The Brain Metabolome Is Modified by Obesity in a Sex-Dependent Manner
Abstract
1. Introduction
2. Results
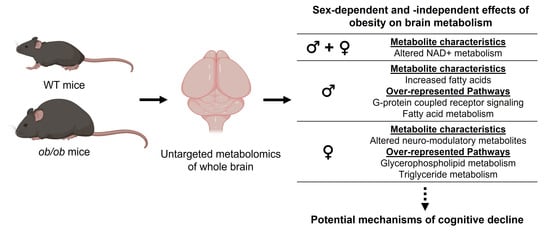
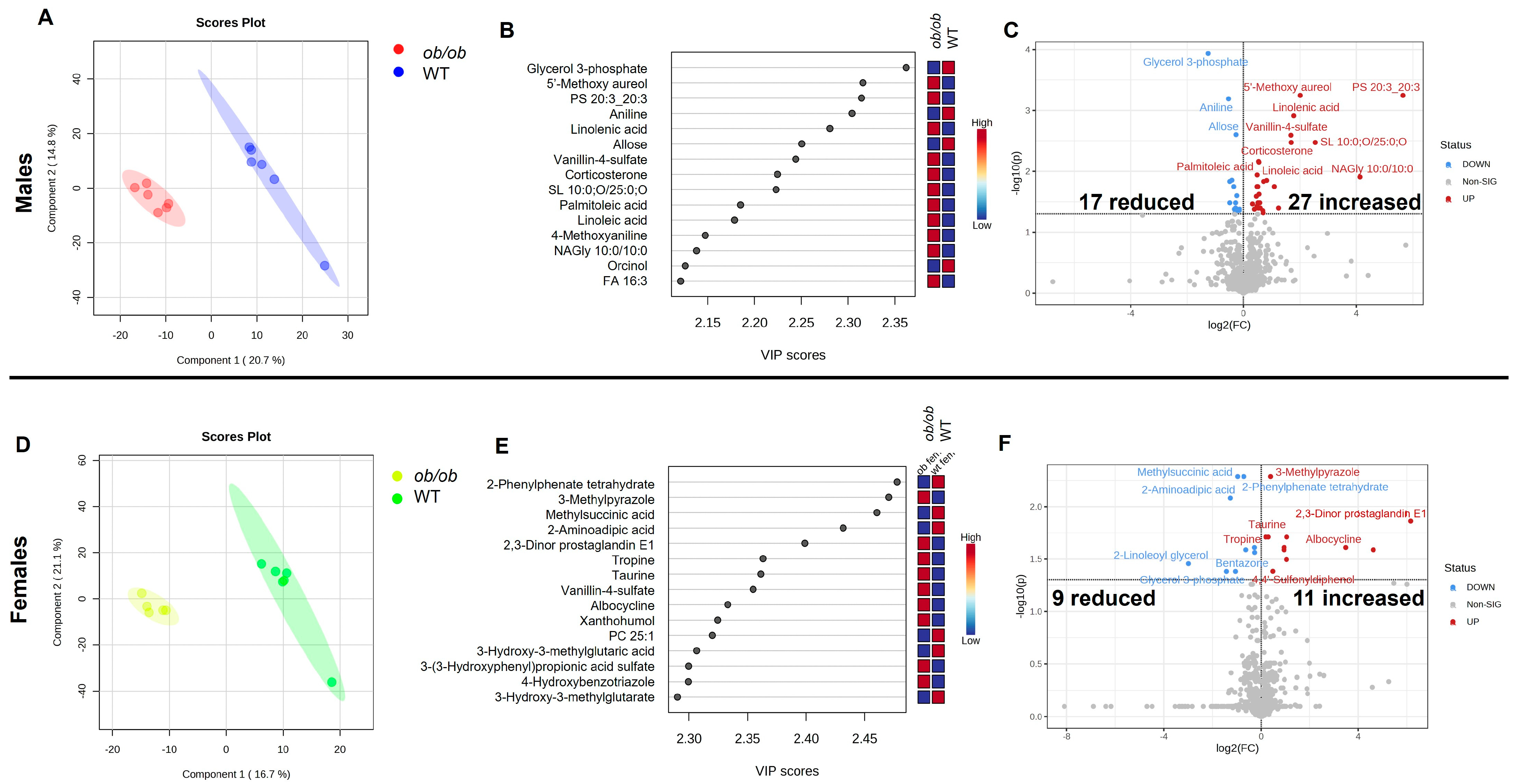
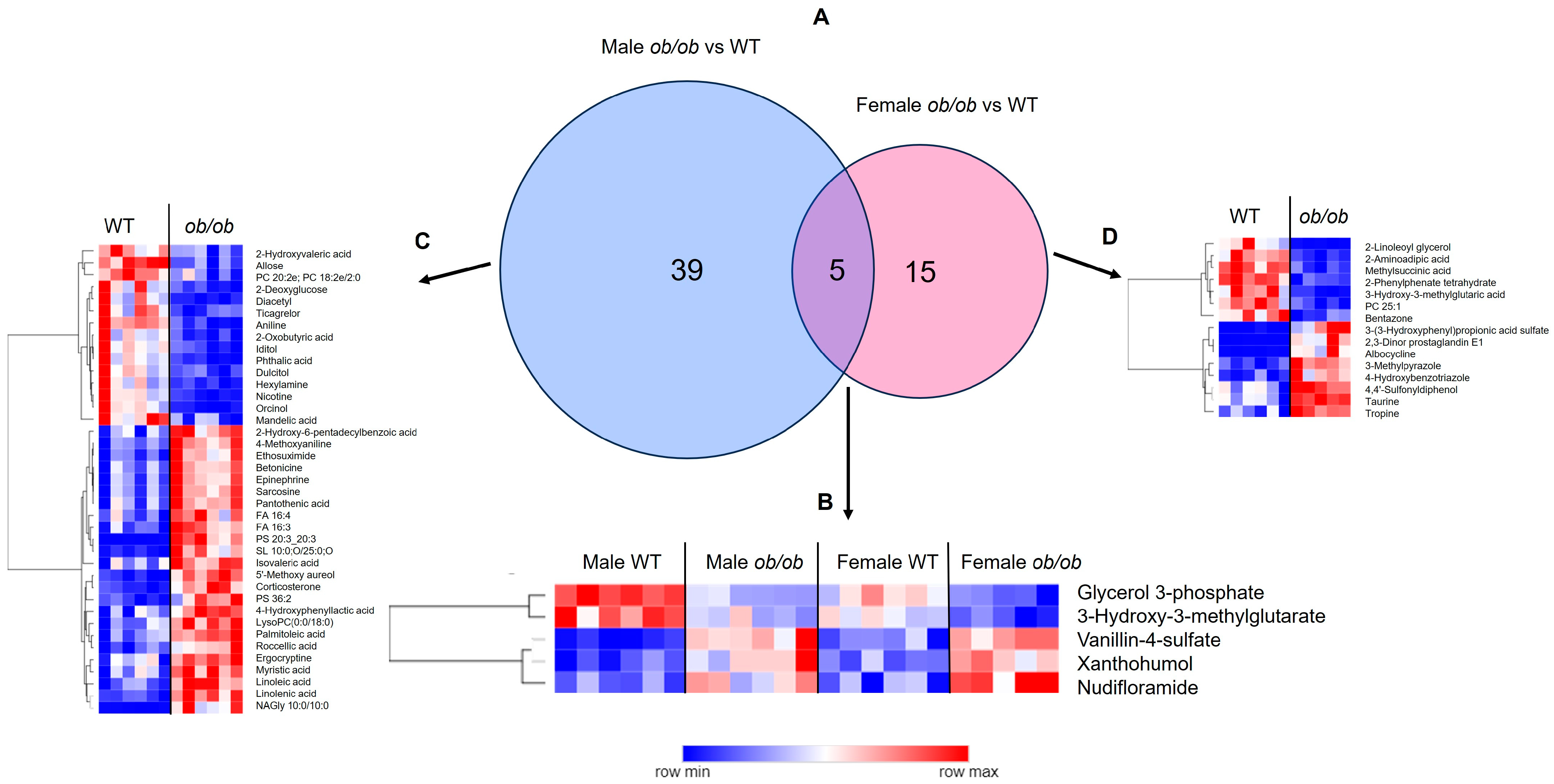
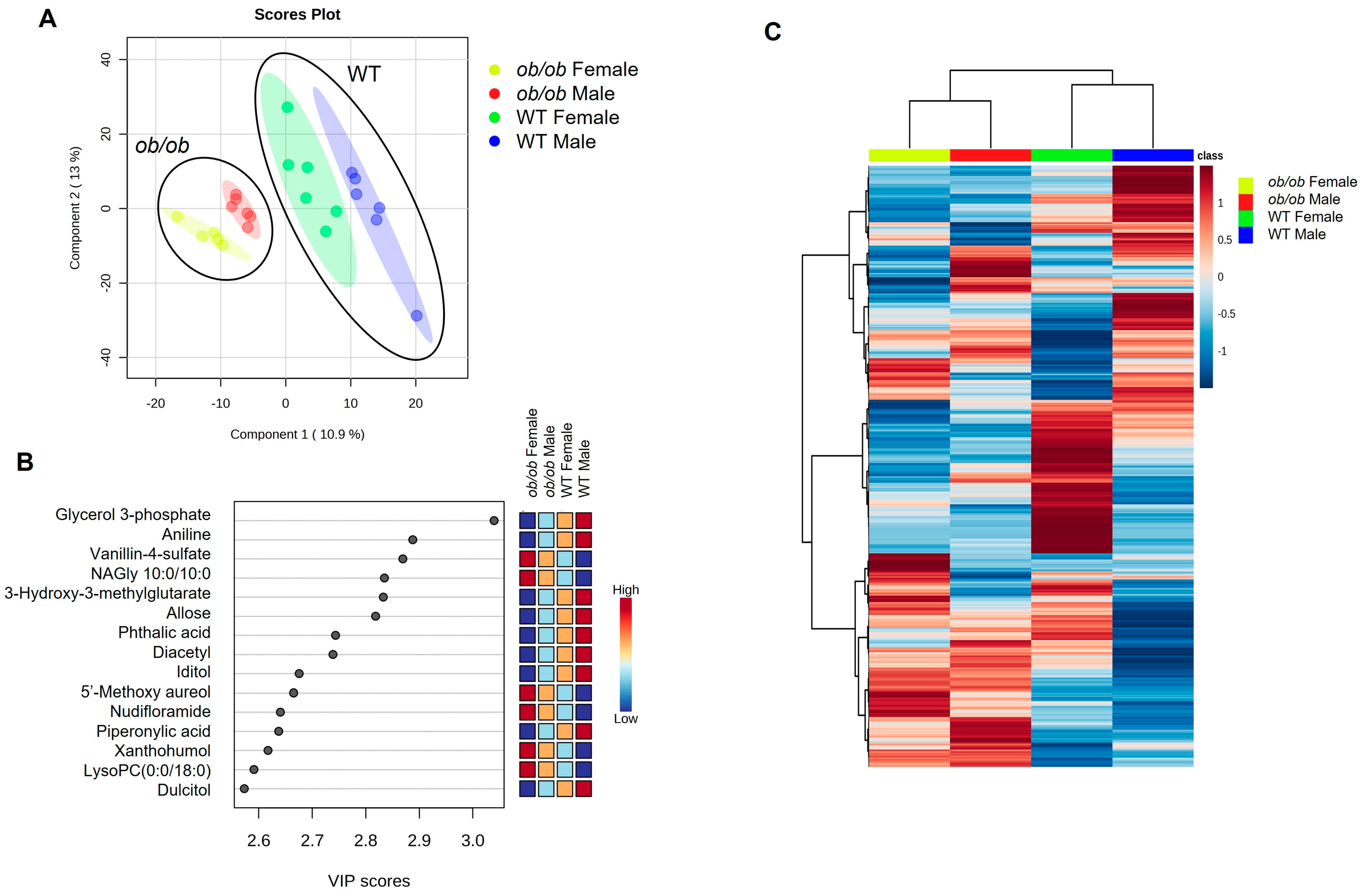
2.1. Obesity Alters the Brain Metabolome in a Sex-Specific Manner
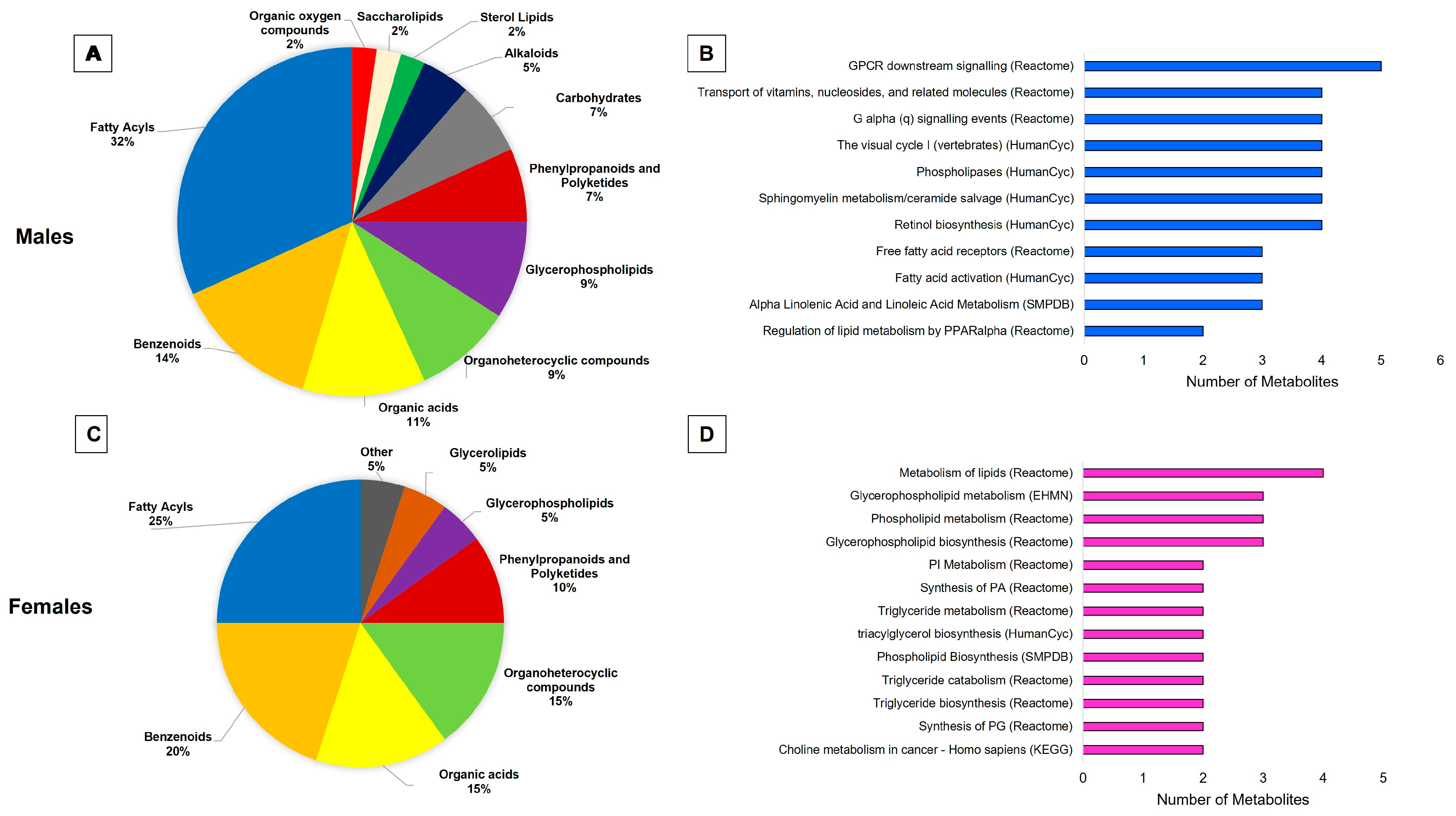
2.2. Lipids Are the Predominant Metabolites Altered by Obesity in the Brain
2.3. Global Comparison Using PLS-DA and Hierarchical Clustering Differentiate the Effects of Obesity and Sex
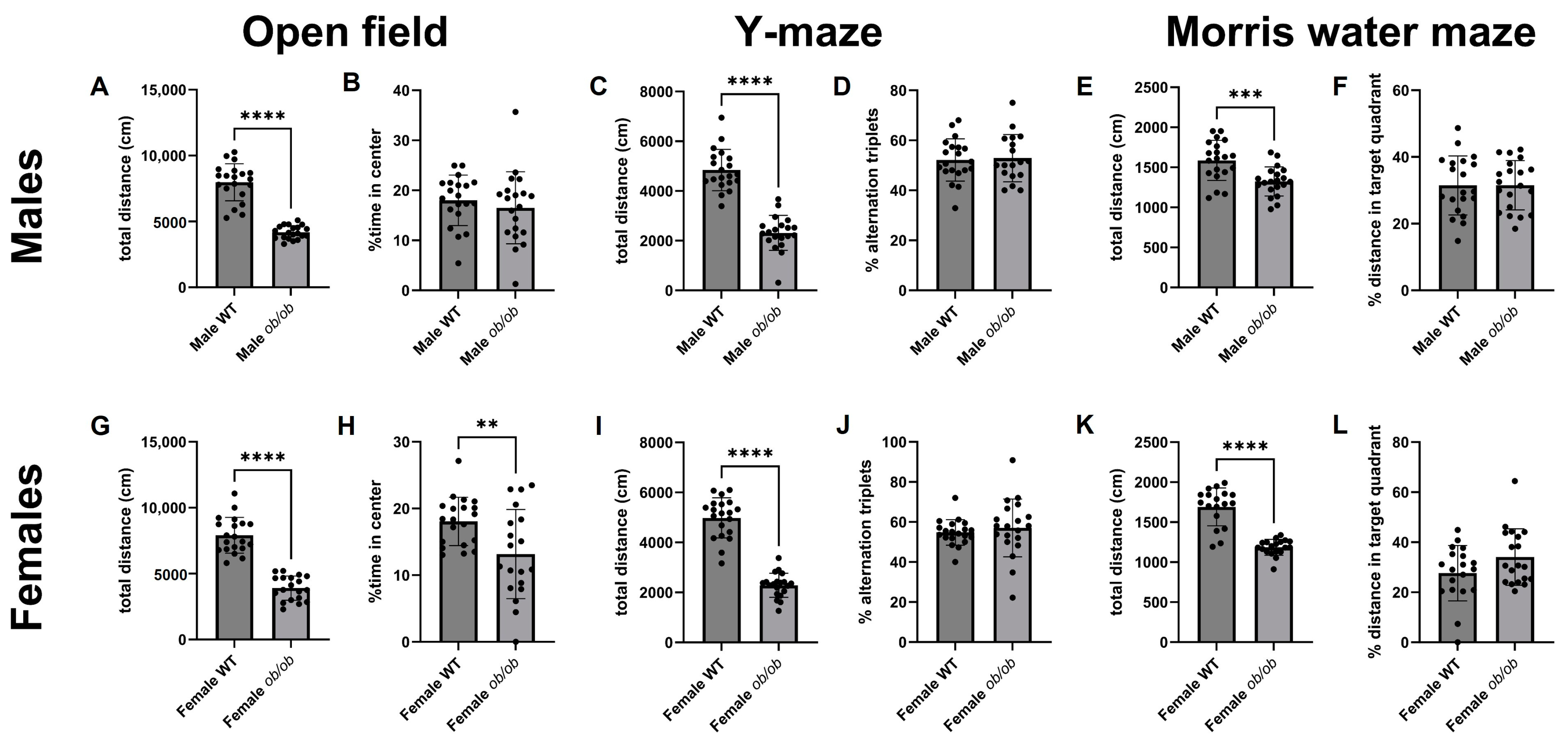
2.4. Obesity Alters Peripheral Metabolic Parameters, Locomotion, and Behavior
2.5. Obesity-Induced Brain Metabolic Changes Correlated with Peripheral Metabolic Characteristics, Behavior, and Cognitive Endpoints
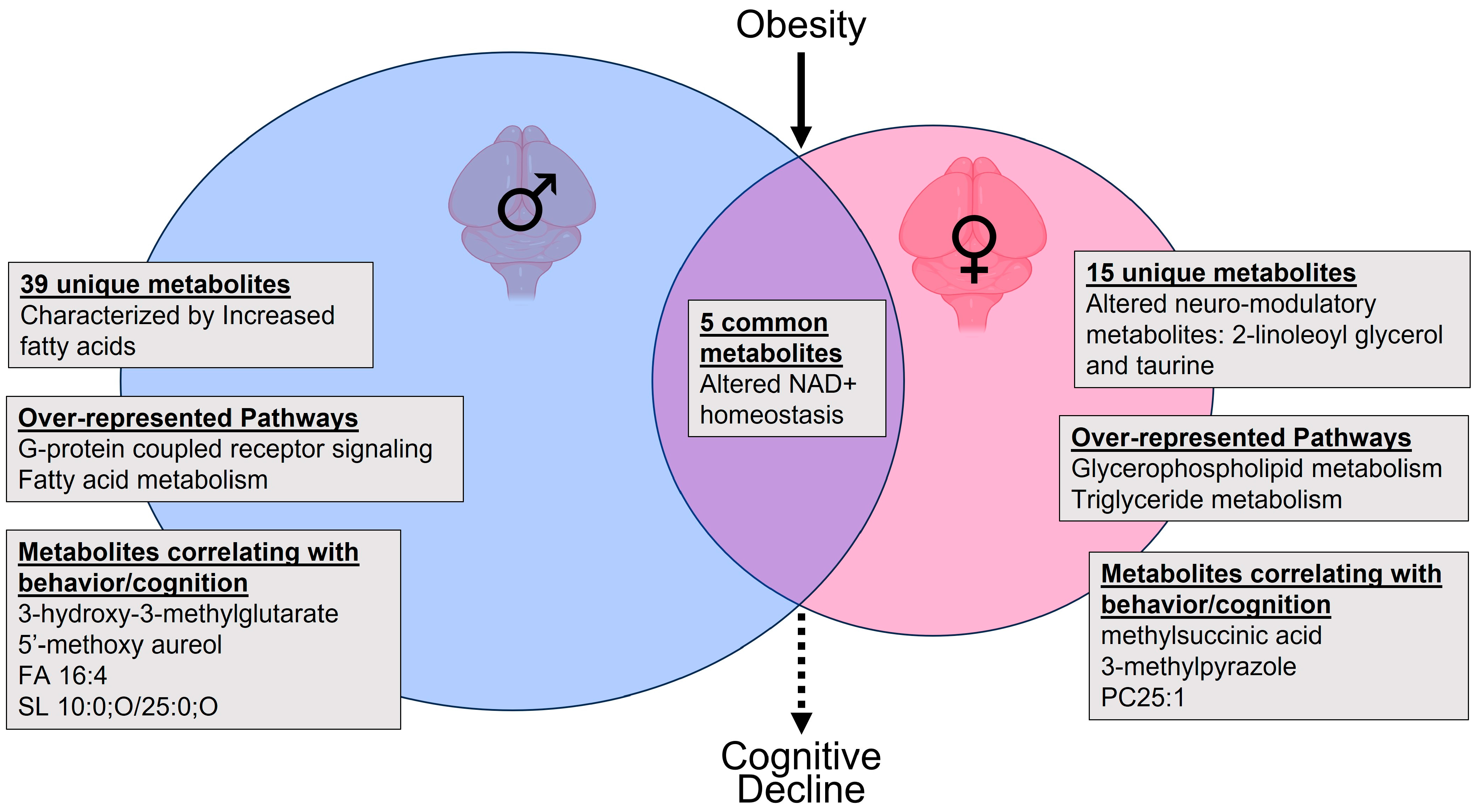
3. Discussion
3.1. The ob/ob Mouse as a Model of Human Obesity
3.2. Sex-Specific Effects of Obesity on the Brain Metabolic Profile
3.3. Sex-Independent Effects of Obesity on the Brain Metabolome
3.4. Potential Implications for Cognitive Decline
3.5. Comparison to Data from Other Obese Murine Models
3.6. Study Limitations
4. Materials and Methods
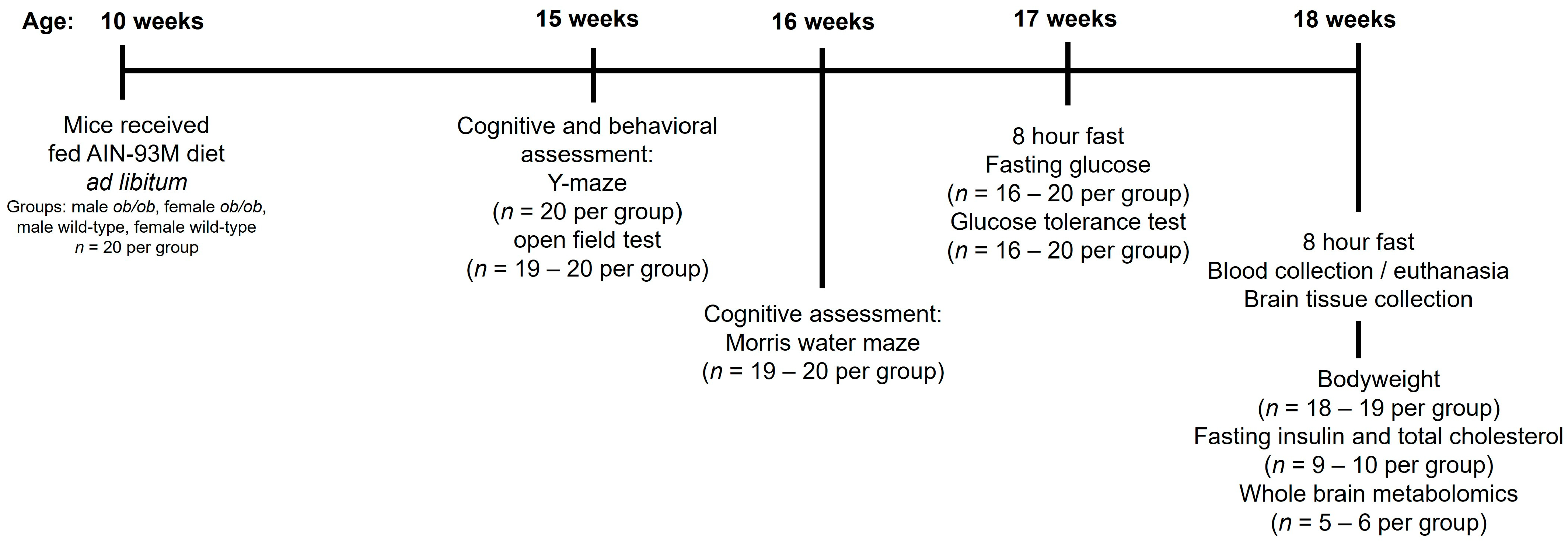
4.1. Experimental Animals
4.2. Peripheral Metabolic Testing for Glucose, Glucose Tolerance, Insulin, and Total Cholesterol
4.3. Behavioral and Cognitive Testing
4.3.1. Y-Maze
4.3.2. Open Field Test
4.3.3. Morris Water Maze (MWM) Test
4.4. Metabolomics
4.5. Metabolite Pathway Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, K.B.; Smith, M.S. Obesity Statistics. Prim. Care Clin. Off. Pract. 2016, 43, 121–135. [Google Scholar] [CrossRef]
- Dye, L.; Boyle, N.B.; Champ, C.; Lawton, C. The Relationship between Obesity and Cognitive Health and Decline. Proc. Nutr. Soc. 2017, 76, 443–454. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Whitmer, R.A.; Gustafson, D.R.; Barrett-Connor, E.; Haan, M.N.; Gunderson, E.P.; Yaffe, K. Central Obesity and Increased Risk of Dementia More than Three Decades Later. Neurology 2008, 71, 1057–1064. [Google Scholar] [CrossRef]
- Whitmer, R.A.; Gunderson, E.P.; Barrett-Connor, E.; Quesenberry, C.P.; Yaffe, K. Obesity in Middle Age and Future Risk of Dementia: A 27 Year Longitudinal Population Based Study. BMJ 2005, 330, 1360. [Google Scholar] [CrossRef]
- Uranga, R.M.; Keller, J.N. The Complex Interactions Between Obesity, Metabolism and the Brain. Front. Neurosci. 2019, 13, 513. [Google Scholar] [CrossRef]
- Anjum, I.; Fayyaz, M.; Wajid, A.; Sohail, W.; Ali, A. Does Obesity Increase the Risk of Dementia: A Literature Review. Cureus 2018, 10, e2660. [Google Scholar] [CrossRef] [PubMed]
- Singh-Manoux, A.; Dugravot, A.; Shipley, M.; Brunner, E.J.; Elbaz, A.; Sabia, S.; Kivimaki, M. Obesity Trajectories and Risk of Dementia: 28 Years of Follow-up in the Whitehall II Study. Alzheimers Dement. 2018, 14, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Val-Laillet, D.; Layec, S.; Guérin, S.; Meurice, P.; Malbert, C.-H. Changes in Brain Activity After a Diet-Induced Obesity. Obesity 2011, 19, 749–756. [Google Scholar] [CrossRef]
- Iozzo, P.; Guzzardi, M.A. Imaging of Brain Glucose Uptake by PET in Obesity and Cognitive Dysfunction: Life-Course Perspective. Endocr. Connect. 2019, 8, R169–R183. [Google Scholar] [CrossRef] [PubMed]
- Rebelos, E.; Hirvonen, J.; Bucci, M.; Pekkarinen, L.; Nyman, M.; Hannukainen, J.C.; Iozzo, P.; Salminen, P.; Nummenmaa, L.; Ferrannini, E.; et al. Brain Free Fatty Acid Uptake Is Elevated in Morbid Obesity, and Is Irreversible 6 Months after Bariatric Surgery: A Positron Emission Tomography Study. Diabetes Obes. Metab. 2020, 22, 1074–1082. [Google Scholar] [CrossRef]
- Morella, I.M.; Brambilla, R.; Morè, L. Emerging Roles of Brain Metabolism in Cognitive Impairment and Neuropsychiatric Disorders. Neurosci. Biobehav. Rev. 2022, 142, 104892. [Google Scholar] [CrossRef]
- Chang, E.; Varghese, M.; Singer, K. Gender and Sex Differences in Adipose Tissue. Curr. Diabetes Rep. 2018, 18, 69. [Google Scholar] [CrossRef]
- Wang, X.-H.; Lin, J.-N.; Liu, G.-Z.; Fan, H.-M.; Huang, Y.-P.; Li, C.-J.; Yan, H.-Y. Women Are at a Higher Risk of Chronic Metabolic Diseases Compared to Men With Increasing Body Mass Index in China. Front. Endocrinol. 2020, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wong, J.; Brooks, V.L. Obesity: Sex and Sympathetics. Biol. Sex Differ. 2020, 11, 10. [Google Scholar] [CrossRef]
- Choleris, E.; Galea, L.A.M.; Sohrabji, F.; Frick, K.M. Sex Differences in the Brain: Implications for Behavioral and Biomedical Research. Neurosci. Biobehav. Rev. 2018, 85, 126–145. [Google Scholar] [CrossRef]
- Hyde, J.S. Sex and Cognition: Gender and Cognitive Functions. Curr. Opin. Neurobiol. 2016, 38, 53–56. [Google Scholar] [CrossRef]
- Grissom, N.M.; Reyes, T.M. Let’s Call the Whole Thing off: Evaluating Gender and Sex Differences in Executive Function. Neuropsychopharmacology 2019, 44, 86–96. [Google Scholar] [CrossRef]
- Zarzar, T.G.; Lee, B.; Coughlin, R.; Kim, D.; Shen, L.; Hall, M.A. Sex Differences in the Metabolome of Alzheimer’s Disease Progression. Front. Radiol. 2022, 2, 782864. [Google Scholar] [CrossRef] [PubMed]
- Gaignard, P.; Fréchou, M.; Liere, P.; Thérond, P.; Schumacher, M.; Slama, A.; Guennoun, R. Sex Differences in Brain Mitochondrial Metabolism: Influence of Endogenous Steroids and Stroke. J. Neuroendocrinol. 2018, 30, e12497. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Profant, M.; Wang, C. Metabolic Sex Dimorphism of the Brain at the Gene, Cell, and Tissue Level. J. Immunol. 2022, 208, 212–220. [Google Scholar] [CrossRef]
- Sala, A.; Malpetti, M.; Ferrulli, A.; Gianolli, L.; Luzi, L.; Perani, D. Alzheimer’s Disease Neuroimaging Initiative (ADNI) database High Body Mass Index, Brain Metabolism and Connectivity: An Unfavorable Effect in Elderly Females. Aging 2019, 11, 8573–8586. [Google Scholar] [CrossRef] [PubMed]
- Ingalls, A.M.; Dickie, M.M.; Snell, G.D. Obese, a New Mutation in the House Mouse. J. Hered. 1950, 41, 317–318. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Ellacott, K.L.J.; King, V.L.; Hasty, A.H. Mouse Models of the Metabolic Syndrome. Dis. Model. Mech. 2010, 3, 156–166. [Google Scholar] [CrossRef]
- Lim, Y.; Boster, J. Obesity and Comorbid Conditions. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- do Carmo, J.M.; da Silva, A.A.; Gava, F.N.; Moak, S.P.; Dai, X.; Hall, J.E. Impact of Leptin Deficiency Compared with Neuronal-Specific Leptin Receptor Deletion on Cardiometabolic Regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R552–R562. [Google Scholar] [CrossRef]
- Faulkner, J.L.; Belin de Chantemèle, E.J. Sex Differences in Mechanisms of Hypertension Associated with Obesity. Hypertension 2018, 71, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Charukeshi Chandrasekera, P.; Pippin, J.J. Leptin- and Leptin Receptor-Deficient Rodent Models: Relevance for Human Type 2 Diabetes. Curr. Diabetes Rev. 2014, 10, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.; Haque, M.E.; Jakaria, M.; Jo, S.-H.; Kim, I.-S.; Choi, D.-K. G-Protein-Coupled Receptors in CNS: A Potential Therapeutic Target for Intervention in Neurodegenerative Disorders and Associated Cognitive Deficits. Cells 2020, 9, 506. [Google Scholar] [CrossRef]
- Snowden, S.G.; Ebshiana, A.A.; Hye, A.; An, Y.; Pletnikova, O.; O’Brien, R.; Troncoso, J.; Legido-Quigley, C.; Thambisetty, M. Association between Fatty Acid Metabolism in the Brain and Alzheimer Disease Neuropathology and Cognitive Performance: A Nontargeted Metabolomic Study. PLoS Med. 2017, 14, e1002266. [Google Scholar] [CrossRef]
- Lu, L.; Williams, G.; Doherty, P. 2-Linoleoylglycerol Is a Partial Agonist of the Human Cannabinoid Type 1 Receptor That Can Suppress 2-Arachidonolyglycerol and Anandamide Activity. Cannabis Cannabinoid Res. 2019, 4, 231–239. [Google Scholar] [CrossRef]
- Walther, S.; Halpern, M. Cannabinoids and Dementia: A Review of Clinical and Preclinical Data. Pharmaceuticals 2010, 3, 2689–2708. [Google Scholar] [CrossRef]
- Ochoa-de la Paz, L.; Zenteno, E.; Gulias-Cañizo, R.; Quiroz-Mercado, H. Taurine and GABA Neurotransmitter Receptors, a Relationship with Therapeutic Potential? Expert Rev. Neurother. 2019, 19, 289–291. [Google Scholar] [CrossRef]
- Jewett, B.E.; Sharma, S. Physiology, GABA. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Jiménez-Balado, J.; Eich, T.S. GABAergic Dysfunction, Neural Network Hyperactivity and Memory Impairments in Human Aging and Alzheimer’s Disease. Semin. Cell Dev. Biol. 2021, 116, 146–159. [Google Scholar] [CrossRef]
- Monaghan, D.T.; Jane, D.E. Pharmacology of NMDA Receptors. In Biology of the NMDA Receptor; Van Dongen, A.M., Ed.; Frontiers in Neuroscience; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009; ISBN 978-1-4200-4414-0. [Google Scholar]
- Waxham, M.N. Chapter 8—Neurotransmitter Receptors. In Fundamental Neuroscience, 4th ed.; Squire, L.R., Berg, D., Bloom, F.E., du Lac, S., Ghosh, A., Spitzer, N.C., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 163–187. ISBN 978-0-12-385870-2. [Google Scholar]
- Chen, C.; Xia, S.; He, J.; Lu, G.; Xie, Z.; Han, H. Roles of Taurine in Cognitive Function of Physiology, Pathologies and Toxication. Life Sci. 2019, 231, 116584. [Google Scholar] [CrossRef]
- Chan, C.Y.; Sun, H.S.; Shah, S.M.; Agovic, M.S.; Friedman, E.; Banerjee, S.P. Modes of Direct Modulation by Taurine of the Glutamate NMDA Receptor in Rat Cortex. Eur. J. Pharmacol. 2014, 728, 167–175. [Google Scholar] [CrossRef]
- Norman, J.E.; Nuthikattu, S.; Milenkovic, D.; Villablanca, A.C. Sex Modifies the Impact of Type 2 Diabetes Mellitus on the Murine Whole Brain Metabolome. Metabolites 2023, 13, 1012. [Google Scholar] [CrossRef]
- Faulkner, J.L.; Belin de Chantemèle, E.J. Sex Hormones, Aging and Cardiometabolic Syndrome. Biol. Sex Differ. 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Gevers Leuven, J.A. Sex Steroids and Lipoprotein Metabolism. Pharmacol. Ther. 1994, 64, 99–126. [Google Scholar] [CrossRef]
- Zore, T.; Palafox, M.; Reue, K. Sex Differences in Obesity, Lipid Metabolism, and Inflammation—A Role for the Sex Chromosomes? Mol. Metab. 2018, 15, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P.; Chen, X. What Does the “Four Core Genotypes” Mouse Model Tell Us about Sex Differences in the Brain and Other Tissues? Front. Neuroendocrinol. 2009, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Seidlitz, J.; Blumenthal, J.D.; Clasen, L.S.; Raznahan, A. Integrative Structural, Functional, and Transcriptomic Analyses of Sex-Biased Brain Organization in Humans. Proc. Natl. Acad. Sci. USA 2020, 117, 18788–18798. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fu, S.; Wang, G.; Cao, Y.; Li, L.; Li, X.; Yang, J.; Li, N.; Shan, Y.; Cao, Y.; et al. Glycerol-3-Phosphate Biosynthesis Regenerates Cytosolic NAD+ to Alleviate Mitochondrial Disease. Cell Metab. 2021, 33, 1974–1987.e9. [Google Scholar] [CrossRef] [PubMed]
- Dhoundiyal, A.; Goeschl, V.; Boehm, S.; Kubista, H.; Hotka, M. Glycerol-3-Phosphate Shuttle Is a Backup System Securing Metabolic Flexibility in Neurons. J. Neurosci. 2022, 42, 7339–7354. [Google Scholar] [CrossRef] [PubMed]
- Martano, G.; Murru, L.; Moretto, E.; Gerosa, L.; Garrone, G.; Krogh, V.; Passafaro, M. Biosynthesis of Glycerol Phosphate Is Associated with Long-Term Potentiation in Hippocampal Neurons. Metabolomics 2016, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Lenglet, A.; Liabeuf, S.; Bodeau, S.; Louvet, L.; Mary, A.; Boullier, A.; Lemaire-Hurtel, A.S.; Jonet, A.; Sonnet, P.; Kamel, S.; et al. N-Methyl-2-Pyridone-5-Carboxamide (2PY)-Major Metabolite of Nicotinamide: An Update on an Old Uremic Toxin. Toxins 2016, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ Metabolism: Pathophysiologic Mechanisms and Therapeutic Potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ Metabolism and Its Roles in Cellular Processes during Ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef]
- Lautrup, S.; Sinclair, D.A.; Mattson, M.P.; Fang, E.F. NAD+ in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019, 30, 630–655. [Google Scholar] [CrossRef]
- Giuliante, R.; Sartini, D.; Bacchetti, T.; Rocchetti, R.; Klöting, I.; Polidori, C.; Ferretti, G.; Emanuelli, M. Potential Involvement of Nicotinamide N-Methyltransferase in the Pathogenesis of Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2015, 13, 165–170. [Google Scholar] [CrossRef]
- Sampson, C.M.; Dimet, A.L.; Neelakantan, H.; Ogunseye, K.O.; Stevenson, H.L.; Hommel, J.D.; Watowich, S.J. Combined Nicotinamide N-Methyltransferase Inhibition and Reduced-Calorie Diet Normalizes Body Composition and Enhances Metabolic Benefits in Obese Mice. Sci. Rep. 2021, 11, 5637. [Google Scholar] [CrossRef]
- Kocinaj, A.; Chaudhury, T.; Uddin, M.S.; Junaid, R.R.; Ramsden, D.B.; Hondhamuni, G.; Klamt, F.; Parsons, L.; Parsons, R.B. High Expression of Nicotinamide N-Methyltransferase in Patients with Sporadic Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 1769–1781. [Google Scholar] [CrossRef]
- Bracke, A.; Domanska, G.; Bracke, K.; Harzsch, S.; van den Brandt, J.; Bröker, B.; von Bohlen und Halbach, O. Obesity Impairs Mobility and Adult Hippocampal Neurogenesis. J. Exp. Neurosci. 2019, 13, 1179069519883580. [Google Scholar] [CrossRef]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Methods Mol. Biol. 2019, 1916, 99–103. [Google Scholar] [CrossRef]
- Rajan, T.M.; Menon, V. Psychiatric Disorders and Obesity: A Review of Association Studies. J. Postgrad. Med. 2017, 63, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Tonini, C.; Segatto, M.; Pallottini, V. Impact of Sex and Age on the Mevalonate Pathway in the Brain: A Focus on Effects Induced by Maternal Exposure to Exogenous Compounds. Metabolites 2020, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.R.; Oommen, A.M.; Varma, S.; Casanova, R.; An, Y.; Andrews, R.M.; O’Brien, R.; Pletnikova, O.; Troncoso, J.C.; Toledo, J.; et al. Brain and Blood Metabolite Signatures of Pathology and Progression in Alzheimer Disease: A Targeted Metabolomics Study. PLoS Med. 2018, 15, e1002482. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Tomassoni, D.; Nittari, G.; Traini, E.; Amenta, F. Effects of Choline Containing Phospholipids on the Neurovascular Unit: A Review. Front. Cell Neurosci. 2022, 16, 988759. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Palavicini, J.P.; Wang, M.; Chen, L.; Yang, K.; Crawford, P.A.; Han, X. Comprehensive and Quantitative Analysis of Polyphosphoinositide Species by Shotgun Lipidomics Revealed Their Alterations in Db/Db Mouse Brain. Anal. Chem. 2016, 88, 12137–12144. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, Y.; Dong, B.; Zheng, H.; Lin, X.; Du, Y.; Li, X.; Zhao, L.; Gao, H. Metabonomic Profiles Delineate Potential Role of Glutamate-Glutamine Cycle in Db/Db Mice with Diabetes-Associated Cognitive Decline. Mol. Brain 2016, 9, 40. [Google Scholar] [CrossRef]
- Zheng, H.; Zheng, Y.; Zhao, L.; Chen, M.; Bai, G.; Hu, Y.; Hu, W.; Yan, Z.; Gao, H. Cognitive Decline in Type 2 Diabetic Db/Db Mice May Be Associated with Brain Region-Specific Metabolic Disorders. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 266–273. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Jeon, B.T.; Heo, R.W.; Jeong, E.A.; Yi, C.-O.; Lee, J.Y.; Kim, K.E.; Kim, H.; Roh, G.S. Effects of Caloric Restriction on O-GlcNAcylation, Ca(2+) Signaling, and Learning Impairment in the Hippocampus of Ob/Ob Mice. Neurobiol. Aging 2016, 44, 127–137. [Google Scholar] [CrossRef]
- Qin, X.; Wang, W.; Wu, H.; Liu, D.; Wang, R.; Xu, J.; Jiang, H.; Pan, F. PPARγ-Mediated Microglial Activation Phenotype Is Involved in Depressive-like Behaviors and Neuroinflammation in Stressed C57BL/6J and Ob/Ob Mice. Psychoneuroendocrinology 2020, 117, 104674. [Google Scholar] [CrossRef]
- Shima, T.; Kawabata-Iwakawa, R.; Onishi, H.; Jesmin, S.; Yoshikawa, T. Light-Intensity Exercise Improves Memory Dysfunction with the Restoration of Hippocampal MCT2 and miRNAs in Type 2 Diabetic Mice. Metab. Brain Dis. 2023, 38, 245–254. [Google Scholar] [CrossRef]
- Zhang, S.; Chai, R.; Yang, Y.-Y.; Guo, S.-Q.; Wang, S.; Guo, T.; Xu, S.-F.; Zhang, Y.-H.; Wang, Z.-Y.; Guo, C. Chronic Diabetic States Worsen Alzheimer Neuropathology and Cognitive Deficits Accompanying Disruption of Calcium Signaling in Leptin-Deficient APP/PS1 Mice. Oncotarget 2017, 8, 43617–43634. [Google Scholar] [CrossRef]
- Hölter, S.M.; Garrett, L.; Einicke, J.; Sperling, B.; Dirscherl, P.; Zimprich, A.; Fuchs, H.; Gailus-Durner, V.; Hrabě de Angelis, M.; Wurst, W. Assessing Cognition in Mice. Curr. Protoc. Mouse Biol. 2015, 5, 331–358. [Google Scholar] [CrossRef]
- Rutkowsky, J.M.; Lee, L.L.; Puchowicz, M.; Golub, M.S.; Befroy, D.E.; Wilson, D.W.; Anderson, S.; Cline, G.; Bini, J.; Borkowski, K.; et al. Reduced Cognitive Function, Increased Blood-Brain-Barrier Transport and Inflammatory Responses, and Altered Brain Metabolites in LDLr -/-and C57BL/6 Mice Fed a Western Diet. PLoS ONE 2018, 13, e0191909. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.Z.; Hassan, Z.; Che Has, A.T. Morris Water Maze: A Versatile and Pertinent Tool for Assessing Spatial Learning and Memory. Exp. Anim. 2022, 71, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a Water-Maze Procedure for Studying Spatial Learning in the Rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Nezami Ranjbar, M.R.; Zhao, Y.; Tadesse, M.G.; Wang, Y.; Ressom, H.W. Evaluation of Normalization Methods for Analysis of LC-MS Data. In Proceedings of the 2012 IEEE International Conference on Bioinformatics and Biomedicine Workshops (BIBMW), Philadelphia, PA, USA, 4–7 October 2012; pp. 610–617. [Google Scholar]
- Pathway Analysis with Transcriptomics and Metabolomics Data. Available online: http://impala.molgen.mpg.de/impala/impala/impala/impala/impala/# (accessed on 24 April 2023).
- Kamburov, A.; Cavill, R.; Ebbels, T.M.D.; Herwig, R.; Keun, H.C. Integrated Pathway-Level Analysis of Transcriptomics and Metabolomics Data with IMPaLA. Bioinformatics 2011, 27, 2917–2918. [Google Scholar] [CrossRef] [PubMed]
- Cavill, R.; Kamburov, A.; Ellis, J.K.; Athersuch, T.J.; Blagrove, M.S.C.; Herwig, R.; Ebbels, T.M.D.; Keun, H.C. Consensus-Phenotype Integration of Transcriptomic and Metabolomic Data Implies a Role for Metabolism in the Chemosensitivity of Tumour Cells. PLoS Comput. Biol. 2011, 7, e1001113. [Google Scholar] [CrossRef] [PubMed]
- MetaboAnalyst. Available online: https://www.metaboanalyst.ca/MetaboAnalyst/ModuleView.xhtml (accessed on 25 August 2021).
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Hulsen, T.; de Vlieg, J.; Alkema, W. BioVenn—A Web Application for the Comparison and Visualization of Biological Lists Using Area-Proportional Venn Diagrams. BMC Genom. 2008, 9, 488. [Google Scholar] [CrossRef] [PubMed]
- Morpheus. Available online: https://software.broadinstitute.org/morpheus/ (accessed on 10 April 2023).
- SRplot-Science and Research Online Plot. Available online: https://www.bioinformatics.com.cn/SRplot (accessed on 4 December 2023).
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A Free Online Platform for Data Visualization and Graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef] [PubMed]

| Males | Females | |||
|---|---|---|---|---|
| WT | ob/ob | WT | ob/ob | |
| Body weight (g) | 29.5 ± 2.7 | 51.5 ± 3.3 * | 20.6 ± 1.4 | 52.4 ± 4.2 * |
| Glucose (mg/dL) | 157.4 ± 32.6 | 175.6 ± 41.6 | 126.3 ± 18.5 | 177.7 ± 54.3 * |
| GTT AUC (mg * min/dL) | 29,500 ± 5380 | 43,307 ± 8706 * | 23,323 ± 2106 | 47,047 ± 8270 * |
| Insulin (pg/mL) | 229.1 ± 131.7 | 3311.4 ± 1625.2 * | 182.2 ± 78.7 | 3769.2 ± 729.6 * |
| Total cholesterol (mg/dL) | 124.1 ± 35.9 | 279.1 ± 25.9 * | 77.0 ± 17.8 | 290.5 ± 15.9 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norman, J.E.; Milenkovic, D.; Nuthikattu, S.; Villablanca, A.C. The Brain Metabolome Is Modified by Obesity in a Sex-Dependent Manner. Int. J. Mol. Sci. 2024, 25, 3475. https://doi.org/10.3390/ijms25063475
Norman JE, Milenkovic D, Nuthikattu S, Villablanca AC. The Brain Metabolome Is Modified by Obesity in a Sex-Dependent Manner. International Journal of Molecular Sciences. 2024; 25(6):3475. https://doi.org/10.3390/ijms25063475
Chicago/Turabian StyleNorman, Jennifer E., Dragan Milenkovic, Saivageethi Nuthikattu, and Amparo C. Villablanca. 2024. "The Brain Metabolome Is Modified by Obesity in a Sex-Dependent Manner" International Journal of Molecular Sciences 25, no. 6: 3475. https://doi.org/10.3390/ijms25063475
APA StyleNorman, J. E., Milenkovic, D., Nuthikattu, S., & Villablanca, A. C. (2024). The Brain Metabolome Is Modified by Obesity in a Sex-Dependent Manner. International Journal of Molecular Sciences, 25(6), 3475. https://doi.org/10.3390/ijms25063475