Therapeutic Applications of Stem Cell-Derived Exosomes
Abstract
1. Introduction
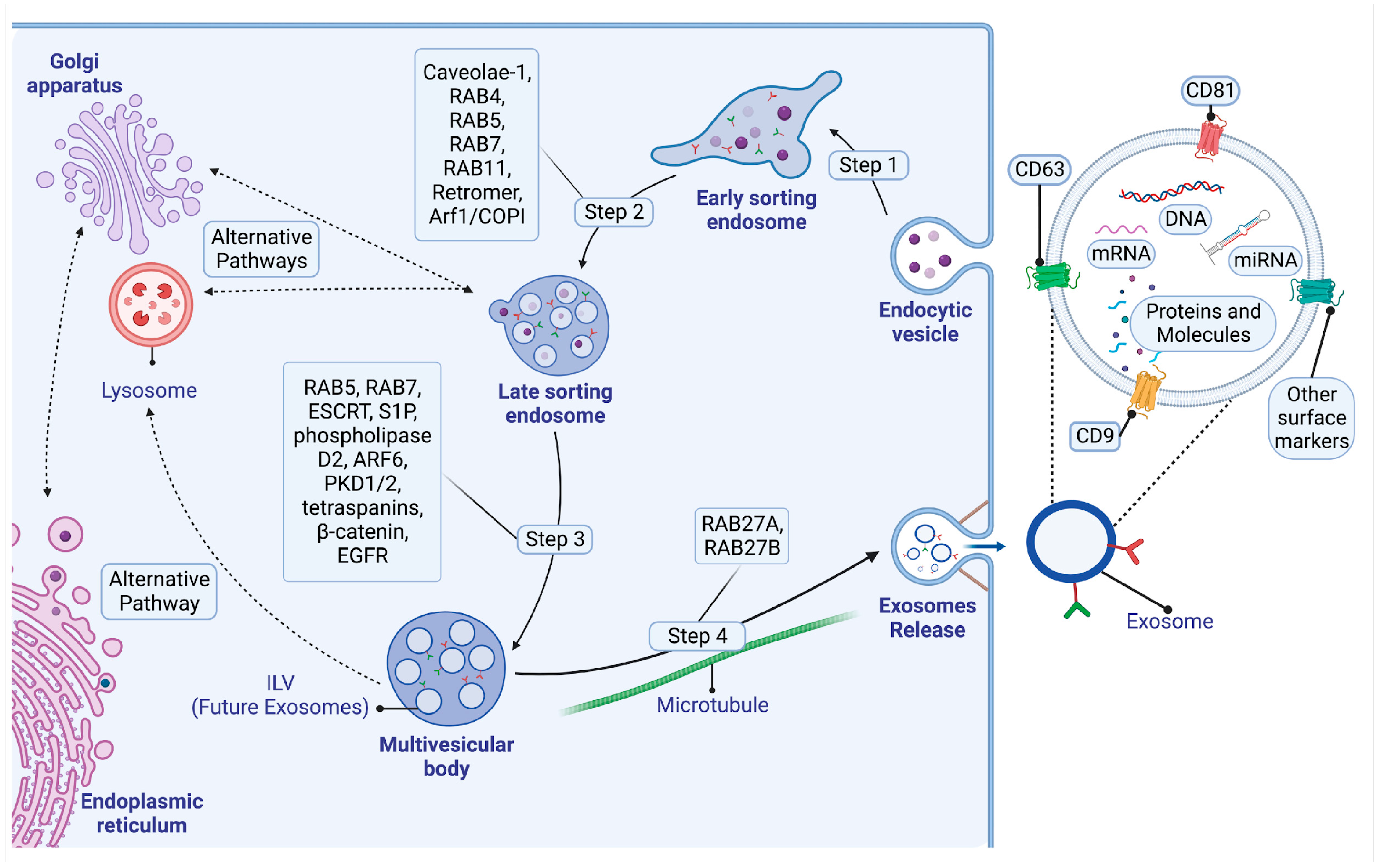
2. Biogenesis, Composition, and Signaling Mechanisms of Exosomes
2.1. Biogenesis of Exosomes
2.1.1. Early Sorting Endosomes
2.1.2. Late-Sorting Endosomes
2.1.3. The Endosomal Sorting Complex Required for Transport (ESCRT) Pathway
2.1.4. The ESCRT-Independent Pathway
2.2. The Structure and Composition of Exosomes
2.3. Cellular Uptake and Signaling of Exosomes: Mechanisms and Implications
2.3.1. Uptake and Internalization of Exosomes by Cells
2.3.2. Clathrin-Mediated Endocytosis
2.3.3. Lipid Raft-Mediated Endocytosis
2.3.4. Phagocytosis
2.3.5. Macropinocytosis
2.3.6. Exosome-Mediated Intercellular Communication
3. Sources of Stem Cell-Derived Exosomes: Molecular Traits, Functionality, and Therapeutic Implications
3.1. Exosomes from Adipose-Derived Stem Cells
3.2. Exosomes from Bone Marrow-Derived Mesenchymal Stem Cells
3.3. Exosomes from Hair Follicle-Derived Mesenchymal Stem Cells
3.4. Exosomes from Induced Pluripotent Stem Cells
3.5. Exosomes from Neural Stem Cells
| Source of Exosome | Therapeutic Application | Surface Markers |
|---|---|---|
| Exosomes from Adipose-Derived Stem Cells | Tissue repair, specifically in bone fracture healing and limb ischemia. Boost angiogenesis in HUVECs. | CD9, CD63, and CD81 [76,77,78,79]. |
| Exosomes from Bone Marrow-Derived Mesenchymal Stem Cells | Cardioprotection against doxorubicin-induced toxicity. | CD63, CD9, and TSG101 [86,87]. |
| Exosomes from Hair Follicle-Derived Mesenchymal Stem Cells | Wound recovery and decreased oxidative stress in dermal fibroblasts. | CD13, CD9, CD63, CD105, CD81, CD29, CD44, CD49e; SSEA-4 [90,91]. |
| Exosomes from Induced Pluripotent Stem Cells | Cardiac tissue repair. Treatment of retina-associated disorders. | CD9, TSG101; SSEA-1 [94,95]. |
| Exosomes from Neural Stem Cells | Increased resilience under oxidative stress and inflammation and support development of synaptic connections. | TSG101, CD9, and Flotillin-1 [97,98]. |
4. Role of Exosomes in Diagnostics and Oncology
4.1. Enhancing Diagnostic Precision: The Emerging Role of Exosomes
4.2. Impact of Cancer Stem Cell-Derived Exosomes on Cancer Aggressiveness and Diagnostic Applications
5. Potential Therapeutic Applications in Disease and Regenerative Medicine
5.1. Endocrine Disorders
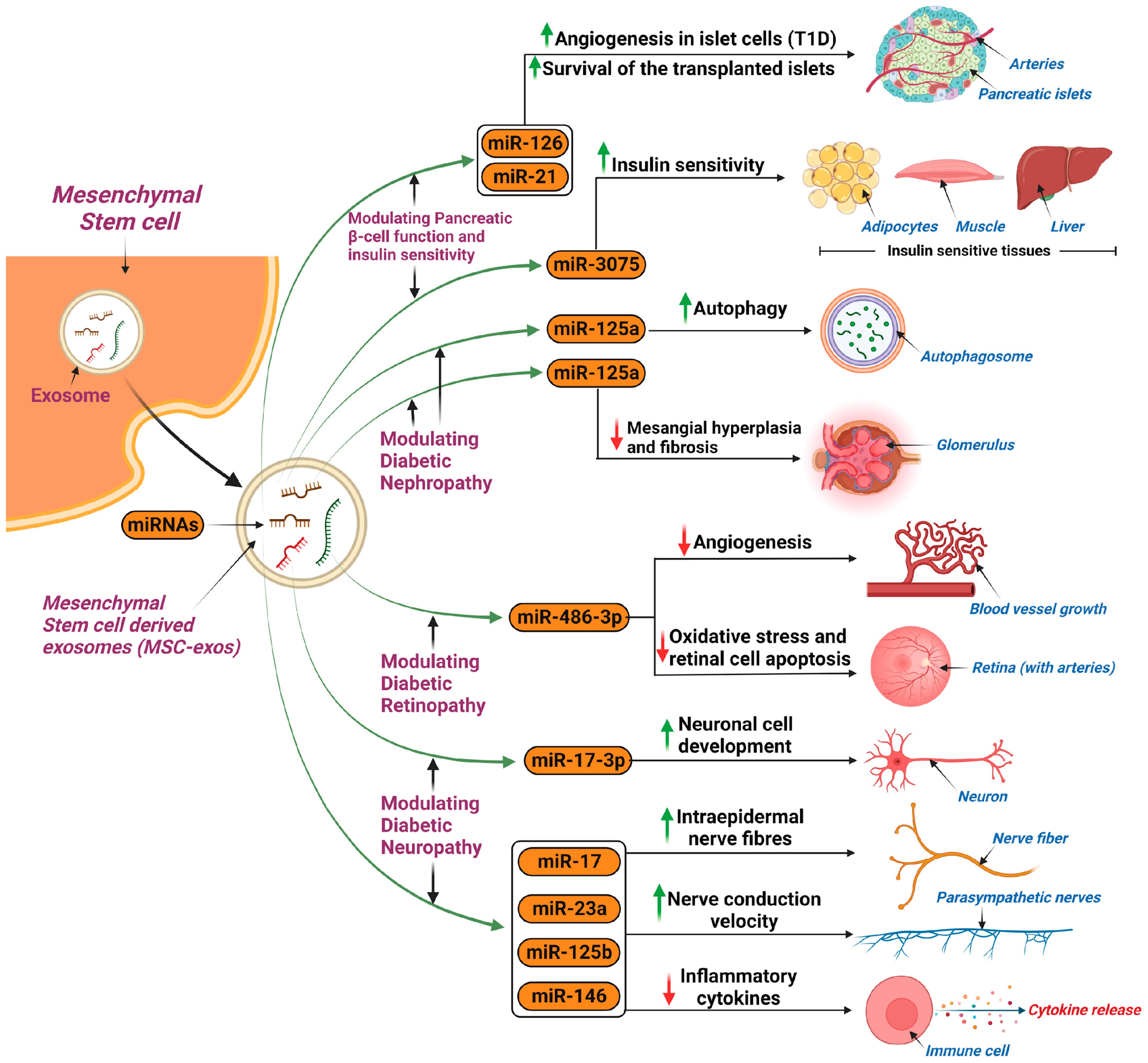
5.1.1. Diabetes Mellitus
Diabetic Nephropathy
Diabetic Retinopathy
Diabetic Peripheral Neuropathy
5.1.2. Polycystic Ovary Syndrome
5.2. Gastrointestinal Disorders (Inflammatory Bowel Disease)
5.3. Cardiovascular Disease (Myocardial Infarction)
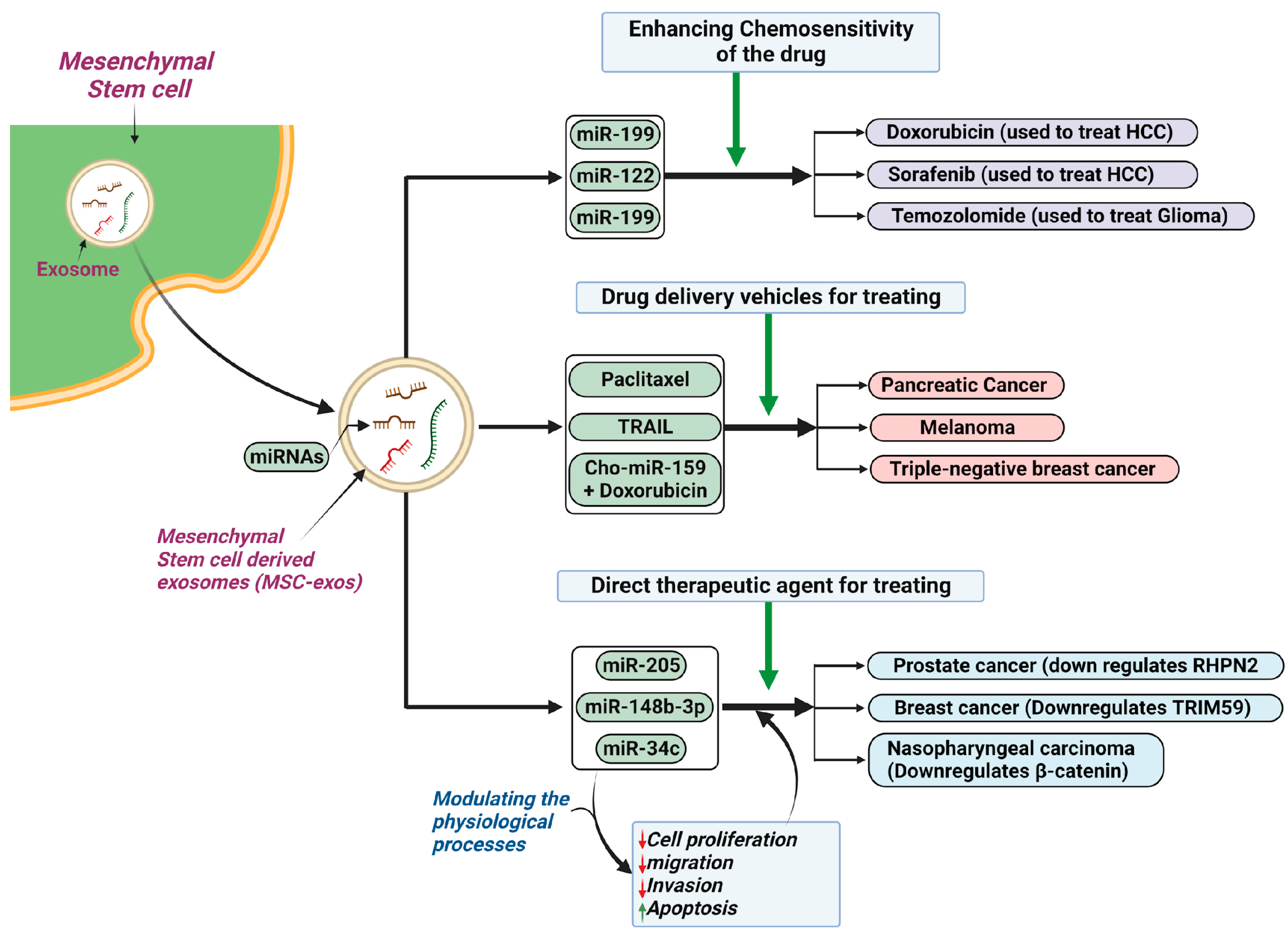
5.4. Cancer
5.5. Plastic Surgery
5.6. Neurodegenerative Disorders
5.7. Lung Diseases
6. Overcoming Challenges of Working with Exosomes in the Laboratory
6.1. Current Methods for Exosome Isolation
6.2. Standardization of Isolation
6.3. Current Methods for Exosome Characterization
6.4. Risk Considerations
7. Exosome Research: From Bench to Bedside and Future Perspectives
7.1. Current Clinical Trials
| Condition Treated | Phase | Patient Group | Type of MSC-Exos | Dosage | Administration Method | Main Outcomes | Trial Reference |
|---|---|---|---|---|---|---|---|
| CKD Stage 3 and 4 | Phase II/III | Stage 3 and 4 CKD patients | HUCMSC-Exos | 100 mcg/kg weekly for two weeks | Intravenous and intra-arterial | Improvement in glomerular filtration rate, reduction in blood creatinine and urea levels | [208] |
| Skin hyperpigmentation | Not specified | Individuals with hyperpigmentation | ADSC-Exos | 0.2 g of ADSC-exos twice a day for 8 weeks | Local | Short-term improvement in hyperpigmentation through reduction in melanin; however, followed by relapses | [207] |
| AIS | Phase I/II | AIS patients | miR-124-transfected MSC-Exos | Not specified | Intraparenchymal or stereotaxis | Incidence of treatment-emergent adverse events and improvement based on the modified Rankin Scale; evaluation of AIS patient disability. | [210] |
| Mild-to-moderate Alzheimer’s, dementia | Phase I/II | Patients with mild-to-moderate Alzheimer’s based on NIA/Aa | Adipose Tissue-Derived Exosomes | Low dose (5 mcg); Medium dose (10 mcg); High dose (20 mcg) twice a week | Nasal drip | Number of participants with abnormal laboratory values and adverse events by CTCAE v4.0 at 12 weeks. Cognitive function, quality of life evaluation, MRI neuroimaging, PET-CT neuroimaging, and changes in AD biomarkers are secondary outcomes. | [211,212] |
| COVID-19 | Phase I | PCR-confirmed COVID-19 patients | MSC-exos | 5 inhalation doses over 5 days | Inhalation | Adverse reaction and severe adverse reaction up to 28 days. | [207,213] |
7.2. Challenges and Limitations of Exosomes
7.3. Emerging Areas of Research and Potential Breakthroughs
7.3.1. Microfluidics
7.3.2. Asymmetric Flow Field Fractionation
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SC | stem cells |
| MSCs | mesenchymal stem cells |
| Sc-exos | stem cell-derived exosomes |
| EVs | extracellular vesicles |
| CSF | cerebrospinal fluid |
| DM | diabetes mellitus |
| hESCs | human embryonic stem cells |
| iPSCs | induced pluripotent stem cells |
| TGF-ß | transforming growth factor beta |
| VEGF | vascular endothelial growth factor |
| IL | interleukin |
| CCL7 | chemokine ligand 7 |
| CxCL2 | chemokine ligand 2 |
| miRNA, miR | microRNA |
| ncRNA | non-coding RNA |
| ESE | endosomes |
| CME | clathrin-mediated endocytosis |
| ClE | clathrin-independent endocytosis |
| ARF6 | ADP-ribosylation factor 6 |
| CLIC | clathrin-independent carriers |
| GEEC | glycosylphosphatidylinositol-enriched endocytic compartment |
| LSE | late-sorting endosome |
| GDP | guanosine diphosphate |
| ILVS | intraluminal vesicles |
| MVBs | multivesicular bodies |
| ESCRT | endosomal sorting complex required for transport |
| S1P | sphingosine 1 phosphate |
| EGFR | epidermal growth factor receptor |
| mRNA | messenger RNAs |
| CRC | colorectal cancer |
| CEA | carcinoembryonic antigen |
| CA19-9 | cancer antigen 19-9 |
| sEV | small EVs |
| NSCLC | non-small-cell lung cancer |
| NSCLC-sEVs | NSCLC-derived sEVs |
| SCLC | smallcell lung cancer |
| GPI | glycosylphosphatidylinositol |
| PI3K | phosphatidylinositol-3-kinase |
| PLC | phospholipase C |
| ADSCs | Adipose-derived stem cells |
| TEM | Transmission Electron Microscopy |
| ADSC-exos | ADSC-derived exosomes |
| ACER2 | alkaline ceramidase 2 |
| HUVECs | human umbilical vein endothelial cells |
| BMSCs | bone marrow-derived stem cells |
| NTA | nanoparticle tracking analysis |
| BMSC-exos | BMSC-derived exosomes |
| HFMSCs | Hair follicle-derived mesenchymal stem cells |
| HFMSC-exos | HFMSC-derived exosomes |
| TFs | transcription factors |
| LV | left ventricular |
| HDAC1 | histone deacetylase 1 |
| HIF | hypoxia inducible factor |
| NSCs | neural stem cells |
| SVZ | subventricular zone |
| NSC-exos | NSC-derived exosomes |
| OPCs | oligodendrocyte precursor cells |
| PSA | Prostate-Specific Antigen |
| CSCs | Cancer stem cells |
| CSC-exos | CSC-derived exosomes |
| ECSCs | esophageal carcinoma stem cell |
| LCSCs | lung cancer stem cells |
| T1DM | type 1 diabetes mellitus |
| Tregs | regulatory T cells |
| IR | insulin resistance |
| PBMCs | peripheral blood mononuclear cells |
| ET-1 | endothelin 1 |
| DR | diabetic retinopathy |
| PCOS | polycystic ovarian syndrome |
| IBD | inflammatory bowel disease |
| Lgr5 | leucine-rich repeat-containing G protein-coupled receptor 5 |
| ERK | extracellular-signal-regulated kinase |
| Mecp2 | methyl CpG binding protein-2 |
| PDGF | platelet-derived growth factor |
| EPCs | endothelial progenitor cells |
| SIRT1 | sirtuin-1 |
| CXCL12 | CXC chemokine-12 |
| Nrf2 | nuclear factor E2 related factor 2 |
| HCC | hepatocellular carcinoma |
| TRAIL | tumor necrosis factor-related apoptosis-inducing ligand |
| TRIM59 | tripartite motif 5 |
| RHPN2 | rhophilin Rho GTPase binding protein 2 |
| SEM | scanning electron microscopy |
| DLS | dynamic light scattering |
| LC-MS-MS | liquid chromatography with tandem mass spectroscopy |
| 2DGE | two-dimensional gel electrophoresis |
| RT-qPCR | reverse transcriptase quantitative polymerase chain reaction |
| FM | fluorescent microscopy |
| CKD | chronic kidney disease |
| AIS | acute ischemic stroke |
| AF4 | asymmetric flow field flow fractionation |
References
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Daneshmandi, L.; Shah, S.; Jafari, T.; Bhattacharjee, M.; Momah, D.; Saveh-Shemshaki, N.; Lo, K.W.-H.; Laurencin, C.T. Emergence of the Stem Cell Secretome in Regenerative Engineering. Trends Biotechnol. 2020, 38, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Parra, D.C.; Motallebnejad, P.; Brocchi, M.; Chen, H.J. Exosomes: Small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact. Mater. 2022, 10, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Milane, L.; Singh, A.; Mattheolabakis, G.; Suresh, M.; Amiji, M.M. Exosome mediated communication within the tumor microenvironment. J. Control. Release 2015, 219, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, D. Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 366. [Google Scholar] [CrossRef]
- Damdimopoulou, P.; Rodin, S.; Stenfelt, S.; Antonsson, L.; Tryggvason, K.; Hovatta, O. Human embryonic stem cells. Best Pr. Res. Clin. Obs. Gynaecol. 2016, 31, 2–12. [Google Scholar] [CrossRef]
- Jaishankar, A.; Barthelery, M.; Freeman, W.M.; Salli, U.; Ritty, T.M.; Vrana, K.E. Human embryonic and mesenchymal stem cells express different nuclear proteomes. Stem Cells Dev. 2009, 18, 793–802. [Google Scholar] [CrossRef]
- Wang, A.Y.L. Human Induced Pluripotent Stem Cell-Derived Exosomes as a New Therapeutic Strategy for Various Diseases. Int. J. Mol. Sci. 2021, 22, 1769. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.M.; Cancemi, P.; Geraci, F. Mesenchymal and Induced Pluripotent Stem Cells-Derived Extracellular Vesicles: The New Frontier for Regenerative Medicine? Cells 2020, 9, 1163. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Lin, S.; Tan, X.; Zhu, S.; Nie, F.; Zhen, Y.; Gu, L.; Zhang, C.; Wang, B.; Wei, W.; et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021, 54, e12993. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, B.; Yang, Y.; Jiang, Q.; Li, T.; Gong, J.; Tang, H.; Zhang, Q. Stem cell-derived exosomes: Emerging therapeutic opportunities for wound healing. Stem Cell Res. Ther. 2023, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, K. Stem cell-derived exosome versus stem cell therapy. Nat. Rev. Bioeng. 2023, 1, 608–609. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Jiang, L. Current Knowledge on Exosome Biogenesis, Cargo-Sorting Mechanism and Therapeutic Implications. Membranes 2022, 12, 498. [Google Scholar] [CrossRef]
- Mayor, S.; Parton, R.G.; Donaldson, J.G. Clathrin-independent pathways of endocytosis. Cold Spring Harb. Perspect. Biol. 2014, 6, a041398. [Google Scholar] [CrossRef]
- McMahon, H.T.; Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011, 12, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Rojas, R.; van Vlijmen, T.; Mardones, G.A.; Prabhu, Y.; Rojas, A.L.; Mohammed, S.; Heck, A.J.; Raposo, G.; van der Sluijs, P.; Bonifacino, J.S. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J. Cell Biol. 2008, 183, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Vonderheit, A.; Helenius, A. Rab7 associates with early endosomes to mediate sorting and transport of Semliki forest virus to late endosomes. PLoS Biol. 2005, 3, e233. [Google Scholar] [CrossRef] [PubMed]
- Hayer, A.; Stoeber, M.; Ritz, D.; Engel, S.; Meyer, H.H.; Helenius, A. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J. Cell Biol. 2010, 191, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Rink, J.; Ghigo, E.; Kalaidzidis, Y.; Zerial, M. Rab conversion as a mechanism of progression from early to late endosomes. Cell 2005, 122, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef]
- Sahu, R.; Kaushik, S.; Clement, C.C.; Cannizzo, E.S.; Scharf, B.; Follenzi, A.; Potolicchio, I.; Nieves, E.; Cuervo, A.M.; Santambrogio, L. Microautophagy of cytosolic proteins by late endosomes. Dev. Cell 2011, 20, 131–139. [Google Scholar] [CrossRef]
- Record, M. Intercellular communication by exosomes in placenta: A possible role in cell fusion? Placenta 2014, 35, 297–302. [Google Scholar] [CrossRef]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef]
- Hurley, J.H. ESCRTs are everywhere. EMBO J. 2015, 34, 2398–2407. [Google Scholar] [CrossRef]
- Villarroya-Beltri, C.; Baixauli, F.; Gutiérrez-Vázquez, C.; Sánchez-Madrid, F.; Mittelbrunn, M. Sorting it out: Regulation of exosome loading. Semin. Cancer Biol. 2014, 28, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Ghossoub, R.; Lembo, F.; Rubio, A.; Gaillard, C.B.; Bouchet, J.; Vitale, N.; Slavík, J.; Machala, M.; Zimmermann, P. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat. Commun. 2014, 5, 3477. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, C.; Calvo, V.; Alonso, R.; Mérida, I.; Izquierdo, M. Protein kinase D1/2 is involved in the maturation of multivesicular bodies and secretion of exosomes in T and B lymphocytes. Cell Death Differ. 2016, 23, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Barreiro, O.; Gordon-Alonso, M.; Sala-Valdés, M.; Sánchez-Madrid, F. Tetraspanin-enriched microdomains: A functional unit in cell plasma membranes. Trends Cell Biol. 2009, 19, 434–446. [Google Scholar] [CrossRef]
- Chairoungdua, A.; Smith, D.L.; Pochard, P.; Hull, M.; Caplan, M.J. Exosome release of β-catenin: A novel mechanism that antagonizes Wnt signaling. J. Cell Biol. 2010, 190, 1079–1091. [Google Scholar] [CrossRef]
- Wei, D.; Zhan, W.; Gao, Y.; Huang, L.; Gong, R.; Wang, W.; Zhang, R.; Wu, Y.; Gao, S.; Kang, T. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021, 31, 157–177. [Google Scholar] [CrossRef]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012, database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012, 40, D1241–D1244. [Google Scholar] [CrossRef]
- Kim, D.-K.; Kang, B.; Kim, O.Y.; Choi, D.-S.; Lee, J.; Kim, S.R.; Go, G.; Yoon, Y.J.; Kim, J.H.; Jang, S.C.; et al. EVpedia: An integrated database of high-throughput data for systemic analyses of extracellular vesicles. J. Extracell Vesicles 2013, 2, 20384. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 2012, 1820, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Sekhavati, N.; Noori, E.; Abbasifard, M.; Butler, A.E.; Sahebkar, A. How statin drugs affect exosomes? J. Cell Biochem. 2023, 124, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Salah, M.; Shaheen, I.; El-Shanawany, P.; Saad, N.E.; Saad, R.; El Guibaly, M.; Momen, N. Detection of miR-1246, miR-23a and miR-451 in sera of colorectal carcinoma patients: A case-control study in Cairo University hospital. Afr. Health Sci. 2020, 20, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-R.; Yan, J.-X.; Wang, L.-N. The diagnostic value of serum carcino-embryonic antigen, alpha fetoprotein and carbohydrate antigen 19-9 for colorectal cancer. J. Cancer Res. Ther. 2014, 10, 307–309. [Google Scholar] [PubMed]
- Wang, M.; Ji, S.; Shao, G.; Zhang, J.; Zhao, K.; Wang, Z.; Wu, A. Effect of exosome biomarkers for diagnosis and prognosis of breast cancer patients. Clin. Transl. Oncol. 2018, 20, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Huang, D.; Li, Z.; Wang, X.; Yang, T.; Zhao, M.; Wu, J.; Zhong, T. The role and application of small extracellular vesicles in breast cancer. Front. Oncol. 2022, 12, 980404. [Google Scholar] [CrossRef]
- Martins, Á.M.; Ramos, C.C.; Freitas, D.; Reis, C.A. Glycosylation of Cancer Extracellular Vesicles: Capture Strategies, Functional Roles and Potential Clinical Applications. Cells 2021, 10, 109. [Google Scholar] [CrossRef]
- Williams, C.; Royo, F.; Aizpurua-Olaizola, O.; Pazos, R.; Boons, G.-J.; Reichardt, N.-C.; Falcon-Perez, J.M. Glycosylation of extracellular vesicles: Current knowledge, tools and clinical perspectives. J. Extracell Vesicles 2018, 7, 1442985. [Google Scholar] [CrossRef]
- Kondo, K.; Harada, Y.; Nakano, M.; Suzuki, T.; Fukushige, T.; Hanzawa, K.; Yagi, H.; Takagi, K.; Mizuno, K.; Miyamoto, Y.; et al. Identification of distinct N-glycosylation patterns on extracellular vesicles from small-cell and non-small-cell lung cancer cells. J. Biol. Chem. 2022, 298, 101950. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Mettlen, M.; Chen, P.-H.; Srinivasan, S.; Danuser, G.; Schmid, S.L. Regulation of Clathrin-Mediated Endocytosis. Annu. Rev. Biochem. 2018, 87, 871–896. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Ashktorab, H.; Smoot, D.T.; Nam, S.W.; Hur, H.; Park, W.S. Uptake and tumor-suppressive pathways of exosome-associated GKN1 protein in gastric epithelial cells. Gastric. Cancer 2020, 23, 848–862. [Google Scholar] [CrossRef] [PubMed]
- Benmerah, A.; Bayrou, M.; Cerf-Bensussan, N.; Dautry-Varsat, A. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 1999, 112 Pt 9, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, S.; Takefuji, M.; Sakaguchi, T.; Ishihama, S.; Mori, Y.; Tsuda, T.; Takikawa, T.; Yoshida, T.; Ohashi, K.; Shimizu, Y.; et al. Cardiomyocytes capture stem cell-derived, anti-apoptotic microRNA-214 via clathrin-mediated endocytosis in acute myocardial infarction. J. Biol. Chem. 2019, 294, 11665–11674. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhao, W.-L.; Ye, Y.-Y.; Bai, X.-C.; Liu, R.-Q.; Chang, L.-F.; Zhou, Q.; Sui, S.-F. Cellular internalization of exosomes occurs through phagocytosis. Traffic 2010, 11, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Delenclos, M.; Trendafilova, T.; Mahesh, D.; Baine, A.M.; Moussaud, S.; Yan, I.K.; Patel, T.; McLean, P.J. Investigation of Endocytic Pathways for the Internalization of Exosome-Associated Oligomeric Alpha-Synuclein. Front. Neurosci. 2017, 11, 172. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Koumangoye, R.B.; Sakwe, A.M.; Goodwin, J.S.; Patel, T.; Ochieng, J. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS ONE 2011, 6, e24234. [Google Scholar] [CrossRef]
- Izquierdo-Useros, N.; Naranjo-Gómez, M.; Archer, J.; Hatch, S.C.; Erkizia, I.; Blanco, J.; Borràs, F.E.; Puertas, M.C.; Connor, J.H.; Fernández-Figueras, M.T.; et al. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood 2009, 113, 2732–2741. [Google Scholar] [CrossRef]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhu, W.; Chen, Q.; Yuan, Y.; Wang, Y.; Wang, J.; Wang, J.; Wu, X. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics 2019, 9, 8206–8220. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef]
- Montecalvo, A.; Larregina, A.T.; Shufesky, W.J.; Stolz, D.B.; Sullivan, M.L.G.; Karlsson, J.M.; Baty, C.J.; Gibson, G.A.; Erdos, G.; Wang, Z.; et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 2012, 119, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.P.; Gleeson, P.A. Macropinocytosis: An endocytic pathway for internalising large gulps. Immunol. Cell Biol. 2011, 89, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.C.; Teasdale, R.D. Defining macropinocytosis. Traffic 2009, 10, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Costa Verdera, H.; Gitz-Francois, J.J.; Schiffelers, R.M.; Vader, P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J. Control. Release 2017, 266, 100–108. [Google Scholar] [CrossRef]
- Fitzner, D.; Schnaars, M.; van Rossum, D.; Krishnamoorthy, G.; Dibaj, P.; Bakhti, M.; Regen, T.; Hanisch, U.-K.; Simons, M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 2011, 124, 447–458. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- Iranpanah, A.; Kooshki, L.; Moradi, S.Z.; Saso, L.; Fakhri, S.; Khan, H. The Exosome-Mediated PI3K/Akt/mTOR Signaling Pathway in Neurological Diseases. Pharmaceutics 2023, 15, 1006. [Google Scholar] [CrossRef]
- Simons, M.; Raposo, G. Exosomes--vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Alsayed, R.K.M.E.; Choudhry, H.; Ahmad, A. Exosome-Mediated Response to Cancer Therapy: Modulation of Epigenetic Machinery. Int. J. Mol. Sci. 2022, 23, 6222. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Gozal, D. Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. J. Transl. Med. 2014, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Wang, J.; Gan, W.; Qin, X.; Yang, R.; Chen, X. Therapeutic potential of exosomes from adipose-derived stem cells in chronic wound healing. Front. Surg. 2022, 9, 1030288. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, X.; Chen, J.; Wang, C.; Sun, Y.; Yan, C.; Ren, S.; Xiong, H.; Xiang, K.; Zhang, M.; et al. Exosomal miR-125b-5p derived from adipose-derived mesenchymal stem cells enhance diabetic hindlimb ischemia repair via targeting alkaline ceramidase 2. J. Nanobiotechnol. 2023, 21, 189. [Google Scholar] [CrossRef]
- Zhang, D.; Xiao, W.; Liu, C.; Wang, Z.; Liu, Y.; Yu, Y.; Jian, C.; Yu, A. Exosomes Derived from Adipose Stem Cells Enhance Bone Fracture Healing via the Activation of the Wnt3a/β-Catenin Signaling Pathway in Rats with Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 4852. [Google Scholar] [CrossRef]
- Hibbs, S. This Is Going to Hurt: Revisiting the Patient Experience of Bone Marrow Biopsies. Hemasphere 2022, 6, e710. [Google Scholar] [CrossRef]
- Hernigou, P.; Homma, Y.; Flouzat Lachaniette, C.H.; Poignard, A.; Allain, J.; Chevallier, N.; Rouard, H. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int. Orthop. 2013, 37, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Gudleviciene, Z.; Kundrotas, G.; Liudkeviciene, R.; Rascon, J.; Jurga, M. Quick and effective method of bone marrow mesenchymal stem cell extraction. Open Med. 2015, 10, 44–49. [Google Scholar] [CrossRef]
- Horn, P.; Bork, S.; Wagner, W. Standardized isolation of human mesenchymal stromal cells with red blood cell lysis. Methods Mol. Biol. 2011, 698, 23–35. [Google Scholar] [PubMed]
- Epah, J.; Spohn, G.; Preiß, K.; Müller, M.M.; Dörr, J.; Bauer, R.; Daqiq-Mirdad, S.; Schwäble, J.; Bernas, S.N.; Schmidt, A.H.; et al. Small volume bone marrow aspirates with high progenitor cell concentrations maximize cell therapy dose manufacture and substantially reduce donor hemoglobin loss. BMC Med. 2023, 21, 360. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Viswanathan, P.; Chandanala, S.; Prasanna, S.J.; Seetharam, R.N. Expansion and characterization of bone marrow derived human mesenchymal stromal cells in serum-free conditions. Sci. Rep. 2021, 11, 3403. [Google Scholar] [CrossRef]
- Han, F.; Wang, C.; Cheng, P.; Liu, T.; Wang, W.-S. Bone marrow mesenchymal stem cells derived exosomal miRNAs can modulate diabetic bone-fat imbalance. Front. Endocrinol. 2023, 14, 1149168. [Google Scholar] [CrossRef]
- Lei, B.; Wu, X.; Xia, K.; Sun, H.; Wang, J. Exosomal Micro-RNA-96 Derived From Bone Marrow Mesenchymal Stem Cells Inhibits Doxorubicin-Induced Myocardial Toxicity by Inhibiting the Rac1/Nuclear Factor-κB Signaling Pathway. J. Am. Heart Assoc. 2021, 10, e020589. [Google Scholar] [CrossRef]
- Hernaez-Estrada, B.; Gonzalez-Pujana, A.; Cuevas, A.; Izeta, A.; Spiller, K.L.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Human Hair Follicle-Derived Mesenchymal Stromal Cells from the Lower Dermal Sheath as a Competitive Alternative for Immunomodulation. Biomedicines 2022, 10, 253. [Google Scholar] [CrossRef]
- Liu, J.Y.; Peng, H.F.; Gopinath, S.; Tian, J.; Andreadis, S.T. Derivation of Functional Smooth Muscle Cells from Multipotent Human Hair Follicle Mesenchymal Stem Cells. Tissue Eng. Part A 2010, 16, 2553. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, S.-J.; Lu, Y.; Tan, X.; Zhang, X.; Yang, M.; Zhang, F.; Li, Y.; Quan, C. Transdifferentiation of Human Hair Follicle Mesenchymal Stem Cells into Red Blood Cells by OCT4. Stem Cells Int. 2015, 2015, 389628. [Google Scholar] [CrossRef]
- Las Heras, K.; Royo, F.; Garcia-Vallicrosa, C.; Igartua, M.; Santos-Vizcaino, E.; Falcon-Perez, J.M.; Hernandez, R.M. Extracellular vesicles from hair follicle-derived mesenchymal stromal cells: Isolation, characterization and therapeutic potential for chronic wound healing. Stem Cell Res. Ther. 2022, 13, 147. [Google Scholar] [CrossRef]
- González, F.; Boué, S.; Belmonte, J.C.I. Methods for making induced pluripotent stem cells: Reprogramming à la carte. Nat. Rev. Genet. 2011, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Yin, Z.; Song, P.; Wu, Y.; He, Y.; Zhu, M.; Wu, Z.; Zhao, S.; Huang, H.; Wang, H.; et al. Safety and biodistribution of exosomes derived from human induced pluripotent stem cells. Front. Bioeng. Biotechnol. 2022, 10, 949724. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.-T.; Skupien-Rabian, B.; Chen, X.; et al. Induced Pluripotent Stem Cell (iPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Li, W.; Li, T.; Feng, T.; Wu, C.; Zhao, D. Induced pluripotent stem cell–derived extracellular vesicles overexpressing SFPQ protect retinal Müller cells against hypoxia-induced injury. Cell Biol. Toxicol. 2023, 39, 2647–2663. [Google Scholar] [CrossRef] [PubMed]
- Galiakberova, A.A.; Dashinimaev, E.B. Neural Stem Cells and Methods for Their Generation From Induced Pluripotent Stem Cells in vitro. Front. Cell Dev. Biol. 2020, 8, 559464. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Ding, L.; Chen, H.; Wang, Y.; Li, C.; Zhao, S.; Yang, X.; Ma, Y.; Zhu, J.; Qi, X.; et al. Neural Stem Cell-Derived Exosomes Regulate Neural Stem Cell Differentiation Through miR-9-Hes1 Axis. Front. Cell Dev. Biol. 2021, 9, 601600. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Z.-H.; Xu, X.; Sun, H.-T.; Zheng, H.-M.; Zhang, J.-L.; Wang, H.-H.; Fang, J.-W.; Liu, Y.-Z.; Huang, L.-L.; et al. Neuron-Derived Exosomes Promote the Recovery of Spinal Cord Injury by Modulating Nerve Cells in the Cellular Microenvironment of the Lesion Area. Mol. Neurobiol. 2023, 60, 4502–4516. [Google Scholar] [CrossRef]
- Mazer, B.L.; Homer, R.J.; Rimm, D.L. False-positive pathology: Improving reproducibility with the next generation of pathologists. Lab. Investig. 2019, 99, 1260–1265. [Google Scholar] [CrossRef]
- Janbaziroudsari, H.; Mirzaei, A.; Maleki, N. Association of serum prostate-specific antigen levels with the results of the prostate needle biopsy. Bull. Cancer 2016, 103, 730–734. [Google Scholar] [CrossRef]
- McCrie, R. Staffing to Meet Protective Goals. In Security Operations Management, 3rd ed.; Butterworth-Heinemann: Oxford, UK, 2016. [Google Scholar] [CrossRef]
- Cai, S.; Sze, J.Y.Y.; Ivanov, A.P.; Edel, J.B. Small molecule electro-optical binding assay using nanopores. Nat. Commun. 2019, 10, 1797. [Google Scholar] [CrossRef]
- Logozzi, M.; Angelini, D.F.; Iessi, E.; Mizzoni, D.; Di Raimo, R.; Federici, C.; Lugini, L.; Borsellino, G.; Gentilucci, A.; Pierella, F.; et al. Increased PSA expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett. 2017, 403, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Hiroš, M.; Selimović, M.; Spahović, H.; Sadović, S.; Spužić-Čelić, E. Transrectal Ultrasound-Guided Prostate Biopsy, Periprostatic Local Anesthesia and Pain Tolerance. Biomol. Biomed. 2010, 10, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Dhar, R.; Gorai, S.; Devi, A.; Jha, S.K.; Rahman, M.A.; Alexiou, A.; Papadakis, M. Exosome: A megastar of future cancer personalized and precision medicine. Clin. Transl. Discov. 2023, 3, e208. [Google Scholar] [CrossRef]
- Liang, H.; Wang, X.; Li, F.; Xie, Y.; Shen, J.; Wang, X.; Huang, Y.; Lin, S.; Chen, J.; Zhang, L.; et al. Label-free plasmonic metasensing of PSA and exosomes in serum for rapid high-sensitivity diagnosis of early prostate cancer. Biosens. Bioelectron. 2023, 235, 115380. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, C. Tissue vs. Liquid Biopsies for Cancer Detection: Ethical Issues. J. Bioeth. Inq. 2019, 16, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.-F.; Yan, L.; Zhang, Z.; Salim, A.; Wang, L.; Hu, T.-T.; Zhao, H.-Y. Accuracy of conventional MRI for preoperative diagnosis of intracranial tumors: A retrospective cohort study of 762 cases. Int. J. Surg. 2016, 36, 109–117. [Google Scholar] [CrossRef]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid biopsy: A step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef]
- Wang, X.; Tian, L.; Lu, J.; Ng, I.O.-L. Exosomes and cancer—Diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis 2022, 11, 54. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Butler, A.E.; Jamialahmadi, T.; Sahebkar, A. The role of exosomal miRNA in nonalcoholic fatty liver disease. J. Cell Physiol. 2022, 237, 2078–2094. [Google Scholar] [CrossRef]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Ju, F.; Atyah, M.M.; Horstmann, N.; Gul, S.; Vago, R.; Bruns, C.J.; Zhao, Y.; Dong, Q.-Z.; Ren, N. Characteristics of the cancer stem cell niche and therapeutic strategies. Stem Cell Res. Ther. 2022, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, L.; Ge, D.; Tan, L.; Cao, B.; Fan, H.; Xue, L. Exosomal O-GlcNAc transferase from esophageal carcinoma stem cell promotes cancer immunosuppression through up-regulation of PD-1 in CD8+ T cells. Cancer Lett. 2021, 500, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, J.; Hu, H.; Tu, L.; Sun, Z.; Liu, Y.; Luo, F. Lung CSC-derived exosomal miR-210-3p contributes to a pro-metastatic phenotype in lung cancer by targeting FGFRL1. J. Cell Mol. Med. 2020, 24, 6324–6339. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhang, J.; Yarden, Y.; Fu, L. The key roles of cancer stem cell-derived extracellular vesicles. Signal Transduct. Target. Ther. 2021, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Bu, N.; Wu, H.-Q.; Zhang, G.-L.; Zhan, S.-Q.; Zhang, R.; Fan, Q.-Y.; Li, Y.-L.; Zhai, Y.-F.; Ren, H.-W. Immature dendritic cell exosomes suppress experimental autoimmune myasthenia gravis. J. Neuroimmunol. 2015, 285, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Shen, Y.; Li, X.; Li, B.; Zhao, S.; Gu, J.; Chen, Y.; Ma, B.; Wei, J.; Han, Q.; et al. Exosomes Derived from Hypoxia-Treated Human Adipose Mesenchymal Stem Cells Enhance Angiogenesis Through the PKA Signaling Pathway. Stem Cells Dev. 2018, 27, 456–465. [Google Scholar] [CrossRef]
- Mattke, J.; Vasu, S.; Darden, C.M.; Kumano, K.; Lawrence, M.C.; Naziruddin, B. Role of Exosomes in Islet Transplantation. Front. Endocrinol. 2021, 12, 681600. [Google Scholar] [CrossRef]
- Wen, D.; Peng, Y.; Liu, D.; Weizmann, Y.; Mahato, R.I. Mesenchymal stem cell and derived exosome as small RNA carrier and Immunomodulator to improve islet transplantation. J. Control. Release 2016, 238, 166–175. [Google Scholar] [CrossRef]
- Sun, Y.; Tao, Q.; Wu, X.; Zhang, L.; Liu, Q.; Wang, L. The Utility of Exosomes in Diagnosis and Therapy of Diabetes Mellitus and Associated Complications. Front. Endocrinol. 2021, 12, 756581. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Kumar, A.P.; Aref, A.R.; Zarrabi, A.; Mostafavi, E. Exosomes as Promising Nanostructures in Diabetes Mellitus: From Insulin Sensitivity to Ameliorating Diabetic Complications. Int. J. Nanomed. 2022, 17, 1229–1253. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, N.; Ahmed, I.A.; Hussien, N.I.; Dessouky, A.A.; Farid, A.S.; Elshazly, A.M.; Mostafa, O.; El Gazzar, W.B.; Sorour, S.M.; Seleem, Y.; et al. Mesenchymal Stem Cell-Derived Exosomes Ameliorated Diabetic Nephropathy by Autophagy Induction through the mTOR Signaling Pathway. Cells 2018, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Liu, T.; Leng, Y.; Yang, W. Stem cell-derived exosomal MicroRNAs: Potential therapies in diabetic kidney disease. Biomed. Pharmacother. 2023, 164, 114961. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Miao, J.; Liu, W.; Cai, K.; Huang, X.; Peng, L. Mesenchymal Stem Cell-Derived Exosomes Carry MicroRNA-125a to Protect Against Diabetic Nephropathy by Targeting Histone Deacetylase 1 and Downregulating Endothelin-1. DMSO 2021, 14, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, J.; Chen, L. The roles of mesenchymal stem cell-derived exosomes in diabetes mellitus and its related complications. Front. Endocrinol. 2022, 13, 1027686. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jin, L.; Cui, Y.; Nie, A.; Xie, N.; Liang, G. Bone marrow mesenchymal stem cells-induced exosomal microRNA-486-3p protects against diabetic retinopathy through TLR4/NF-κB axis repression. J. Endocrinol. Investig. 2020, 44, 1193–1207. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jin, L.-Y.; Cui, Y.-B.; Xie, N. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-17-3p ameliorates inflammatory reaction and antioxidant injury of mice with diabetic retinopathy via targeting STAT1. Int. Immunopharmacol. 2021, 90, 107010. [Google Scholar] [CrossRef]
- Fan, B.; Li, C.; Szalad, A.; Wang, L.; Pan, W.; Zhang, R.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia 2019, 63, 431–443. [Google Scholar] [CrossRef]
- Fan, B.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Treatment of diabetic peripheral neuropathy with engineered mesenchymal stromal cell-derived exosomes enriched with microRNA-146a provide amplified therapeutic efficacy. Exp. Neurol. 2021, 341, 113694. [Google Scholar] [CrossRef]
- Cao, M.; Zhao, Y.; Chen, T.; Zhao, Z.; Zhang, B.; Yuan, C.; Wang, X.; Chen, L.; Wang, N.; Li, C.; et al. Adipose mesenchymal stem cell-derived exosomal microRNAs ameliorate polycystic ovary syndrome by protecting against metabolic disturbances. Biomaterials 2022, 288, 121739. [Google Scholar] [CrossRef]
- Kim, Y.D.; Kim, S.-G.; Hwang, S.-L.; Choi, H.-S.; Bae, J.-H.; Song, D.-K.; Im, S.-S. B-cell translocation gene 2 regulates hepatic glucose homeostasis via induction of orphan nuclear receptor Nur77 in diabetic mouse model. Diabetes 2014, 63, 1870–1880. [Google Scholar] [CrossRef] [PubMed]
- Ocansey, D.K.W.; Zhang, L.; Wang, Y.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Exosome-mediated effects and applications in inflammatory bowel disease. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1287–1307. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liang, Z.; Wang, F.; Zhou, C.; Zheng, X.; Hu, T.; He, X.; Wu, X.; Lan, P. Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight 2019, 4, 131273. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Peng, Y.; Li, Y.; Shi, T.; Luan, Y.; Yin, C. Role of stem cell derivatives in inflammatory diseases. Front. Immunol. 2023, 14, 1153901. [Google Scholar] [CrossRef] [PubMed]
- Soontararak, S.; Chow, L.; Johnson, V.; Coy, J.; Wheat, W.; Regan, D.; Dow, S. Mesenchymal Stem Cells (MSC) Derived from Induced Pluripotent Stem Cells (iPSC) Equivalent to Adipose-Derived MSC in Promoting Intestinal Healing and Microbiome Normalization in Mouse Inflammatory Bowel Disease Model. Stem Cells Transl. Med. 2018, 7, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Lu, B.; Xi, J.; Ocansey, D.K.W.; Mao, F.; Hao, D.; Yan, Y. hucMSC-Ex Alleviates IBD-Associated Intestinal Fibrosis by Inhibiting ERK Phosphorylation in Intestinal Fibroblasts. Stem Cells Int. 2023, 2023, 2828981. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, Y.; Liu, Z.; Yin, Y.; Li, Q.; Liu, G.; Yan, B. The Application Potential and Advance of Mesenchymal Stem Cell-Derived Exosomes in Myocardial Infarction. Stem Cells Int. 2021, 2021, 5579904. [Google Scholar] [CrossRef] [PubMed]
- Zamani, P.; Fereydouni, N.; Butler, A.E.; Navashenaq, J.G.; Sahebkar, A. The therapeutic and diagnostic role of exosomes in cardiovascular diseases. Trends Cardiovasc. Med. 2019, 29, 313–323. [Google Scholar] [CrossRef]
- Zhu, L.-P.; Tian, T.; Wang, J.-Y.; He, J.-N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.-X.; Qiu, X.-T.; Li, C.-C.; et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, W.; Wani, M.; Yu, X.; Ashraf, M. Ischemic Preconditioning Potentiates the Protective Effect of Stem Cells through Secretion of Exosomes by Targeting Mecp2 via miR-22. PLoS ONE 2014, 9, e88685. [Google Scholar] [CrossRef]
- Ma, T.; Chen, Y.; Chen, Y.; Meng, Q.; Sun, J.; Shao, L.; Yu, Y.; Huang, H.; Hu, Y.; Yang, Z.; et al. MicroRNA-132, Delivered by Mesenchymal Stem Cell-Derived Exosomes, Promote Angiogenesis in Myocardial Infarction. Stem Cells Int. 2018, 2018, 3290372. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, C.; Yang, D.; Liao, Q.; Luo, H.; Wang, X.; Zhou, F.; Yang, X.; Yang, J.; Zeng, C.; et al. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim. Biophys. Acta—Mol. Basis Dis. 2017, 1863, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhao, Y.; Sun, L.; Sun, X.; Zhao, X.; Sun, X.; Qian, H.; Xu, W.; Zhu, W. Exosomes Derived from Akt-Modified Human Umbilical Cord Mesenchymal Stem Cells Improve Cardiac Regeneration Promote Angiogenesis via Activating Platelet-Derived Growth Factor, D. Stem Cells Transl. Med. 2016, 6, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, Z.; Qi, Y.; Zhang, W.; Zhang, C.; Jiang, M.; Deng, S.; Wang, H. Exosomes from SIRT1-Overexpressing ADSCs Restore Cardiac Function by Improving Angiogenic Function of EPCs. Mol. Ther. Nucleic Acids 2020, 21, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Qiao, S.; Zhao, J.; Liu, Y.; Li, Q.; Wei, Z.; Dai, Q.; Kang, L.; Xu, B. miRNA-181a over-expression in mesenchymal stem cell-derived exosomes influenced inflammatory response after myocardial ischemia-reperfusion injury. Life Sci. 2019, 232, 116632. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wu, Y.; Xu, Y.; Li, G.; Li, Z.; Liu, T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: Recent advances and therapeutic potential. Mol. Cancer 2022, 21, 179. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Song, X.; Yang, F.; Wu, S.; Wang, J.; Chen, Z.; Liu, Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015, 8, 122. [Google Scholar] [CrossRef]
- Lou, G.; Chen, L.; Xia, C.; Wang, W.; Qi, J.; Li, A.; Zhao, L.; Chen, Z.; Zheng, M.; Liu, Y. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J. Exp. Clin. Cancer Res. 2020, 39, 4. [Google Scholar] [CrossRef]
- Yu, L.; Gui, S.; Liu, Y.; Qiu, X.; Zhang, G.; Zhang, X.; Pan, J.; Fan, J.; Qi, S.; Qiu, B. Exosomes derived from microRNA-199a-overexpressing mesenchymal stem cells inhibit glioma progression by down-regulating AGAP2. Aging 2019, 11, 5300–5318. [Google Scholar] [CrossRef]
- Luo, T.; von der Ohe, J.; Hass, R. MSC-Derived Extracellular Vesicles in Tumors and Therapy. Cancers 2021, 13, 5212. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, G.; Zhang, K.; Cao, Q.; Liu, T.; Li, J. Mesenchymal stem cells-derived exosomes for drug delivery. Stem Cell Res. Ther. 2021, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Shamili, F.H.; Bayegi, H.R.; Salmasi, Z.; Sadri, K.; Mahmoudi, M.; Kalantari, M.; Ramezani, M.; Abnous, K. Exosomes derived from TRAIL-engineered mesenchymal stem cells with effective anti-tumor activity in a mouse melanoma model. Int. J. Pharm. 2018, 549, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Tian, J.; Wang, Z.; Gao, Y.; Wu, X.; Ding, X.; Qiang, L.; Li, G.; Han, Z.; Yuan, Y.; et al. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J. Nanobiotechnol. 2019, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, J.; Li, J.; Li, M.; Ge, J.; Wu, P.; You, B.; Qian, H. Roles of Mesenchymal Stem Cell-Derived Exosomes in Cancer Development and Targeted Therapy. Stem Cells Int. 2021, 2021, 9962194. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liu, Y.; Qu, Y.; Liu, L.; Li, H. Exosomes Derived From MicroRNA-148b-3p-Overexpressing Human Umbilical Cord Mesenchymal Stem Cells Restrain Breast Cancer Progression. Front. Oncol. 2019, 9, 459965. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Mo, C.; Guo, S.; Zhuang, J.; Huang, B.; Mao, X. Human bone marrow mesenchymal stem cells-derived microRNA-205-containing exosomes impede the progression of prostate cancer through suppression of RHPN2. J. Exp. Clin. Cancer Res. 2019, 38, 495. [Google Scholar] [CrossRef]
- Wan, F.-Z.; Chen, K.-H.; Sun, Y.-C.; Chen, X.-C.; Liang, R.-B.; Chen, L.; Zhu, X.-D. Exosomes overexpressing miR-34c inhibit malignant behavior and reverse the radioresistance of nasopharyngeal carcinoma. J. Transl. Med. 2020, 18, 12. [Google Scholar] [CrossRef]
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Almadani, Y.H.; Vorstenbosch, J.; Davison, P.G.; Murphy, A.M. Wound Healing: A Comprehensive Review. Semin. Plast. Surg. 2021, 35, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, Y.; Zhang, Q. Human mesenchymal stem cell-derived exosomes accelerate wound healing of mice eczema. J. Dermatol. Treat 2022, 33, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.S.; Kim, J.O.; Ha, D.H.; Yi, Y.W. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res. Ther. 2018, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Liu, J.; Zheng, C.; Su, Y.; Bao, L.; Zhu, B.; Liu, S.; Wang, L.; Wang, X.; Wang, Y.; et al. Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis. Cell Prolif. 2020, 53, e12830. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liu, W.; Li, J.; Lu, J.; Lu, H.; Jia, W.; Liu, F. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res. Ther. 2020, 11, 350. [Google Scholar] [CrossRef]
- Gao, S.; Chen, T.; Hao, Y.; Zhang, F.; Tang, X.; Wang, D.; Wei, Z.; Qi, J. Exosomal miR-135a derived from human amnion mesenchymal stem cells promotes cutaneous wound healing in rats and fibroblast migration by directly inhibiting LATS2 expression. Stem Cell Res. Ther. 2020, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hong, G.; Zhan, W.; Deng, M.; Tu, C.; Wei, J.; Lin, H. Endothelial progenitor cell derived exosomes mediated miR-182-5p delivery accelerate diabetic wound healing via down-regulating, P.P.A.R.G. Int. J. Med. Sci. 2023, 20, 468–481. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, F.; Liu, Z.; Zuo, K.; Wang, B.; Zhang, Y.; Han, X.; Lian, A.; Wang, Y.; Liu, M.; et al. MSC-derived exosomes attenuate cell death through suppressing AIF nucleus translocation and enhance cutaneous wound healing. Stem Cell Res. Ther. 2020, 11, 174. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, F.; Gu, L.; Ji, P.; Yang, X.; Liu, M.; Tao, K.; Hu, D. Adipose mesenchymal stem cell exosomes promote wound healing through accelerated keratinocyte migration and proliferation by activating the AKT/HIF-1α axis. J. Mol. Histol. 2020, 51, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.K.; Kim, H.; Kim, T.M. Exosomes Secreted from Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Accelerate Skin Cell Proliferation. Int. J. Mol. Sci. 2018, 19, 3119. [Google Scholar] [CrossRef]
- He, L.; Zhu, C.; Jia, J.; Hao, X.-Y.; Yu, X.-Y.; Liu, X.-Y.; Shu, M.-G. ADSC-Exos containing MALAT1 promotes wound healing by targeting miR-124 through activating Wnt/β-catenin pathway. Biosci. Rep. 2020, 40, BSR20192549. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-β/SMAD2 Pathway During Wound Healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, Y.; Huang, Y.; Su, Y. Human umbilical cord mesenchymal stem cell-derived exosomes suppress dermal fibroblasts-myofibroblats transition via inhibiting the TGF-β1/Smad 2/3 signaling pathway. Exp. Mol. Pathol. 2020, 115, 104468. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Zhang, S.; Barthe, S.; Kumar, P.; Pivetti, C.; Kreutzberg, N.; Reed, C.; Wang, Y.; Paxton, Z.; Farmer, D.; et al. Placental Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Myelin Regeneration in an Animal Model of Multiple Sclerosis. Cells 2019, 8, 1497. [Google Scholar] [CrossRef]
- Li, Z.; Liu, F.; He, X.; Yang, X.; Shan, F.; Feng, J. Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int. Immunopharmacol. 2019, 67, 268–280. [Google Scholar] [CrossRef]
- Mokarizadeh, A.; Delirezh, N.; Morshedi, A.; Mosayebi, G.; Farshid, A.-A.; Mardani, K. Microvesicles derived from mesenchymal stem cells: Potent organelles for induction of tolerogenic signaling. Immunol. Lett. 2012, 147, 47–54. [Google Scholar] [CrossRef]
- Shen, Z.; Huang, W.; Liu, J.; Tian, J.; Wang, S.; Rui, K. Effects of Mesenchymal Stem Cell-Derived Exosomes on Autoimmune Diseases. Front. Immunol. 2021, 12, 749192. [Google Scholar] [CrossRef]
- Wang, H.; Huber, C.C.; Li, X.-P. Mesenchymal and Neural Stem Cell-Derived Exosomes in Treating Alzheimer’s Disease. Bioengineering 2023, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Reza-Zaldivar, E.E.; Hernández-Sapiéns, M.A.; Gutiérrez-Mercado, Y.K.; Sandoval-Ávila, S.; Gomez-Pinedo, U.; Márquez-Aguirre, A.L.; Méndez, E.; Padilla-Camberos, E. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural. Regen. Res. 2019, 14, 1626–1634. [Google Scholar] [PubMed]
- Xiong, W.-P.; Yao, W.-Q.; Wang, B.; Liu, K. BMSCs-exosomes containing GDF-15 alleviated SH-SY5Y cell injury model of Alzheimer’s disease via AKT/GSK-3β/β-catenin. Brain Res. Bull. 2021, 177, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-Q.; Pu, J.-L.; Zheng, R.; Fang, Y.; Gu, L.-Y.; Guo, T.; Si, X.; Zhou, C.; Chen, Y.; Liu, Y.; et al. Different patterns of exosomal α-synuclein between Parkinson’s disease and probable rapid eye movement sleep behavior disorder. Eur. J. Neurol. 2022, 29, 3590–3599. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xie, Z.; Zhang, X.; Mao, J.; Wang, M.; Wei, S.; Fu, Y.; Zheng, H.; He, Y.; Chen, H.; et al. Investigation of α-Synuclein Species in Plasma Exosomes and the Oligomeric and Phosphorylated α-Synuclein as Potential Peripheral Biomarker of Parkinson’s Disease. Neuroscience 2021, 469, 79–90. [Google Scholar] [CrossRef]
- Si, X.; Tian, J.; Chen, Y.; Yan, Y.; Pu, J.; Zhang, B. Central Nervous System-Derived Exosomal Alpha-Synuclein in Serum May Be a Biomarker in Parkinson’s Disease. Neuroscience 2019, 413, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Kang, W.; Liu, Z.; Zhu, X. The role of exosomes in the diagnosis of Parkinson’s disease. Heliyon 2023, 9, e20595. [Google Scholar] [CrossRef] [PubMed]
- Jarmalavičiūtė, A.; Tunaitis, V.; Pivoraitė, U.; Venalis, A.; Pivoriūnas, A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy 2015, 17, 932–939. [Google Scholar] [CrossRef]
- Xue, C.; Li, X.; Ba, L.; Zhang, M.; Yang, Y.; Gao, Y.; Sun, Z.; Han, Q.; Zhao, R.C. MSC-Derived Exosomes can Enhance the Angiogenesis of Human Brain MECs and Show Therapeutic Potential in a Mouse Model of Parkinson’s Disease. Aging Dis. 2021, 12, 1211–1222. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, K.; Jiang, J.; Lin, S. Mesenchymal stem cell-derived exosomes as delivery vehicles for non-coding RNAs in lung diseases. Biomed. Pharmacother. 2024, 170, 116008. [Google Scholar] [CrossRef]
- Azhdari, M.H.; Goodarzi, N.; Doroudian, M.; MacLoughlin, R. Molecular Insight into the Therapeutic Effects of Stem Cell-Derived Exosomes in Respiratory Diseases and the Potential for Pulmonary Delivery. Int. J. Mol. Sci. 2022, 23, 6273. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Cui, T.; Chen, J.; Mao, X.; Zhang, T. Advancements in engineered mesenchymal stem cell exosomes for chronic lung disease treatment. J. Transl. Med. 2023, 21, 895. [Google Scholar] [CrossRef] [PubMed]
- Shapira, S.; Schwartz, R.; Tsiodras, S.; Bar-Shai, A.; Melloul, A.; Borsekofsky, S.; Peer, M.; Adi, N.; MacLoughlin, R.; Arber, N. Inhaled CD24-Enriched Exosomes (EXO-CD24) as a Novel Immune Modulator in Respiratory Disease. Int. J. Mol. Sci. 2023, 25, 77. [Google Scholar] [CrossRef]
- Shapira, S.; Ben Shimon, M.; Hay-Levi, M.; Shenberg, G.; Choshen, G.; Bannon, L.; Tepper, M.; Kazanov, D.; Seni, J.; Lev-Ari, S.; et al. A novel platform for attenuating immune hyperactivity using EXO-CD24 in COVID-19 and beyond. EMBO Mol. Med. 2022, 14, e15997. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Cheng, Z.; Qin, W.; Jiang, L. Exosomes as a liquid biopsy for lung cancer. Lung Cancer 2018, 116, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Review on Strategies and Technologies for Exosome Isolation and Purification. Front. Bioeng. Biotechnol. 2022, 9, 811971. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, opportunity, and perspective on exosome isolation—Efforts for efficient exosome-based theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef]
- Zarovni, N.; Corrado, A.; Guazzi, P.; Zocco, D.; Lari, E.; Radano, G.; Muhhina, J.; Fondelli, C.; Gavrilova, J.; Chiesi, A. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods 2015, 87, 46–58. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3.22.1–3.22.29. [Google Scholar] [CrossRef]
- Gandham, S.; Su, X.; Wood, J.; Nocera, A.L.; Alli, S.C.; Milane, L.; Zimmerman, A.; Amiji, M.; Ivanov, A.R. Technologies and Standardization in Research on Extracellular Vesicles. Trends Biotechnol. 2020, 38, 1066. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, K.P.; Rossi, I.; Abdullahi, M.; Ramirez, M.I.; Stratton, D.; Inal, J.M. Isolation and characterization of extracellular vesicles and future directions in diagnosis and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1835. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Crow, J.; Roth, M.; Zeng, Y.; Godwin, A.K. Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology. Lab Chip 2014, 14, 3773–3780. [Google Scholar] [CrossRef]
- Engin, A. Dark-Side of Exosomes. Protein Kinase-Mediated Decisions Between Life and Death; Springer: Cham, Switzerland, 2021; pp. 101–131. [Google Scholar]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A review on exosomes application in clinical trials: Perspective, questions, and challenges. Cell Commun. Signal. 2022, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Mostafa, M.A.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.-N.; Essa, W.; Adel, H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 2016, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, A.; Wairkar, S. Management of hyperpigmentation: Current treatments and emerging therapies. Pigment Cell Melanoma Res. 2021, 34, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Allogenic Mesenchymal Stem Cell Derived Exosome in Patients with Acute Ischemic Stroke. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03384433 (accessed on 4 September 2023).
- Chen, Y.-A.; Lu, C.-H.; Ke, C.-C.; Liu, R.-S. Mesenchymal Stem Cell-Derived Extracellular Vesicle-Based Therapy for Alzheimer’s Disease: Progress and Opportunity. Membranes 2021, 11, 796. [Google Scholar] [CrossRef]
- ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT04388982 (accessed on 15 March 2024).
- Case Medical Research. A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia. Case Medical Research. 2020. Available online: https://clinicaltrials.gov/study/NCT04276987#publications (accessed on 10 September 2023).
- Pinky; Gupta, S.; Krishnakumar, V.; Sharma, Y.; Dinda, A.K.; Mohanty, S. Mesenchymal Stem Cell Derived Exosomes: A Nano Platform for Therapeutics and Drug Delivery in Combating COVID-19. Stem Cell Rev. Rep. 2021, 17, 33–43. [Google Scholar] [CrossRef]
- Plava, J.; Cihova, M.; Burikova, M.; Matuskova, M.; Kucerova, L.; Miklikova, S. Recent advances in understanding tumor stroma-mediated chemoresistance in breast cancer. Mol. Cancer 2019, 18, 67. [Google Scholar] [CrossRef]
- Karn, V.; Ahmed, S.; Tsai, L.-W.; Dubey, R.; Ojha, S.; Singh, H.N.; Kumar, M.; Gupta, P.K.; Sadhu, S.; Jha, N.K.; et al. Extracellular Vesicle-Based Therapy for COVID-19, Promises, Challenges and Future Prospects. Biomedicines 2021, 9, 1373. [Google Scholar] [CrossRef] [PubMed]
- Lőrincz, Á.M.; Timár, C.I.; Marosvári, K.A.; Veres, D.S.; Otrokocsi, L.; Kittel, Á.; Ligeti, E. Effect of storage on physical and functional properties of extracellular vesicles derived from neutrophilic granulocytes. J. Extracell Vesicles 2014, 3, 25465. [Google Scholar] [CrossRef] [PubMed]
- Scarfe, L.; Taylor, A.; Sharkey, J.; Harwood, R.; Barrow, M.; Comenge, J.; Beeken, L.; Astley, C.; Santeramo, I.; Hutchinson, C.; et al. Non-invasive imaging reveals conditions that impact distribution and persistence of cells after in vivo administration. Stem Cell Res. Ther. 2018, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Rezabakhsh, A.; Sokullu, E.; Rahbarghazi, R. Applications, challenges and prospects of mesenchymal stem cell exosomes in regenerative medicine. Stem Cell Res. Ther. 2021, 12, 521. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Defourny, K.A.Y.; Smid, E.J.; Abee, T. Gram-Positive Bacterial Extracellular Vesicles and Their Impact on Health and Disease. Front. Microbiol. 2018, 9, 1502. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, J.; Aslan, C.; Ahmadi, M.; Zolbanin, N.M.; Kashanchi, F.; Jafari, R. The versatile role of exosomes in human retroviral infections: From immunopathogenesis to clinical application. Cell Biosci. 2021, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research—Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef]
- Woo, H.-K.; Sunkara, V.; Park, J.; Kim, T.-H.; Han, J.-R.; Kim, C.-J.; Choi, H.-I.; Kim, Y.-K.; Cho, Y.-K. Exodisc for Rapid, Size-Selective, and Efficient Isolation and Analysis of Nanoscale Extracellular Vesicles from Biological Samples. ACS Nano 2017, 11, 1360–1370. [Google Scholar] [CrossRef]

| Method | Description | Advantages | Limitations |
|---|---|---|---|
| Ultrafiltration | Separates based on size/weight [197]. | Easy with no need for special equipment. | Time -consuming, and may not be suitable for blood samples, and clogging of filtration membrane. |
| Chromatography | Uses molecular size for separation [198]. | Gentle isolation, preserves exosome integrity. | Limited from blood (1–5%) [199]. |
| Ultracentrifugation | Relies on density/size differences [200]. | Effective for large samples. | Labor-intensive, potential damage to exosomes [201]. |
| Immunoaffinity | Targets specific proteins for isolation [197]. | High specificity. | Requires specific antibodies and validation, which is expensive. Requires concentrated small volumes [197]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulmalek, O.A.A.Y.; Husain, K.H.; AlKhalifa, H.K.A.A.; Alturani, M.M.A.B.; Butler, A.E.; Moin, A.S.M. Therapeutic Applications of Stem Cell-Derived Exosomes. Int. J. Mol. Sci. 2024, 25, 3562. https://doi.org/10.3390/ijms25063562
Abdulmalek OAAY, Husain KH, AlKhalifa HKAA, Alturani MMAB, Butler AE, Moin ASM. Therapeutic Applications of Stem Cell-Derived Exosomes. International Journal of Molecular Sciences. 2024; 25(6):3562. https://doi.org/10.3390/ijms25063562
Chicago/Turabian StyleAbdulmalek, Omar Abdulhakeem Ahmed Yusuf, Khaled Hameed Husain, Haya Khaled Ali Abdulla AlKhalifa, Mariam Masood Abdulkarim Bahrooz Alturani, Alexandra E. Butler, and Abu Saleh Md Moin. 2024. "Therapeutic Applications of Stem Cell-Derived Exosomes" International Journal of Molecular Sciences 25, no. 6: 3562. https://doi.org/10.3390/ijms25063562
APA StyleAbdulmalek, O. A. A. Y., Husain, K. H., AlKhalifa, H. K. A. A., Alturani, M. M. A. B., Butler, A. E., & Moin, A. S. M. (2024). Therapeutic Applications of Stem Cell-Derived Exosomes. International Journal of Molecular Sciences, 25(6), 3562. https://doi.org/10.3390/ijms25063562







