Steroid-Induced Ocular Hypertension in Mice Is Differentially Reduced by Selective EP2, EP3, EP4, and IP Prostanoid Receptor Agonists
Abstract
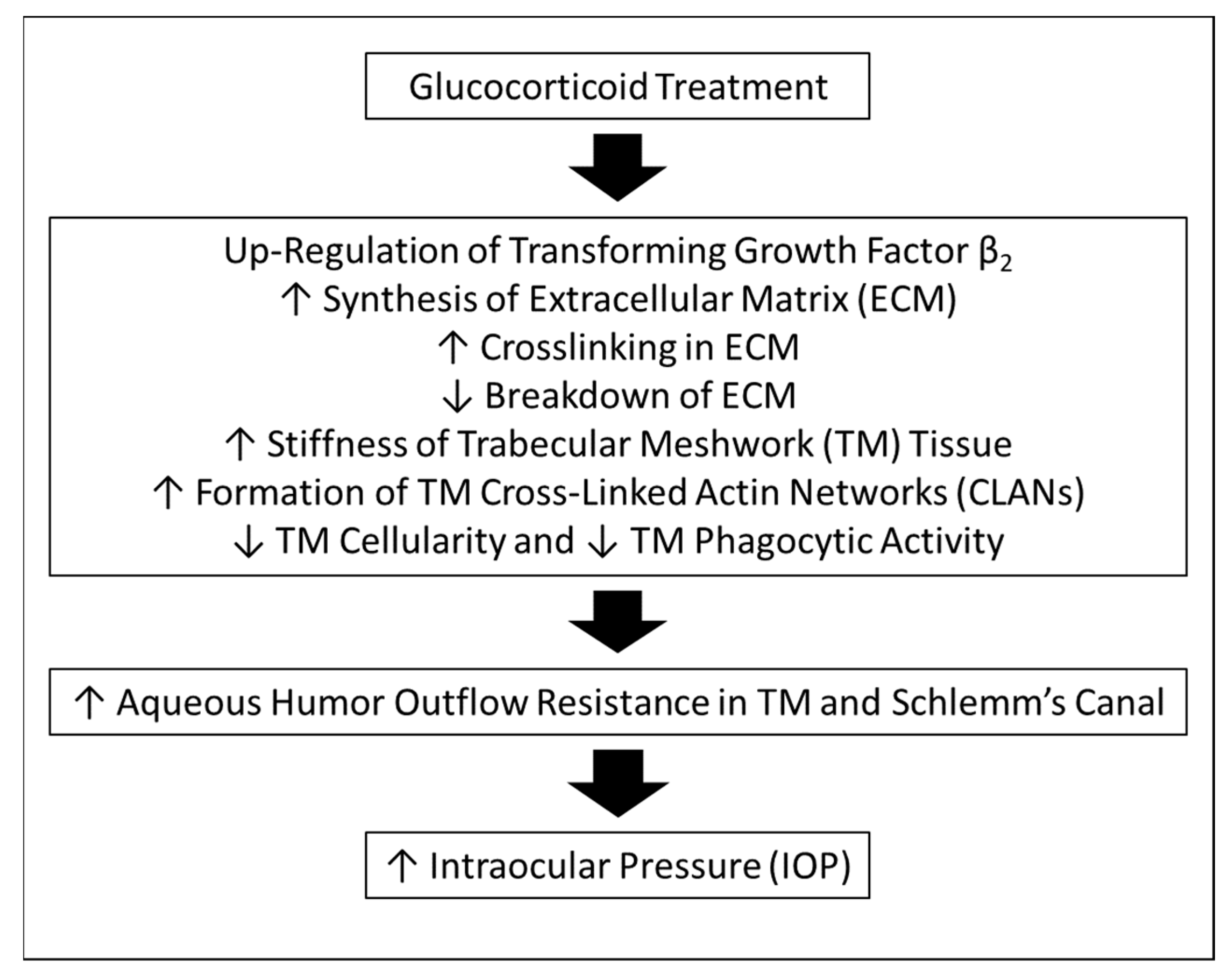
:1. Introduction
| Animal Model or Human Subjects | Route of Administration of Steroid | Steroid Used | Test Compound | Max. IOP Reduction | Reference |
|---|---|---|---|---|---|
| C57BL/6 mice | Topical ocular | Dexamethasone | Sodium 4-phenylacetate (Chaperone) | 16% | [24] |
| C57BL/6J mice | Periocular fornix | Dexamethasone | Cosopt + latanoprost (CAI inhibitor + Timolol + FP–PGR agonist) | 187% | [20] |
| C57BL/6J mice | Topical ocular | Metformin (anti-diabetic/glycemic) | 14% | [59] | |
| C57BL/6J mice | Topical ocular | Dexamethasone | HL-3501 (Adenosine-3R antagonist) Latanoprost (FP–PGR agonist) | 37% 36% | [58] |
| C57BL/6J mice | Subconjunctival (Fornix) | Dexamethasone | CKLP1 (Cromakalim analog (K+-channel activator)) Diazoxide (K+-channel activator; anti-glycemic) | 24% 32% | [56] |
| C57BL/6J mice | Subconjunctival (Fornix) | Dexamethasone | Stanniocalcin-1 (hormone) | 26% | [57] |
| C57BL/6J mice | Mini-osmotic pumps (systemic) | Dexamethasone | siRNA to zonula occluddens-1 and tricellulin | ~20% | [63] |
| C57BL/6J mice | Topical ocular | Dexamethasone | Netarsudil (Rho kinase inhibitor) | 20% | [60] |
| C57BL/6J mice | Mini-osmotic pumps | Dexamethasone | Cabergoline (D3 and 5HT2 receptor agonist) | 40% | [62] |
| 129.B6 mice | Mini-osmotic pumps | Dexamethasone | Timolol (β-blocker) Latanoprost (FP–PGR agonist) Y39983 (Rho kinase inhibitor) | 16% 27% 33% | [61] |
| C57BL/6J mice | Subconjunctival (Fornix) | Dexamethasone | Misoprostol (EP3-EP4-EP2R agonist) PF-04217329 (EP2 R agonist) Butaprost (EP2R agonist) Rivenprost (EP4R agonist) NS-304 (IPR-agonist) | 74.5% 74.3% 65.2% 58.4% 55.3% | The current studies |
| Sprague-Dawley Rats | Topical ocular | Dexamethasone | Timolol (βR-blocker) Pilocarpine (Muscarinic R agonist) Dorzolamide (CAI) Brimonidine (α2-R agonist) Latanoprost (FP–PGR agonist) | 20% 16% 19% 17% 27% | [45] |
| Sprague-Dawley Rats | Topical ocular | Dexamethasone | Losartan (Ang. IIR antagonist) | 27% | [47] |
| Sprague-Dawley Rats | Subconjunctival (Fornix) | Betamethasone | C. cicadae mycelia extract | 50% | [48] |
| Rabbits | Topical ocular | Prednisolone | Ocimum bascilicum seed extract | 32% | [51] |
| Rabbits | Topical ocular | Prednisolone | Aegle Marmelos fruit extract | 28% | [50] |
| Rabbits | Topical ocular | Prednisolone | Pilocarpine (Muscarinic R agonist) Timolol (βR-blocker) Latanoprost (FP–PGR agonist) | 26% 34% 35% | [49] |
| Sheep | Topical ocular | Prednisolone | Tissue plasminogen activator (tPA) (intracameral) | 36% | [53] |
| Sheep | Sub-tenon | Triamcinolone | MMP-1 gene therapy | 70% | [52] |
| Sheep | Topical ocular or Intravitreal | Prednisolone or Difluprednate Triamcinolone | MMP-1 gene therapy | 38% | [54] |
| Human | Topical/systemic | Dexamethasone | Latanoprost (FP–PGR agonist) | 28% | [30] |
2. Results
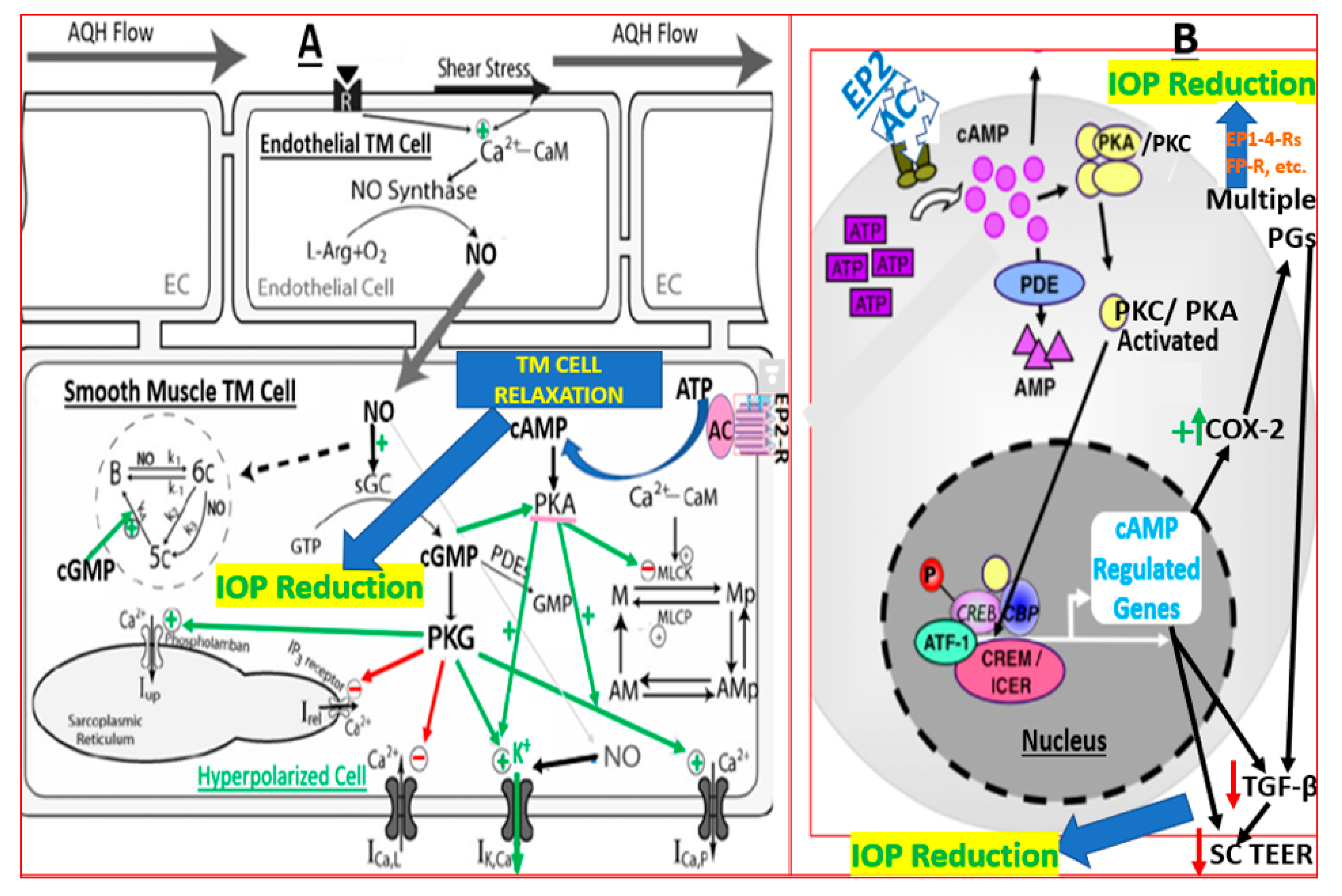
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ophthalmic Formulations
4.3. Induction of Ocular Hypertension Using Dexamethasone (DEX)
4.4. Preparation of DEX for Periocular Injection
4.5. IOP Measurement/Direct Ophthalmoscopy
4.6. Topical Ocular Dosing
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bucolo, C.; Platania, C.B.M.; Drago, F.; Bonfiglio, V.; Reibaldi, M.; Avitabile, T.; Uva, M. Novel Therapeutics in Glaucoma Management. Curr. Neuropharmacol. 2018, 16, 978–992. [Google Scholar] [CrossRef]
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Adornetto, A.; Russo, R.; Parisi, V. Neuroinflammation as a Target for Glaucoma Therapy. Neural Regen. Res. 2019, 14, 391–394. [Google Scholar] [CrossRef]
- Keller, K.E.; Peters, D.M. Pathogenesis of Glaucoma: Extracellular Matrix Dysfunction in the Trabecular Meshwork-A Review. Clin. Exp. Ophthalmol. 2022, 50, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Evangelho, K.; Mogilevskaya, M.; Losada-Barragan, M.; Vargas-Sanchez, J.K. Pathophysiology of Primary Open-Angle Glaucoma from a Neuroinflammatory and Neurotoxicity Perspective: A Review of the Literature. Int. Ophthalmol. 2019, 39, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Calkins, D.J.; Horner, P.J. The Cell and Molecular Biology of Glaucoma: Axonopathy and the Brain. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2482–2484. [Google Scholar] [CrossRef] [PubMed]
- Soto, I.; Howell, G.R. The Complex Role of Neuroinflammation in Glaucoma. Cold Spring Harb. Perspect. Med. 2014, 4, a017269. [Google Scholar] [CrossRef]
- Tsai, J.C. Innovative IOP-Independent Neuroprotection and Neuroregeneration Strategies in the Pipeline for Glaucoma. J. Ophthalmol. 2020, 2020, 9329310. [Google Scholar] [CrossRef]
- Mokhles, P.; Schouten, J.S.; Beckers, H.J.; Azuara-Blanco, A.; Tuulonen, A.; Webers, C.A. Glaucoma Blindness at the End of Life. Acta Ophthalmol. 2017, 95, 10–11. [Google Scholar] [CrossRef]
- Yücel, Y.H.; Zhang, Q.; Weinreb, R.N.; Kaufman, P.L.; Gupta, N. Atrophy of Relay Neurons in Magno- and Parvocellular Layers in the Lateral Geniculate Nucleus in Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3216–3222. [Google Scholar]
- Lee, E.J.; Kim, T.-W.; Weinreb, R.N. Reversal of Lamina Cribrosa Displacement and Thickness after Trabeculectomy in Glaucoma. Ophthalmology 2012, 119, 1359–1366. [Google Scholar] [CrossRef]
- Waisbourd, M.; Ahmed, O.M.; Molineaux, J.; Gonzalez, A.; Spaeth, G.L.; Katz, L.J. Reversible Structural and Functional Changes after Intraocular Pressure Reduction in Patients with Glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Sigireddi, R.R.; Westenskow, P.D.; Channa, R.; Frankfort, B.J. Single Transient Intraocular Pressure Elevations Cause Prolonged Retinal Ganglion Cell Dysfunction and Retinal Capillary Abnormalities in Mice. Exp. Eye Res. 2020, 201, 108296. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, M.E.; Shah, P.; Cowan, C.S.; Yang, Z.; Wu, S.M.; Frankfort, B.J. Effects of Chronic and Acute Intraocular Pressure Elevation on Scotopic and Photopic Contrast Sensitivity in Mice. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3077–3087. [Google Scholar] [CrossRef]
- Sharif, N.A. Identifying New Drugs and Targets to Treat Rapidly Elevated Intraocular Pressure for Angle Closure and Secondary Glaucomas to Curb Visual Impairment and Prevent Blindness. Exp. Eye Res. 2023, 232, 109444. [Google Scholar] [CrossRef] [PubMed]
- Danford, I.D.; Verkuil, L.D.; Choi, D.J.; Collins, D.W.; Gudiseva, H.V.; Uyhazi, K.E.; Lau, M.K.; Kanu, L.N.; Grant, G.R.; Chavali, V.R.M.; et al. Characterizing the “POAGome”: A Bioinformatics-Driven Approach to Primary Open-Angle Glaucoma. Prog. Retin. Eye Res. 2017, 58, 89–114. [Google Scholar] [CrossRef]
- Wykrota, A.A.; Abdin, A.D.; Munteanu, C.; Löw, U.; Seitz, B. Incidence and Treatment Approach of Intraocular Pressure Elevation after Various Types of Local Steroids for Retinal Diseases. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 3569–3579. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.E.; Schwartz, S.G.; Gao, X.; Jeong, S.; Patel, N.; Itakura, T.; Price, M.O.; Price, F.W.; Varma, R.; Stamer, W.D. Steroid-Induced Ocular Hypertension/Glaucoma: Focus on Pharmacogenomics and Implications for Precision Medicine. Prog. Retin. Eye Res. 2017, 56, 58–83. [Google Scholar] [CrossRef]
- Maddineni, P.; Kasetti, R.B.; Patel, P.D.; Millar, J.C.; Kiehlbauch, C.; Clark, A.F.; Zode, G.S. CNS Axonal Degeneration and Transport Deficits at the Optic Nerve Head Precede Structural and Functional Loss of Retinal Ganglion Cells in a Mouse Model of Glaucoma. Mol. Neurodegener. 2020, 15, 48. [Google Scholar] [CrossRef]
- Clark, A.F.; Wilson, K.; McCartney, M.D.; Miggans, S.T.; Kunkle, M.; Howe, W. Glucocorticoid-Induced Formation of Cross-Linked Actin Networks in Cultured Human Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 1994, 35, 281–294. [Google Scholar]
- Jones, R.; Rhee, D.J. Corticosteroid-Induced Ocular Hypertension and Glaucoma: A Brief Review and Update of the Literature. Curr. Opin. Ophthalmol. 2006, 17, 163–167. [Google Scholar] [CrossRef]
- Mao, W.; Tovar-Vidales, T.; Yorio, T.; Wordinger, R.J.; Clark, A.F. Perfusion-Cultured Bovine Anterior Segments as an Ex Vivo Model for Studying Glucocorticoid-Induced Ocular Hypertension and Glaucoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8068–8075. [Google Scholar] [CrossRef]
- Zode, G.S.; Sharma, A.B.; Lin, X.; Searby, C.C.; Bugge, K.; Kim, G.H.; Clark, A.F.; Sheffield, V.C. Ocular-Specific ER Stress Reduction Rescues Glaucoma in Murine Glucocorticoid-Induced Glaucoma. J. Clin. Investig. 2014, 124, 1956–1965. [Google Scholar] [CrossRef]
- Zode, G.S.; Kuehn, M.H.; Nishimura, D.Y.; Searby, C.C.; Mohan, K.; Grozdanic, S.D.; Bugge, K.; Anderson, M.G.; Clark, A.F.; Stone, E.M.; et al. Reduction of ER Stress via a Chemical Chaperone Prevents Disease Phenotypes in a Mouse Model of Primary Open Angle Glaucoma. J. Clin. Investig. 2011, 121, 3542–3553. [Google Scholar] [CrossRef]
- Kasetti, R.B.; Maddineni, P.; Patel, P.D.; Searby, C.; Sheffield, V.C.; Zode, G.S. Transforming Growth Factor Β2 (TGFβ2) Signaling Plays a Key Role in Glucocorticoid-Induced Ocular Hypertension. J. Biol. Chem. 2018, 293, 9854–9868. [Google Scholar] [CrossRef]
- Li, G.; Lee, C.; Agrahari, V.; Wang, K.; Navarro, I.; Sherwood, J.M.; Crews, K.; Farsiu, S.; Gonzalez, P.; Lin, C.-W.; et al. In Vivo Measurement of Trabecular Meshwork Stiffness in a Corticosteroid-Induced Ocular Hypertensive Mouse Model. Proc. Natl. Acad. Sci. USA 2019, 116, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Kersey, J.P.; Broadway, D.C. Corticosteroid-Induced Glaucoma: A Review of the Literature. Eye 2006, 20, 407–416. [Google Scholar] [CrossRef]
- Zhang, X.; Ognibene, C.M.; Clark, A.F.; Yorio, T. Dexamethasone Inhibition of Trabecular Meshwork Cell Phagocytosis and Its Modulation by Glucocorticoid Receptor Beta. Exp. Eye Res. 2007, 84, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Scherer, W.J.; Hauber, F.A. Effect of Latanoprost on Intraocular Pressure in Steroid-Induced Glaucoma. J. Glaucoma 2000, 9, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Cimolai, N. A Review of Neuropsychiatric Adverse Events from Topical Ophthalmic Brimonidine. Hum. Exp. Toxicol. 2020, 39, 1279–1290. [Google Scholar] [CrossRef]
- Arbabi, A.; Bao, X.; Shalaby, W.S.; Razeghinejad, R. Systemic Side Effects of Glaucoma Medications. Clin. Exp. Optom. 2022, 105, 157–165. [Google Scholar] [CrossRef]
- Patchinsky, A.; Petitpain, N.; Gillet, P.; Angioi-Duprez, K.; Schmutz, J.L.; Bursztejn, A.C. Dermatological Adverse Effects of Anti-Glaucoma Eye Drops: A Review. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 661–670. [Google Scholar] [CrossRef]
- Sharif, N.A.; Odani-Kawabata, N.; Lu, F.; Pinchuk, L. FP and EP2 Prostanoid Receptor Agonist Drugs and Aqueous Humor Outflow Devices for Treating Ocular Hypertension and Glaucoma. Exp. Eye Res. 2023, 229, 109415. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Kong, Y.X.G.; Tao, L.W.; Lim, L.L.; Martin, K.R.; Green, C.; Ruddle, J.; Crowston, J.G. Surgical Outcomes of Trabeculectomy and Glaucoma Drainage Implant for Uveitic Glaucoma and Relationship with Uveitis Activity. Clin. Exp. Ophthalmol. 2017, 45, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Abramovitz, M.; Adam, M.; Boie, Y.; Carrière, M.; Denis, D.; Godbout, C.; Lamontagne, S.; Rochette, C.; Sawyer, N.; Tremblay, N.M.; et al. The Utilization of Recombinant Prostanoid Receptors to Determine the Affinities and Selectivities of Prostaglandins and Related Analogs. Biochim. Biophys. Acta 2000, 1483, 285–293. [Google Scholar] [CrossRef]
- Prasanna, G.; Carreiro, S.; Anderson, S.; Gukasyan, H.; Sartnurak, S.; Younis, H.; Gale, D.; Xiang, C.; Wells, P.; Dinh, D.; et al. Effect of PF-04217329 a Prodrug of a Selective Prostaglandin EP(2) Agonist on Intraocular Pressure in Preclinical Models of Glaucoma. Exp. Eye Res. 2011, 93, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Asaki, T.; Kuwano, K.; Morrison, K.; Gatfield, J.; Hamamoto, T.; Clozel, M. Selexipag: An Oral and Selective IP Prostacyclin Receptor Agonist for the Treatment of Pulmonary Arterial Hypertension. J. Med. Chem. 2015, 58, 7128–7137. [Google Scholar] [CrossRef]
- Biswas, S.; Wan, K.H. Review of Rodent Hypertensive Glaucoma Models. Acta Ophthalmol. 2019, 97, e331–e340. [Google Scholar] [CrossRef]
- Evangelho, K.; Mastronardi, C.A.; de-la-Torre, A. Experimental Models of Glaucoma: A Powerful Translational Tool for the Future Development of New Therapies for Glaucoma in Humans-A Review of the Literature. Medicina 2019, 55, 280. [Google Scholar] [CrossRef]
- Agarwal, R.; Agarwal, P.; Iezhitsa, I. Exploring the Current Use of Animal Models in Glaucoma Drug Discovery: Where Are We in 2023? Expert Opin. Drug. Discov. 2023, 18, 1287–1300. [Google Scholar] [CrossRef]
- McDowell, C.M.; Kizhatil, K.; Elliott, M.H.; Overby, D.R.; van Batenburg-Sherwood, J.; Millar, J.C.; Kuehn, M.H.; Zode, G.; Acott, T.S.; Anderson, M.G.; et al. Consensus Recommendation for Mouse Models of Ocular Hypertension to Study Aqueous Humor Outflow and Its Mechanisms. Investig. Ophthalmol. Vis. Sci. 2022, 63, 12. [Google Scholar] [CrossRef]
- Lee, K.M.; Song, D.Y.; Kim, S.H. Effect of Strain on Rodent Glaucoma Models: Magnetic Bead Injection Versus Hydrogel Injection Versus Circumlimbal Suture. Transl. Vis. Sci. Technol. 2022, 11, 31. [Google Scholar] [CrossRef]
- Fernandes, K.A.; Harder, J.M.; Williams, P.A.; Rausch, R.L.; Kiernan, A.E.; Nair, K.S.; Anderson, M.G.; John, S.W.M.; Howell, G.R.; Libby, R.T. Using Genetic Mouse Models to Gain Insight into Glaucoma: Past Results and Future Possibilities. Exp. Eye Res. 2015, 141, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Razali, N.; Agarwal, R.; Agarwal, P.; Kapitonova, M.Y.; Kannan Kutty, M.; Smirnov, A.; Salmah Bakar, N.; Ismail, N.M. Anterior and Posterior Segment Changes in Rat Eyes with Chronic Steroid Administration and Their Responsiveness to Antiglaucoma Drugs. Eur. J. Pharmacol. 2015, 749, 73–80. [Google Scholar] [CrossRef]
- Razali, N.; Agarwal, R.; Agarwal, P.; Tripathy, M.; Kapitonova, M.Y.; Kutty, M.K.; Smirnov, A.; Khalid, Z.; Ismail, N.M. Topical Trans-Resveratrol Ameliorates Steroid-Induced Anterior and Posterior Segment Changes in Rats. Exp. Eye Res. 2016, 143, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Krasilnikova, A.; Mohamed, S.N.L.; Agarwal, P.; Bakar, N.; Zainudin, L.D.B.; Iezhitsa, I.; Mohd Ismail, N. Topical Losartan Reduces IOP by Altering TM Morphology in Rats with Steroid-Induced Ocular Hypertension. Indian J. Physiol. Pharmacol. 2018, 62, 238–248. [Google Scholar]
- Horng, C.-T.; Yang, Y.-L.; Chen, C.-C.; Huang, Y.-S.; Chen, C.; Chen, F.-A. Intraocular Pressure-Lowering Effect of Cordyceps Cicadae Mycelia Extract in a Glaucoma Rat Model. Int. J. Med. Sci. 2021, 18, 1007–1014. [Google Scholar] [CrossRef]
- Gupta, S.K.; Agarwal, R.; Galpalli, N.D.; Srivastava, S.; Agrawal, S.S.; Saxena, R. Comparative Efficacy of Pilocarpine, Timolol and Latanoprost in Experimental Models of Glaucoma. Methods Find Exp. Clin. Pharmacol. 2007, 29, 665–671. [Google Scholar] [CrossRef]
- Agarwal, R.; Gupta, S.K.; Srivastava, S.; Saxena, R.; Agrawal, S.S. Intraocular Pressure-Lowering Activity of Topical Application of Aegle Marmelos Fruit Extract in Experimental Animal Models. Ophthalmic Res. 2009, 42, 112–116. [Google Scholar] [CrossRef]
- Agarwal, R.; Gupta, S.; Srivastava, S.; Saxena, R. IOP Lowering Effects of Ocimum Basilicum Seed Extract in Two Rabbit Models of Ocular Hypertension. J. Clin. Health Sci. 2019, 4, 39–46. [Google Scholar] [CrossRef]
- Gerometta, R.; Spiga, M.-G.; Borrás, T.; Candia, O.A. Treatment of Sheep Steroid-Induced Ocular Hypertension with a Glucocorticoid-Inducible MMP1 Gene Therapy Virus. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3042–3048. [Google Scholar] [CrossRef] [PubMed]
- Candia, O.A.; Gerometta, R.M.; Danias, J. Tissue Plasminogen Activator Reduces the Elevated Intraocular Pressure Induced by Prednisolone in Sheep. Exp. Eye Res. 2014, 128, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Borrás, T.; Buie, L.K.; Spiga, M.G. Inducible ScAAV2.GRE.MMP1 Lowers IOP Long-Term in a Large Animal Model for Steroid-Induced Glaucoma Gene Therapy. Gene Ther. 2016, 23, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Rybkin, I.; Gerometta, R.; Fridman, G.; Candia, O.; Danias, J. Model Systems for the Study of Steroid-Induced IOP Elevation. Exp. Eye Res. 2017, 158, 51–58. [Google Scholar] [CrossRef]
- Roy Chowdhury, U.; Millar, J.C.; Holman, B.H.; Anderson, K.J.; Dosa, P.I.; Roddy, G.W.; Fautsch, M.P. Effect of ATP-Sensitive Potassium Channel Openers on Intraocular Pressure in Ocular Hypertensive Animal Models. Investig. Ophthalmol. Vis. Sci. 2022, 63, 15. [Google Scholar] [CrossRef]
- Roddy, G.W.; Chowdhury, U.R.; Monson, K.J.; Fautsch, M.P. Stanniocalcin-1 Reduced Intraocular Pressure in Two Models of Ocular Hypertension. Curr. Eye Res. 2021, 46, 1525–1530. [Google Scholar] [CrossRef]
- Kim, Y.; Yang, J.; Kim, J.Y.; Lee, J.M.; Son, W.C.; Moon, B.-G. HL3501, a Novel Selective A3 Adenosine Receptor Antagonist, Lowers Intraocular Pressure (IOP) in Animal Glaucoma Models. Transl. Vis. Sci. Technol. 2022, 11, 30. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Zhao, Y.; Gang, X.; Zhou, T.; Han, J.; Cao, Y.; Qi, B.; Song, S.; Wang, X.; et al. Metformin Protects Trabecular Meshwork against Oxidative Injury via Activating Integrin/ROCK Signals. eLife 2023, 12, e81198. [Google Scholar] [CrossRef]
- Ren, R.; Humphrey, A.A.; Kopczynski, C.; Gong, H. Rho Kinase Inhibitor AR-12286 Reverses Steroid-Induced Changes in Intraocular Pressure, Effective Filtration Areas, and Morphology in Mouse Eyes. Investig. Ophthalmol. Vis. Sci. 2023, 64, 7. [Google Scholar] [CrossRef]
- Whitlock, N.A.; McKnight, B.; Corcoran, K.N.; Rodriguez, L.A.; Rice, D.S. Increased Intraocular Pressure in Mice Treated with Dexamethasone. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6496–6503. [Google Scholar] [CrossRef] [PubMed]
- Platania, C.B.M.; Leggio, G.M.; Drago, F.; Salomone, S.; Bucolo, C. Regulation of Intraocular Pressure in Mice: Structural Analysis of Dopaminergic and Serotonergic Systems in Response to Cabergoline. Biochem. Pharmacol. 2013, 86, 1347–1356. [Google Scholar] [CrossRef]
- Cassidy, P.S.; Kelly, R.A.; Reina-Torres, E.; Sherwood, J.M.; Humphries, M.M.; Kiang, A.-S.; Farrar, G.J.; O’Brien, C.; Campbell, M.; Stamer, W.D.; et al. SiRNA Targeting Schlemm’s Canal Endothelial Tight Junctions Enhances Outflow Facility and Reduces IOP in a Steroid-Induced OHT Rodent Model. Mol. Ther. Methods Clin. Dev. 2021, 20, 86–94. [Google Scholar] [CrossRef]
- Arcuri, J.; Elbaz, A.; Sharif, N.A.; Bhattacharya, S.K. Ocular Treatments Targeting Separate Prostaglandin Receptors in Mice Exhibit Alterations in Intraocular Pressure and Optic Nerve Lipidome. J. Ocul. Pharmacol. Ther. 2023, 39, 541–550. [Google Scholar] [CrossRef]
- Ota, T.; Murata, H.; Sugimoto, E.; Aihara, M.; Araie, M. Prostaglandin Analogues and Mouse Intraocular Pressure: Effects of Tafluprost, Latanoprost, Travoprost, and Unoprostone, Considering 24-Hour Variation. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2006–2011. [Google Scholar] [CrossRef] [PubMed]
- Akaishi, T.; Odani-kawabata, N.; Ishida, N.; Nakamura, M. Ocular Hypotensive Effects of Anti-Glaucoma Agents in Mice. J. Ocul. Pharmacol. Ther. 2009, 25, 401–408. [Google Scholar] [CrossRef]
- Gabelt, B.T.; Hennes, E.A.; Bendel, M.A.; Constant, C.E.; Okka, M.; Kaufman, P.L. Prostaglandin Subtype-Selective and Non-Selective IOP-Lowering Comparison in Monkeys. J. Ocul. Pharmacol. Ther. 2009, 25, 1–8. [Google Scholar] [CrossRef]
- Waterbury, L.D.; Eglen, R.M.; Faurot, G.F.; Cooper, G.F. EP3, but Not EP2, FP, or TP Prostanoid-Receptor Stimulation May Reduce Intraocular Pressure. Investig. Ophthalmol. Vis. Sci. 1990, 31, 2560–2567. [Google Scholar]
- Hoyng, P.F.; de Jong, N. Iloprost, a Stable Prostacyclin Analog, Reduces Intraocular Pressure. Investig. Ophthalmol. Vis. Sci. 1987, 28, 470–476. [Google Scholar]
- Prasanna, G.; Fortner, J.; Xiang, C.; Zhang, E.; Carreiro, S.; Anderson, S.; Sartnurak, S.; Wu, G.; Gukasyan, H.; Niesman, M.; et al. Ocular Pharmacokinetics and Hypotensive Activity of PF-04475270, an EP4 Prostaglandin Agonist in Preclinical Models. Exp. Eye Res. 2009, 89, 608–617. [Google Scholar] [CrossRef]
- Nilsson, S.F.E.; Drecoll, E.; Lütjen-Drecoll, E.; Toris, C.B.; Krauss, A.H.-P.; Kharlamb, A.; Nieves, A.; Guerra, T.; Woodward, D.F. The Prostanoid EP2 Receptor Agonist Butaprost Increases Uveoscleral Outflow in the Cynomolgus Monkey. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4042–4049. [Google Scholar] [CrossRef]
- Doucette, L.P.; Walter, M.A. Prostaglandins in the Eye: Function, Expression, and Roles in Glaucoma. Ophthalmic Genet. 2017, 38, 108–116. [Google Scholar] [CrossRef]
- Kirihara, T.; Taniguchi, T.; Yamamura, K.; Iwamura, R.; Yoneda, K.; Odani-Kawabata, N.; Shimazaki, A.; Matsugi, T.; Shams, N.; Zhang, J.-Z. Pharmacologic Characterization of Omidenepag Isopropyl, a Novel Selective EP2 Receptor Agonist, as an Ocular Hypotensive Agent. Investig. Ophthalmol. Vis. Sci. 2018, 59, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Ota, T.; Aihara, M.; Saeki, T.; Narumiya, S.; Araie, M. The Effects of Prostaglandin Analogues on Prostanoid EP1, EP2, and EP3 Receptor-Deficient Mice. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3395–3399. [Google Scholar] [CrossRef]
- Aihara, M. Prostanoid Receptor Agonists for Glaucoma Treatment. Jpn. J. Ophthalmol. 2021, 65, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.C.; Clark, A.F.; Pang, I.-H. Assessment of Aqueous Humor Dynamics in the Mouse by a Novel Method of Constant-Flow Infusion. Investig. Ophthalmol. Vis. Sci. 2011, 52, 685–694. [Google Scholar] [CrossRef]
- Saeki, T.; Ota, T.; Aihara, M.; Araie, M. Effects of Prostanoid EP Agonists on Mouse Intraocular Pressure. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2201–2208. [Google Scholar] [CrossRef]
- Woodward, D.F.; Nilsson, S.F.E.; Toris, C.B.; Kharlamb, A.B.; Nieves, A.L.; Krauss, A.H.-P. Prostanoid EP4 Receptor Stimulation Produces Ocular Hypotension by a Mechanism That Does Not Appear to Involve Uveoscleral Outflow. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3320–3328. [Google Scholar] [CrossRef]
- Woodward, D.F.; Bogardus, A.M.; Donello, J.E.; Fairbairn, C.E.; Gil, D.W.; Kedzie, K.M.; Burke, J.A.; Kharlamb, A.; Runde, E.; Andrews, S.W. Molecular Characterization and Ocular Hypotensive Properties of the Prostanoid EP2 Receptor. J. Ocul. Pharmacol. Ther. 1995, 11, 447–454. [Google Scholar] [CrossRef]
- Miki, A.; Miyamoto, E.; Ishida, N.; Shii, D.; Hori, K. LESPOIR Research Group Efficacy and Safety of Omidenepag Isopropyl 0.002% Ophthalmic Solution: A Retrospective Analysis of Real-World Data in Japan. Adv. Ther. 2022, 39, 2085–2095. [Google Scholar] [CrossRef]
- Yang, M.C.; Lin, K.Y. Drug-Induced Acute Angle-Closure Glaucoma: A Review. J. Curr. Glaucoma Pract. 2019, 13, 104–109. [Google Scholar] [CrossRef]
- Wu, R.-Y.; Ma, N. Expression of Nitric Oxide Synthase and Guanylate Cyclase in the Human Ciliary Body and Trabecular Meshwork. Chin. Med. J. 2012, 125, 129–133. [Google Scholar]
- Schneemann, A.; Leusink-Muis, A.; van den Berg, T.; Hoyng, P.F.J.; Kamphuis, W. Elevation of Nitric Oxide Production in Human Trabecular Meshwork by Increased Pressure. Graefe’s Arch. Clin. Exp. Ophthalmol. 2003, 241, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Wiederholt, M.; Sturm, A.; Lepple-Wienhues, A. Relaxation of Trabecular Meshwork and Ciliary Muscle by Release of Nitric Oxide. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2515–2520. [Google Scholar]
- Buys, E.S.; Zimmer, D.P.; Chickering, J.; Graul, R.; Chien, Y.T.; Profy, A.; Hadcock, J.R.; Masferrer, J.L.; Milne, G.T. Discovery and Development of next Generation SGC Stimulators with Diverse Multidimensional Pharmacology and Broad Therapeutic Potential. Nitric Oxide 2018, 78, 72–80. [Google Scholar] [CrossRef]
- Yousufzai, S.Y.; Ye, Z.; Abdel-Latif, A.A. Prostaglandin F2 Alpha and Its Analogs Induce Release of Endogenous Prostaglandins in Iris and Ciliary Muscles Isolated from Cat and Other Mammalian Species. Exp. Eye Res. 1996, 63, 305–310. [Google Scholar] [CrossRef]
- Zhu, T.; Gobeil, F.; Vazquez-Tello, A.; Leduc, M.; Rihakova, L.; Bossolasco, M.; Bkaily, G.; Peri, K.; Varma, D.R.; Orvoine, R.; et al. Intracrine Signaling through Lipid Mediators and Their Cognate Nuclear G-Protein-Coupled Receptors: A Paradigm Based on PGE2, PAF, and LPA1 Receptors. Can. J. Physiol. Pharmacol. 2006, 84, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Rösch, S.; Ramer, R.; Brune, K.; Hinz, B. Prostaglandin E2 Induces Cyclooxygenase-2 Expression in Human Non-Pigmented Ciliary Epithelial Cells through Activation of P38 and P42/44 Mitogen-Activated Protein Kinases. Biochem. Biophys. Res. Commun. 2005, 338, 1171–1178. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Zenkel, M.; Nüsing, R.M. Expression and Localization of FP and EP Prostanoid Receptor Subtypes in Human Ocular Tissues. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1475–1487. [Google Scholar]
- Husain, S.; Jafri, F.; Crosson, C.E. Acute Effects of PGF2alpha on MMP-2 Secretion from Human Ciliary Muscle Cells: A PKC- and ERK-Dependent Process. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1706–1713. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Geoghegan, T.E.; Patil, R.V.; Bhattacherjee, P.; Paterson, C.A. Detection of EP2, EP4, and FP Receptors in Human Ciliary Epithelial and Ciliary Muscle Cells. Biochem. Pharmacol. 1997, 53, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, M.; Toris, C.B.; Fan, S.; Taniguchi, T.; Ichikawa, M.; Odani-Kawabata, N.; Iwamura, R.; Yoneda, K.; Matsugi, T.; Shams, N.K.; et al. Effects of a Novel Selective EP2 Receptor Agonist, Omidenepag Isopropyl, on Aqueous Humor Dynamics in Laser-Induced Ocular Hypertensive Monkeys. J. Ocul. Pharmacol. Ther. 2018, 34, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Kalouche, G.; Beguier, F.; Bakria, M.; Melik-Parsadaniantz, S.; Leriche, C.; Debeir, T.; Rostène, W.; Baudouin, C.; Vigé, X. Activation of Prostaglandin FP and EP2 Receptors Differently Modulates Myofibroblast Transition in a Model of Adult Primary Human Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-H.; Millar, J.C.; Pang, I.-H.; Wax, M.B.; Clark, A.F. Noninvasive Measurement of Rodent Intraocular Pressure with a Rebound Tonometer. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4617–4621. [Google Scholar] [CrossRef]
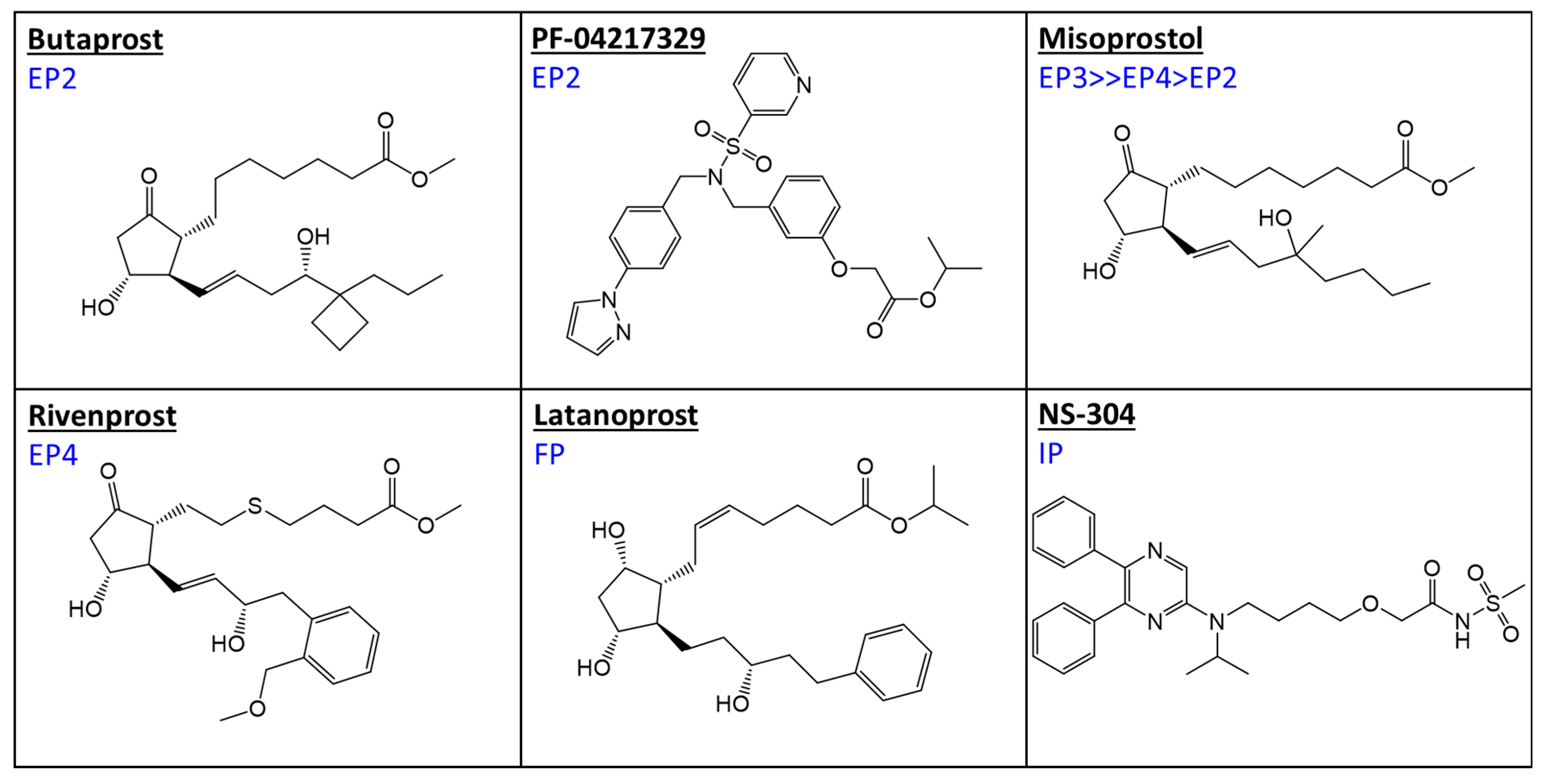

| Major Prostanoid Receptor Types and Some Sub-Types | ||||||||
|---|---|---|---|---|---|---|---|---|
| DP1 | EP1 | EP2 | EP3 | EP4 | FP | IP | TP | |
| Receptor Binding Inhibition Constants (Ki, nM) | ||||||||
| Latanoprost free acid | 54,567 | 1750 | 39,667 | 6503 | 100,000 | 3 | 100,000 | 16,300 |
| Butaprost free acid | 12,097 | 27,721 | 91 | 1643 | 19,104 | 100,000 | 54,836 | 19,667 |
| PF-04217329 free acid | 3200 | 15 | 3200 | 3200 | ||||
| Misoprostol free acid | 15,876 | 11,935 | 34 | 8 | 23 | 9382 | 100,000 | 53,047 |
| Rivenprost free acid | 10,000 | 620 | 56 | 1 | ||||
| NS-304 free acid (MRE-269) | >10,000 | 5800 | >10,000 | 4900 | >10,000 | 20 | >10,000 | |
| Compound | Receptor/Enzyme Target | Maximum IOP-Lowering Observed (% From Baseline) | Reference |
|---|---|---|---|
| Tafluprost | FP–PGR agonist | 24% | [65] |
| Latanoprost | FP–PGR agonist | 20% | |
| Travoprost | FP–PGR agonist | 21% | |
| Unoprostone | FP–PGR agonist | 11% | |
| Latanoprost | FP–PGR agonist | 21% (16%) | [74] |
| Travoprost | FP–PGR agonist | 25% (19%) | |
| Bimatoprost | FP–PGR agonist | 20% (13%) | |
| Unoprostone | FP–PGR agonist | 14% (11%) | |
| Tafluprost | FP–PGR agonist | 22% | [66] |
| Latanoprost | FP–PGR agonist | 22% | |
| Bunazosin | α-1R antagonist | 22% | |
| Dipivefrin | α-2R agonist | 27% | |
| Timolol | β-blocker | 17% | |
| Dorzolamide | Carbonic anhydrase inhibitor | 11% | |
| Pilocarpine | Muscarinic receptor agonist | 11% | |
| Betaxolol | β-blocker | 38% | [76] |
| Latanoprost | FP–PGR agonist | 34% | |
| Brimonidine | α-2R agonist | 16% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharif, N.A.; Millar, J.C.; Zode, G.; Ota, T. Steroid-Induced Ocular Hypertension in Mice Is Differentially Reduced by Selective EP2, EP3, EP4, and IP Prostanoid Receptor Agonists. Int. J. Mol. Sci. 2024, 25, 3328. https://doi.org/10.3390/ijms25063328
Sharif NA, Millar JC, Zode G, Ota T. Steroid-Induced Ocular Hypertension in Mice Is Differentially Reduced by Selective EP2, EP3, EP4, and IP Prostanoid Receptor Agonists. International Journal of Molecular Sciences. 2024; 25(6):3328. https://doi.org/10.3390/ijms25063328
Chicago/Turabian StyleSharif, Najam A., J. Cameron Millar, Gulab Zode, and Takashi Ota. 2024. "Steroid-Induced Ocular Hypertension in Mice Is Differentially Reduced by Selective EP2, EP3, EP4, and IP Prostanoid Receptor Agonists" International Journal of Molecular Sciences 25, no. 6: 3328. https://doi.org/10.3390/ijms25063328
APA StyleSharif, N. A., Millar, J. C., Zode, G., & Ota, T. (2024). Steroid-Induced Ocular Hypertension in Mice Is Differentially Reduced by Selective EP2, EP3, EP4, and IP Prostanoid Receptor Agonists. International Journal of Molecular Sciences, 25(6), 3328. https://doi.org/10.3390/ijms25063328







