Osmotic Pressure and Its Biological Implications
Abstract
:1. Introduction
2. Theories of Osmotic Pressure
2.1. Ideal Dilute Mixtures
2.1.1. van ‘t Hoff Equation
2.1.2. Morse Equation
2.2. Non-Ideal Dilute Solutions
Virial Expansion Model
2.3. Non-Ideal Concentrated Solutions
2.3.1. van Laar Equation
2.3.2. Free Solvent Model
3. Roles of Osmotic Pressure
3.1. Impact of Osmotic Pressure on the Symmetry of Cell Division
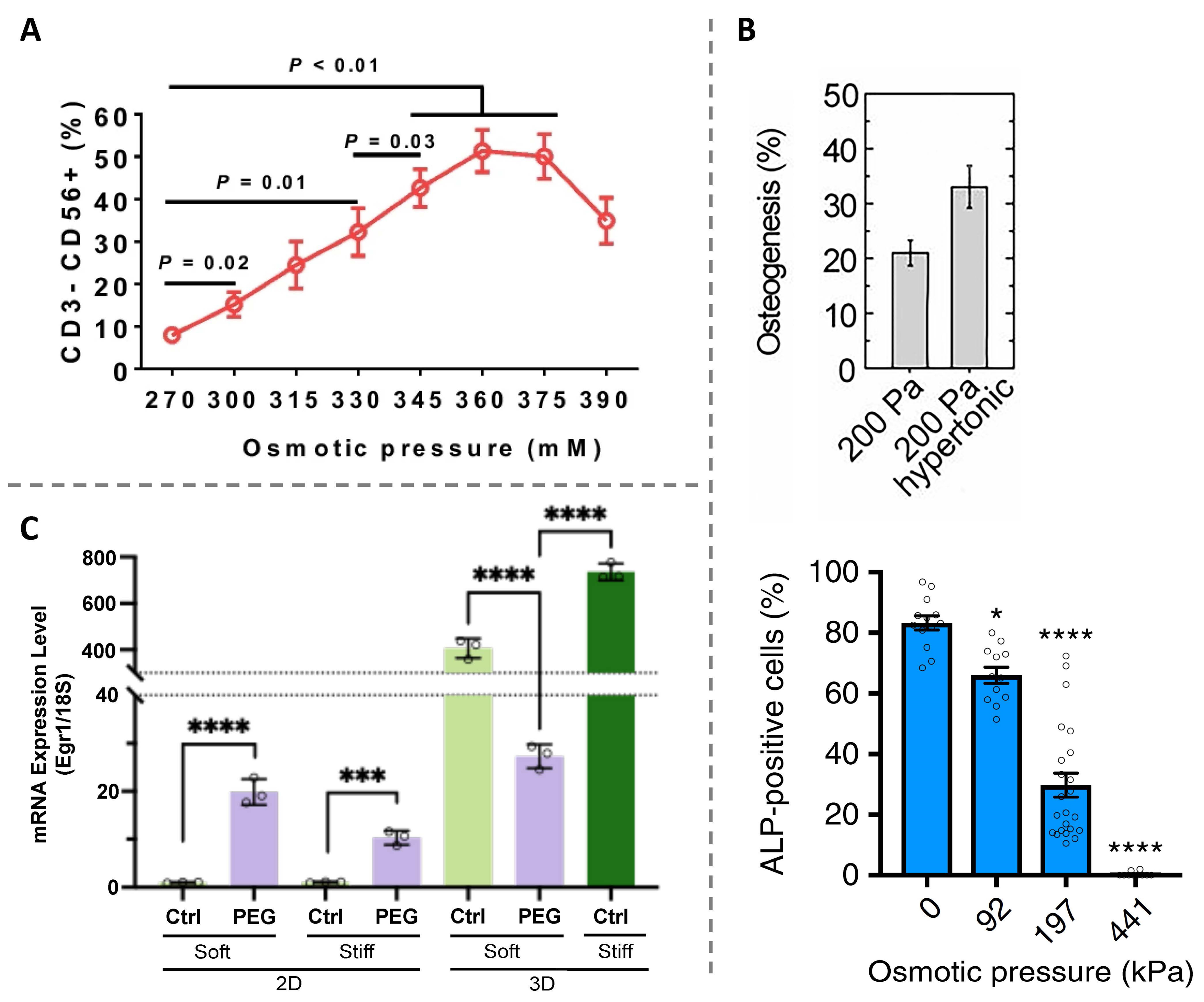
3.2. Impact of Osmotic Pressure on Cell Differentiation
3.3. Impact of Osmotic Pressure on the Lipid Phase Behavior
4. Conclusions and Future Prospectives
Author Contributions
Funding
Conflicts of Interest
References
- Raghunathan, A.V.; Aluru, N.R. Molecular understanding of osmosis in semipermeable membranes. Phys. Rev. Lett. 2006, 97, 024501. [Google Scholar] [CrossRef]
- van’t Hoff, J.H. The role of osmotic pressure in the analogy between solutions and gases. J. Membr. Sci. 1995, 100, 39–44. [Google Scholar] [CrossRef]
- Hosseni, A.; Ashbaugh, H.S. Osmotic force balance evaluation of aqueous electrolyte osmotic pressures and chemical potentials. J. Chem. Theory Comput. 2023, 19, 8826–8838. [Google Scholar] [CrossRef]
- van Rensburg, E.J.J. Osmotic pressure of compressed lattice knots. Phys. Rev. E 2019, 100, 012501. [Google Scholar] [CrossRef]
- Bowler, M.G. The physics of osmotic pressure. Eur. J. Phys. 2017, 38, 055102. [Google Scholar] [CrossRef]
- Yasuda, T.; Sakumichi, N.; Chung, U.I.; Sakai, T. Universal equation of state describes osmotic pressure throughout gelation process. Phys. Rev. Lett. 2020, 125, 267801. [Google Scholar] [CrossRef]
- Doan-Nguyen, T.P.; Mantala, K.; Atithep, T.; Crespy, D. Osmotic pressure as driving force for reducing the size of nanoparticles in emulsions. ACS Nano 2022, 17, 940–954. [Google Scholar] [CrossRef]
- Liu, C.; An, Y.P.; Yang, J.; Guo, B.B.; Yu, H.H.; Xu, Z.K. Osmotic pressure as driving force for recovering ionic liquids from aqueous solutions. J. Membr. Sci. 2020, 599, 117835. [Google Scholar] [CrossRef]
- Skilhagen, S.E.; Dugstad, J.E.; Aaberg, R.J. Osmotic power–power production based on the osmotic pressure difference between waters with varying salt gradients. Desalination 2008, 220, 476–482. [Google Scholar] [CrossRef]
- Obruca, S.; Sedlacek, P.; Mravec, F.; Krzyzanek, V.; Nebesarova, J.; Samek, O.; Kucera, D.; Benesova, P.; Hrubanova, K.; Milerova, M.; et al. The presence of PHB granules in cytoplasm protects non-halophilic bacterial cells against the harmful impact of hypertonic environments. New Biotechnol. 2017, 39, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Di Ciano, C.; Rotstein, O.D.; Kapus, A. Osmotic stress activates Rac and Cdc42 in neutrophils: Role in hypertonicity-induced actin polymerization. Am. J. Physiol. Cell Physiol. 2002, 282, C271–C279. [Google Scholar] [CrossRef]
- Conner, M.T.; Conner, A.C.; Bland, C.E.; Taylor, L.H.; Brown, J.E.; Parri, H.R.; Bill, R.M. Rapid aquaporin translocation regulates cellular water flow: Mechanism of hypotonicity-induced subcellular localization of aquaporin 1 water channel. J. Biol. Chem. 2012, 287, 11516–11525. [Google Scholar] [CrossRef]
- Márquez-Islas, R.; Pérez-Pacheco, A.; Salazar-Nieva, L.B.; Acevedo-Barrera, A.; Mendoza-García, E.; García-Valenzuela, A. Optical device and methodology for optical sensing of hemolysis in hypotonic media. Meas. Sci. Technol. 2020, 31, 095701. [Google Scholar] [CrossRef]
- Rowlands, D.S.; Kopetschny, B.H.; Badenhorst, C.E. The hydrating effects of hypertonic, isotonic and hypotonic sports drinks and waters on central hydration during continuous exercise: A systematic meta-analysis and perspective. Sports Med. 2022, 52, 349–375. [Google Scholar] [CrossRef]
- Paarvanova, B.; Tacheva, B.; Savova, G.; Karabaliev, M.; Georgieva, R. Hemolysis by saponin Is accelerated at hypertonic conditions. Molecules 2023, 28, 7096. [Google Scholar] [CrossRef] [PubMed]
- Brauer, A.M.; Shi, H.D.; Levin, P.A.; Huang, K.C. Physiological and regulatory convergence between osmotic and nutrient stress responses in microbes. Curr. Opin. Cell Biol. 2023, 81, 102170. [Google Scholar] [CrossRef]
- Rojas, E.; Theriot, J.A.; Huang, K.C. Response of Escherichia coli growth rate to osmotic shock. Proc. Natl. Acad. Sci. USA 2014, 111, 7807–7812. [Google Scholar] [CrossRef]
- Alric, B.; Formosa-Dague, C.; Dague, E.; Holt, L.J.; Delarue, M. Macromolecular crowding limits growth under pressure. Nat. Phys. 2022, 18, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.S.; Zhang, Q.K.; Liu, M.Y.; Zhou, H.P.; Ma, C.L.; Wang, P.P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, E.J.; Rodan, A.R. Intracellular ion control of WNK signaling. Annu. Rev. Physiol. 2023, 85, 383–406. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Li, B.; Zhao, Z.L.; Xu, G.K.; Feng, X.Q.; Gao, H.J. Mesoscopic dynamic model of epithelial cell division with cell-cell junction effects. Phys. Rev. E 2020, 102, 012405. [Google Scholar] [CrossRef]
- Efremov, Y.M.; Grebenik, E.A.; Sharipov, R.R.; Krasilnikova, I.A.; Kotova, S.L.; Akovantseva, A.A.; Bakaeva, Z.V.; Pinelis, V.G.; Surin, A.M.; Timashev, P.S. Viscoelasticity and volume of cortical neurons under glutamate excitotoxicity and osmotic challenges. Biophys. J. 2020, 119, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Koushki, N.; Ghagre, A.; Srivastava, L.K.; Molter, C.; Ehrlicher, A.J. Nuclear compression regulates YAP spatiotemporal fluctuations in living cells. Proc. Natl. Acad. Sci. USA 2023, 120, e2301285120. [Google Scholar] [CrossRef] [PubMed]
- Deviri, D.; Safran, S.A. Balance of osmotic pressures determines the nuclear-to-cytoplasmic volume ratio of the cell. Proc. Natl. Acad. Sci. USA 2022, 119, e2118301119. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Hou, J.X. Shape transformation of red blood cells induced by the osmotic pressure. Eur. J. Phys. 2020, 41, 065501. [Google Scholar] [CrossRef]
- Lew, V.L.; Macdonald, L.; Ginsburg, H.; Krugliak, M.; Tiffert, T. Excess haemoglobin digestion by malaria parasites: A strategy to prevent premature host cell lysis. Blood Cells Mol. Dis. 2004, 32, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, Y.W.; Han, L.C.; Xu, F.; Li, F. Cell mechanical microenvironment for cell volume regulation. J. Cell. Physiol. 2020, 235, 4070–4081. [Google Scholar] [CrossRef]
- Liu, Y.M.; Wu, W.J.; Feng, S.; Chen, Y.; Wu, X.P.; Zhang, Q.C.; Wu, S.Q. Dynamic response of the cell traction force to osmotic shock. Microsystems Nanoeng. 2023, 9, 131. [Google Scholar] [CrossRef]
- Fajrial, A.K.; Liu, K.; Gao, Y.; Gu, J.H.; Lakerveld, R.; Ding, X.Y. Characterization of single-cell osmotic swelling dynamics for new physical biomarkers. Anal. Chem. 2021, 93, 1317–1325. [Google Scholar] [CrossRef]
- Kanias, T.; Sinchar, D.; Osei-Hwedieh, D.; Baust, J.J.; Jordan, A.; Zimring, J.C.; Waterman, H.R.; de Wolski, K.S.; Acker, J.P.; Gladwin, M.T. Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease. Transfusion 2016, 56, 2571–2583. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Anastasiadi, A.T.; Valsami, S.I.; Stamoulis, K.E.; Papageorgiou, E.G.; Politou, M.; Papassideri, I.S.; Kriebardis, A.G.; Antonelou, M.H. Osmotic hemolysis is a donor-specific feature of red blood cells under various storage conditions and genetic backgrounds. Transfusion 2021, 61, 2538–2544. [Google Scholar] [CrossRef]
- Gabaj, N.N.; Miler, M.; Vrtaric, A.; Celap, I.; Bocan, M.; Filipi, P.; Biljak, V.R.; Simundic, A.M.; Smolcic, V.S.; Kocijancic, M. Comparison of three different protocols for obtaining hemolysis. Clin. Chem. Lab. Med. 2022, 60, 714–725. [Google Scholar] [CrossRef]
- Delano, M.D. Simple physical constraints in hemolysis. J. Theor. Biol. 1995, 175, 517–524. [Google Scholar] [CrossRef]
- Hao, L.M.; Chen, Q.; Lu, J.K.; Li, Z.Y.; Guo, C.J.; Qian, P.; Yu, J.Y.; Xing, X.H. A novel hypotonic sports drink containing a high molecular weight polysaccharide. Food Funct. 2014, 5, 961–965. [Google Scholar] [CrossRef]
- Stewart, M.P.; Helenius, J.; Toyoda, Y.; Ramanathan, S.P.; Muller, D.J.; Hyman, A.A. Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding. Nature 2011, 469, 226–230. [Google Scholar] [CrossRef]
- Ramkumar, N.; Baum, B. Coupling changes in cell shape to chromosome segregation. Nat. Rev. Mol. Cell Biol. 2016, 17, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Kang, J.H.; Oh, S.; Kirschner, M.W.; Mitchison, T.J.; Manalis, S. Resonant microchannel volume and mass measurements show that suspended cells swell during mitosis. J. Cell Biol. 2015, 211, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, A.V.; Baum, B.; Matthews, H.K. The Mechanics of Mitotic Cell Rounding. Front. Cell Dev. Biol. 2020, 8, 687. [Google Scholar] [CrossRef]
- Flegler, V.J.; Rasmussen, T.; Bottcher, B. How functional lipids affect the structure and gating of mechanosensitive MscS-like channels. Int. J. Mol. Sci. 2022, 23, 15071. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.J.; Hiiragi, T. Integration of luminal pressure and signalling in tissue self-organization. Development 2020, 147, dev181297. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, L.; Shao, Y.F.; Wei, J.C.; Song, R.P.; Zheng, S.J.; Li, Y.Q.; Song, F. Effects of the Laplace pressure on the cells during cytokinesis. iScience 2021, 24, 102945. [Google Scholar] [CrossRef]
- La Porta, C.A.M.; Ghilardi, A.; Pasini, M.; Laurson, L.; Alava, M.J.; Zapperi, S.; Ben Amar, M. Osmotic stress affects functional properties of human melanoma cell lines. Eur. Phys. J. Plus. 2015, 130, 64. [Google Scholar] [CrossRef]
- Ortiz, A.; Sanz, A.B. A pathway of osmotic stress-induced necroptosis. Nat. Rev. Nephrol. 2022, 18, 609–610. [Google Scholar] [CrossRef]
- Yamagishi, A.; Ito, F.; Nakamura, C. Study on cancer cell invasiveness via application of mechanical force to induce chloride ion efflux. Anal. Chem. 2021, 93, 9032–9035. [Google Scholar] [CrossRef]
- Raimann, J.G.; Tzamaloukas, A.H.; Levin, N.W.; Ing, T.S. Osmotic Pressure in Clinical Medicine with an Emphasis on Dialysis. Semin. Dial. 2017, 30, 69–79. [Google Scholar] [CrossRef]
- Wang, Z.; Irianto, J.; Kazun, S.; Wang, W.; Knight, M.M. The rate of hypo-osmotic challenge influences regulatory volume decrease (RVD) and mechanical properties of articular chondrocytes. Osteoarthr. Cartil. 2015, 23, 289–299. [Google Scholar] [CrossRef]
- Boucher, R.C. Muco-Obstructive Lung Diseases. N. Engl. J. Med. 2019, 380, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Orozco, D.; Quigley, R. Dialysis disequilibrium syndrome. Pediatr. Nephrol. 2012, 27, 2205–2211. [Google Scholar] [CrossRef]
- Otvos, B.; Kshettry, V.R.; Benzel, E.C. The history of urea as a hyperosmolar agent to decrease brain swelling. Neurosurg. Focus 2014, 36, E3. [Google Scholar] [CrossRef] [PubMed]
- Popli, S.; Tzamaloukas, A.H.; Ing, T.S. Osmotic diuresis-induced hypernatremia: Better explained by solute-free water clearance or electrolyte-free water clearance? Int. Urol. Nephrol. 2014, 46, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Laar, J.J.v. Über die genauen Formeln für den osmotischen Druck, für die Änderungen der Löslichkeit, für Gefrierpunkts- und Siedepunktsänderungen, und für die Lösungs- und Verdünnungswärmen bei in Lösung dissociierten Körpern. Z. Phys. Chem. 1894, 15U, 457–497. [Google Scholar] [CrossRef]
- Dao, V.N.T.; Morris, P.H.; Dux, P.F. On equations for the total suction and its matric and osmotic components. Cem. Concr. Res. 2008, 38, 1302–1305. [Google Scholar] [CrossRef]
- Chialvo, A.A. Molecular-based description of the osmotic second virial coefficients of electrolytes: Rigorous formal links to solute-solvent interaction asymmetry, virial expansion paths, and experimental evidence. J. Phys. Chem. B 2022, 126, 4339–4353. [Google Scholar] [CrossRef]
- Yousef, M.; Datta, R.; Rodgers, V. Free-solvent model of osmotic pressure revisited: Application to concentrated IgG solution under physiological conditions. J. Colloid Interface Sci. 1998, 197, 108–118. [Google Scholar] [CrossRef]
- Passamonti, F.J.; de Chialvo, M.R.G.; Chialvo, A.C. Application of the multicomponent Gibbs-Duhem equation for the activity coefficient evaluation of a ternary aqueous solution with an electrolyte and a molecular solute from osmotic coefficient data. Fluid Phase Equilib. 2023, 568, 113740. [Google Scholar] [CrossRef]
- Smolko, A.; Bauer, N.; Pavlovic, I.; Pencik, A.; Novak, O.; Salopek-Sondi, B. Altered rootgrowth, auxin metabolism and distribution in arabidopsis thaliana exposed to salt and osmotic stress. Int. J. Mol. Sci. 2021, 22, 7993. [Google Scholar] [CrossRef]
- Iqbal, A.; Dong, Q.; Wang, X.R.; Gui, H.P.; Zhang, H.H.; Zhang, X.L.; Song, M.Z. High nitrogen enhance drought tolerance in cotton through antioxidant enzymatic activities, nitrogen metabolism and osmotic adjustment. Plants 2020, 9, 178. [Google Scholar] [CrossRef]
- Takahashi, Y.; Zhang, J.B.; Hsu, P.K.; Ceciliato, P.H.O.; Zhang, L.; Dubeaux, G.; Munemasa, S.; Ge, C.N.; Zhao, Y.D.; Hauser, F.; et al. MAP3Kinase-dependent SnRK2-kinase activation is required for abscisic acid signal transduction and rapid osmotic stress response. Nat. Commun. 2020, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Wang, D.; Chen, J.; Long, T.H.; Zhong, C.J.; Li, Y.T. Effects of glucose and osmotic pressure on the proliferation and cell cycle of human chorionic trophoblast cells. Open Life Sci. 2022, 17, 1418–1428. [Google Scholar] [CrossRef]
- Freeman, S.A.; Uderhardt, S.; Saric, A.; Collins, R.F.; Buckley, C.M.; Mylvaganam, S.; Boroumand, P.; Plumb, J.; Germain, R.N.; Ren, D.J.; et al. Lipid-gated monovalent ion fluxes regulate endocytic traffic and support immune surveillance. Science 2020, 367, 301–305. [Google Scholar] [CrossRef]
- Kuo, S.P.; Chiang, P.P.; Nippert, A.R.; Newman, E.A. Spatial organization and dynamics of the extracellular space in the mouse retina. J. Neurosci. 2020, 40, 7785–7794. [Google Scholar] [CrossRef]
- Hoff, J.H.v.t. Die Rolle des osmotischen Druckes in der Analogie zwischen Lösungen und Gasen. Z. Phys. Chem. 1887, 1U, 481–508. [Google Scholar] [CrossRef]
- Van Houten, J. Nobel Prize in Chemistry 1901—Jacobus Henricus van’t Hoff (1852–1911)—For his pioneering work on chemical dynamics and osmotic pressure in solutions. J. Chem. Educ. 2001, 78, 1572–1573. [Google Scholar]
- Nagy, E.; Hegedus, I.; Rehman, D.; Wei, Q.T.J.; Ahdab, Y.D.; Lienhard, J.H. The need for accurate osmotic pressure and mass transfer resistances in modeling osmotically driven membrane processes. Membranes 2021, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Hammel, H.T. Colligative properties of a solution. Science 1976, 192, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Olver, D.J.; Benson, J.D. Meta-analysis of the Boyle van ‘t Hoff relation: Turgor and leak models explain non-ideal volume equilibrium. Cryobiology 2023, 113, 104581. [Google Scholar] [CrossRef] [PubMed]
- Suarez, D.L. Thermodynamics of the Soil Solution; CRC Press: Boca Raton, FL, USA, 2018; pp. 97–134. [Google Scholar]
- Salmani, H.J.; Karkhanechi, H.; Jeon, S.; Matsuyama, H. Calculating osmotic pressure of liquid mixtures by association theory for sustainable separating of solvents by membrane processes. J. Ind. Eng. Chem. 2022, 109, 137–146. [Google Scholar] [CrossRef]
- Spagnoli, C.; Beyder, A.; Besch, S.; Sachs, F. Atomic force microscopy analysis of cell volume regulation. Phys. Rev. E 2008, 78, 031916. [Google Scholar] [CrossRef] [PubMed]
- Fritz, C.J.; Scalia, J.; Shackelford, C.D.; Malusis, M.A. Determining maximum chemico-osmotic pressure difference across clay membranes. J. Geotech. Geoenviron. 2020, 146, 06019018. [Google Scholar] [CrossRef]
- Granik, V.T.; Smith, B.R.; Lee, S.C.; Ferrari, M. Osmotic pressures for binary solutions of non-electrolytes. Biomed. Microdevices 2002, 4, 309–321. [Google Scholar] [CrossRef]
- Liu, S.B.; Yang, H.Q.; Bian, Z.T.; Tao, R.; Chen, X.; Lu, T.J. Regulation on mechanical properties of spherically cellular fruits under osmotic stress. J. Mech. Phys. Solids 2019, 127, 182–190. [Google Scholar] [CrossRef]
- Moggia, E. Osmotic coefficients of electrolyte solutions. J. Phys. Chem. B 2008, 112, 1212–1217. [Google Scholar] [CrossRef]
- Zargarzadeh, L.; Elliott, J.A.W. Comparison of the osmotic virial equation with the margules activity model for solid-liquid equilibrium. J. Phys. Chem. B 2019, 123, 1099–1107. [Google Scholar] [CrossRef]
- Horkay, F.; Douglas, J.F. Evidence of many-body interactions in the virial coefficients of polyelectrolyte gels. Gels 2022, 8, 96. [Google Scholar] [CrossRef]
- Chremos, A.; Douglas, J.F.; Basser, P.J.; Horkay, F. Molecular dynamics study of the swelling and osmotic properties of compact nanogel particles. Soft Matter 2022, 18, 6278–6290. [Google Scholar] [CrossRef]
- Casassa, E.F.; Eisenberg, H. Thermodynamic analysis of multicomponent solutions. Adv. Protein Chem. 1964, 19, 287–395. [Google Scholar]
- Passamonti, F.J.; de Chialvo, M.R.G.; Chialvo, A.C. Evaluation of the activity coefficients of ternary molecular solutions from osmotic coefficient data. Fluid Phase Equilib. 2022, 559, 113464. [Google Scholar] [CrossRef]
- Peyrovedin, H.; Khorram, M.; Shariati, A. Application of hard-core Exponential-6 intermolecular potential function to determine the second osmotic virial coefficients of polymer solutions. Polym. Bull. 2021, 78, 931–950. [Google Scholar] [CrossRef]
- Caracciolo, S.; Mognetti, B.M.; Pelissetto, A. Virial coefficients and osmotic pressure in polymer solutions in good-solvent conditions. J. Chem. Phys. 2006, 125, 094903. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, S.; Mognetti, B.M.; Pelissetto, A. Third virial coefficient for 4-arm and 6-arm star polymers. Macromol. Theory Simul. 2008, 17, 67–72. [Google Scholar] [CrossRef]
- Mussel, M.; Basser, P.J.; Horkay, F. Effects of mono- and divalent cations on the structure and thermodynamic properties of polyelectrolyte gels. Soft Matter 2019, 15, 4153–4161. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, M.; Sharif, A.O.; Derwish, G.; Al-Aibi, S.; Altaee, A. Draw solutions for Forward Osmosis process: Osmotic pressure of binary and ternary aqueous solutions of magnesium chloride, sodium chloride, sucrose and maltose. J. Food Eng. 2015, 155, 10–15. [Google Scholar] [CrossRef]
- Smith, W.R.; Moucka, F.; Nezbeda, I. Osmotic pressure of aqueous electrolyte solutions via molecular simulations of chemical potentials: Application to NaCl. Fluid Phase Equilib. 2016, 407, 76–83. [Google Scholar] [CrossRef]
- Smith, W.R.; Nezbeda, I.; Kolafa, J.; Moucka, F. Recent progress in the molecular simulation of thermodynamic properties of aqueous electrolyte solutions. Fluid Phase Equilib. 2018, 466, 19–30. [Google Scholar] [CrossRef]
- Wang, Y.; Rodgers, V. Free-solvent model shows osmotic pressure is the dominant factor in limiting flux during protein ultrafiltration. J. Membr. Sci. 2008, 320, 335–343. [Google Scholar] [CrossRef]
- Yousef, M.; Datta, R.; Rodgers, V. Understanding nonidealities of the osmotic pressure of concentrated bovine serum albumin. J. Colloid Interface Sci. 1998, 207, 273–282. [Google Scholar] [CrossRef]
- Yousef, M.A.; Datta, R.; Rodgers, V.G.J. Confirmation of free solvent model assumptions in predicting the osmotic pressure of concentrated globular proteins. J. Colloid Interface Sci. 2001, 243, 321–325. [Google Scholar] [CrossRef]
- Yousef, M.A.; Datta, R.; Rodgers, V.G.J. Model of osmotic pressure for high concentrated binary protein solutions. Aiche J. 2002, 48, 913–917. [Google Scholar] [CrossRef]
- McBride, D.W.; Rodgers, V.G.J. Interpretation of negative second virial coefficients from non-attractive protein solution osmotic pressure data: An alternate perspective. Biophys. Chem. 2013, 184, 79–86. [Google Scholar] [CrossRef]
- Robinson, M.; Filice, C.T.; McRae, D.M.; Leonenko, Z. Atomic force microscopy and other scanning probe microscopy methods to study nanoscale domains in model lipid membranes. Adv. Phys. X 2023, 8, 2197623. [Google Scholar] [CrossRef]
- Sakuragi, T.; Nagata, S. Regulation of phospholipid distribution in the lipid bilayer by flippases and scramblases. Nat. Rev. Mol. Cell Biol. 2023, 24, 576–596. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. Elucidation of complex dynamic intermolecular interactions in membranes. Chem. Pharm. Bull. 2022, 70, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lapaquette, P.; Ducreux, A.; Morel, E.; Dalle, F. You shall not pass! Protective role of autophagic machinery in response to plasma membrane damage triggered by Candida albicans invasion. Autophagy 2022, 18, 2761–2762. [Google Scholar] [CrossRef] [PubMed]
- Batori, R.K.; Chen, F.; Bordan, Z.; Haigh, S.; Su, Y.C.; Verin, A.D.; Barman, S.A.; Stepp, D.W.; Chakraborty, T.; Lucas, R.; et al. Protective role of Cav-1 in pneumolysin-induced endothelial barrier dysfunction. Front. Immunol. 2022, 13, 945656. [Google Scholar] [CrossRef] [PubMed]
- Leonard, T.A.; Loose, M.; Martens, S. The membrane surface as a platform that organizes cellular and biochemical processes. Dev. Cell 2023, 58, 1315–1332. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, R.H. Physiology of extracorporeal gas exchange. Compr. Physiol. 2020, 10, 879–891. [Google Scholar] [PubMed]
- Chan, E.P.; Frieberg, B.R.; Ito, K.; Tarver, J.; Tyagi, M.; Zhang, W.X.; Coughlin, E.B.; Stafford, C.M.; Roy, A.; Rosenberg, S.; et al. Insights into the water transport mechanism in polymeric membranes from neutron scattering. Macromolecules 2020, 53, 1443–1450. [Google Scholar] [CrossRef]
- Shen, J.; Cai, Y.C.; Zhang, C.H.; Wei, W.; Chen, C.L.; Liu, L.M.; Yang, K.W.; Ma, Y.C.; Wang, Y.G.; Tseng, C.C.; et al. Fast water transport and molecular sieving through ultrathin ordered conjugated-polymer-framework membranes. Nat. Mater. 2022, 21, 1183–1190. [Google Scholar] [CrossRef]
- Hossain, M.; Blanchard, G.J. Effects of ethanol and n-butanol on the fluidity of supported lipid bilayers. Chem. Phys. Lipids 2021, 238, 105091. [Google Scholar] [CrossRef]
- Wu, D.; Grund, T.N.; Welsch, S.; Mills, D.J.; Michel, M.; Safarian, S.; Michel, H. Structural basis for amino acid exchange by a human heteromeric amino acid transporter. Proc. Natl. Acad. Sci. USA 2020, 117, 21281–21287. [Google Scholar] [CrossRef]
- Carbó, R.; Rodríguez, E. Relevance of sugar transport across the cell membrane. Int. J. Mol. Sci. 2023, 24, 6085. [Google Scholar] [CrossRef] [PubMed]
- Sorce, B.; Escobedo, C.; Toyoda, Y.; Stewart, M.P.; Cattin, C.J.; Newton, R.; Banerjee, I.; Stettler, A.; Roska, B.; Eaton, S.; et al. Mitotic cells contract actomyosin cortex and generate pressure to round against or escape epithelial confinement. Nat. Commun. 2015, 6, 8872. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, G.C.; Paridaen, J. The symmetry of neural stem cell and progenitor divisions in the vertebrate brain. Front. Cell Dev. Biol. 2022, 10, 885269. [Google Scholar]
- Manzano-López, J.; Monje-Casas, F. Asymmetric cell division and replicative aging: A new perspective from the spindle poles. Curr. Genet. 2020, 66, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Kimble, J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 2006, 441, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Hirani, N.; Bland, T.; Borrego-Pinto, J.; Wagner, S.; Kreysing, M.; Goehring, N.W. Cleavage furrow-directed cortical flows bias PAR polarization pathways to link cell polarity to cell division. Curr. Biol. 2023, 33, 4298–4311. [Google Scholar] [CrossRef]
- Chann, A.S.; Chen, Y.; Kinwel, T.; Humbert, P.O.; Russell, S.M. Scribble and E-cadherin cooperate to control symmetric daughter cell positioning by multiple mechanisms. J. Cell Sci. 2023, 136, 260547. [Google Scholar] [CrossRef]
- Garcia, A.G.; Soleilhac, M.; Minc, N.; Delacour, D. Regulation and functions of cell division in the intestinal tissue. Semin. Cell Dev. Biol. 2023, 150, 3–14. [Google Scholar] [CrossRef]
- Pomp, O.; Lim, H.Y.G.; Skory, R.M.; Moverley, A.A.; Tetlak, P.; Bissiere, S.; Plachta, N. A monoastral mitotic spindle determines lineage fate and position in the mouse embryo. Nat. Cell Biol. 2022, 24, 155–167. [Google Scholar] [CrossRef]
- Cuvelier, M.; Vangheel, J.; Thiels, W.; Ramon, H.; Jelier, R.; Smeets, B. Stability of asymmetric cell division: A deformable cell model of cytokinesis applied to C. elegans. Biophys. J. 2023, 122, 1858–1867. [Google Scholar] [CrossRef]
- Gupta, V.K.; Chaudhuri, O. Mechanical regulation of cell-cycle progression and division. Trends Cell Biol. 2022, 32, 773–785. [Google Scholar] [CrossRef]
- Leguay, K.; Decelle, B.; Elkholi, I.E.; Bouvier, M.; Cote, J.-F.; Carreno, S. Interphase microtubule disassembly is a signaling cue that drives cell rounding at mitotic entry. J. Cell Biol. 2022, 221, e202109065. [Google Scholar] [CrossRef]
- Chen, N.P.; Aretz, J.; Fässler, R. CDK1-cyclin-B1-induced kindlin degradation drives focal adhesion disassembly at mitotic entry. Nat. Cell Biol. 2022, 24, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Sarkar, A.; Zhang, H.; Agashe, A.; Wang, J.; Paul, R.; Gov, N.S.; DeLuca, J.G.; Nain, A.S. Mitotic outcomes and errors in fibrous environments. Proc. Natl. Acad. Sci. USA 2023, 120, e2120536120. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, O.M.; Le Berre, M.; Dimitracopoulos, A.; Bonazzi, D.; Zlotek-Zlotkiewicz, E.; Picone, R.; Duke, T.; Piel, M.; Baum, B. Mitotic rounding alters cell geometry to ensure efficient bipolar spindle formation. Dev. Cell 2013, 25, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Cadart, C.; Monnier, S.; Grilli, J.; Sáez, P.J.; Srivastava, N.; Attia, R.; Terriac, E.; Baum, B.; Cosentino-Lagomarsino, M.; Piel, M. Size control in mammalian cells involves modulation of both growth rate and cell cycle duration. Nat. Commun. 2018, 9, 3275. [Google Scholar] [CrossRef] [PubMed]
- Cadart, C.; Zlotek-Zlotkiewicz, E.; Le Berre, M.; Piel, M.; Matthews, H.K. Exploring the function of cell shape and size during mitosis. Dev. Cell 2014, 29, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Song, F. Measuring Laplace pressure and imaging the actin cortex during cytokinesis in cultured cells. STAR Protoc. 2022, 3, 101239. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Fletcher, R.B.; Ngai, J. Cellular mechanisms of epithelial stem cell self-renewal and differentiation during homeostasis and repair. WIREs Dev. Biol. 2020, 9, e361. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.M.; Gaitsch, H.; Alomari, S.; Lubelski, D.; Tyler, B.M. Molecular pathways and genomic landscape of glioblastoma stem cells: Opportunities for targeted therapy. Cancers 2022, 14, 3743. [Google Scholar] [CrossRef]
- Ayaz, G.; Yan, H.L.; Malik, N.; Huang, J. An updated view of the roles of p53 in embryonic stem cells. Stem Cells 2022, 40, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Goodell, M.A.; Rando, T.A. Ageing and rejuvenation of tissue stem cells and their niches. Nat. Rev. Mol. Cell Biol. 2023, 24, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.W.; Xu, R.S.; Lei, K.X.; Yuan, Q. Insights into skeletal stem cells. Bone Res. 2022, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Huang, H.C.; Li, G.X.; Yu, J.Y.; Fang, F.C.; Qiu, W. Dental-derived mesenchymal stem cell sheets: A prospective tissue engineering for regenerative medicine. Stem Cell Res. Ther. 2022, 13, 38. [Google Scholar] [CrossRef]
- Deus, I.A.; Mano, J.F.; Custódio, C.A. Perinatal tissues and cells in tissue engineering and regenerative medicine. Acta Biomater. 2020, 110, 1–14. [Google Scholar] [CrossRef]
- Fan, Y.L.; Zhao, H.C.; Feng, X.Q. Hypertonic pressure affects the pluripotency and self-renewal of mouse embryonic stem cells. Stem Cell Res. 2021, 56, 102537. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Gilbert, P.M.; Blau, H.M. Designing materials to direct stem-cell fate. Nature 2009, 462, 433–441. [Google Scholar] [CrossRef]
- Driskill, J.H.; Pan, D.J. Control of stem cell renewal and fate by YAP and TAZ. Nat. Rev. Mol. Cell Bio. 2023, 24, 895–911. [Google Scholar] [CrossRef]
- Skylar-Scott, M.A.; Huang, J.Y.; Lu, A.; Ng, A.H.M.; Duenki, T.; Liu, S.L.; Nam, L.L.; Damaraju, S.; Church, G.M.; Lewis, J.A. Orthogonally induced differentiation of stem cells for the programmatic patterning of vascularized organoids and bioprinted tissues. Nat. Biomed. Eng. 2022, 6, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Krauss, R.S.; Kann, A.P. Muscle stem cells get a new look: Dynamic cellular projections as sensors of the stem cell niche. Bioessays 2023, 45, 2200249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Chong, L.H.; Woon, J.Y.X.; Chua, T.X.; Cheruba, E.; Yip, A.; Li, H.Y.; Chiam, K.H.; Koh, C.G. Zyxin regulates embryonic stem cell fate by modulating mechanical and biochemical signaling interface. Commun. Biol. 2023, 6, 62. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, Y.Y.; Zhou, X.P.; Wang, J.K.; Shi, M.M.; Wang, J.; Li, F.C.; Chen, Q.X. Osmolarity controls the differentiation of adipose-derived stem cells into nucleus pulposus cells via histone demethylase KDM4B. Mol. Cell. Biochem. 2020, 472, 157–171. [Google Scholar] [CrossRef]
- Wen, W.; Chen, X.; Shen, X.Y.; Li, H.Y.; Zhang, F.; Fang, F.Q.; Zhang, X.B. Enhancing cord blood stem cell-derived NK cell growth and differentiation through hyperosmosis. Stem Cell Res. Ther. 2023, 14, 295. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Pegoraro, A.F.; Mao, A.; Zhou, E.H.; Arany, P.R.; Han, Y.; Burnette, D.T.; Jensen, M.H.; Kasza, K.E.; Moore, J.R.; et al. Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proc. Natl. Acad. Sci. USA 2017, 114, E8618–E8627. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Stowers, R.; Chaudhuri, O. Volume expansion and TRPV4 activation regulate stem cell fate in three-dimensional microenvironments. Nat. Commun. 2019, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Xie, J.; Piruska, A.; Huck, W.T.S. 3D microniches reveal the importance of cell size and shape. Nat. Commun. 2017, 8, 1962. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Lopez, P.A.; Lee, S.; Kim, T.S.; Kumar, S.; Schaffer, D.V. Egr1 is a 3D matrix-specific mediator of mechanosensitive stem cell lineage commitment. Sci. Adv. 2022, 8, eabm4646. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L.; de Mattos, G.F. Fifty years of the fluid-mosaic model of biomembrane structure and organization and Its importance in biomedicine with particular emphasis on membrane lipid replacement. Biomedicines 2022, 10, 1711. [Google Scholar] [CrossRef]
- Löwe, M.; Kalacheva, M.; Boersma, A.J.; Kedrov, A. The more the merrier: Effects of macromolecular crowding on the structure and dynamics of biological membranes. FEBS J. 2020, 287, 5039–5067. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Lipid rafts as major platforms for signaling regulation in cancer. Adv. Biol. Regul. 2015, 57, 130–146. [Google Scholar] [CrossRef]
- Li, L.; Hu, J.L.; Rózycki, B.; Wang, X.H.; Wu, H.L.; Song, F. Influence of lipid rafts on pattern formation during T-cell adhesion. New J. Phys. 2021, 23, 043052. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef]
- Li, L.; Hu, J.L.; Shi, X.H.; Shao, Y.F.; Song, F. Lipid rafts enhance the binding constant of membrane-anchored receptors and ligands. Soft Matter 2017, 13, 4294–4304. [Google Scholar] [CrossRef]
- Ayuyan, A.G.; Cohen, F.S. Raft composition at physiological temperature and pH in the absence of detergents. Biophys. J. 2008, 94, 2654–2666. [Google Scholar] [CrossRef]
- Hamada, T.; Kishimoto, Y.; Nagasaki, T.; Takagi, M. Lateral phase separation in tense membranes. Soft Matter 2011, 7, 9061–9068. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ando, J. Vascular endothelial cell membranes differentiate between stretch and shear stress through transitions in their lipid phases. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1178–H1185. [Google Scholar] [CrossRef] [PubMed]
- Wongsirojkul, N.; Shimokawa, N.; Opaprakasit, P.; Takagi, M.; Hamada, T. Osmotic-tension-induced membrane lateral organization. Langmuir 2020, 36, 2937–2945. [Google Scholar] [CrossRef] [PubMed]
- Wongsirojkul, N.; Masuta, A.; Shimokawa, N.; Takagi, M. Control of line tension at phase-separated lipid domain boundaries: Monounsaturated fatty acids with different chain lengths and osmotic pressure. Membranes 2022, 12, 781. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Su, W.C.; Purushothaman, S.; Ngassam, V.N.; Parikh, A.N. Permeability and line-tension-dependent response of polyunsaturated membranes to osmotic stresses. Biophys. J. 2018, 115, 1942–1955. [Google Scholar] [CrossRef] [PubMed]
- Colom, A.; Derivery, E.; Soleimanpour, S.; Tomba, C.; Dal Molin, M.; Sakai, N.; González-Gaitán, M.; Matile, S.; Roux, A. A fluorescent membrane tension probe. Nat. Chem. 2018, 10, 1118–1125. [Google Scholar] [CrossRef]
- Oglecka, K.; Rangamani, P.; Liedberg, B.; Kraut, R.S.; Parikh, A.N. Oscillatory phase separation in giant lipid vesicles induced by transmembrane osmotic differentials. eLife 2014, 3, e03695. [Google Scholar] [CrossRef] [PubMed]
- Riggi, M.; Niewola-Staszkowska, K.; Chiaruttini, N.; Colom, A.; Kusmider, B.; Mercier, V.; Soleimanpour, S.; Stahl, M.; Matile, S.; Roux, A.; et al. Decrease in plasma membrane tension triggers PtdIns(4,5)P2 phase separation to inactivate TORC2. Nat. Cell Biol. 2018, 20, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, J.L.; Rózycki, B.; Song, F. Intercellular receptor–ligand binding and thermal fluctuations facilitate receptor aggregation in adhering membranes. Nano Lett. 2020, 20, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, J.L.; Shi, X.H.; Rozycki, B.; Song, F. Interplay between cooperativity of intercellular receptor-ligand binding and coalescence of nanoscale lipid clusters in adhering membranes. Soft Matter 2021, 17, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, J.L.; Rózycki, B.; Ji, J.; Song, F. Interplay of receptor-ligand binding and lipid domain formation during cell adhesion. Front. Mol. Biosci. 2022, 9, 1019477. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ji, J.; Song, F.; Hu, J.L. Intercellular receptor-ligand binding: Effect of protein-membrane Interaction. J. Mol. Biol. 2023, 435, 167787. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, J.L.; Wu, H.P.; Song, F. Cis-interaction of ligands on a supported lipid bilayer affects their binding to cell adhesion receptors. Sci. China Phys. Mech. 2021, 64, 108712. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.H.; Wu, H.L.; Shao, Y.F.; Wu, H.P.; Song, F. Interplay between receptor-ligand binding and lipid domain formation depends on the mobility of ligands in cell-substrate adhesion. Front. Mol. Biosci. 2021, 8, 655662. [Google Scholar] [CrossRef]
- Li, L.; Hu, J.L.; Xu, G.K.; Song, F. Binding constant of cell adhesion receptors and substrate-immobilized ligands depends on the distribution of ligands. Phys. Rev. E 2018, 97, 012405. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.H.; Shao, Y.F.; Li, W.; Song, F. Entropic pressure between fluctuating membranes in multilayer systems. Sci. China Phys. Mech. 2018, 61, 128711. [Google Scholar] [CrossRef]
- Li, L.; Song, F. Entropic force between biomembranes. Acta Mech. Sin. 2016, 32, 970–975. [Google Scholar] [CrossRef]
- Roffay, C.; Molinard, G.; Kim, K.; Urbanska, M.; Andrade, V.; Barbarasa, V.; Nowak, P.; Mercier, V.; García-Calvo, J.; Matile, S.; et al. Passive coupling of membrane tension and cell volume during active response of cells to osmosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2103228118. [Google Scholar] [CrossRef]
- Saha, S.K.; Shibly, S.U.; Yamazaki, M. Membrane Tension in Negatively Charged Lipid Bilayers in a Buffer under Osmotic Pressure. J. Phys. Chem. B 2020, 124, 5588–5599. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, G.K.; Song, F. Impact of lipid rafts on the T-cell-receptor and peptide-major-histocompatibility-complex interactions under different measurement conditions. Phys. Rev. E 2017, 95, 012403. [Google Scholar] [CrossRef] [PubMed]
- Vian, A.; Pochitaloff, M.; Yen, S.-T.; Kim, S.; Pollock, J.; Liu, Y.; Sletten, E.M.; Campas, O. In situ quantification of osmotic pressure within living embryonic tissues. Nat. Commun. 2023, 14, 7023. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Li, Y.; Shao, Y.; Li, L.; Song, F. Osmotic Pressure and Its Biological Implications. Int. J. Mol. Sci. 2024, 25, 3310. https://doi.org/10.3390/ijms25063310
Zheng S, Li Y, Shao Y, Li L, Song F. Osmotic Pressure and Its Biological Implications. International Journal of Molecular Sciences. 2024; 25(6):3310. https://doi.org/10.3390/ijms25063310
Chicago/Turabian StyleZheng, Songjie, Yan Li, Yingfeng Shao, Long Li, and Fan Song. 2024. "Osmotic Pressure and Its Biological Implications" International Journal of Molecular Sciences 25, no. 6: 3310. https://doi.org/10.3390/ijms25063310
APA StyleZheng, S., Li, Y., Shao, Y., Li, L., & Song, F. (2024). Osmotic Pressure and Its Biological Implications. International Journal of Molecular Sciences, 25(6), 3310. https://doi.org/10.3390/ijms25063310






