Radiation Dermatitis: Radiation-Induced Effects on the Structural and Immunological Barrier Function of the Epidermis
Abstract
:1. Clinical Background
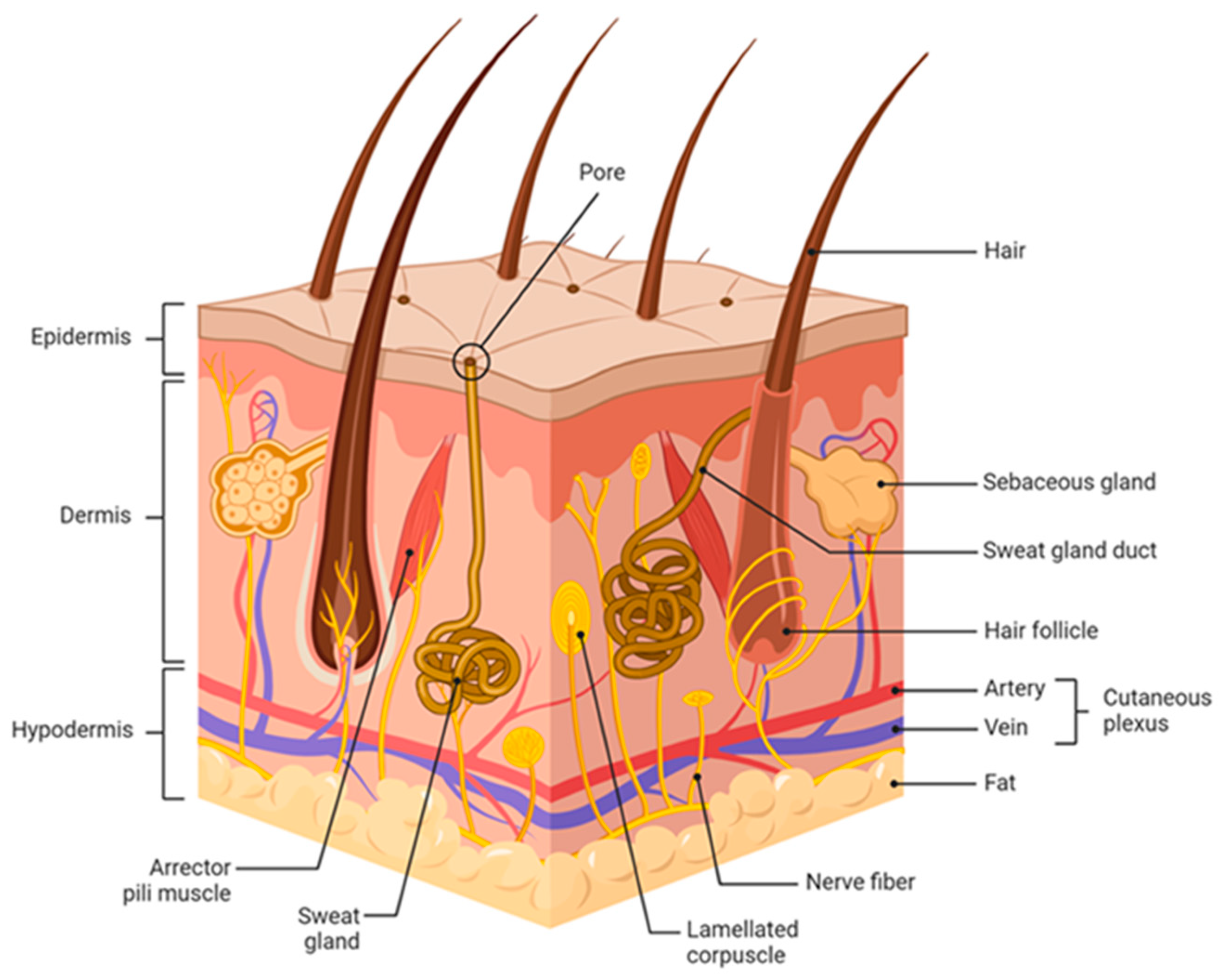
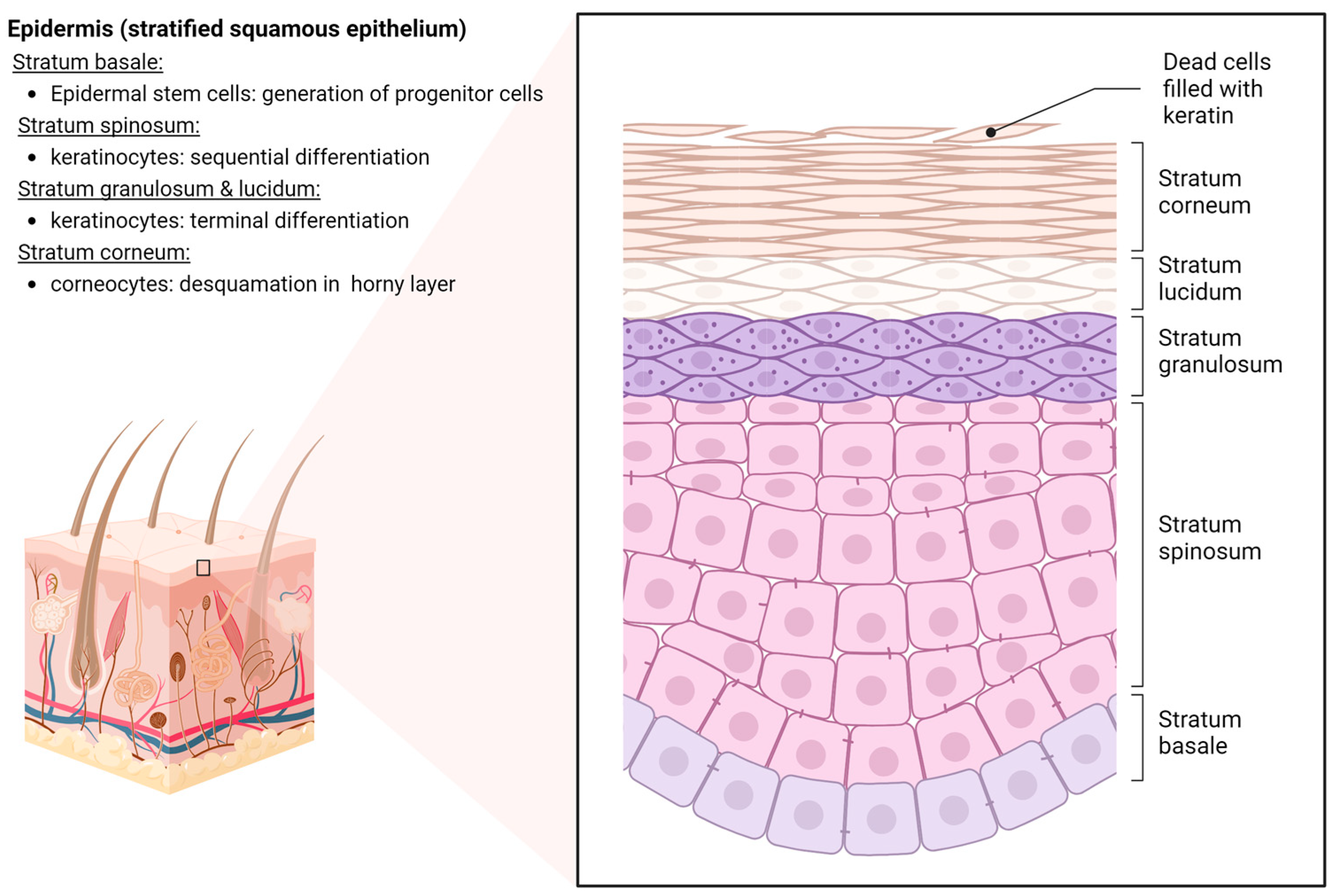
2. Anatomy and Physiology of Human Skin
3. Clinical Features of Radiation-Induced Skin Reactions
4. Pathophysiology of Radiation-Induced Skin Reactions
4.1. Suppressed Proliferation of Basal Cells following IR Exposure
4.2. Radiation-Induced Senescence in Keratinocytes
4.3. Secretion of Pro-Inflammatory Mediators by Senescent Keratinocytes
4.4. Immune Reactions of the Skin following IR Exposure
4.5. Structural Barrier Dysfunction of the Epidermis
5. Radiation-Induced Alopecia
5.1. Clinical Features
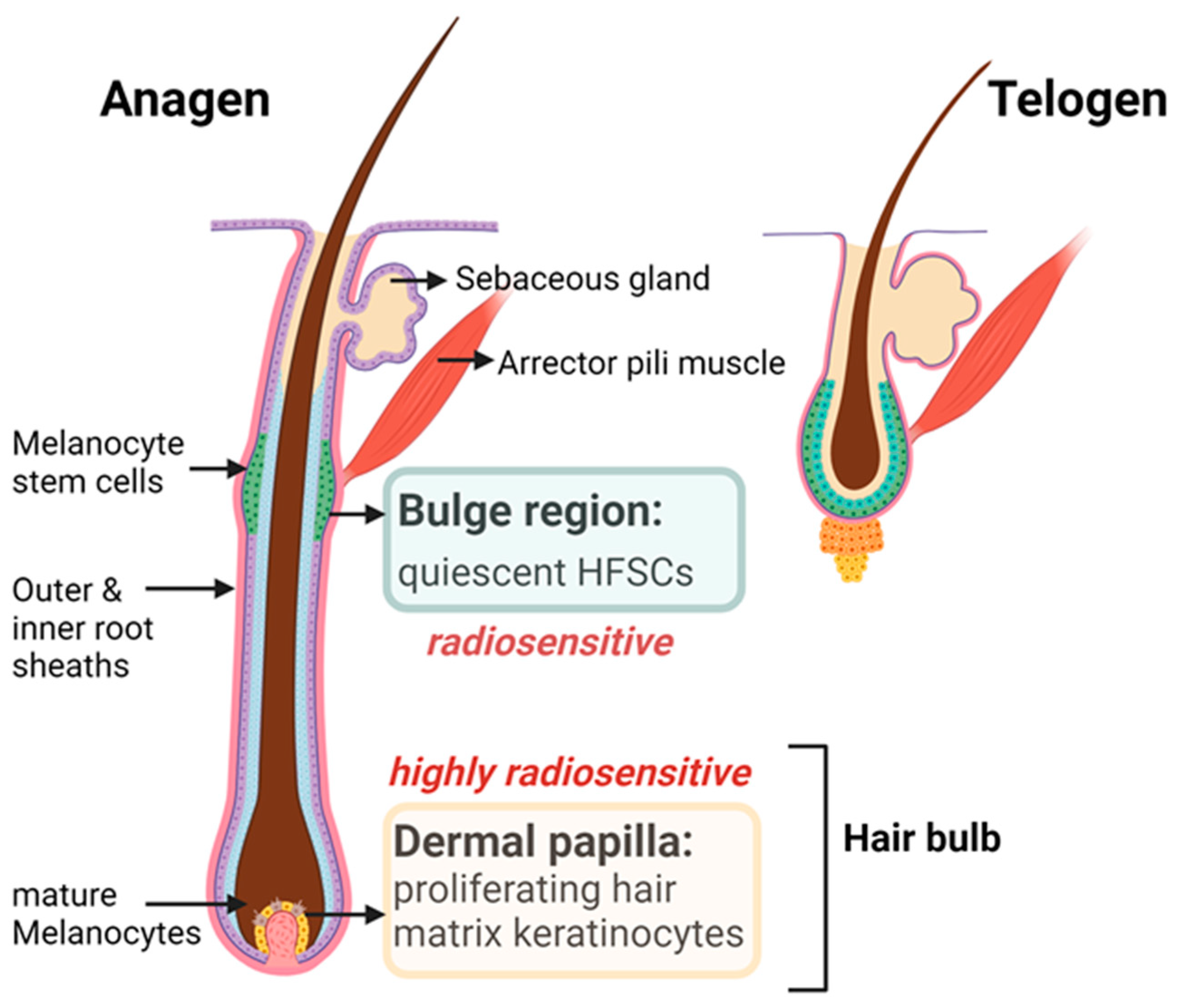
5.2. Anatomy and Cyclic Remodeling of the Hair Follicles
5.3. Pathobiology of HF Cycling in Response to IR
5.4. Radiation Injury to the Hair Pigmentary System
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ryan, J.L. Ionizing radiation: The good, the bad, and the ugly. J. Investig. Dermatol. 2012, 132, 985–993. [Google Scholar] [CrossRef]
- Yee, C.; Wang, K.; Asthana, R.; Drost, L.; Lam, H.; Lee, J.; Vesprini, D.; Leung, E.; DeAngelis, C.; Chow, E. Radiation-induced Skin Toxicity in Breast Cancer Patients: A Systematic Review of Randomized Trials. Clin. Breast Cancer 2018, 18, e825–e840. [Google Scholar] [CrossRef]
- Ramseier, J.Y.; Ferreira, M.N.; Leventhal, J.S. Dermatologic toxicities associated with radiation therapy in women with breast cancer. Int. J. Women’s Dermatol. 2020, 6, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Hecht, M.; Eckstein, M.; Rutzner, S.; von der Grün, J.; Illmer, T.; Klautke, G.; Laban, S.; Hautmann, M.G.; Brunner, T.B.; Tamaskovics, B.; et al. Induction chemoimmunotherapy followed by CD8+ immune cell-based patient selection for chemotherapy-free radioimmunotherapy in locally advanced head and neck cancer. J. Immunother. Cancer 2022, 10, e003747. [Google Scholar] [CrossRef]
- Allais, B.S.; Fay, C.J.; Kim, D.Y.; Semenov, Y.R.; LeBoeuf, N.R. Cutaneous immune-related adverse events from immune checkpoint inhibitor therapy: Moving beyond “maculopapular rash”. Immunol. Rev. 2023, 318, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.Y.; Wasilewski, G.; Lacouture, M.E.; Barker, C.A. Incidence of dermatologic adverse events in patients with cancer treated with concurrent immune checkpoint inhibitors and radiation therapy: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2021, 84, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef]
- Quaresma, J.A.S. Organization of the Skin Immune System and Compartmentalized Immune Responses in Infectious Diseases. Clin. Microbiol. Rev. 2019, 32, e00034-18. [Google Scholar] [CrossRef]
- DiCarlo, A.L.; Bandremer, A.C.; Hollingsworth, B.A.; Kasim, S.; Laniyonu, A.; Todd, N.F.; Wang, S.-J.; Wertheimer, E.R.; Rios, C.I. Cutaneous Radiation Injuries: Models, Assessment and Treatments. Radiat. Res. 2020, 194, 315–344. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef]
- Lopez-Ojeda, W.; Pandey, A.; Alhajj, M.; Oakley, A.M. Anatomy, Skin (Integument); StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Rajkumar, J.; Chandan, N.; Lio, P.; Shi, V. The Skin Barrier and Moisturization: Function, Disruption, and Mechanisms of Repair. Ski. Pharmacol. Physiol. 2023, 36, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Persa, O.D.; Koester, J.; Niessen, C.M. Regulation of Cell Polarity and Tissue Architecture in Epidermal Aging and Cancer. J. Investig. Dermatol. 2021, 141, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Botchkarev, V.A.; Gdula, M.R.; Mardaryev, A.N.; Sharov, A.A.; Fessing, M.Y. Epigenetic regulation of gene expression in keratinocytes. J. Investig. Dermatol. 2012, 132, 2505–2521. [Google Scholar] [CrossRef]
- Évora, A.S.; Adams, M.J.; Johnson, S.A.; Zhang, Z. Corneocytes: Relationship between Structural and Biomechanical Properties. Ski. Pharmacol. Physiol. 2021, 34, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Schwab, H.; Flora, J.; Mayrovitz, H.N. Impacts of Skin Eccrine Glands on the Measured Values of Transepidermal Water Loss. Cureus 2022, 14, e32266. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef]
- Weinzierl, A.; Harder, Y.; Schmauss, D.; Menger, M.D.; Laschke, M.W. Boosting Tissue Vascularization: Nanofat as a Potential Source of Functional Microvessel Segments. Front. Bioeng. Biotechnol. 2022, 10, 820835. [Google Scholar] [CrossRef]
- Varricchi, G.; Granata, F.; Loffredo, S.; Genovese, A.; Marone, G. Angiogenesis and lymphangiogenesis in inflammatory skin disorders. J. Am. Acad. Dermatol. 2015, 73, 144–153. [Google Scholar] [CrossRef]
- Schuler, N.; Timm, S.; Rübe, C.E. Hair Follicle Stem Cell Faith Is Dependent on Chromatin Remodeling Capacity following Low-Dose Radiation. Stem Cells 2018, 36, 574–588. [Google Scholar] [CrossRef]
- Zomer, H.D.; Trentin, A.G. Skin wound healing in humans and mice: Challenges in translational research. J. Dermatol. Sci. 2018, 90, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.N.; Simmons, B.J.; Wolfson, A.H.; Nouri, K. Acute and Chronic Cutaneous Reactions to Ionizing Radiation Therapy. Dermatol. Ther. 2016, 6, 185–206. [Google Scholar] [CrossRef]
- Delfino, S.; Brunetti, B.; Toto, V.; Persichetti, P. Burn after breast reconstruction. Burns 2008, 34, 873–877. [Google Scholar] [CrossRef]
- Richardson, B.N.; Lin, J.; Buchwald, Z.S.; Bai, J. Skin Microbiome and Treatment-Related Skin Toxicities in Patients with Cancer: A Mini-Review. Front. Oncol. 2022, 12, 924849. [Google Scholar] [CrossRef]
- Schuler, N.; Palm, J.; Kaiser, M.; Betten, D.; Furtwängler, R.; Rübe, C.; Graf, N.; Rübe, C.E. DNA-Damage Foci to Detect and Characterize DNA Repair Alterations in Children Treated for Pediatric Malignancies. PLoS ONE 2014, 9, e91319. [Google Scholar] [CrossRef]
- Toledano, A.; Garaud, P.; Serin, D.; Fourquet, A.; Bosset, J.-F.; Breteau, N.; Body, G.; Azria, D.; Le Floch, O.; Calais, G. Concurrent administration of adjuvant chemotherapy and radiotherapy after breast-conserving surgery enhances late toxicities: Long-term results of the ARCOSEIN multicenter randomized study. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 324–332. [Google Scholar] [CrossRef]
- Satzger, I.; Degen, A.; Asper, H.; Kapp, A.; Hauschild, A.; Gutzmer, R. Serious skin toxicity with the combination of BRAF inhibitors and radiotherapy. J. Clin. Oncol. 2013, 31, e220–e222. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Overgaard, J. Patient-to-Patient Variability in the Expression of Radiation-Induced Normal Tissue Injury. Semin. Radiat. Oncol. 1994, 4, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Dekoninck, S.; Rulands, S.; Lenglez, S.; Mascré, G.; Simons, B.D.; Blanpain, C. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat. Commun. 2017, 8, 14684. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Shimada, M.; Matsumoto, Y.; Okino, A. DNA Damage Response after Ionizing Radiation Exposure in Skin Keratinocytes Derived from Human-Induced Pluripotent Stem Cells. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Cell biology: The beginning of the end. Nature 2014, 505, 35–36. [Google Scholar] [CrossRef]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Contrepois, K.; Coudereau, C.; Benayoun, B.A.; Schuler, N.; Roux, P.-F.; Bischof, O.; Courbeyrette, R.; Carvalho, C.; Thuret, J.-Y.; Ma, Z.; et al. Histone variant H2A.J accumulates in senescent cells and promotes inflammatory gene expression. Nat. Commun. 2017, 8, 14995. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The role of cellular senescence in skin aging and age-related skin pathologies. Front. Physiol. 2023, 14, 1297637. [Google Scholar] [CrossRef] [PubMed]
- Rübe, C.E.; Bäumert, C.; Schuler, N.; Isermann, A.; Schmal, Z.; Glanemann, M.; Mann, C.; Scherthan, H. Human skin aging is associated with increased expression of the histone variant H2A.J in the epidermis. NPJ Aging Mech. Dis. 2021, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Tewary, G.; Freyter, B.; Al-Razaq, M.A.; Auerbach, H.; Laschke, M.W.; Kübelbeck, T.; Kolb, A.; Mangelinck, A.; Mann, C.; Kramer, D.; et al. Immunomodulatory Effects of Histone Variant H2A.J in Ionizing Radiation Dermatitis. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 801–816. [Google Scholar] [CrossRef] [PubMed]
- Hippchen, Y.; Tewary, G.; Jung, D.; Schmal, Z.; Meessen, S.; Palm, J.; Rübe, C.E. Cultured Human Foreskin as a Model System for Evaluating Ionizing Radiation-Induced Skin Injury. Int. J. Mol. Sci. 2022, 23, 9830. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.S.; Ong, P.F.; Chojnowski, A.; Clavel, C.; Dreesen, O. Loss of lamin B1 is a biomarker to quantify cellular senescence in photoaged skin. Sci. Rep. 2017, 7, 15678. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef]
- Kobayashi, T.; Naik, S.; Nagao, K. Choreographing Immunity in the Skin Epithelial Barrier. Immunity 2019, 50, 552–565. [Google Scholar] [CrossRef]
- Müller, K.; Meineke, V. Radiation-induced alterations in cytokine production by skin cells. Exp. Hematol. 2007, 35, 96–104. [Google Scholar] [CrossRef]
- Harjunpää, H.; Asens, M.L.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Yan, B.; Liu, N.; Li, J.; Li, J.; Zhu, W.; Kuang, Y.; Chen, X.; Peng, C. The role of Langerhans cells in epidermal homeostasis and pathogenesis of psoriasis. J. Cell. Mol. Med. 2020, 24, 11646–11655. [Google Scholar] [CrossRef]
- Krohn, I.K.; Aerts, J.L.; Breckpot, K.; Goyvaerts, C.; Knol, E.; Van Wijk, F.; Gutermuth, J. T-cell subsets in the skin and their role in inflammatory skin disorders. Allergy 2022, 77, 827–842. [Google Scholar] [CrossRef]
- Zhang, C.; Merana, G.R.; Harris-Tryon, T.; Scharschmidt, T.C. Skin immunity: Dissecting the complex biology of our body’s outer barrier. Mucosal. Immunol. 2022, 15, 551–561. [Google Scholar] [CrossRef]
- Cruz, M.S.; Diamond, A.; Russell, A.; Jameson, J.M. Human alphabeta and gammadelta T Cells in Skin Immunity and Disease. Front. Immunol. 2018, 9, 1304. [Google Scholar] [CrossRef]
- Yanez, D.A.; Lacher, R.K.; Vidyarthi, A.; Colegio, O.R. The role of macrophages in skin homeostasis. Pflugers Arch. 2017, 469, 455–463. [Google Scholar] [CrossRef]
- Nakabo, S.; Romo-Tena, J.; Kaplan, M.J. Neutrophils as Drivers of Immune Dysregulation in Autoimmune Diseases with Skin Manifestations. J. Investig. Dermatol. 2022, 142, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Muto, Y.; Kinoshita, M.; Shimada, S.; Kawamura, T. Neutrophil Extracellular Traps in Skin Diseases. Biomedicines 2021, 9, 1888. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Yang, Y.J.; Nagarajan, P.; Ge, Y. Transcriptional and signalling regulation of skin epithelial stem cells in homeostasis, wounds and cancer. Exp. Dermatol. 2021, 30, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Hei, T.K.; Cheng, S.K. Radiation-Induced Dermatitis is Mediated by IL17-Expressing γδ T Cells. Radiat. Res. 2017, 187, 464–474. [Google Scholar] [CrossRef]
- Müller, A.; Hennig, A.; Lorscheid, S.; Grondona, P.; Schulze-Osthoff, K.; Hailfinger, S.; Kramer, D. IκBζ is a key transcriptional regulator of IL-36-driven psoriasis-related gene expression in keratinocytes. Proc. Natl. Acad. Sci. USA 2018, 115, 10088–10093. [Google Scholar] [CrossRef]
- Sumigray, K.D.; Lechler, T. Cell adhesion in epidermal development and barrier formation. Curr. Top Dev. Biol. 2015, 112, 383–414. [Google Scholar]
- Lawenda, B.D.; Gagne, H.M.; Gierga, D.P.; Niemierko, A.; Wong, W.M.; Tarbell, N.J.; Chen, G.T.; Hochberg, F.H.; Loeffler, J.S. Permanent alopecia after cranial irradiation: Dose-response relationship. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 879–887. [Google Scholar] [CrossRef]
- Phillips, G.S.; Freret, M.E.; Friedman, D.N.; Trelles, S.; Kukoyi, O.; Freites-Martinez, A.; Unger, R.H.; Disa, J.J.; Wexler, L.H.; Tinkle, C.L.; et al. Assessment and Treatment Outcomes of Persistent Radiation-Induced Alopecia in Patients with Cancer. JAMA Dermatol. 2020, 156, 963–972. [Google Scholar] [CrossRef]
- Balter, S.; Hopewell, J.W.; Miller, D.L.; Wagner, L.K.; Zelefsky, M.J. Fluoroscopically guided interventional procedures: A review of radiation effects on patients’ skin and hair. Radiology 2010, 254, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Huang, W.-Y.; Wang, E.H.C.; Tai, K.-Y.; Lin, S.-J. Functional complexity of hair follicle stem cell niche and therapeutic targeting of niche dysfunction for hair regeneration. J. Biomed. Sci. 2020, 27, 43. [Google Scholar] [CrossRef] [PubMed]
- Nicu, C.; O’sullivan, J.D.; Ramos, R.; Timperi, L.; Lai, T.; Farjo, N.; Farjo, B.; Pople, J.; Bhogal, R.; Hardman, J.A.; et al. Dermal Adipose Tissue Secretes HGF to Promote Human Hair Growth and Pigmentation. J. Investig. Dermatol. 2021, 141, 1633–1645.e13. [Google Scholar] [CrossRef] [PubMed]
- Al-Nuaimi, Y.; Hardman, J.A.; Bíró, T.; Haslam, I.S.; Philpott, M.P.; Tóth, B.I.; Farjo, N.; Farjo, B.; Baier, G.; Watson, R.E.; et al. A meeting of two chronobiological systems: Circadian proteins Period1 and BMAL1 modulate the human hair cycle clock. J. Investig. Dermatol. 2014, 134, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Geyfman, M.; Plikus, M.V.; Treffeisen, E.; Andersen, B.; Paus, R. Resting no more: Re-defining telogen, the maintenance stage of the hair growth cycle. Biol. Rev. Camb. Philos. Soc. 2015, 90, 1179–1196. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Cotsarelis, G. The Biology of Hair Follicles. N. Engl. J. Med. 1999, 341, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.W.; Kloepper, J.; Langan, E.A.; Kim, Y.; Yeo, J.; Kim, M.J.; Hsi, T.-C.; Rose, C.; Yoon, G.S.; Lee, S.-J.; et al. A Guide to Studying Human Hair Follicle Cycling In Vivo. J. Investig. Dermatol. 2016, 136, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Hadam, S.; Heiderhoff, M.; Audring, H.; Lademann, J.; Sterry, W.; Blume-Peytavi, U. Morphometry of human terminal and vellus hair follicles. Exp. Dermatol. 2007, 16, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Lai, S.F.; Chiu, H.Y.; Chang, M.; Plikus, M.V.; Chan, C.C.; Chen, Y.T.; Tsao, P.N.; Yang, T.L.; Lee, H.S.; et al. Mobilizing Transit-Amplifying Cell-Derived Ectopic Progenitors Prevents Hair Loss from Chemotherapy or Radiation Therapy. Cancer Res. 2017, 77, 6083–6096. [Google Scholar] [CrossRef] [PubMed]
- Müller-Röver, S.; Foitzik, K.; Paus, R.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; McKay, I.A.; Stenn, K.S. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Chabanon, R.M.; Rouanne, M.; Lord, C.J.; Soria, J.-C.; Pasero, P.; Postel-Vinay, S. Targeting the DNA damage response in immuno-oncology: Developments and opportunities. Nat. Rev. Cancer 2021, 21, 701–717. [Google Scholar] [CrossRef] [PubMed]
- Goodell, M.A.; Nguyen, H.; Shroyer, N. Somatic stem cell heterogeneity: Diversity in the blood, skin and intestinal stem cell compartments. Nat. Rev. Mol. Cell Biol. 2015, 16, 299–309. [Google Scholar] [CrossRef]
- Sotiropoulou, P.A.; Candi, A.; Mascré, G.; De Clercq, S.; Youssef, K.K.; Lapouge, G.; Dahl, E.; Semeraro, C.; Denecker, G.; Marine, J.C.; et al. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat. Cell Biol. 2010, 12, 572–582. [Google Scholar] [CrossRef]
- O’Sullivan, J.D.; Nicu, C.; Picard, M.; Chéret, J.; Bedogni, B.; Tobin, D.J.; Paus, R. The biology of human hair greying. Biol. Rev. Camb. Philos. Soc. 2021, 96, 107–128. [Google Scholar] [CrossRef]
- Suzuki, T.; Chéret, J.; Scala, F.D.; Akhundlu, A.; Gherardini, J.; Demetrius, D.; O’Sullivan, J.D.B.; Epstein, G.K.; Bauman, A.J.; Demetriades, C.; et al. mTORC1 activity negatively regulates human hair follicle growth and pigmentation. Embo Rep. 2023, 24, e56574. [Google Scholar] [CrossRef]
- Aoki, H.; Hara, A.; Motohashi, T.; Kunisada, T. Keratinocyte stem cells but not melanocyte stem cells are the primary target for radiation-induced hair graying. J. Investig. Dermatol. 2013, 133, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Inomata, K.; Aoto, T.; Binh, N.T.; Okamoto, N.; Tanimura, S.; Wakayama, T.; Iseki, S.; Hara, E.; Masunaga, T.; Shimizu, H.; et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell 2009, 137, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, E.; Di Maio, R.; Caccavale, S.; Siano, M.; Schiavo, A.L. Radiation dermatitis, burns, and recall phenomena: Meaningful instances of immunocompromised district. Clin. Dermatol. 2014, 32, 660–669. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rübe, C.E.; Freyter, B.M.; Tewary, G.; Roemer, K.; Hecht, M.; Rübe, C. Radiation Dermatitis: Radiation-Induced Effects on the Structural and Immunological Barrier Function of the Epidermis. Int. J. Mol. Sci. 2024, 25, 3320. https://doi.org/10.3390/ijms25063320
Rübe CE, Freyter BM, Tewary G, Roemer K, Hecht M, Rübe C. Radiation Dermatitis: Radiation-Induced Effects on the Structural and Immunological Barrier Function of the Epidermis. International Journal of Molecular Sciences. 2024; 25(6):3320. https://doi.org/10.3390/ijms25063320
Chicago/Turabian StyleRübe, Claudia E., Benjamin M. Freyter, Gargi Tewary, Klaus Roemer, Markus Hecht, and Christian Rübe. 2024. "Radiation Dermatitis: Radiation-Induced Effects on the Structural and Immunological Barrier Function of the Epidermis" International Journal of Molecular Sciences 25, no. 6: 3320. https://doi.org/10.3390/ijms25063320
APA StyleRübe, C. E., Freyter, B. M., Tewary, G., Roemer, K., Hecht, M., & Rübe, C. (2024). Radiation Dermatitis: Radiation-Induced Effects on the Structural and Immunological Barrier Function of the Epidermis. International Journal of Molecular Sciences, 25(6), 3320. https://doi.org/10.3390/ijms25063320




