Cell Heterogeneity and Variability in Peripheral Nerve after Injury
Abstract
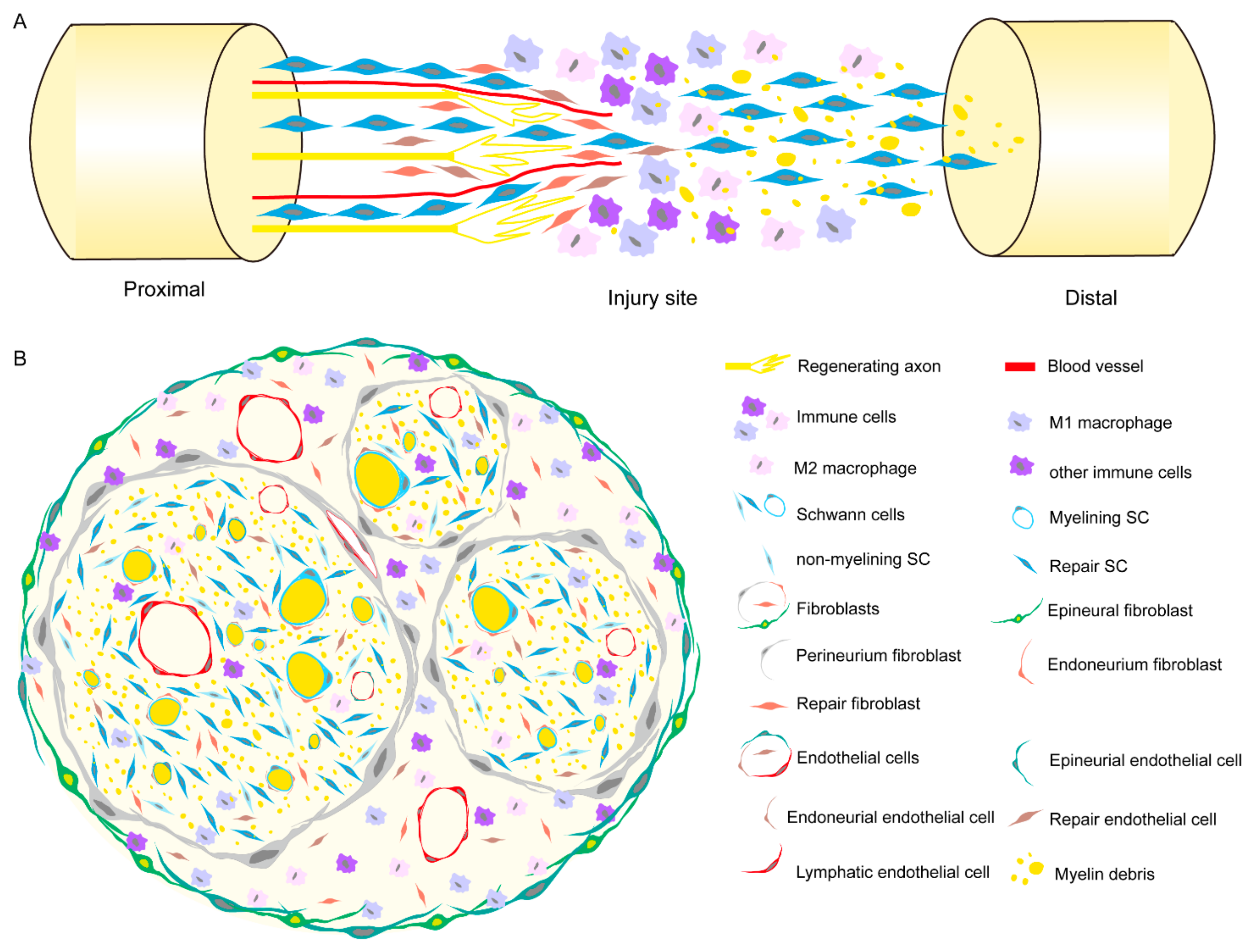
1. Introduction
2. Cell Composition in Peripheral Nerve
3. Schwann Cells
3.1. Cell Heterogeneity of SCs during Development
3.2. Unique Repaired SC Subtype Appeared after Injury
4. Fibroblast
5. Immune Cells
5.1. Macrophage
5.2. Other Immune Cells
6. Endothelial Cells
7. Prospect
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wood, M.D.; Kemp, S.W.; Weber, C.; Borschel, G.H.; Gordon, T. Outcome measures of peripheral nerve regeneration. Ann. Anat. 2011, 193, 321–333. [Google Scholar] [CrossRef]
- Saheb-Al-Zamani, M.; Yan, Y.; Farber, S.J.; Hunter, D.A.; Newton, P.; Wood, M.D.; Stewart, S.A.; Johnson, P.J.; Mackinnon, S.E. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp. Neurol. 2013, 247, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Renthal, W.; Tochitsky, I.; Yang, L.; Cheng, Y.C.; Li, E.; Kawaguchi, R.; Geschwind, D.H.; Woolf, C.J. Transcriptional Reprogramming of Distinct Peripheral Sensory Neuron Subtypes after Axonal Injury. Neuron 2020, 108, 128–144.e9. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Queralt, M.; Fledrich, R.; Stassart, R.M. Schwann cell functions in peripheral nerve development and repair. Neurobiol. Dis. 2023, 176, 105952. [Google Scholar] [CrossRef] [PubMed]
- Stratton, J.A.; Holmes, A.; Rosin, N.L.; Sinha, S.; Vohra, M.; Burma, N.E.; Trang, T.; Midha, R.; Biernaskie, J. Macrophages Regulate Schwann Cell Maturation after Nerve Injury. Cell Rep. 2018, 24, 2561–2572.e6. [Google Scholar] [CrossRef] [PubMed]
- Lubetzki, C.; Sol-Foulon, N.; Desmazieres, A. Nodes of Ranvier during development and repair in the CNS. Nat. Rev. Neurol. 2020, 16, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Peng, J.; Han, G.H.; Ding, X.; Wei, S.; Gao, G.; Huang, K.; Chang, F.; Wang, Y. Role of macrophages in peripheral nerve injury and repair. Neural Regen. Res. 2019, 14, 1335–1342. [Google Scholar] [PubMed]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Macri, R.; Scicchitano, M.; Bosco, F.; Scarano, F.; Ruga, S.; et al. The Role of Endothelial Dysfunction in Peripheral Blood Nerve Barrier: Molecular Mechanisms and Pathophysiological Implications. Int. J. Mol. Sci. 2019, 20, 3022. [Google Scholar] [CrossRef]
- Ydens, E.; Amann, L.; Asselbergh, B.; Scott, C.L.; Martens, L.; Sichien, D.; Mossad, O.; Blank, T.; De Prijck, S.; Low, D.; et al. Profiling peripheral nerve macrophages reveals two macrophage subsets with distinct localization, transcriptome and response to injury. Nat. Neurosci. 2020, 23, 676–689. [Google Scholar] [CrossRef]
- Jovic, D.; Liang, X.; Zeng, H.; Lin, L.; Xu, F.; Luo, Y. Single-cell RNA sequencing technologies and applications: A brief overview. Clin. Transl. Med. 2022, 12, e694. [Google Scholar] [CrossRef]
- Stahl, P.L.; Salmen, F.; Vickovic, S.; Lundmark, A.; Navarro, J.F.; Magnusson, J.; Giacomello, S.; Asp, M.; Westholm, J.O.; Huss, M.; et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 2016, 353, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, A.; Hochgerner, H.; Lonnerberg, P.; Johnsson, A.; Memic, F.; van der Zwan, J.; Haring, M.; Braun, E.; Borm, L.E.; La Manno, G.; et al. Molecular Architecture of the Mouse Nervous System. Cell 2018, 174, 999–1014.e22. [Google Scholar] [CrossRef] [PubMed]
- Wolbert, J.; Li, X.; Heming, M.; Mausberg, A.K.; Akkermann, D.; Frydrychowicz, C.; Fledrich, R.; Groeneweg, L.; Schulz, C.; Stettner, M.; et al. Redefining the heterogeneity of peripheral nerve cells in health and autoimmunity. Proc. Natl. Acad. Sci. USA 2020, 117, 9466–9476. [Google Scholar] [CrossRef] [PubMed]
- Toma, J.S.; Karamboulas, K.; Carr, M.J.; Kolaj, A.; Yuzwa, S.A.; Mahmud, N.; Storer, M.A.; Kaplan, D.R.; Miller, F.D. Peripheral Nerve Single-Cell Analysis Identifies Mesenchymal Ligands that Promote Axonal Growth. eNeuro 2020, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.J.; Toma, J.S.; Johnston, A.P.W.; Steadman, P.E.; Yuzwa, S.A.; Mahmud, N.; Frankland, P.W.; Kaplan, D.R.; Miller, F.D. Mesenchymal Precursor Cells in Adult Nerves Contribute to Mammalian Tissue Repair and Regeneration. Cell Stem Cell 2019, 24, 240–256.e9. [Google Scholar] [CrossRef] [PubMed]
- Yim, A.K.Y.; Wang, P.L.; Bermingham, J.R., Jr.; Hackett, A.; Strickland, A.; Miller, T.M.; Ly, C.; Mitra, R.D.; Milbrandt, J. Disentangling glial diversity in peripheral nerves at single-nuclei resolution. Nat. Neurosci. 2022, 25, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Banton, M.C.; Singh, L.; Parkinson, D.B.; Dun, X.P. Single Cell Transcriptome Data Analysis Defines the Heterogeneity of Peripheral Nerve Cells in Homeostasis and Regeneration. Front. Cell Neurosci. 2021, 15, 624826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, S.; Wang, X.; Gu, X.; Yi, S. Cell populations in neonatal rat peripheral nerves identified by single-cell transcriptomics. Glia 2021, 69, 765–778. [Google Scholar] [CrossRef]
- Gerber, D.; Pereira, J.A.; Gerber, J.; Tan, G.; Dimitrieva, S.; Yanguez, E.; Suter, U. Transcriptional profiling of mouse peripheral nerves to the single-cell level to build a sciatic nerve ATlas (SNAT). Elife 2021, 10, e58591. [Google Scholar] [CrossRef]
- Zhang, F.; Miao, Y.; Liu, Q.; Li, S.; He, J. Changes of pro-inflammatory and anti-inflammatory macrophages after peripheral nerve injury. RSC Adv. 2020, 10, 38767–38773. [Google Scholar] [CrossRef]
- Reed, C.B.; Feltri, M.L.; Wilson, E.R. Peripheral glia diversity. J. Anat. 2022, 241, 1219–1234. [Google Scholar] [CrossRef] [PubMed]
- Monk, K.R.; Feltri, M.L.; Taveggia, C. New insights on Schwann cell development. Glia 2015, 63, 1376–1393. [Google Scholar] [CrossRef] [PubMed]
- Kastriti, M.E.; Faure, L.; Von Ahsen, D.; Bouderlique, T.G.; Bostrom, J.; Solovieva, T.; Jackson, C.; Bronner, M.; Meijer, D.; Hadjab, S.; et al. Schwann cell precursors represent a neural crest-like state with biased multipotency. EMBO J. 2022, 41, e108780. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Woodhoo, A.; Alonso, M.B.; Droggiti, A.; Turmaine, M.; D'Antonio, M.; Parkinson, D.B.; Wilton, D.K.; Al-Shawi, R.; Simons, P.; Shen, J.; et al. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat. Neurosci. 2009, 12, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Wanner, I.B.; Guerra, N.K.; Mahoney, J.; Kumar, A.; Wood, P.M.; Mirsky, R.; Jessen, K.R. Role of N-cadherin in Schwann cell precursors of growing nerves. Glia 2006, 54, 439–459. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. Schwann Cell Precursors; Multipotent Glial Cells in Embryonic Nerves. Front. Mol. Neurosci. 2019, 12, 69. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, W.; Yi, S. Cellular complexity of the peripheral nervous system: Insights from single-cell resolution. Front. Neurosci. 2023, 17, 1098612. [Google Scholar] [CrossRef]
- Lindsay, R.M. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J. Neurosci. 1988, 8, 2394–2405. [Google Scholar] [CrossRef]
- Naveilhan, P.; ElShamy, W.M.; Ernfors, P. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur. J. Neurosci. 1997, 9, 1450–1460. [Google Scholar] [CrossRef]
- Johnston, A.P.; Yuzwa, S.A.; Carr, M.J.; Mahmud, N.; Storer, M.A.; Krause, M.P.; Jones, K.; Paul, S.; Kaplan, D.R.; Miller, F.D. Dedifferentiated Schwann Cell Precursors Secreting Paracrine Factors Are Required for Regeneration of the Mammalian Digit Tip. Cell Stem Cell 2016, 19, 433–448. [Google Scholar] [CrossRef]
- Ou, M.Y.; Tan, P.C.; Xie, Y.; Liu, K.; Gao, Y.M.; Yang, X.S.; Zhou, S.B.; Li, Q.F. Dedifferentiated Schwann cell-derived TGF-beta3 is essential for the neural system to promote wound healing. Theranostics 2022, 12, 5470–5487. [Google Scholar] [CrossRef]
- Direder, M.; Wielscher, M.; Weiss, T.; Laggner, M.; Copic, D.; Klas, K.; Bormann, D.; Vorstandlechner, V.; Tschachler, E.; Jan Ankersmit, H.; et al. The transcriptional profile of keloidal Schwann cells. Exp. Mol. Med. 2022, 54, 1886–1900. [Google Scholar] [CrossRef] [PubMed]
- Direder, M.; Weiss, T.; Copic, D.; Vorstandlechner, V.; Laggner, M.; Pfisterer, K.; Mildner, C.S.; Klas, K.; Bormann, D.; Haslik, W.; et al. Schwann cells contribute to keloid formation. Matrix Biol. 2022, 108, 55–76. [Google Scholar] [CrossRef]
- Chen, Y.L.; Tsai, Y.T.; Chao, T.T.; Wu, Y.N.; Chen, M.C.; Lin, Y.H.; Liao, C.H.; Chou, S.P.; Chiang, H.S. DAPK and CIP2A are involved in GAS6/AXL-mediated Schwann cell proliferation in a rat model of bilateral cavernous nerve injury. Oncotarget 2018, 9, 6402–6415. [Google Scholar] [CrossRef] [PubMed]
- May, F.; Buchner, A.; Matiasek, K.; Schlenker, B.; Stief, C.; Weidner, N. Recovery of erectile function comparing autologous nerve grafts, unseeded conduits, Schwann-cell-seeded guidance tubes and GDNF-overexpressing Schwann cell grafts. Dis. Model. Mech. 2016, 9, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.M.; Mukouyama, Y.S.; Mosher, J.T.; Jaegle, M.; Crone, S.A.; Dormand, E.L.; Lee, K.F.; Meijer, D.; Anderson, D.J.; Morrison, S.J. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development 2004, 131, 5599–5612. [Google Scholar] [CrossRef]
- Woodhoo, A.; Sommer, L. Development of the Schwann cell lineage: From the neural crest to the myelinated nerve. Glia 2008, 56, 1481–1490. [Google Scholar] [CrossRef]
- Min, Q.; Parkinson, D.B.; Dun, X.P. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia 2021, 69, 235–254. [Google Scholar] [CrossRef]
- Zilic, L.; Garner, P.E.; Yu, T.; Roman, S.; Haycock, J.W.; Wilshaw, S.P. An anatomical study of porcine peripheral nerve and its potential use in nerve tissue engineering. J. Anat. 2015, 227, 302–314. [Google Scholar] [CrossRef]
- Mueller, M.; Leonhard, C.; Wacker, K.; Ringelstein, E.B.; Okabe, M.; Hickey, W.F.; Kiefer, R. Macrophage response to peripheral nerve injury: The quantitative contribution of resident and hematogenous macrophages. Lab. Investig. 2003, 83, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Dun, X.P.; Carr, L.; Woodley, P.K.; Barry, R.W.; Drake, L.K.; Mindos, T.; Roberts, S.L.; Lloyd, A.C.; Parkinson, D.B. Macrophage-Derived Slit3 Controls Cell Migration and Axon Pathfinding in the Peripheral Nerve Bridge. Cell Rep. 2019, 26, 1458–1472.e4. [Google Scholar] [CrossRef]
- Zigmond, R.E.; Echevarria, F.D. Macrophage biology in the peripheral nervous system after injury. Prog. Neurobiol. 2019, 173, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Wang, P.; Chen, Q.; Yi, S.; Liu, Q.; Wang, H.; Wang, S.; Geng, W.; Liu, Z.; Li, S. The dynamic changes of main cell types in the microenvironment of sciatic nerves following sciatic nerve injury and the influence of let-7 on their distribution. RSC Adv. 2018, 8, 41181–41191. [Google Scholar] [CrossRef] [PubMed]
- Bruck, W. The role of macrophages in Wallerian degeneration. Brain Pathol. 1997, 7, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Cescon, M.; Zuccolotto, G.; Nobbio, L.; Colombelli, C.; Filaferro, M.; Vitale, G.; Feltri, M.L.; Bonaldo, P. Collagen VI regulates peripheral nerve regeneration by modulating macrophage recruitment and polarization. Acta Neuropathol. 2015, 129, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Mokarram, N.; Dymanus, K.; Srinivasan, A.; Lyon, J.G.; Tipton, J.; Chu, J.; English, A.W.; Bellamkonda, R.V. Immunoengineering nerve repair. Proc. Natl. Acad. Sci. USA 2017, 114, E5077–E5084. [Google Scholar] [CrossRef]
- Zhang, B.; Su, Y.; Zhou, J.; Zheng, Y.; Zhu, D. Toward a Better Regeneration through Implant-Mediated Immunomodulation: Harnessing the Immune Responses. Adv. Sci. 2021, 8, e2100446. [Google Scholar] [CrossRef]
- Perkins, N.M.; Tracey, D.J. Hyperalgesia due to nerve injury: Role of neutrophils. Neuroscience 2000, 101, 745–757. [Google Scholar] [CrossRef]
- Nadeau, S.; Filali, M.; Zhang, J.; Kerr, B.J.; Rivest, S.; Soulet, D.; Iwakura, Y.; de Rivero Vaccari, J.P.; Keane, R.W.; Lacroix, S. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1beta and TNF: Implications for neuropathic pain. J. Neurosci. 2011, 31, 12533–12542. [Google Scholar] [CrossRef]
- Palmer, M.T.; Weaver, C.T. Autoimmunity: Increasing suspects in the CD4+ T cell lineup. Nat. Immunol. 2010, 11, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.; Bennett, D.L. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zheng, Y.; Sun, L.; Badea, S.R.; Jin, Y.; Liu, Y.; Rolfe, A.J.; Sun, H.; Wang, X.; Cheng, Z.; et al. Microvascular endothelial cells engulf myelin debris and promote macrophage recruitment and fibrosis after neural injury. Nat. Neurosci. 2019, 22, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Muangsanit, P.; Roberton, V.; Costa, E.; Phillips, J.B. Engineered aligned endothelial cell structures in tethered collagen hydrogels promote peripheral nerve regeneration. Acta Biomater. 2021, 126, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Bakken, T.E.; Hodge, R.D.; Miller, J.A.; Yao, Z.; Nguyen, T.N.; Aevermann, B.; Barkan, E.; Bertagnolli, D.; Casper, T.; Dee, N.; et al. Single-nucleus and single-cell transcriptomes compared in matched cortical cell types. PLoS ONE 2018, 13, e0209648. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Barbacioru, C.; Wang, Y.; Nordman, E.; Lee, C.; Xu, N.; Wang, X.; Bodeau, J.; Tuch, B.B.; Siddiqui, A.; et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 2009, 6, 377–382. [Google Scholar] [CrossRef]
- Maniatis, S.; Aijo, T.; Vickovic, S.; Braine, C.; Kang, K.; Mollbrink, A.; Fagegaltier, D.; Andrusivova, Z.; Saarenpaa, S.; Saiz-Castro, G.; et al. Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science 2019, 364, 89–93. [Google Scholar] [CrossRef]
- Gong, L.; Gu, Y.; Han, X.; Luan, C.; Liu, C.; Wang, X.; Sun, Y.; Zheng, M.; Fang, M.; Yang, S.; et al. Spatiotemporal Dynamics of the Molecular Expression Pattern and Intercellular Interactions in the Glial Scar Response to Spinal Cord Injury. Neurosci. Bull. 2023, 39, 213–244. [Google Scholar] [CrossRef]
- Yi, S.; Zhang, Y.; Gu, X.; Huang, L.; Zhang, K.; Qian, T.; Gu, X. Application of stem cells in peripheral nerve regeneration. Burns Trauma. 2020, 8, tkaa002. [Google Scholar]
- Dong, X.; Yang, Y.; Bao, Z.; Midgley, A.C.; Li, F.; Dai, S.; Yang, Z.; Wang, J.; Liu, L.; Li, W.; et al. Micro-nanofiber composite biomimetic conduits promote long-gap peripheral nerve regeneration in canine models. Bioact. Mater. 2023, 30, 98–115. [Google Scholar] [CrossRef]
- Yang, P.; Peng, Y.; Dai, X.; Jie, J.; Kong, D.; Gu, X.; Yang, Y. Bionic peptide scaffold in situ polarization and recruitment of M2 macrophages to promote peripheral nerve regeneration. Bioact. Mater. 2023, 30, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Efremova, M.; Vento-Tormo, M.; Teichmann, S.A.; Vento-Tormo, R. CellPhoneDB: Inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat. Protoc. 2020, 15, 1484–1506. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, M.; Richard, L.; Vallat, J.M.; Desmouliere, A.; Billet, F. Peripheral nerve regeneration and intraneural revascularization. Neural Regen. Res. 2019, 14, 24–33. [Google Scholar] [PubMed]
- Mueller, M.; Wacker, K.; Ringelstein, E.B.; Hickey, W.F.; Imai, Y.; Kiefer, R. Rapid response of identified resident endoneurial macrophages to nerve injury. Am. J. Pathol. 2001, 159, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Klaver, D.; Thurnher, M. P2Y(11)/IL-1 receptor crosstalk controls macrophage inflammation: A novel target for anti-inflammatory strategies? Purinergic Signal 2023, 19, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, F.; Udina, E.; Navarro, X. Extracellular matrix components in peripheral nerve regeneration. Int. Rev. Neurobiol. 2013, 108, 257–275. [Google Scholar]
| Cell Type | Sample Source | Methods | Subtype and Related Markers | Reference |
|---|---|---|---|---|
| Schwann cell | Mouse sciatic nerves | scRNA-seq and bulk-seq (Smart-seq2 and 10X genomics | prol. SC: Mki67, Top2a nm(R)SC: Ngfr, Ncam1, L1cam iSC: Ngfr, Ncam1, L1cam tSC: Ngfr, Ncam1, L1cam pmSCs: Pou3f1, Cdkn1c mSCs: Mpz, Mbp, Ncmap | [19] |
| Sciatic nerves and DRG from rat | snRNA-seq (10X genomics) | subtype 1: Hbb, LOC100134871 subtype 2: Cldn19, Emid1 subtype 3: Timp3, Col5a3 subtype 4: Cenpf, Mki67 | [18] | |
| Mouse sciatic nerves | snRNA-seq (10X genomics) | Schwann_M: Prx, Qk, Mbp Schwann_N: Ncam1, Slc35f1, Scn7a21, Csmd1 | [16] | |
| Mouse sciatic nerves | scRNA-seq | mSCs: Mbp, Mpz, Mag, Egr2 nmSCs: Cdh2c, L1cam Repair Schwann: Mki67, Top2a, Prc1, Ccna2 | [17] | |
| Mouse DRGs | snRNA-seq | Repair Schwann: Shh | [3] | |
| Fibroblast | Mouse sciatic nerves | scRNA-seq | Mesenchymal cells: Pdgfra Perineurial cells: Slc2a1/Glut1, Itgb4, Msln, Lmo7 Epineurial cells: Dpt, Pcolce2, Ly6c1 Endoneurial cells: Osr2, Sox9, Ccl9, Wif1, Cdkn2a | [15] |
| Mouse sciatic nerves | snRNA-seq (10X Genomics) scRNA-seq | Epineurial fibroblast: Pdgfra, Pcolce2 Perineurial fibroblast: Itgb4, Slc2a1 Endoneurial fibroblast: Ccbe1, Adamts3 | [16] | |
| Mouse sciatic nerves | scRNA-seq | Epineurial fibroblast: Sfrp2, Dpt, Pcolce2 Endoneurial fibroblast: Sox9, Osr2, Wif1 Differentiating fibroblast: Dlk1, Mest, Cilp | [17] | |
| Mouse DRGs | snRNA-seq | Repair fibroblasts: Wif1 | [3] | |
| Mouse sciatic nerves | scRNA-seq (10X genomics) | Fibroblast: Dpt, Gsn, Col1a1, Col1a2, Pi16, Sfrp4, Col3a1, Clec3b, Cygb | [13] | |
| Immune cell | Mouse sciatic nerves | snRNA-seq (10X genomics) scRNA-seq | Endoneurial macrophages: Cx3cr1, Trem2 Epineurial macrophages: Clec10a, Cd209a Monocytes: Ccr2 B cells: Bank1 NK cells: Nkg7, Klrb1c T cells: Cd3g, Cd3e CD8+ cytotoxic T cells: Cd8a, Trac CD4+ helper T cells: Trac, Cd4 | [16] |
| Mouse sciatic nerves | scRNA-seq | Epineurial macrophages: Retnla, Clec10a Macrophages: Aif1/Iba1, Cd68, Mrc1/Cd206 Monocytes: Ccl6, Fcgr3, Cx3cr1, Csf1r, Cd300a, Clec4e Neutrophils: S100a8, S100a9, Cxcr2, Cxcl2 Mast cells: Cma1, Mcpt4, Mcpt1, Kit T cells: Cd3g, Cxcr6, Trac, Cd3e NK cells: Nkg7, Klrk1, Ncr1 B cells: Bank1, Cbfa2t3, Taok, Ms4a1, Cd19, Cd79a | [17] | |
| Sciatic nerves and DRG from rat | RNA-seq | M1 macrophages: CD38, Grp18 M2 macrophages: Egr2, Myc, Myc | [20] | |
| Mouse sciatic nerves | scRNA-seq (10X genomics) | Epineurial Relmα Mgl1 snMacs: Cc18, Cd209a, Cd209d, Fxyd2, Tslp, Mmp9 Endoneurial Relmα Mgl1 snMacs: Ccr2, Cxcl1, ll1rl1, Selm, Pla2g2d, Qpct, Tnfsf9 | [9] | |
| Endothelial cell | Sciatic nerves and DRG from rat | snRNA-seq (10X genomics) | Endothelial cells: Plvap | [18] |
| Mouse sciatic nerves | scRNA-seq and Bulk-seq (Smart-seq2 and 10X genomics) | Endothelial cells-1: Cldn5, Slc2a1, Pecam1 Endothelial cells-2: Cd300lg, Pecam1 | [19] | |
| Mouse sciatic nerves | scRNA-seq | Endothelial cell: Pecam1/Cd31, Plvap, Esam | [14] | |
| Mouse sciatic nerves | scRNA-seq | Lymphatic endothelial cells: Lyve1, Mmrn1, Prox1, Flt4 Epineurial endothelial cells: Sox17, Spock2, Rgcc Endoneurial endothelial cells: Lrg1, Icam1 | [17] | |
| Mouse sciatic nerves | snRNA-seq (10X genomics) scRNA-seq | Microvascular endothelial cells: Cldn5 Lymphatic endothelial cells: Prox1, Lyve1 | [16] | |
| Vasculature-associated smooth muscle cells (VSMCs) and pericyte | Mouse sciatic nerves | snRNA-seq (10X genomics) scRNA-seq | VSMCs: Acta2a Pericytes: Pdgfrb | [16] |
| Mouse sciatic nerves | scRNA-seq | VSMCs: Des, Tpm2, Myh11, Acta2, Mylk, Myom1, Myocd Pericytes: Rgs5, Kcnj8, Pdgfrb | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Tan, Y.; Zhao, L. Cell Heterogeneity and Variability in Peripheral Nerve after Injury. Int. J. Mol. Sci. 2024, 25, 3511. https://doi.org/10.3390/ijms25063511
Ren Z, Tan Y, Zhao L. Cell Heterogeneity and Variability in Peripheral Nerve after Injury. International Journal of Molecular Sciences. 2024; 25(6):3511. https://doi.org/10.3390/ijms25063511
Chicago/Turabian StyleRen, Zhixian, Ya Tan, and Lili Zhao. 2024. "Cell Heterogeneity and Variability in Peripheral Nerve after Injury" International Journal of Molecular Sciences 25, no. 6: 3511. https://doi.org/10.3390/ijms25063511
APA StyleRen, Z., Tan, Y., & Zhao, L. (2024). Cell Heterogeneity and Variability in Peripheral Nerve after Injury. International Journal of Molecular Sciences, 25(6), 3511. https://doi.org/10.3390/ijms25063511






