Effect of Centhaquine on the Coagulation Cascade in Normal State and Uncontrolled Hemorrhage: A Multiphase Study Combining Ex Vivo and In Vivo Experiments in Different Species
Abstract
1. Introduction
2. Results
2.1. Effect of Centhaquine on Coagulation by Ex Vivo Thromboelastography (Rat Model)
2.2. Development of a Translational Rabbit Model of Uncontrolled Hemorrhagic Shock
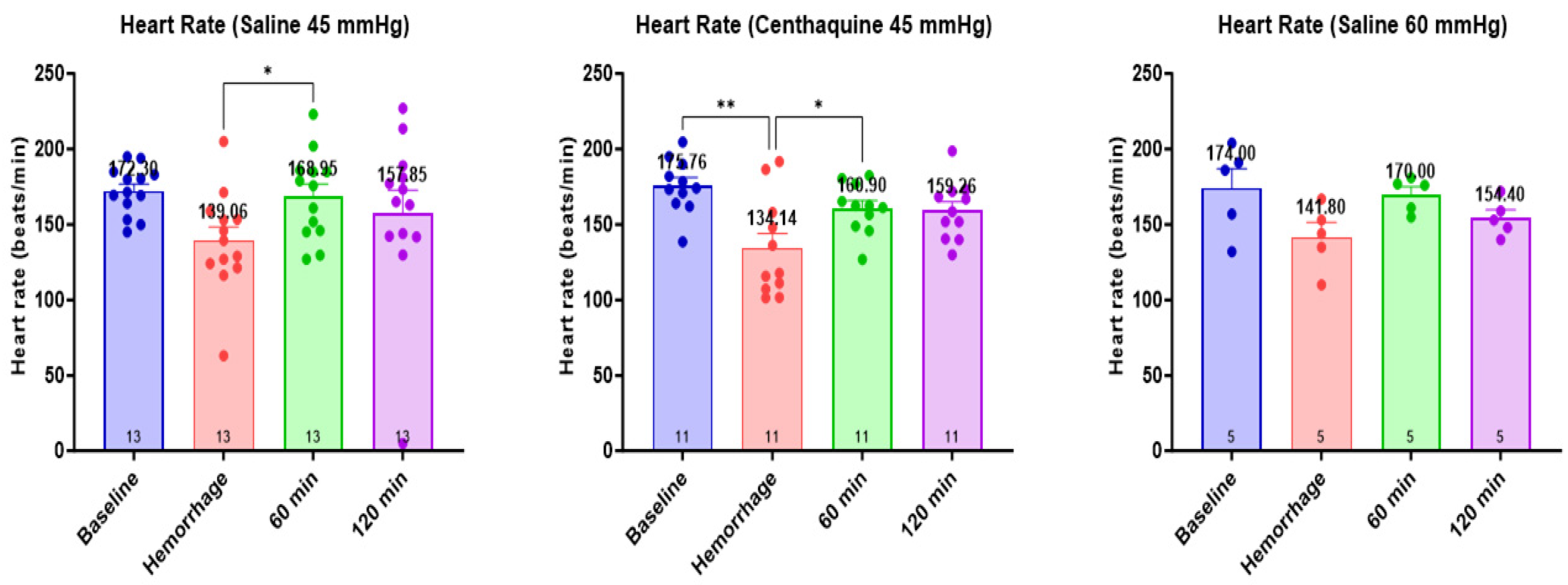
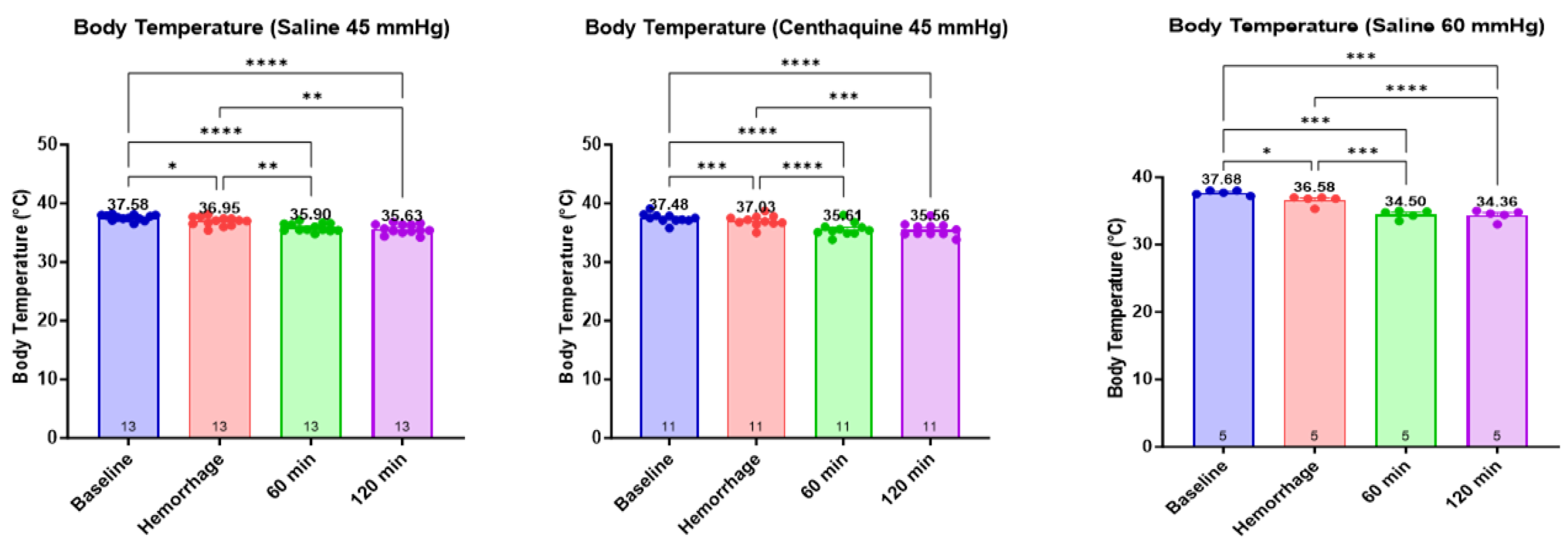
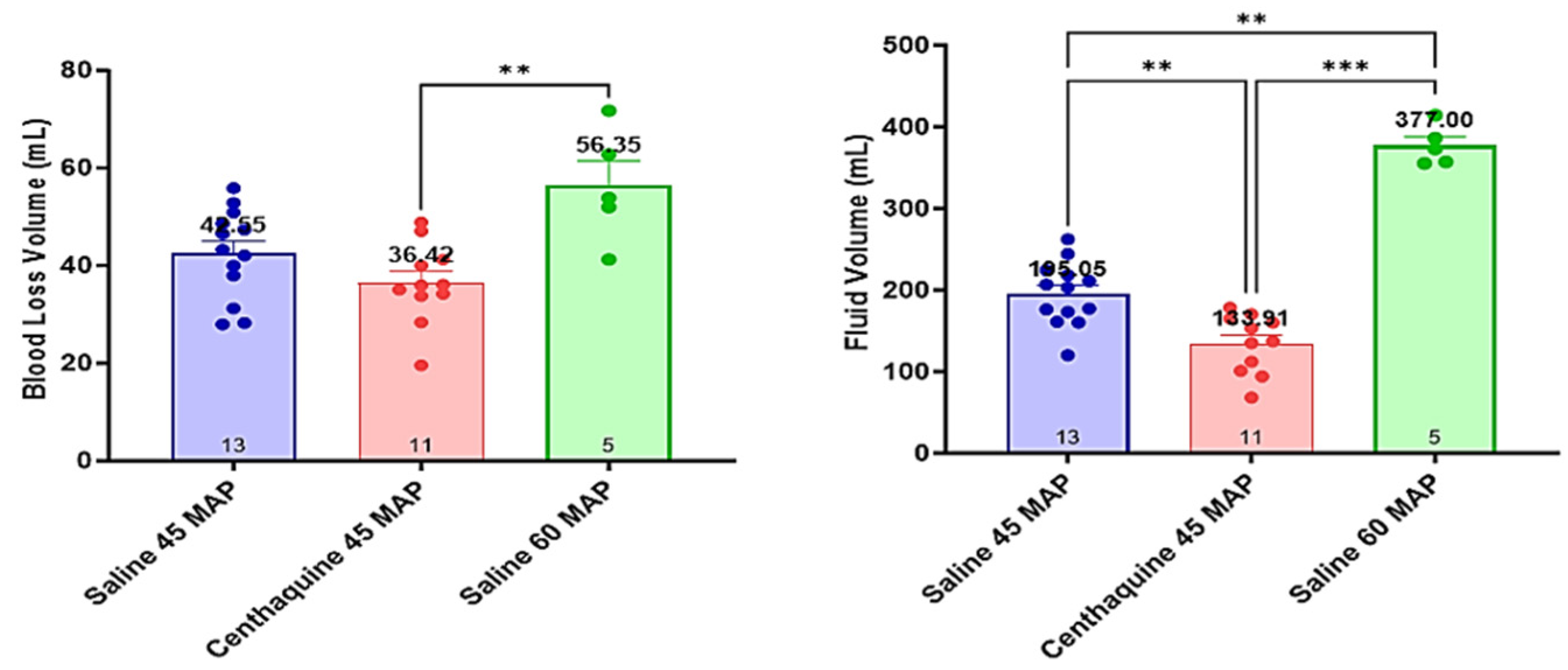
2.3. Effect of Hypotensive Resuscitation with Centhaquine Versus a Higher Blood Pressure Target on Blood Coagulation
3. Discussion
4. Materials and Methods
4.1. The Rationale for Choosing Translational Models and Ethics Approval
4.2. Effects of Centhaquine on Thromboelastometry Analysis of Rat Whole Blood
4.2.1. Animals
4.2.2. Drugs and Experimental Protocol
4.2.3. Blood Collection
4.2.4. Thromboelastography
4.3. Development of a Translational Rabbit Model of Uncontrolled Hemorrhagic Shock
4.3.1. Animal Preparation
4.3.2. Experimental Protocol
- Group 1: injury leading to uncontrolled hemorrhage + no resuscitation; n = 10 (no resuscitation group)
- Group 2: injury leading to uncontrolled hemorrhage + normal saline infused to maintain a MAP of 45 mmHg for 60 min; n = 10 (saline 45 mmHg group)
- Group 3: injury leading to uncontrolled hemorrhage + centhaquine (0.05 mg kg−1) along with normal saline to maintain a MAP of 45 mmHg for 60 min; n = 10 (centhaquine 45 mmHg group)
4.4. Assessment of the Effect of Hypotensive Resuscitation with Centhaquine Versus a Higher Blood Pressure Target on Blood Coagulation
- Group 1: injury leading to uncontrolled hemorrhage + normal saline infused to maintain a MAP of 45 mmHg; n = 13 (Sal-MAP 45 group)
- Group 2: injury leading to uncontrolled hemorrhage + centhaquine (0.05 mg kg−1) along with normal saline to maintain a MAP of 45 mmHg; n = 11 (Centh-MAP 45 group)
- Group 3: injury leading to uncontrolled hemorrhage + normal saline infused to maintain a MAP of 60 mmHg; n = 5 (Sal-MAP 60 group)
4.5. Statistical Analysis
4.5.1. Rat Study
4.5.2. Rabbit Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toung, T.; Reilly, P.M.; Fuh, K.C.; Ferris, R.; Bulkley, G.B. Mesenteric vasoconstriction in response to hemorrhagic shock. Shock 2000, 13, 267–273. [Google Scholar] [CrossRef]
- Gupta, B.; Garg, N.; Ramachandran, R. Vasopressors: Do they have any role in hemorrhagic shock? J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 3–8. [Google Scholar] [CrossRef]
- Fecher, A.; Stimpson, A.; Ferrigno, L.; Pohlman, T.H. The Pathophysiology and Management of Hemorrhagic Shock in the Polytrauma Patient. J. Clin. Med. 2021, 10, 4793. [Google Scholar] [CrossRef]
- Galbraith, C.M.; Wagener, B.M.; Chalkias, A.; Siddiqui, S.; Douin, D.J. Massive Trauma and Resuscitation Strategies. Anesth. Clin. 2023, 41, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.E.; Moore, H.B.; Kornblith, L.Z.; Neal, M.D.; Hoffman, M.; Mutch, N.J.; Schöchl, H.; Hunt, B.J.; Sauaia, A. Trauma-induced coagulopathy. Nat. Rev. Dis. Primers 2021, 7, 30. [Google Scholar] [CrossRef]
- Gonzalez, E.; Moore, E.E.; Moore, H.B.; Chapman, M.P.; Chin, T.L.; Ghasabyan, A.; Wohlauer, M.V.; Barnett, C.C.; Bensard, D.D.; Biffl, W.L.; et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Ann. Surg. 2016, 263, 1051–1059. [Google Scholar] [CrossRef]
- Rossaint, R.; Afshari, A.; Bouillon, B.; Cerny, V.; Cimpoesu, D.; Curry, N.; Duranteau, J.; Filipescu, D.; Grottke, O.; Grønlykke, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Sixth edition. Crit. Care 2023, 27, 80. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A. The interplay between ‘rest volume’, mean circulatory filling pressure, and cardiac output that drives venous return. Eur. J. Anaesthesiol. 2023, 40, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Gelman, S. Venous circulation: A few challenging concepts in goal-directed hemodynamic therapy (GDHT). In Perioperative Fluid Management; Farag, E., Kurz, A., Troianos, C., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 365–385. [Google Scholar]
- Hartmann, C.; Radermacher, P.; Wepler, M.; Nußbaum, B. Non-Hemodynamic Effects of Catecholamines. Shock 2017, 48, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Von Känel, R.; Dimsdale, J.E. Effects of sympathetic activation by adrenergic infusions on hemostasis in vivo. Eur. J. Haematol. 2000, 65, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Vosburgh, C.H.; Richards, A.N. An experimental study of the sugar content and extra vascular coagulation of the blood after administration of adrenalin. Am. J. Physiol. 1903, 9, 35–51. [Google Scholar] [CrossRef][Green Version]
- Cannon, W.B.; Gray, H. Factors affecting the coagulation time of blood. II. The hastening or retarding of coagulation by adrenalin injections. Am. J. Physiol. 1914, 34, 232–242. [Google Scholar] [CrossRef]
- Rutlen, D.; Supple, E.W.; Powell, P.W., Jr. Adrenergic regulation of total systemic distensibility. Venous distensibility effects of norepinephrine and isoproterenol before and after selective adrenergic blockade. Am. J. Cardiol. 1981, 47, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.K.; Gulati, A. Controls of Central and Peripheral Blood Pressure and Hemorrhagic/Hypovolemic Shock. J. Clin. Med. 2023, 12, 1108. [Google Scholar] [CrossRef]
- Gulati, A.; Choudhuri, R.; Gupta, A.; Singh, S.; Ali, S.K.N.; Sidhu, G.K.; Haque, P.D.; Rahate, P.; Bothra, A.R.; Singh, G.P.; et al. A Multicentric, Randomized, Controlled Phase III Study of Centhaquine (Lyfaquin®) as a Resuscitative Agent in Hypovolemic Shock Patients. Drugs 2021, 81, 1079–1100. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Jain, D.; Agrawal, N.R.; Rahate, P.; Choudhuri, R.; Das, S.; Dhibar, D.P.; Prabhu, M.; Haveri, S.; Agarwal, R.; et al. Resuscitative Effect of Centhaquine (Lyfaquin®) in Hypovolemic Shock Patients: A Randomized, Multicentric, Controlled Trial. Adv. Ther. 2021, 38, 3223–3265. [Google Scholar] [CrossRef]
- Sumislawski, J.J.; Christie, S.A.; Kornblith, L.Z.; Stettler, G.R.; Nunns, G.R.; Moore, H.B.; Moore, E.E.; Silliman, C.C.; Sauaia, A.; Callcut, R.A.; et al. Discrepancies between conventional and viscoelastic assays in identifying trauma-induced coagulopathy. Am. J. Surg. 2019, 217, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- McCully, S.P.; Fabricant, L.J.; Kunio, N.R.; Groat, T.L.; Watson, K.M.; Differding, J.A.; Deloughery, T.G.; Schreiber, M.A. The International Normalized Ratio overestimates coagulopathy in stable trauma and surgical patients. J. Trauma Acute Care Surg. 2013, 75, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Stettler, G.R.; Moore, E.E.; Moore, H.B.; Nunns, G.R.; Coleman, J.R.; Colvis, A.; Ghasabyan, A.; Cohen, M.J.; Silliman, C.C.; Banerjee, A.; et al. Variability in international normalized ratio and activated partial thromboplastin time after injury are not explained by coagulation factor deficits. J. Trauma Acute Care Surg. 2019, 87, 582–589. [Google Scholar] [CrossRef]
- Wool, G.D.; Carll, T. Viscoelastic testing: Critical appraisal of new methodologies and current literature. Int. J. Lab. Hematol. 2023, 45, 643–658. [Google Scholar] [CrossRef]
- Lloyd-Donald, P.; Vasudevan, A.; Angus, P.; Gow, P.; Mårtensson, J.; Glassford, N.; Eastwood, G.M.; Hart, G.K.; Jones, D.; Weinberg, L.; et al. Comparison of Thromboelastography and Conventional Coagulation Tests in Patients With Severe Liver Disease. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620925915. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, M.; Di Dedda, U.; Baryshnikova, E. Trials and Tribulations of Viscoelastic-Based Determination of Fibrinogen Concentration. Anesth. Analg. 2020, 130, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Erdoes, G.; Faraoni, D.; Koster, A.; Steiner, M.E.; Ghadimi, K.; Levy, J.H. Perioperative Considerations in Management of the Severely Bleeding Coagulopathic Patient. Anesthesiology 2023, 138, 535–560. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.E.; Barnes, G.D. A call to action for anticoagulation stewardship. Res. Pr. Thromb. Haemost. 2022, 6, e12757. [Google Scholar] [CrossRef] [PubMed]
- Grymonprez, M.; Simoens, C.; Steurbaut, S.; De Backer, T.L.; Lahousse, L. Worldwide trends in oral anticoagulant use in patients with atrial fibrillation from 2010 to 2018: A systematic review and meta-analysis. Europace 2022, 24, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Galvagno, S.M., Jr.; Nahmias, J.T.; Young, D.A. Advanced Trauma Life Support((R)) update 2019: Management and applications for adults and special populations. Anesth. Clin. 2019, 37, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.Y.; Ko, A.; Barmparas, G.; Smith, E.J.; Patel, B.K.; Dhillon, N.K.; Thomsen, G.M.; Ley, E.J. 10-Year trend in crystalloid resuscitation: Reduced volume and lower mortality. Int. J. Surg. 2017, 38, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Crochemore, T.; Görlinger, K.; Lance, M.D. Early Goal-Directed Hemostatic Therapy for Severe Acute Bleeding Management in the Intensive Care Unit: A Narrative Review. Anesth. Analg. 2024, 138, 499–513. [Google Scholar] [CrossRef]
- Forwell, G.D.; Ingram, G.I. The effect of adrenaline infusion on human blood coagulation. J. Physiol. 1957, 135, 371–383. [Google Scholar] [CrossRef]
- Shimamoto, T.; Sunaga, T. Edematous arterial reaction by adrenaline and cholesterol and its prevention by MAO Inhibitor observed by electron microscopic technique. Jpn Heart J. 1962, 3, 581–601. [Google Scholar] [CrossRef][Green Version]
- Cash, J.D.; Lind, A.R.; McNicol, G.W.; Woodfield, D.G. Fibrinolytic and forearm blood flow responses to intravenous adrenaline in healthy subjects. Life Sci. 1969, 8, 207–213. [Google Scholar] [CrossRef]
- Aster, R.H. Pooling of platelets in the spleen: Role in the pathogenesis of “hypersplenic” thrombocytopenia. J. Clin. Investig. 1966, 45, 645–657. [Google Scholar] [CrossRef]
- Libre, E.P.; Cowan, D.H.; Watkins, S.P., Jr.; Shulman, N.R. Relationships between spleen, platelets and factor 8 levels. Blood 1968, 31, 358–368. [Google Scholar] [CrossRef]
- Kral, J.G.; Åblad, B.; Johnsson, G.; Korsan-Bengtsen, K. Effects of adrenaline and alprenolol (Aptin®) on blood coagulation and fibrinolysis in man. Eur. J. Clin. Pharmacol. 1971, 3, 144–147. [Google Scholar] [CrossRef]
- Richards, J.E.; Harris, T.; Dünser, M.W.; Bouzat, P.; Gauss, T. Vasopressors in Trauma: A Never Event? Anesth. Analg. 2021, 133, 68–79. [Google Scholar] [CrossRef]
- Larsson, P.T.; Wallén, N.H.; Hjemdahl, P. Norepinephrine-induced human platelet activation in vivo is only partly counteracted by aspirin. Circulation 1994, 89, 1951–1957. [Google Scholar] [CrossRef]
- von Känel, R.; Heimgartner, N.; Stutz, M.; Zuccarella-Hackl, C.; Hänsel, A.; Ehlert, U.; Wirtz, P.H. Prothrombotic response to norepinephrine infusion, mimicking norepinephrine stress-reactivity effects, is partly mediated by α-adrenergic mechanisms. Psychoneuroendocrinology 2019, 105, 44–50. [Google Scholar] [CrossRef]
- Bracht, H.; Calzia, E.; Georgieff, M.; Singer, J.; Radermacher, P.; Russell, J.A. Inotropes and vasopressors: More than haemodynamics! Br. J. Pharmacol. 2012, 165, 2009–2011. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, S.R.; Berg, R.M.; Windeløv, N.A.; Meyer, M.A.; Plovsing, R.R.; Møller, K.; Johansson, P.I. Coagulopathy, catecholamines, and biomarkers of endothelial damage in experimental human endotoxemia and in patients with severe sepsis: A prospective study. J. Crit. Care 2013, 28, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A. Shear Stress and Endothelial Mechanotransduction in Trauma Patients with Hemorrhagic Shock: Hidden Coagulopathy Pathways and Novel Therapeutic Strategies. Int. J. Mol. Sci. 2023, 24, 17522. [Google Scholar] [CrossRef] [PubMed]
- Siller-Matula, J.M.; Plasenzotti, R.; Spiel, A.; Quehenberger, P.; Jilma, B. Interspecies differences in coagulation profile. Thromb. Haemost. 2008, 100, 397–404. [Google Scholar] [CrossRef]
- Mohammed, B.M.; Monroe, D.M.; Gailani, D. Mouse models of hemostasis. Platelets 2020, 31, 417–422. [Google Scholar] [CrossRef]
- Badimon, L.; Martínez-González, J.; Royo, T.; Lassila, R.; Badimon, J.J. A sudden increase in plasma epinephrine levels transiently enhances platelet deposition on severely damaged arterial wall studies in a porcine model. Thromb. Haemost. 1999, 82, 1736–1742. [Google Scholar]
- Goto, S.; Handa, S.; Takahashi, E.; Abe, S.; Handa, M.; Ikeda, Y. Synergistic effect of epinephrine and shearing on platelet activation. Thromb. Res. 1996, 84, 351–359. [Google Scholar] [CrossRef]
- Wagner, C.T.; Kroll, M.H.; Chow, T.W.; Hellums, J.D.; Schafer, A.I. Epinephrine and shear stress synergistically induce platelet aggregation via a mechanism that partially bypasses VWF-GP IB interactions. Biorheology 1996, 33, 209–229. [Google Scholar] [CrossRef]
- Geenen, I.L.; Molin, D.G.; van den Akker, N.M.; Jeukens, F.; Spronk, H.M.; Schurink, G.W.; Post, M.J. Endothelial cells (ECs) for vascular tissue engineering: Venous ECs are less thrombogenic than arterial ECs. J. Tissue Eng. Regen. Med. 2015, 9, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Paszkowiak, J.J.; Dardik, A. Arterial wall shear stress: Observations from the bench to the bedside. Vasc. Endovasc. Surg. 2003, 37, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Frueh, J.; Maimari, N.; Homma, T.; Bovens, S.M.; Pedrigi, R.M.; Towhidi, L.; Krams, R. Systems biology of the functional and dysfunctional endothelium. Cardiovasc. Res. 2013, 99, 334–341. [Google Scholar] [CrossRef]
- Ranjan, A.K.; Zhang, Z.; Briyal, S.; Gulati, A. Centhaquine Restores Renal Blood Flow and Protects Tissue Damage After Hemorrhagic Shock and Renal Ischemia. Front. Pharm. 2021, 12, 616253. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Briyal, S.; Zhang, Z.; Marwah, M.; Posen, M.; Cherian, V.; Kotsko, M.; Gulati, A. Centhaquine Upregulates Hif1-a and Protects Hemorrhage-Induced Acute Kidney Injury. Crit. Care Med. 2020, 48, 690. [Google Scholar] [CrossRef]
- Tarandovskiy, I.D.; Shin, H.K.H.; Baek, J.H.; Karnaukhova, E.; Buehler, P.W. Interspecies comparison of simultaneous thrombin and plasmin generation. Sci. Rep. 2020, 10, 3885. [Google Scholar] [CrossRef]
- Ruiz, S.M.; Sridhara, S.; Blajchman, M.A.; Clarke, B.J. Expression and purification of recombinant rabbit factor VII. Thromb. Res. 2000, 98, 203–211. [Google Scholar] [CrossRef]
- Mei, H.; Hu, Y.; Wang, H.; Shi, W.; Deng, J.; Guo, T. Binding of EGF1 domain peptide in coagulation factor VII with tissue factor and its implications for the triggering of coagulation. J. Huazhong. Univ. Sci. Technol. Med. Sci. 2010, 30, 42–47. [Google Scholar] [CrossRef]
- Brooks, M.B.; Stablein, A.P.; Johnson, L.; Schultze, A.E. Preanalytic processing of rat plasma influences thrombin generation and fibrinolysis assays. Vet. Clin. Pathol. 2017, 46, 496–507. [Google Scholar] [CrossRef]
- Nielsen, V.G. Hemodilution modulates the time of onset and rate of fibrinolysis in human and rabbit plasma. J. Heart Lung. Transpl. 2006, 25, 1344–1352. [Google Scholar] [CrossRef]
- Fraga, G.P.; Bansal, V.; Coimbra, R. Transfusion of blood products in trauma: An update. J. Emerg. Med. 2010, 39, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef] [PubMed]
- Lavhale, M.S.; Havalad, S.; Gulati, A. Resuscitative effect of centhaquin after hemorrhagic shock in rats. J. Surg. Res. 2013, 179, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Lavhale, M.S.; Garcia, D.J.; Havalad, S. Centhaquin improves resuscitative effect of hypertonic saline in hemorrhaged rats. J. Surg. Res. 2012, 178, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Bakar, S.K.; Niazi, S. Stability of aspirin in different media. J. Pharm. Sci. 1983, 72, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Guidance for industry. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. 2005. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/estimating-maximum-safe-starting-dose-initial-clinical-trials-therapeutics-adult-healthy-volunteers (accessed on 10 December 2023).
- Lee, H.B.; Blaufox, M.D. Blood volume in the rat. J. Nucl. Med. 1985, 26, 72–76. [Google Scholar] [PubMed]
- Rezende-Neto, J.B.; Rizoli, S.B.; Andrade, M.V.; Lisboa, T.A.; Cunha-Melo, J.R. Rabbit model of uncontrolled hemorrhagic shock and hypotensive resuscitation. Braz. J. Med. Biol. Res. 2010, 43, 1153–1159. [Google Scholar] [CrossRef]
- Lee, M.H.; Ingvertsen, B.T.; Kirpensteijn, J.; Jensen, A.L.; Kristensen, A.T. Quantification of surgical blood loss. Vet. Surg. 2006, 35, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Angele, M.K.; Schneider, C.P.; Chaudry, I.H. Bench-to-bedside review: Latest results in hemorrhagic shock. Crit. Care 2008, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Lin, X.; Guan, R.; Xu, J.G. The effects of hydroxyethyl starch on lung capillary permeability in endotoxic rats and possible mechanisms. Anesth. Analg. 2004, 98, 768–774. [Google Scholar] [CrossRef]
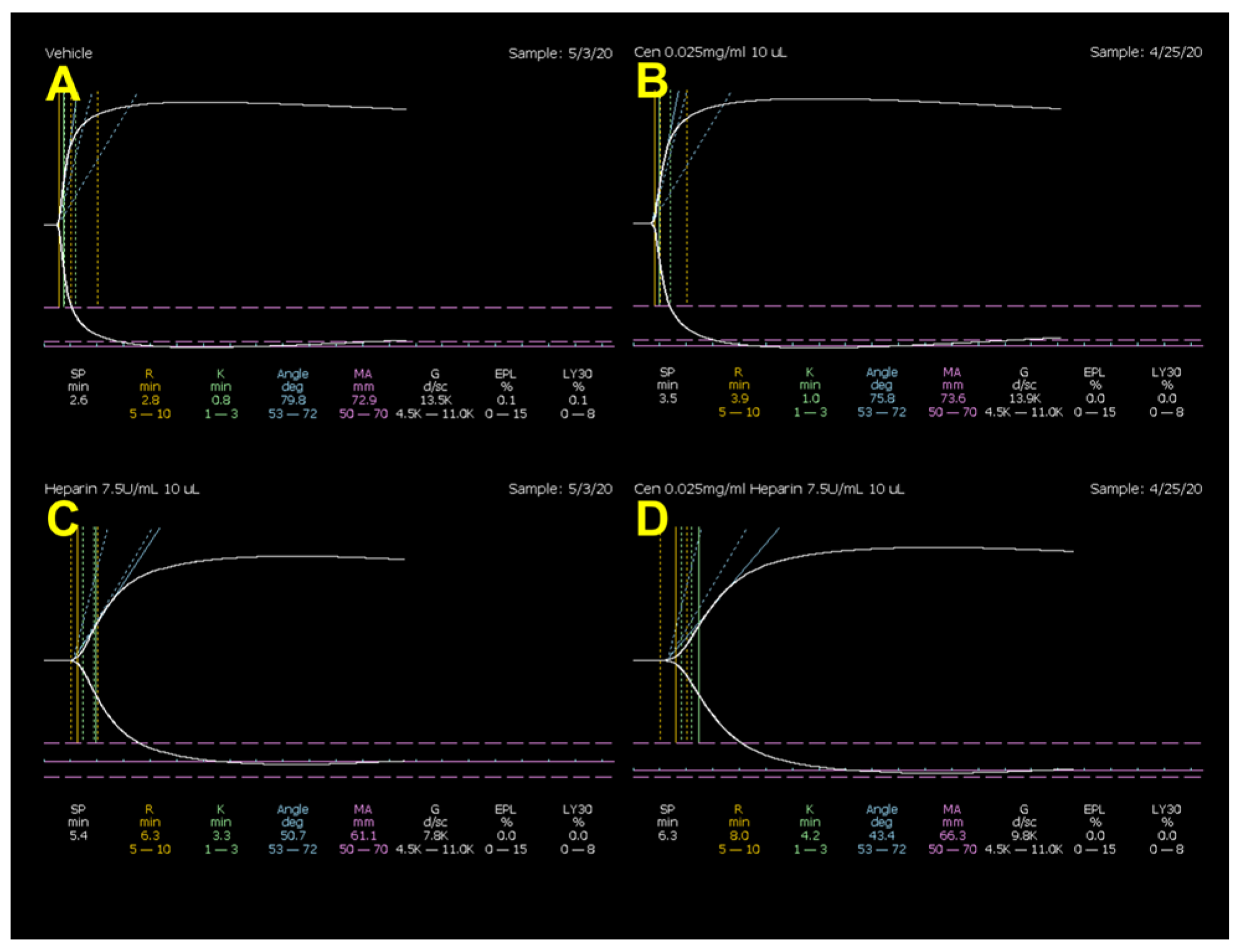
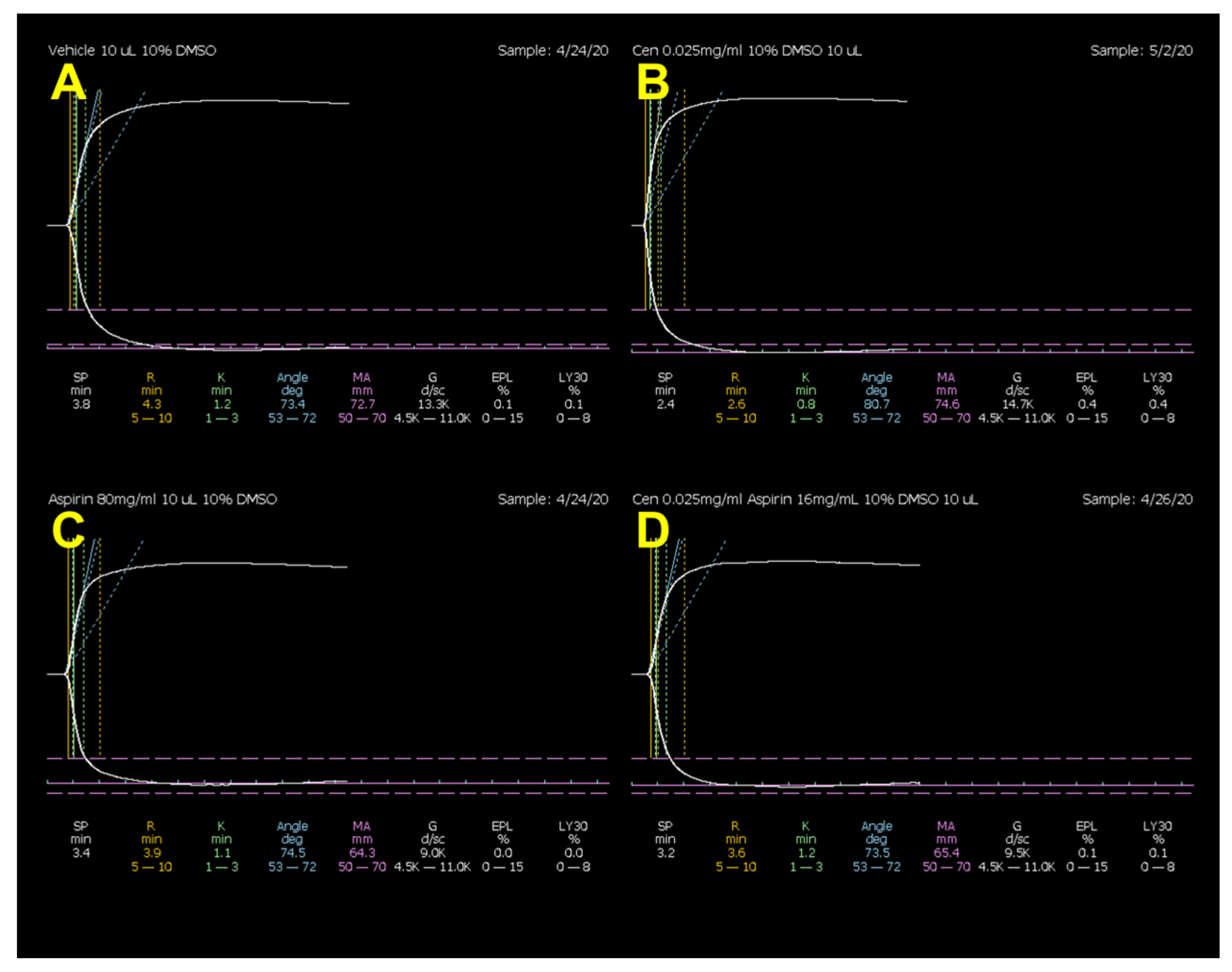




| R (min) | K (min) | α (°) | MA (mm) | LY30 (%) | |
|---|---|---|---|---|---|
| Vehicle # | 2.9 ± 0.2 | 0.96 ± 0.0 | 76.0 ± 0.7 | 71.2 ± 0.7 | 0.14 ± 0.0 |
| Centhaquine | 3.8 ± 0.4 | 1.11 ± 0.1 | 74.6 ± 1.1 | 72.1 ± 0.4 | 0.22 ± 0.1 |
| % change | 23.8 | 13.2 | −1.9 | 1.2 | 37.5 |
| Vehicle # | 2.9 ± 0.2 | 0.96 ± 0.0 | 76.0 ± 0.7 | 71.2 ± 0.7 | 0.14 ± 0.0 |
| Heparin | 6.5 ± 0.7 * | 2.68 ± 0.3 * | 57.6 ± 3.0 * | 64.2 ± 1.0 * | 0.42 ± 0.3 |
| % change | 55.5 | 64.1 | −32.0 | −11.0 | 67.0 |
| Heparin | 6.5 ± 0.7 | 2.68 ± 0.3 | 57.6 ± 3.0 | 64.2 ± 1.0 | 0.42 ± 0.3 |
| Centhaquine + heparin | 8.2 ± 1.0 | 4.37 ± 1.0 | 46.1 ± 4.9 | 65.3 ± 0.9 | 0.02 ± 0.0 |
| % change | 21.0 | 38.5 | −24.8 | 1.8 | −2400 |
| R (min) | K (min) | α (°) | MA (mm) | LY30 (%) | |
|---|---|---|---|---|---|
| Vehicle # | 2.9 ± 0.2 | 0.9 ± 0.1 | 77.9 ± 1.3 | 74.6 ± 1.8 | 0.3 ± 0.1 |
| Centhaquine | 2.6 ± 0.2 | 0.8 ± 0.0 | 79.2 ± 0.6 | 74.4 ± 1.4 | 0.1 ± 0.1 |
| % change | −9.8 | −15.0 | 1.7 | −0.3 | −100.0 |
| Vehicle # | 2.9 ± 0.2 | 0.9 ± 0.1 | 77.9 ± 1.3 | 74.6 ± 1.8 | 0.3 ± 0.1 |
| Aspirin | 2.9 ± 0.1 | 0.8 ± 0.0 | 79.0 ± 0.7 | 64.4 ± 2.0 * | 0.8 ± 0.4 |
| % change | −0.7 | −9.5 | 1.4 | −15.8 | 65.0 |
| Aspirin | 2.9 ± 0.1 | 0.8 ± 0.0 | 79.0 ± 0.7 | 64.4 ± 2.0 | 0.8 ± 0.4 |
| Centhaquine + aspirin | 2.7 ± 0.3 | 0.8 ± 0.0 | 78.8 ± 0.7 | 65.4 ± 1.5 | 1.1 ± 0.5 |
| % change | −8.3 | −5.0 | −0.3 | 1.5 | 28.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalkias, A.; Pais, G.; Gulati, A. Effect of Centhaquine on the Coagulation Cascade in Normal State and Uncontrolled Hemorrhage: A Multiphase Study Combining Ex Vivo and In Vivo Experiments in Different Species. Int. J. Mol. Sci. 2024, 25, 3494. https://doi.org/10.3390/ijms25063494
Chalkias A, Pais G, Gulati A. Effect of Centhaquine on the Coagulation Cascade in Normal State and Uncontrolled Hemorrhage: A Multiphase Study Combining Ex Vivo and In Vivo Experiments in Different Species. International Journal of Molecular Sciences. 2024; 25(6):3494. https://doi.org/10.3390/ijms25063494
Chicago/Turabian StyleChalkias, Athanasios, Gwendolyn Pais, and Anil Gulati. 2024. "Effect of Centhaquine on the Coagulation Cascade in Normal State and Uncontrolled Hemorrhage: A Multiphase Study Combining Ex Vivo and In Vivo Experiments in Different Species" International Journal of Molecular Sciences 25, no. 6: 3494. https://doi.org/10.3390/ijms25063494
APA StyleChalkias, A., Pais, G., & Gulati, A. (2024). Effect of Centhaquine on the Coagulation Cascade in Normal State and Uncontrolled Hemorrhage: A Multiphase Study Combining Ex Vivo and In Vivo Experiments in Different Species. International Journal of Molecular Sciences, 25(6), 3494. https://doi.org/10.3390/ijms25063494






