Role of Sensory Nerves in Pulmonary Fibrosis
Abstract
1. Introduction
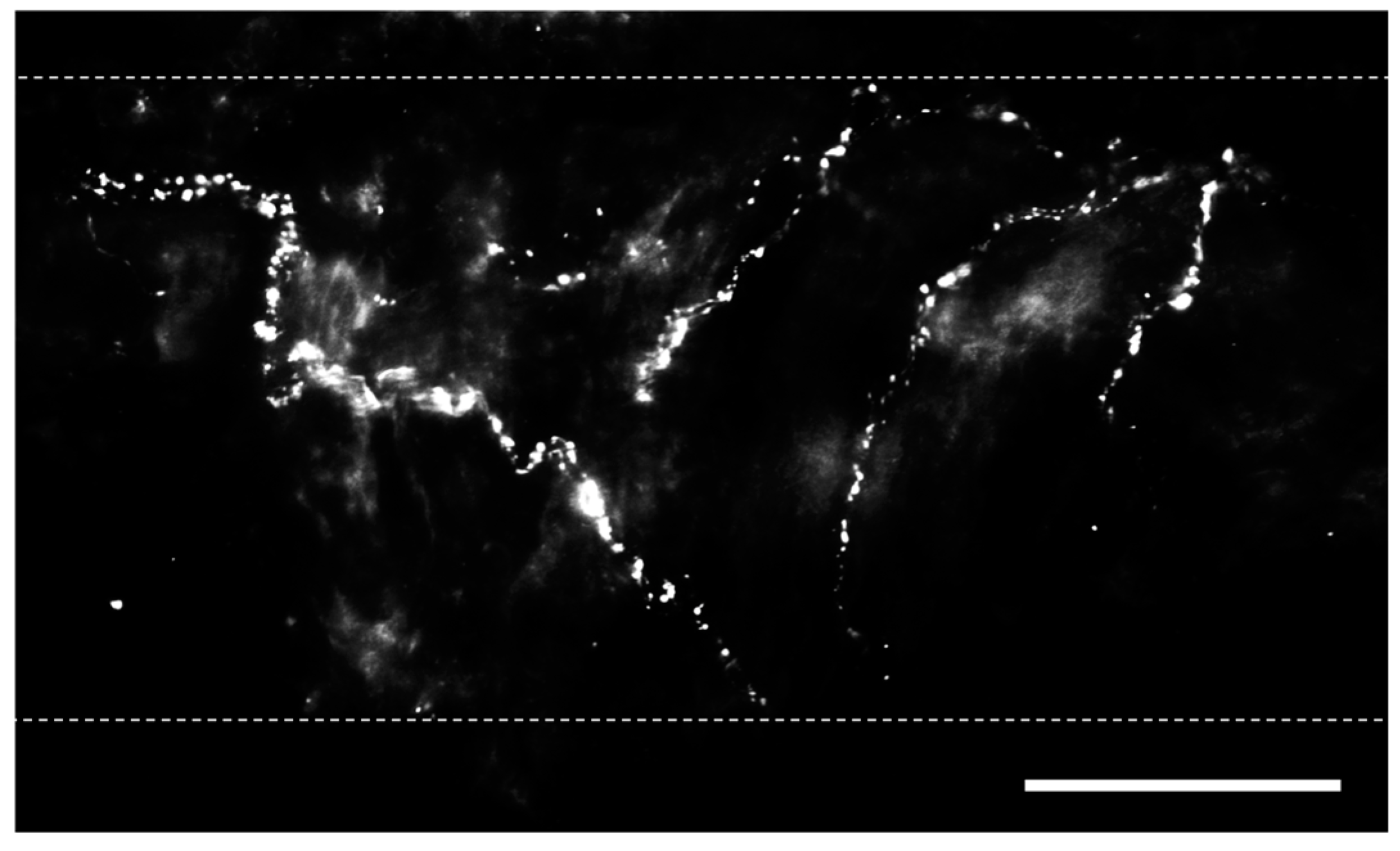
2. Sensory Nerves in the Lungs
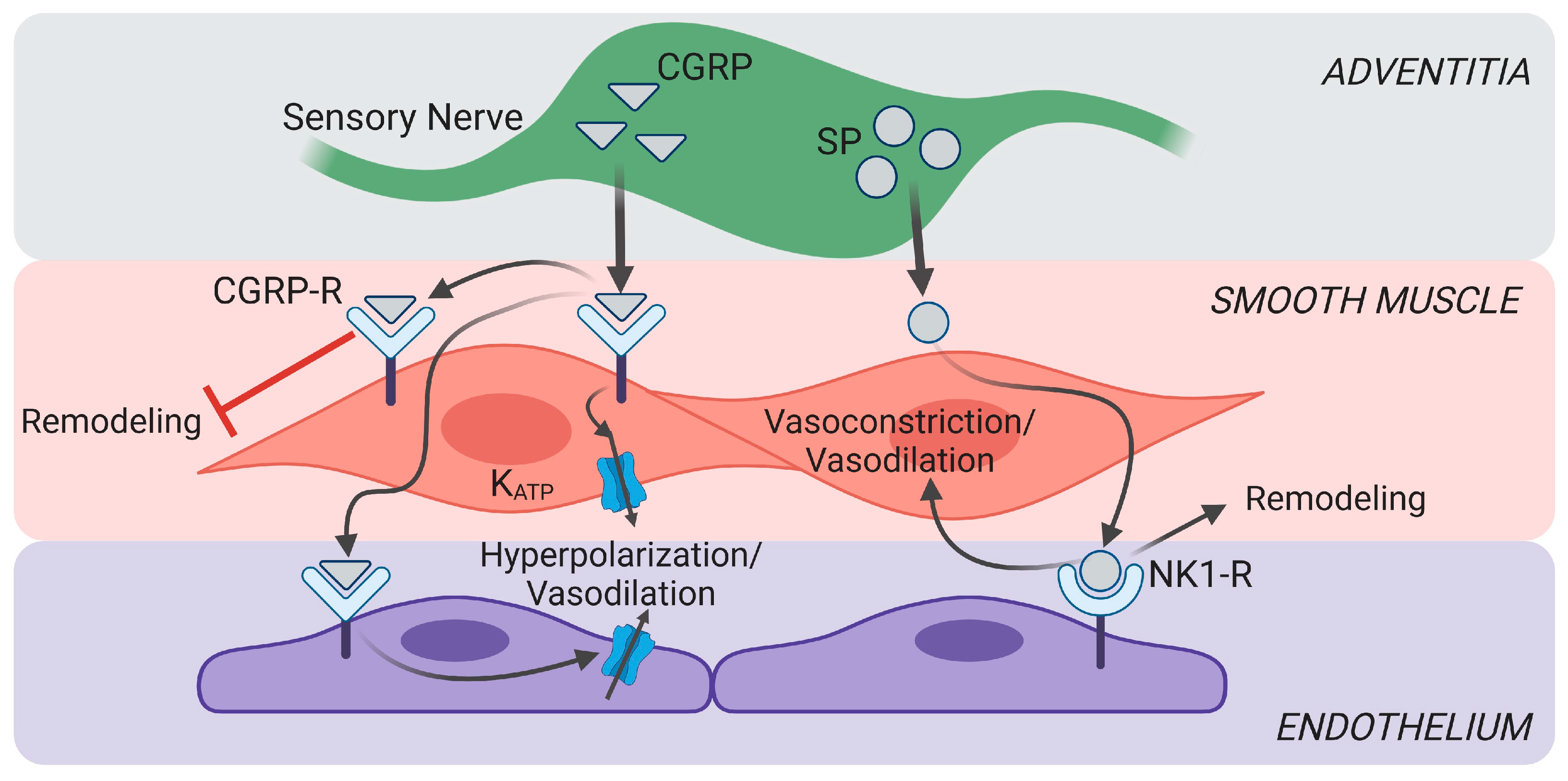
2.1. Calcitonin Gene-Related Peptide
2.2. Substance P
2.3. Neurokinin A
2.4. Interaction with Sympathetic Nerves
3. Sensory Nerves in Airway and Cough
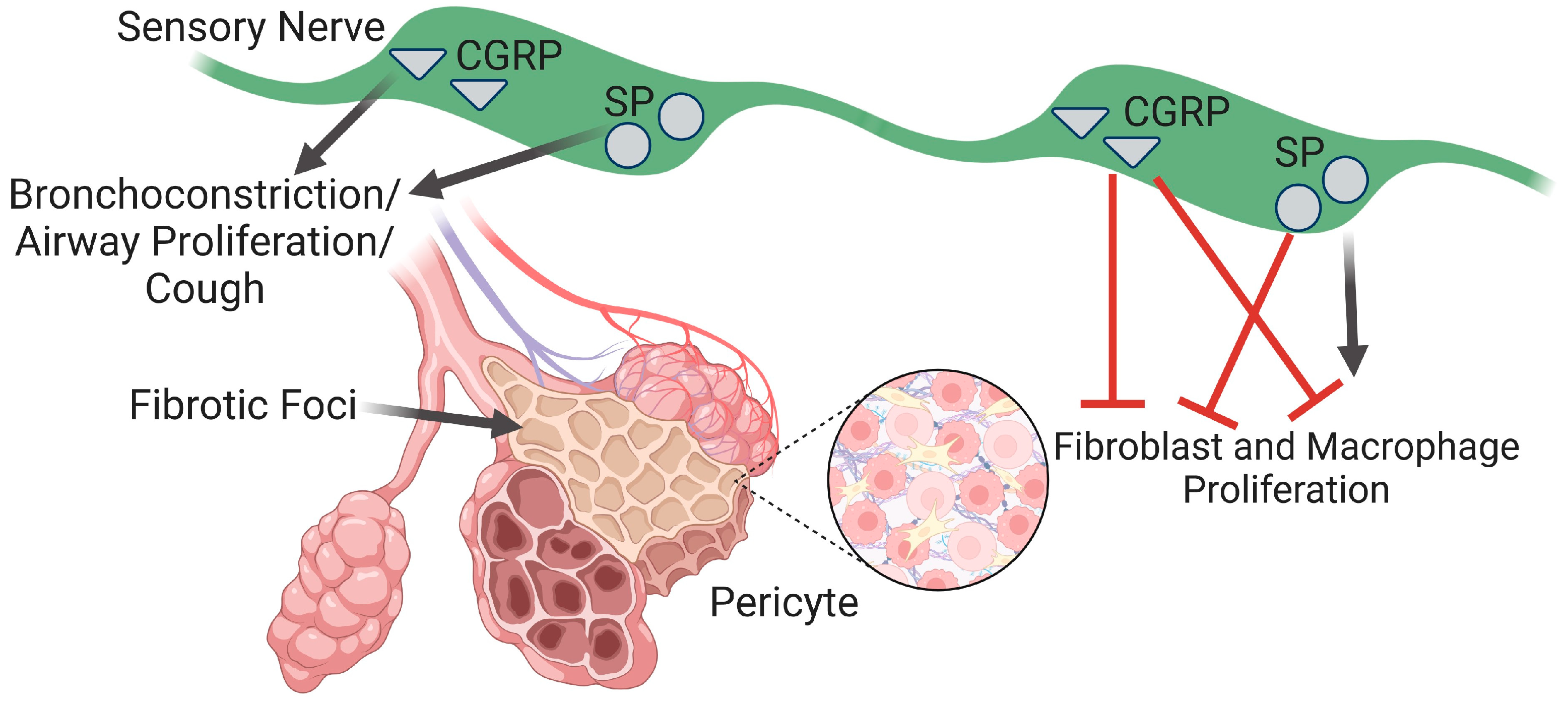
3.1. Sensory Nerves in Airway Structure and Function during PF
3.2. Pulmonary Fibrosis and Cough
4. Sensory Nerves in Vascular Dysfunction
4.1. Pulmonary Fibrosis and Vascular Remodeling
4.2. Changes in Vascular Tone in Pulmonary Hypertension and Pulmonary Fibrosis
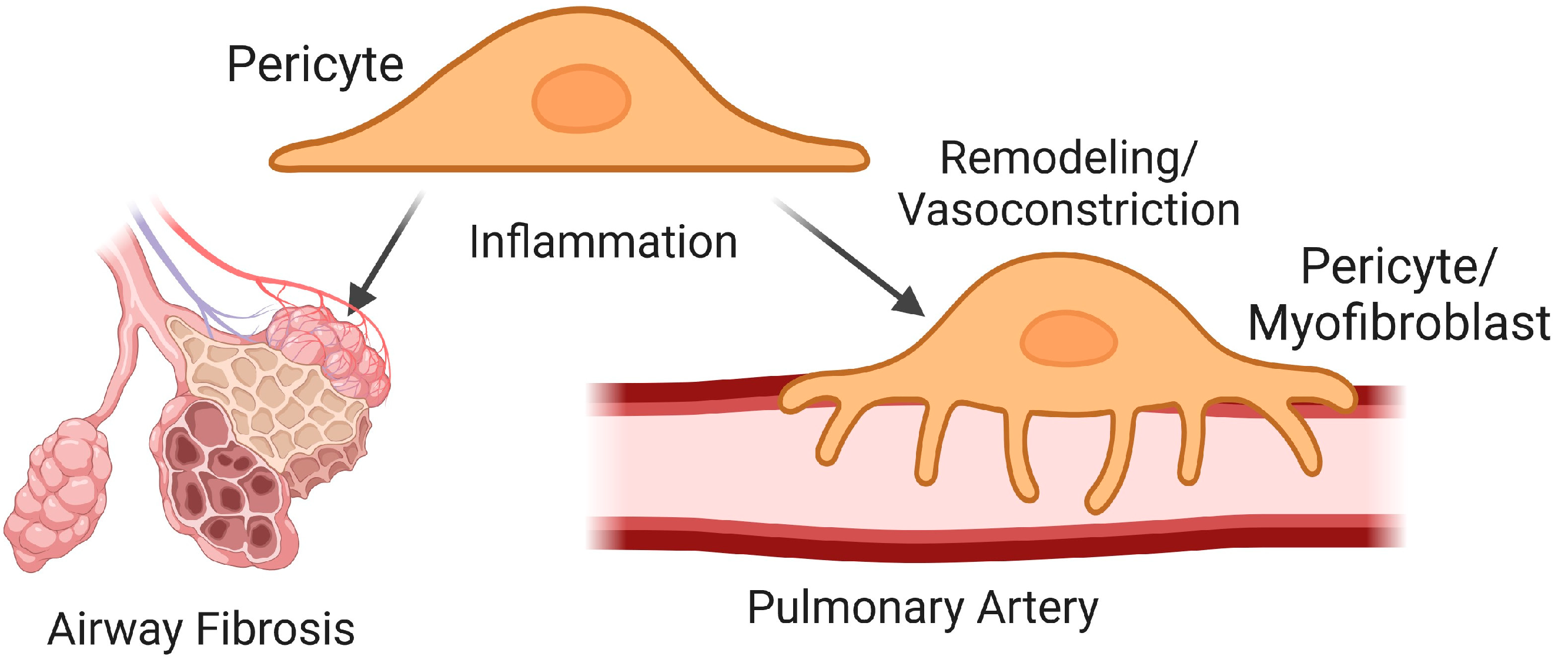
5. Vascular Pericytes in Pulmonary Fibrosis
5.1. Pericytes in Airway Fibrosis
5.2. Pericytes in Vascular Remodeling
5.3. Pericytes in Vasomotor Control
5.4. Sensory Nerve Modulation of Pericytes
6. Pulmonary Fibrosis and Fluid Clearance
6.1. Pulmonary Lymphatics
6.2. Lymphangiogenesis in PF
6.3. Lymphatic function in PF
6.4. Contribution of Lymphatic Cells to PF
6.5. Sensory Nerves and Lymphatic Function
7. Sleep Apnea and Pulmonary Fibrosis
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mann, J.; Goh, N.S.L.; Holland, A.E.; Khor, Y.H. Cough in Idiopathic Pulmonary Fibrosis. Front. Rehabil. Sci. 2021, 2, 751798. [Google Scholar] [CrossRef]
- van Manen, M.J.G.; Birring, S.S.; Vancheri, C.; Cottin, V.; Renzoni, E.A.; Russel, A.; Wijsenbeek, M.S. Cough in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2016, 25, 278–286. [Google Scholar] [CrossRef]
- Behr, J.; Ryu, J.H. Pulmonary hypertension in interstitial lung disease. Eur. Respir. J. 2008, 31, 1357–1367. [Google Scholar] [CrossRef]
- Marshall, D.C.; Salciccioli, J.D.; Shea, B.S.; Akuthota, P. Trends in mortality from idiopathic pulmonary fibrosis in the European Union: An observational study of the WHO mortality database from 2001–2013. Eur. Respir. J. 2018, 51, 1701603. [Google Scholar] [CrossRef]
- Ryu, J.H.; Colby, T.V.; Hartman, T.E. Idiopathic pulmonary fibrosis: Current concepts. Mayo Clin. Proc. 1998, 73, 1085–1101. [Google Scholar] [CrossRef]
- Mazzone, S.B.; Undem, B.J. Vagal afferent innervation of the airways in health and disease. Physiol. Rev. 2016, 96, 975–1024. [Google Scholar] [CrossRef] [PubMed]
- Yegen, C.H.; Marchant, D.; Bernaudin, J.F.; Planes, C.; Boncoeur, E.; Voituron, N. Chronic pulmonary fibrosis alters the functioning of the respiratory neural network. Front. Physiol. 2023, 14, 1205924. [Google Scholar] [CrossRef] [PubMed]
- Hartopo, A.B.; Emoto, N.; Vignon-Zellweger, N.; Suzuki, Y.; Yagi, K.; Nakayama, K.; Hirata, K. Endothelin-converting enzyme-1 gene ablation attenuates pulmonary fibrosis via CGRP-cAMP/EPAC1 pathway. Am. J. Respir. Cell Mol. Biol. 2013, 48, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Li, X.H.; Du, J.; Li, D.; Li, Y.J.; Hu, C.P. Calcitonin gene-related peptide down-regulates bleomycin-induced pulmonary fibrosis. Can. J. Physiol. Pharmacol. 2016, 94, 1315–1324. [Google Scholar] [CrossRef]
- El-Bermani, A.W.; Bloomquist, E.I.; Montvilo, J.A. Distribution of pulmonary cholinergic nerves in the rabbit. Thorax 1982, 37, 703–710. [Google Scholar] [CrossRef]
- Fisher, A.W.F. The intrinsic innervation of the pulmonary vessels. Acta Anat. 1965, 60, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Haberberger, R.; Schemann, R.; Sann, M.; Kummer, W. Innervation pattern of guinea pig pulmonary vasculature depends of vascular diameter. J. Appl. Physiol. 1997, 82, 426–434. [Google Scholar] [CrossRef]
- Cech, S. Cholinesterase-containing nerve fibres on blood vessels in lungs of some laboratory mammals. Z. Zellforsch. Mikrosk. Anat. 1973, 140, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Barr, J.; Jaquish, A.; Xu, J.; Verheyden, J.M.; Sun, X. Identification of lung innervating sensory neurons and their target specificity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 322, L50–L63. [Google Scholar] [CrossRef]
- Cadieux, A.; Springall, D.R.; Mulderry, P.K.; Rodrigo, J.; Ghatei, M.A.; Terenghi, G.; Bloom, S.R.; Polak, J.M. Occurrence, distribution and ontogeny of CGRP immunoreactivity in the rat lower respiratory tract: Effect of capsaicin treatment and surgical denervations. Neuroscience 1986, 19, 605–627. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Yamamoto, M.; Shimokata, K.; Nagura, H. Distribution of substance P-immunoreactive and calcitonin gene-related peptide-immunoreactive nerve in normal human lungs. Int. Arch. Allergy Immunol. 1991, 95, 23–28. [Google Scholar] [CrossRef]
- Kummer, W.A.; Fischer, A.; Kurkowski, R.; Heym, C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience 1992, 49, 715–737. [Google Scholar] [CrossRef]
- Martling, C.R.; Matran, R.; Alving, K.; Hökfelt; Lundberg, J.M. Innervation of lower airways and neuropeptide effects on bronchial and vascular tone in the pig. Cell Tissue Res. 1990, 260, 223–233. [Google Scholar] [CrossRef]
- Norton, C.E.; Segal, S.S. Calcitonin gene-related peptide hyperpolarizes mouse pulmonary artery endothelial tubes through KATP activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L212–L226. [Google Scholar] [CrossRef]
- Hobara, N.; Nakamura, A.; Ohtsuka, A.; Narasaki, M.; Shibata, K.; Bomoita, Y.; Kawasaki, H. Distribution of adrenomedullin-containing perivascular nerves in the rat mesenteric artery. Peptided 2004, 25, 589–599. [Google Scholar] [CrossRef]
- Westcott, E.B.; Segal, S.S. Perivascular innervation: A multiplicity of roles in vasomotor control and myoendothelial signaling. Microcirculation 2013, 20, 217–238. [Google Scholar] [CrossRef]
- Boerman, E.M.; Segal, S.S. Depressed perivascular sensory innervation of mouse mesenteric arteries with advanced age. J. Physiol. 2016, 594, 2323–2338. [Google Scholar] [CrossRef]
- Yabrak, M. The axon reflex. Neuroanatomy 2008, 7, 17–19. [Google Scholar]
- Undem, B.J.; Kollarik, M. The role of vagal afferent nerves in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2005, 2, 355–360. [Google Scholar] [CrossRef]
- Sant’Ambrogio, G.; Widdicombe, J.G. Reflexes from airway rapidly adapting receptors. Respir. Physiol. 2001, 125, 33–45. [Google Scholar] [CrossRef]
- Kummer, W.A. Pulmonary vascular innervation and its role in responses to hypoxia: Size matters! Proc. Am. Thorac. Soc. 2011, 8, 471–476. [Google Scholar] [CrossRef]
- Grasby, D.J.; Morris, J.L.; Segal, S.S. Heterogeneity of vascular innervation in hamster cheek pouch and retractor muscle. J. Vasc. Res. 1999, 36, 465–476. [Google Scholar] [CrossRef]
- Brain, S.D.; Grant, A.D. Vascular action of calcitonin gene-related peptide and adrenomedullin. Physiol. Rev. 2004, 84, 903–934. [Google Scholar] [CrossRef]
- Brain, S.D.; Williams, T.J.; Tippins, J.R.; Morris, H.R.; MacIntyre, I. Calcitonin gene-related peptide is a potent vasodilator. Nature 1985, 313, 54–56. [Google Scholar] [CrossRef]
- Kashihara, Y.; Sakaguchi, M.; Kuno, M. Axonal transport and distribution of endogenous calcitonin gene-related peptide in rat peripheral nerve. J. Neurosci. 1989, 9, 3796–3802. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; McDermott, B.J. Calcitonin gene-related peptide in the cardiovascular system: Characterizations of receptor populations and their (patho)physiological signaificance. Am. J. Physiol. Lung Cell. Mol. Physiol. 1996, 290, L661–L673. [Google Scholar]
- Dakhama, A.; Larsen, G.L.; Gelfand, E.W. Calcitonin gene-related peptide: Role in airway homeostasis. Curr. Opin. Pharmacol. 2004, 4, 215–220. [Google Scholar] [CrossRef]
- McLatchie, L.M.; Fraser, N.J.; Main, M.J.; Wise, A.; Brown, J.; Thompson, N.; Solari, R.; Lee, M.G.; Foord, S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 1998, 393, 333–339. [Google Scholar] [CrossRef]
- Nelson, M.T.; Huang, Y.; Brayden, J.E.; Herscheler, J.; Standen, N.B. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature 1990, 344, 770–773. [Google Scholar] [CrossRef]
- Wellman, G.C.; Quayle, J.M.; Standen, N.B. ATP-sensitive K+ channel activation by calcitonin gene related peptide and protein kinase A in pig coronary arterial smooth muscle. J. Physiol. 1998, 507, 117–129. [Google Scholar] [CrossRef]
- Wisskirchen, F.M.; Burt, R.P.; Marshall, I. Pharmacological characterization of CGRP receptors mediating relaxation of the rat pulmonary artery and inhibition of twitch responses of the rat vas deferens. Br. J. Pharmacol. 1998, 123, 1673–1683. [Google Scholar] [CrossRef]
- Kawasaki, H.; Takasaki, S.; Saito, A.; Goto, K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature 1988, 335, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Holton, P. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J. Physiol. 1959, 145, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 2007, 87, 659–797. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.J.; Sayers, I.; Kuokkanen, K.; Hall, I.P. Purinergic receptors in the airways: Potential therapeutic targets for asthma? Front. Allergy 2021, 2, 677677. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic regulation of vascular tone and remodelling. Auton. Autacoid Pharmacol. 2009, 29, 63–72. [Google Scholar] [CrossRef]
- Le, T.T.T.; Berg, N.K.; Harting, M.T.; Li, X. Purinergic signaling in pulmonary inflammation. Front. Immunol. 2019, 10, 1633. [Google Scholar] [CrossRef]
- Kennedy, C.; Saville, V.L.; Burnstock, G. The contributions of noradrenaline and ATP to the responses of the rabbit central ear artery to sympathetic nerve stimulation depend on the parameters of stimulation. Eur. J. Pharmacol. 1986, 122, 291–300. [Google Scholar] [CrossRef]
- Lohman, A.W.; Billaud, M.; Isakson, B.E. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc. Res. 2012, 95, 269–280. [Google Scholar] [CrossRef]
- White, J.D.; Stewart, K.D.; Krause, J.E.; McKelvy, J.F. Biochemistry of peptide-secreting neurons. Physiol. Rev. 1985, 65, 553–606. [Google Scholar] [CrossRef]
- Sponchiado, M.; Liao, Y.S.; Atanasova, K.R.; Collins, E.N.; Schurmann, V.; Bravo, L.; Reznikov, L.R. Overexpression of Substance P in pig airways increases MUC5AC through an NF-kβ pathway. Physiol. Rep. 2021, 9, e14749. [Google Scholar] [CrossRef]
- Norton, C.E.; Boerman, E.M.; Segal, S.S. Differential hyperpolarization to substance P and calcitonin gene related peptide in smooth muscle versus endothelium of mouse mesenteric artery. Microcirculation 2021, 28, e12733. [Google Scholar] [CrossRef] [PubMed]
- Brain, S.D.; Cox, H.M. Neuropeptides and their receptors: Innovative science providing novel therapeutic targets. Br. J. Pharmacol. 2006, 147, S202–S211. [Google Scholar] [CrossRef] [PubMed]
- Brain, S.D. Sensory neuropeptides: Their role in inflammation and wound healing. Immunopharmacology 1997, 37, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Duckles, S.P. Effect of endothelium on the actions of sympathetic and sensory nerves in the perfused rat mesentery. Eur. J. Pharmacol. 1992, 210, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.K.; Hickey, H.; Hill, C.E. Heterogeneity in mechanisms underlying vasodilatory responses in small arteries of the rat hepatic mesentery. Auton. Neurosci. 2000, 83, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L.; Kulik, T.J.; Chesler, E.; Weir, E.K. The effects of substance P on the preconstricted pulmonary vasculature of the anesthetized dog. Proc. Soc. Exp. Biol. Med. 1986, 183, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Regoli, D.; Drapeau, G.; Dorleans-Just, P. Pharmacological receptors for substance P and neurokinins. Life Sci. 1987, 40, 109–117. [Google Scholar] [CrossRef]
- Sung, J.; Ramnath, D.; Tamizhselvi, R.; Bhatia, M. Neurokinin A engages neurokinin-1 receptor to induce NF-κB-dependent gene expression in murine macrophages: Implications of ERK1/2 and PI 3-kinase/Akt pathways. Am. J. Physiol. Cell Physiol. 2008, 295, C679–C691. [Google Scholar]
- Hernandez, J.M.; Cox, G.; Janssen, L.J. Involvement of the neurokinin-2 receptor in airway smooth muscle stretch-activated contractions assessed in perfused intact bovine bronchial segments. J. Pharmacol. Exp. Ther. 2008, 327, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Dion, S.; Rouissi, N.; Nantel, F.; Jukic, D.; Rhaleb, N.E.; Tousignant, C.; Telemaque, S.; Drapeau, G.; Regoli, D.; Naline, E.; et al. Structure-activity study of neurokinins: Antagonists for the neurokinin-2 receptor. Pharmacology 1990, 41, 184–194. [Google Scholar] [CrossRef]
- Gallicchio, M.; Rosa, A.C.; Benetti, E.; Collino, M.; Dianzani, C.; Fantozzi, R. Substance P-induced cyclooxygenase-2 expression in human umbilical vein endothelial cells. Br. J. Pharmacol. 2006, 147, 681–689. [Google Scholar] [CrossRef]
- Haley, K.J.; Sunday, M.E.; Osathanondh, R.; Du, J.; Vathanaprida, C.; Karpitsky, V.V.; Krause, J.E.; Lilly, C.M. Developmental expression of neurokinin A and functional neurokinin-2 receptors in lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L1348–L1358. [Google Scholar] [CrossRef]
- Couture, R.; Laneuville, O.; Guimond, C.; Drapeau, G.; Regoli, D. Characterization of the peripheral action of neurokinins and neurokinin receptor selective agonists on the rat cardiovascular system. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1989, 340, 547–557. [Google Scholar] [CrossRef]
- Kotecha, N.; Neild, T.O. Actions of vasodilator nerves on arteriolar smooth muscle and neurotransmitter release from sympathetic nerves in the guinea-pig small intestine. J. Physiol. 1995, 489, 849–855. [Google Scholar] [CrossRef]
- Kawasaki, H. Regulation of vascular function by perivascular calcitonin gene-related peptide-containing nerves. Jpn. J. Pharmacol. 2002, 88, 39–43. [Google Scholar] [CrossRef]
- Kawasaki, H.; Nuki, C.; Saito, A.; Takasaki, K. Adrenergic modulation of calcitonin gene-related peptide (CGRP)-containing nerve-mediated vasodilation in the rat mesenteric resistance vessel. Brain Res. 1990, 506, 287–290. [Google Scholar] [CrossRef]
- Donoso, M.V.; Hermosilla, D.; Navarrete, C.; Alzvarez, P.; Lillo, J.G.; Huidobro-Toro, J.P. Reciprocal sympatho-sensory control: Functional role of nucleotides and calcitonin gene-related peptide in a peripheral neuroeffector junction. Neuroscience 2012, 203, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Leslie, K.O. Idiopathic fibrosis may be a disease of recurrent, tractional injury to the periphery of the aging lung: A unifying hypothesis regarding etiology and pathogenesis. Arch. Pathol. Lab. Med. 2012, 136, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Shichino, S.; Ueha, S.; Matsushima, K. Macrophages in lung fibrosis. Int. Immunol. 2021, 33, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Verleden, S.E.; Tanabe, N.; McDonough, J.E.; Vasilescu, D.M.; Xu, F.; Wuyts, W.A.; Piloni, D.; De Sadeleer, L.; Willems, S.; Mai, C.; et al. Small airways pathology in idiopathic pulmonary fibrosis: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Narula, M.; McGovern, A.E.; Yang, S.; Farrell, M.J.; Mazzone, S.B. Afferent neural pathways mediating cough in animals and humans. J. Thorac. Dis. 2014, 6, S712–S719. [Google Scholar] [PubMed]
- Ochoa-Callejero, L.; Garcia-Sanmarin, J.; Villoslada-Blanco, P.; Iniguez, M.; Perez-Matute, P.; Pujada, E.; Fowkes, M.E.; Brody, R.; Oteo, J.A.; Martinez, A. Circulating levels of calcitonin gene-related peptide are lower in COVID-19 patients. J. Endoc Soc. 2021, 5, bvaa199. [Google Scholar] [CrossRef] [PubMed]
- Undem, B.J.; Nassenstein, C. Airway nerves and dyspnea associated with inflammatory airway disease. Respir. Physiol. Neurobiol. 2009, 30, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, M.; Sun, G.Y.; Liu, Y.P.; Ran, W.Z.; Peng, L.; Guan, C.X. Calcitonin gene-related peptide promotes the wound healing of human bronchial epithelial cells via PKC and MAPK pathways. Regul. Pept. 2013, 184, 22–29. [Google Scholar] [CrossRef]
- Kawanami, Y.; Morimoto, Y.; Kim, H.; Nakamura, T.; Machida, K.; Kido, T.; Asonuma, E.; Yatera, K.; Yoshii, C.; Kido, M. Calcitonin gene-related peptide stimulates proliferation of alveolar epithelial cells. Respir. Res. 2009, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Sun, M.; Shang, Y.; Zhang, C.; Jiao, X. Neurokinin 1 receptor promotes rat airway smooth muscle cell migration in asthmatic airway remodelling by enhancing tubulin expression. J. Thorac. Dis. 2018, 10, 4849–4857. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Funayama, H.; Mizuta, K.; Emala, C.; Gallos, G. Anti-proliferative effects of the neurokinin 1 receptor in human airway smooth muscle cells. Am. J. Respir. Crit. Care Med. 2013, 187, A1993. [Google Scholar]
- Widdicombe, J.G. Overview of neural pathways in allergy and asthma. Pulm. Pharmacol. Ther. 2003, 16, 23–30. [Google Scholar] [CrossRef]
- Herrera, J.A.; Dingle, L.A.; Angeles Monetero, M.; Venkateswaran, R.V.; Blaikley, J.F.; Branato, F.; Pearson, S.; Lawless, C.; Thronton, D.J. Morphologically intact airways in lung fibrosis have an abnormal proteome. Respir. Res. 2023, 24, 99. [Google Scholar] [CrossRef]
- Cadieux, A.; Monast, N.P.; Pomerleau, F.; Fournier, A.; Lanoue, C. Bronchoprotector properties of calcitonin gene-related peptide in guinea pig and human airways. Am. J. Respir. Crit. Care Med. 1999, 159, 235–243. [Google Scholar] [CrossRef]
- Tanaka, D.T.; Grunstein, M.M. Mechanisms of substance P-induced contraction of rabbit airway smooth muscle. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 1551–1557. [Google Scholar] [CrossRef]
- Guan, M.; Ying, S.; Wang, Y. Increased expression of transient receptor potential channels and neurogenic factors associates with cough severity in a guinea pig model. BMC Pulm. Med. 2021, 21, 187. [Google Scholar] [CrossRef] [PubMed]
- Ojiaku, C.A.; Yoo, E.J.; Panettieri, R.A. Transforming growth factor β1 function in airway remodeling and hyperresponsiveness. The missing link? Am. J. Respir. Cell Mol. Biol. 2017, 56, 432–442. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef]
- Shochet, G.E.; Brook, E.; Barndenstein-Wald, B.; Shitrit, D. TGF-β pathway activation by idiopathic pulmonary fibrosis (IPF) fibroblast derived soluble factors is mediated by IL-6 trans-signaling. Respir. Res. 2020, 21, 56. [Google Scholar] [CrossRef]
- Jia, M.; Rosas, L.; Kapetanaki, M.G.; Tabib, T.; Sebrat, J.; Cruz, T.; Bondonese, A.; Mora, A.L.; Lafyatis, R.; Rojas, M.; et al. Early events marking lung fibroblast transition to profibrotic state in idiopathic pulmonary fibrosis. Respir. Res. 2023, 24, 116. [Google Scholar] [CrossRef] [PubMed]
- Yule, K.A.; White, S.R. Migration of 3T3 and lung fibroblasts in response to calcitonin gene-related peptide and bombesin. Exp. Lung Res. 1999, 25, 261–273. [Google Scholar]
- Ramos, C.; Montano, M.; Cisneros, J.; Sommer, B.; Delgado, J.; Gonzalez-Avila, G. Substance P up-regulates matrix metalloproteinase-1 and down-regulates collagen in human lung fibroblast. Exp. Lung Res. 2007, 33, 151–167. [Google Scholar] [CrossRef]
- Gu, Y.; Lawrence, T.; Mohamed, R.; Liang, Y.; Yahaya, B.H. The emerging roles of interstitial macrophages in pulmonary fibrosis: A perspective from scRNA-seq analyses. Front. Immunol. 2022, 13, 923235. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.X.; Zhou, Y.; Zhou, A.Y.; Guan, X.X.; Liu, T.; Yang, H.H.; Xie, H.; Chen, P. Calcitonin gene-related peptide exerts anti-inflammatory property through regulating murine macrophages polarization in vitro. Mol. Immunol. 2017, 91, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Yu, D.; Qin, X.; Qian, Y.; Ma, J.; Li, W.; Liu, C.; Wang, C.; Zhang, Y.; Li, Y.; et al. The neuropeptide CGRP enters the macrophage cytosol to suppress the NLRP3 inflammasome during pulmonary infection. Cell. Mol. Immunol. 2023, 20, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Yang, L.; Zheng, Z.; Li, Y.; Ren, W.; Wu, C.; Guo, L. Calcitonin gene-related peptide induces IL-6 expression in RAW264.7 macrophages mediated by mmu_circRNA_007893. Mol. Med. Rep. 2017, 16, 93367–99374. [Google Scholar] [CrossRef]
- Joos, G.F.; Germonpre, P.R.; Kips, J.C.; Peleman, R.A.; Pauwels, R.A. Sensory neuropeptides and the human lower airways: Present state and future directions. Eur. Respir. J. 1994, 7, 1161–1171. [Google Scholar] [CrossRef]
- Shen, W.; Wang, X.; Xiang, H.; Shichi, S.; Nakamoto, H.; Kimura, S.; Sugiyama, K.; Taketomi, A.; Kitamura, H. IFN-γ–STAT1-mediated NK2R expression is involved in the induction of antitumor effector CD8+ T cells in vivo. Cancer Sci. 2023, 114, 1816–1829. [Google Scholar] [CrossRef]
- Zhao, X.N.; Bai, Z.Z.; Li, C.H.; Sheng, C.L.; Li, H.Y. The NK-1R antagonist Aprepitant prevents LPS-induced oxidative stress and inflammation in RAW264.7 macrophages. Drug Des. Dev. Ther. 2020, 14, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Network, I.P.F.C.R.; Raghu, G.; Anstrom, K.J.; King, T.E.; Lasky, J.A.; Martinez, F.J. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N. Engl. J. Med. 2012, 366, 1968–1977. [Google Scholar]
- Polverino, M.; Polverino, F.; Fasolino, M.; Ando, F.; Alfieri, A.; De Blasio, F. Anatomy and neuro-pathophysiology of the cough reflex arc. Multidiscip. Respir. Med. 2012, 7, 5. [Google Scholar] [CrossRef]
- Belvisi, M.G.; Birrell, M.A. The emerging role of transient receptor potential channels in chronic lung disease. Eur. Respir. J. 2017, 50, 1601357. [Google Scholar] [CrossRef] [PubMed]
- Young, E.C.; Smith, J.A. Pharmacologic therapy for cough. Curr. Opin. Pharmacol. 2011, 11, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Ryerson, C.J.; Abbritti, M.; Ley, B.; Elicker, B.M.; Jones, K.D.; Collard, H.R. Cough predicts prognosis in idiopathic pulmonary fibrosis. Respirology 2011, 16, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Hope-Gill, B.D.M.; Hilldrup, S.; Davies, C.; Newton, R.P.; Harrison, N.K. A study of the cough reflex in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 995–1002. [Google Scholar] [CrossRef]
- Harrison, N.K. Idiopathic pulmonary fibrosis: A nervous cough? Pulm. Pharmacol. Ther. 2004, 17, 347–350. [Google Scholar] [CrossRef]
- Jones, R.M.; Hilldrup, S.; Hope-Gill, B.D.M.; Eccles, R.; Harrison, N.K. Mechanical induction of cough in idiopathic pulmary fibrosis. Cough 2011, 7, 2. [Google Scholar] [CrossRef]
- Ricci, A.; Graziano, P.; Bronzetti, E.; Saltini, C.; Sciancchitano, S.; Cherubini, E.; Renzoni, E.; Du Bois, R.M.; Grutters, J.C.; Mariotta, S. Increased pulmonary neurotrophin protein expression in idiopathic interstitial pheumonias. Sarcoidosis Vasc. Diffus. Lung Dis. 2007, 24, 13–23. [Google Scholar]
- Wakwaya, Y.; Ramdurai, D.; Swigris, J.J. Managing cough in idiopathic pulmonary fibrosis. Chest 2021, 160, 1774–1782. [Google Scholar] [CrossRef]
- George, P.M.; Mitchell, J.A. Defining a pathological role for the vasculature in the development of fibrosis and pulmonary hypertension in interstitial lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L431–L433. [Google Scholar] [CrossRef]
- Ryu, J.H.; Krowka, M.J.; Pellikka, P.A.; Swanson, K.L.; McGoon, M.D. Pulmonary hypertension in patients with interstitial lung diseases. Mayo Clin. Proc. 2007, 82, 342–350. [Google Scholar] [CrossRef]
- Strange, C.; Highland, K.B. Pulmonary hypertension in interstitial lung disease. Curr. Opin. Pulm. Med. 2005, 11, 452–455. [Google Scholar] [CrossRef]
- Lettieri, C.J.; Nathan, S.D.; Barnett, S.D.; Ahmed, S.; Shorr, A.F. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest 2018, 129, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Nadrous, H.F.; Pellikka, P.A.; Krowka, M.J.; Swanson, K.L.; Chaowalit, N.; Decker, P.A.; Ryu, J.H. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest 2005, 128, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Luff, S.E. Ultrastructure of sympathetic axons and their structural relationship with vascular smooth muscle. Anat. Embryol. 1996, 193, 515–531. [Google Scholar] [CrossRef]
- Tuder, R.M. Pulmonary vascular remodeling in pulmonary hypertension. Cell Tissue Res. 2016, 367, 643–649. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, S.; Yan, H.; Cao, Y.; Zhang, X.; Wang, L.; Zhang, Z.; Lin, S.; Wang, X.; Mao, J. Pulmonary vascular remodeling in pulmonary hypertension. J. Pers. Med. 2023, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, T.; Kato, S.; Ogura, T.; Kusakawa, Y.; Iso, S.; Baba, T.; Fukui, K.; Oba, M.S. Low-normal lung volume correlates with pulmonary hypertension in fibrotic idiopathic interstitial pneumonia: Computer-aided 3D quantitative analysis of chest CT. AJR Am. J. Roentgenol. 2014, 203, W166–W173. [Google Scholar] [CrossRef]
- Shekerdemian, L.; Bohn, D. Cardiovascular effects of mechanical ventilation. Arch. Dis. Child. 1999, 80, 475–480. [Google Scholar] [CrossRef]
- Sakao, S.; Tanabe, N.; Tatsumi, K. Hypoxic pulmonary vasoconstriction and the diffusing capacity in pulmonary hypertension secondary to idiopathic pulmonary fibrosis. J. Am. Heart Assoc. 2019, 8, e013310. [Google Scholar] [CrossRef]
- Turner-Warwick, M. Precapillary systemic-pulmonary anastomoses. Thorax 1963, 18, 225–237. [Google Scholar] [CrossRef]
- Keane, M.P.; Arenberg, D.A.; Lynch, J.P.; Whyte, R.I.; Iannettoni, M.D.; Burdick, M.D.; Wilke, C.A.; Morris, S.B.; Glass, M.C.; DiGiovine, B.; et al. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J. Immunol. 1997, 159, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Golden, A.; Bronk, T.T. Diffuse interstitial fibrosis of lungs. Arch. Intern. Med. 1953, 92, 606–614. [Google Scholar] [CrossRef]
- Coalson, J.J. The ultrastructure of human fibrosing alveolitis. Virchows Arch. 1982, 395, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Gracey, D.R.; Divertie, M.B.; Brown, A.L. Alveolar-capillary membrane in idiopathic interstitial pulmonary fibrosis. Am. Rev. Respir. Dis. 1968, 98, 16–21. [Google Scholar] [PubMed]
- Ebina, M.; Shimizukawa, M.; Shibata, N.; Kimura, Y.; Suzuki, T.; Endo, M.; Sasano, H.; Kondo, T.; Nukiwa, T. Heterogeneous increase in CD34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2004, 169, 1203–1208. [Google Scholar] [CrossRef]
- Renzoni, E.A.; Walsh, D.A.; Salmon, M.; Wells, E.A.; Sestini, P.; Nicholson, A.G.; Veeraraghyayan, S.; Bishop, A.E.; Romanska, H.M.; Pantelidids, P.; et al. Interstitial vascularity in fibrosing alveolitis. Am. J. Respir. Crit. Care Med. 2003, 167, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Burdick, M.D.; Murray, L.A.; Keane, M.P.; Xue, Y.Y.; Zisman, D.A.; Belperio, J.A.; Strieter, R.M. CXCL11 attenuates bleomycin-induced pulmonary fibrosis via inhibition of vascular remodeling. Am. J. Respir. Crit. Care Med. 2004, 171, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Stockman, C.; Kerdiles, Y.; Nomaksteinsky, M.; Weidemann, A.; Takeda, N.; Doedens, A.; Torres-Collado, A.X.; Iruela-Arispe, L.; Nizet, V.; Johnson, R.S. Loss of myeloid cell-derived vascular endothelial growth factor accelerates fibrosis. Proc. Natl. Acad. Sci. USA 2010, 107, 4329–4334. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Fu, C.; Lu, Y.; Williams, M.A.; Pi, L.; Brantly, M.L.; Ventetuolo, C.E.; Raizada, M.K.; Mehrad, B.; Scott, E.W.; et al. Chemokine signaling axis between endothelial and myeloid cells regulates development of pulmonary hypertension associated with pulmonary fibrosis and hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L434–L444. [Google Scholar] [CrossRef]
- Suga, M.; Iyonaga, K.; Okamoto, T.; Gushima, Y.; Miyakawa, H.; Akaike, T.; Ando, M. Characteristic elevation of matrix metalloproteinase activity inidiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2000, 162, 1949–1956. [Google Scholar] [CrossRef]
- Champion, H.C.; Bivalacqua, T.J.; Toyoda, K.; Heistad, D.D.; Hyman, A.L.; Kadowitz, P.J. In vivo gene transfer of prepro-calcitonin gene-related peptide to the lung attenuates chronic hypoxia-induced pulmonary hypertension in the mouse. Circulation 2000, 101, 923–930. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Z.; Wang, Z.; Yang, C.; Lui, J.; Lu, J. Effect of prepro-calcitonin gene related peptide-expressing endothelial progenitor cells on pulmonary hypertension. Ann. Thorac. Surg. 2007, 84, 544–552. [Google Scholar] [CrossRef]
- Chen, K.H.; Lai, Y.L.; Chen, M.J. Oxygen radicals and substance P in perinatal hypoxia-exaggerated, monocrotaline-induced pulmonary hypertension. Chin. J. Physiol. 2012, 55, 82–90. [Google Scholar]
- Chen, L.W.; Chen, C.F.; Lai, Y.L. Chronic activation of neurokinin-1 receptor induces pulmonary hypertension in rats. Am. J. Physiol. 1999, 276, H1543–H1551. [Google Scholar] [CrossRef]
- Springer, J.; Fisher, A. Substance P-induced pulmonary vascular remodelling in precision cut lung slices. Eur. Respir. J. 2003, 22, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Tjen-a-looi, S.; Ekman, R.; Lippton, H.; Cary, J.; Keith, I. CGRP and somatostatin modulate chronic hypoxic pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 1992, 263, H681–H690. [Google Scholar] [CrossRef] [PubMed]
- Bartosik, I.; Eskilsson, J.; Ekman, R.; Akesson, A.; Scheja, A. Correlation between plasma concentrations of calcitonin gene related peptide and pulmonary pressure in patients with systemic schlerosis. Ann. Rheum. Dis. 2002, 61, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Maggi, C.A.; Giachetti, A.; Rey, R.D.; Said, S.I. Neuropeptides as regulators of airway function: Vasoactive intestinal peptide and the tachykinins. Physiol. Rev. 1995, 75, 277–322. [Google Scholar] [CrossRef]
- Mannan, M.M.; Springall, D.R.; Denard, C.; Moradoghli-Haftvani, A.; Eddahibi, S.; Adnot, S.; Polak, J.M. Decreased endothelium-dependent pulmonary vasodilator effect of calcitonin gene-related peptide in hypoxic rats contrasts with increased binding sites. Eur. Respir. J. 1995, 8, 2029–2037. [Google Scholar] [CrossRef]
- Lo, C.C.W.; Moosavi, S.M.; Bubb, K.J. The regulation of pulmonary vascular tone by neuropeptides and the implications for pulmonary hypertension. Front. Physiol. 2018, 9, 1167. [Google Scholar] [CrossRef] [PubMed]
- Norton, C.E.; Grunz-Borgmann, E.A.; Hart, M.L.; Jones, B.W.; Franklin, C.L.; Boerman, E.M. Role of perivascular nerve and sensory neurotransmitter dysfunction in inflammatory bowel disease. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1887–H1902. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, K.R.; Deng, Y.; Yang, A.; Cober, N.D.; Stewart, D.J. Penetrance of Severe Pulmonary Arterial Hypertension in Response to Vascular Endothelial Growth Factor Receptor 2 Blockade in a Genetically Prone Rat Model Is Reduced by Female Sex. J. Am. Heart Assoc. 2021, 10, e019488. [Google Scholar] [CrossRef] [PubMed]
- Avona, A.; Burgos-Vera, C.; Burton, M.D.; Akopian, A.N.; Price, T.J.; Dussor, G. Dural calcitonin gene-related peptide produces female-specific responses in rodent migraine models. J. Neurosci. 2019, 39, 4323–4331. [Google Scholar] [CrossRef] [PubMed]
- Shammout, B.; Johnson, J.R. Pericytes in Chronic Lung Disease. Adv. Exp. Med. Biol. 2019, 1147, 299–317. [Google Scholar] [PubMed]
- Hung, C.F.; Wilson, C.L.; Schnapp, L.M. Pericyte Biology in Different Organs; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Zhang, Z.; Zhou, H.N.; He, S.S.; Xue, M.Y.; Li, T.; Liu, L.M. Research advances in pericyte function and their roles in diseases. Chin. J. Traumatol. 2020, 23, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Garrison, A.T.; Bignold, R.E.; Wu, X.; Johnson, J.R. Pericytes: The lung-forgotten cell type. Front. Physiol. 2023, 14, 1150028. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Q. Gross pathology and histopathology of asthma. J. Allergy Clin. Immunol. 2003, 111, 431–432. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.J.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Crnkovic, S.; Valzano, F.; Flieber, E.; Gindlhuber, J.; Thekkekara Puthenparampil, H.; Basil, M.; Morley, M.P.; Katzen, J.; Gschwandtner, E.; Klepetko, W.; et al. Single-cell transcriptomics reveals skewed cellular communication and phenotypic shift in pulmonary artery remodeling. JCI Insight 2022, 7, e153471. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Liu, Y.; Zhang, Y.; Nathan, A.; Tian, W.; Yu, J.; Sweatt, A.J.; Shamshou, E.A.; Condon, D.; Chakraborty, A.; et al. Mural Cell SDF1 Signaling Is Associated with the Pathogenesis of Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol. 2020, 62, 474–759. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Hirai, S.; Tanaka, Y.; Sumi, T.; Tada, M.; Takahashi, H.; Watanabe, A.; Sakuma, Y. Pericyte-myofibroblast transition in the human lung. Biochem. Biophys. Res. Commun. 2020, 528, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Savai, R.; Pullamsetti, S.; Kolbe, J.; Bieniek, E.; Fink, L.; Scheed, A.; Ritter, C.; Dahal, B.K.; Vater, A.; Klussmannn, S.; et al. Immune/inflammatory cell involvement in the pathology of idiopathic pulmonary arterieal hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Folestad, E.; Rowley, J.E.; Noll, E.M.; Walker, S.A.; Lloyd, C.M.; Rankin, S.M.; Pietras, K.; Eriksson, U.; Fuxe, J. Pericytes contribute to airway remodeling in a mouse model of chronic allergic asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L658–L671. [Google Scholar] [CrossRef] [PubMed]
- Bignold, R.E.; Shammout, B.; Rowley, J.E.; Repici, M.; Simms, J.; Johnson, J.R. Chemokine CXCL12 drives pericyte accumulation and airway remodeling in allergic airway disease. Respir. Res. 2022, 23, 183. [Google Scholar] [CrossRef] [PubMed]
- Butsabong, T.; Felippe, M.; Campagnolo, P.; Maringer, K. The emerging role of perivascular cells (pericytes) in viral pathogenesis. J. Gen. Virol. 2021, 102, 001634. [Google Scholar] [CrossRef] [PubMed]
- Ricard, N.; Tu, L.; Le Hiress, L.; Huerta, A.; Phan, C.; Thuillet, R.; Sattler, C.; Fadel, E.; Seferian, A.; Montani, D.; et al. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation 2014, 129, 1586–1597. [Google Scholar] [CrossRef]
- Bordenave, J.; Thuillet, R.; Tu, L.; Phan, C.; Cumont, A.; Marsol, C.; Huertas, A.; Savale, L.; Hibert, M.; Galzi, J.L.; et al. Neutralization of CXCL12 attenuates established pulmonary hypertension in rats. Cardiovasc. Res. 2020, 116, 686–697. [Google Scholar] [CrossRef]
- Hung, C.; Linn, G.; Chow, Y.H.; Kobayashi, A.; Mittelsteadt, K.; Altemeier, W.A.; Sgharib, S.A.; Schnapp, L.M.; Duffield, J.S. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2013, 188, 820–830. [Google Scholar] [CrossRef]
- Tannenberg, P.; Chang, Y.T.; Muhl, L.; Lavina, B.; Gladh, H.; Genove, G.; Betsholtz, C.; Folestad, E.; Tran-Lundmark, K. Extracellular retention of PDGF-B directs vascular remodeling in mouse hypoxia-induced pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L593–L605. [Google Scholar] [CrossRef] [PubMed]
- Chetty, A.; Nielsen, H.C. Targeting airway smooth muscle hypertrophy in asthma: An approach whose time has come. J. Asthma Allergy 2021, 14, 539–556. [Google Scholar] [CrossRef]
- Hamilton, N.B.; Attwell, D.; Hall, C.N. Pericyte-mediated regulation of capillary diameter: A component of neurovascular coupling in health and disease. Front. Neuroenergetics 2010, 2, 5. [Google Scholar] [CrossRef]
- Gong, C.X.; Zhang, Q.; Xiong, X.Y.; Yuan, J.J.; Yang, G.Q.; Huang, J.C.; Liu, J.; Duan, C.M.; Xu, R.; Qui, Z.M.; et al. Pericytes Regulate Cerebral Perfusion through VEGFR1 in Ischemic Stroke. Cell. Mol. Neurobiol. 2022, 42, 1897–1908. [Google Scholar] [CrossRef]
- Meng, Y.M.; Jiang, X.; Zhao, X.; Meng, Q.; Wu, S.; Chen, Y.; Kong, X.; Qiu, X.; Su, L.; Huang, C.; et al. Hexokinase 2-driven glycolysis in pericytes activates their contractility leading to tumor blood vessel abnormalities. Nat. Commun. 2021, 12, 6011. [Google Scholar] [CrossRef] [PubMed]
- Friebe, A.; Englert, N. NO-sensitive guanylyl cyclase in the lung. Br. J. Pharmacol. 2022, 179, 2328–2343. [Google Scholar] [CrossRef] [PubMed]
- Aue, A.; Englert, N.; Harrer, L.; Schwiering, F.; Gaab, A.; Konig, P.; Adams, R.; Schmitko, A.; Friebe, A. NO-sensitive guanylyl cyclase discriminates pericyte-derived interstitial from intra-alveolar myofibroblasts in murine pulmonary fibrosis. Respir. Res. 2023, 24, 167. [Google Scholar] [CrossRef]
- Brown, L.S.; Foster, C.G.; Courtney, J.M.; King, N.E.; Howells, D.W.; Sutherland, B.A. Pericytes and Neurovascular Function in the Healthy and Diseased Brain. Front. Cell. Neurosci. 2019, 13, 282. [Google Scholar] [CrossRef]
- Shimizu, F.; Sano, Y.; Saito, K.; Abe, M.A.; Maeda, T.; Haruki, H.; Kanda, T. Pericyte-derived glial cell line-derived neurotrophic factor increase the expression of claudin-5 in the blood-brain barrier and the blood-nerve barrier. Neurochem. Res. 2012, 37, 401–409. [Google Scholar] [CrossRef]
- Hong, H.S.; Kim, S.; Jin, Y.; Son, Y. Substance P enhances the therapeutic effect of MSCs by modulating their angiogenic potential. J. Cell. Mol. Med. 2020, 24, 12560–12571. [Google Scholar] [CrossRef]
- Gao, X.; Bayraktutan, U. Substance P reversibly compromises the integrity and function of blood-brain barrier. Peptides 2023, 167, 171048. [Google Scholar] [CrossRef]
- Almaca, J.; Weitz, J.; Rodriguez-Diaz, R.; Pereira, E.; Caicedo, A. The Pericyte of the Pancreatic Islet Regulates Capillary Diameter and Local Blood Flow. Cell Metab. 2018, 27, 630–644. [Google Scholar] [CrossRef]
- Traber, D.L.; Lentz, C.W.; Traber, L.D.; Herndon, D.N. Lymph and blood flow responses in central airways. Am. Rev. Respir. Dis. 1992, 146, S15–S18. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Enkhbaatar, P.; Traber, D.L.; Cox, R.A.; Jacob, S.; Mathew, B.P.; Hamahata, A.; Traber, L.D.; Herndon, D.N.; Hawkins, H.K. Role of calcitonin gene-related peptide (CGRP) in ovine burn and smoke inhalation injury. J. Appl. Physiol. 2009, 107, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Ghabriel, M.N.; Lu, M.X.; Leigh, C.; Cheung, W.C.; Allt, G. Substance P-induced enhanced permeability of dura mater microvessels is accompanied by pronounced ultrastructural changes, but is not dependent on the density of endothelial cell anionic sites. Acta Neuropathol. 1999, 97, 297–305. [Google Scholar] [CrossRef]
- Nguyen, L.S.; Villablanca, A.C.; Rutledge, J.C. Substance P increases microvascular permeability via nitric oxide-mediated convective pathways. Am. J. Physiol. 1995, 368, R1060–R1068. [Google Scholar]
- Kato, K.; Dieguez-Hurtado, R.; Park, D.Y.; Hong, S.P.; Kato-Azuma, S.; Adams, S.; Stehling, M.; Trappmann, B.; Wrana, J.L.; Koh, G.Y.; et al. Pulmonary pericytes regulate lung morphogenesis. Nat. Commun. 2018, 9, 2448. [Google Scholar] [CrossRef] [PubMed]
- Keith, I.M. The role of endogenous lung neuropeptides in regulation of the pulmonary circulation. Physiol. Rev. 2000, 49, 519–537. [Google Scholar]
- Hainis, K.D.; Sznajder, J.I.; Schraufnagel, D.E. Lung lymphatics cast from the airspace. Am. J. Physiol. 1994, 267, L199–L205. [Google Scholar] [CrossRef] [PubMed]
- Peao, M.N.; Aguas, A.P.; de Sa, C.M.; Pereira, A.S.; Grande, N.R. Scanning electron microscopy of the deep lymphatic network of the murine lung as viewed in corrosion casts. Lymphology 1993, 26, 42–48. [Google Scholar]
- Marchetti, C.; Poggi, P.; Clement, M.G.; Aguggini, G.; Piacentini, C.; Icaro-Cornaglia, A. Lymphatic capillaries of the pig lung: TEM and SEM observations. Anat. Rec. 1994, 238, 368–373. [Google Scholar] [CrossRef]
- Leak, L.V. Lymphatic removal of fluids and particles in the mammalian lung. Environ. Health Perspect. 1980, 35, 55–76. [Google Scholar] [CrossRef]
- Solari, E.; Marcozzi, C.; Ottaviani, C.; Negrini, D.; Moriondo, A. Draining the pleural space: Lymphatic vessels facing the most challenging task. Biology 2022, 11, 419. [Google Scholar] [CrossRef]
- Stump, B.; Cui, Y.; Kidambi, P.; Lamattina, A.M.; El-Chemaly, S. Lymphatic changes in respiratory diseases: More than just remodeling of the lung? Am. J. Respir. Cell Mol. Biol. 2017, 57, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Meinecke, A.K.; Nagy, N.; Lago, G.D.; Kirmse, S.; Klose, R.; Schrodter, K.; Zimmermann, A.; Helfrich, I.; Rundqvist, H.; Theegarten, D.; et al. Aberrant mural cell recruitment to lymphatic vessels and impaired lymphatic drainage in a murine model of pulmonary fibrosis. Blood 2012, 119, 5931–5942. [Google Scholar] [CrossRef] [PubMed]
- El-Chemaly, S.; Malide, D.; Zudaire, E.; Ikeda, Y.; Wieinberg, B.A.; Pacheco-Rodriguez, G.; Rosas, I.O.; Aparicio, M.; Ren, P.; MacDonald, S.D.; et al. Abnormal lymphangiogenesis in idiopathic pulmonary fibrosis with insights into cellular and molecular mechanisms. Proc. Natl. Acad. Sci. USA 2009, 106, 3958–3963. [Google Scholar] [CrossRef] [PubMed]
- Ebina, M.; Shibata, N.; Ohta, H.; Hisata, S.; Tamada, T.; Ono, M.; Okaya, K.; Kondo, T.; Nukiwa, T. The disappearance of subpleural and interlobular lymphatics in idiopathic pulmonary fibrosis. Lymphat. Res. Biol. 2010, 8, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Karpanen, T.; Alitalo, K. Molecular biology and pathology of lymphangiogenesis. Annu. Rev. Pathol. 2008, 3, 367–397. [Google Scholar] [CrossRef] [PubMed]
- Baluk, P.; Naikawadi, R.P.; Kim, S.; Rodriguez, F.; Choi, D.; Hong, Y.K.; Wolters, P.J.; McDonald, D.M. Lymphatic proliferation ameliorates pulmonary fibrosis after lung injury. Am. J. Pathol. 2020, 190, 2355–2375. [Google Scholar] [CrossRef] [PubMed]
- Baluk, P.; Tammela, T.; Ator, E.; Lubynska, N.; Achen, M.G.; Hicklin, D.J.; Jeltxch, M.; Petraova, T.V.; Pytowski, B.; Stacker, S.A.; et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J. Clin. Investig. 2005, 115, 247–257. [Google Scholar] [CrossRef]
- Reed, H.O.; Wang, L.; Sonett, J.; Chen, M.; Yang, J.; Li, L.; Aradi, P.; Jakus, Z.; D’Armiento, J.; Hancock, W.W.; et al. Lymphatic impairment leads to pulmonary tertiary lymphoid organ formation and alveolar damage. J. Clin. Investig. 2019, 129, 2514–2526. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, A.; Reed, H.O. The lymphatic vasculature in lung function and respiratory disease. Front. Med. 2023, 10, 1118583. [Google Scholar] [CrossRef]
- Jannaway, M.; Iyer, D.; Mastrogiacomo, D.M.; Li, K.; Sung, D.C.; Yang, Y.; Kahn, M.L.; Scallan, J.P. VEGFR3 is required for button junction formation in lymphatic vessels. Cell Rep. 2023, 42, 112777. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, K. The lymphatic vasculature in disease. Nat. Med. 2011, 17, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Oliver, G.; Kipnis, J.; Randolph, G.J.; Harvey, N.L. The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease. Cell 2020, 182, 270–296. [Google Scholar] [CrossRef]
- Scallan, J.P.; Zawieja, S.D.; Castorena-Gonzalez, J.A.; Davis, M.J. Lymphatic pumping: Mechanics, mechanisms and malfunction. J. Physiol. 2016, 594, 5749–5768. [Google Scholar] [CrossRef]
- Olszewski, W.L. Contractility patterns of human leg lymphatics in various stages of obstructive lymphedema. Ann. N. Y Acad. Sci. 2008, 1131, 110–118. [Google Scholar] [CrossRef]
- Zawieja, S.D.; Castorena-Gonzalez, J.A.; Gui, P.; Li, M.; Bulley, S.A.; Jaggar, J.H.; Rock, J.R.; Davis, M.J. Ano1 mediates pressure-sensitive contraction frequency changes in mouse lymphatic collecting vessels. J. Gen. Physiol. 2019, 151, 532–554. [Google Scholar] [CrossRef]
- Zawieja, S.D.; Pea, G.A.; Broyhill, S.E.; Patro, A.; Bromert, K.H.; Li, M.; Norton, C.E.; Castorena-Gonzalez, J.A.; Hancock, E.J.; Bertram, C.D.; et al. IP3R1 underlies diastolic ANO1 activation and pressure-dependent chronotropy in lymphatic collecting vessels. J. Gen. Physiol. 2023, 155, e202313358. [Google Scholar] [CrossRef]
- Papp, R.; Nagaraj, C.; Zabini, D.; Nagy, B.M.; Lengyel, M.; Mauurer, D.S.; Sharma, N.; Egemnazarov, B.; Kovacs, G.; Kwapiszewska, G.; et al. Targeting TMEM16A to reverse vasoconstriction and remodelling in idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1800965. [Google Scholar] [CrossRef]
- Xie, J.; Liu, W.; Lv, W.; Han, X.; Kong, Q.; Wu, Y.; Liu, X.; Han, Y.; Shi, C.; Jia, X. Transmembrane protein 16A/anoctamin 1 inhibitor T16Ainh-A01 reversed monocrotaline-induced rat pulmonary arterial hypertension. Pulm. Circ. 2020, 10, 2045894020946670. [Google Scholar] [CrossRef]
- Shang, L.; Wang, K.; Liu, D.; Qin, S.; Huang, J.; Zhao, Y.; Pang, Y. TMEM16A regulates the cell cycle of pulmonary artery smooth muscle cells in high-flow-induced pulmonary arterial hypertension rat model. Exp. Ther. Med. 2020, 19, 3275–3281. [Google Scholar] [CrossRef]
- McHale, N.G.; Thornbury, K. A method for studying lymphatic pumping activity in conscious and anaesthetized sheep. J. Physiol. 1986, 378, 109–118. [Google Scholar] [CrossRef]
- Datar, S.A.; Johnson, E.G.; Oishi, P.E.; Johengen, M.; Tang, E.; Aramburo, A.; Barton, J.; Kuo, H.C.; Bennet, S.; Xoinis, K.; et al. Altered lymphatics in an ovine model of congenital heart disease with increased pulmonary blood flow. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L530–L540. [Google Scholar] [CrossRef] [PubMed]
- Scallan, J.P.; Knauer, L.A.; Hou, H.; Castorena-Gonzalez, J.A.; Davis, M.J.; Yang, Y. Foxo1 deletion promotes the growth of new lymphatic valves. J. Clin. Investig. 2021, 131, e142341. [Google Scholar] [CrossRef]
- Castorena-Gonzalez, J.A. Lymphatic valve dysfunction in western diet-fed mice: New insights into obesity-induced lymphedema. Front. Pharmacol. 2022, 13, 823266. [Google Scholar] [CrossRef]
- Bromley, S.K.; Thomas, S.Y.; Luster, A.D. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 2005, 6, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Marchal-Somme, J.; Uzunhan, Y.; Marchan-Adam, S.; Valeyre, D.; Soumelis, V.; Crestani, B.; Soler, P. Cutting edge: Nonproliferating mature immune cells form a novel type of organized lymphoid structure in idiopathic pulmonary fibrosis. J. Immunol. 2006, 176, 5735–5739. [Google Scholar] [CrossRef] [PubMed]
- Pierce, E.M.; Carpenter, K.; Jakubzick, C.; Kunkel, S.L.; Evanoff, H.; Flaherty, K.R.; Martinez, F.J.; Toews, G.B.; Hogaboam, C.M. Idiopathic pulmonary fibrosis fibroblasts migrate and proliferate to CC chemokine ligand 21. Eur. Respir. J. 2007, 29, 1082–1093. [Google Scholar] [CrossRef]
- Yamada, K.; Hoshino, T. An examination of the close relationship between lymphatic vessels and nerve fibers containing calcitonin gene-related peptide and substance P in rat skin. Nagoya J. Med. Sci. 1996, 59, 143–150. [Google Scholar] [PubMed]
- Huang, S.; Ziegler, C.G.K.; Austin, J.; Mannoun, N.; Vukovic, M.; Ordovas-Montanes, J.; Shalek, A.K.; von Andrian, U.H. Lymph nodes are innervated by a unique population of sensory neurons with immunomodulatory potential. Cell 2021, 184, 441–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L.; Czepielewski, R.S.; Randolph, G.J. Sensory nerves regulate transcriptional dynamics of lymph node cells. Trends Immunol. 2021, 42, 180–182. [Google Scholar] [CrossRef]
- Matsui, S.; Tanaka, M.; Kamiyoshi, A.; Sakurai, T.; Ichikawa-Shindo, Y.; Kawate, H.; Dai, K.; Cui, N.; Wei, Y.; Tanaka, M.; et al. Endogenous calcitonin gene-related peptide deficiency exacerbates postoperative lymphedema by suppressing lymphatic capillary formation and M2 macrophage accumulation. Am. J. Pathol. 2019, 189, 2487–2502. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Li, Y.; Chang, W.; Gao, F.; Ding, X.; Xu, W.; Han, D. Quantitative Evaluation of the Function of the Sensory Nerve Fibers of the Palate in Patients with Obstructive Sleep Apnea. J. Clin. Sleep Med. 2019, 15, 1347–1353. [Google Scholar] [CrossRef]
- Karuga, F.F.; Kaczmarski, P.; Szmyd, B.; Bialasiewicz, P.; Sochal, M.; Gabryelska, A. The association between idiopathic pulmonary fibrosis and obstructive sleep apnea: A systematic review and meta-analysis. J. Clin. Med. 2022, 11, 5008. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, L.H.; Mason, W.R.; Parnell, J.A.; Rice, T.W.; Loyd, J.E.; Milstone, A.P.; Collard, H.R.; Malow, B.A. Obstructive Sleep Apnea Is Common in Idiopathic Pulmonary Fibrosis. Chest 2009, 136, 772–778. [Google Scholar] [CrossRef]
- Tudorache, V.; Traila, D.; Marc, M.; Oancea, C.; Manolescu, D.; Tudorache, E.; Timar, B.; Albai, A.; Fira-Mladinescu, O. Impact of moderate to severe obstructive sleep apnea on the cognition in idiopathic pulmonary fibrosis. PLoS ONE 2019, 14, e0211455. [Google Scholar] [CrossRef]
- Mermigkis, C.; Bouloukaki, I.; Antoniou, K.; Papadogiannis, G.; Giannarakis, I.; Varouchakis, G.; Siafakas, N.; Schiza, S.E. Obstructive sleep apnea should be treated in patients with idiopathic pulmonary fibrosis. Sleep Breath. 2015, 19, 385–391. [Google Scholar] [CrossRef]
- Perlman, C.E.; Bhattacharya, J. Alveolar expansion imaged by optical sectioning microscopy. J. Appl. Physiol. 2007, 103, 1037–1044. [Google Scholar] [CrossRef]
- Ichimura, H.; Parthasarathi, K.; Quadri, S.; Issekutz, A.C.; Bhattacharya, J. Mechano-oxidative coupling by mitochondria induces proinflammatory responses in lung venular capillaries. J. Clin. Investig. 2003, 111, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Heo, I.R.; Kim, C.K. Impact of high-risk of obstructive sleep apnea on chronic cough: Data from the Korea National Health and Nutrition Examination Survey. BMC Pulm. Med. 2022, 22, 419. [Google Scholar] [CrossRef] [PubMed]
- Snow, J.B.; Norton, C.E.; Sands, M.A.; Weise Cross, L.; Yan, S.; Herbert, L.M.; Sheak, J.R.; Gonzales Bosc, L.V.; Walker, B.R.; Kanagy, N.L.; et al. Intermittent hypoxia aurments pulmonary vasoconstrictor reactivity through PCKB/mitochondrial oxidant signaling. Am. J. Respir. Cell Mol. Biol. 2020, 62, 732–746. [Google Scholar] [CrossRef] [PubMed]
- Snow, J.B.; Kitzis, V.; Norton, C.E.; Torres, S.N.; Johnson, K.D.; Kanagy, N.L.; Walker, B.R.; Resta, T.C. Differential effects of chronic hypoxia and intermittent hypocapnic and eucapnic hypoxia on pulmonary vasoreactivity. J. Appl. Physiol. 2008, 104, 110–118. [Google Scholar] [CrossRef]
- Glass, D.S.; Grossfield, D.; Renna, H.A.; Agarwala, P.; Spiegler, P.; DeLeon, J.; Reiss, A.B. Idiopathic pulmonary fibrosis: Current and future treatment. Clin. Respir. J. 2022, 16, 84–96. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norton, C.E. Role of Sensory Nerves in Pulmonary Fibrosis. Int. J. Mol. Sci. 2024, 25, 3538. https://doi.org/10.3390/ijms25063538
Norton CE. Role of Sensory Nerves in Pulmonary Fibrosis. International Journal of Molecular Sciences. 2024; 25(6):3538. https://doi.org/10.3390/ijms25063538
Chicago/Turabian StyleNorton, Charles E. 2024. "Role of Sensory Nerves in Pulmonary Fibrosis" International Journal of Molecular Sciences 25, no. 6: 3538. https://doi.org/10.3390/ijms25063538
APA StyleNorton, C. E. (2024). Role of Sensory Nerves in Pulmonary Fibrosis. International Journal of Molecular Sciences, 25(6), 3538. https://doi.org/10.3390/ijms25063538





