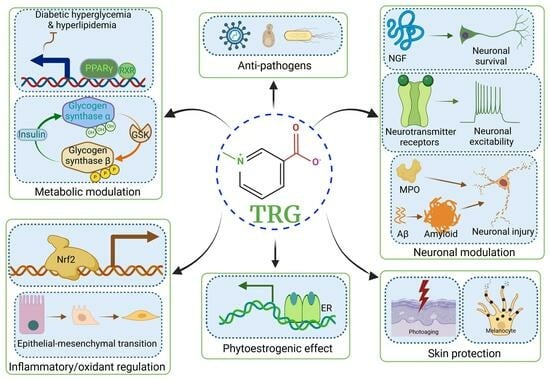
Pharmacological Activities, Therapeutic Effects, and Mechanistic Actions of Trigonelline
Abstract
1. Introduction
1.1. Safety Profile of TRG
1.2. Pharmacokinetics of TRG
2. Regulatory Role of TRG in Glucose and Lipid Metabolism
2.1. TRG Regulates Glucose Synthesis and Transport (Figure 1A)
2.2. TRG Modulates Lipogenesis and Fatty Acid Metabolism (Figure 1A)
3. Anti-Diabetic Mellitus (DM) Effects of TRG
3.1. Improvements in β-Cell Function and Mitigation of β-Cell Apoptosis (Figure 1A)
3.2. Effects on Oxidative Stress (Figure 1A,B)
3.3. Hypoglycemic Effect
3.4. Improvements in Insulin Sensitivity (Figure 1A)
4. Neuroprotective Effects of TRG
4.1. Anti-Diabetic Peripheral Neuropathy (Figure 1C)
4.2. Effects on Alzheimer’s Disease (AD) (Figure 1C)
4.3. Effects on Parkinson’s Disease (PD) (Figure 1C)
4.4. Effects on Cognition, Learning, and Memory (Figure 1C)
4.5. Effects on Stroke (Figure 1C)
4.6. Anti-Depression and Anti-Epilepsy Effects (Figure 1C)
4.7. Neuromodulation (Figure 1C)
5. Liver Protection Effects of TRG
5.1. Alleviation of Liver Steatosis and NAFLD Injury (Figure 1D)
5.2. Improving Liver Function (Figure 1B,D)
6. Cardiovascular Protection Effects of TRG
6.1. Anti-Cardiomyopathy Effect (Figure 1E)
6.2. Mitigation of Myocardial Injury (Figure 1E)
6.3. Alleviation of Fibrosis (Figure 1B,E)
6.4. Improving Endothelial Function (Figure 1E)
7. Anti-Nephropathy Effects of TRG
7.1. Anti-Diabetic Nephropathy (Figure 1F)
7.2. Effects on Metal Exposure-Induced Renal Tubular Injury (Figure 1F)
7.3. Preventive Effect against Kidney Stone Formation (Figure 1F)
8. Anti-Cancer Effects of TRG
8.1. Anti-Head-and-Neck Cancer (HNC) Effects (Figure 1G)
8.2. Anti-Lung Cancer and -Colon Cancer Effects (Figure 1G)
8.3. Anti-Cancer Cell Migration and Anti-Lipoblastoma Effects (Figure 1G)
9. Antiviral, Antimicrobial, and Antifungal Effects of TRG
9.1. Antiviral Effects (Figure 1H)
9.2. Antimicrobial Effects (Figure 1H)
9.3. Antifungal and Antiparasitic Effects (Figure 1H)
10. Other Protective Effects of TRG
10.1. Skin Protection Effects (Figure 1I)
10.2. Anti-Allergic Inflammation Effect
10.3. Gastroprotective Effects (Figure 1J)
10.4. Phytoestrogenic Effects (Figure 1K)
10.5. Bone Density Regulation (Figure 1L)
10.6. Extending the Lifespan (Figure 1M)
11. Discrepancy Regarding TRG Effects in the Literature
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Johns, E. Ueber die alkaloide des bockshornsamens. Berdeutchem. Ges. 1885, 15, 2518–2523. [Google Scholar] [CrossRef]
- Taguchi, H.; Sakaguchi, M.; Shimabayashi, Y. Trigonelline content in coffee beans and the thermal conversion of trigonelline into nicotinic acid during the roasting of coffee beans. Agric. Biol. Chem. 1985, 49, 3467–3471. [Google Scholar]
- Ashihara, H. Chapter 3—Plant biochemistry: Trigonelline biosynthesis in coffea arabica and coffea canephora. In Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Yoshinari, O.; Takenake, A.; Igarashi, K. Trigonelline ameliorates oxidative stress in type 2 diabetic goto-kakizaki rats. J. Med. Food 2013, 16, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ilavenil, S.; Arasu, M.V.; Lee, J.C.; Kim, D.H.; Roh, S.G.; Park, H.S.; Choi, G.J.; Mayakrishnan, V.; Choi, K.C. Trigonelline attenuates the adipocyte differentiation and lipid accumulation in 3t3-l1 cells. Phytomedicine 2014, 21, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Hamden, K.; Mnafgui, K.; Amri, Z.; Aloulou, A.; Elfeki, A. Inhibition of key digestive enzymes related to diabetes and hyperlipidemia and protection of liver-kidney functions by trigonelline in diabetic rats. Sci. Pharm. 2013, 81, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Devi, V.R.; Sharmila, C.; Subramanian, S. Molecular docking studies involving the inhibitory effect of gymnemic acid, trigonelline and ferulic acid, the phytochemicals with antidiabetic properties, on glycogen synthase kinase 3 (α and β). J. Appl. Pharm. Sci. 2018, 8, 150–160. [Google Scholar]
- Vellai, R.D.; Chandiran, S.; Pillai, S.S. Gtf-231, a mixture of gymnemic acid, trigonelline and ferulic acid significantly ameliorates oxidative stress in experimental type 2 diabetes in rats. Can. J. Diabetes 2018, 42, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, O.; Igarashi, K. Anti-diabetic effect of trigonelline and nicotinic acid, on kk-a(y) mice. Curr. Med. Chem. 2010, 17, 2196–2202. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.; Lima, T.F.O.; Arcaro, C.A.; Inacio, M.D.; Batista-Duharte, A.; Carlos, I.Z.; Spolidorio, L.C.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Trigonelline and curcumin alone, but not in combination, counteract oxidative stress and inflammation and increase glycation product detoxification in the liver and kidney of mice with high-fat diet-induced obesity. J. Nutr. Biochem. 2020, 76, 108303. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, S.; Zeng, S. Experimental diabetes treated with trigonelline: Effect on beta cell and pancreatic oxidative parameters. Fundam. Clin. Pharmacol. 2013, 27, 279–287. [Google Scholar] [CrossRef]
- Hamadi, S.A. Effect of trigonelline and ethanol extract of iraqi fenugreek seeds on oxidative stress in alloxan diabetic rabbits. J. Assoc. Arab Univ. Basic Appl. Sci. 2012, 12, 23–26. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, C.; Lou, Z.; Li, Q. Trigonelline reduced diabetic nephropathy and insulin resistance in type 2 diabetic rats through peroxisome proliferator-activated receptor-gamma. Exp. Ther. Med. 2019, 18, 1331–1337. [Google Scholar]
- Sheweita, S.A.; ElHady, S.A.; Hammoda, H.M. Trigonella stellata reduced the deleterious effects of diabetes mellitus through alleviation of oxidative stress, antioxidant- and drug-metabolizing enzymes activities. J. Ethnopharmacol. 2020, 256, 112821. [Google Scholar] [CrossRef] [PubMed]
- Makowska, J.; Szczesny, D.; Lichucka, A.; Gieldon, A.; Chmurzynski, L.; Kaliszan, R. Preliminary studies on trigonelline as potential anti-alzheimer disease agent: Determination by hydrophilic interaction liquid chromatography and modeling of interactions with beta-amyloid. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 968, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.M.; Yang, X.; Kuboyama, T.; Tohda, C. Trigonelline recovers memory function in alzheimer’s disease model mice: Evidence of brain penetration and target molecule. Sci. Rep. 2020, 10, 16424. [Google Scholar] [CrossRef]
- Fahanik-Babaei, J.; Baluchnejadmojarad, T.; Nikbakht, F.; Roghani, M. Trigonelline protects hippocampus against intracerebral aβ(1–40) as a model of alzheimer’s disease in the rat: Insights into underlying mechanisms. Metab. Brain Dis. 2019, 34, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Mirzaie, M.; Khalili, M.; Kiasalari, Z.; Roghani, M. Neuroprotective and antiapoptotic potential of trigonelline in a striatal 6-hydroxydopamine rat model of parkinson’s disease. Neurophysiology 2016, 48, 176–183. [Google Scholar] [CrossRef]
- Pravalika, K.; Sarmah, D.; Kaur, H.; Vats, K.; Saraf, J.; Wanve, M.; Kalia, K.; Borah, A.; Yavagal, D.R.; Dave, K.R.; et al. Trigonelline therapy confers neuroprotection by reduced glutathione mediated myeloperoxidase expression in animal model of ischemic stroke. Life Sci. 2019, 216, 49–58. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, K.; Jiang, C.; Su, Y.; Fan, X.; Li, J.; Xue, S.; Yao, L. Trigonelline protects hippocampal neurons from oxygen-glucose deprivation-induced injury through activating the pi3k/akt pathway. Chem. Biol. Interact. 2020, 317, 108946. [Google Scholar] [CrossRef]
- Chowdhury, A.A.; Gawali, N.B.; Munshi, R.; Juvekar, A.R. Trigonelline insulates against oxidative stress, proinflammatory cytokines and restores bdnf levels in lipopolysaccharide induced cognitive impairment in adult mice. Metab. Brain Dis. 2018, 33, 681–691. [Google Scholar] [CrossRef]
- Chowdhury, A.A.; Gawali, N.B.; Bulani, V.D.; Kothavade, P.S.; Mestry, S.N.; Deshpande, P.S.; Juvekar, A.R. In vitro antiglycating effect and in vivo neuroprotective activity of trigonelline in d-galactose induced cognitive impairment. Pharmacol. Rep. 2018, 70, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Aktar, S.; Ferdousi, F.; Kondo, S.; Kagawa, T.; Isoda, H. Transcriptomics and biochemical evidence of trigonelline ameliorating learning and memory decline in the senescence-accelerated mouse prone 8 (samp8) model by suppressing proinflammatory cytokines and elevating neurotransmitter release. Geroscience 2023, 46, 1671–1691. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Zhou, S.W. Protection of trigonelline on experimental diabetic peripheral neuropathy. Evid. Based Complement. Altern. Med. 2012, 2012, 164219. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, R.; Rodriguez, I.; Nam, Y.H.; Hong, B.N.; Kang, T.H. Trigonelline promotes auditory function through nerve growth factor signaling on diabetic animal models. Phytomedicine 2017, 36, 128–136. [Google Scholar] [CrossRef]
- Anjomshoa, M.; Boroujeni, S.N.; Bagheri, E.; Lorigooini, Z.; Amini-Khoei, H. Possible involvement of n-methyl-d-aspartate receptor (nmda-r) in the antidepressant- like effect of trigonelline in male mice. Curr. Pharm. Des. 2020, 26, 5067–5071. [Google Scholar] [CrossRef]
- Lorigooini, Z.; Sadeghi Dehsahraei, K.; Bijad, E.; Habibian Dehkordi, S.; Amini-Khoei, H. Trigonelline through the attenuation of oxidative stress exerts antidepressant- and anxiolytic-like effects in a mouse model of maternal separation stress. Pharmacology 2020, 105, 289–299. [Google Scholar] [CrossRef]
- Faizan, M.; Jahan, I.; Ishaq, M.; Alhalmi, A.; Khan, R.; Noman, O.M.; Hasson, S.; Mothana, R.A. Neuroprotective effects of trigonelline in kainic acid-induced epilepsy: Behavioral, biochemical, and functional insights. Saudi Pharm. J. 2023, 31, 101843. [Google Scholar] [CrossRef]
- Liu, L.; Du, X.; Zhang, Z.; Zhou, J. Trigonelline inhibits caspase 3 to protect beta cells apoptosis in streptozotocin-induced type 1 diabetic mice. Eur. J. Pharmacol. 2018, 836, 115–121. [Google Scholar] [CrossRef]
- Tharaheswari, M.; Jayachandra Reddy, N.; Kumar, R.; Varshney, K.C.; Kannan, M.; Sudha Rani, S. Trigonelline and diosgenin attenuate er stress, oxidative stress-mediated damage in pancreas and enhance adipose tissue ppargamma activity in type 2 diabetic rats. Mol. Cell. Biochem. 2014, 396, 161–174. [Google Scholar] [CrossRef]
- Hamden, K.; Bengara, A.; Amri, Z.; Elfeki, A. Experimental diabetes treated with trigonelline: Effect on key enzymes related to diabetes and hypertension, beta-cell and liver function. Mol. Cell. Biochem. 2013, 381, 85–94. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Du, X.H.; Zhang, Z.; Qian, G.S. Trigonelline inhibits inflammation and protects beta cells to prevent fetal growth restriction during pregnancy in a mouse model of diabetes. Pharmacology 2017, 100, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Kamble, H.V.; Bodhankar, S.L. Cardioprotective effect of concomitant administration of trigonelline and sitagliptin on cardiac biomarkers, lipid levels, electrocardiographic and heamodynamic modulation on cardiomyopathy in diabetic wistar rats. Biomed. Aging Pathol. 2014, 4, 335–342. [Google Scholar] [CrossRef]
- Panda, S.; Biswas, S.; Kar, A. Trigonelline isolated from fenugreek seed protects against isoproterenol-induced myocardial injury through down-regulation of hsp27 and alphab-crystallin. Nutrition 2013, 29, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Afifi, N.A.; Ramadan, A.; Erian, E.Y.; Saleh, D.O.; Sedik, A.A.; Badawi, M.; El Hotaby, W. Trigonelline attenuates hepatic complications and molecular alterations in high-fat high-fructose diet-induced insulin resistance in rats. Can. J. Physiol. Pharmacol. 2017, 95, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Peerapen, P.; Chanthick, C.; Thongboonkerd, V. Quantitative proteomics reveals common and unique molecular mechanisms underlying beneficial effects of caffeine and trigonelline on human hepatocytes. Biomed. Pharmacother. 2023, 158, 114124. [Google Scholar] [CrossRef] [PubMed]
- Peerapen, P.; Thongboonkerd, V. Protective roles of trigonelline against oxalate-induced epithelial-to-mesenchymal transition in renal tubular epithelial cells: An in vitro study. Food Chem. Toxicol. 2020, 135, 110915. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Guo, Y.; Dong, H.; Wu, W.; Wu, F.; Lu, F. Trigonelline inhibits tubular epithelial-mesenchymal transformation in diabetic kidney disease via targeting smad7. Biomed. Pharmacother. 2023, 168, 115747. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ma, J.; Miao, C.S.; Zhang, H.; Zhang, M.; Cao, X.; Shi, Y. Trigonelline induces autophagy to protect mesangial cells in response to high glucose via activating the mir-5189-5p-ampk pathway. Phytomedicine 2021, 92, 153614. [Google Scholar] [CrossRef]
- Ghule, A.E.; Jadhav, S.S.; Bodhankar, S.L. Trigonelline ameliorates diabetic hypertensive nephropathy by suppression of oxidative stress in kidney and reduction in renal cell apoptosis and fibrosis in streptozotocin induced neonatal diabetic (nstz) rats. Int. Immunopharmacol. 2012, 14, 740–748. [Google Scholar] [CrossRef]
- Shao, X.; Chen, C.; Miao, C.; Yu, X.; Li, X.; Geng, J.; Fan, D.; Lin, X.; Chen, Z.; Shi, Y. Expression analysis of micrornas and their target genes during experimental diabetic renal lesions in rats administered with ginsenoside rb1 and trigonelline. Pharmazie 2019, 74, 492–498. [Google Scholar]
- Zhao, S.; Ghosh, A.; Lo, C.S.; Chenier, I.; Scholey, J.W.; Filep, J.G.; Ingelfinger, J.R.; Zhang, S.L.; Chan, J.S.D. Nrf2 deficiency upregulates intrarenal angiotensin-converting enzyme-2 and angiotensin 1–7 receptor expression and attenuates hypertension and nephropathy in diabetic mice. Endocrinology 2018, 159, 836–852. [Google Scholar] [CrossRef] [PubMed]
- Antonisamy, P.; Arasu, M.V.; Dhanasekaran, M.; Choi, K.C.; Aravinthan, A.; Kim, N.S.; Kang, C.W.; Kim, J.H. Protective effects of trigonelline against indomethacin-induced gastric ulcer in rats and potential underlying mechanisms. Food Funct. 2016, 7, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Omidi-Ardali, H.; Lorigooini, Z.; Soltani, A.; Balali-Dehkordi, S.; Amini-Khoei, H. Inflammatory responses bridge comorbid cardiac disorder in experimental model of ibd induced by dss: Protective effect of the trigonelline. Inflammopharmacology 2019, 27, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Nazir, L.A.; Tanveer, M.A.; Shahid, N.H.; Sharma, R.R.; Tasduq, S.A. Trigonelline, a naturally occurring alkaloidal agent protects ultraviolet-b (uv-b) irradiation induced apoptotic cell death in human skin fibroblasts via attenuation of oxidative stress, restoration of cellular calcium homeostasis and prevention of endoplasmic reticulum (er) stress. J. Photochem. Photobiol. B 2020, 202, 111720. [Google Scholar]
- Tanveer, M.A.; Rashid, H.; Nazir, L.A.; Archoo, S.; Shahid, N.H.; Ragni, G.; Umar, S.A.; Tasduq, S.A. Trigonelline, a plant derived alkaloid prevents ultraviolet-b-induced oxidative DNA damage in primary human dermal fibroblasts and balb/c mice via modulation of phosphoinositide 3-kinase-akt-nrf2 signalling axis. Exp. Gerontol. 2023, 171, 112028. [Google Scholar] [CrossRef]
- Nazir, L.A.; Tanveer, M.A.; Umar, S.A.; Love, S.; Divya, G.; Tasduq, S.A. Inhibition of ultraviolet-b radiation induced photodamage by trigonelline through modulation of mitogen activating protein kinases and nuclear factor-kappab signaling axis in skin. Photochem. Photobiol. 2021, 97, 785–794. [Google Scholar] [CrossRef]
- Hamzawy, M.A.; Abo-Youssef, A.M.; Malak, M.N.; Khalaf, M.M. Multiple targets of nrf 2 inhibitor; trigonelline in combating urethane-induced lung cancer by caspase-executioner apoptosis, cgmp and limitation of cyclin d1 and bcl2. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 9393–9408. [Google Scholar]
- Pirpour Tazehkand, A.; Salehi, R.; Velaei, K.; Samadi, N. The potential impact of trigonelline loaded micelles on nrf2 suppression to overcome oxaliplatin resistance in colon cancer cells. Mol. Biol. Rep. 2020, 47, 5817–5829. [Google Scholar] [CrossRef]
- Arlt, A.; Sebens, S.; Krebs, S.; Geismann, C.; Grossmann, M.; Kruse, M.L.; Schreiber, S.; Schafer, H. Inhibition of the nrf2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity. Oncogene 2013, 32, 4825–4835. [Google Scholar] [CrossRef]
- Zia, S.R.; Wasim, M.; Ahmad, S. Unlocking therapeutic potential of trigonelline through molecular docking as a promising approach for treating diverse neurological disorders. Metab. Brain Dis. 2023, 38, 2721–2733. [Google Scholar] [CrossRef]
- Allred, K.F.; Yackley, K.M.; Vanamala, J.; Allred, C.D. Trigonelline is a novel phytoestrogen in coffee beans. J. Nutr. 2009, 139, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Zeyada, M.S.; Eraky, S.M.; El-Shishtawy, M.M. Trigonelline mitigates bleomycin-induced pulmonary inflammation and fibrosis: Insight into nlrp3 inflammasome and sphk1/s1p/hippo signaling modulation. Life Sci. 2023, 336, 122272. [Google Scholar] [CrossRef] [PubMed]
- Peerapen, P.; Boonmark, W.; Putpeerawit, P.; Sassanarakkit, S.; Thongboonkerd, V. Proteomic and computational analyses followed by functional validation of protective effects of trigonelline against calcium oxalate-induced renal cell deteriorations. Comput. Struct. Biotechnol. J. 2023, 21, 5851–5867. [Google Scholar] [CrossRef]
- Masjedi, M.; Solhjoo, A. Does trigonelline help skin tone? Molecular docking studies of trigonelline on the human tyrosinase, formulation, optimization, and characterization of an emulgel-containing Trigonella foenum-graecum L. Fenugreek standardized hydroalcoholic extract. J. Cosmet. Dermatol. 2022, 21, 7178–7193. [Google Scholar] [CrossRef]
- Aswar, U.; Mohan, V.; Bodhankar, S. Effect of trigonelline on fertility in female rats. Int. J. Green Pharm. 2009, 3, 220–223. [Google Scholar]
- Deshpande, P.; Mohan, V.; Reddy, K.; Manjunath, V.; Thakurdesai, P. Prenatal developmental toxicity evaluation of idm01, a botanical composition of 4-hydroxyisoleucine and trigonelline based standardized fenugreek seed extract, during organogenesis period of pregnancy in rats. J. Appl. Pharm. Sci. 2017, 7, 62–69. [Google Scholar]
- Fouzder, C.; Mukhuty, A.; Mukherjee, S.; Malick, C.; Kundu, R. Trigonelline inhibits nrf2 via egfr signalling pathway and augments efficacy of cisplatin and etoposide in nsclc cells. Toxicol. In Vitro 2021, 70, 105038. [Google Scholar] [CrossRef]
- Konstantinidis, N.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk assessment of trigonelline in coffee and coffee by-products. Molecules 2023, 28, 3460. [Google Scholar] [CrossRef]
- Cheng, Z.X.; Wu, J.J.; Liu, Z.Q.; Lin, N. Development of a hydrophilic interaction chromatography-uplc assay to determine trigonelline in rat plasma and its application in a pharmacokinetic study. Chin. J. Nat. Med. 2013, 11, 164–170. [Google Scholar] [CrossRef]
- Mohamadi, N.; Sharififar, F.; Ansari, M.; Pournamdari, M.; Rezaei, M.; Hassanabadi, N. Pharmacokinetic profile of diosgenin and trigonelline following intravenous and oral administration of fenugreek seed extract and pure compound in rabbit. J. Asian Nat. Prod. Res. 2021, 23, 466–477. [Google Scholar] [CrossRef]
- van Dijk, A.E.; Olthof, M.R.; Meeuse, J.C.; Seebus, E.; Heine, R.J.; van Dam, R.M. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care 2009, 32, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, O.; Sato, H.; Igarashi, K. Anti-diabetic effects of pumpkin and its components, trigonelline and nicotinic acid, on goto-kakizaki rats. Biosci. Biotechnol. Biochem. 2009, 73, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Mukherjee, S.; Yun, J.W. Trigonelline induces browning in 3t3-l1 white adipocytes. Phytother. Res. 2021, 35, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, O.; Igarashi, K. Chapter 85—Antidiabetic effects of trigonelline: Comparison with nicotinic acid. In Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- MacAulay, K.; Doble, B.W.; Patel, S.; Hansotia, T.; Sinclair, E.M.; Drucker, D.J.; Nagy, A.; Woodgett, J.R. Glycogen synthase kinase 3alpha-specific regulation of murine hepatic glycogen metabolism. Cell Metab. 2007, 6, 329–337. [Google Scholar] [CrossRef]
- Friedrich, N.; Skaaby, T.; Pietzner, M.; Budde, K.; Thuesen, B.H.; Nauck, M.; Linneberg, A. Identification of urine metabolites associated with 5-year changes in biomarkers of glucose homoeostasis. Diabetes Metab. 2018, 44, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.P.; Prasath, G.S. Antidiabetic and antidyslipidemic nature of trigonelline, a major alkaloid of fenugreek seeds studied in high-fat-fed and low-dose streptozotocin-induced experimental diabetic rats. Biomed. Prev. Nutr. 2014, 4, 475–480. [Google Scholar] [CrossRef]
- Aldakinah, A.A.; Al-Shorbagy, M.Y.; Abdallah, D.M.; El-Abhar, H.S. Trigonelline and vildagliptin antidiabetic effect: Improvement of insulin signalling pathway. J. Pharm. Pharmacol. 2017, 69, 856–864. [Google Scholar] [CrossRef]
- Najdi, R.A.; Hagras, M.M.; Kamel, F.O.; Magadmi, R.M. A randomized controlled clinical trial evaluating the effect of trigonella foenum-graecum (fenugreek) versus glibenclamide in patients with diabetes. Afr. Health Sci. 2019, 19, 1594–1601. [Google Scholar] [CrossRef]
- Khalili, M.; Alavi, M.; Esmaeil-Jamaat, E.; Baluchnejadmojarad, T.; Roghani, M. Trigonelline mitigates lipopolysaccharide-induced learning and memory impairment in the rat due to its anti-oxidative and anti-inflammatory effect. Int. Immunopharmacol. 2018, 61, 355–362. [Google Scholar] [CrossRef]
- Pravalika, K.; Sarmah, D.; Kaur, H.; Wanve, M.; Saraf, J.; Kalia, K.; Borah, A.; Yavagal, D.R.; Dave, K.R.; Bhattacharya, P. Myeloperoxidase and neurological disorder: A crosstalk. ACS Chem. Neurosci. 2018, 9, 421–430. [Google Scholar] [CrossRef]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef]
- Sharma, L.; Lone, N.A.; Knott, R.M.; Hassan, A.; Abdullah, T. Trigonelline prevents high cholesterol and high fat diet induced hepatic lipid accumulation and lipo-toxicity in c57bl/6j mice, via restoration of hepatic autophagy. Food Chem. Toxicol. 2018, 121, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.F.; Zhang, F.; Zhang, J.; Zhang, R.M.; Li, R. Protection effect of trigonelline on liver of rats with non-alcoholic fatty liver diseases. Asian Pac. J. Trop. Med. 2015, 8, 651–654. [Google Scholar] [CrossRef]
- Ilavenil, S.; Kim, D.H.; Jeong, Y.I.; Arasu, M.V.; Vijayakumar, M.; Prabhu, P.N.; Srigopalram, S.; Choi, K.C. Trigonelline protects the cardiocyte from hydrogen peroxide induced apoptosis in h9c2 cells. Asian Pac. J. Trop. Med. 2015, 8, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Warrier, M. Trimethylamine n-oxide, the microbiome, and heart and kidney disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Bhandari, U.; Panda, B.P.; Dubey, K.; Khan, W.; Ahmad, S. Trigonelline inhibits intestinal microbial metabolism of choline and its associated cardiovascular risk. J. Pharm. Biomed. Anal. 2018, 159, 100–112. [Google Scholar] [CrossRef]
- Rasheeda, K.; Fathima, N.N. Trigonelline hydrochloride: A promising inhibitor for type i collagen fibrillation. Colloids Surf. B Biointerfaces 2018, 170, 273–279. [Google Scholar] [CrossRef]
- Sasaki, M.; Nonoshita, Y.; Kajiya, T.; Atsuchi, N.; Kido, M.; Chu, D.C.; Juneja, L.R.; Minami, Y.; Kajiya, K. Characteristic analysis of trigonelline contained in Raphanus sativus cv. Sakurajima daikon and results from the first trial examining its vasodilator properties in humans. Nutrients 2020, 12, 1872. [Google Scholar]
- Zhao, S.; Lo, C.S.; Miyata, K.N.; Ghosh, A.; Zhao, X.P.; Chenier, I.; Cailhier, J.F.; Ethier, J.; Lattouf, J.B.; Filep, J.G.; et al. Overexpression of nrf2 in renal proximal tubular cells stimulates sodium-glucose cotransporter 2 expression and exacerbates dysglycemia and kidney injury in diabetic mice. Diabetes 2021, 70, 1388–1403. [Google Scholar] [CrossRef]
- van Raaij, S.E.G.; Masereeuw, R.; Swinkels, D.W.; van Swelm, R.P.L. Inhibition of nrf2 alters cell stress induced by chronic iron exposure in human proximal tubular epithelial cells. Toxicol. Lett. 2018, 295, 179–186. [Google Scholar] [CrossRef]
- Peerapen, P.; Boonmark, W.; Thongboonkerd, V. Trigonelline prevents kidney stone formation processes by inhibiting calcium oxalate crystallization, growth and crystal-cell adhesion, and downregulating crystal receptors. Biomed. Pharmacother. 2022, 149, 112876. [Google Scholar] [CrossRef]
- Gjorgieva Ackova, D.; Maksimova, V.; Smilkov, K.; Buttari, B.; Arese, M.; Saso, L. Alkaloids as natural nrf2 inhibitors: Chemoprevention and cytotoxic action in cancer. Pharmaceuticals 2023, 16, 850. [Google Scholar] [CrossRef]
- Pouremamali, F.; Pouremamali, A.; Dadashpour, M.; Soozangar, N.; Jeddi, F. An update of nrf2 activators and inhibitors in cancer prevention/promotion. Cell Commun. Signal. 2022, 20, 100. [Google Scholar] [CrossRef]
- Cirone, M.; D’Orazi, G. Nrf2 in cancer: Cross-talk with oncogenic pathways and involvement in gammaherpesvirus-driven carcinogenesis. Int. J. Mol. Sci. 2022, 24, 595. [Google Scholar] [CrossRef]
- Lisek, K.; Campaner, E.; Ciani, Y.; Walerych, D.; Del Sal, G. Mutant p53 tunes the nrf2-dependent antioxidant response to support survival of cancer cells. Oncotarget 2018, 9, 20508–20523. [Google Scholar] [CrossRef]
- Hamada, S.; Taguchi, K.; Masamune, A.; Yamamoto, M.; Shimosegawa, T. Nrf2 promotes mutant k-ras/p53-driven pancreatic carcinogenesis. Carcinogenesis 2017, 38, 661–670. [Google Scholar] [CrossRef]
- Gilardini Montani, M.S.; Cecere, N.; Granato, M.; Romeo, M.A.; Falcinelli, L.; Ciciarelli, U.; D’Orazi, G.; Faggioni, A.; Cirone, M. Mutant p53, stabilized by its interplay with hsp90, activates a positive feed-back loop between nrf2 and p62 that induces chemo-resistance to apigenin in pancreatic cancer cells. Cancers 2019, 11, 703. [Google Scholar] [CrossRef]
- Shin, D.; Kim, E.H.; Lee, J.; Roh, J.L. Nrf2 inhibition reverses resistance to gpx4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic. Biol. Med. 2018, 129, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Kim, E.H.; Jang, H.; Shin, D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017, 11, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Jang, H.; Kim, E.H.; Shin, D. Targeting of the glutathione, thioredoxin, and nrf2 antioxidant systems in head and neck cancer. Antioxid. Redox Signal. 2017, 27, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.I.; Kim, D.H.; Chung, K.D.; Kim, Y.H.; Lee, Y.S.; Choi, K.C. Antitumor activity of trigonelline-incorporated chitosan nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 5633–5637. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C.; Lee, K.T.; You, B.J.; Lee, C.L.; Chang, W.T.; Wu, Y.C.; Lee, H.Z. Raf/erk/nrf2 signaling pathway and mmp-7 expression involvement in the trigonelline-mediated inhibition of hepatocarcinoma cell migration. Food Nutr. Res. 2015, 59, 29884. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Saravanakumar, K.; Jayalakshmi, J.; Gopi, M.; Shajahan, A.; Barathikannan, K.; Kalaichelvan, P.T.; Wang, M.H. Trigonelline-loaded chitosan nanoparticles prompted antitumor activity on glioma cells and biocompatibility with pheochromocytoma cells. Int. J. Biol. Macromol. 2020, 163, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yu, H.Y.; Zhang, C.L.; Zhu, T.N.; Huang, S.H. Respiratory syncytial virus infection up-regulates tlr7 expression by inducing oxidative stress via the nrf2/are pathway in a549 cells. Arch. Virol. 2018, 163, 1209–1217. [Google Scholar] [CrossRef]
- Ozcelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Zhang, Y.; Gao, C.; Cao, X.; Tian, Y.; Carver, W.; Kiaris, H.; Cui, T.; Tan, W. The spike protein of SARS-CoV-2 impairs lipid metabolism and increases susceptibility to lipotoxicity: Implication for a role of nrf2. Cells 2022, 11, 1916. [Google Scholar] [CrossRef]
- More, G.K.; Vervoort, J.; Steenkamp, P.A.; Prinsloo, G. Metabolomic profile of medicinal plants with anti-rvfv activity. Heliyon 2022, 8, e08936. [Google Scholar] [CrossRef]
- Gilardini Montani, M.S.; Santarelli, R.; Granato, M.; Gonnella, R.; Torrisi, M.R.; Faggioni, A.; Cirone, M. Ebv reduces autophagy, intracellular ros and mitochondria to impair monocyte survival and differentiation. Autophagy 2019, 15, 652–667. [Google Scholar] [CrossRef]
- Gjyshi, O.; Bottero, V.; Veettil, M.V.; Dutta, S.; Singh, V.V.; Chikoti, L.; Chandran, B. Kaposi’s sarcoma-associated herpesvirus induces nrf2 during de novo infection of endothelial cells to create a microenvironment conducive to infection. PLoS Pathog. 2014, 10, e1004460. [Google Scholar] [CrossRef]
- Gjyshi, O.; Flaherty, S.; Veettil, M.V.; Johnson, K.E.; Chandran, B.; Bottero, V. Kaposi’s sarcoma-associated herpesvirus induces nrf2 activation in latently infected endothelial cells through sqstm1 phosphorylation and interaction with polyubiquitinated keap1. J. Virol. 2015, 89, 2268–2286. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Guan, D.; Ma, R.; Yang, R.; Xing, G.; Shi, H.; Tang, G.; Li, J.; Lv, H.; Jiang, Y. Effects of trigonelline inhibition of the nrf2 transcription factor in vitro on echinococcus granulosus. Acta Biochim. Biophys. Sin. 2017, 49, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Chen, S.; Zhang, H.; Yuan, M.; Xiao, L.; Lu, Y.; Xu, H. Trigonelline, an alkaloid from leonurus japonicus houtt., suppresses mast cell activation and ova-induced allergic asthma. Front. Pharmacol. 2021, 12, 687970. [Google Scholar] [CrossRef] [PubMed]
- Nugrahini, A.D.; Ishida, M.; Nakagawa, T.; Nishi, K.; Sugahara, T. Trigonelline: An alkaloid with anti-degranulation properties. Mol. Immunol. 2020, 118, 201–209. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, K.S.; Lee, E.K.; Park, N.C. Efficacy and safety of a mixed extract of trigonella foenum-graecum seed and lespedeza cuneata in the treatment of testosterone deficiency syndrome: A randomized, double-blind, placebo-controlled clinical trial. World J. Mens Health 2018, 36, 230–238. [Google Scholar] [CrossRef]
- Yoo, G.; Allred, C.D. The estrogenic effect of trigonelline and 3,3-diindolymethane on cell growth in non-malignant colonocytes. Food Chem. Toxicol. 2016, 87, 23–30. [Google Scholar] [CrossRef]
- Rathi, A.; Ishaq, M.; Najmi, A.K.; Akhtar, M. Trigonelline demonstrated ameliorative effects in dexamethasone induced osteoporotic rats. Drug Res. 2020, 70, 257–264. [Google Scholar] [CrossRef]
- Folwarczna, J.; Janas, A.; Pytlik, M.; Cegiela, U.; Sliwinski, L.; Krivosikova, Z.; Stefikova, K.; Gajdos, M. Effects of trigonelline, an alkaloid present in coffee, on diabetes-induced disorders in the rat skeletal system. Nutrients 2016, 8, 133. [Google Scholar] [CrossRef]
- Zeng, W.Y.; Tan, L.; Han, C.; Zheng, Z.Y.; Wu, G.S.; Luo, H.R.; Li, S.L. Trigonelline extends the lifespan of c. Elegans and delays the progression of age-related diseases by activating ampk, daf-16, and hsf-1. Oxid. Med. Cell. Longev. 2021, 2021, 7656834. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Nguyen, V.T.; Tamaoki, J.; Endo, Y.; Dong, G.; Sato, A.; Kobayashi, M. Genetic hyperactivation of nrf2 causes larval lethality in keap1a and keap1b-double-knockout zebrafish. Redox Biol. 2023, 62, 102673. [Google Scholar] [CrossRef] [PubMed]
- Nakhate, K.T.; Bharne, A.P.; Verma, V.S.; Aru, D.N.; Kokare, D.M. Plumbagin ameliorates memory dysfunction in streptozotocin induced alzheimer’s disease via activation of nrf2/are pathway and inhibition of beta-secretase. Biomed. Pharmacother. 2018, 101, 379–390. [Google Scholar] [CrossRef]
- Shaw, P.; Sen, A.; Mondal, P.; Dey Bhowmik, A.; Rath, J.; Chattopadhyay, A. Shinorine ameliorates chromium induced toxicity in zebrafish hepatocytes through the facultative activation of nrf2-keap1-are pathway. Aquat. Toxicol. 2020, 228, 105622. [Google Scholar] [CrossRef]
- Vaglienti, M.V.; Subirada, P.V.; Joray, M.B.; Bonacci, G.; Sanchez, M.C. Protective effect of no(2)-oa on oxidative stress, gliosis, and pro-angiogenic response in muller glial cells. Cells 2023, 12, 494. [Google Scholar] [CrossRef] [PubMed]
- Varshney, V.; Garabadu, D. Ang(1–7) exerts nrf2-mediated neuroprotection against amyloid beta-induced cognitive deficits in rodents. Mol. Biol. Rep. 2021, 48, 4319–4331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Q.; Liu, D.Q.; Chen, S.P.; Chen, N.; Sun, J.; Wang, X.M.; Li, D.Y.; Tian, Y.K.; Ye, D.W. Ppargamma activation mitigates mechanical allodynia in paclitaxel-induced neuropathic pain via induction of nrf2/ho-1 signaling pathway. Biomed. Pharmacother. 2020, 129, 110356. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liao, J.; Luo, Y.; Li, M.; Su, X.; Yu, B.; Teng, J.; Wang, H.; Lv, X. Berberine alleviates doxorubicin-induced myocardial injury and fibrosis by eliminating oxidative stress and mitochondrial damage via promoting nrf-2 pathway activation. Int. J. Mol. Sci. 2023, 24, 3257. [Google Scholar] [CrossRef]
- Eslami, M.; Esfandyari, S.; Aghahosseini, M.; Rashidi, Z.; Hosseinishental, S.H.; Brenjian, S.; Sobhani, A.; Amidi, F. Astaxanthin protects human granulosa cells against oxidative stress through activation of nrf2/are pathway and its downstream phase ii enzymes. Cell J. 2021, 23, 319–328. [Google Scholar] [PubMed]
- Campolo, M.; Casili, G.; Biundo, F.; Crupi, R.; Cordaro, M.; Cuzzocrea, S.; Esposito, E. The neuroprotective effect of dimethyl fumarate in an mptp-mouse model of parkinson’s disease: Involvement of reactive oxygen species/nuclear factor-kappab/nuclear transcription factor related to nf-e2. Antioxid. Redox Signal. 2017, 27, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Qu, C.; Niu, T.; Zang, H.; Qi, L.; Lyu, L.; Wang, X.; Nagarkatti, M.; Nagarkatti, P.; Janicki, J.S.; et al. Nrf2-mediated cardiac maladaptive remodeling and dysfunction in a setting of autophagy insufficiency. Hypertension 2016, 67, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Wu, W.; Qi, L.; Tan, W.; Nagarkatti, P.; Nagarkatti, M.; Wang, X.; Cui, T. Autophagy inhibition enables nrf2 to exaggerate the progression of diabetic cardiomyopathy in mice. Diabetes 2020, 69, 2720–2734. [Google Scholar] [CrossRef]
- Zang, H.; Mathew, R.O.; Cui, T. The dark side of nrf2 in the heart. Front. Physiol. 2020, 11, 722. [Google Scholar] [CrossRef]
- Desangles, F. Diagnosis of fanconi’s anemia by nitrogen mustard induction of chromosome breakage in fibroblasts. Pathol. Biol. 1991, 39, 99–104. [Google Scholar] [PubMed]


| Organs/Pathological Conditions | Pharmacological Effects | Experimental Models | Potential Signaling Pathways/Targets | Compound/Natural Sources | Refs |
|---|---|---|---|---|---|
| Aging | Mitochondria protection (Section 10.6); increasing lifespan (Section 10.6) | C. elegans; Zebrafish | AMPK; DAF-16; Suppressing hyperactivation of Nrf2 | TRG | [110,111] |
| Cardiovascular system | Decreasing cardiomyopathy (Section 6.1) | H9C2 cells; isolated gut microbe; DSS-induced IBD mouse model | Anti-apoptotic pathway; FMOs | TRG; TRG extracted from Trigonella foenum-graecum seeds | [44,76,78] |
| Decreasing myocardial injury (Section 6.2) | NICO/STZ-induced DM rats; ISO-induced rats | Downregulation of Hsp27, alphaB-crystallin, and CaMKII delta | TRG; TRG isolated from fenugreek seeds | [33,34] | |
| Decreasing fibrosis/inhibiting EMT (Section 6.3) | In vitro turbidity assay; BLM-induced pulmonary fibrosis | Inhibiting NF-κB/NLRP3/IL-1β | TRG | [53,79] | |
| Improving endothelial cell function (Section 6.4) | Human | n.a. | TRG-enriched Sakurajima radish | [80] | |
| Inflammation | Anti-inflammatory effects (Section 5.2 and Section 6.3) | BLM-induced pulmonary fibrosis; HFHF IR rats | Inhibiting NF-κB/NLRP3/IL-1β | TRG | [35,53] |
| Anti-allergic effects (Section 10.2) | RBL-2H3 cells; PCA reaction, mice; OVA-induced asthma model | Inhibiting intracellular calcium-dependent and -independent pathways; HIF-1alpha | TRG | [104,105] | |
| Kidney | Decreasing DM nephropathy (Section 7.1) | Oxalate-induced EMT; db/db DKD mice; HMCs; neonatal diabetic rats; STZ-induced T2DM rats | Anti-EMT pathway; inhibiting TNF-α signaling; anti-Wnt/b-catenin signaling; AMPK; Smad7 | TRG | [37,38,39,40,41,42] |
| Decreasing metal-induced kidney injury (Section 7.2) | PTCs | Inhibiting hyperactivated Nrf2 signaling | TRG | [82] | |
| Decreasing stone formation (Section 7.3) | MDCK renal tubular cells | n.a. | TRG | [54,83] | |
| Liver | Decreasing steatosis (Section 5.1) | HC-HFD mice | Modulating autophagy | TRG | [74] |
| Decreasing NAFLD injury (Section 5.1) | HFD rats | Anti-apoptotic pathway | TRG | [75] | |
| Improving liver function (Section 5.2) | HFHF IR rats; HepG2 cells | Anti-inflammatory and antioxidative pathways | TRG | [35,36] | |
| Glucose and lipid metabolism | Decreasing glucose synthesis and transport (Section 2.1); hypoglycemic effects (Section 3.3) | T2DM-GK rats; overweight men; T2DM KK-Ay obese mouse; molecular docking simulation; HFD mice | GSK-3a; GSK-3b | TRG; GTF-231 (gymnemic acid, TRG, and ferulic acid in the ratio of 2:3:1); TRG-enriched yogurt | [4,5,6,7,8,9,10] |
| Decreasing lipogenesis and fatty acid levels (Section 2.2) | T2DM-GK rats; 3T3-L1 cells | PPARγ; p38/ATF-2; inhibiting TNF-α signaling | TRG | [4,5,63,64] | |
| Nervous system | Peripheral neuropathy (Section 4.1) | STZ HCHF T2DM rats; LepR(db/db) mice; docking simulation; alloxan-induced diabetic zebrafish | p38 MAPK; NGF | TRG | [24,25] |
| Neuronal protection in AD and PD (Section 4.2 and Section 4.3) | Aβ-induced AD rat model; 5XFAD mouse model; HILIC; 6-OHDA-induced PD rats; | Aβ; CKB | TRG | [15,16,17,18] | |
| Cognitive improvement (Section 4.4) | LPS-induced cognitive dysfunction; D-gal-induced amnesia model; SAMP8 mice | TLR4/NF-kB; Traf6-NF-kB | TRG | [21,22,23] | |
| Decreasing stroke-induced brain injury (Section 4.5) | MCAo ischemic stroke rat model; OGD/R mouse model | MPO; PI3K/Akt | TRG | [19,20] | |
| Anti-depression and anti-epilepsy (Section 4.6) | FST-induced mice; MS stress-induced depressive- and anxiety-like mouse model; kainic acid-induced epileptic model | Anti-inflammatory and antioxidative pathways | TRG | [26,27,28] | |
| Neuromodulation effects (Section 4.7) | Molecular docking simulation | GABARS, mAChR, 5HTRs, NMDAR, AMPAR | TRG | [51] | |
| Oxidation | Antioxidative stress (Section 3.2) | Alloxan diabetic rabbits; STZ HCHF T2DM rats; STZ–HFD rats | PPARγ; inhibiting TNF-α signaling; increasing SOD, CAT, GSH | TRG; Iraqi fenugreek seed extracts; Trigonella stellata | [11,12,13,14] |
| Pancreas | Increasing insulin sensitivity (Section 3.4) | STZ HFD T2DM rats; DM patients | Insulin receptor | TRG; fenugreek seed | [68,69,70] |
| Protecting β-cells and improving β-cell function (Section 3.1 and Section 3.2) | STZ-induced DM mice; T2DM rats; alloxan-induced diabetic rats; diabetic pregnant mice | PPARγ; anti-apoptotic pathway | TRG | [6,11,29,30,31,32] | |
| Pathogen infections | Anti-viral effects (Section 9.1) | RSV, HSV-1, PI-3, RVFV, EBV, human gammaherpesvirus, spike protein of SARS-CoV-2 | Inhibiting TLR7 signaling; inhibiting hyperactivated Nrf2 signaling | TRG | [96,97,98,99,100,101,102] |
| Anti-bacterial effects (Section 9.2) | A. baumannii, B. subtilis, E. coli, E. faecalis, K. pneumoniae, P. mirabilis, P. aeruginosa, and S. aureus | n.a. | TRG | [97] | |
| Antifungal effects (Section 9.3) | C. albicans and C. parapsilosis | n.a. | TRG | [97] | |
| Antiparasitic effects (Section 9.3) | Echinococcus granulosus | Inhibiting hyperactivated Nrf2 signaling | TRG | [103] | |
| Skin | Anti-melanogenic effects (Section 10.1) | Molecular docking simulation; in vitro kinetic assay | Inhibiting tyrosinase | Emulgels containing fenugreek extract and fenugreek extract-entrapped niosomes | [55] |
| Decreasing UVB-induced photoaging (Section 10.1) | human skin fibroblasts; UVB-exposed mouse skin; Hs68 cells; | Inhibiting ROS/MAPK/NF-kB | TRG | [45,46,47] | |
| Tumor | Inhibition of tumor cell proliferation/increasing chemo-sensitivity (Section 8.1, Section 8.2 and Section 8.3) | HNC, NSCLC, colon cancer cells | Inhibiting hyperactivated Nrf2 signaling | TRG, TRG-loaded micelles | [48,49,50] |
| Inhibition of tumor cell migration (Section 8.3) | Hepatoma cell | Inhibiting Raf/ERK/Nrf2 | TRG-loaded water-soluble chitosan nanoparticles | [93,94] | |
| Others | Bone density regulation (Section 10.5) | Nicotinamide/STZ rats; STZ rats; dexamethasone-induced osteoporosis; | n.a. | TRG | [108,109] |
| Phytoestrogen effects (Section 10.4) | Human subjects with TDS; MCF-7 cells; YAMCs | ER | TRG-enriched extract of TFGL (Trigonella foenum-graecum seed and lespedeza cuneata); TRG | [52,106,107] | |
| Mitigation of gastric ulcer and IBD (Section 10.3) | Indomethacin-induced gastric ulcer rat model; DSS-induced IBD mouse model | anti-inflammatory, antioxidant, and anti-apoptotic pathways | TRG | [43,44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Pharmacological Activities, Therapeutic Effects, and Mechanistic Actions of Trigonelline. Int. J. Mol. Sci. 2024, 25, 3385. https://doi.org/10.3390/ijms25063385
Nguyen V, Taine EG, Meng D, Cui T, Tan W. Pharmacological Activities, Therapeutic Effects, and Mechanistic Actions of Trigonelline. International Journal of Molecular Sciences. 2024; 25(6):3385. https://doi.org/10.3390/ijms25063385
Chicago/Turabian StyleNguyen, Vi, Elaine G. Taine, Dehao Meng, Taixing Cui, and Wenbin Tan. 2024. "Pharmacological Activities, Therapeutic Effects, and Mechanistic Actions of Trigonelline" International Journal of Molecular Sciences 25, no. 6: 3385. https://doi.org/10.3390/ijms25063385
APA StyleNguyen, V., Taine, E. G., Meng, D., Cui, T., & Tan, W. (2024). Pharmacological Activities, Therapeutic Effects, and Mechanistic Actions of Trigonelline. International Journal of Molecular Sciences, 25(6), 3385. https://doi.org/10.3390/ijms25063385