State-of-the-Art Differentiation Protocols for Patient-Derived Cardiac Pacemaker Cells
Abstract
:1. Introduction
2. Genesis of SAN: Molecular Determinants
2.1. Development of Cardiac Mesoderm
2.2. First and Second Heart Fields
2.3. SAN Generates from Posterior SHF
2.4. Map of SAN Markers
3. Electrical Activity of the Native SAN
4. Protocols to Differentiate hiPSC-PMs
4.1. Transgene-Dependent Methods to Obtain hiPSC-PMs
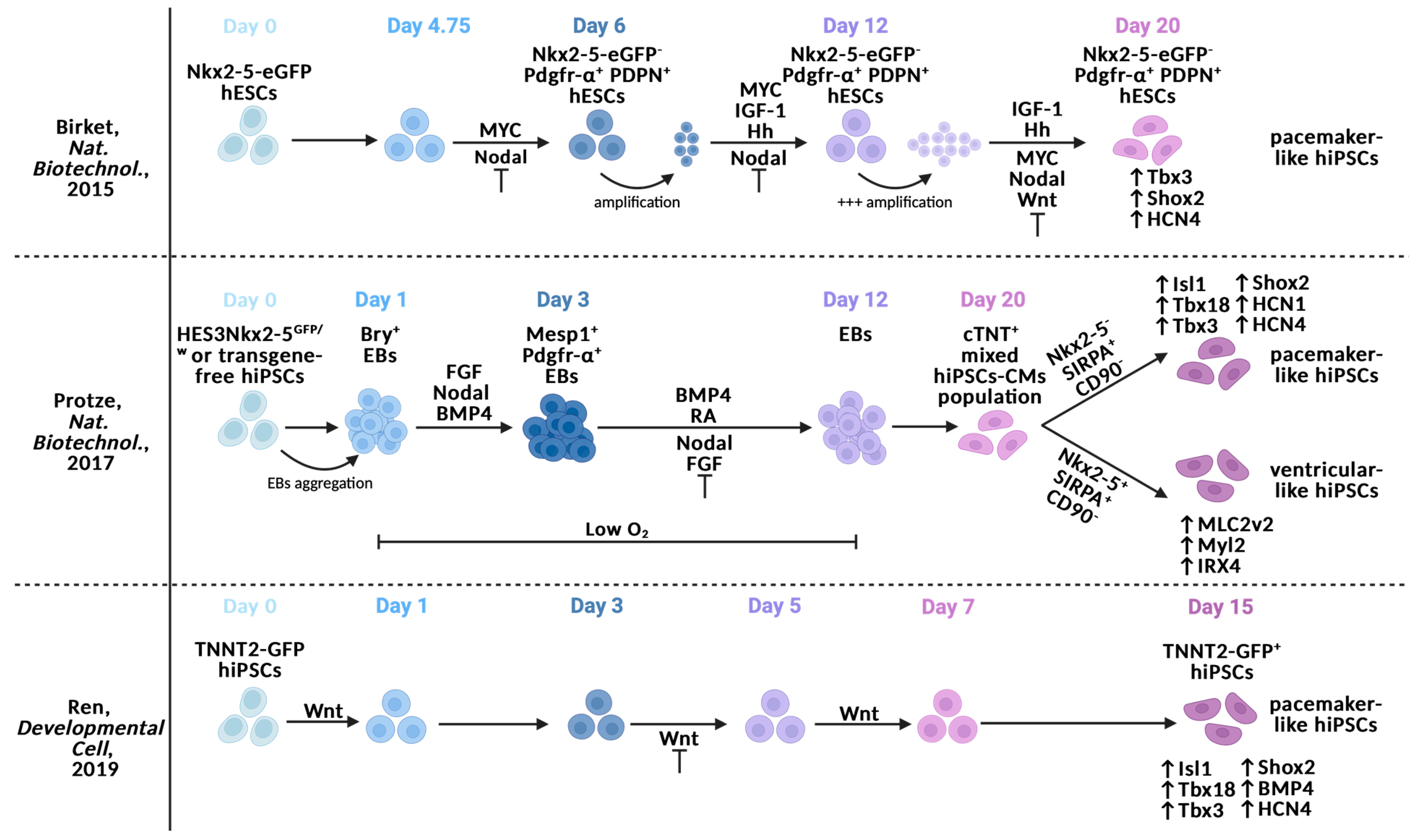
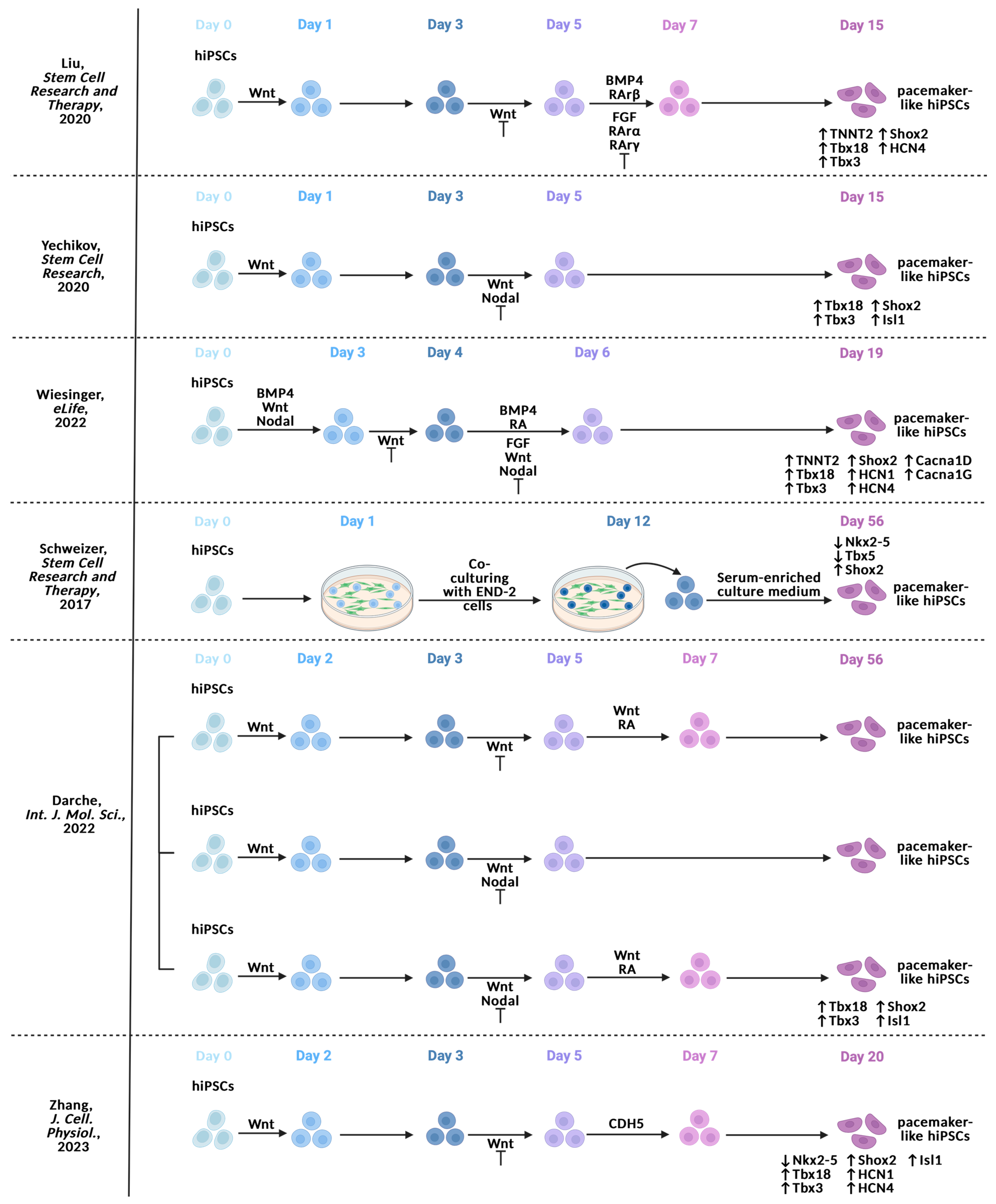
4.2. Transgene-Free Methods to Obtain hiPSC-PMs
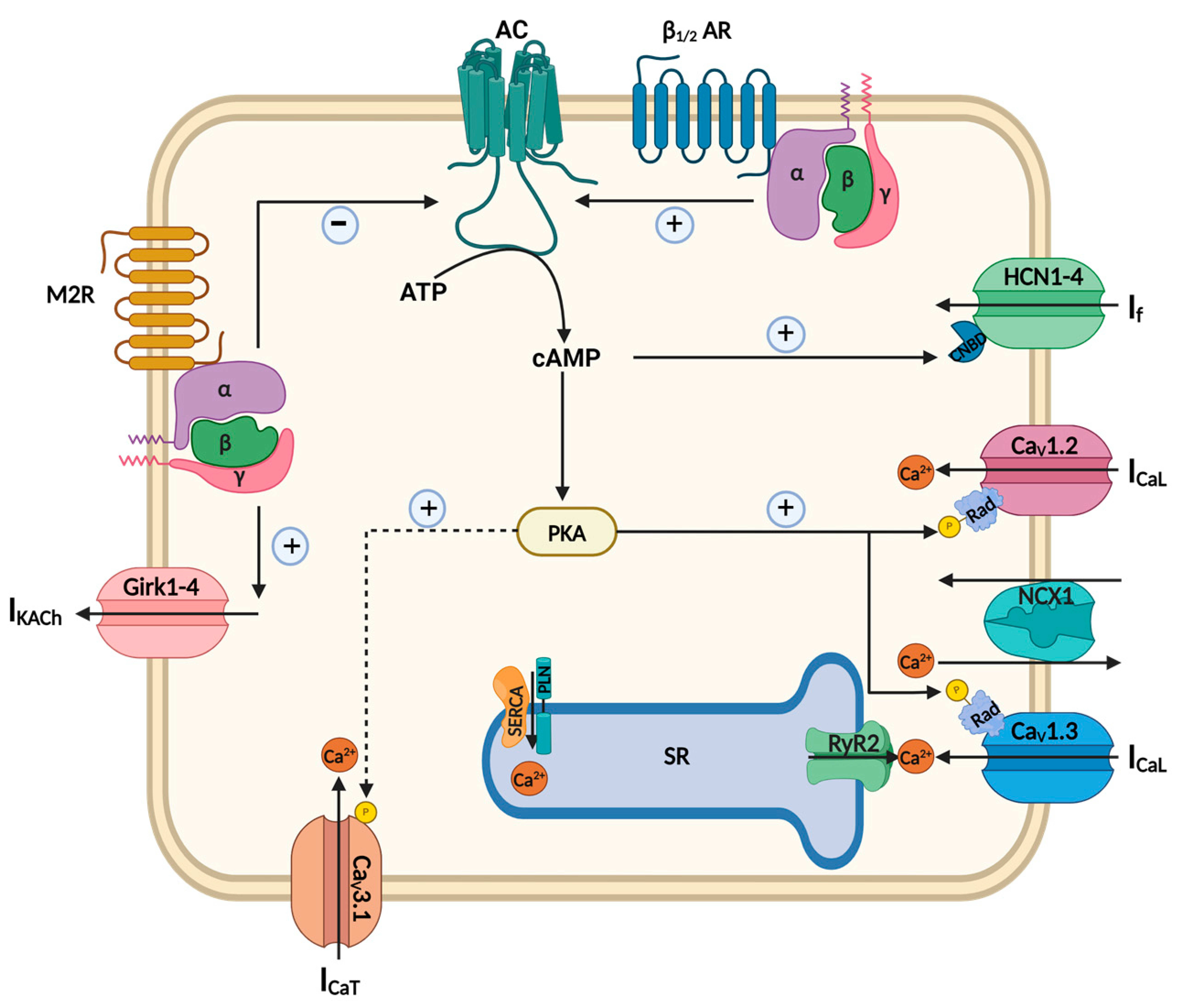
5. Electrophysiological Properties of hiPSC-PMs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mangoni, M.E.; Nargeot, J. Genesis and Regulation of the Heart Automaticity. Physiol. Rev. 2008, 88, 919–982. [Google Scholar] [CrossRef]
- Mangoni, M.E.; Couette, B.; Bourinet, E.; Platzer, J.; Reimer, D.; Striessnig, J.; Nargeot, J. Functional Role of L-Type Cav1.3 Ca2+ Channels in Cardiac Pacemaker Activity. Proc. Natl. Acad. Sci. USA 2003, 100, 5543–5548. [Google Scholar] [CrossRef]
- DiFrancesco, D. The Role of the Funny Current in Pacemaker Activity. Circ. Res. 2010, 106, 434–446. [Google Scholar] [CrossRef]
- Mesirca, P.; Bidaud, I.; Briec, F.; Evain, S.; Torrente, A.G.; Le Quang, K.; Leoni, A.-L.; Baudot, M.; Marger, L.; Chung You Chong, A.; et al. G Protein-Gated IKACh Channels as Therapeutic Targets for Treatment of Sick Sinus Syndrome and Heart Block. Proc. Natl. Acad. Sci. USA 2016, 113, E932–E941. [Google Scholar] [CrossRef]
- Odening, K.E.; Gomez, A.-M.; Dobrev, D.; Fabritz, L.; Heinzel, F.R.; Mangoni, M.E.; Molina, C.E.; Sacconi, L.; Smith, G.; Stengl, M.; et al. ESC Working Group on Cardiac Cellular Electrophysiology Position Paper: Relevance, Opportunities, and Limitations of Experimental Models for Cardiac Electrophysiology Research. EP Eur. 2021, 23, 1795–1814. [Google Scholar] [CrossRef]
- Lodrini, A.M.; Barile, L.; Rocchetti, M.; Altomare, C. Human Induced Pluripotent Stem Cells Derived from a Cardiac Somatic Source: Insights for an In-Vitro Cardiomyocyte Platform. Int. J. Mol. Sci. 2020, 21, 507. [Google Scholar] [CrossRef]
- Gnecchi, M.; Sala, L.; Schwartz, P.J. Precision Medicine and Cardiac Channelopathies: When Dreams Meet Reality. Eur. Heart J. 2021, 42, 1661–1675. [Google Scholar] [CrossRef]
- Reisqs, J.; Moreau, A.; Charrabi, A.; Sleiman, Y.; Meli, A.C.; Millat, G.; Briand, V.; Beauverger, P.; Richard, S.; Chevalier, P. The PPARγ Pathway Determines Electrophysiological Remodelling and Arrhythmia Risks in DSC2 Arrhythmogenic Cardiomyopathy. Clin. Transl. Med. 2022, 12, e748. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Sala, L.; Mura, M.; Rocchetti, M.; Pedrazzini, M.; Ran, X.; Mak, T.S.H.; Crotti, L.; Sham, P.C.; Torre, E.; et al. MTMR4 SNVs Modulate Ion Channel Degradation and Clinical Severity in Congenital Long QT Syndrome: Insights in the Mechanism of Action of Protective Modifier Genes. Cardiovasc. Res. 2021, 117, 767–779. [Google Scholar] [CrossRef]
- Acimovic, I.; Refaat, M.; Moreau, A.; Salykin, A.; Reiken, S.; Sleiman, Y.; Souidi, M.; Přibyl, J.; Kajava, A.; Richard, S.; et al. Post-Translational Modifications and Diastolic Calcium Leak Associated to the Novel RyR2-D3638A Mutation Lead to CPVT in Patient-Specific hiPSC-Derived Cardiomyocytes. J. Clin. Med. 2018, 7, 423. [Google Scholar] [CrossRef]
- Sheng, G.; Martinez Arias, A.; Sutherland, A. The Primitive Streak and Cellular Principles of Building an Amniote Body through Gastrulation. Science 2021, 374, abg1727. [Google Scholar] [CrossRef]
- Evans, S.M.; Yelon, D.; Conlon, F.L.; Kirby, M.L. Myocardial Lineage Development. Circ. Res. 2010, 107, 1428–1444. [Google Scholar] [CrossRef]
- Harvey, R.P. Patterning the Vertebrate Heart. Nat. Rev. Genet. 2002, 3, 544–556. [Google Scholar] [CrossRef]
- Paige, S.L.; Plonowska, K.; Xu, A.; Wu, S.M. Molecular Regulation of Cardiomyocyte Differentiation. Circ. Res. 2015, 116, 341–353. [Google Scholar] [CrossRef]
- Tam, P.P.L.; Parameswaran, M.; Kinder, S.J.; Weinberger, R.P. The Allocation of Epiblast Cells to the Embryonic Heart and Other Mesodermal Lineages: The Role of Ingression and Tissue Movement during Gastrulation. Development 1997, 124, 1631–1642. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- David, R.; Jarsch, V.B.; Schwarz, F.; Nathan, P.; Gegg, M.; Lickert, H.; Franz, W.-M. Induction of MesP1 by Brachyury(T) Generates the Common Multipotent Cardiovascular Stem Cell. Cardiovasc. Res. 2011, 92, 115–122. [Google Scholar] [CrossRef]
- Costello, I.; Pimeisl, I.-M.; Dräger, S.; Bikoff, E.K.; Robertson, E.J.; Arnold, S.J. The T-Box Transcription Factor Eomesodermin Acts Upstream of Mesp1 to Specify Cardiac Mesoderm during Mouse Gastrulation. Nat. Cell Biol. 2011, 13, 1084–1091. [Google Scholar] [CrossRef]
- Pfeiffer, M.J.; Quaranta, R.; Piccini, I.; Fell, J.; Rao, J.; Röpke, A.; Seebohm, G.; Greber, B. Cardiogenic Programming of Human Pluripotent Stem Cells by Dose-Controlled Activation of EOMES. Nat. Commun. 2018, 9, 440. [Google Scholar] [CrossRef]
- Bondue, A.; Lapouge, G.; Paulissen, C.; Semeraro, C.; Iacovino, M.; Kyba, M.; Blanpain, C. Mesp1 Acts as a Master Regulator of Multipotent Cardiovascular Progenitor Specification. Cell Stem Cell 2008, 3, 69–84. [Google Scholar] [CrossRef]
- David, R.; Brenner, C.; Stieber, J.; Schwarz, F.; Brunner, S.; Vollmer, M.; Mentele, E.; Müller-Höcker, J.; Kitajima, S.; Lickert, H.; et al. MesP1 Drives Vertebrate Cardiovascular Differentiation through Dkk-1-Mediated Blockade of Wnt-Signalling. Nat. Cell Biol. 2008, 10, 338–345. [Google Scholar] [CrossRef]
- Saga, Y.; Kitajima, S.; Miyagawa-Tomita, S. Mesp1 Expression Is the Earliest Sign of Cardiovascular Development. Trends Cardiovasc. Med. 2000, 10, 345–352. [Google Scholar] [CrossRef]
- Tsaytler, P.; Liu, J.; Blaess, G.; Schifferl, D.; Veenvliet, J.V.; Wittler, L.; Timmermann, B.; Herrmann, B.G.; Koch, F. BMP4 Triggers Regulatory Circuits Specifying the Cardiac Mesoderm Lineage. Development 2023, 150, dev201450. [Google Scholar] [CrossRef]
- Gaarenstroom, T.; Hill, C.S. TGF-β Signaling to Chromatin: How Smads Regulate Transcription during Self-Renewal and Differentiation. Semin. Cell Dev. Biol. 2014, 32, 107–118. [Google Scholar] [CrossRef]
- Kattman, S.J.; Witty, A.D.; Gagliardi, M.; Dubois, N.C.; Niapour, M.; Hotta, A.; Ellis, J.; Keller, G. Stage-Specific Optimization of Activin/Nodal and BMP Signaling Promotes Cardiac Differentiation of Mouse and Human Pluripotent Stem Cell Lines. Cell Stem Cell 2011, 8, 228–240. [Google Scholar] [CrossRef]
- Lin, X.; Swedlund, B.; Ton, M.-L.N.; Ghazanfar, S.; Guibentif, C.; Paulissen, C.; Baudelet, E.; Plaindoux, E.; Achouri, Y.; Calonne, E.; et al. Mesp1 Controls the Chromatin and Enhancer Landscapes Essential for Spatiotemporal Patterning of Early Cardiovascular Progenitors. Nat. Cell Biol. 2022, 24, 1114–1128. [Google Scholar] [CrossRef]
- Guzzetta, A.; Koska, M.; Rowton, M.; Sullivan, K.R.; Jacobs-Li, J.; Kweon, J.; Hidalgo, H.; Eckart, H.; Hoffmann, A.D.; Back, R.; et al. Hedgehog–FGF Signaling Axis Patterns Anterior Mesoderm during Gastrulation. Proc. Natl. Acad. Sci. USA 2020, 117, 15712–15723. [Google Scholar] [CrossRef]
- Li, D.; Sun, J.; Zhong, T.P. Wnt Signaling in Heart Development and Regeneration. Curr. Cardiol. Rep. 2022, 24, 1425–1438. [Google Scholar] [CrossRef]
- Choi, M.; Stottmann, R.W.; Yang, Y.-P.; Meyers, E.N.; Klingensmith, J. The Bone Morphogenetic Protein Antagonist Noggin Regulates Mammalian Cardiac Morphogenesis. Circ. Res. 2007, 100, 220–228. [Google Scholar] [CrossRef]
- Zhang, Q.; Carlin, D.; Zhu, F.; Cattaneo, P.; Ideker, T.; Evans, S.M.; Bloomekatz, J.; Chi, N.C. Unveiling Complexity and Multipotentiality of Early Heart Fields. Circ. Res. 2021, 129, 474–487. [Google Scholar] [CrossRef]
- Yao, Y.; Gupta, D.; Yelon, D. The MEK-ERK Signaling Pathway Promotes Maintenance of Cardiac Chamber Identity. Development 2024, 151, dev.202183. [Google Scholar] [CrossRef]
- Pradhan, A.; Zeng, X.-X.I.; Sidhwani, P.; Marques, S.R.; George, V.; Targoff, K.L.; Chi, N.C.; Yelon, D. FGF Signaling Enforces Cardiac Chamber Identity in the Developing Ventricle. Development 2017, 144, 1328–1338. [Google Scholar] [CrossRef]
- Miyamoto, M.; Kannan, S.; Anderson, M.J.; Liu, X.; Suh, D.; Htet, M.; Li, B.; Kakani, T.; Murphy, S.; Tampakakis, E.; et al. Cardiac Progenitors Instruct Second Heart Field Fate through Wnts. Proc. Natl. Acad. Sci. USA 2023, 120, e2217687120. [Google Scholar] [CrossRef]
- Keegan, B.R.; Feldman, J.L.; Begemann, G.; Ingham, P.W.; Yelon, D. Retinoic Acid Signaling Restricts the Cardiac Progenitor Pool. Science 2005, 307, 247–249. [Google Scholar] [CrossRef]
- Ryckebusch, L.; Wang, Z.; Bertrand, N.; Lin, S.-C.; Chi, X.; Schwartz, R.; Zaffran, S.; Niederreither, K. Retinoic Acid Deficiency Alters Second Heart Field Formation. Proc. Natl. Acad. Sci. USA 2008, 105, 2913–2918. [Google Scholar] [CrossRef]
- Kirchhof, P.; Kahr, P.C.; Kaese, S.; Piccini, I.; Vokshi, I.; Scheld, H.-H.; Rotering, H.; Fortmueller, L.; Laakmann, S.; Verheule, S.; et al. PITX2c Is Expressed in the Adult Left Atrium, and Reducing Pitx2c Expression Promotes Atrial Fibrillation Inducibility and Complex Changes in Gene Expression. Circ. Cardiovasc. Genet. 2011, 4, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Mommersteeg, M.T.M.; Hoogaars, W.M.H.; Prall, O.W.J.; de Gier-de Vries, C.; Wiese, C.; Clout, D.E.W.; Papaioannou, V.E.; Brown, N.A.; Harvey, R.P.; Moorman, A.F.M.; et al. Molecular Pathway for the Localized Formation of the Sinoatrial Node. Circ. Res. 2007, 100, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Clauss, S.; Kääb, S. Is Pitx2 Growing Up? Circ. Cardiovasc. Genet. 2011, 4, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Hoogaars, W.M.H.; Engel, A.; Brons, J.F.; Verkerk, A.O.; de Lange, F.J.; Wong, L.Y.E.; Bakker, M.L.; Clout, D.E.; Wakker, V.; Barnett, P.; et al. Tbx3 Controls the Sinoatrial Node Gene Program and Imposes Pacemaker Function on the Atria. Genes Dev. 2007, 21, 1098–1112. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.M.; Pu, W.T. HCN4 Charges up the First Heart Field. Circ. Res. 2013, 113, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Christoffels, V.M.; Mommersteeg, M.T.M.; Trowe, M.-O.; Prall, O.W.J.; de Gier-de Vries, C.; Soufan, A.T.; Bussen, M.; Schuster-Gossler, K.; Harvey, R.P.; Moorman, A.F.M.; et al. Formation of the Venous Pole of the Heart from an Nkx2–5–Negative Precursor Population Requires Tbx18. Circ. Res. 2006, 98, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Mommersteeg, M.T.M.; Domínguez, J.N.; Wiese, C.; Norden, J.; de Gier-de Vries, C.; Burch, J.B.E.; Kispert, A.; Brown, N.A.; Moorman, A.F.M.; Christoffels, V.M. The Sinus Venosus Progenitors Separate and Diversify from the First and Second Heart Fields Early in Development. Cardiovasc. Res. 2010, 87, 92–101. [Google Scholar] [CrossRef]
- Puskaric, S.; Schmitteckert, S.; Mori, A.D.; Glaser, A.; Schneider, K.U.; Bruneau, B.G.; Blaschke, R.J.; Steinbeisser, H.; Rappold, G. Shox2 Mediates Tbx5 Activity by Regulating Bmp4 in the Pacemaker Region of the Developing Heart. Hum. Mol. Genet. 2010, 19, 4625–4633. [Google Scholar] [CrossRef]
- Hoffmann, S.; Berger, I.M.; Glaser, A.; Bacon, C.; Li, L.; Gretz, N.; Steinbeisser, H.; Rottbauer, W.; Just, S.; Rappold, G. Islet1 Is a Direct Transcriptional Target of the Homeodomain Transcription Factor Shox2 and Rescues the Shox2-Mediated Bradycardia. Basic Res. Cardiol. 2013, 108, 339. [Google Scholar] [CrossRef]
- Blaschke, R.J.; Hahurij, N.D.; Kuijper, S.; Just, S.; Wisse, L.J.; Deissler, K.; Maxelon, T.; Anastassiadis, K.; Spitzer, J.; Hardt, S.E.; et al. Targeted Mutation Reveals Essential Functions of the Homeodomain Transcription Factor Shox2 in Sinoatrial and Pacemaking Development. Circulation 2007, 115, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, C.; Ye, W.; Espinoza-Lewis, R.A.; Hu, X.; Zhang, Y.; Chen, Y. Phosphorylation of Shox2 Is Required for Its Function to Control Sinoatrial Node Formation. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2014, 3, e000796. [Google Scholar] [CrossRef]
- Klaus, A.; Saga, Y.; Taketo, M.M.; Tzahor, E.; Birchmeier, W. Distinct Roles of Wnt/β-Catenin and Bmp Signaling during Early Cardiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 18531. [Google Scholar] [CrossRef]
- Wu, L.; Du, J.; Jing, X.; Yan, Y.; Deng, S.; Hao, Z.; She, Q. Bone Morphogenetic Protein 4 Promotes the Differentiation of Tbx18-Positive Epicardial Progenitor Cells to Pacemaker-like Cells. Exp. Ther. Med. 2019, 17, 2648–2656. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, A.; Li, N.; Augostini, R.S.; Weiss, R.; Hummel, J.D.; Fedorov, V.V. Three-Dimensional Functional Anatomy of the Human Sinoatrial Node for Epicardial and Endocardial Mapping and Ablation. Heart Rhythm 2023, 20, 122–133. [Google Scholar] [CrossRef]
- Goodyer, W.R.; Beyersdorf, B.M.; Paik, D.T.; Tian, L.; Li, G.; Buikema, J.W.; Chirikian, O.; Choi, S.; Venkatraman, S.; Adams, E.L.; et al. Transcriptomic Profiling of the Developing Cardiac Conduction System at Single-Cell Resolution. Circ. Res. 2019, 125, 379–397. [Google Scholar] [CrossRef]
- Li, N.; Csepe, T.A.; Hansen, B.J.; Dobrzynski, H.; Higgins, R.S.D.; Kilic, A.; Mohler, P.J.; Janssen, P.M.L.; Rosen, M.R.; Biesiadecki, B.J.; et al. Molecular Mapping of Sinoatrial Node HCN Channel Expression in the Human Heart. Circ. Arrhythm. Electrophysiol. 2015, 8, 1219–1227. [Google Scholar] [CrossRef]
- Bers, D.M. Calcium Cycling and Signaling in Cardiac Myocytes. Annu. Rev. Physiol. 2008, 70, 23–49. [Google Scholar] [CrossRef]
- Mesirca, P.; Torrente, A.G.; Mangoni, M.E. T-Type Channels in the Sino-Atrial and Atrioventricular Pacemaker Mechanism. Pflugers Arch. 2014, 466, 791–799. [Google Scholar] [CrossRef]
- Irisawa, H.; Brown, H.F.; Giles, W. Cardiac Pacemaking in the Sinoatrial Node. Physiol. Rev. 1993, 73, 197–227. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Jones, S.A.; Liu, J.; Lancaster, M.K.; Fung, S.S.-M.; Dobrzynski, H.; Camelliti, P.; Maier, S.K.G.; Noble, D.; Boyett, M.R. Requirement of Neuronal- and Cardiac-Type Sodium Channels for Murine Sinoatrial Node Pacemaking. J. Physiol. 2004, 559, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Torrente, A.G.; Mesirca, P.; Neco, P.; Rizzetto, R.; Dubel, S.; Barrere, C.; Sinegger-Brauns, M.; Striessnig, J.; Richard, S.; Nargeot, J.; et al. L-Type Cav1.3 Channels Regulate Ryanodine Receptor-Dependent Ca2+ Release during Sino-Atrial Node Pacemaker Activity. Cardiovasc. Res. 2016, 109, 451–461. [Google Scholar] [CrossRef]
- Papa, A.; Kushner, J.; Marx, S.O. Adrenergic Regulation of Calcium Channels in the Heart. Annu. Rev. Physiol. 2022, 84, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Louradour, J.; Bortolotti, O.; Torre, E.; Bidaud, I.; Lamb, N.; Fernandez, A.; Le Guennec, J.-Y.; Mangoni, M.E.; Mesirca, P. L-Type Cav1.3 Calcium Channels Are Required for Beta-Adrenergic Triggered Automaticity in Dormant Mouse Sinoatrial Pacemaker Cells. Cells 2022, 11, 1114. [Google Scholar] [CrossRef]
- Papa, A.; Zakharov, S.I.; Katchman, A.N.; Kushner, J.S.; Chen, B.; Yang, L.; Liu, G.; Jimenez, A.S.; Eisert, R.J.; Bradshaw, G.A.; et al. Rad Regulation of CaV1.2 Channels Controls Cardiac Fight-or-Flight Response. Nat. Cardiovasc. Res. 2022, 1, 1022–1038. [Google Scholar] [CrossRef]
- Mesirca, P.; Marger, L.; Toyoda, F.; Rizzetto, R.; Audoubert, M.; Dubel, S.; Torrente, A.G.; Difrancesco, M.L.; Muller, J.C.; Leoni, A.-L.; et al. The G-Protein-Gated K+ Channel, IKACh, Is Required for Regulation of Pacemaker Activity and Recovery of Resting Heart Rate after Sympathetic Stimulation. J. Gen. Physiol. 2013, 142, 113–126. [Google Scholar] [CrossRef]
- Krapivinsky, G.; Gordon, E.A.; Wickman, K.; Velimirović, B.; Krapivinsky, L.; Clapham, D.E. The G-Protein-Gated Atrial K+ Channel IKAch Is a Heteromultimer of Two Inwardly Rectifying K+-Channel Proteins. Nature 1995, 374, 135–141. [Google Scholar] [CrossRef]
- Jung, J.J.; Husse, B.; Rimmbach, C.; Krebs, S.; Stieber, J.; Steinhoff, G.; Dendorfer, A.; Franz, W.-M.; David, R. Programming and Isolation of Highly Pure Physiologically and Pharmacologically Functional Sinus-Nodal Bodies from Pluripotent Stem Cells. Stem Cell Rep. 2014, 2, 592–605. [Google Scholar] [CrossRef]
- Ionta, V.; Liang, W.; Kim, E.H.; Rafie, R.; Giacomello, A.; Marbán, E.; Cho, H.C. SHOX2 Overexpression Favors Differentiation of Embryonic Stem Cells into Cardiac Pacemaker Cells, Improving Biological Pacing Ability. Stem Cell Rep. 2015, 4, 129–142. [Google Scholar] [CrossRef]
- Zhu, W.-Z.; Xie, Y.; Moyes, K.W.; Gold, J.D.; Askari, B.; Laflamme, M.A. Neuregulin/ErbB Signaling Regulates Cardiac Subtype Specification in Differentiating Human Embryonic Stem Cells. Circ. Res. 2010, 107, 776–786. [Google Scholar] [CrossRef]
- Birket, M.J.; Ribeiro, M.C.; Verkerk, A.O.; Ward, D.; Leitoguinho, A.R.; den Hartogh, S.C.; Orlova, V.V.; Devalla, H.D.; Schwach, V.; Bellin, M.; et al. Expansion and Patterning of Cardiovascular Progenitors Derived from Human Pluripotent Stem Cells. Nat. Biotechnol. 2015, 33, 970–979. [Google Scholar] [CrossRef]
- Gittenberger-De Groot, A.C.; Mahtab, E.A.F.; Hahurij, N.D.; Wisse, L.J.; Deruiter, M.C.; Wijffels, M.C.E.F.; Poelmann, R.E. Nkx2.5-Negative Myocardium of the Posterior Heart Field and Its Correlation with Podoplanin Expression in Cells from the Developing Cardiac Pacemaking and Conduction System. Anat. Rec. 2007, 290, 115–122. [Google Scholar] [CrossRef]
- Protze, S.I.; Liu, J.; Nussinovitch, U.; Ohana, L.; Backx, P.H.; Gepstein, L.; Keller, G.M. Sinoatrial Node Cardiomyocytes Derived from Human Pluripotent Cells Function as a Biological Pacemaker. Nat. Biotechnol. 2017, 35, 56–68. [Google Scholar] [CrossRef]
- Ren, J.; Han, P.; Ma, X.; Farah, E.N.; Bloomekatz, J.; Zeng, X.-X.I.; Zhang, R.; Swim, M.M.; Witty, A.D.; Knight, H.G.; et al. Canonical Wnt5b Signaling Directs Outlying Nkx2.5+ Mesoderm into Pacemaker Cardiomyocytes. Dev. Cell 2019, 50, 729–743.e5. [Google Scholar] [CrossRef]
- Liu, F.; Fang, Y.; Hou, X.; Yan, Y.; Xiao, H.; Zuo, D.; Wen, J.; Wang, L.; Zhou, Z.; Dang, X.; et al. Enrichment Differentiation of Human Induced Pluripotent Stem Cells into Sinoatrial Node-like Cells by Combined Modulation of BMP, FGF, and RA Signaling Pathways. Stem Cell Res. Ther. 2020, 11, 284. [Google Scholar] [CrossRef]
- Dollé, P. Developmental Expression of Retinoic Acid Receptors (RARs). Nucl. Recept. Signal. 2009, 7, e006. [Google Scholar] [CrossRef]
- Ghyselinck, N.B.; Duester, G. Retinoic Acid Signaling Pathways. Development 2019, 146, dev167502. [Google Scholar] [CrossRef]
- Mollard, R.; Viville, S.; Ward, S.J.; Décimo, D.; Chambon, P.; Dollé, P. Tissue-Specific Expression of Retinoic Acid Receptor Isoform Transcripts in the Mouse Embryo. Mech. Dev. 2000, 94, 223–232. [Google Scholar] [CrossRef]
- Ghyselinck, N.B.; Wendling, O.; Messaddeq, N.; Dierich, A.; Lampron, C.; Décimo, D.; Viville, S.; Chambon, P.; Mark, M. Contribution of Retinoic Acid Receptor Beta Isoforms to the Formation of the Conotruncal Septum of the Embryonic Heart. Dev. Biol. 1998, 198, 303–318. [Google Scholar] [CrossRef]
- Schwach, V.; Cofiño-Fabres, C.; Ten Den, S.A.; Passier, R. Improved Atrial Differentiation of Human Pluripotent Stem Cells by Activation of Retinoic Acid Receptor Alpha (RARα). J. Pers. Med. 2022, 12, 628. [Google Scholar] [CrossRef]
- Yechikov, S.; Kao, H.K.J.; Chang, C.-W.; Pretto, D.; Zhang, X.-D.; Sun, Y.-H.; Smithers, R.; Sirish, P.; Nolta, J.A.; Chan, J.W.; et al. NODAL Inhibition Promotes Differentiation of Pacemaker-like Cardiomyocytes from Human Induced Pluripotent Stem Cells. Stem Cell Res. 2020, 49, 102043. [Google Scholar] [CrossRef]
- Wiesinger, A.; Li, J.; Fokkert, L.; Bakker, P.; Verkerk, A.O.; Christoffels, V.M.; Boink, G.J.; Devalla, H.D. A Single Cell Transcriptional Roadmap of Human Pacemaker Cell Differentiation. eLife 2022, 11, e76781. [Google Scholar] [CrossRef]
- Schweizer, P.A.; Darche, F.F.; Ullrich, N.D.; Geschwill, P.; Greber, B.; Rivinius, R.; Seyler, C.; Müller-Decker, K.; Draguhn, A.; Utikal, J.; et al. Subtype-Specific Differentiation of Cardiac Pacemaker Cell Clusters from Human Induced Pluripotent Stem Cells. Stem Cell Res. Ther. 2017, 8, 229. [Google Scholar] [CrossRef]
- Darche, F.F.; Ullrich, N.D.; Huang, Z.; Koenen, M.; Rivinius, R.; Frey, N.; Schweizer, P.A. Improved Generation of Human Induced Pluripotent Stem Cell-Derived Cardiac Pacemaker Cells Using Novel Differentiation Protocols. Int. J. Mol. Sci. 2022, 23, 7318. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, F.; Yin, L.; Tang, Y.; Wang, X.; Huang, C. Cadherin-5 Facilitated the Differentiation of Human Induced Pluripotent Stem Cells into Sinoatrial Node-like Pacemaker Cells by Regulating β-Catenin. J. Cell. Physiol. 2024, 239, e31161. [Google Scholar] [CrossRef]
- Zawada, D.; Kornherr, J.; Meier, A.B.; Santamaria, G.; Dorn, T.; Nowak-Imialek, M.; Ortmann, D.; Zhang, F.; Lachmann, M.; Dreßen, M.; et al. Retinoic Acid Signaling Modulation Guides In Vitro Specification of Human Heart Field-Specific Progenitor Pools. Nat. Commun. 2023, 14, 1722. [Google Scholar] [CrossRef]
- Delgado-Bellido, D.; Zamudio-Martínez, E.; Fernández-Cortés, M.; Herrera-Campos, A.B.; Olmedo-Pelayo, J.; Perez, C.J.; Expósito, J.; de Álava, E.; Amaral, A.T.; Valle, F.O.; et al. VE-Cadherin Modulates β-Catenin/TCF-4 to Enhance Vasculogenic Mimicry. Cell Death Dis. 2023, 14, 135. [Google Scholar] [CrossRef]
- Nelson, W.J.; Nusse, R. Convergence of Wnt, ß-Catenin, and Cadherin Pathways. Science 2004, 303, 1483–1487. [Google Scholar] [CrossRef]
- Zhao, M.; Tang, Y.; Zhou, Y.; Zhang, J. Deciphering Role of Wnt Signalling in Cardiac Mesoderm and Cardiomyocyte Differentiation from Human iPSCs: Four-Dimensional Control of Wnt Pathway for hiPSC-CMs Differentiation. Sci. Rep. 2019, 9, 19389. [Google Scholar] [CrossRef]
- Devalla, H.D.; Schwach, V.; Ford, J.W.; Milnes, J.T.; El-Haou, S.; Jackson, C.; Gkatzis, K.; Elliott, D.A.; Chuva de Sousa Lopes, S.M.; Mummery, C.L.; et al. Atrial-like Cardiomyocytes from Human Pluripotent Stem Cells Are a Robust Preclinical Model for Assessing Atrial-selective Pharmacology. EMBO Mol. Med. 2015, 7, 394–410. [Google Scholar] [CrossRef]
- Mesirca, P.; Chemin, J.; Barrère, C.; Torre, E.; Gallot, L.; Monteil, A.; Bidaud, I.; Diochot, S.; Lazdunski, M.; Soong, T.W.; et al. Selective Blockade of Cav1.2 (α1C) versus Cav1.3 (α1D) L-Type Calcium Channels by the Black Mamba Toxin Calciseptine. Nat. Commun. 2024, 15, 54. [Google Scholar] [CrossRef]
- Schmidt, C.; Deyett, A.; Ilmer, T.; Haendeler, S.; Caballero, A.T.; Novatchkova, M.; Netzer, M.A.; Ginistrelli, L.C.; Juncosa, E.M.; Bhattacharya, T.; et al. Multi-Chamber Cardioids Unravel Human Heart Development and Cardiac Defects. Cell 2023, 186, 5587–5605.e27. [Google Scholar] [CrossRef]

| Birket, Nat. Biotechnol., 2015 [65] | Protze, Nat. Biotechnol., 2017 [67] | Schweizer, Stem Cell Research and Therapy, 2017 [77] | Ren, Developmental Cell, 2019 [68] | Liu, Stem Cell Research and Therapy, 2020 [69] | Yechikov, Stem Cell Research, 2020 [75] | Wiesinger, eLife, 2022 [76] | Zhang, J. Cell. Physiol., 2023 [79] | |
|---|---|---|---|---|---|---|---|---|
| AP rate | + | + | + | + | + | + | + | + |
| dV/dtmax | + | + | + | + | + | |||
| If | + | + | + (indirectly) | + (indirectly) | + | |||
| ICaL | ||||||||
| ICaT | + | |||||||
| INa | + | |||||||
| IKACh | + | |||||||
| Adrenergic response | + | + | ||||||
| Muscarinic response | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torre, E.; Mangoni, M.E.; Lacampagne, A.; Meli, A.C.; Mesirca, P. State-of-the-Art Differentiation Protocols for Patient-Derived Cardiac Pacemaker Cells. Int. J. Mol. Sci. 2024, 25, 3387. https://doi.org/10.3390/ijms25063387
Torre E, Mangoni ME, Lacampagne A, Meli AC, Mesirca P. State-of-the-Art Differentiation Protocols for Patient-Derived Cardiac Pacemaker Cells. International Journal of Molecular Sciences. 2024; 25(6):3387. https://doi.org/10.3390/ijms25063387
Chicago/Turabian StyleTorre, Eleonora, Matteo E. Mangoni, Alain Lacampagne, Albano C. Meli, and Pietro Mesirca. 2024. "State-of-the-Art Differentiation Protocols for Patient-Derived Cardiac Pacemaker Cells" International Journal of Molecular Sciences 25, no. 6: 3387. https://doi.org/10.3390/ijms25063387
APA StyleTorre, E., Mangoni, M. E., Lacampagne, A., Meli, A. C., & Mesirca, P. (2024). State-of-the-Art Differentiation Protocols for Patient-Derived Cardiac Pacemaker Cells. International Journal of Molecular Sciences, 25(6), 3387. https://doi.org/10.3390/ijms25063387






