Hepatoprotective Effect of Allium ochotense Extracts on Chronic Alcohol-Induced Fatty Liver and Hepatic Inflammation in C57BL/6 Mice
Abstract
1. Introduction
2. Results
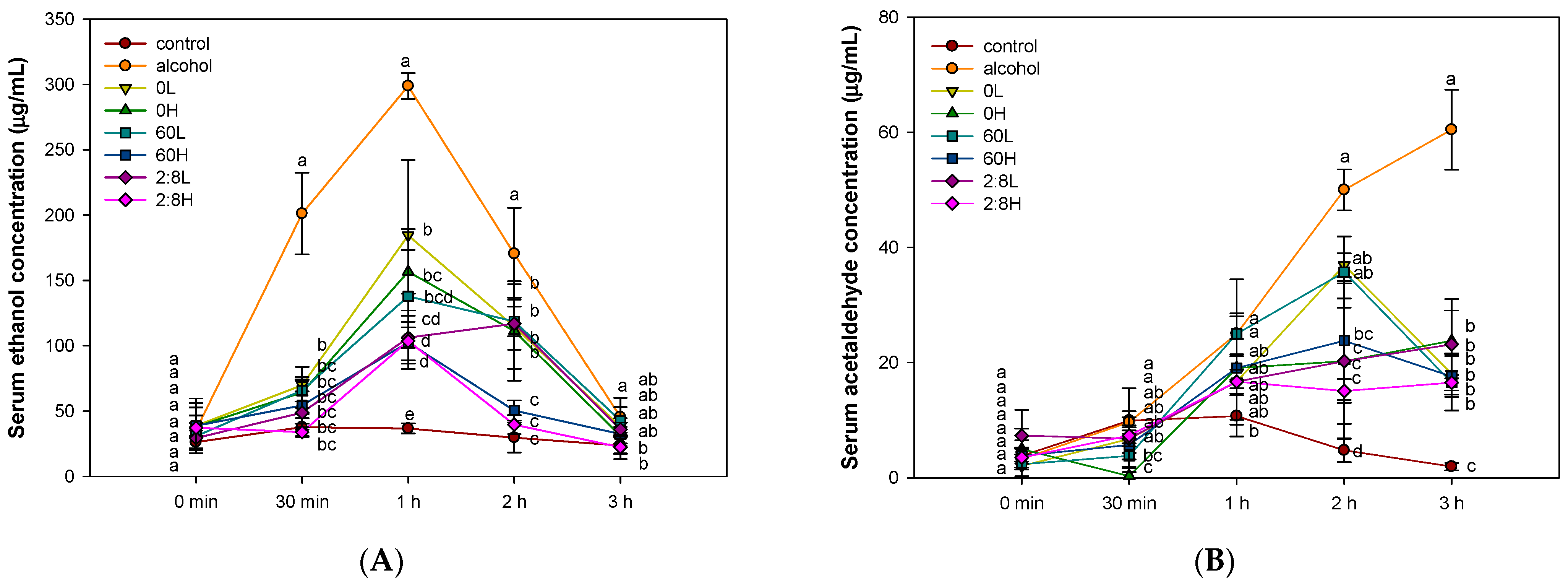
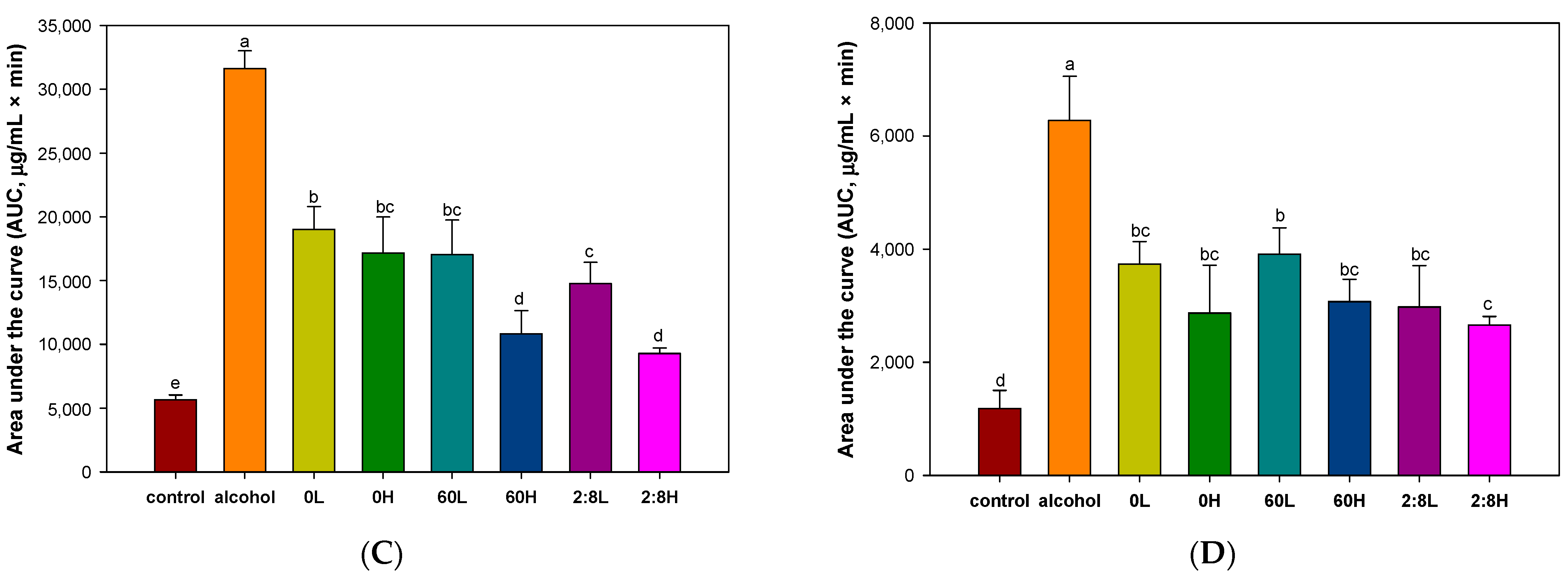
2.1. The Activity of Alcohol Degradation in Serum
2.2. Biologically Active Substances in the Mixture of Aqueous and 60% Ethanol Extracts of Allium ochotense (EA)
2.3. The Regulation of Fatty Liver and Hepatotoxicity Biomarkers in Serum
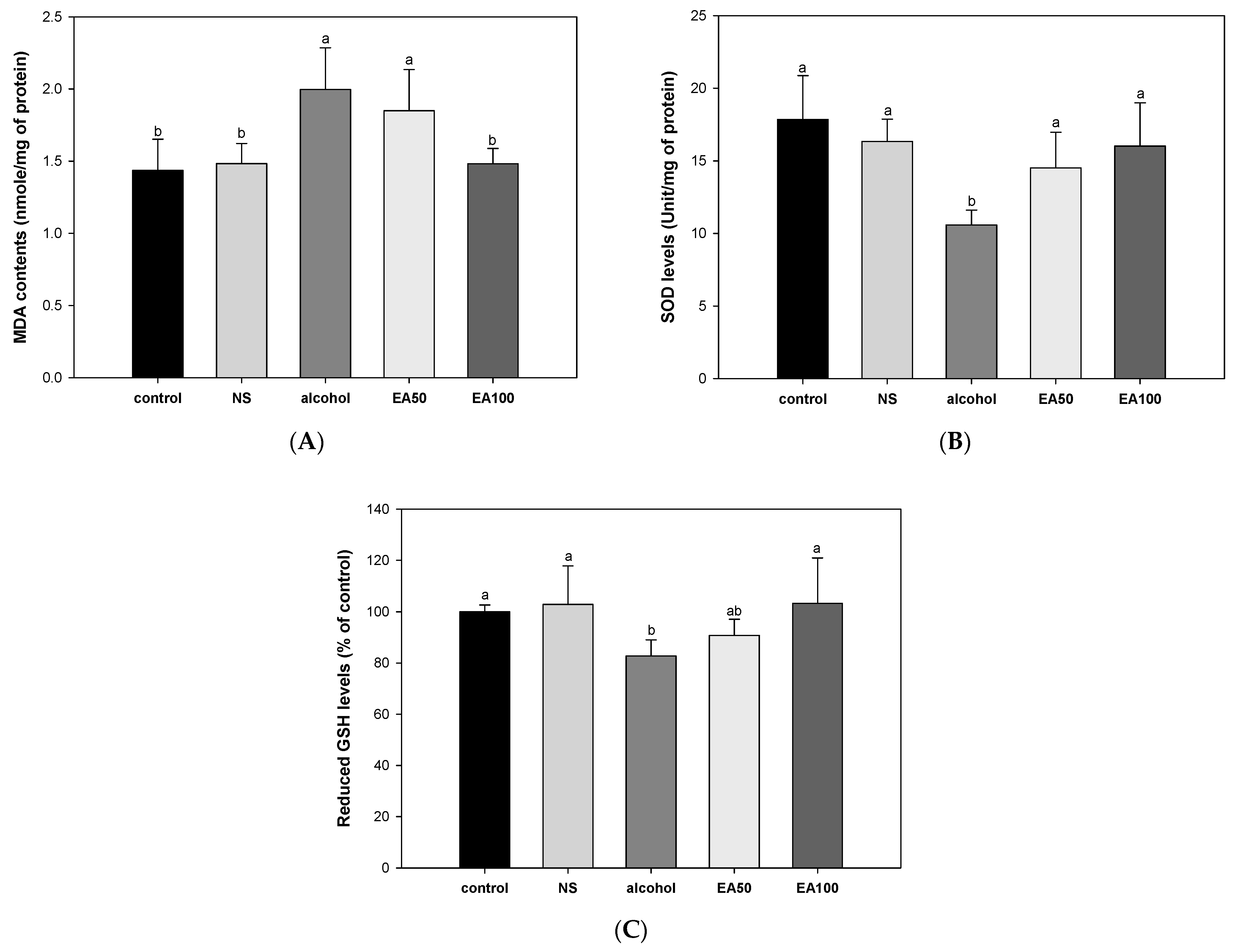
2.4. The Activity of Antioxidant System in Liver Tissue
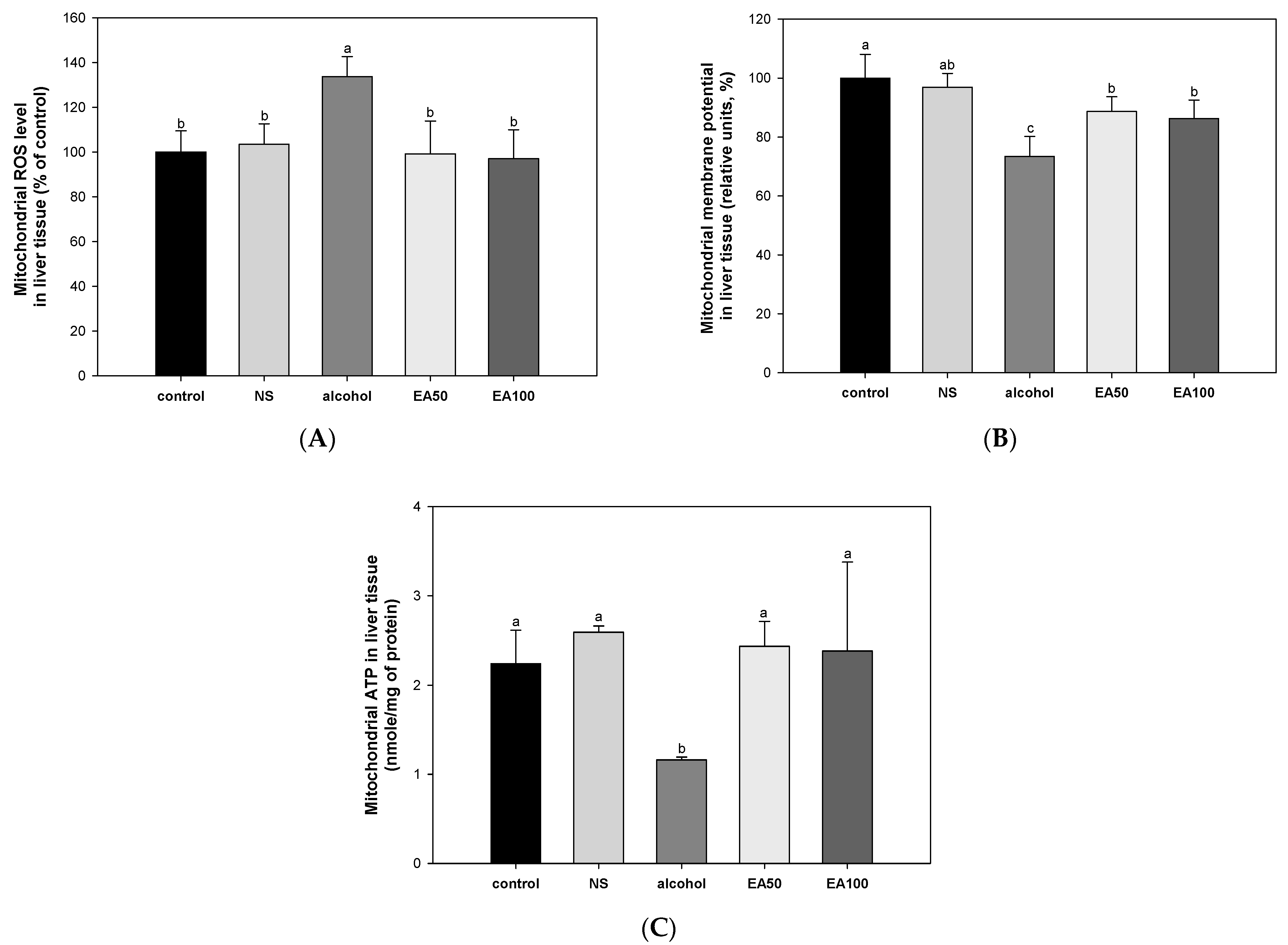
2.5. The Activity of Extracted Mitochondrial Function in Liver Tissue
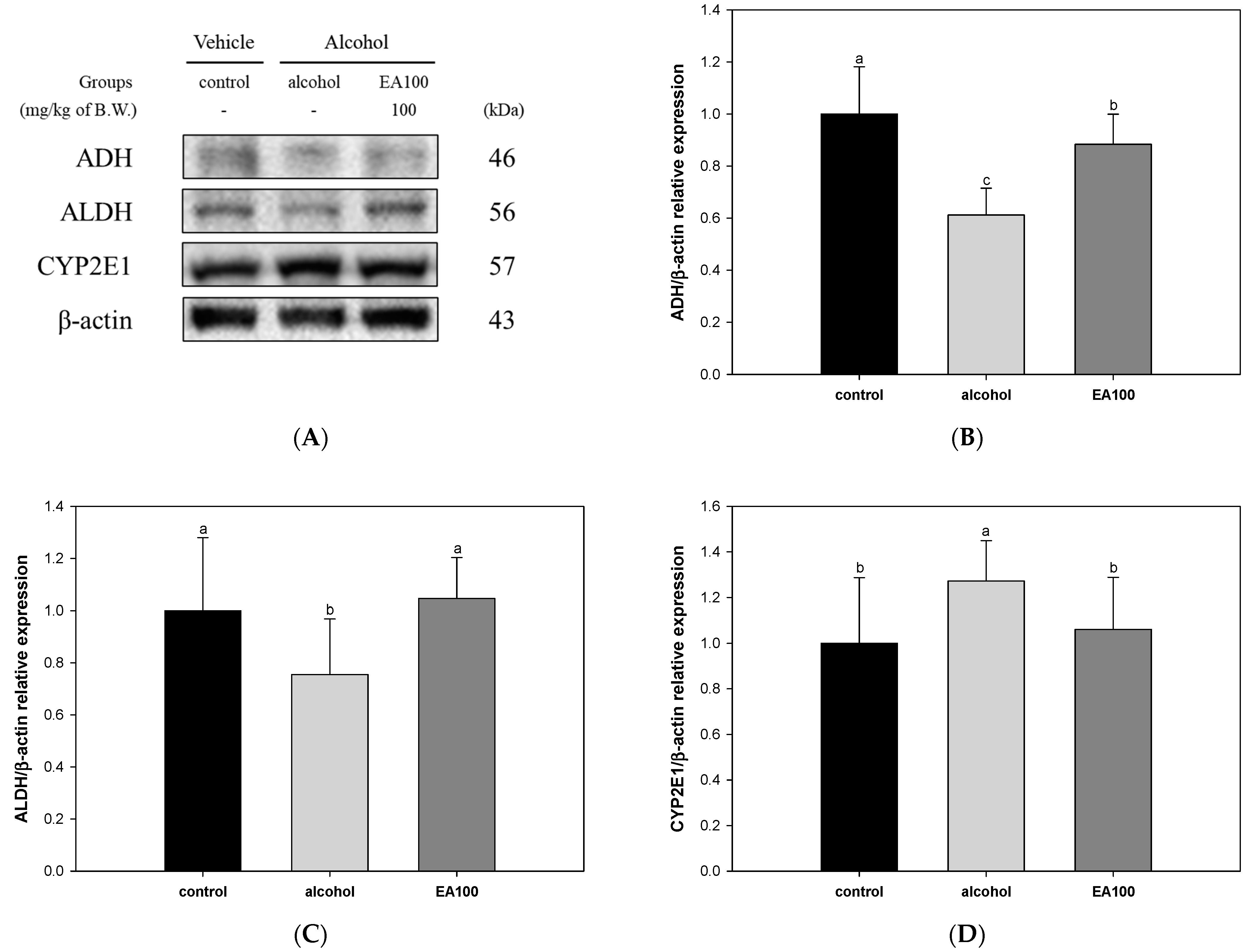
2.6. The Expression Levels of Alcohol Degradation Enzyme Related Biomarkers
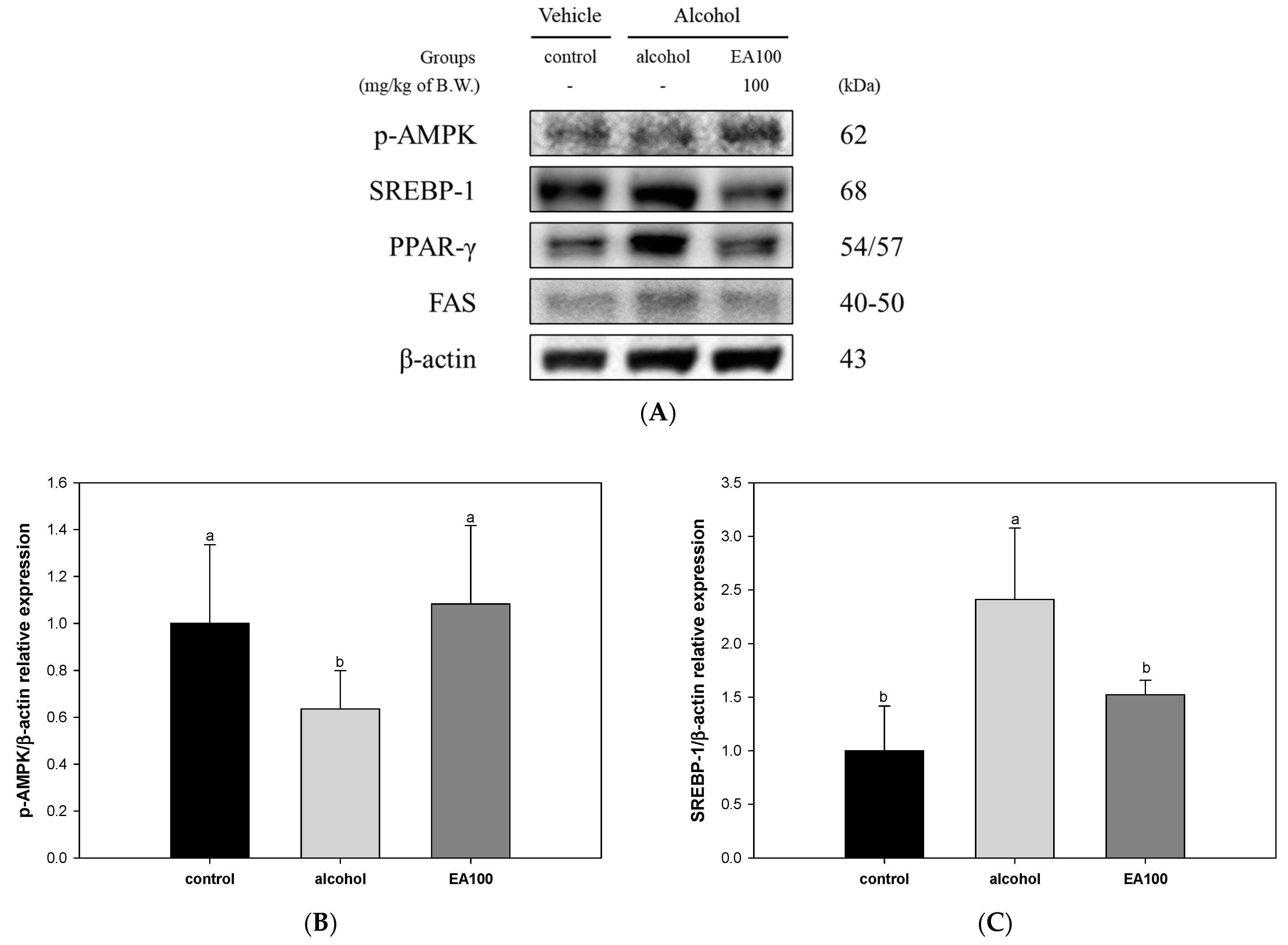
2.7. The Expression Levels of Lipid Metabolism Related Biomarkers
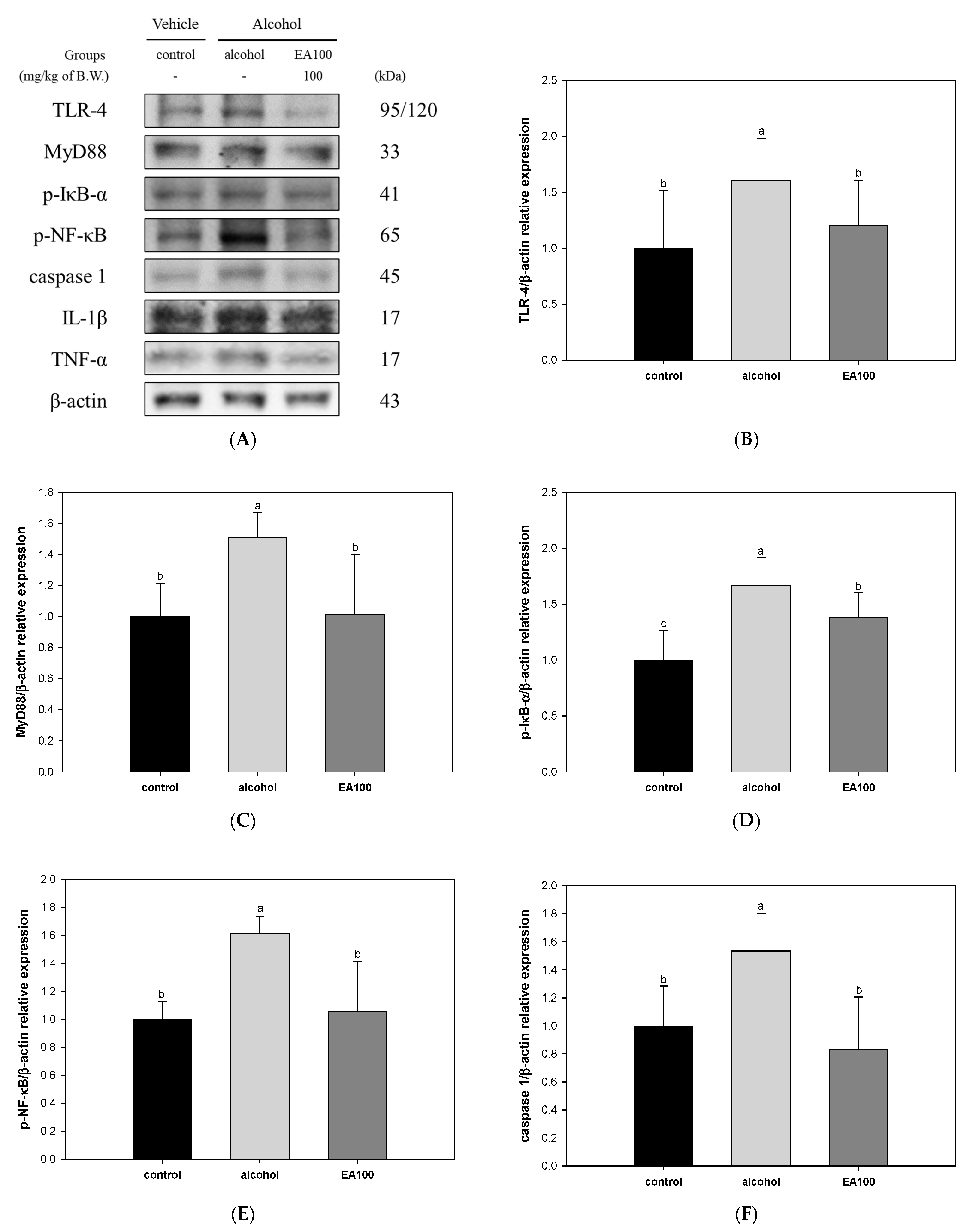
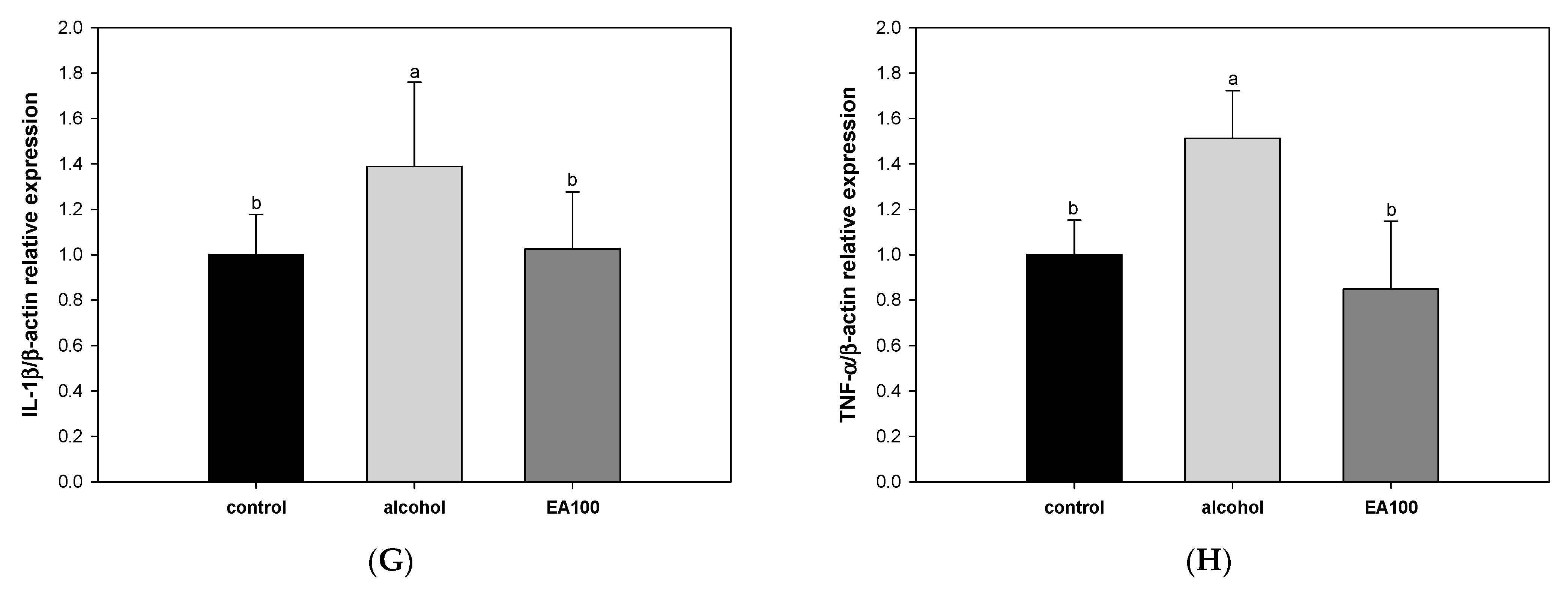
2.8. The Expression Levels of Alcoholic Hepatitis Related Biomarkers
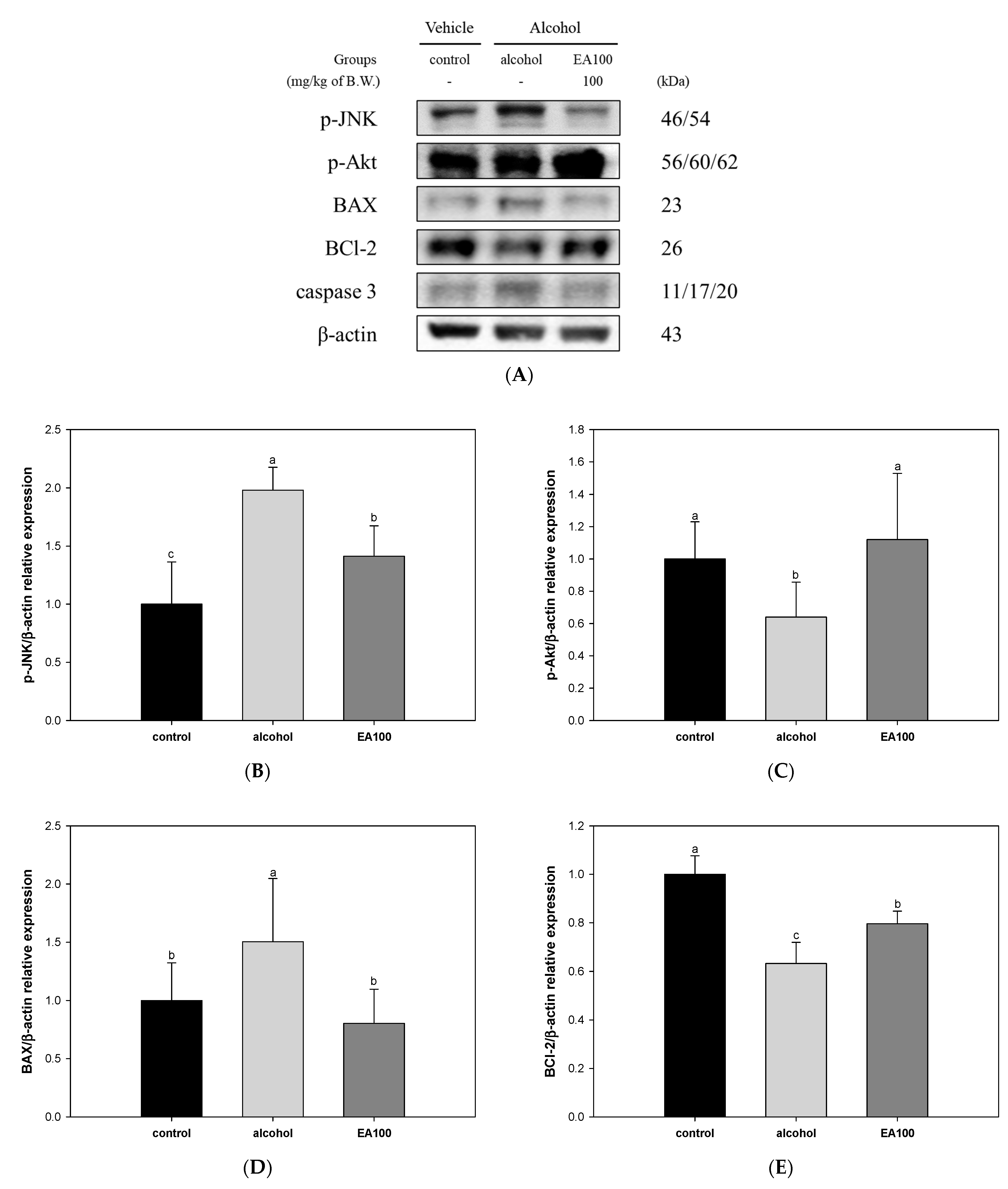
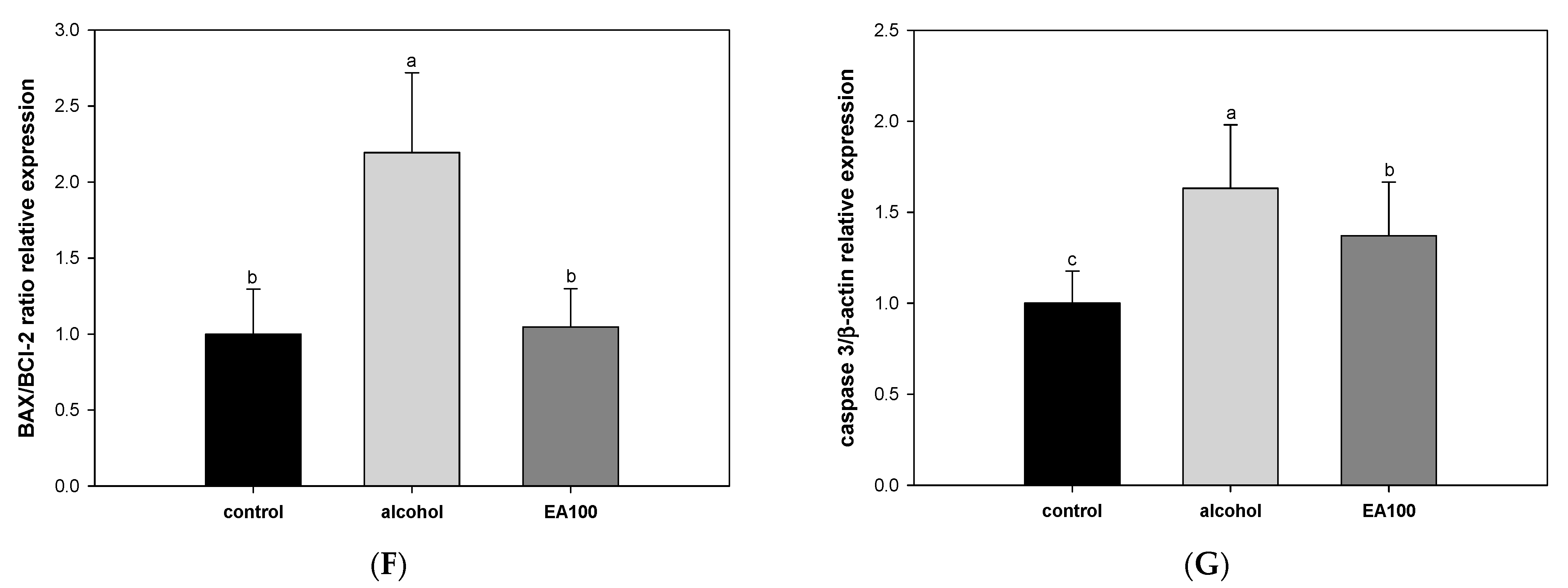
2.9. The Expression Levels of Alcohol-Induced Hepatocytes Apoptosis-Related Biomarker
2.10. The Expression Levels of Liver Fibrosis Related Biomarkers
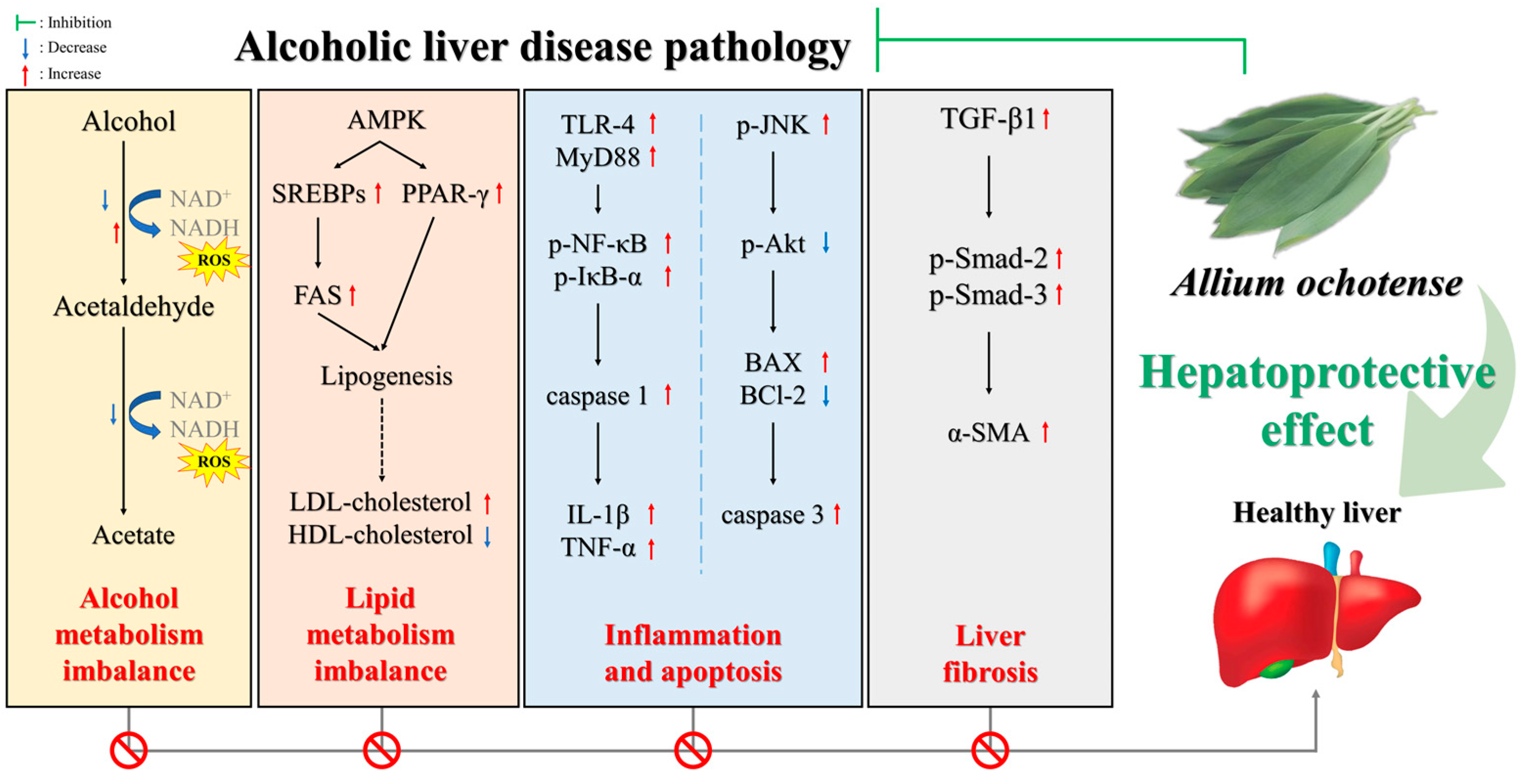
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Identification of Bioactive Substance in Allium ochotense
4.3. Animal Experimental Design
4.3.1. In Vivo Alcohol Degradation Experimental Model
4.3.2. Alcohol-Induced Fatty Liver Experimental Model
4.4. Measurement of Fatty Liver and Hepatotoxicity Biomarkers in Serum
4.5. Antioxidant System
4.5.1. Preparation of Tissue
4.5.2. Measurement of Antioxidant System Biomarkers
4.6. Mitochondrial Function
4.6.1. Extraction of Mitochondrial Sample
4.6.2. Measurement of Mitochondrial Function Biomarkers
4.7. Investigation of Protein Expression Levels in Liver Tissue Using Western Blot Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osna, N.A.; Donohue, T.M., Jr.; Kharbanda, K.K. Alcoholic liver disease: Pathogenesis and current management. Alcohol Res. 2017, 38, 147. [Google Scholar]
- Hosseini, N.; Shor, J.; Szabo, G. Alcoholic hepatitis: A review. Alcohol Alcohol. 2019, 54, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Y.; Tsai, I.-T.; Hsu, Y.-C. Alcohol-related liver disease: Basic mechanisms and clinical perspectives. Int. J. Mol. Sci. 2021, 22, 5170. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Ethanol and liver: Recent insights into the mechanisms of ethanol-induced fatty liver. World J. Gastroenterol. 2014, 20, 14672. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.-Y.; Han, X.; Jiang, Y.-C.; Dou, J.-Y.; Yao, K.-C.; Hu, Z.-H.; Yuan, M.-H.; Bao, X.-X.; Zhou, M.-J.; Liu, Y. Allium victorialis L. extracts promote activity of FXR to ameliorate alcoholic liver disease: Targeting liver lipid deposition and inflammation. Front. Pharmacol. 2021, 12, 738689. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Cho, Y.E.; Hwang, S. Crosstalk between oxidative stress and inflammatory liver injury in the pathogenesis of alcoholic liver disease. Int. J. Mol. Sci. 2022, 23, 774. [Google Scholar] [CrossRef] [PubMed]
- Subramaiyam, N. Insights of mitochondrial involvement in alcoholic fatty liver disease. J. Cell. Physiol. 2023, 238, 2175–2190. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Masarone, M.; Federico, A.; Rosato, V.; Dallio, M.; Loguercio, C.; Persico, M. Alcoholic hepatitis: Pathogenesis, diagnosis and treatment. Rev. Recent Clin. Trials 2016, 11, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Kim, H.-S.; Lee, S.-M.; Park, G.-S. Analysis of vegetation and vegetation-environment relationships in main wild vegetables of Ulleungdo in Korea-vegetation of herb layer of the Aster glehni, Allium ochotense, and Aruncus sylvester. J. Korean Soc. Environ. Res. Technol. 2018, 21, 71–82. [Google Scholar]
- Woo, K.W.; Moon, E.; Park, S.Y.; Kim, S.Y.; Lee, K.R. Flavonoid glycosides from the leaves of Allium victorialis var. platyphyllum and their anti-neuroinflammatory effects. Bioorg. Med. Chem. Lett. 2012, 22, 7465–7470. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, J.M.; Lee, H.L.; Go, M.J.; Joo, S.G.; Kim, J.H.; Lee, H.S.; Sim, S.J.; Heo, H.J. Protective effect of Allium ochotense Prokh. extract against ethanol-induced cytotoxicity. Food Sci. Preserv. 2023, 30, 526–537. [Google Scholar]
- Teschke, R. Alcoholic liver disease: Alcohol metabolism, cascade of molecular mechanisms, cellular targets, and clinical aspects. Biomedicines 2018, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Sferrazza, G.; Brusotti, G.; Zonfrillo, M.; Temporini, C.; Tengattini, S.; Bononi, M.; Tateo, F.; Calleri, E.; Pierimarchi, P. Hovenia dulcis Thumberg: Phytochemistry, pharmacology, toxicology and regulatory framework for its use in the European Union. Molecules 2021, 26, 903. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, L.; Yang, C.-F.; Zhong, Y.-J.; Wu, D.; Shi, L.; Chen, L.; Li, Y.-W. Hepatoprotective effects of methyl ferulic acid on alcohol-induced liver oxidative injury in mice by inhibiting the NOX4/ROS-MAPK pathway. Biochem. Biophys. Res. Commun. 2017, 493, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Vasudevan, D. Alcohol-induced oxidative stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.; Lee, H.; Sung, J. Relative protective activities of quercetin, quercetin-3-glucoside, and rutin in alcohol-induced liver injury. J. Food Biochem. 2019, 43, e13002. [Google Scholar] [CrossRef]
- Noh, J.-R.; Kim, Y.-H.; Gang, G.-T.; Hwang, J.-H.; Kim, S.-K.; Ryu, S.-Y.; Kim, Y.-S.; Lee, H.-S.; Lee, C.-H. Hepatoprotective effect of Platycodon grandiflorum against chronic ethanol-induced oxidative stress in C57BL/6 mice. Ann. Nutr. Metab. 2011, 58, 224–231. [Google Scholar] [CrossRef]
- Khlifi, R.; Dhaouefi, Z.; Toumia, I.B.; Lahmar, A.; Sioud, F.; Bouhajeb, R.; Bellalah, A.; Chekir-Ghedira, L. Erica multiflora extract rich in quercetin-3-O-glucoside and kaempferol-3-O-glucoside alleviates high fat and fructose diet-induced fatty liver disease by modulating metabolic and inflammatory pathways in Wistar rats. J. Nutr. Biochem. 2020, 86, 108490. [Google Scholar] [CrossRef]
- Li, F.; Zhang, X.; Ameer, K.; Eun, J.-B. Protective effect of victory onion (Allium victorialis L.) extract on reproductive system injury in male rats. Food Sci. Technol. 2020, 41, 591–602. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Singal, A.K. Mitochondrial dysfunction and alcohol-associated liver disease: A novel pathway and therapeutic target. Signal Transduct. Target. Ther. 2020, 5, 26. [Google Scholar] [CrossRef]
- Michalak, A.; Lach, T.; Cichoż-Lach, H. Oxidative stress—A key player in the course of alcohol-related liver disease. J. Clin. Med. 2021, 10, 3011. [Google Scholar] [CrossRef]
- Tan, H.K.; Yates, E.; Lilly, K.; Dhanda, A.D. Oxidative stress in alcohol-related liver disease. World J. Hepatol. 2020, 12, 332. [Google Scholar] [CrossRef]
- Zavodnik, I.; Buko, V.; Lukivskaya, O.; Lapshina, E.; Ilyich, T.; Belonovskaya, E.; Kirko, S.; Naruta, E.; Kuzmitskaya, I.; Budryn, G. Cranberry (Vaccinium macrocarpon) peel polyphenol-rich extract attenuates rat liver mitochondria impairments in alcoholic steatohepatitis in vivo and after oxidative treatment in vitro. J. Funct. Foods 2019, 57, 83–94. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, C.; Dai, S.; Liu, Y.; Zhang, F.; Peng, C.; Li, Y. Quercetin protects ethanol-induced hepatocyte pyroptosis via scavenging mitochondrial ROS and promoting PGC-1α-regulated mitochondrial homeostasis in L02 cells. Oxid. Med. Cell. Longev. 2022, 2022, 4591134. [Google Scholar] [CrossRef]
- Chen, J.-L.; Duan, W.-J.; Luo, S.; Li, S.; Ma, X.-H.; Hou, B.-N.; Cheng, S.-Y.; Fang, S.-H.; Wang, Q.; Huang, S.-Q. Ferulic acid attenuates brain microvascular endothelial cells damage caused by oxygen-glucose deprivation via punctate-mitochondria-dependent mitophagy. Brain Res. 2017, 1666, 17–26. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, R.; Pu, Q.; Jiang, S.; Yu, F.; Yang, Z.; Han, T. Investigation of nephrotoxicity on mice exposed to polystyrene nanoplastics and the potential amelioration effects of DHA-enriched phosphatidylserine. Sci. Total Environ. 2023, 892, 164808. [Google Scholar] [CrossRef]
- Chyau, C.-C.; Wang, H.-F.; Zhang, W.-J.; Chen, C.-C.; Huang, S.-H.; Chang, C.-C.; Peng, R.Y. Antrodan alleviates high-fat and high-fructose diet-induced fatty liver disease in C57BL/6 mice model via AMPK/Sirt1/SREBP-1c/PPARγ pathway. Int. J. Mol. Sci. 2020, 21, 360. [Google Scholar] [CrossRef] [PubMed]
- Abozid, M.M.; Farid, H. The anti-fatty liver effects of guava leaves and pomegranate peel extracts on ethanol-exposed rats. J. Biol. Chem. Environ. Sci. 2013, 8, 83–104. [Google Scholar]
- Yan, Q.-J.; Chen, Y.-Y.; Wu, M.-X.; Yang, H.; Cao, J.-P.; Sun, C.-D.; Wang, Y. Phenolics and terpenoids profiling in diverse loquat fruit varieties and systematic assessment of their mitigation of alcohol-induced oxidative stress. Antioxidants 2023, 12, 1795. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaran, P.; Thiyagarajan, G.; Babu, R.A.; Lakshmi, B.S. Raffinose from Costus speciosus attenuates lipid synthesis through modulation of PPARs/SREBP1c and improves insulin sensitivity through PI3K/AKT. Chem. Biol. Interact. 2018, 284, 80–89. [Google Scholar] [CrossRef]
- Bede, D.; Zaixiang, L. Dietary Polysaccharides from Allium species: A critical review in dietary polysaccharides from Allium Species: Extraction, characterization, bioactivity, and potential utilization. Acta Sci. Agric. 2020, 4, 1–15. [Google Scholar] [CrossRef]
- Gao, Y.; Xi, B.; Li, J.; Li, Z.; Xu, J.; Zhong, M.; Xu, Q.; Lian, Y.; Wei, R.; Wang, L. Scoparone alleviates hepatic fibrosis by inhibiting the TLR-4/NF-κB pathway. J. Cell. Physiol. 2021, 236, 3044–3058. [Google Scholar] [CrossRef]
- Slevin, E.; Baiocchi, L.; Wu, N.; Ekser, B.; Sato, K.; Lin, E.; Ceci, L.; Chen, L.; Lorenzo, S.R.; Xu, W. Kupffer cells: Inflammation pathways and cell-cell interactions in alcohol-associated liver disease. Am. J. Pathol. 2020, 190, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, P.; Yang, B.; Wang, T.; Li, J.; Song, X.; Sun, W. Kaempferol prevents the progression from simple steatosis to non-alcoholic steatohepatitis by inhibiting the NF-κB pathway in oleic acid-induced HepG2 cells and high-fat diet-induced rats. J. Funct. Foods 2021, 85, 104655. [Google Scholar] [CrossRef]
- Miyata, T.; Nagy, L.E. Programmed cell death in alcohol-associated liver disease. Clin. Mol. Hepatol. 2020, 26, 618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-F.; Bu, F.-T.; Yin, N.-N.; You, H.-M.; Wang, L.; Jia, W.-Q.; Huang, C.; Li, J. NLRP12 negatively regulates EtOH-induced liver macrophage activation via NF-κB pathway and mediates hepatocyte apoptosis in alcoholic liver injury. Int. Immunopharmacol. 2020, 88, 106968. [Google Scholar] [CrossRef]
- Kwon, B.S.; Kim, J.M.; Park, S.K.; Kang, J.Y.; Kang, J.E.; Lee, C.J.; Park, S.H.; Park, S.B.; Yoo, S.K.; Lee, U. Chronic alcohol exposure induced neuroapoptosis: Diminishing effect of ethyl acetate fraction from Aralia elata. Oxid. Med. Cell. Longev. 2019, 2019, 7849876. [Google Scholar] [CrossRef]
- Dong, B.; Shi, Z.; Dong, Y.; Chen, J.; Wu, Z.-X.; Wu, W.; Chen, Z.-S.; Han, C. Quercetin ameliorates oxidative stress-induced cell apoptosis of seminal vesicles via activating Nrf2 in type 1 diabetic rats. Biomed. Pharmacother. 2022, 151, 113108. [Google Scholar] [CrossRef]
- Xiang, D.; Zou, J.; Zhu, X.; Chen, X.; Luo, J.; Kong, L.; Zhang, H. Physalin D attenuates hepatic stellate cell activation and liver fibrosis by blocking TGF-β/Smad and YAP signaling. Phytomedicine 2020, 78, 153294. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Jung, D.H.; Lee, I.S.; Choi, S.-J.; Yu, S.Y.; Ku, S.-K.; Kim, M.-H.; Kim, J.S. Effects of Allium victorialis leaf extracts and its single compounds on aldose reductase, advanced glycation end products and TGF-β1 expression in mesangial cells. BMC Complement. Altern. Med. 2013, 13, 1–7. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.-X.; Guo, S.-D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Albadrani, G.M.; BinMowyna, M.N.; Bin-Jumah, M.N.; El-Akabawy, G.; Aldera, H.; Al-Farga, A.M. Quercetin prevents myocardial infarction adverse remodeling in rats by attenuating TGF-β1/Smad3 signaling: Different mechanisms of action. Saudi J. Biol. Sci. 2021, 28, 2772–2782. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Kim, J.M.; Go, M.J.; Joo, S.G.; Kim, T.Y.; Lee, H.S.; Kim, J.H.; Son, J.-S.; Heo, H.J. Fermented Protaetia brevitarsis Larvae ameliorates chronic ethanol-induced hepatotoxicity in mice via AMPK and TLR-4/TGF-β1 pathways. J. Microbiol. Biotechnol. 2023, 34, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.I.; Choi, Y.-J.; Kim, J.; Baek, K.-S.; Kim, E.J. Aqueous extract of Laurus nobilis leaf accelerates the alcohol metabolism and prevents liver damage in single-ethanol binge rats. Nutr. Res. Pract. 2023, 17, 1113. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; An, M.; Kim, J.-K.; Lim, Y.-H. Antiobesity effect of ethanolic extract of Ramulus mori in differentiated 3T3-L1 adipocytes and high-fat diet-induced obese mice. J. Ethnopharmacol. 2020, 251, 112542. [Google Scholar] [CrossRef] [PubMed]
- Kondaparthi, P.; Deore, M.; Naqvi, S.; Flora, S.J.S. Dose-dependent hepatic toxicity and oxidative stress on exposure to nano and bulk selenium in mice. Environ. Sci. Pollut. Res. 2021, 28, 53034–53044. [Google Scholar] [CrossRef]
- Fu, J.; Hu, F.; Ma, T.; Zhao, W.J.; Tian, H.; Zhang, Y.; Hu, M.; Zhou, J.; Zhang, Y.; Jian, C. A conventional immune regulator mitochondrial antiviral signaling protein blocks hepatic steatosis by maintaining mitochondrial homeostasis. Hepatology 2022, 75, 403–418. [Google Scholar] [CrossRef]
- Azevedo, R.D.; Falcão, K.V.; Assis, C.R.; Martins, R.M.; Araújo, M.C.; Yogui, G.T.; Neves, J.L.; Seabra, G.M.; Maia, M.B.; Amaral, I.P. Effects of pyriproxyfen on zebrafish brain mitochondria and acetylcholinesterase. Chemosphere 2021, 263, 128029. [Google Scholar] [CrossRef]
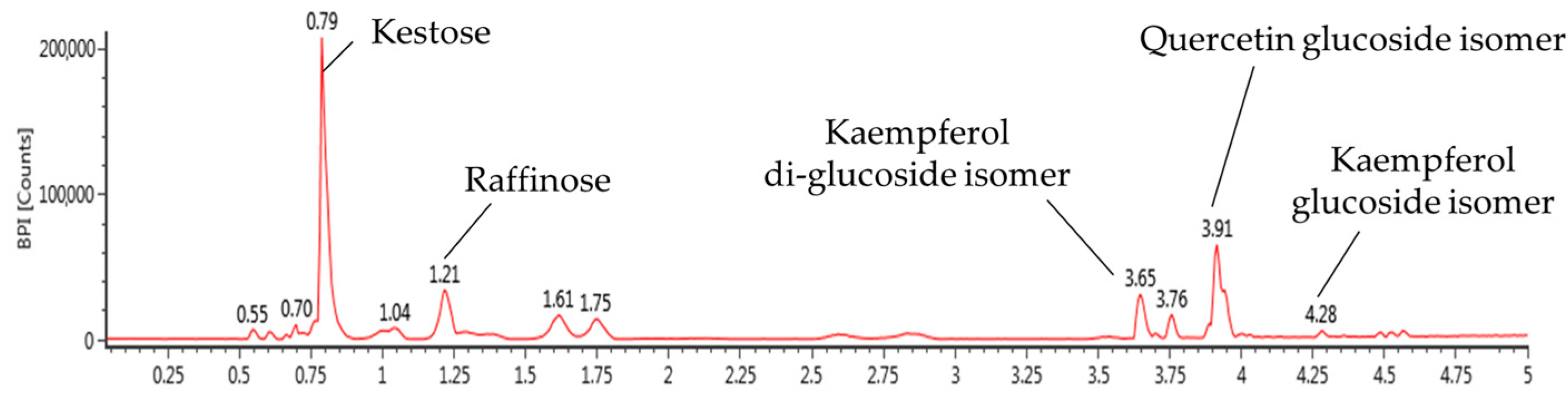


| No. | RT (min) | m/z [M − H]− | Fragment (m/z) | Proposed Compounds |
|---|---|---|---|---|
| 1 | 0.79 | 503 | 179, 341, 101, 323, 221, 113 | Ketose |
| 2 | 1.21 | 503 | 179, 341, 323, 221, 179 | Raffinose |
| 3 | 3.65 | 609 | 447, 285 | Kaempferol di-glucoside isomer |
| 4 | 3.91 | 463 | 301, 285, 464 | Quercetin glucoside isomer |
| 5 | 4.28 | 447 | 174, 285, 229 | Kaempferol glucoside isomer |
| TCHO (mg/dL) | TG (mg/dL) | HDLC (mg/dL) | LDLC (mg/dL) | GOT (U/L) | GPT (U/L) | TBIL (mg/dL) | |
|---|---|---|---|---|---|---|---|
| Control | 94.50 ± 3.42 c | 115.50 ± 5.20 b | 74.00 ± 4.16 bc | 3.45 ± 1.59 b | 45.40 ± 4.45 b | 29.40 ± 1.52 b | 0.50 ± 0.07 b |
| NS | 95.75 ± 7.89 c | 101.75 ± 12.66 b | 76.25 ± 2.06 bc | 4.00 ± 0.59 b | 44.80 ± 1.30 b | 29.20 ± 4.76 b | 0.52 ± 0.22 b |
| Alcohol | 110.25 ± 1.50 a | 143.50 ± 12.50 a | 70.50 ± 3.70 c | 11.40 ± 3.98 a | 54.00 ± 10.12 a | 35.40 ± 4.92 a | 0.70 ± 0.07 a |
| EA50 | 97.25 ± 5.68 bc | 104.25 ± 16.94 b | 82.25 ± 11.90 ab | 0.90 ± 4.67 b | 47.40 ± 2.30 ab | 34.20 ± 4.09 a | 0.38 ± 0.04 b |
| EA100 | 105.00 ± 9.70 ab | 113.25 ± 2.50 b | 91.00 ± 4.76 a | 0.55 ± 0.91 b | 46.00 ± 2.00 b | 29.40 ± 1.82 b | 0.42 ± 0.04 b |
| Antibody | Catalog No. | Concentration | Manufacturer |
|---|---|---|---|
| β-actin | sc-69879 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| ADH | sc-133207 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| ALDH | sc-374076 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| p-AMPK | sc-33524 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| SREBP-1 | sc-13551 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| PPAR-γ | sc-7273 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| p-JNK | sc-6254 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| p-Akt 1/2/3 | sc-393887 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| BAX | sc-7480 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| BCl-2 | sc-509 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| caspase 3 | sc-56053 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| TLR-4 | sc-52962 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| MyD88 | sc-74532 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| p-NF-κB | sc-136548 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| p-IκB-α | sc-8404 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| caspase 1 | sc-392736 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| IL-1β | sc-515598 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| TNF-α | sc-33639 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| TGFβ-1 | sc-130348 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| p-Smad-3 | sc-517575 | 1:1000 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| CYP2E1 | CSB-PA006425EA01HU | 1:1000 | Cusabio (Wuhan, China) |
| FAS | CSB-PA06354A0Rb | 1:1000 | Cusabio (Wuhan, China) |
| α-SMA | Ab5694 | 1:1000 | Abcam (Cambridge, UK) |
| p-Smad-2 | #3108 | 1:1000 | Cell Signaling Technology (Danvers MA, USA) |
| Anti-rabbit | 7074S | 1:5000 | Cell Signaling Technology (Danvers, MA, USA) |
| Anti-mouse | #1706516 | 1:5000 | Bio-Rad (Richmond, CA, USA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Go, M.J.; Kim, J.M.; Lee, H.L.; Kim, T.Y.; Kim, J.H.; Lee, H.S.; Kim, I.Y.; Sim, S.J.; Heo, H.J. Hepatoprotective Effect of Allium ochotense Extracts on Chronic Alcohol-Induced Fatty Liver and Hepatic Inflammation in C57BL/6 Mice. Int. J. Mol. Sci. 2024, 25, 3496. https://doi.org/10.3390/ijms25063496
Go MJ, Kim JM, Lee HL, Kim TY, Kim JH, Lee HS, Kim IY, Sim SJ, Heo HJ. Hepatoprotective Effect of Allium ochotense Extracts on Chronic Alcohol-Induced Fatty Liver and Hepatic Inflammation in C57BL/6 Mice. International Journal of Molecular Sciences. 2024; 25(6):3496. https://doi.org/10.3390/ijms25063496
Chicago/Turabian StyleGo, Min Ji, Jong Min Kim, Hyo Lim Lee, Tae Yoon Kim, Ju Hui Kim, Han Su Lee, In Young Kim, Seon Jeong Sim, and Ho Jin Heo. 2024. "Hepatoprotective Effect of Allium ochotense Extracts on Chronic Alcohol-Induced Fatty Liver and Hepatic Inflammation in C57BL/6 Mice" International Journal of Molecular Sciences 25, no. 6: 3496. https://doi.org/10.3390/ijms25063496
APA StyleGo, M. J., Kim, J. M., Lee, H. L., Kim, T. Y., Kim, J. H., Lee, H. S., Kim, I. Y., Sim, S. J., & Heo, H. J. (2024). Hepatoprotective Effect of Allium ochotense Extracts on Chronic Alcohol-Induced Fatty Liver and Hepatic Inflammation in C57BL/6 Mice. International Journal of Molecular Sciences, 25(6), 3496. https://doi.org/10.3390/ijms25063496





