On the Possibility of Using 5-Aminolevulinic Acid in the Light-Induced Destruction of Microorganisms
Abstract
1. Introduction
1.1. Photodynamic Therapy
Mechanism of aPDI and the Possibility of Developing Tolerance
2. Methods
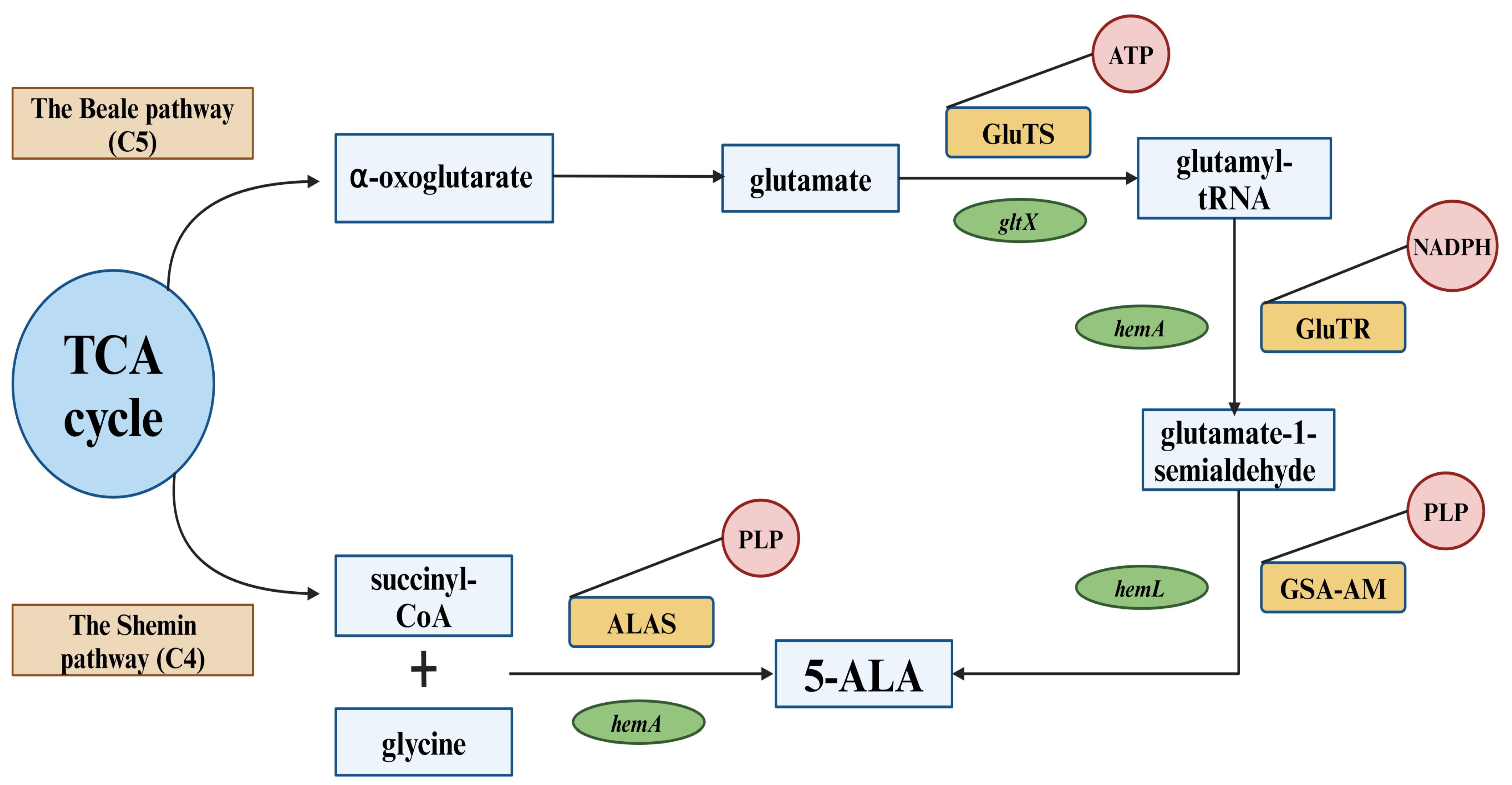
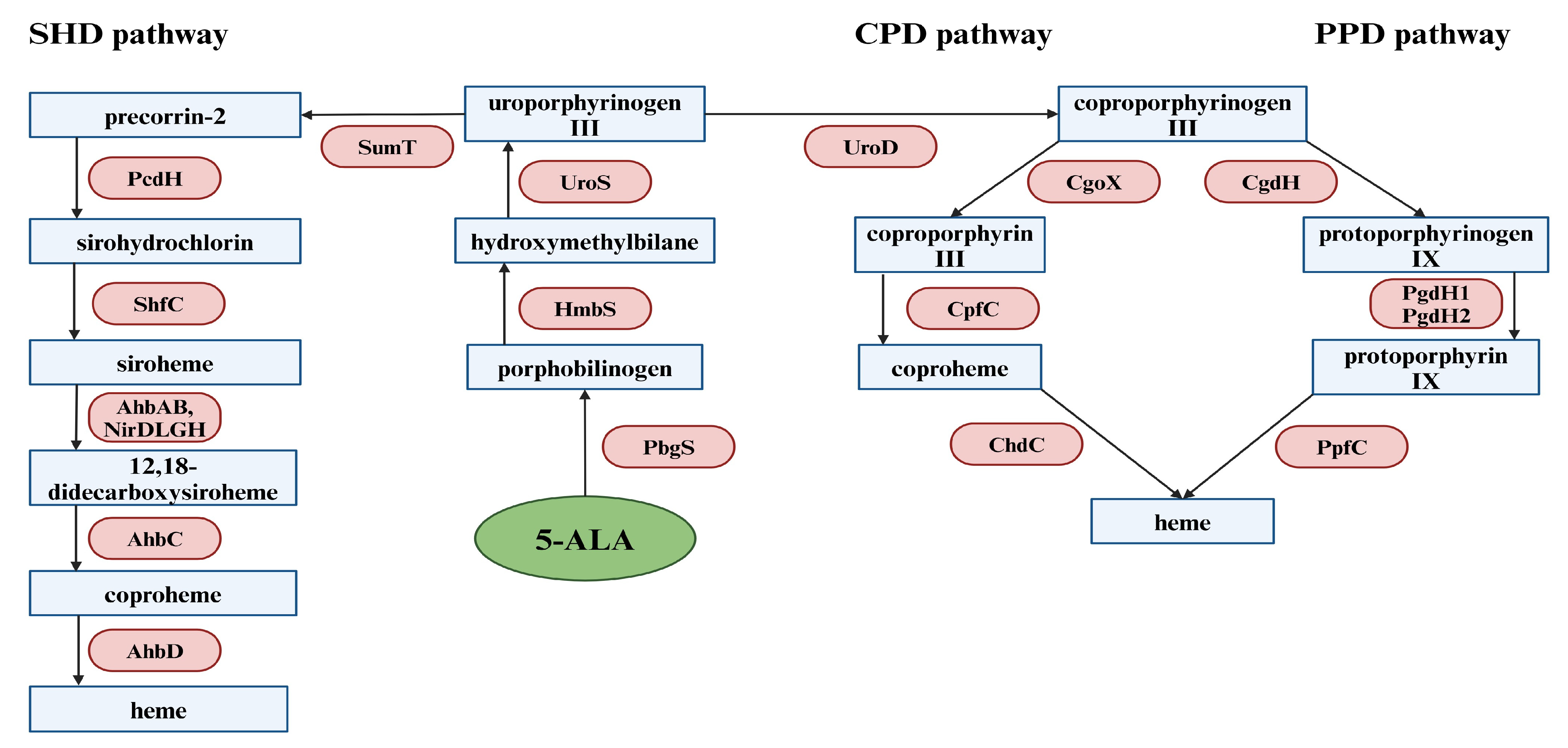
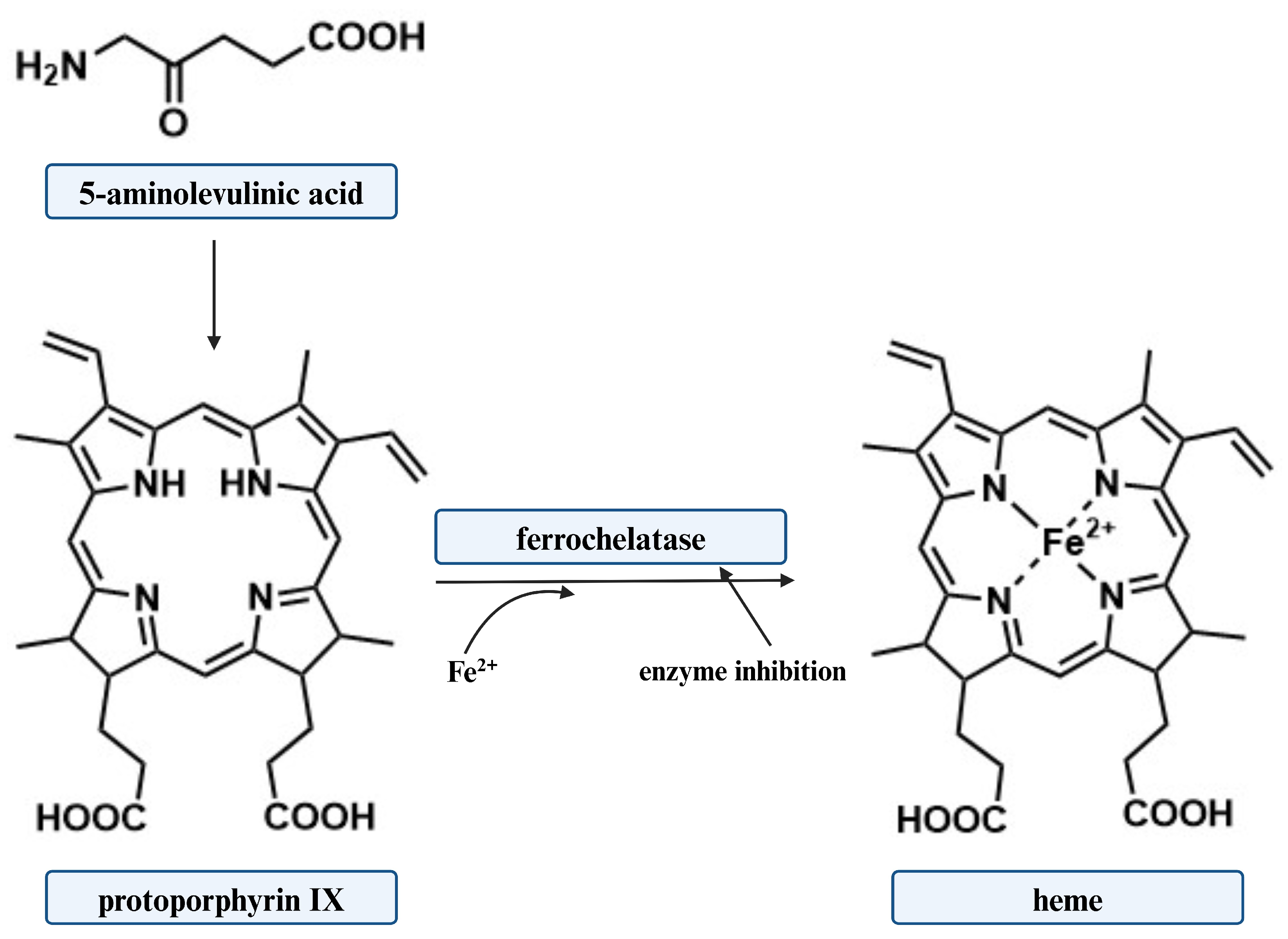
3. Biosynthesis of 5-ALA
3.1. The Shemin Pathway (C4)
3.2. The C5 Pathway (The Beale Pathway)
4. 5-ALA in Antimicrobial Photodynamic Inactivation
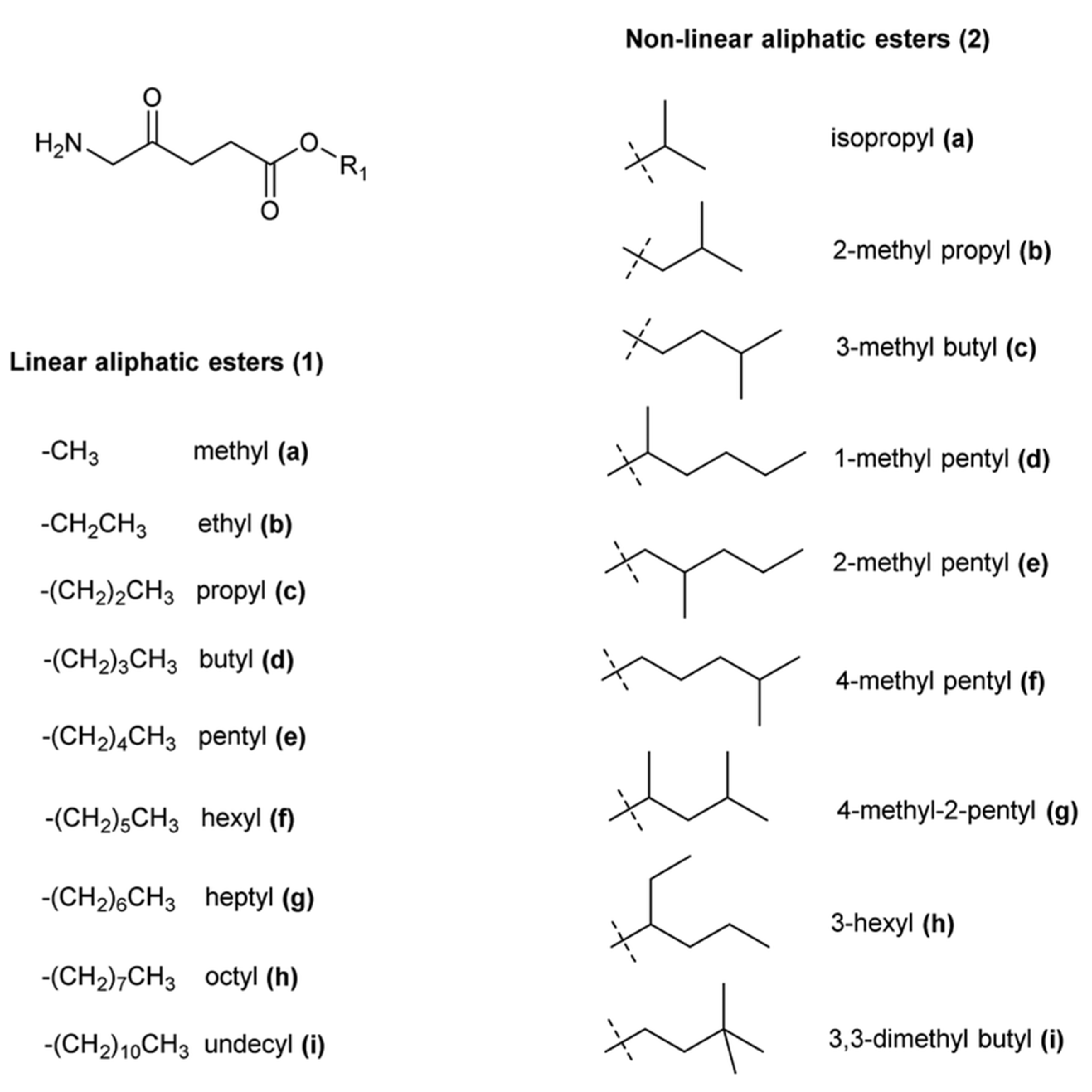
4.1. Strategies to Improve the Effectiveness of 5-ALA
| Bacterial Strain | Concentration of 5-ALA Derivative | Light Dose | Light Source | Pre-Incubation Time with Derivative | Viability Reduction | Ref. |
|---|---|---|---|---|---|---|
| Klebsiella pneumoniae (ESBL-producing clinical isolate) planktonic | 10.00 mM ALA-methyl ester | 360 Jcm−2 | 150 W xenon lamp A wavelength range between 400 and 780 nm | 4 h | 4.52 log10 of CFUcm−3 | [118] |
| Klebsiella pneumoniae (ESBL-producing clinical isolate) biofilm | 10.00 mM ALA-methyl ester | 360 Jcm−2 | 150 W xenon lamp A wavelength range between 400 and 780 nm | 4 h | 3.91 log10 of CFUcm−3 | [118] |
| Klebsiella pneumoniae (non-ESBL-producing clinical isolate) planktonic | 10.00 mM ALA-methyl ester | 360 Jcm−2 | 150 W xenon lamp A wavelength range between 400 and 780 nm | 4 h | 4.32 log10 of CFUcm−3 | [118] |
| Klebsiella pneumoniae (non-ESBL-producing clinical isolate) biofilm | 10.00 mM ALA-methyl ester | 360 Jcm−2 | 150 W xenon lamp A wavelength range between 400 and 780 nm | 4 h | 3.49 log10 of CFUcm−3 | [118] |
| Klebsiella pneumoniae ATCC 700603 planktonic | 10.00 mM ALA-methyl ester | 360 Jcm−2 | 150 W xenon lamp A wavelength range between 400 and 780 nm | 4 h | 4.80 log10 of CFUcm−3 | [118] |
| Klebsiella pneumoniae ATCC 700603 biofilm | 10.00 mM ALA-methyl ester | 360 Jcm−2 | 150 W xenon lamp A wavelength range between 400 and 780 nm | 4 h | 4.25 log10 of CFUcm−3 | [118] |
| Staphylococcus aureus ATCC 6538 planktonic | 3.90 µM ALA-methyl ester | 20 Jcm−2 | Laser light 600 nm | 0.5 h | Almost complete inactivation | [143] |
| Staphylococcus aureus ATCC 6538 planktonic | 3.90 µM ALA-hexyl ester | 20 Jcm−2 | Laser light 600 nm | 0.5 h | Almost complete inactivation | [143] |
| Escherichia coli ATCC 35218 planktonic | 3.90 µM ALA-methyl ester | 20 Jcm−2 | Laser light 600 nm | 0.5 h | Almost complete inactivation | [143] |
| Escherichia coli ATCC 35218 planktonic | 3.90 µM ALA-hexyl ester | 20 Jcm−2 | Laser light 600 nm | 0.5 h | Almost complete inactivation | [143] |
| Escherichia coli K-12 planktonic | 10.00 mM ALA-methyl ester | 120 Jcm−2 | 400 W-halogen lamp | 4 h | 4.0 log10 of CFUcm−3 | [119] |
| Escherichia coli K-12 planktonic | 10.00 mM ALA-butyl ester | 120 Jcm−2 | 400 W-halogen lamp | 4 h | 5.50 log10 of CFUcm−3 | [119] |
| Escherichia coli Ti05 planktonic | 10.00 mM ALA-methyl ester | 120 Jcm−2 | 400 W-halogen lamp | 4 h | 5.00 log10 of CFUcm−3 | [119] |
| Pseudomonas aeruginosa planktonic | 10.00 mM ALA-methyl ester | 120 Jcm−2 | 400 W-halogen lamp | 4 h | 2.00 log10 of CFUcm−3 | [119] |
| Staphylococcus aureus planktonic | 10.00 mM ALA-methyl ester | 120 Jcm−2 | 400 W-halogen lamp | 4 h | 3.00 log10 of CFUcm−3 | [119] |
4.2. 5-ALA Mediated aPDI against Bacteria
| Bacterial Strain Gram (+) | Concentration of 5-ALA | Light Dose | Light Source | Pre-incubation Time with 5-ALA | Viability Reduction | Ref. |
|---|---|---|---|---|---|---|
| Staphylococcus epidermidis (clinical isolate) planktonic | 1.00 mM | 35 Jcm−2 | ELH tungsten-halogen GE Quartzline lamps 25.2 mWcm−2 | 3 h | 5 log10 of CFUcm−3 | [148] |
| Staphylococcus epidermidis (clinical isolate) biofilm | 1.00 mM | 210 Jcm−2 | ELH tungsten-halogen GE Quartzline lamps 25.2 mWcm−2 | 3 h | 5 log10 of CFUcm−3 | [148] |
| Staphylococcus aureus ATCC 25923 planktonic | 2.00 mM | 35 to 143 Jcm−2 | ELH tungsten-halogen GE Quartzline lamps 25.2 mWcm−2 | 1 h | 6 log10 of CFUcm−3 | [148] |
| Staphylococcus aureus ATCC 25923 biofilm | 2.00 mM | 143 Jcm−2 | ELH tungsten-halogen GE Quartzline lamps 25.2 mWcm−2 | 1 h | 6 log10 of CFUcm−3 | [148] |
| Staphylococcus aureus MRSA SA325 planktonic | 0.05 mM | 384 Jcm−2 | Light-emitting diode 633 nm | 4 h | Complete elimination | [147] |
| Staphylococcus aureus MRSA SA325 planktonic | 10.00% | 25 Jcm−2 | Light-emitting diode 635 nm | 3 h | 2.05 log10 of CFUcm−3 | [150] |
| Staphylococcus aureus MRSA (clinical isolate) biofilm | 10.00 mM | 360 Jcm−2 | Light-emitting diode 633 nm | 2 h | 2.56 log10 of CFUcm−3 | [146] |
| Staphylococcus aureus MSSA (clinical isolate) biofilm | 10.00 mM | 360 Jcm−2 | Light-emitting diode 633 nm | 2 h | 2.71 log10 of CFUcm−3 | [146] |
| Staphylococcus pseudintermedius (isolated from canine skin) planktonic | 10.00% | 55.2 Jcm−2 | Light-emitting diode 465–470 nm | 24 h | 97.86% | [149] |
| Corynebacterium jeikeium K411 planktonic | 1.00 mM | 522 Jcm−2 | Light-emitting diode 565 nm | - | 4.5 log10 of CFUcm−3 | [151] |
| Mycobacterium abscessus ATCC19977 planktonic | 100.00 µg/mL | 80 Jcm−2 | Red light 585–635 nm | 12 h | Approximately 50% | [152] |
| Mycobacterium abscessus ATCC19977 planktonic | 100.00 µg/mL | 160 Jcm−2 | Red light 585–635 nm | 12 h | Approximately 80% | [152] |
| Streptococcus mutans (isolate) biofilm | 125.00 mM | - | Light-emitting diode 635 nm | 30 s | 2.23 log10 of CFUcm−3 | [153] |
| Streptococcus sobrinus (isolate) biofilm | 62.50 mM | - | Light-emitting diode 635 nm | 30 s | 2.87 log10 of CFUcm−3 | [153] |
| Enterococcus faecalis (clinical isolate) planktonic | 10.00 mM | 288 Jcm−2 | Light-emitting diode 633 nm | 4 h | 5.37 log10 of CFUcm−3 | [144] |
| Enterococcus faecalis ATCC 51299 planktonic | 10.00 mM | 288 Jcm−2 | Light-emitting diode 633 nm | 4 h | 5.22 log10 of CFUcm−3 | [144] |
| Listeria monocytogenes ATCL3C 7644 planktonic | 7.50 mM | 18 Jcm−2 | Light-emitting diode 400 nm | 2 h | 2.3 log10 of CFUcm−3 | [154] |
| Listeria monocytogenes ATCL3C 7644 planktonic | 10.00 mM | 18 Jcm−2 | Light-emitting diode 400 nm | 2 h | 3.7 log10 of CFUcm−3 | [154] |
| Listeria monocytogenes ATCL3C 7644 biofilm | 7.50 mM | 18 Jcm−2 | Light-emitting diode 400 nm | 2 h | 1.7 log10 of CFUcm−3 | [154] |
| Listeria monocytogenes ATCL3C 7644 biofilm | 10.00 mM | 18 Jcm−2 | Light-emitting diode 400 nm | 2 h | 3 log10 of CFUcm−3 | [154] |
| Bacterial Strain Gram (-) | Concentration of 5-ALA | Light Dose | Light Source | Pre-Incubation Time with 5-ALA | Viability Reduction | Ref. |
|---|---|---|---|---|---|---|
| Escherichia coli (clinical isolate) Planktonic | 40.00 mM | 142 Jcm−2 | ELH tungsten-halogen GE Quartzline lamps 25.2 mWcm−2 | 4 h | Not responsive to aPDI | [148] |
| Escherichia coli (clinical isolate) Biofilm | 40.00 mM | 142 Jcm−2 | ELH tungsten-halogen GE Quartzline lamps 25.2 mWcm−2 | 4 h | Not responsive to aPDI | [148] |
| Pseudomonas aeruginosa ATCC 27853 Planktonic | 40.00 mM | 142 Jcm−2 | ELH tungsten-halogen GE Quartzline lamps 25.2 mWcm−2 | 4 h | 4.00 log10 of CFUcm−3 | [148] |
| Pseudomonas aeruginosa ATCC 27853 Biofilm | 40.00 mM | 142 Jcm−2 | ELH tungsten-halogen GE Quartzline lamps 25.2 mWcm−2 | 4 h | Not responsive to aPDI | [148] |
| Escherichia coli K-12 Planktonic | 0.10 mM | 120 Jcm−2 | 400 W-halogen lamp | 4 h | 3.31 log10 of CFUcm−3 | [119] |
| Escherichia coli K-12 Planktonic | 1.00 mM | 120 Jcm−2 | 400 W-halogen lamp | 4 h | 4.30 log10 of CFUcm−3 | [119] |
| Pseudomonas aeruginosa ATCC 2338 biofilm | 0.50% | 9 Jcm−2 | Light-emitting diode 410 nm | 4 h | 5.00 log10 of CFUcm−3 | [167] |
| Pseudomonas aeruginosa ATCC 27853 Biofilm | 1.408 M | 54 Jcm−2 | Light-emitting diode 630 nm | 0.5 h | No growth of bacteria after 14 days of wound healing | [176] |
| Pseudomonas aeruginosa PAO1 Biofilm | 20.00 mM | 240 Jcm−2 | Light-emitting diode 630 nm | 1 h | Complete inactivation | [168] |
| Pseudomonas aeruginosa PAO1 Planktonic | 10.00 mM 7.50 mM | 240 Jcm−2 360 Jcm−2 | Light-emitting diode 630 nm | 1 h | Complete inactivation | [168] |
| Salmonella enterica DS88 planktonic | 0.50 mM | 10 Jcm−2 20 Jcm−2 | Light-emitting diode 400 nm | 4 h | Approximately 2.2 log10 of CFUcm−3 Approximately 1.7 log10 of CFUcm−3 | [171] |
| Salmonella enterica DS88 Planktonic | 0.50 mM | 10 Jcm−2 20 Jcm−2 | Light-emitting diode 400 nm | 20 h | Approximately 1.75 log10 of CFUcm−3 Approximately 1.35 log10 of CFUcm−3 | [171] |
| Klebsiella pneumoniae ATCC 700603 Planktonic | 10.00 mM | 360 Jcm−2 | 150 W xenon lamp A wavelength range between 400 and 780 nm | 4 h | 3.68 log10 of CFUcm−3 | [118] |
| Klebsiella pneumoniae ATCC 700603 Biofilm | 10.00 mM | 360 Jcm−2 | 150 W xenon lamp A wavelength range between 400 and 780 nm | 4 h | 3.09 log10 of CFUcm−3 | [118] |
| Klebsiella pneumoniae (non-ESBL-producing clinical isolate) Planktonic | 10.00 mM | 360 Jcm−2 | 150 W xenon lamp A wavelength range between 400 and 780 nm | 4 h | 3.17 log10 of CFUcm−3 | [118] |
| Klebsiella pneumoniae (non-ESBL-producing clinical isolate) Biofilm | 10.00 mM | 360 Jcm−2 | 150 W xenon lamp A wavelength range between 400 and 780 nm | 4 h | 1.92 log10 of CFUcm−3 | [118] |
| Klebsiella pneumoniae (ESBL-producing clinical isolate) Planktonic | 10.00 mM | 360 Jcm−2 | 150 W xenon lamp A wavelength range between 400 and 780 nm | 4 h | 3.20 log10 of CFUcm−3 | [118] |
| Klebsiella pneumoniae (ESBL-producing clinical isolate) Biofilm | 10.00 mM | 360 Jcm−2 | 150 W xenon lamp A wavelength range between 400 and 780 nm | 4 h | 2.28 log10 of CFUcm−3 | [118] |
| Escherichia coli IQ0245 Planktonic | 50.00 µgmL−1 | 80 Jcm−2 | Light-emitting diode 405 nm | 0.5 h | 5.00 log10 of CFUcm−3 | [158] |
| Klebsiella pneumoniae IQ0035 Planktonic | 50.00 µgmL−1 | 80 Jcm−2 | Light-emitting diode 405 nm | 0.5 h | 3.00 log10 of CFUcm−3 | [158] |
| Acinetobacter baumannii ATCC 19606 Planktonic | 1.25 mM | 32 Jcm−2 | Diode laser 405 nm | 4 h | Lethal effect | [163] |
| Acinetobacter baumannii ATCC 19606 Planktonic | 1.25 mM | 102 Jcm−2 | Diode laser 635 nm | 4 h | Lethal effect | [163] |
4.3. 5-ALA Mediated PDI of Viruses, Fungi, Yeasts, and Parasites
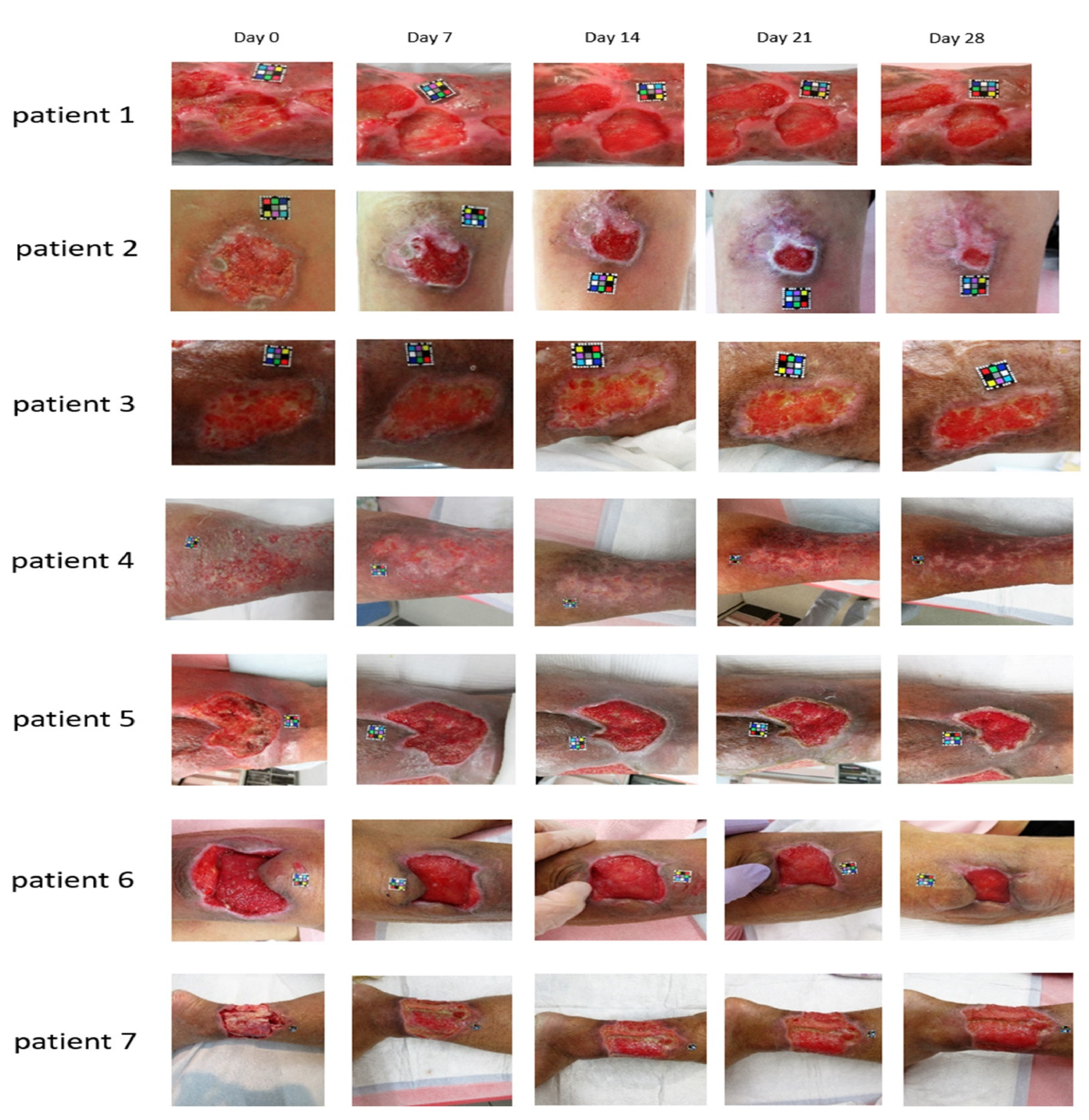
4.4. Applications of 5-ALA in Chronic Infectious Leg Ulcers
4.5. 5-ALA-Mediated Photodynamic Therapy in Endodontics
4.6. The Combined Effect of 5-ALA-aPDI with Other Agents
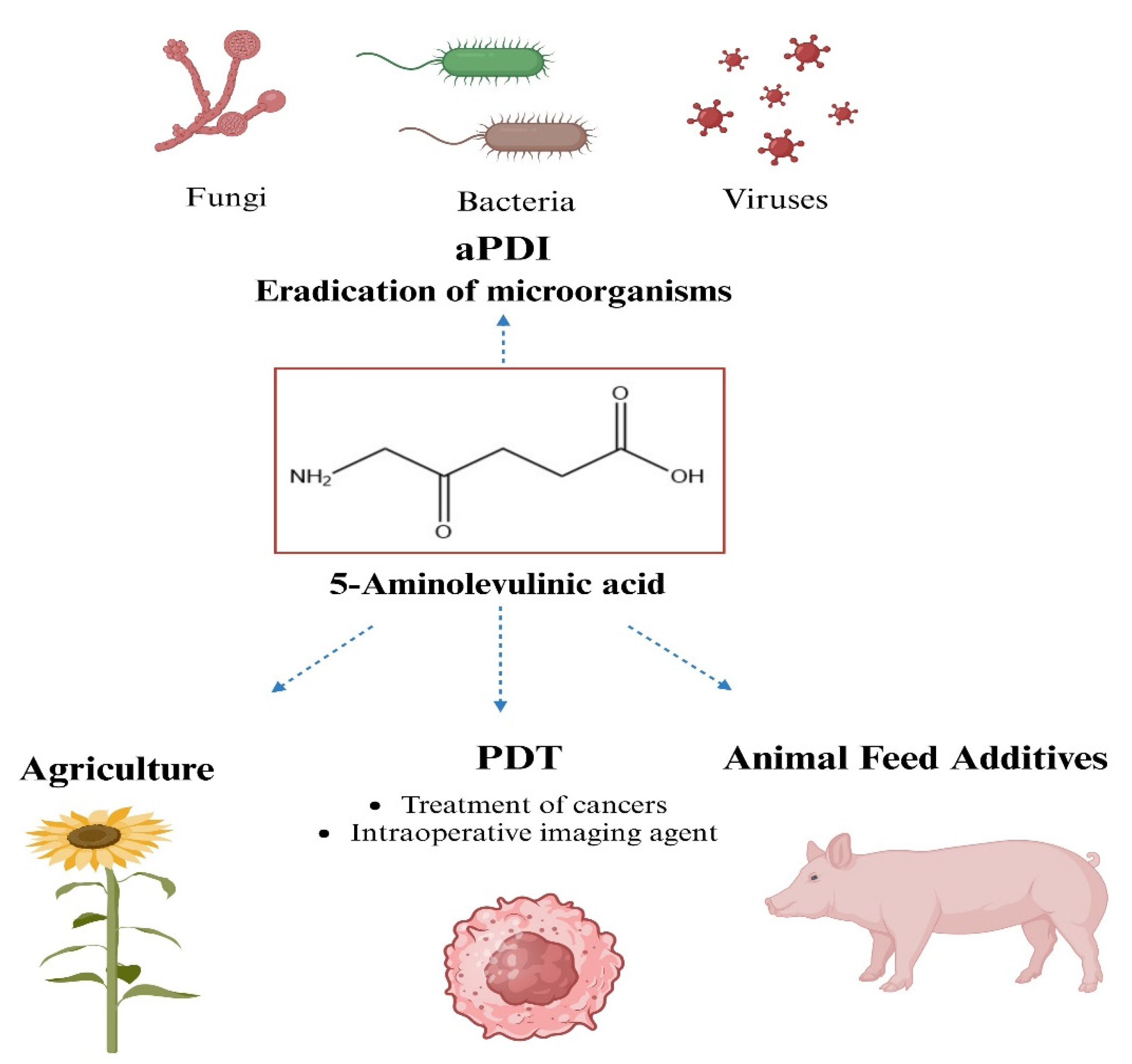
5. Other Applications of 5-ALA
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Deniell, M.D.; Hill, J.S. A history of Photodynamic therapy. Aust. N. Z. J. Surg. 1991, 61, 340–348. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaardm, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef]
- Raab, O. The effect of fluorescent agents on infusoria. Ztg. Biol. 1900, 39, 524–526. (In German) [Google Scholar]
- Dougherty, T.J.; Marcus, S.L. Photodynamic therapy. Eur. J. Cancer 1992, 28, 1734–1742. [Google Scholar] [CrossRef]
- Lui, H.; Anderson, R.R. Photodynamic therapy in dermatology: Shedding a different light on skin disease. Arch. Dermatol. 1992, 128, 1631–1636. [Google Scholar] [CrossRef]
- Maisch, T. Anti-microbial photodynamic therapy: Useful in the future? Lasers Med. Sci. 2007, 22, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, A.A.; Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Wang, C.Y.; Koshy, G.; Romanos, G.; Ishikawa, I.; Izumi, Y. Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontology 2000 2009, 51, 109–140. [Google Scholar] [CrossRef] [PubMed]
- Sharman, W.M.; Allen, C.M.; Van Lier, J.E. Photodynamic therapeutics: Basic principles and clinical applications. Drug. Discov. Today 1999, 4, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Berg, K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef]
- Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef]
- Zhou, W.; Jiang, X.; Zhen, X. Development of organic photosensitizers for antimicrobial photodynamic therapy. Biomater. Sci. 2023, 11, 5108–5128. [Google Scholar] [CrossRef] [PubMed]
- Lena, A.; Marino, M.; Manzano, M.; Comuzzi, C.; Mainfreni, M. An overview of the application of blue light-emitting diodes as a non-thermic green technology for microbial inactivation in the food sector. Food. Eng. Rev. 2024, 16, 59–84. [Google Scholar] [CrossRef]
- Piksa, K.; Lian, C.; Pawlik, K.J.; Samuel, I.D.W.; Matczyszyn, K. The role of the light source in antimicrobial photodynamic therapy. Chem. Soc. Rev. 2023, 52, 1697–1722. [Google Scholar] [CrossRef]
- Kashef, N.; Hamblin, M.R. Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist. Updat. 2017, 31, 31–42. [Google Scholar] [CrossRef]
- Mackay, A.M. Microbial Resistance to Photodynamic Therapy. J. Cell. Immunol. 2022, 4, 117–120. [Google Scholar]
- Rapacka-Zdonczyk, A.; Wozniak, A.; Nakonieczna, J.; Grinholc, M. Development of antimicrobial phototreatment tolerance: Why the methodology matters. Int. J. Mol. Sci. 2021, 22, 2224. [Google Scholar] [CrossRef] [PubMed]
- Shemin, D.; Russell, C.S. δ-Aminolevulinic acid, its role in the biosynthesis of porphyrins and purines. J. Am. Chem. Soc. 1953, 75, 4873–4874. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, J.; Zhou, J.; Qi, Q.; Du, G.; Chen, J. Recent advances in microbial production of δ-aminolevulinic acid and vitamin B 12. Biotechnol. Adv. 2012, 30, 1533–1542. [Google Scholar] [CrossRef]
- Jiang, M.; Hong, K.; Mao, Y.; Ma, H.; Chen, T.; Wang, Z. Natural 5-aminolevulinic acid: Sources, biosynthesis, detection and applications. Front. Bioeng. Biotechnol. 2022, 10, 841443. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, G.; Li, J.; Li, X.; Zhang, J. Optimization of biomass and 5-aminolevulinic acid production by Rhodobacter sphaeroides ATCC17023 via response surface methodology. Appl. Biochem. Biotechnol. 2016, 179, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Tian, Y.; Liao, X.; Tang, Y.X.; Ni, Q.Q.; Sun, J.; Zhao, Y.; Zhang, J.; Teng, Z.; Lu, G. Enhancing selective photosensitizer accumulation and oxygen supply for high-efficacy photodynamic therapy toward glioma by 5-aminolevulinic acid loaded nanoplatform. J. Colloid Interface Sci. 2020, 565, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kang, Z.; Chen, J.; Du, G. Optimization of the heme biosynthesis pathway for the production of 5-aminolevulinic acid in Escherichia coli. Sci. Rep. 2015, 5, 8584. [Google Scholar] [CrossRef]
- Burnham, B.F. δ-Aminolevulinic acid synthase (Rhodopseudomonas sphaeroides). Methods Enzymol. 1970, 17A, 195–204. [Google Scholar]
- Sasaki, K.; Watanabe, M.; Tanaka, T. Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid. Appl. Microbiol. Biotechnol. 2002, 58, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Shemin, D.; Rittenberg, D. The biological utilization of glycine for the synthesis of the protoporphyrin of hemoglobin. J. Biol. Chem. 1946, 166, 621–625. [Google Scholar] [CrossRef]
- Radin, N.S.; Rittenberg, D.; Shemin, D. The role of acetic acid in the biosynthesis of heme. J. Biol. Chem. 1950, 184, 755–767. [Google Scholar] [CrossRef]
- Gibson, K.D.; Laver, W.G.; Neuberger, A. Initial stages in the biosynthesis of porphyrins. 2. The formation of δ-aminolaevulic acid from glycine and succinyl-coenzyme A by particles from chicken erythrocytes. Biochem. J. 1958, 70, 71–81. [Google Scholar] [CrossRef]
- Shemin, D.; Kumin, S. The mechanism of porphyrin formation: The formation of a succinyl intermediate from succinate. J. Biol. Chem. 1952, 198, 827–837. [Google Scholar] [CrossRef]
- Neuberger, A.; Scott, J.J. Aminolaevulinic acid and porphyrin biosynthesis. Nature 1953, 172, 1093–1094. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.A.; Hunter, C.N.; Warren, M.J. Biosynthesis of the modified tetrapyrroles-the pigments of life. J. Biol. Chem. 2020, 295, 6888–6925. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, G.; Kumar, A.; Talmage, P.; Shemin, D. The enzymatic synthesis of δ-aminolevulinic acid. J. Biol. Chem. 1958, 233, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Astner, I.; Schulze, J.O.; van den Heuvel, J.; Jahn, D.; Schubert, W.D.; Heinz, D.W. Crystal structure of 5-aminolevulinate synthase, the first enzyme of heme biosynthesis, and its link to XLSA in humans. EMBO J. 2005, 24, 3166–3177. [Google Scholar] [CrossRef]
- Tai, T.N.; Moore, M.D.; Kaplan, S. Cloning and characterization of the 5-aminolevulinate synthase gene(s) from Rhodobacter sphaeroides. Gene 1988, 70, 139–151. [Google Scholar]
- Neidle, E.L.; Kaplan, S. Expression of the Rhodobacter sphaeroides hemA and hemT genes, encoding two 5-aminolevulinic acid synthase isozymes. J. Bacteriol. 1993, 175, 2292–2303. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, G.; Li, X.; Zhang, J. Microbial production and applications of 5-aminolevulinic acid. Appl. Microbiol. Biotechnol. 2014, 98, 7349–7357. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Ding, W.; Gong, X.; Liu, Q.; Du, G.; Chen, J. Recent advances in production of 5-aminolevulinic acid using biological strategies. World J. Microbiol. Biotechnol. 2017, 33, 200. [Google Scholar] [CrossRef]
- Choi, C.; Hong, B.S.; Sung, H.C.; Lee, H.S.; Kim, J.H. Optimization of extracellular 5-aminolevulinic acid production from Escherichia coli transformed with ALA synthase gene of Bradyrhizobium japonicum. Biotechnol. Lett. 1999, 21, 551–554. [Google Scholar] [CrossRef]
- Xie, L.; Hall, D.; Eiteman, M.A.; Altman, E. Optimization of recombinant aminolevulinate synthase production in Escherichia coli using factorial design. Appl. Microbiol. Biotechnol. 2003, 63, 267–273. [Google Scholar] [CrossRef]
- Jung, S.; Yang, K.; Lee, D.E.; Back, K. Expression of Bradyrhizobium japonicum 5-aminolevulinic acid synthase induces severe photodynamic damage in transgenic rice. Plant Sci. 2004, 167, 789–795. [Google Scholar] [CrossRef]
- Chung, S.Y.; Seo, K.H.; Rhee, J.I. Influence of culture conditions on the production of extra-cellular 5-aminolevulinic acid (ALA) by recombinant E. coli. Process Biochem. 2005, 40, 385–394. [Google Scholar] [CrossRef]
- Fu, W.; Lin, J.; Cen, P. 5-Aminolevulinate production with recombinant Escherichia coli using a rare codon optimizer host strain. Appl. Microbiol. Biotechnol. 2007, 75, 777–782. [Google Scholar] [CrossRef]
- Fu, W.; Lin, J.; Cen, P. Enhancement of 5-aminolevulinate production with recombinant Escherichia coli using batch and fed-batch culture system. Bioresour. Technol. 2008, 99, 4864–4870. [Google Scholar] [CrossRef]
- Lin, J.; Fu, W.; Cen, P. Characterization of 5-aminolevulinate synthase from Agrobacterium radiobacter, screening new inhibitors for 5-aminolevulinate dehydratase from Escherichia coli and their potential use for high 5-aminolevulinate production. Bioresour. Technol. 2009, 100, 2293–2297. [Google Scholar] [CrossRef]
- Fu, W.; Lin, J.; Cen, P. Expression of a hemA gene from Agrobacterium radiobacter in a rare codon optimizing Escherichia coli for improving 5-aminolevulinate production. Appl. Biochem. Biotechnol. 2010, 160, 456–466. [Google Scholar] [CrossRef]
- Liu, X.X.; Wang, L.; Wang, Y.J.; Cai, L.L. D-glucose enhanced 5-aminolevulinic acid production in recombinant Escherichia coli culture. Appl. Biochem. Biotechnol. 2010, 160, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, L.; Fu, W.Q.; Lin, Y.J.; Lin, J.P.; Cen, P.L. Improved 5-aminolevulinic acid production with recombinant Escherichia coli by a short-term dissolved oxygen shock in fed-batch fermentation. Chin. J. Chem. Eng. 2013, 21, 1291–1295. [Google Scholar] [CrossRef]
- Lou, J.W.; Zhu, L.; Wu, M.B.; Yang, L.R.; Lin, J.P.; Cen, P.L. High-level soluble expression of the hemA gene from Rhodobacter capsulatus and comparative study of its enzymatic properties. J. Zhejiang Univ. Sci. B 2014, 15, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Weng, H.; Du, G.; Chen, J.; Kang, Z. 5-Aminolevulinic acid production from inexpensive glucose by engineering the C4 pathway in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2017, 44, 1127–1135. [Google Scholar] [CrossRef]
- Ren, J.; Zhou, L.; Wang, C.; Lin, C.; Li, Z.; Zeng, A.-P. An Unnatural Pathway for Efficient 5-aminolevulinic acid Biosynthesis with glycine from glyoxylate based on retrobiosynthetic design. ACS Synth. Biol. 2018, 7, 2750–2757. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, J.; Wang, Y.; Wang, L.; Guo, X.; Chen, N.; Zheng, P.; Sun, J.; Ma, Y. Enhancing 5-aminolevulinic acid tolerance and production by engineering the antioxidant defense system of Escherichia coli. Biotechnol. Bioeng. 2019, 116, 2018–2028. [Google Scholar] [CrossRef]
- Zhou, L.; Ren, J.; Li, Z.; Nie, J.; Wang, C.; Zeng, A.P. Characterization and engineering of a Clostridium glycine riboswitch and its use to control a novel metabolic pathway for 5-aminolevulinic acid production in Escherichia coli. ACS Synth. Biol. 2019, 8, 2327–2335. [Google Scholar] [CrossRef]
- Miscevic, D.; Mao, J.Y.; Kefale, T.; Abedi, D.; Moo-Young, M.; Perry Chou, C. Strain engineering for high-level 5-aminolevulinic acid production in Escherichia coli. Biotechnol. Bioeng. 2021, 118, 30–42. [Google Scholar] [CrossRef]
- Tan, S.I.; You, S.C.; Shih, I.T.; Ng, I.S. Quantification, regulation and production of 5-aminolevulinic acid by green fluorescent protein in recombinant Escherichia coli. J. Biosci. Bioeng. 2019, 129, 387–394. [Google Scholar] [CrossRef]
- Yu, T.H.; Yi, Y.C.; Shih, I.T.; Ng, I.S. Enhanced 5-aminolevulinic acid production by co-expression of codon-optimized hemA gene with chaperone in genetic engineered Escherichia coli. Appl. Biochem. Biotechnol. 2020, 191, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Yu, T.H.; Ng, I.S. Engineering pyridoxal kinase PdxY-integrated Escherichia coli strain and optimization for high-level 5-aminolevulinic acid production. J. Taiwan Inst. Chem. Eng. 2021, 120, 49–58. [Google Scholar] [CrossRef]
- Ge, F.; Wen, D.; Ren, Y.; Chen, G.; He, B.; Li, X. Downregulating of hemB via synthetic antisense RNAs for improving 5-aminolevulinic acid production in Escherichia coli. 3 Biotech 2021, 11, 230. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.-C.; Xue, C.; Ng, I.S. Low-carbon-footprint production of high-end 5-aminolevulinic acid via integrative strain engineering and RuBisCo-equipped Escherichia coli. ACS Sus. Chem. Eng. 2021, 9, 15623–15633. [Google Scholar] [CrossRef]
- Yu, T.-H.; Tan, S.-I.; Yi, Y.-C.; Xue, C.; Ting, W.-W.; Chang, J.-J.; Ng, I.S. New insight into the codon usage and medium optimization toward stable and high-level 5-aminolevulinic acid production in Escherichia coli. Biochem. Eng. J. 2022, 177, 108259. [Google Scholar] [CrossRef]
- Pu, W.; Chen, J.; Zhou, Y.; Qiu, H.; Shi, T.; Zhou, W.; Guo, X.; Cai, N.; Tan, Z.; Feng, J.; et al. Systems metabolic engineering of Escherichia coli for hyper-production of 5-aminolevulinic acid. Biotechnol. Biofuels Bioprod. 2023, 16, 31. [Google Scholar]
- Feng, L.; Zhang, Y.; Fu, J.; Mao, Y.; Chen, T.; Zhao, X.; Wang, Z. Metabolic engineering of Corynebacterium glutamicum for efficient production of 5-aminolevulinic acid. Biotechnol. Bioeng. 2016, 113, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, S.; Yang, T.; Xu, M.; Zhang, X.; Shao, M.; Li, H.; Rao, Z. Engineering the C4 pathway of Corynebacterium glutamicum for efficient production of 5-aminolevulinic acid. Sheng Wu Gong Cheng Xue Bao 2021, 37, 4314–4328. [Google Scholar]
- Yang, P.; Liu, W.; Cheng, X.; Wang, J.; Wang, Q.; Qi, Q. A new strategy for production of 5-aminolevulinic acid in recombinant Corynebacterium glutamicum with high yield. Appl. Environ. Microbiol. 2016, 82, 2709–2717. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, T.; Feng, L.; Zhang, S.; Xing, D.; Wang, Z. Enhancement of 5-aminolevulinic acid production by metabolic engineering of the glycine biosynthesis pathway in Corynebacterium glutamicum. Biotechnol. Lett. 2017, 39, 1369–1374. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Guo, X.; Rao, D.; Zhou, W.; Zheng, P.; Sun, J.; Ma, Y. Efficient bioproduction of 5-aminolevulinic acid, a promising biostimulant and nutrient, from renewable bioresources by engineered Corynebacterium glutamicum. Biotechnol. Biofuels 2020, 13, 41. [Google Scholar] [CrossRef]
- Ge, F.; Li, X.; Ge, Q.; Zhu, D.; Li, W.; Shi, F. Modular control of multiple pathways of Corynebacterium glutamicum for 5-aminolevulinic acid production. AMB Express 2021, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Beale, S.I. The biosynthesis of δ-aminolevulinic acid in Chlorella. Plant Physiol. 1970, 45, 504–506. [Google Scholar] [CrossRef]
- Moser, J.; Lorenz, S.; Hubschwerlen, C.; Rompf, A.; Jahn, D. Methanopyrus kandleri glutamyl-tRNA-reductase. J. Biol. Chem. 1999, 274, 30679–30685. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Kannangara, C.G.; Grimm, B. Glutamate 1-semialdehyde aminotransferase: Anomalous enantiomeric reaction and enzyme mechanism. Biochemistry 1992, 31, 11249–11254. [Google Scholar] [CrossRef]
- Moser, J.; Schubert, W.D.; Beier, V.; Bringemeier, I.; Jahn, D.; Heinz, D.W. V-shaped structure of glutamyl-tRNA reductase, the first enzyme of tRNA-dependent tetrapyrrole biosynthesis. EMBO J. 2001, 20, 6583–6590. [Google Scholar] [CrossRef] [PubMed]
- Schulze, J.O.; Schubert, W.D.; Moser, J.; Jahn, D.; Heinz, D.W. Evolutionary relationship between initial enzymes of tetrapyrrole biosynthesis. J. Mol. Biol. 2006, 358, 1212–1220. [Google Scholar] [CrossRef]
- Ge, H.; Lv, X.; Fan, J.; Gao, Y.; Teng, M.; Niu, L. Crystal structure of glutamate-1-semialdehyde aminotransferase from Bacillus subtilis with bound pyridoxamine-5’-phosphate. Biochem. Biophys. Res. Commun. 2010, 402, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Wang, Y.; Gu, P.; Wang, Q.; Qi, Q. Engineering Escherichia coli for efficient production of 5-aminolevulinic acid from glucose. Metabol. Eng. 2011, 13, 492–498. [Google Scholar] [CrossRef]
- Ramzi, A.B.; Hyeon, J.E.; Kim, S.W.; Park, C.; Han, S.O. 5-Aminolevulinic acid production in engineered Corynebacterium glutamicum via C5 biosynthesis pathway. Enzyme Microb. Technol. 2015, 81, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.J.; You, S.K.; Kim, M.; Lee, E.; Shin, S.K.; Park, H.M.; Oh, Y.; Hn, S.O. Enhanced production of 5-aminolevulinic acid via flux redistribution of TCA cycle toward l-Glutamate in Corynebacterium glutamicum. Biotechnol. Bioprocess Eng. 2019, 24, 915–923. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Zhu, F.; Li, Z.; Lu, N.; Li, Y.; Xu, Q.; Chen, N. Metabolic engineering of an auto-regulated Corynebacterium glutamicum chassis for biosynthesis of 5-aminolevulinic acid. Bioresour. Technol. 2020, 318, 124064. [Google Scholar] [CrossRef]
- Kang, Z.; Wang, Y.; Wang, Q.; Qi, Q. Metabolic engineering to improve 5-aminolevulinic acid production. Bioeng. Bugs 2011, 2, 342–345. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, Z.; Ding, W.; Chen, J.; Du, G. Integrated optimization of the in vivo heme biosynthesis pathway and the in vitro iron concentration for 5-aminolevulinate production. Appl. Biochem. 2016, 178, 1252–1262. [Google Scholar] [CrossRef]
- Yu, X.L.; Jin, H.Y.; Liu, W.L.; Wang, Q.; Qi, Q.S. Engineering Corynebacterium glutamicum to produce 5-aminolevulinic acid from glucose. Microb. Cell Fact. 2015, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.H.; Lim, H.G.; Park, S.; Seo, S.W.; Jung, G.Y. Precise flux redistribution to glyoxylate cycle for 5-aminolevulinic acid production in Escherichia coli. Metab. Eng. 2017, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Xu, J.S.; Zhao, Q.; Wang, Q.; Qi, Q.S. Engineering Escherichia coli for efficient coproduction of polyhydroxyalkanoates and 5-aminolevulinic Acid. J. Ind. Microbiol. Biotechnol. 2018, 45, 43–51. [Google Scholar] [CrossRef]
- Zhang, B.; Ye, B.C. Pathway Engineering in Corynebacterium glutamicum S9114 for 5-aminolevulinic acid production. 3 Biotech 2018, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Weng, H.J.; Zhou, Z.X.; Du, G.C.; Kang, Z. Engineering of multiple modular pathways for high-yield production of 5-aminolevulinic acid in Escherichia coli. Bioresour. Technol. 2019, 274, 353–360. [Google Scholar] [CrossRef]
- Su, T.; Guo, Q.; Zheng, Y.; Liang, Q.; Wang, Q.; Qi, Q. Fine-tuning of hemB using CRISPRi for increasing 5-aminolevulinic acid production in Escherichia coli. Front. Microbiol. 2019, 10, 1731. [Google Scholar] [CrossRef]
- Cui, Z.; Jiang, Z.; Zhang, J.; Zheng, H.; Jiang, X.; Gong, K.; Liang, Q.; Wang, Q.; Qi, Q. Stable and efficient biosynthesis of 5-aminolevulinic acid using plasmid-free Escherichia coli. J. Agric. Food Chem. 2019, 67, 1478–1483. [Google Scholar] [CrossRef]
- Aiguo, Z.; Meizhi, Z. Production of 5-aminolevulinic acid from glutamate by overexpressing HemA1 and pgr7 from Arabidopsis thaliana in Escherichia coli. World J. Microbiol. Biotechnol. 2019, 35, 175. [Google Scholar] [CrossRef]
- Layer, G. Heme biosynthesis in prokaryotes. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118861. [Google Scholar] [CrossRef]
- Dailey, H.A.; Fleming, J.E. Bovine ferrochelatase. Kinetic analysis of inhibition by N-methylprotoporphyrin, manganese, and heme. J. Biol. Chem. 1983, 258, 11453–11459. [Google Scholar]
- Sishtla, K.; Lambert-Cheatham, N.; Lee, B.; Han, D.H.; Park, J.; Sardar Pasha, S.P.B.; Lee, S.; Kwon, S.; Muniyandi, A.; Park, B.; et al. Small-molecule inhibitors of ferrochelatase are antiangiogenic agents. Cell Chem. Biol. 2022, 29, 1010–1023. [Google Scholar] [CrossRef]
- Shistla, K.; Lee, S.; Seo, S.-Y.; Corson, W.T. Discovery of ferrochelatase inhibitors as antiangiogenic agents. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5405. [Google Scholar]
- Van Hillegersberg, R.; Van den Berg, J.W.; Kort, W.J.; Terpstra, O.T.; Wilson, J.H. Selective accumulation of endogenously produced porphyrins in a liver metastasis model in rats. Gastroenterology 1992, 103, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Navone, N.M.; Polo, C.F.; Frisardi, A.L.; Batlle, A.M. Mouse mammary carcinoma porphobilinogenase and hydroxymethylbilane synthetase. Comp. Biochem. Physiol. B 1991, 98, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, Y.; Salmon-Divon, M.; Shporen, E.; Malik, Z. ALA induced photodynamic effects on gram positive and negative bacteria. Photochem. Photobiol. Sci. 2004, 3, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Viveiros, J.; Yang, C.; Ahmadi, A.; Ganz, R.A.; Tolkoff, M.J. Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob. Agents Chemother. 2005, 49, 2822–2827. [Google Scholar] [CrossRef]
- Kim, M.-J.; Yuk, H.-G. Antibacterial mechanism of 405-nanometer light-emitting diode against Salmonella at refrigeration temperature. Appl. Environ. Microb. 2017, 83, e02582-16. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chu, Z.; Ruan, Z.; Wang, X.; Dai, T.; Hu, X. Changes of Intracellular Porphyrin, Reactive Oxygen Species, and Fatty Acids Profiles During Inactivation of Methicillin-Resistant Staphylococcus aureus by Antimicrobial Blue Light. Front. Physiol. 2018, 9, 1658. [Google Scholar] [CrossRef]
- Cruz-Oliveira, C.; Almeida, A.F.; Freire, J.M.; Caruso, M.B.; Morando, M.A.; Ferreira, V.N.S.; Assunção-Miranda, I.; Gomes, A.M.O.; Castanho, M.A.R.B.; Da Poian, A.T. Mechanisms of Vesicular Stomatitis Virus Inactivation by Protoporphyrin IX, Zinc-Protoporphyrin IX, and Mesoporphyrin IX. Antimicrob. Agents Chemother. 2017, 61, e00053-17. [Google Scholar] [CrossRef]
- Leanse, L.G.; dos Anjos, C.; Mushtaq, S.; Dai, T. Antimicrobial blue light: A ‘Magic Bullet’ for the 21st century and beyond? Adv. Drug Deliv. Rev. 2022, 18, 114057. [Google Scholar] [CrossRef]
- Guffey, J.S.; Payne, W.; Jones, T.; Martin, K. Evidence of resistance development by Staphylococcus aureus to an in vitro, multiple stage application of 405 nm light from a supraluminous diode array. Photomed. Laser Surg. 2013, 31, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Guffey, J.S.; Payne, W.; Jones, T.; Martin, K.; Dodson, C. Delaying the onset of resistance formation: Effect of manipulating dose, wavelength, and rate of energy delivery of 405-, 464-, and 850-nanometer light for Staphylococcus aureus. Wounds Compend. Clin. Res. Pract. 2014, 26, 95–100. [Google Scholar]
- Amin, R.M.; Bhayana, B.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies. Lasers Surg. Med. 2016, 48, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Rapacka-Zdonczyk, A.; Wozniak, A.; Pieranski, M.; Woziwodzka, A.; Bielawski, K.P.; Grinholc, M. Development of Staphylococcus aureus tolerance to antimicrobial photodynamic inactivation and antimicrobial blue light upon sub-lethal treatment. Sci. Rep. 2019, 9, 9423. [Google Scholar] [CrossRef] [PubMed]
- Rapacka-Zdonczyk, A.; Wozniak, A.; Kruszewska, B.; Waleron, K.; Grinholc, M. Can Gram-Negative Bacteria Develop Resistance to Antimicrobial Blue light Treatment? Int. J. Mol. Sci. 2021, 22, 11579. [Google Scholar] [CrossRef]
- Novo, M.; Huttmann, G.; Diddens, H. Chemical instability of 5-aminolevulinic acid used in the fluorescence diagnosis of bladder tumours, J. Photochem. Photobiol. B 1996, 34, 143–148. [Google Scholar] [CrossRef]
- Bunke, A.; Zerbe, O.; Schmid, H.; Burmeister, G.; Merkle, H.P.; Gander, B. Degradation mechanism and stability of 5-aminolevulinic acid. J. Pharm. Sci. 2000, 89, 1335–1341. [Google Scholar] [CrossRef]
- Franck, B.; Stratmann, H. Condensation products of the porphyrin precursor, 5-aminolevulinic acid. Heterocycles 1981, 15, 919–923. [Google Scholar] [CrossRef]
- Scott, J.J. Synthesis of crystallisable porphobilinogen. Biochem. J. 1956, 62, P6. [Google Scholar]
- Fotinos, N.; Campo, M.A.; Popowycz, F.; Gurny, R.; Lange, N. 5-Aminolevulinic acid derivatives in photomedicine: Characteristics, application and perspectives. Photochem. Photobiol. 2006, 82, 994–1015. [Google Scholar] [CrossRef]
- Malik, Z.; Kostenich, G.; Roitman, L.; Ehrenberg, B.; Orenstein, A. Topical application of 5-aminolevulinic acid, DMSO and EDTA—Protoporphyrin IX accumulation in skin and tumors of mice. J. Photochem. Photobiol. B 1995, 28, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Van Den Akker, J.T.; Iani, V.; Star, W.M.; Sterenborg, H.J.; Moan, J. Topical application of 5-aminolevulinic acid hexyl ester and 5-aminolevulinic acid to normal nude mouse skin: Differences in protoporphyrin IX fluorescence kinetics and the role of the stratum corneum. Photochem. Photobiol. 2000, 72, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Steluti, R.; DeRosa, F.S.; Collett, J.; Tedesco, A.C.; Bentley, M.V.L.B. Topical glycerol monooleate/propylene glycol formulations enhance 5-aminolevulinic acid in vitro skin delivery and in vivo protophorphyrin IX accumulation in hairless mouse skin. Eur. J. Pharm. Biopharm. Sci. 2005, 60, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Auner, B.G.; Petzenhauser, E.; Valenta, C. Influence of 6-ketocholestanol on skin permeation of 5-aminolevulinic acid and evaluation of chemical stability. J. Pharm. Sci. 2004, 93, 2780–2787. [Google Scholar] [CrossRef]
- Pierre, M.B.R.; Ricci, E., Jr.; Tedesco, A.C.; Bentley, M.V.L.B. Oleic acid as optimizer of the skin delivery of 5-aminolevulinic acid in photodynamic therapy. Pharm. Res. 2006, 23, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Thunshelle, C.; Yin, R.; Chen, Q.; Hamblin, M.R. Current advances in 5-aminolevulinic acid mediated photodynamic therapy. Curr. Dermatol. Rep. 2016, 5, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.M.; Eggleston, I.M. Chemical approaches for the enhancement of 5-aminolevulinic acid-based photodynamic therapy and photodiagnosis. Photochem. Photobiol. Sci. 2018, 17, 1553–1572. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, Y.; Wang, L.; Han, L.; Lei, J.; Ishaq, H.M.; Nair, S.P.; Xu, J. Photodynamic inactivation of Klebsiella pneumoniae biofilms and planktonic cells by 5-aminolevulinic acid and 5-aminolevulinic acid methyl ester. Lasers Med. Sci. 2016, 31, 557–565. [Google Scholar] [CrossRef]
- Fotinos, N.; Convert, M.; Piffaretti, J.; Gurny, R.; Lange, N. Effects on Gram-Negative and Gram-Positive Bacteria Mediated by 5-Aminolevulinic Acid and 5-Aminolevulinic Acid Derivatives. Antimicrob. Agents Chemother. 2008, 52, 4. [Google Scholar] [CrossRef]
- Kloek, J.; van Henegouwen, G.M.J.B. Prodrugs for 5-aminolevulinic acid for photodynamic therapy. Photochem. Photobiol. 1996, 64, 994–1000. [Google Scholar] [CrossRef]
- Godal, A.; Nilsen, N.O.; Klaveness, J.; Branden, J.E.; Nesland, J.M.; Peng, Q. New derivatives of 5-aminolevulinic acid for photodynamic therapy: Chemical synthesis and porphyrin production in in vitro and in vivo biological systems. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Godal’, A.; Klaveness, J.; Morris, K. Acne Treatment with Using 5-Aminolevulinic Acid Derivatives. Patent RU2385718C2, 10 April 2010. [Google Scholar]
- Yang, C.; Wu, T.; Qi, Y.; Zhang, Z. Recent advances in the application of vitamin E TPGS for drug delivery. Theranostics 2018, 8, 464–485. [Google Scholar] [CrossRef] [PubMed]
- Vallinayagam, R.; Schmitt, F.; Barge, J.; Wagnieres, G.; Wenger, V.; Neier, R.; Juillerat-Jeanneret, L. Glycoside esters of 5-aminolevulinic acid for photodynamic therapy of cancer. Bioconjugate Chem. 2008, 19, 821–839. [Google Scholar] [CrossRef]
- Gurba, P.; Vallinayagam, R.; Schmitt, F.; Furrer, J.; Juillerat-Jeanneret, L.; Neier, R. Novel bioconjugates of aminolevulinic acid with nucleosides. Synthesis 2008, 24, 3957–3962. [Google Scholar]
- Berger, Y.; Ingrassia, L.; Neier, R.; Juillerat-Jeanneret, L. Evaluation of dipeptide-derivatives of 5-aminolevulinic acid as precursors for photosensitizers in photodynamic therapy. Bioorg. Med. Chem. 2003, 11, 1343–1351. [Google Scholar] [CrossRef][Green Version]
- Rogers, L.M.-A.; McGivern, P.G.; Butler, A.R.; MacRobert, A.J.; Eggleston, I.M. An efficient synthesis of 5-aminolaevulinic acid (ALA)-containing peptides for use in photodynamic therapy. Tetrahedron 2005, 61, 6951–6958. [Google Scholar] [CrossRef]
- Bourré, L.; Giuntini, F.; Eggleston, I.M.; Wilson, M.; MacRobert, A.J. Protoporphyrin IX enhancement by 5-aminolaevulinic acid peptide derivatives and the effect of RNA silencing on intracellular metabolism. Br. J. Cancer 2009, 100, 723–731. [Google Scholar] [CrossRef]
- Di Venosa, G.; Vallecorsa, P.; Giuntini, F.; Mamone, L.; Batlle, A.; Vanzuli, S.; Juarranz, A.; MacRobert, A.J.; Eggleston, I.M.; Casas, A. The use of dipeptide derivatives of 5-aminolaevulinic acid promotes their entry to tumor cells and improves tumor selectivity of photodynamic Therapy. Mol. Cancer Ther. 2015, 14, 440–451. [Google Scholar] [CrossRef]
- Dixon, M.J.; Bourré, L.; MacRobert, A.J.; Eggleston, I.M. Novel prodrug approach to photodynamic therapy: Fmoc solid-phase synthesis of a cell permeable peptide incorporating 5-aminolaevulinic acid. Bioorg. Med. Chem. Lett. 2007, 17, 4518–4522. [Google Scholar] [CrossRef]
- Abd-Elgaliel, W.R.; Cruz-Monserrate, Z.; Wang, H.; Logsdon, C.D.; Tung, C.H. Pancreatic cancer-associated Cathpesin E as a drug activator. J. Control. Release 2013, 167, 221–227. [Google Scholar] [CrossRef]
- Ji, B.; Wei, M.; Yang, B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics 2022, 12, 434–458. [Google Scholar] [CrossRef] [PubMed]
- Aggelidou, C.; Theosossiou, T.A.; Goncalves, A.R.; Lampropoulu, M.; Yannakopoulou, K. A vesatile δ-aminolevulinic acid (ALA)-cyclodextrin bimodal conjugate-prodrug for PDT applications with the help of intracellular chemistry. Beilstein J. Org. Chem. 2014, 10, 2414–2420. [Google Scholar] [CrossRef] [PubMed]
- Battah, S.H.; Chee, C.E.; Nakanishi, H.; Gerscher, S.; MacRobert, A.J.; Edwards, C. Synthesis and biological studies of 5-aminolevulinic acid-containing dendrimers for photodynamic therapy. Bioconjugate Chem. 2001, 12, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Battah, S.; Balaratnam, S.; Casas, A.; O’Neill, S.; Edwards, C.; Batlle, A.; Dobbin, P.; MacRobert, A.J. Macromolecular delivery of 5-aminolaevulinic acid for photodynamic therapy using dendrimer conjugates. Mol. Cancer Ther. 2007, 6, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, X.; Zhao, F.; Luan, H.; Tu, Q.; Huang, Z.; Wang, H. In vitro evaluationof 5-aminolevulinic acid (ALA) loaded PLGA nanoparticles. Int. J. Nanomed. 2013, 8, 2669–2676. [Google Scholar] [CrossRef]
- Goncalves, K.D.; de Oliveira, K.; Viera, D.P.; Courrol, L.C. Synthesis and characterisation of aminolevulinic acid gold nanoparticles: Photo and sonosensitizer agent for artherosclerosis. J. Lumin. 2018, 197, 317–323. [Google Scholar] [CrossRef]
- Babic, A.; Herceg, V.; Bastien, E.; Lassalle, H.P.; Bzdetnaya, L.; Lange, N. 5-Aminolevulinic acid-squalene nanoasemblies for tumor photodetection and therapy: In vitro studies. Nanoscale Res. Lett. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Y.; Lin, Z.; Wei, Q.; Qian, J.; Ruan, R.; Jiang, X.; Hou, L.; Song, J.; Ding, J.; et al. Stimuli-responsive nanoparticles for controlled drug delivery in synergistic cancer immunotherapy. Adv. Sci. 2022, 9, e2103444. [Google Scholar] [CrossRef]
- Lou, L.; Zhou, S.; Tan, S.; Xiang, M.; Wang, W.; Yuan, C.; Gao, L.; Xiao, Q. Amplifying the efficacy of ALA-based prodrugs for photodynamic therapy using nanotechnology. Front. Pharmacol. 2023, 14, 1137707. [Google Scholar] [CrossRef]
- Berg, K.; Anholt, H.; Bech, O.; Moan, J. The influence of iron chelators on the accumulation of protoporphyrin IX in 5-aminolaevulinic acid-treated cells. Br. J. Cancer 1996, 74, 688–697. [Google Scholar] [CrossRef]
- Hunter, G.A.; Sampson, M.P.; Ferreira, G.C. Metal Ion Substrate Inhibition of Ferrochelatase. J. Biol. Chem. 2008, 283, 23685–23691. [Google Scholar] [CrossRef]
- Sajjad, F.; Sun, N.-N.; Chen, T.; Yan, Y.-J.; Margetić, D.; Chen, Z.-L. Evaluation of antimicrobial photodynamic activities of 5-aminolevulinic acid derivatives. Photodermatol. Photoimmunol. Photomed. 2021, 37, 296–305. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, Y.; Wang, L.; Han, L.; Lei, J.; Ishaq, H.M.; Xu, J. Mechanistic aspects of the photodynamic iactivation of vancomycin-resistant Enterococci mediated by 5-aminolevulinic acid and 5-aminolevulinic acid methyl ester. Curr. Microbiol. 2015, 70, 528–535. [Google Scholar] [CrossRef]
- Boucher, H.W.; Corey, G.R. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2008, 46, S344–S349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Z.; Zhao, K.Q.; Wu, Y.; Li, X.H.; Yang, C.; Guo, L.M.; Liu, C.H.; Qu, D.; Zheng, C.Q. 5-aminolevulinic acid-mediated photodynamic therapy and its strain-dependent combined effect with antibiotics on Staphylococcus aureus biofilm. PLoS ONE 2017, 12, e0174627. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guo, M.; Jin, S.; Wu, M.; Yang, C.; Zhang, G.; Wang, P.; Ji, J.; Zeng, Q.; Wang, X.; et al. Antibacterial photodynamic therapy mediated by 5-aminolevulinic acid on methicillin-resistant Staphylococcus aureus. Photodiagn. Photodyn. Ther. 2019, 28, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Bohm, G.C.; Gándara, L.; Di Venosa, G.; Mamone, L.; Buzzola, F.; Casas, A. Photodynamic inactivation mediated by 5-aminolevulinic acid of bacteria in planktonic and biofilm forms. Biochem. Pharmacol. 2020, 177, 114016. [Google Scholar] [CrossRef]
- Bae, S.; Oh, T. In vitro bactericidal activity of 465–470 nm blue light phototherapy and aminolevulinic acid on Staphylococcus pseudintermedius. Vet. Dermat. 2018, 29, 296-e102. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guo, M.; Wu, M.; Shen, S.; Shi, L.; Cao, Z.; Wang, X.; Wang, H. Effectiveness of a single treatment of photodynamic therapy using topical administration of 5-aminolevulinic acid on methicillin-resistant Staphylococcus aureus-infected wounds of diabetic mice. Photodiagn. Photodyn. Ther. 2020, 30, 101748. [Google Scholar] [CrossRef]
- Shleeva, M.; Savitsky, A.; Kaprelyants, A. Corynebacterium jeikeium dormant cell formation and photodynamic inactivation. Front. Microbiol. 2020, 11, 605899. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wan, M.; Zhang, L.; Dai, Y.; Hai, Y.; Yue, C.; Xu, J.; Ding, Y.; Wang, M.; Xie, J.; et al. ALA_PDT promotes ferroptosis-like death of Mycobacterium abscessus and antibiotic sterilization via oxidative stress. Antioxidants 2022, 11, 546. [Google Scholar] [CrossRef]
- Saeed, H.M.M.; Faraj, B.M.; Mirdan, B.M. Evaluation of antibacterial effects of 5-aminolevulinic acid in combination with light emitting diode (LED: 635 nm) with different disinfection methods. Photodiagn. Photodyn. Ther. 2020, 29, 101615. [Google Scholar] [CrossRef]
- Buchovec, I.; Paskeviciute, E.; Luksiene, Z. Photosensitization-based inactivation of food pathogen Listeria monocytogenes in vitro and on the surface of packaging material. J. Photochem. Photobiol. B Biol. 2010, 99, 9–14. [Google Scholar] [CrossRef]
- Ballén, V.; Gabasa, Y.; Ratia, C.; Ortega, R.; Tejero, M.; Soto, S. Antibiotic resistance and virulence profiles of Klebsiella pneumoniae strains isolated from different clinical sources. Front. Cell. Infect. Microbiol. 2021, 11, 738223. [Google Scholar] [CrossRef]
- Pitout, J.D.; Nordmann, P.; Poirel, L. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob. Agents Chem. 2015, 59, 5873–5884. [Google Scholar] [CrossRef]
- Fils, P.E.L.; Cholley, P.; Gbaguidi-Haore, H.; Hocquet, D.; Sauget, M.; Bertrand, X. ESBL-producing Klebsiella pneumoniae in a University hospital: Molecular features, diffusion of epidemic clones and evaluation of cross-transmission. PLoS ONE 2021, 16, e0247875. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.X. Visualization and elimination of polymicrobial biofilms by a combination of ALA-carvacrol-blue light. J. Photochem. Photobiol. B Biol. 2022, 234, 112525. [Google Scholar] [CrossRef]
- Antunes, L.C.S.; Towner, K.J.; Visca, P. Acientobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef]
- Evans, B.A.; Hamouda, A.; Amyes, S.G. The rise of carbapenem-resistant Acinetobacter baumannii. Curr. Pharm. Des. 2013, 19, 223–238. [Google Scholar] [CrossRef]
- Huang, L.; Krayer, M.; Roubil, J.G.; Huang, Y.Y.; Holten, D.; Lindsey, J.S.; Hamblin, M.R. Stable synthetic mono-substituted cationic bacteriochlorins mediate selective broad-spectrum photoinactivation of drug-resistant pathogens at nanomolar concentrations. J. Photochem. Photobiol. B 2014, 141, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Halstead, F.D.; Thwaite, J.E.; Burt, R.; Laws, T.R.; Raguse, M.; Moeller, R.; Webber, M.A.; Oppenheim, B.A. Antibacterial Activity of Blue Light against Nosocomial Wound Pathogens Growing Planktonically and as Mature Biofilms. Appl. Environ. Microbiol. 2016, 82, 4006–4016. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, I.; Goldeman, W. Pentamidine enhances photosensitization of Acinetobacter baumannii using diode lasers with emission of light at wavelength of ʎ = 405 nm and ʎ = 635 nm. Photodiagn. Photodyn. Ther. 2021, 34, 102242. [Google Scholar] [CrossRef] [PubMed]
- Cantón, R.; Ruiz-Garbajosa, P. Epidemiology of antibiotic resistance in Pseudomonas aeruginosa. Impications for empiric and definitive therapy. Rev. Esp. Quimioter. 2017, 30, 8–12. [Google Scholar]
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Strateva, T.; Yordanov, D. Pseudomonas aeruginosa—A phenomen of bacterial resistance. J. Med. Microbiol. 2009, 58, 1133–1148. [Google Scholar] [CrossRef]
- Katayama, B.; Ozawa, T.; Morimoto, K.; Awazu, K.; Ito, N.; Honda, N.; Oiso, N.; Tsuruta, D. Enhanced sterilization and healing of cutaneous pseudomonas infection using 5-aminolevulinic acid as a photosensitizer with 410-nm LED light. J. Dermatol. Sci. 2018, 90, 323–331. [Google Scholar] [CrossRef]
- Lee, C.F.; Lee, C.J.; Chen, C.T.; Huang, C.T. delta-Aminolaevulinic acid mediated photodynamic antimicrobial chemotherapy on Pseudomonas aeruginosa planktonic and biofilm cultures. J. Photochem. Photobiol. B. 2004, 75, 21–25. [Google Scholar] [CrossRef]
- Alqahtani, M.A. Decontamination of a siloxane impression material by using 5-aminolevulinic acid activated by photodynamic therapy, microwave irradiation, and hydrogen peroxide. Photodiagn. Photodyn. Ther. 2022, 38, 102867. [Google Scholar] [CrossRef]
- Chu, E.S.; Wu, R.W.; Yow, C.M.; Wong, T.K.; Chen, J.Y. The cytotoxic and genomic potential of 5-aminolevulinic acid on lymphocytes: A comet assay study. Cancer Chemother. Pharmacol. 2006, 58, 408–414. [Google Scholar] [CrossRef]
- Polmickaitė-Smirnova, E.; Buchovec, I.; Bagdonas, S.; Sužiedėlienė, E.; Ramanavičius, A.; Anusevičius, Ž. Photoinactivation of Salmonella enterica exposed to 5-aminolevulinic acid: Impact of sensitization conditions and irradiation time. J. Photochem. Photobiol. B Biol. 2022, 231, 112446. [Google Scholar] [CrossRef]
- Lim, C.K.; Razzaque, M.A.; Luo, J.; Farmer, P.B. Isolation and characterization of protoporphyrin glycoconjugates from rat harderian gland by HPLC, capillary electrophoresis and HPLC/electrospray ionization MS. Biochem. J. 2000, 1, 757–761. [Google Scholar] [CrossRef]
- Dan, M.; Song, W.; Liu, M.; Zhang, Y.; Gao, F. Three-dimensional quantification of protoporphyrin IX in photodynqamic therapy using SFDI/DFT: A pilot experimental validation. J. Inn. Opt. Health Sci. 2022, 15, 2240008. [Google Scholar] [CrossRef]
- Lee, C.N.; Hsu, R.; Chen, H.; Wong, T.W. Daylight Photodynamic Therapy: An Update. Molecules 2020, 25, 5195. [Google Scholar] [CrossRef] [PubMed]
- Myrzakhmetov, B.; Arnoux, P.; Mordon, S.; Acherar, S.; Tsoy, I.; Frochot, C. Photophysical Properties of Protoporphyrin IX, Pyropheophorbide-a, and Photofrin® in Different Conditions. Pharmaceuticals 2021, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Tan, Y.; Zhang, W.; Yang, W.; Luo, J.; Chen, L.; Liu, H.; Yang, G.; Lei, X. Effects of ALA-PDT on the healing of mouse skin wounds infected with Pseudomonas aeruginosa and its related mechanisms. Front. Cell Dev. Biol. 2020, 8, 585132. [Google Scholar] [CrossRef] [PubMed]
- Mohr, H.; Lambrecht, B.; Selz, A. Photodynamic virus inactivation of blood components. Immunol. Investig. 1995, 24, 73–85. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, Z.; Gao, T.; Fan, L.; Wang, W.; Zeng, X.; Qin, L. Topical 5-aminolevulinic acid photodynamic therapy for recalcitrant facial flat warts. Photodiagn. Photodyn. Ther. 2024, 45, 103934. [Google Scholar] [CrossRef]
- Chen, Y.; Mei, Y.; Gu, L.; Li, X.; Guo, P.; Chen, L.; He, D. A novel PDT: 5-aminolevulinic acid combined 450 nm blue laser photodynamic therapy significantly promotes cell death of HR-HPV infected cells. Artif. Cells Nanomed. Biotechnol. 2023, 51, 22–32. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef]
- Lan, Y.; Zheng, B.; Tang, Z.; Li, J.; Zhang, J. Combinatory Effect of ALA-PDT and Itraconazole Treatment for Trichosporon asahii. Lasers Surg. Med. 2020, 53, 722–730. [Google Scholar] [CrossRef]
- Souza, D.M.; Alves, P.M.; Silva, M.L.; Paulino, T.P.; Coraspe, H.O.; Mendonça, M.M.; Ribeiro, B.M.; da Silva, M.V.; Rodrigues Júnior, V.; Rodrigues, D.B. 5-ALA-mediated photodynamic therapy reduces the parasite load in mice infected with Leishmania braziliensis. Parasite Immunol. 2017, 39, e12403. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-H.; Lee, J.Y.-Y.; Pan, S.-C.; Wong, T.-W. Enhancing wound healing in recalcitrant leg ulcers with aminolevulinic acid-mediated antimicrobial photodynamic therapy. Photodiagn. Photodyn. Ther. 2021, 33, 102149. [Google Scholar] [CrossRef]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef]
- Shiratori, M.; Ozawa, T.; Ito, N.; Awazu, K.; Tsuruta, D. Open study of photodynamic therapy for skin ulcers infected with MRSA and Pseudomonas aeruginosa. Photodiagn. Photodyn. Ther. 2021, 36, 102484. [Google Scholar] [CrossRef]
- Lei, X.; Liu, B.; Huang, Z.; Wu, J. A clinical study of photodynamic therapy for chronic skin ulcers in lower limbs infected with Pseudomonas aeruginosa. Arch. Dermatol. Res. 2015, 307, 49–55. [Google Scholar] [CrossRef]
- Nakano, A.; Tamada, Y.; Watanabe, D.; Ishida, N.; Yamashita, N.; Kuhara, Y.; Yanagishita, T.; Kawamura, C.; Akita, Y.; Matsumoto, Y. A pilot study to assess the efficacy of photodynamic therapy for Japanese patients with actinic keratosis in relation to lesion size and histological severity. Photoder. Photoimm. Photomed. 2009, 25, 37–40. [Google Scholar] [CrossRef]
- Krupka, M.; Bożek, A.; Bartusik-Aebisher, D.; Cieślar, G.; Kawczyk-Krupka, A. Photodynamic therapy for the treatment of infected leg ulcers-A pilot study. Antibiotics 2021, 10, 506. [Google Scholar] [CrossRef]
- Plotino, G.; Grande, N.M.; Mercade, M. Photodynamic Therapy in endodontics. Int. Endod. J. 2019, 52, 760–774. [Google Scholar] [CrossRef]
- D’Ercole, S.; Carlesi, T.; Dotta, T.C.; Pierfelice, T.V.; D’Amico, E.; Tripodi, D.; Iezzi, G.; Piattelli, A.; Petrini, M. 5-Aminolevulinic acid and red led in endodontics: A narrative review and a case report. Gels 2022, 8, 697. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, L.L.; Vaishnavi, C. Endodontic microbiology. J. Conserv. Dent. 2010, 13, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, S.; Kangarlou, A.; Shahbazi, R.; Nazari Nasab, A.; Naseri, M. Comparison of the Bactericidal efficacy of photodynamic therapy, 2.5% sodium hypochlorite, and 2% chlorhexidine against Enterococcus faecalis in root canals; an in vitro study. Dent. Res. J. 2012, 9, 613. [Google Scholar] [CrossRef]
- Silva Garcez, A.; Núñez, S.C.; Lage-Marques, J.L.; Jorge, A.O.C.; Ribeiro, M.S. Efficiency of NaOCl and laser-assisted photosensitization on the reduction of Enterococcus faecalis in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, e93–e98. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.; He, J.; Glickman, G.N.; Spears, R.; Schneiderman, E.D.; Honeyman, A.L. Evaluation of photodynamic therapy using a light-emitting diode lamp against Enterococcus faecalis in extracted human teeth. J. Endod. 2011, 37, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.; Cheng, J.L.; Lim, T.W.; Teo, E.G.; Wong, J.; George, S.; Kishen, A. Light activated disinfection: An alternative endodontic disinfection strategy. Aust. Dent. J. 2009, 54, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.C.; Brito, P.R.R.; Machado de Oliveira, J.C.; Alves, F.R.F.; Moreira, E.J.L.; Sampaio-Filho, H.R.; Rôças, I.N.; Siqueira, J.F. Photodynamic therapy with two different photosensitizers as a supplement to instrumentation/irrigation procedures in promoting intracanal reduction of Enterococcus faecalis. J. Endod. 2010, 36, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, Y.Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Front. Microbiol. 2018, 27, 1299. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, H.; Wang, Y.; Shiu, B.-C.; Lin, J.-H.; Zhang, S.; Lou, C.-W.; Li, T.-T. Synergistic antibacterial strategy based on photodynamic therapy: Progress and perspectives. Chem. Eng. J. 2022, 450, 138129. [Google Scholar] [CrossRef]
- Di Lodovico, S.; Diban, F.; Di Fermo, P.; Petrini, M.; Fontana, A.; Di Giulio, M.; Piattelli, A.; D’Ercole, S.; Cellini, L. Antimicrobial combined action of graphene oxide and light emitting diodes for chronic wound management. Int. J. Mol. Sci. 2022, 23, 6942. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, W.B.; Dawuda, M.M.; Hu, L.; Yu, J.H. 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants: A review. Plant Growth Regul. 2018, 87, 357–374. [Google Scholar] [CrossRef]
- Hendawy, A.O.; Khattab, M.S.; Sugimura, S.; Sato, K. Effects of 5-aminolevulinic acid as a supplement on animal performance, iron status, and immune response in farm animals: A review. Animals 2020, 10, 1352. [Google Scholar] [CrossRef]
- Itoh, Y.; Ninomiya, Y.; Tajima, S.; Ishibashi, A. Photodynamic therapy for acne vulgaris with topical 5-aminolevulinic acid. Arch. Dermatol. 2000, 136, 1093–1095. [Google Scholar] [CrossRef]
- Tan, S.; Cao, J.; Xia, X.; Li, Z. Advances in 5-aminolevulinic acid priming to enhance plant tolerance to abiotic stress. Int. J. Mol. Sci. 2022, 23, 702. [Google Scholar] [CrossRef] [PubMed]
- Rapacka-Zdończyk, A.; Woźniak, A.; Michalska, K.; Pierański, M.; Ogonowska, P.; Grinholc, M.; Nakonieczna, J. Factors Determining the Susceptibility of Bacteria to Antibacterial Photodynamic Inactivation. Front. Med. 2021, 12, 642609. [Google Scholar] [CrossRef]
- Valle, R.D.; Hadjipanayis, C.G.; Stummer, W. Established and emerging uses of 5-ALA in the brain: An overview. J. Neuro-Oncol. 2019, 141, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Arits, A.H.; van de Weert, M.M.; Nelemans, P.J.; Kelleners-Smeets, N.W. Pain during topical photodynamic therapy: Uncomfortable and unpredictable. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1452–1457. [Google Scholar] [CrossRef]
- Wang, B.; Shi, L.; Zhang, Y.F.; Zhou, Q.; Zheng, J.; Szeimies, R.M.; Wang, X.L. Gain with no pain? Pain management in dermatological photodynamic therapy. Br. J. Dermatol. 2017, 177, 656–665. [Google Scholar] [CrossRef]
- Zeng, L.; Zou, Q.; Huang, P.; Xiong, L.; Cheng, Y.; Chen, Q.; Li, Y.; He, H.; Yi, W.; Wei, W. Inhibition of autophagy with Chloroquine enhanced apoptosis induced by 5-aminolevulinic acidphotodynamic therapy in secondary hyperparathyroidism primary cells and organoids. Biomed. Pharmacother. 2021, 142, 111994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shi, L.; Zhang, Y.; Wang, P.; Zhang, G.; Cao, Y.; Zhou, Z.; Wang, X. Modified photodynamic therapy to minimize pain in the treatment of condylomata acuminata: A prospective, randomized, self-controlled study. Photodiagn. Photodyn. Ther. 2020, 32, 101915. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhou, C.; Zhang, Y.; Yan, G.; Wang, X. Modified 5-aminolevulinic acid photodynamic therapy reduces pain and improves therapeutic effects in cutaneous squamous cell carcinoma mouse model. Lasers Surg. Med. 2022, 54, 804–812. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdubek, A.; Maliszewska, I. On the Possibility of Using 5-Aminolevulinic Acid in the Light-Induced Destruction of Microorganisms. Int. J. Mol. Sci. 2024, 25, 3590. https://doi.org/10.3390/ijms25073590
Zdubek A, Maliszewska I. On the Possibility of Using 5-Aminolevulinic Acid in the Light-Induced Destruction of Microorganisms. International Journal of Molecular Sciences. 2024; 25(7):3590. https://doi.org/10.3390/ijms25073590
Chicago/Turabian StyleZdubek, Anna, and Irena Maliszewska. 2024. "On the Possibility of Using 5-Aminolevulinic Acid in the Light-Induced Destruction of Microorganisms" International Journal of Molecular Sciences 25, no. 7: 3590. https://doi.org/10.3390/ijms25073590
APA StyleZdubek, A., & Maliszewska, I. (2024). On the Possibility of Using 5-Aminolevulinic Acid in the Light-Induced Destruction of Microorganisms. International Journal of Molecular Sciences, 25(7), 3590. https://doi.org/10.3390/ijms25073590






