The Senescent Heart—“Age Doth Wither Its Infinite Variety”
Abstract
1. Introduction
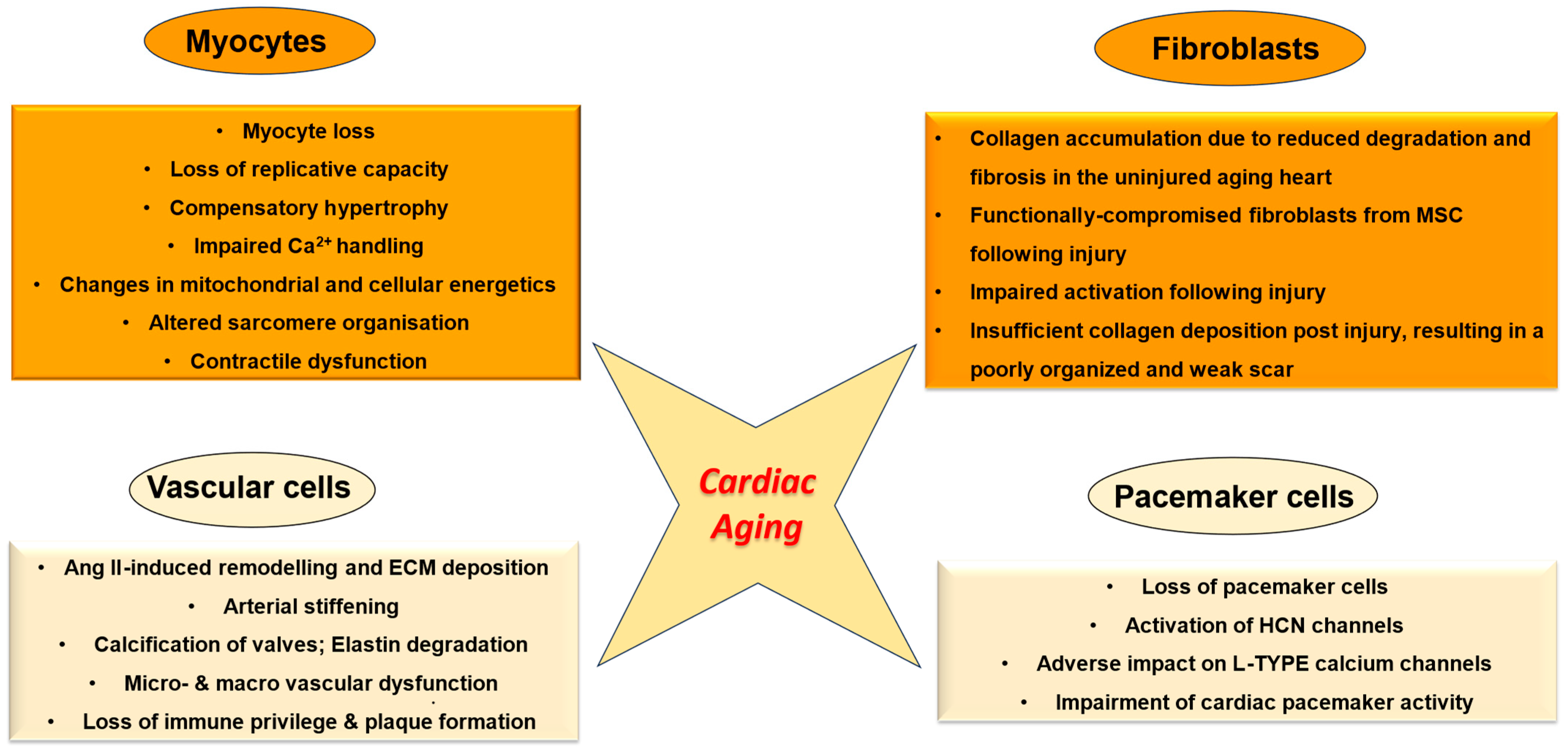
2. The Aging Heart—An Overview
3. Myocytes in Cardiac Aging
3.1. Increased Cell Death and Loss of Replicative Capacity
3.2. Myocyte Hypertrophy
3.3. Ca2+ Handling
3.4. Mitochondrial Changes and Cellular Energetics
3.5. Impairment of Cardiac Pacemaker Activity with Aging
4. Role of Non-Myocyte Cells in Cardiac Aging
4.1. Vascular Changes
4.2. Pericytes in the Aging Heart
5. Cardiac Fibroblasts and Aging
5.1. Origin of Cardiac Fibroblasts
5.2. Mechanisms of Cardiac Fibroblast-Mediated Wound Healing in the Myocardium
5.3. Impaired Wound Healing and Fibrosis in the Aged Myocardium
5.4. Dysregulated Collagen Turnover Promotes Excessive Collagen Deposition in the Aged Myocardium
5.5. Dysregulation of Cardiac Fibroblast Response to Injury in the Aging Heart
5.6. Aging and a Poorly Organised Scar in the Injured Heart
5.7. Myocardial Aging and Impaired Immune Response to Injury
5.8. Molecular Changes in Fibroblasts with Aging
6. Signalling Pathways in the Aging Myocardium
6.1. The TGF-β Signalling Pathway in the Aging Heart
6.2. MAPK Signalling in the Aging Heart
6.3. The Renin–Angiotensin–Aldosterone Pathway
6.4. MicroRNAs in Aging
7. Senescence-Associated Secretory Phenotype in Cardiac Aging
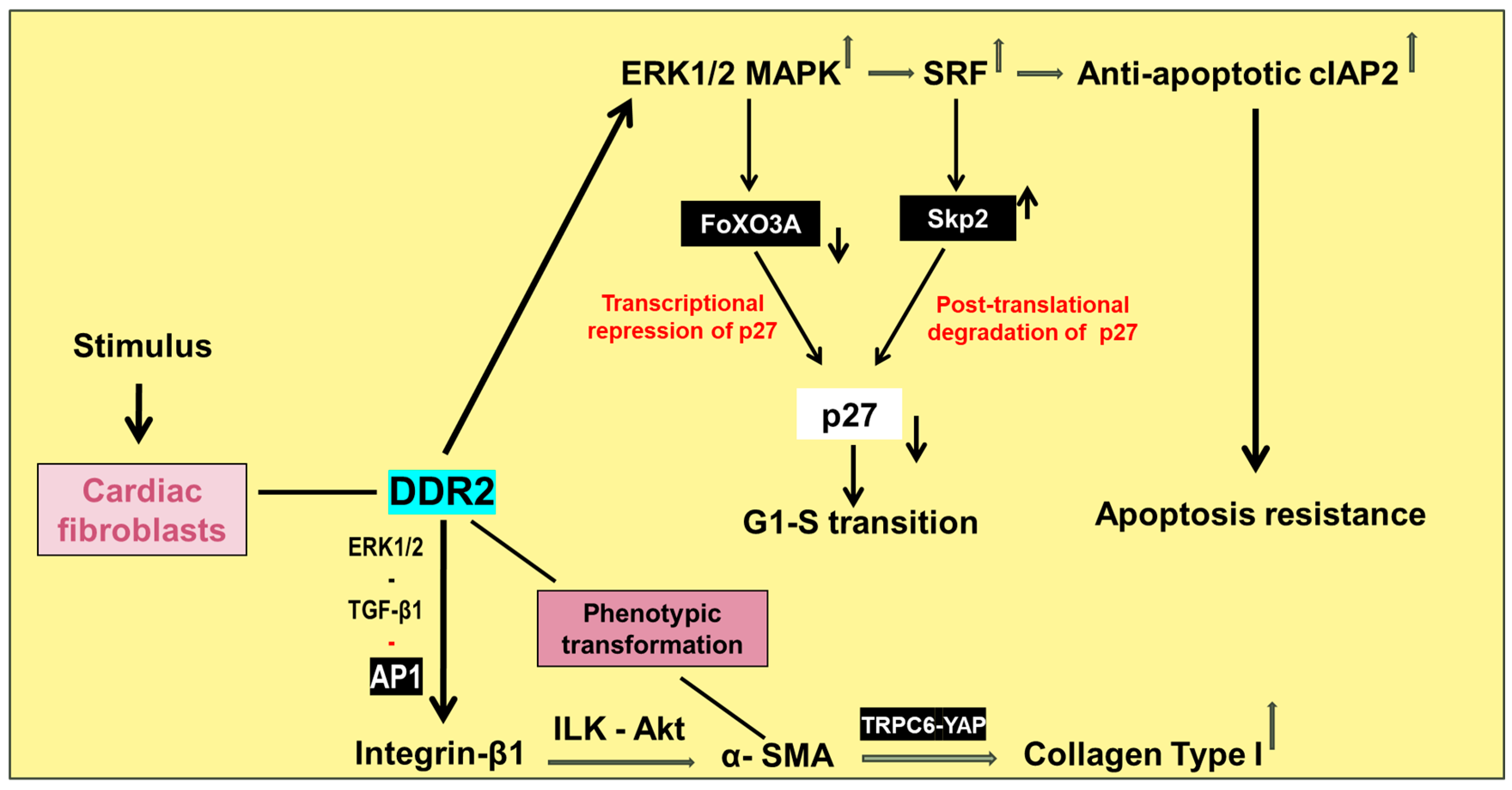
8. Discoidin Domain Receptor 2—A Potential Therapeutic Target to Mitigate Cardiac Fibrosis
9. Summary and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balcombe, N.R.; Sinclair, A. Ageing: Definitions, Mechanisms and the Magnitude of the Problem. Best Pract. Res. Clin. Gastroenterol. 2001, 15, 835–849. [Google Scholar] [CrossRef]
- Todhunter, M.E.; Sayaman, R.W.; Miyano, M.; LaBarge, M.A. Tissue Aging: The Integration of Collective and Variant Responses of Cells to Entropic Forces over Time. Curr. Opin. Cell Biol. 2018, 54, 121–129. [Google Scholar] [CrossRef]
- Harman, D. The Aging Process: Major Risk Factor for Disease and Death. Proc. Natl. Acad. Sci. USA 1991, 88, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Li, Y.; Li, R.; Yan, Y.; Zhang, D.; Li, T.; Li, Z.; Sun, Y.; Zhen, H.; Ding, J.; et al. Distinct Biological Ages of Organs and Systems Identified from a Multi-Omics Study. Cell Rep. 2022, 38, 110459. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, L.; Sirago, G.; Giacomello, E. Experimental Models for Ageing Research. Histol. Histopathol. 2023, 38, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T.; Sun, Y.; Katwa, L.C.; Cleutjens, J.P. Connective Tissue: A Metabolic Entity? J. Mol. Cell. Cardiol. 1995, 27, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Souders, C.A.; Bowers, S.L.K.; Baudino, T.A. Cardiac Fibroblast: The Renaissance Cell. Circ. Res. 2009, 105, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, D.; Laufer, G. The Aging Cardiomyocyte: A Mini-Review. Gerontology 2008, 54, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.-F.; Chen, T.; Johnson, S.C.; Szeto, H.; Rabinovitch, P.S. Cardiac Aging: From Molecular Mechanisms to Significance in Human Health and Disease. Antioxid. Redox Signal 2012, 16, 1492–1526. [Google Scholar] [CrossRef]
- Strait, J.B.; Lakatta, E.G. Aging-Associated Cardiovascular Changes and Their Relationship to Heart Failure. Heart Fail. Clin. 2012, 8, 143–164. [Google Scholar] [CrossRef]
- Fajemiroye, J.O.; da Cunha, L.C.; Saavedra-Rodríguez, R.; Rodrigues, K.L.; Naves, L.M.; Mourão, A.A.; da Silva, E.F.; Williams, N.E.E.; Martins, J.L.R.; Sousa, R.B.; et al. Aging-Induced Biological Changes and Cardiovascular Diseases. BioMed Res. Int. 2018, 2018, e7156435. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Levy, D. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises. Circulation 2003, 107, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Nakou, E.S.; Parthenakis, F.I.; Kallergis, E.M.; Marketou, M.E.; Nakos, K.S.; Vardas, P.E. Healthy Aging and Myocardium: A Complicated Process with Various Effects in Cardiac Structure and Physiology. Int. J. Cardiol. 2016, 209, 167–175. [Google Scholar] [CrossRef]
- Cheng, S.; Fernandes, V.R.; Bluemke, D.A.; McClelland, R.L.; Kronmal, R.A.; Lima, J.A. Age-Related Left Ventricular Remodeling and Associated Risk for Cardiovascular Outcomes: The Multi-Ethnic Study of Atherosclerosis. Circ. Cardiovasc. Imaging 2009, 2, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Singam, N.S.V.; Fine, C.; Fleg, J.L. Cardiac Changes Associated with Vascular Aging. Clin. Cardiol. 2019, 43, 92–98. [Google Scholar] [CrossRef]
- Lakatta, E.G. Cardiovascular Aging Research: The Next Horizons. J. Am. Geriatr. Soc. 1999, 47, 613–625. [Google Scholar] [CrossRef]
- Tallquist, M.D.; Molkentin, J.D. Redefining the Identity of Cardiac Fibroblasts. Nat. Rev. Cardiol. 2017, 14, 484–491. [Google Scholar] [CrossRef]
- Biernacka, A.; Frangogiannis, N.G. Aging and Cardiac Fibrosis. Aging Dis. 2011, 2, 158–173. [Google Scholar]
- Sheydina, A.; Riordon, D.R.; Boheler, K.R. Molecular Mechanisms of Cardiomyocyte Aging. Clin. Sci. 2011, 121, 315–329. [Google Scholar] [CrossRef]
- de Wit, L.; Fang, J.; Neef, K.; Xiao, J.; Doevendans, P.A.; Schiffelers, R.M.; Lei, Z.; Sluijter, J.P.G. Cellular and Molecular Mechanism of Cardiac Regeneration: A Comparison of Newts, Zebrafish, and Mammals. Biomolecules 2020, 10, 1204. [Google Scholar] [CrossRef]
- Anversa, P.; Leri, A.; Kajstura, J.; Nadal-Ginard, B. Myocyte Growth and Cardiac Repair. J. Mol. Cell. Cardiol. 2002, 34, 91–105. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for Cardiomyocyte Renewal in Humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Oh, H.; Taffet, G.E.; Youker, K.A.; Entman, M.L.; Overbeek, P.A.; Michael, L.H.; Schneider, M.D. Telomerase Reverse Transcriptase Promotes Cardiac Muscle Cell Proliferation, Hypertrophy, and Survival. Proc. Natl. Acad. Sci. USA 2001, 98, 10308–10313. [Google Scholar] [CrossRef]
- Anversa, P.; Palackal, T.; Sonnenblick, E.H.; Olivetti, G.; Meggs, L.G.; Capasso, J.M. Myocyte Cell Loss and Myocyte Cellular Hyperplasia in the Hypertrophied Aging Rat Heart. Circ. Res. 1990, 67, 871–885. [Google Scholar] [CrossRef]
- Anversa, P.; Hiler, B.; Ricci, R.; Guideri, G.; Olivetti, G. Myocyte Cell Loss and Myocyte Hypertrophy in the Aging Rat Heart. J. Am. Coll. Cardiol. 1986, 8, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Eduardo Carreño, J.; Apablaza, F.; Ocaranza, M.P.; Jalil, J.E. Cardiac Hypertrophy: Molecular and Cellular Events. Rev. Esp. Cardiol. 2006, 59, 473–486. [Google Scholar] [CrossRef]
- Frey, N.; Katus, H.A.; Olson, E.N.; Hill, J.A. Hypertrophy of the Heart. Circulation 2004, 109, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Sessions, A.O.; Engler, A.J. Mechanical Regulation of Cardiac Aging in Model Systems. Circ. Res. 2016, 118, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Heineke, J.; Molkentin, J.D. Regulation of Cardiac Hypertrophy by Intracellular Signalling Pathways. Nat. Rev. Mol. Cell Biol. 2006, 7, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Muslin, A.J. MAPK Signalling in Cardiovascular Health and Disease: Molecular Mechanisms and Therapeutic Targets. Clin. Sci. 2008, 115, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Zhang, Y.; Ceylan-Isik, A.F.; Wold, L.E.; Nunn, J.M.; Ren, J. Chronic Akt Activation Accentuates Aging-Induced Cardiac Hypertrophy and Myocardial Contractile Dysfunction: Role of Autophagy. Basic. Res. Cardiol. 2011, 106, 1173–1191. [Google Scholar] [CrossRef]
- Weichhart, T. mTOR as Regulator of Lifespan, Aging and Cellular Senescence. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Chiao, Y.A.; Rabinovitch, P.S. The Aging Heart. Cold Spring Harb. Perspect. Med. 2015, 5, a025148. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Yang, Z.; Fang, H.; Xiang, J.; Xu, C.; Zhou, Y.; Wu, Q.; Liu, J. Aging Attenuates Cardiac Contractility and Affects Therapeutic Consequences for Myocardial Infarction. Aging Dis. 2020, 11, 365–376. [Google Scholar] [CrossRef]
- Fares, E.; Howlett, S.E. Effect of Age on Cardiac Excitation–Contraction Coupling. Clin. Exp. Pharmacol. Physiol. 2010, 37, 1–7. [Google Scholar] [CrossRef]
- Saeed, Y.; Temple, I.P.; Borbas, Z.; Atkinson, A.; Yanni, J.; Maczewski, M.; Mackiewicz, U.; Aly, M.; Logantha, S.J.R.J.; Garratt, C.J.; et al. Structural and Functional Remodeling of the Atrioventricular Node with Aging in Rats: The Role of Hyperpolarization-Activated Cyclic Nucleotide–Gated and Ryanodine 2 Channels. Heart Rhythm. 2018, 15, 752–760. [Google Scholar] [CrossRef]
- Lompre, A.M.; Mercadier, J.J.; Wisnewsky, C.; Bouveret, P.; Pantaloni, C.; D’Albis, A.; Schwartz, K. Species- and Age-Dependent Changes in the Relative Amounts of Cardiac Myosin Isoenzymes in Mammals. Dev. Biol. 1981, 84, 286–290. [Google Scholar] [CrossRef]
- Long, X.; Boluyt, M.O.; O’Neill, L.; Zheng, J.S.; Wu, G.; Nitta, Y.K.; Crow, M.T.; Lakatta, E.G. Myocardial Retinoid X Receptor, Thyroid Hormone Receptor, and Myosin Heavy Chain Gene Expression in the Rat during Adult Aging. J. Gerontol. A Biol. Sci. Med. Sci. 1999, 54, B23–B27. [Google Scholar] [CrossRef] [PubMed]
- Mercadier, J.J.; de la Bastie, D.; Ménasché, P.; Van Cao, A.N.; Bouveret, P.; Lorente, P.; Piwnica, A.; Slama, R.; Schwartz, K. Alpha-Myosin Heavy Chain Isoform and Atrial Size in Patients with Various Types of Mitral Valve Dysfunction: A Quantitative Study. J. Am. Coll. Cardiol. 1987, 9, 1024–1030. [Google Scholar] [CrossRef]
- Carrier, L.; Boheler, K.R.; Chassagne, C.; de la Bastie, D.; Wisnewsky, C.; Lakatta, E.G.; Schwartz, K. Expression of the Sarcomeric Actin Isogenes in the Rat Heart with Development and Senescence. Circ. Res. 1992, 70, 999–1005. [Google Scholar] [CrossRef]
- Cain, B.S.; Meldrum, D.R.; Joo, K.S.; Wang, J.-F.; Meng, X.; Cleveland, J.C.; Banerjee, A.; Harken, A.H. Human SERCA2a Levels Correlate Inversely with Age in Senescent Human Myocardium. J. Am. Coll. Cardiol. 1998, 32, 458–467. [Google Scholar] [CrossRef]
- Koban, M.U.; Brugh, S.A.; Riordon, D.R.; Dellow, K.A.; Yang, H.-T.; Tweedie, D.; Boheler, K.R. A Distant Upstream Region of the Rat Multipartite Na+–Ca2+ Exchanger NCX1 Gene Promoter Is Sufficient to Confer Cardiac-Specific Expression. Mech. Dev. 2001, 109, 267–279. [Google Scholar] [CrossRef]
- Del Ry, S.; Maltinti, M.; Giannessi, D.; Cavallini, G.; Bergamini, E. Age-Related Changes in Endothelin-1 Receptor Subtypes in Rat Heart. Exp. Aging Res. 2008, 34, 251–266. [Google Scholar] [CrossRef]
- Heymes, C.; Silvestre, J.S.; Llorens-Cortes, C.; Chevalier, B.; Marotte, F.; Levy, B.I.; Swynghedauw, B.; Samuel, J.L. Cardiac Senescence Is Associated with Enhanced Expression of Angiotensin II Receptor Subtypes. Endocrinology 1998, 139, 2579–2587. [Google Scholar] [CrossRef] [PubMed]
- Wenz, T. Mitochondria and PGC-1α in Aging and Age-Associated Diseases. J. Aging Res. 2011, 2011, 810619. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.-F.; Santana, L.F.; Vermulst, M.; Tomazela, D.M.; Emond, M.J.; MacCoss, M.J.; Gollahon, K.; Martin, G.M.; Loeb, L.A.; Ladiges, W.C.; et al. Overexpression of Catalase Targeted to Mitochondria Attenuates Murine Cardiac Aging. Circulation 2009, 119, 2789–2797. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.-F.; Chen, T.; Wanagat, J.; Laflamme, M.; Marcinek, D.J.; Emond, M.J.; Ngo, C.P.; Prolla, T.A.; Rabinovitch, P.S. Age-Dependent Cardiomyopathy in Mitochondrial Mutator Mice Is Attenuated by Overexpression of Catalase Targeted to Mitochondria. Aging Cell 2010, 9, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Sithara, T.; Drosatos, K. Metabolic Complications in Cardiac Aging. Front. Physiol. 2021, 12, 669497. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.L.; Jaswal, J.S.; Stanley, W.C. Myocardial Fatty Acid Metabolism in Health and Disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.B.; Newgard, C.B.; et al. Mitochondrial Overload and Incomplete Fatty Acid Oxidation Contribute to Skeletal Muscle Insulin Resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Drosatos, K.; Schulze, P.C. Cardiac Lipotoxicity: Molecular Pathways and Therapeutic Implications. Curr. Heart Fail. Rep. 2013, 10, 109–121. [Google Scholar] [CrossRef]
- Kates, A.M.; Herrero, P.; Dence, C.; Soto, P.; Srinivasan, M.; Delano, D.G.; Ehsani, A.; Gropler, R.J. Impact of Aging on Substrate Metabolism by the Human Heart. J. Am. Coll. Cardiol. 2003, 41, 293–299. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Yin, F.C. Myocardial Aging: Functional Alterations and Related Cellular Mechanisms. Am. J. Physiol. 1982, 242, H927–H941. [Google Scholar] [CrossRef] [PubMed]
- Galassi, A.; Reynolds, K.; He, J. Metabolic Syndrome and Risk of Cardiovascular Disease: A Meta-Analysis. Am. J. Med. 2006, 119, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Takahara, S.; Soni, S.; Maayah, Z.H.; Ferdaoussi, M.; Dyck, J.R.B. Ketone Therapy for Heart Failure: Current Evidence for Clinical Use. Cardiovasc. Res. 2021, 118, cvab068. [Google Scholar] [CrossRef] [PubMed]
- Mallet, R.T.; Sun, J. Antioxidant Properties of Myocardial Fuels. Mol. Cell Biochem. 2003, 253, 103–111. [Google Scholar] [CrossRef]
- Ostchega, Y.; Porter, K.S.; Hughes, J.; Dillon, C.F.; Nwankwo, T. Resting Pulse Rate Reference Data for Children, Adolescents, and Adults: United States, 1999–2008. Natl. Health Stat. Rep. 2011, 41, 1–16. [Google Scholar]
- Choi, S.; Vivas, O.; Baudot, M.; Moreno, C.M. Aging Alters the Formation and Functionality of Signaling Microdomains between L-Type Calcium Channels and Β2-Adrenergic Receptors in Cardiac Pacemaker Cells. Front. Physiol. 2022, 13, 805909. [Google Scholar] [CrossRef]
- Peters, C.H.; Sharpe, E.J.; Proenza, C. Cardiac Pacemaker Activity and Aging. Annu. Rev. Physiol. 2020, 82, 21–43. [Google Scholar] [CrossRef]
- Alghamdi, A.M.; Boyett, M.R.; Hancox, J.C.; Zhang, H. Cardiac Pacemaker Dysfunction Arising from Different Studies of Ion Channel Remodeling in the Aging Rat Heart. Front. Physiol. 2020, 11, 546508. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.A.; George, S.J. The Effect of Ageing on Vascular Smooth Muscle Cell Behaviour—A Mini-Review. Gerontology 2015, 61, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Hariri, R.J.; Alonso, D.R.; Hajjar, D.P.; Coletti, D.; Weksler, M.E. Aging and Arteriosclerosis. I. Development of Myointimal Hyperplasia after Endothelial Injury. J. Exp. Med. 1986, 164, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Andrés, V.; Castiglioni, S.; Giudici, A.; Lau, E.S.; Nemcsik, J.; Seta, F.; Zaninotto, P.; Catalano, M.; Hamburg, N.M. Aging and Vascular Disease: A Multidisciplinary Overview. J. Clin. Med. 2023, 12, 5512. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. Nitric Oxide Signaling in Health and Disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef] [PubMed]
- Bachschmid, M.M.; Schildknecht, S.; Matsui, R.; Zee, R.; Haeussler, D.; Cohen, R.A.; Pimental, D.; Loo, B. van der Vascular Aging: Chronic Oxidative Stress and Impairment of Redox Signaling-Consequences for Vascular Homeostasis and Disease. Ann. Med. 2013, 45, 17–36. [Google Scholar] [CrossRef]
- Morgan, R.G.; Donato, A.J.; Walker, A.E. Telomere Uncapping and Vascular Aging. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1–H5. [Google Scholar] [CrossRef]
- Ding, Y.-N.; Tang, X.; Chen, H.-Z.; Liu, D.-P. Epigenetic Regulation of Vascular Aging and Age-Related Vascular Diseases. Adv. Exp. Med. Biol. 2018, 1086, 55–75. [Google Scholar] [CrossRef]
- Lin, Z.; Ding, Q.; Li, X.; Feng, Y.; He, H.; Huang, C.; Zhu, Y. Targeting Epigenetic Mechanisms in Vascular Aging. Front. Cardiovasc. Med. 2021, 8, 806988. [Google Scholar] [CrossRef]
- Ponticos, M.; Smith, B.D. Extracellular Matrix Synthesis in Vascular Disease: Hypertension, and Atherosclerosis. J. Biomed. Res. 2014, 28, 25–39. [Google Scholar] [CrossRef]
- Monfredi, O.; Lakatta, E.G. Complexities in Cardiovascular Rhythmicity: Perspectives on Circadian Normality, Ageing and Disease. Cardiovasc. Res. 2019, 115, 1576–1595. [Google Scholar] [CrossRef]
- Xu, J.; Shi, G.-P. Vascular Wall Extracellular Matrix Proteins and Vascular Diseases. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2014, 1842, 2106–2119. [Google Scholar] [CrossRef]
- Wang, M.; Monticone, R.E.; McGraw, K.R. Proinflammation, Profibrosis, and Arterial Aging. Aging Med. 2020, 3, 159–168. [Google Scholar] [CrossRef]
- Coen, M.; Gabbiani, G.; Bochaton-Piallat, M.-L.; Chen, Y.E. Myofibroblast-Mediated Adventitial Remodeling. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2391–2396. [Google Scholar] [CrossRef]
- Selvin, E.; Najjar, S.S.; Cornish, T.C.; Halushka, M.K. A Comprehensive Histopathological Evaluation of Vascular Medial Fibrosis: Insights into the Pathophysiology of Arterial Stiffening. Atherosclerosis 2010, 208, 69–74. [Google Scholar] [CrossRef]
- AlGhatrif, M.; Strait, J.B.; Morrell, C.H.; Canepa, M.; Wright, J.; Elango, P.; Scuteri, A.; Najjar, S.S.; Ferrucci, L.; Lakatta, E.G. Longitudinal Trajectories of Arterial Stiffness and the Role of Blood Pressure. Hypertension 2013, 62, 934–941. [Google Scholar] [CrossRef]
- Kovacic, J.C.; Moreno, P.; Nabel, E.G.; Hachinski, V.; Fuster, V. Cellular Senescence, Vascular Disease, and Aging. Circulation 2011, 123, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, J.C.; Moreno, P.; Hachinski, V.; Nabel, E.G.; Fuster, V. Cellular Senescence, Vascular Disease, and Aging: Part 1 of a 2-Part Review. Circulation 2011, 123, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, X.; Liu, T.; Zhu, X.; Pan, X. The Multifaceted Role of the SASP in Atherosclerosis: From Mechanisms to Therapeutic Opportunities. Cell Biosci. 2022, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, D.; Spinetti, G.; Zhang, J.; Jiang, L.-Q.; Pintus, G.; Monticone, R.; Lakatta, E.G. Matrix Metalloproteinase 2 Activation of Transforming Growth Factor-Β1 (TGF-Β1) and TGF-Β1–Type II Receptor Signaling Within the Aged Arterial Wall. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1503–1509. [Google Scholar] [CrossRef]
- Wang, M.; Lakatta, E.G. Altered Regulation of Matrix Metalloproteinase-2 in Aortic Remodeling During Aging. Hypertension 2002, 39, 865–873. [Google Scholar] [CrossRef]
- Li, Z.; Froehlich, J.; Galis, Z.S.; Lakatta, E.G. Increased Expression of Matrix Metalloproteinase-2 in the Thickened Intima of Aged Rats. Hypertension 1999, 33, 116–123. [Google Scholar] [CrossRef]
- Nanayakkara, S.; Marwick, T.H.; Kaye, D.M. The Ageing Heart: The Systemic and Coronary Circulation. Heart 2018, 104, 370–376. [Google Scholar] [CrossRef]
- Chung, H.Y.; Sung, B.; Jung, K.J.; Zou, Y.; Yu, B.P. The Molecular Inflammatory Process in Aging. Antioxid. Redox Signal 2006, 8, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Lakatta, E.G. Cardiovascular Ageing in Health Sets the Stage for Cardiovascular Disease. Heart Lung Circ. 2002, 11, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.C.; Chobanian, A.V.; Brecher, P. Angiotensin II Induces Fibronectin Expression Associated with Cardiac Fibrosis in the Rat. Circ. Res. 1994, 74, 727–739. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, T.A.; Falcone, D.J. Evidence for an Age-Related Dysfunction in the Antiproliferative Response to Transforming Growth Factor-Beta in Vascular Smooth Muscle Cells. Mol. Biol. Cell 1993, 4, 315–322. [Google Scholar] [CrossRef][Green Version]
- Miller, A.J.; Arnold, A.C. The Renin-Angiotensin System and Cardiovascular Autonomic Control in Aging. Peptides 2022, 150, 170733. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.E.; Kim, E.N.; Kim, M.Y.; Lim, J.H.; Jang, I.-A.; Ban, T.H.; Shin, S.J.; Park, C.W.; Chang, Y.S.; Choi, B.S. Age-Associated Changes in the Vascular Renin-Angiotensin System in Mice. Oxidative Med. Cell. Longev. 2016, 2016, 6731093. [Google Scholar] [CrossRef]
- Ghebre, Y.T.; Yakubov, E.; Wong, W.T.; Krishnamurthy, P.; Sayed, N.; Sikora, A.G.; Bonnen, M.D. Vascular Aging: Implications for Cardiovascular Disease and Therapy. Transl. Med. 2016, 6, 183. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Lakatta, E.G. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises: Part III: Cellular and Molecular Clues to Heart and Arterial Aging. Circulation 2003, 107, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Luxan, G.; Tamiato, A.; Tombor, L.; Nicin, L.; Neitz, J.; Wagner, J.U.G.; John, D.; Dimmeler, S. The Role of Pericytes in Cardiac Ageing and Disease. Eur. Heart J. 2022, 43, ehac544.3012. [Google Scholar] [CrossRef]
- Camelliti, P.; Borg, T.K.; Kohl, P. Structural and Functional Characterisation of Cardiac Fibroblasts. Cardiovasc. Res. 2005, 65, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Baudino, T.A.; Carver, W.; Giles, W.; Borg, T.K. Cardiac Fibroblasts: Friend or Foe? Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H1015–H1026. [Google Scholar] [CrossRef]
- Fan, D.; Takawale, A.; Lee, J.; Kassiri, Z. Cardiac Fibroblasts, Fibrosis and Extracellular Matrix Remodeling in Heart Disease. Fibrogen. Tissue Repair 2012, 5, 15. [Google Scholar] [CrossRef]
- Porter, K.E.; Turner, N.A. Cardiac Fibroblasts: At the Heart of Myocardial Remodeling. Pharmacol. Ther. 2009, 123, 255–278. [Google Scholar] [CrossRef]
- Nicin, L.; Wagner, J.U.G.; Luxán, G.; Dimmeler, S. Fibroblast-Mediated Intercellular Crosstalk in the Healthy and Diseased Heart. FEBS Lett. 2021, 596, 638–654. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Kalluri, R. Origins of Cardiac Fibroblasts. Circ. Res. 2010, 107, 1304–1312. [Google Scholar] [CrossRef]
- Lajiness, J.D.; Conway, S.J. Origin, Development, and Differentiation of Cardiac Fibroblasts. J. Mol. Cell. Cardiol. 2014, 70, 2–8. [Google Scholar] [CrossRef]
- Furtado, M.B.; Nim, H.T.; Boyd, S.E.; Rosenthal, N.A. View from the Heart: Cardiac Fibroblasts in Development, Scarring and Regeneration. Development 2016, 143, 387–397. [Google Scholar] [CrossRef]
- Ali, S.R.; Ranjbarvaziri, S.; Talkhabi, M.; Zhao, P.; Subat, A.; Hojjat, A.; Kamran, P.; Müller, A.M.S.; Volz, K.S.; Tang, Z.; et al. Developmental Heterogeneity of Cardiac Fibroblasts Does Not Predict Pathological Proliferation and Activation. Circ. Res. 2014, 115, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Moore-Morris, T.; Guimarães-Camboa, N.; Banerjee, I.; Zambon, A.C.; Kisseleva, T.; Velayoudon, A.; Stallcup, W.B.; Gu, Y.; Dalton, N.D.; Cedenilla, M.; et al. Resident Fibroblast Lineages Mediate Pressure Overload–Induced Cardiac Fibrosis. J. Clin. Investig. 2014, 124, 2921–2934. [Google Scholar] [CrossRef] [PubMed]
- Snider, P.; Standley, K.N.; Wang, J.; Azhar, M.; Doetschman, T.; Conway, S.J. Origin of Cardiac Fibroblasts and the Role of Periostin. Circ. Res. 2009, 105, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, E.C.; Bradshaw, A.D.; Zile, M.R.; Spinale, F.G. Myocardial Fibroblast-Matrix Interactions and Potential Therapeutic Targets. J. Mol. Cell. Cardiol. 2014, 70, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.D.; Ambler, S.K.; Mitchell, M.D.; Long, C.S. The Cardiac Fibroblast: Therapeutic Target in Myocardial Remodeling and Failure. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 657–687. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G. Myocardial Matrix Remodeling and the Matrix Metalloproteinases: Influence on Cardiac Form and Function. Physiol. Rev. 2007, 87, 1285–1342. [Google Scholar] [CrossRef] [PubMed]
- Gabbiani, G.; Ryan, G.B.; Majne, G. Presence of Modified Fibroblasts in Granulation Tissue and Their Possible Role in Wound Contraction. Experientia 1971, 27, 549–550. [Google Scholar] [CrossRef]
- Lodi, R.S.; Xia, L.; Zhang, Y.; Liu, F. Evolving Roles of Cardiac Fibroblasts in Cardiogenesis and Immunology, Electrophysiology, and Aging. RCM 2021, 22, 1173–1183. [Google Scholar] [CrossRef]
- Thomas, T.P.; Grisanti, L.A. The Dynamic Interplay between Cardiac Inflammation and Fibrosis. Front. Physiol. 2020, 11, 529075. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and Mechano-Regulation of Connective Tissue Remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Hinz, B.; Celetta, G.; Tomasek, J.J.; Gabbiani, G.; Chaponnier, C. Alpha-Smooth Muscle Actin Expression Upregulates Fibroblast Contractile Activity. Mol. Biol. Cell 2001, 12, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T. Cardiac Interstitium in Health and Disease: The Fibrillar Collagen Network. J. Am. Coll. Cardiol. 1989, 13, 1637–1652. [Google Scholar] [CrossRef]
- Anderson, K.R.; Sutton, M.G.; Lie, J.T. Histopathological Types of Cardiac Fibrosis in Myocardial Disease. J. Pathol. 1979, 128, 79–85. [Google Scholar] [CrossRef]
- Lu, L.; Guo, J.; Hua, Y.; Huang, K.; Magaye, R.; Cornell, J.; Kelly, D.J.; Reid, C.; Liew, D.; Zhou, Y.; et al. Cardiac Fibrosis in the Ageing Heart: Contributors and Mechanisms. Clin. Exp. Pharmacol. Physiol. 2017, 44, 55–63. [Google Scholar] [CrossRef]
- Horn, M.A.; Trafford, A.W. Aging and the Cardiac Collagen Matrix: Novel Mediators of Fibrotic Remodelling. J. Mol. Cell. Cardiol. 2016, 93, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Chiao, Y.A.; Ramirez, T.A.; Zamilpa, R.; Okoronkwo, S.M.; Dai, Q.; Zhang, J.; Jin, Y.-F.; Lindsey, M.L. Matrix Metalloproteinase-9 Deletion Attenuates Myocardial Fibrosis and Diastolic Dysfunction in Ageing Mice. Cardiovasc. Res. 2012, 96, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Eghbali, M.; Eghbali, M.; Robinson, T.F.; Seifter, S.; Blumenfeld, O.O. Collagen Accumulation in Heart Ventricles as a Function of Growth and Aging. Cardiovasc. Res. 1989, 23, 723–729. [Google Scholar] [CrossRef]
- Gazoti Debessa, C.R.; Mesiano Maifrino, L.B.; Rodrigues de Souza, R. Age Related Changes of the Collagen Network of the Human Heart. Mech. Ageing Dev. 2001, 122, 1049–1058. [Google Scholar] [CrossRef]
- Lin, J.; Lopez, E.F.; Jin, Y.; Van Remmen, H.; Bauch, T.; Han, H.-C.; Lindsey, M.L. Age-Related Cardiac Muscle Sarcopenia: Combining Experimental and Mathematical Modeling to Identify Mechanisms. Exp. Gerontol. 2008, 43, 296–306. [Google Scholar] [CrossRef]
- Mendes, A.B.L.; Ferro, M.; Rodrigues, B.; de Souza, M.R.; Araujo, R.C.; de Souza, R.R. Quantification of Left Ventricular Myocardial Collagen System in Children, Young Adults, and the Elderly. Medicina 2012, 72, 216–220. [Google Scholar] [PubMed]
- Salcan, S.; Bongardt, S.; Monteiro Barbosa, D.; Efimov, I.R.; Rassaf, T.; Krüger, M.; Kötter, S. Elastic Titin Properties and Protein Quality Control in the Aging Heart. Biochim. Biophys. Acta (BBA) -Mol. Cell Res. 2020, 1867, 118532. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.A.; Graham, H.K.; Richards, M.A.; Clarke, J.D.; Greensmith, D.J.; Briston, S.J.; Hall, M.C.S.; Dibb, K.M.; Trafford, A.W. Age-Related Divergent Remodeling of the Cardiac Extracellular Matrix in Heart Failure: Collagen Accumulation in the Young and Loss in the Aged. J. Mol. Cell. Cardiol. 2012, 53, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Besse, S.; Robert, V.; Assayag, P.; Delcayre, C.; Swynghedauw, B. Nonsynchronous Changes in Myocardial Collagen mRNA and Protein during Aging: Effect of DOCA-Salt Hypertension. Am. J. Physiol. 1994, 267, H2237–H2244. [Google Scholar] [CrossRef] [PubMed]
- Annoni, G.; Luvarà, G.; Arosio, B.; Gagliano, N.; Fiordaliso, F.; Santambrogio, D.; Jeremic, G.; Mircoli, L.; Latini, R.; Vergani, C.; et al. Age-Dependent Expression of Fibrosis-Related Genes and Collagen Deposition in the Rat Myocardium. Mech. Ageing Dev. 1998, 101, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Mays, P.K.; McAnulty, R.J.; Campa, J.S.; Laurent, G.J. Age-Related Changes in Collagen Synthesis and Degradation in Rat Tissues. Importance of Degradation of Newly Synthesized Collagen in Regulating Collagen Production. Biochem. J. 1991, 276 Pt 2, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Robert, V.; Besse, S.; Sabri, A.; Silvestre, J.S.; Assayag, P.; Nguyen, V.T.; Swynghedauw, B.; Delcayre, C. Differential Regulation of Matrix Metalloproteinases Associated with Aging and Hypertension in the Rat Heart. Lab. Invest 1997, 76, 729–738. [Google Scholar] [PubMed]
- Herrmann, K.L.; McCulloch, A.D.; Omens, J.H. Glycated Collagen Cross-Linking Alters Cardiac Mechanics in Volume-Overload Hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1277–H1284. [Google Scholar] [CrossRef]
- Bailey, A.J.; Sims, T.J.; Avery, N.C.; Halligan, E.P. Non-Enzymic Glycation of Fibrous Collagen: Reaction Products of Glucose and Ribose. Biochem. J. 1995, 305, 385–390. [Google Scholar] [CrossRef]
- Achkar, A.; Saliba, Y.; Fares, N. Differential Gender-Dependent Patterns of Cardiac Fibrosis and Fibroblast Phenotypes in Aging Mice. Oxidative Med. Cell. Longev. 2020, 2020, 8282157. [Google Scholar] [CrossRef]
- Pappritz, K.; Puhl, S.-L.; Matz, I.; Brauer, E.; Shia, Y.X.; El-Shafeey, M.; Koch, S.E.; Miteva, K.; Mucha, C.; Duda, G.N.; et al. Sex- and Age-Related Differences in the Inflammatory Properties of Cardiac Fibroblasts: Impact on the Cardiosplenic Axis and Cardiac Fibrosis. Front. Cardiovasc. Med. 2023, 10, 1117419. [Google Scholar] [CrossRef]
- Trial, J.; Cieslik, K.A. Changes in Cardiac Resident Fibroblast Physiology and Phenotype in Aging. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H745–H755. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Trial, J.; Ortiz-Urbina, J.; Cieslik, K.A. Mechanosensing Dysregulation in the Fibroblast: A Hallmark of the Aging Heart. Ageing Res. Rev. 2020, 63, 101150. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Trial, J.; Saltzman, A.B.; Malovannaya, A.; Cieslik, K.A. A Defective Mechanosensing Pathway Affects Fibroblast-to-Myofibroblast Transition in the Old Male Mouse Heart. iScience 2023, 26, 107283. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, K.A.; Trial, J.; Entman, M.L. Defective Myofibroblast Formation from Mesenchymal Stem Cells in the Aging Murine Heart Rescue by Activation of the AMPK Pathway. Am. J. Pathol. 2011, 179, 1792–1806. [Google Scholar] [CrossRef] [PubMed]
- Bujak, M.; Kweon, H.J.; Chatila, K.; Li, N.; Taffet, G.; Frangogiannis, N.G. Aging-Related Defects Are Associated With Adverse Cardiac Remodeling in a Mouse Model of Reperfused Myocardial Infarction. J. Am. Coll. Cardiol. 2008, 51, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Trial, J.; Entman, M.L.; Cieslik, K.A. Mesenchymal Stem Cell-Derived Inflammatory Fibroblasts Mediate Interstitial Fibrosis in the Aging Heart. J. Mol. Cell. Cardiol. 2016, 91, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, K.; Dostal, D.E.; Boheler, K.; Baker, K.M.; Lakatta, E.G. Differential Response of Cardiac Fibroblasts from Young Adult and Senescent Rats to ANG II. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1454–H1459. [Google Scholar] [CrossRef] [PubMed]
- Izzo, C.; Vitillo, P.; Di Pietro, P.; Visco, V.; Strianese, A.; Virtuoso, N.; Ciccarelli, M.; Galasso, G.; Carrizzo, A.; Vecchione, C. The Role of Oxidative Stress in Cardiovascular Aging and Cardiovascular Diseases. Life 2021, 11, 60. [Google Scholar] [CrossRef]
- Anupama, V.; George, M.; Dhanesh, S.B.; Chandran, A.; James, J.; Shivakumar, K. Molecular Mechanisms in H2O2-Induced Increase in AT1 Receptor Gene Expression in Cardiac Fibroblasts: A Role for Endogenously Generated Angiotensin II. J. Mol. Cell. Cardiol. 2016, 97, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Xu, J.; Kaiser, R.A.; Melendez, J.; Hambleton, M.; Sargent, M.A.; Lorts, A.; Brunskill, E.W.; Dorn, G.W.; Conway, S.J.; et al. Genetic Manipulation of Periostin Expression Reveals a Role in Cardiac Hypertrophy and Ventricular Remodeling. Circ. Res. 2007, 101, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, M.; Nakamura, K.; Kii, I.; Kashima, T.; Amizuka, N.; Li, M.; Saito, M.; Fukuda, K.; Nishiyama, T.; Kitajima, S.; et al. Periostin Is Essential for Cardiac Healing after Acute Myocardial Infarction. J. Exp. Med. 2008, 205, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Ma, F.; Tosevska, A.; Farrell, C.; Pellegrini, M.; Deb, A. Cardiac Fibroblast Proliferation Rates and Collagen Expression Mature Early and Are Unaltered with Advancing Age. JCI Insight 2020, 5, e140628. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Cimini, M.; Fedak, P.W.M.; Altamentova, S.; Fazel, S.; Huang, M.-L.; Weisel, R.D.; Li, R.-K. TIMP-3 Deficiency Accelerates Cardiac Remodeling after Myocardial Infarction. J. Mol. Cell. Cardiol. 2007, 43, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Foster, C.R.; Dalal, S.; Singh, K. Osteopontin: Role in Extracellular Matrix Deposition and Myocardial Remodeling Post-MI. J. Mol. Cell Cardiol. 2010, 48, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Yabluchanskiy, A.; Ma, Y.; DeLeon-Pennell, K.Y.; Altara, R.; Halade, G.V.; Voorhees, A.P.; Nguyen, N.T.; Jin, Y.-F.; Winniford, M.D.; Hall, M.E.; et al. Myocardial Infarction Superimposed on Aging: MMP-9 Deletion Promotes M2 Macrophage Polarization. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 475–483. [Google Scholar] [CrossRef]
- Jugdutt, B.I.; Jelani, A. Aging and Defective Healing, Adverse Remodeling, and Blunted Post-Conditioning in the Reperfused Wounded Heart*. J. Am. Coll. Cardiol. 2008, 51, 1399–1403. [Google Scholar] [CrossRef]
- Jugdutt, B.I. Ventricular Remodeling after Infarction and the Extracellular Collagen Matrix: When Is Enough Enough? Circulation 2003, 108, 1395–1403. [Google Scholar] [CrossRef]
- Ertl, G.; Frantz, S. Healing after Myocardial Infarction. Cardiovasc. Res. 2005, 66, 22–32. [Google Scholar] [CrossRef]
- Tobin, S.W.; Alibhai, F.J.; Weisel, R.D.; Li, R.-K. Considering Cause and Effect of Immune Cell Aging on Cardiac Repair after Myocardial Infarction. Cells 2020, 9, 1894. [Google Scholar] [CrossRef]
- Reuter, H.; Perner, B.; Wahl, F.; Rohde, L.; Koch, P.; Groth, M.; Buder, K.; Englert, C. Aging Activates the Immune System and Alters the Regenerative Capacity in the Zebrafish Heart. Cells 2022, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-Aging. An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, D.; Añé-Kourí, A.L.; Barzilai, N.; Caruso, C.; Cho, K.-H.; Fontana, L.; Franceschi, C.; Frasca, D.; Ledón, N.; Niedernhofer, L.J.; et al. Aging and Chronic Inflammation: Highlights from a Multidisciplinary Workshop. Immun. Ageing 2023, 20, 25. [Google Scholar] [CrossRef]
- Sudo, K.; Ema, H.; Morita, Y.; Nakauchi, H. Age-Associated Characteristics of Murine Hematopoietic Stem Cells. J. Exp. Med. 2000, 192, 1273–1280. [Google Scholar] [CrossRef]
- Pang, W.W.; Price, E.A.; Sahoo, D.; Beerman, I.; Maloney, W.J.; Rossi, D.J.; Schrier, S.L.; Weissman, I.L. Human Bone Marrow Hematopoietic Stem Cells Are Increased in Frequency and Myeloid-Biased with Age. Proc. Natl. Acad. Sci. USA 2011, 108, 20012–20017. [Google Scholar] [CrossRef]
- Gekas, C.; Graf, T. CD41 Expression Marks Myeloid-Biased Adult Hematopoietic Stem Cells and Increases with Age. Blood 2013, 121, 4463–4472. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.; Sanjuan-Pla, A.; Thongjuea, S.; Carrelha, J.; Giustacchini, A.; Gambardella, A.; Macaulay, I.; Mancini, E.; Luis, T.C.; Mead, A.; et al. Single-Cell RNA Sequencing Reveals Molecular and Functional Platelet Bias of Aged Haematopoietic Stem Cells. Nat. Commun. 2016, 7, 11075. [Google Scholar] [CrossRef]
- Oliver, G.; Kipnis, J.; Randolph, G.J.; Harvey, N.L. The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease. Cell 2020, 182, 270–296. [Google Scholar] [CrossRef]
- Perreault, L.R.; Le, T.T.; Oudin, M.J.; Black, L.D. RNA Sequencing Indicates Age-Dependent Shifts in the Cardiac Fibroblast Transcriptome between Fetal, Neonatal, and Adult Developmental Ages. Physiol. Genom. 2021, 53, 414–429. [Google Scholar] [CrossRef]
- Vidal, R.; Wagner, J.U.G.; Braeuning, C.; Fischer, C.; Patrick, R.; Tombor, L.; Muhly-Reinholz, M.; John, D.; Kliem, M.; Conrad, T.; et al. Transcriptional Heterogeneity of Fibroblasts Is a Hallmark of the Aging Heart. JCI Insight 2019, 4, e131092. [Google Scholar] [CrossRef]
- Wagner, J.U.G.; Dimmeler, S. Cellular Cross-Talks in the Diseased and Aging Heart. J. Mol. Cell. Cardiol. 2020, 138, 136–146. [Google Scholar] [CrossRef]
- Sangaralingham, S.J.; Huntley, B.K.; Martin, F.L.; McKie, P.M.; Bellavia, D.; Ichiki, T.; Harders, G.E.; Chen, H.H.; Burnett, J.C. The Aging Heart, Myocardial Fibrosis, and Its Relationship to Circulating C-Type Natriuretic Peptide. Hypertension 2011, 57, 201–207. [Google Scholar] [CrossRef]
- Sun, S.-N.; Ni, S.-H.; Li, Y.; Liu, X.; Deng, J.-P.; Chen, Z.-X.; Li, H.; Feng, W.-J.; Huang, Y.-S.; Li, D.-N.; et al. G-MDSCs Promote Aging-Related Cardiac Fibrosis by Activating Myofibroblasts and Preventing Senescence. Cell Death Dis. 2021, 12, 594. [Google Scholar] [CrossRef]
- Bagchi, R.A.; Roche, P.; Aroutiounova, N.; Espira, L.; Abrenica, B.; Schweitzer, R.; Czubryt, M.P. The Transcription Factor Scleraxis Is a Critical Regulator of Cardiac Fibroblast Phenotype. BMC Biol. 2016, 14, 21. [Google Scholar] [CrossRef]
- Hanna, A.; Frangogiannis, N.G. The Role of the TGF-β Superfamily in Myocardial Infarction. Front. Cardiovasc. Med. 2019, 6, 140. [Google Scholar] [CrossRef]
- Desmoulière, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming Growth Factor-Beta 1 Induces Alpha-Smooth Muscle Actin Expression in Granulation Tissue Myofibroblasts and in Quiescent and Growing Cultured Fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Eghbali, M.; Tomek, R.; Sukhatme, V.P.; Woods, C.; Bhambi, B. Differential Effects of Transforming Growth Factor-Beta 1 and Phorbol Myristate Acetate on Cardiac Fibroblasts. Regulation of Fibrillar Collagen mRNAs and Expression of Early Transcription Factors. Circ. Res. 1991, 69, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Meng, X.; Wang, L.; Dai, X. Mechanism of Action of Non-Coding RNAs and Traditional Chinese Medicine in Myocardial Fibrosis: Focus on the TGF-β/Smad Signaling Pathway. Front. Pharmacol. 2023, 14, 1092148. [Google Scholar] [CrossRef] [PubMed]
- Mauviel, A. Transforming Growth Factor-Beta: A Key Mediator of Fibrosis. Methods Mol. Med. 2005, 117, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Baugé, C.; Cauvard, O.; Leclercq, S.; Galéra, P.; Boumédiene, K. Modulation of Transforming Growth Factor Beta Signalling Pathway Genes by Transforming Growth Factor Beta in Human Osteoarthritic Chondrocytes: Involvement of Sp1 in Both Early and Late Response Cells to Transforming Growth Factor Beta. Arthritis Res. Ther. 2011, 13, R23. [Google Scholar] [CrossRef]
- Cieslik, K.A.; Trial, J.; Crawford, J.R.; Taffet, G.E.; Entman, M.L. Adverse Fibrosis in the Aging Heart Depends on Signaling between Myeloid and Mesenchymal Cells; Role of Inflammatory Fibroblasts. J. Mol. Cell. Cardiol. 2014, 70, 56–63. [Google Scholar] [CrossRef]
- Chiao, Y.A.; Dai, Q.; Zhang, J.; Lin, J.; Lopez, E.F.; Ahuja, S.S.; Chou, Y.-M.; Lindsey, M.L.; Jin, Y.-F. Multi-Analyte Profiling Reveals Matrix Metalloproteinase-9 and Monocyte Chemotactic Protein-1 as Plasma Biomarkers of Cardiac Aging. Circ. Cardiovasc. Genet. 2011, 4, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Bonnema, D.D.; Webb, C.S.; Pennington, W.R.; Stroud, R.E.; Leonardi, A.E.; Clark, L.L.; McClure, C.D.; Finklea, L.; Spinale, F.G.; Zile, M.R. Effects of Age on Plasma Matrix Metalloproteinases (MMPs) and Tissue Inhibitor of Metalloproteinases (TIMPs). J. Card. Fail. 2007, 13, 530–540. [Google Scholar] [CrossRef]
- Sarrazy, V.; Koehler, A.; Chow, M.L.; Zimina, E.; Li, C.X.; Kato, H.; Caldarone, C.A.; Hinz, B. Integrins Avβ5 and Avβ3 Promote Latent TGF-Β1 Activation by Human Cardiac Fibroblast Contraction. Cardiovasc. Res. 2014, 102, 407–417. [Google Scholar] [CrossRef]
- Cieslik, K.A.; Trial, J.; Carlson, S.; Taffet, G.E.; Entman, M.L. Aberrant Differentiation of Fibroblast Progenitors Contributes to Fibrosis in the Aged Murine Heart: Role of Elevated Circulating Insulin Levels. FASEB J. 2013, 27, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, K.A.; Taffet, G.E.; Carlson, S.; Hermosillo, J.; Trial, J.; Entman, M.L. Immune-Inflammatory Dysregulation Modulates the Incidence of Progressive Fibrosis and Diastolic Stiffness in the Aging Heart. J. Mol. Cell. Cardiol. 2011, 50, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.; Berk, B.C. Fyn and JAK2 Mediate Ras Activation by Reactive Oxygen Species. J. Biol. Chem. 1999, 274, 21003–21010. [Google Scholar] [CrossRef]
- Lijnen, P.; Papparella, I.; Petrov, V.; Semplicini, A.; Fagard, R. Angiotensin II-Stimulated Collagen Production in Cardiac Fibroblasts Is Mediated by Reactive Oxygen Species. J. Hypertens. 2006, 24, 757–766. [Google Scholar] [CrossRef]
- Sreedhar, R.; Giridharan, V.V.; Arumugam, S.; Karuppagounder, V.; Palaniyandi, S.S.; Krishnamurthy, P.; Quevedo, J.; Watanabe, K.; Konishi, T.; Thandavarayan, R.A. Role of MAPK-Mediated Endoplasmic Reticulum Stress Signaling in the Heart during Aging in Senescence-Accelerated Prone Mice. Biofactors 2016, 42, 368–375. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Kuro-o, M.; Rosenblatt, K.P.; Brobey, R.; Papaconstantinou, J. The ASK1-Signalosome Regulates P38 MAPK Activity in Response to Levels of Endogenous Oxidative Stress in the Klotho Mouse Models of Aging. Aging 2010, 2, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Auger-Messier, M.; Accornero, F.; Goonasekera, S.A.; Bueno, O.F.; Lorenz, J.N.; van Berlo, J.H.; Willette, R.N.; Molkentin, J.D. Unrestrained P38 MAPK Activation in Dusp1/4 Double-Null Mice Induces Cardiomyopathy. Circ. Res. 2013, 112, 48–56. [Google Scholar] [CrossRef]
- Jazwinski, S.M.; Kim, S. Metabolic and Genetic Markers of Biological Age. Front. Genet. 2017, 8, 64. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.; Xu, Y.; Li, C.; Meng, X.; Fu, F. Protective Effect of RA on Myocardial Infarction-Induced Cardiac Fibrosis via AT1R/P38 MAPK Pathway Signaling and Modulation of the ACE2/ACE Ratio. J. Agric. Food Chem. 2016, 64, 6716–6722. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Zhao, W.; Zhang, X.; Wang, B.; Wang, J.; Sun, X.; Liu, X.; Feng, S.; Yang, B.; Lu, Y. Scutellarin Alleviates Interstitial Fibrosis and Cardiac Dysfunction of Infarct Rats by Inhibiting TGFβ1 Expression and Activation of P38-MAPK and ERK1/2. Br. J. Pharmacol. 2011, 162, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Fei, A.-H.; Wang, F.-C.; Wu, Z.-B.; Pan, S.-M. Phosphocreatine Attenuates Angiotensin II-Induced Cardiac Fibrosis in Rat Cardiomyocytes through Modulation of MAPK and NF-κB Pathway. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2726–2733. [Google Scholar]
- Adler, A.S.; Sinha, S.; Kawahara, T.L.A.; Zhang, J.Y.; Segal, E.; Chang, H.Y. Motif Module Map Reveals Enforcement of Aging by Continual NF-κB Activity. Genes. Dev. 2007, 21, 3244–3257. [Google Scholar] [CrossRef]
- Bernal, G.M.; Wahlstrom, J.S.; Crawley, C.D.; Cahill, K.E.; Pytel, P.; Liang, H.; Kang, S.; Weichselbaum, R.R.; Yamini, B. Loss of Nfkb1 Leads to Early Onset Aging. Aging 2014, 6, 931–943. [Google Scholar] [CrossRef][Green Version]
- Wu, J.; Xia, S.; Kalionis, B.; Wan, W.; Sun, T. The Role of Oxidative Stress and Inflammation in Cardiovascular Aging. Biomed. Res. Int. 2014, 2014, 615312. [Google Scholar] [CrossRef]
- Ungvari, Z.; Bailey-Downs, L.; Sosnowska, D.; Gautam, T.; Koncz, P.; Losonczy, G.; Ballabh, P.; de Cabo, R.; Sonntag, W.E.; Csiszar, A. Vascular Oxidative Stress in Aging: A Homeostatic Failure Due to Dysregulation of NRF2-Mediated Antioxidant Response. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H363–H372. [Google Scholar] [CrossRef]
- Karagianni, C.; Bazopoulou, D. Redox Regulation in Lifespan Determination. J. Biol. Chem. 2024, 300, 105761. [Google Scholar] [CrossRef]
- Jugdutt, B.I. Aging and Remodeling of the RAS and RAAS and Related Pathways: Implications for Heart Failure Therapy. In Aging and Heart Failure: Mechanisms and Management; Jugdutt, B.I., Ed.; Springer: New York, NY, USA, 2014; pp. 259–289. ISBN 978-1-4939-0268-2. [Google Scholar]
- Matsoukas, M.-T.; Potamitis, C.; Plotas, P.; Androutsou, M.-E.; Agelis, G.; Matsoukas, J.; Zoumpoulakis, P. Insights into AT1 Receptor Activation through AngII Binding Studies. J. Chem. Inf. Model. 2013, 53, 2798–2811. [Google Scholar] [CrossRef] [PubMed]
- Dostal, D.E.; Baker, K.M. The Cardiac Renin-Angiotensin System: Conceptual, or a Regulator of Cardiac Function? Circ. Res. 1999, 85, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Kanugula, A.K.; Kaur, J.; Batra, J.; Ankireddypalli, A.R.; Velagapudi, R. Renin-Angiotensin System: Updated Understanding and Role in Physiological and Pathophysiological States. Cureus 2023, 15, e40725. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, Y. Alteration of Messenger RNA and Protein Levels of Cardiac Alpha(1)-Adrenergic Receptor and Angiotensin II Receptor Subtypes during Aging in Rats. Can. J. Cardiol. 2009, 25, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Heymes, C.; Swynghedauw, B.; Chevalier, B. Activation of Angiotensinogen and Angiotensin-Converting Enzyme Gene Expression in the Left Ventricle of Senescent Rats. Circulation 1994, 90, 1328–1333. [Google Scholar] [CrossRef]
- Neumann, S.; Huse, K.; Semrau, R.; Diegeler, A.; Gebhardt, R.; Buniatian, G.H.; Scholz, G.H. Aldosterone and D-Glucose Stimulate the Proliferation of Human Cardiac Myofibroblasts In Vitro. Hypertension 2002, 39, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Stockand, J.D.; Meszaros, J.G. Aldosterone Stimulates Proliferation of Cardiac Fibroblasts by Activating Ki-RasA and MAPK1/2 Signaling. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H176–H184. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac Fibrosis. Cardiovasc. Res. 2020, 117, 1450–1488. [Google Scholar] [CrossRef]
- Somanna, N.K.; Yariswamy, M.; Garagliano, J.M.; Siebenlist, U.; Mummidi, S.; Valente, A.J.; Chandrasekar, B. Aldosterone-Induced Cardiomyocyte Growth, and Fibroblast Migration and Proliferation Are Mediated by TRAF3IP2. Cell. Signal. 2015, 27, 1928–1938. [Google Scholar] [CrossRef]
- Brilla, C.G.; Zhou, G.; Matsubara, L.; Weber, K.T. Collagen Metabolism in Cultured Adult Rat Cardiac Fibroblasts: Response to Angiotensin II and Aldosterone. J. Mol. Cell. Cardiol. 1994, 26, 809–820. [Google Scholar] [CrossRef]
- de Lucia, C.; Komici, K.; Borghetti, G.; Femminella, G.D.; Bencivenga, L.; Cannavo, A.; Corbi, G.; Ferrara, N.; Houser, S.R.; Koch, W.J.; et al. microRNA in Cardiovascular Aging and Age-Related Cardiovascular Diseases. Front. Med. 2017, 4, 74. [Google Scholar] [CrossRef]
- Kramna, D.; Riedlova, P.; Jirik, V. MicroRNAs as a Potential Biomarker in the Diagnosis of Cardiovascular Diseases. Medicina 2023, 59, 1329. [Google Scholar] [CrossRef]
- Zhou, S.; Jin, J.; Wang, J.; Zhang, Z.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in Cardiovascular Diseases: Potential Biomarkers, Therapeutic Targets and Challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef]
- Rupani, H.; Sanchez-Elsner, T.; Howarth, P. MicroRNAs and Respiratory Diseases. Eur. Respir J. 2013, 41, 695–705. [Google Scholar] [CrossRef]
- Zhang, X.; Azhar, G.; Wei, J.Y. The Expression of microRNA and microRNA Clusters in the Aging Heart. PLoS ONE 2012, 7, e34688. [Google Scholar] [CrossRef]
- Schulte, C.; Zeller, T. microRNA-Based Diagnostics and Therapy in Cardiovascular Disease—Summing up the Facts. Cardiovasc. Diagn. Ther. 2015, 5, 17. [Google Scholar] [CrossRef]
- Boon, R.A.; Iekushi, K.; Lechner, S.; Seeger, T.; Fischer, A.; Heydt, S.; Kaluza, D.; Tréguer, K.; Carmona, G.; Bonauer, A.; et al. MicroRNA-34a Regulates Cardiac Ageing and Function. Nature 2013, 495, 107–110. [Google Scholar] [CrossRef]
- Seeger, T.; Boon, R.A. MicroRNAs in Cardiovascular Ageing. J. Physiol. 2016, 594, 2085–2094. [Google Scholar] [CrossRef]
- van Almen, G.C.; Verhesen, W.; van Leeuwen, R.E.W.; van de Vrie, M.; Eurlings, C.; Schellings, M.W.M.; Swinnen, M.; Cleutjens, J.P.M.; van Zandvoort, M.A.M.J.; Heymans, S.; et al. MicroRNA-18 and microRNA-19 Regulate CTGF and TSP-1 Expression in Age-Related Heart Failure. Aging Cell 2011, 10, 769–779. [Google Scholar] [CrossRef]
- Jazbutyte, V.; Fiedler, J.; Kneitz, S.; Galuppo, P.; Just, A.; Holzmann, A.; Bauersachs, J.; Thum, T. MicroRNA-22 Increases Senescence and Activates Cardiac Fibroblasts in the Aging Heart. AGE 2013, 35, 747–762. [Google Scholar] [CrossRef]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 Contributes to Myocardial Disease by Stimulating MAP Kinase Signalling in Fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef]
- Lin, R.; Rahtu-Korpela, L.; Magga, J.; Ulvila, J.; Swan, J.; Kemppi, A.; Pakanen, L.; Porvari, K.; Huikuri, H.; Junttila, J.; et al. miR-1468-3p Promotes Aging-Related Cardiac Fibrosis. Mol. Ther.-Nucleic Acids 2020, 20, 589–605. [Google Scholar] [CrossRef]
- Tchkonia, T.; Zhu, Y.; van Deursen, J.; Campisi, J.; Kirkland, J.L. Cellular Senescence and the Senescent Secretory Phenotype: Therapeutic Opportunities. J. Clin. Investig. 2013, 123, 966–972. [Google Scholar] [CrossRef]
- Yan, C.; Xu, Z.; Huang, W. Cellular Senescence Affects Cardiac Regeneration and Repair in Ischemic Heart Disease. Aging Dis. 2021, 12, 552–569. [Google Scholar] [CrossRef]
- Cianflone, E.; Torella, M.; Biamonte, F.; De Angelis, A.; Urbanek, K.; Costanzo, F.S.; Rota, M.; Ellison-Hughes, G.M.; Torella, D. Targeting Cardiac Stem Cell Senescence to Treat Cardiac Aging and Disease. Cells 2020, 9, 1558. [Google Scholar] [CrossRef]
- Lewis-McDougall, F.C.; Ruchaya, P.J.; Domenjo-Vila, E.; Shin Teoh, T.; Prata, L.; Cottle, B.J.; Clark, J.E.; Punjabi, P.P.; Awad, W.; Torella, D.; et al. Aged-senescent Cells Contribute to Impaired Heart Regeneration. Aging Cell 2019, 18, e12931. [Google Scholar] [CrossRef]
- Kakkar, R.; Lee, R.T. Intramyocardial Fibroblast Myocyte Communication. Circ. Res. 2010, 106, 47–57. [Google Scholar] [CrossRef]
- Suda, M.; Paul, K.H.; Minamino, T.; Miller, J.D.; Lerman, A.; Ellison-Hughes, G.M.; Tchkonia, T.; Kirkland, J.L. Senescent Cells: A Therapeutic Target in Cardiovascular Diseases. Cells 2023, 12, 1296. [Google Scholar] [CrossRef]
- Valiathan, R.R.; Marco, M.; Leitinger, B.; Kleer, C.G.; Fridman, R. Discoidin Domain Receptor Tyrosine Kinases: New Players in Cancer Progression. Cancer Metastasis Rev. 2012, 31, 295–321. [Google Scholar] [CrossRef]
- Labrador, J.P.; Azcoitia, V.; Tuckermann, J.; Lin, C.; Olaso, E.; Mañes, S.; Brückner, K.; Goergen, J.L.; Lemke, G.; Yancopoulos, G.; et al. The Collagen Receptor DDR2 Regulates Proliferation and Its Elimination Leads to Dwarfism. EMBO Rep. 2001, 2, 446–452. [Google Scholar] [CrossRef]
- Kawai, I.; Hisaki, T.; Sugiura, K.; Naito, K.; Kano, K. Discoidin Domain Receptor 2 (DDR2) Regulates Proliferation of Endochondral Cells in Mice. Biochem. Biophys. Res. Commun. 2012, 427, 611–617. [Google Scholar] [CrossRef]
- Olaso, E.; Lin, H.-C.; Wang, L.-H.; Friedman, S.L. Impaired Dermal Wound Healing in Discoidin Domain Receptor 2-Deficient Mice Associated with Defective Extracellular Matrix Remodeling. Fibrogenes. Tissue Repair 2011, 4, 5. [Google Scholar] [CrossRef]
- Badiola, I.; Villacé, P.; Basaldua, I.; Olaso, E. Downregulation of Discoidin Domain Receptor 2 in A375 Human Melanoma Cells Reduces Its Experimental Liver Metastasis Ability. Oncol. Rep. 2011, 26, 971–978. [Google Scholar] [CrossRef][Green Version]
- Toy, K.A.; Valiathan, R.R.; Núñez, F.; Kidwell, K.M.; Gonzalez, M.E.; Fridman, R.; Kleer, C.G. Tyrosine Kinase Discoidin Domain Receptors DDR1 and DDR2 Are Coordinately Deregulated in Triple-Negative Breast Cancer. Breast Cancer Res. Treat. 2015, 150, 9–18. [Google Scholar] [CrossRef]
- Ushakumary, M.G.; Wang, M.V.H.; Titus, A.S.; Zhang, J.; Liu, L.; Monticone, R.; Wang, Y.; Mattison, J.A.; de Cabo, R.; Lakatta, E.G.; et al. Discoidin Domain Receptor 2: A Determinant of Metabolic Syndrome-Associated Arterial Fibrosis in Non-Human Primates. PLoS ONE 2019, 14, e0225911. [Google Scholar] [CrossRef]
- George, M.; Vijayakumar, A.; Dhanesh, S.B.; James, J.; Shivakumar, K. Molecular Basis and Functional Significance of Angiotensin II-Induced Increase in Discoidin Domain Receptor 2 Gene Expression in Cardiac Fibroblasts. J. Mol. Cell. Cardiol. 2016, 90, 59–69. [Google Scholar] [CrossRef]
- Harikrishnan, V.; Titus, A.S.; Cowling, R.T.; Kailasam, S. Collagen Receptor Cross-Talk Determines α-Smooth Muscle Actin-Dependent Collagen Gene Expression in Angiotensin II-Stimulated Cardiac Fibroblasts. J. Biol. Chem. 2019, 294, 19723–19739. [Google Scholar] [CrossRef]
- Titus, A.S.V.H.; Kailasam, S. Coordinated Regulation of Cell Survival and Cell Cycle Pathways by DDR2-Dependent SRF Transcription Factor in Cardiac Fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1538–H1558. [Google Scholar] [CrossRef]
- Doppler, S.A.; Carvalho, C.; Lahm, H.; Deutsch, M.-A.; Dreßen, M.; Puluca, N.; Lange, R.; Krane, M. Cardiac Fibroblasts: More than Mechanical Support. J. Thorac. Dis. 2017, 9, S36. [Google Scholar] [CrossRef]
- Bertaud, A.; Joshkon, A.; Heim, X.; Bachelier, R.; Bardin, N.; Leroyer, A.S.; Blot-Chabaud, M. Signaling Pathways and Potential Therapeutic Strategies in Cardiac Fibrosis. Int. J. Mol. Sci. 2023, 24, 1756. [Google Scholar] [CrossRef]
- Sawaki, D.; Czibik, G.; Pini, M.; Ternacle, J.; Suffee, N.; Mercedes, R.; Marcelin, G.; Surenaud, M.; Marcos, E.; Gual, P.; et al. Visceral Adipose Tissue Drives Cardiac Aging through Modulation of Fibroblast Senescence by Osteopontin Production. Circulation 2018, 138, 809–822. [Google Scholar] [CrossRef]
- Dookun, E.; Passos, J.F.; Arthur, H.M.; Richardson, G.D. Therapeutic Potential of Senolytics in Cardiovascular Disease. Cardiovasc. Drugs Ther. 2022, 36, 187–196. [Google Scholar] [CrossRef]
- Sweeney, M.; Cook, S.A.; Gil, J. Therapeutic Opportunities for Senolysis in Cardiovascular Disease. FEBS J. 2023, 290, 1235–1255. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T.; Zhu, Y.; Niedernhofer, L.J.; Robbins, P.D. The Clinical Potential of Senolytic Drugs. J. Am. Geriatr. Soc. 2017, 65, 2297–2301. [Google Scholar] [CrossRef]
- Evangelou, K.; Vasileiou, P.V.S.; Papaspyropoulos, A.; Hazapis, O.; Petty, R.; Demaria, M.; Gorgoulis, V.G. Cellular Senescence and Cardiovascular Diseases: Moving to the “Heart” of the Problem. Physiol. Rev. 2023, 103, 609–647. [Google Scholar] [CrossRef]
- Tse, C.; Shoemaker, A.R.; Adickes, J.; Anderson, M.G.; Chen, J.; Jin, S.; Johnson, E.F.; Marsh, K.C.; Mitten, M.J.; Nimmer, P.; et al. ABT-263: A Potent and Orally Bioavailable Bcl-2 Family Inhibitor. Cancer Res. 2008, 68, 3421–3428. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ Heel of Senescent Cells: From Transcriptome to Senolytic Drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting Cellular Senescence with Senotherapeutics: Senolytics and Senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef] [PubMed]
- Mourad, O.; Mastikhina, O.; Khan, S.; Sun, X.; Hatkar, R.; Williams, K.; Nunes, S.S. Antisenescence Therapy Improves Function in a Human Model of Cardiac Fibrosis-on-a-Chip. ACS Mater. Au 2023, 3, 360–370. [Google Scholar] [CrossRef]
- Claridge, B.; Drack, A.; Pinto, A.R.; Greening, D.W. Defining Cardiac Fibrosis Complexity and Regulation towards Therapeutic Development. Clin. Transl. Discov. 2023, 3, e163. [Google Scholar] [CrossRef]
- Linscheid, N.; Poulsen, P.C.; Pedersen, I.D.; Gregers, E.; Svendsen, J.H.; Olesen, M.S.; Olsen, J.V.; Delmar, M.; Lundby, A. Quantitative Proteomics of Human Heart Samples Collected In Vivo Reveal the Remodeled Protein Landscape of Dilated Left Atrium Without Atrial Fibrillation. Mol. Cell Proteom. 2020, 19, 1132–1144. [Google Scholar] [CrossRef] [PubMed]
- Laggerbauer, B.; Engelhardt, S. MicroRNAs as Therapeutic Targets in Cardiovascular Disease. J. Clin. Investig. 2022, 132, e159179. [Google Scholar] [CrossRef] [PubMed]

|
|
|
|
|
|
| |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijayakumar, A.; Wang, M.; Kailasam, S. The Senescent Heart—“Age Doth Wither Its Infinite Variety”. Int. J. Mol. Sci. 2024, 25, 3581. https://doi.org/10.3390/ijms25073581
Vijayakumar A, Wang M, Kailasam S. The Senescent Heart—“Age Doth Wither Its Infinite Variety”. International Journal of Molecular Sciences. 2024; 25(7):3581. https://doi.org/10.3390/ijms25073581
Chicago/Turabian StyleVijayakumar, Anupama, Mingyi Wang, and Shivakumar Kailasam. 2024. "The Senescent Heart—“Age Doth Wither Its Infinite Variety”" International Journal of Molecular Sciences 25, no. 7: 3581. https://doi.org/10.3390/ijms25073581
APA StyleVijayakumar, A., Wang, M., & Kailasam, S. (2024). The Senescent Heart—“Age Doth Wither Its Infinite Variety”. International Journal of Molecular Sciences, 25(7), 3581. https://doi.org/10.3390/ijms25073581






