||||||
| HQP1351-CML/Ph+ ALL (NCT04260022) | Olverembatinib (30 mg, 40 mg, 50 mg QD) | Monotherapy +Blinatumomab | Safety and efficacy of olverembatinib against CML-CP, AP and BP or Ph+ ALL in patients resistant to PON and ASC | I | 57 CML-CP 19 Ph+ ALL | 12 CML-CP and 7 Ph+ ALL patients discontinued treatment due to adverse events | Recruiting |
| CML-CP: | |||||||
| 57% CCyR | |||||||
| 43% MMR | |||||||
| CML-CP patients with ≥4 TKI failures: | |||||||
| 57% CCyR | |||||||
| 42% MMR | |||||||
| CML-CP with T315I: | |||||||
| Previously treated and resistant to PON and/or ASC | 60% CCyR | ||||||
| 44% MMR | |||||||
| CML-CP with prior PON failure: | |||||||
| 53% CCyR | |||||||
| 38% MMR | |||||||
| CML-CP with prior ASC failure: | |||||||
| 43% CCyR | |||||||
| 38% MMR | |||||||
| CML-CP with prior PON and ASC failure: | |||||||
| 25% MMR | |||||||
| Ph+ ALL: | |||||||
| 23% MMR | |||||||
| HQP1351-VEN (NCT05594784) | Olverembatinib (40 mg QD) | +Venetoclax (100 mg d1, 200 mg d2, 400 mg d3–d28) +Chemotherapy (10 mg methotrexate, 50 mg cytarabine, 10 mg dexamethasone) | Safety and efficacy of olverembatinib combined with venetoclax and chemotherapy against Ph+ ALL | II | 31 Ph+ ALL patients with relapsed or refractory disease | 32% (10) MMR 6.5% (2) < MMR 61% (19) MR4.5 | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, A.; Liu, X.; Fruhstorfer, C.; Jiang, X. Clinical Insights into Structure, Regulation, and Targeting of ABL Kinases in Human Leukemia. Int. J. Mol. Sci. 2024, 25, 3307. https://doi.org/10.3390/ijms25063307
Wu A, Liu X, Fruhstorfer C, Jiang X. Clinical Insights into Structure, Regulation, and Targeting of ABL Kinases in Human Leukemia. International Journal of Molecular Sciences. 2024; 25(6):3307. https://doi.org/10.3390/ijms25063307
Chicago/Turabian StyleWu, Andrew, Xiaohu Liu, Clark Fruhstorfer, and Xiaoyan Jiang. 2024. "Clinical Insights into Structure, Regulation, and Targeting of ABL Kinases in Human Leukemia" International Journal of Molecular Sciences 25, no. 6: 3307. https://doi.org/10.3390/ijms25063307
APA StyleWu, A., Liu, X., Fruhstorfer, C., & Jiang, X. (2024). Clinical Insights into Structure, Regulation, and Targeting of ABL Kinases in Human Leukemia. International Journal of Molecular Sciences, 25(6), 3307. https://doi.org/10.3390/ijms25063307





