Abstract
Limonoids are extremely diversified in plants, with many categories of products bearing an intact, rearranged or fragmented oxygenated scaffold. A specific subgroup of fragmented or degraded limonoids derives from the tetranortriterpenoid prieurianin, initially isolated from the tree Trichilia prieuriana but also found in other plants of the Meliaceae family, including the more abundant species Aphanamixis polystachya. Prieurianin-type limonoids include about seventy compounds, among which are dregeanin and rohitukin. Prieurianin and analogs exhibit insecticidal, antimicrobial, antiadipogenic and/or antiparasitic properties but their mechanism of action remains ill-defined at present. Previous studies have shown that prieurianin, initially known as endosidin 1, stabilizes the actin cytoskeleton in plant and mammalian cells via the modulation of the architecture and dynamic of the actin network, most likely via interference with actin-binding proteins. A new mechanistic hypothesis is advanced here based on the recent discovery of the targeting of the chaperone protein Hsp47 by the fragmented limonoid fraxinellone. Molecular modeling suggested that prieurianin and, to a lesser extent dregeanin, can form very stable complexes with Hsp47 at the protein–collagen interface. Hsp-binding may account for the insecticidal action of the product. The present review draws up a new mechanistic portrait of prieurianin and provides an overview of the pharmacological properties of this atypical limonoid and its chemical family.
Keywords:
dregeanin; fraxinellone; Hsp47; limonoids; prieurianin; Trichilia prieuriana; Trichilia species 1. Introduction
Limonoids are highly oxygenated modified triterpenoids well represented in plants. They are largely present in the Meliaceae family and designated meliacins. They are also frequently encountered in Rutaceae and less frequently in Cneoraceae [1,2,3]. The tetranortriterpenoid limonin was the first limonoid identified as the bitter constituent of citrus fruits in 1841 [4,5]. Citrus, oranges, lemons and grapefruits contain limonin (1) and other bioactive limonoids such as nomilin (2) and obacunone (3), endowed with antioxidative, anti-inflammatory, antimicrobial, antiviral, insecticidal, immunomodulatory and antiproliferative properties [6,7,8,9]. Limonin is extensively studied for its antioxidant and anti-inflammatory properties and is considered of interest for the treatment of liver diseases and as a cytoprotective agent to protect against organ damage [10,11,12]. However, this compound presents limited bioavailability and can lead to renal and hepatic toxicities [13]. It provides a useful starting material for elaborating rearranged bioactive molecules [14].
The furanolactone core structure is the signature of limonoids (Figure 1). They are biosynthesized from a 30-carbon precursor (protolimonoid) via a scaffold rearrangement process implicating many enzymes [15]. Complex modifications or remodeling of the initial scaffold can occur [16,17]. The modifications lead to so-called deformed or rearranged limonoids. The modifications include B-ring cleavage reactions, B/C-ring rearrangements and various types of cyclization, altogether leading to many different scaffolds and ring systems [14]. The structural diversity is large among limonoids [18,19].
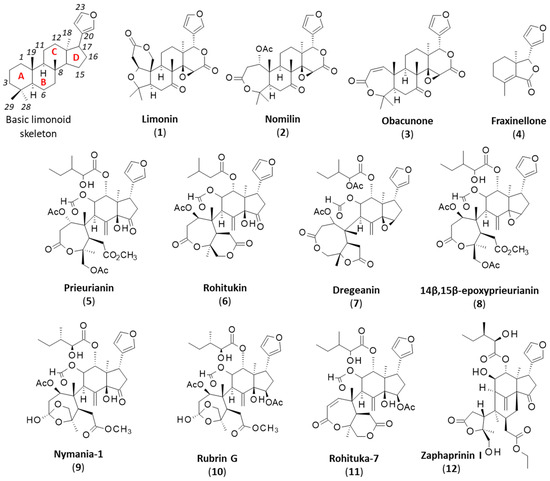
Figure 1.
Chemical structures of the basic limonoid skeleton (with the four cycles A-B-C-D and numbering system) and various intact or fragmented (degraded) limonoid cores.
There are also fragmented limonoids, often called degraded limonoids, corresponding to smaller products with ring openings associated with the loss of a skeletal fragment [20]. In general, these smaller molecules are easier to access by chemical synthesis than complex full limonoids, and as such, they provide starting structures for the total or semi-synthesis of natural products or original derivatives derived from naturally occurring limonoids. Degraded limonoids offer convenient scaffolds for the design of bioactive molecules, such as novel antibacterial agents [21]. This is typically the case for fraxinellone (4) isolated from the root bark of Dictamnus and Melia plants. This compound displays marked insecticidal and anticancer activities, linked to its anti-inflammatory and immuno-modulatory properties [22]. Fraxinellone (4) and congeners (e.g., fraxinellonone and isofraxinellone) have led to the synthesis of derivatives and novel insecticide candidates [23,24,25].
Several types of limonoids can be found in Meliaceae plants, with a highly complex structure (e.g., azadirachtin) or a simpler scaffold (such as the cedrelone and azadirone classes). Potent insecticidal agents can be found in each subgroup of compounds [26,27,28]. A less-known subgroup of limonoids is the prieurianin type, which includes three main members: prieurianin (5), rohitukin (6) and dregeanin (7) (Figure 1). The present review provides an analysis of prieurianin-type limonoids to highlight the structural diversity within the family and the origins of these compounds and, especially, to discuss their mechanisms of action. A novel direction is proposed for prieurianin based on the mechanism of the degraded limonoid fraxinellone.
2. Prieurianin-Type Limonoids: Structure and Origins
Prieurianin (5) is a tetranortriterpenoid that was first isolated from the timber of the tree Trichilia prieuriana A. Juss. (synonym: Trichilia senegalensis C.DC.) collected in Nigeria, together with many other limonoids [29]. T. prieuriana (Meliaceae) is generally a tall tree (up to 30 m) that is well fluted (up to 100 cm in diameter) with a dense crown. It is found in different parts of tropical Africa. The wood is used for the construction of local houses, tool handles and kitchen utensils. All parts of the plant (leaves, bark, twigs and roots) can be used for diverse medicinal usage. For example, a decoction of leafy twigs is taken to treat bronchitis and edema, whereas the pulverized roots are used as a treatment against ascariasis and as purgatives. It is a multi-purpose medicinal tree [30]. Trichilia species are commonly used in traditional medicine in Africa, not only T. prieuriana but also T. dregeana and T. emetica [31]. Extracts prepared from these plants are considered active and safe. An ethanolic leaf extract of T. prieuriana has not revealed any major toxicity, even when administered to rats at a high dose (LD50 > 5000 mg/kg) [32].
Prieurianin was discovered in 1965, but the complex highly oxidized structure of the molecule was elucidated only ten years later based on a precise NMR analysis. It is an A,B-seco-type degraded limonoid derived from the cleavage of the C-3/C-4 and C-7/C-8 bonds of the canonical limonoid framework, with a rearranged new oxo-ring formed by recyclization [33]. Other compounds of interest have been isolated from T. prieuriana, notably from the roots of a plant collected in Cameroon (Africa), such as the classical (ring-intact) limonoids flindissone and picraquassin E [34,35]. The protolimonoid glucoside prieurianoside and the limonoid prieurone have also been isolated from the leaves of the same plant [36,37], but these compounds are structurally distinct from prieurianin (5) (Figure 2).
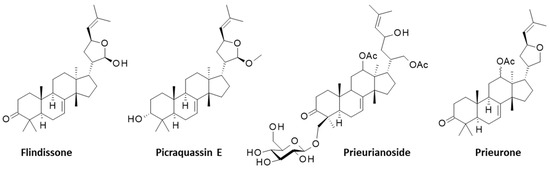
Figure 2.
Four other limonoids isolated from Trichilia prieuriana A. Juss. together with prieurianin.
Prieurianin has been found in a few other species, notably the root bark of the medicinal tree Guarea guidona (L.) Sleumer (Meliaceae, found in French Guiana, South America), together with its analog 14β,15β-epoxyprieurianin (8) [38]. Prieurianin has been found in four other Meliaceae: (i) Turraea obtusifolia Hochst. [39], (ii) Nymania capensis (Thunb.) Lindb. [40], (iii) Aphanamixis polystachya (Wall.) R. Parker [41] and (iv) Entandrophragma candolei Harms. [42,43] (Figure 3). The bark of this latter African plant was shown to contain the same epoxy–prieurianin derivative (8) as in Guarea guidona [42]. The bark and timber of N. capensis afforded prieurianin (5) and the related anti-plasmodial product nymania-1 (9) lack the C29-acetate of prieurianin and bear an ortho ester (Figure 1) [40,44]. Prieurianin can be isolated from the bark of A. polystachya but also from the seeds of the plant, together with rohitukin (6) and other rohituka limonoids [45].

Figure 3.
Plants containing prieurianin. An illustration of the leaves, twigs and fruits of T. prieuriana is presented (from M. Simo-Droissart, https://identify.plantnet.org/fr/k-world-flora/species/Trichilia%20prieuriana%20A.Juss./data (accessed on 18 February 2024)).
An appropriate plant to obtain quantities of prieurianin is the medicinal plant Aphanamixis polystachya (Wall.) R. Parker (also known as Amoora rohituka (Roxb.) Wight and Arn.) for at least three reasons: First, a specific procedure (depicted in Figure 4) has been described to obtain and purify the compound. The multistep process is long and tedious, with successive solvent extractions and chromatographic steps, but the global yield is satisfactory. The authors previously reported the isolation of 443 mg of (5) starting with 6.6 kg of air-dried roots, which corresponds to a correct yield of 67 mg/kg. The process was used to obtain prieurianin (5) in addition to triterpenoids such as aphataiwanin A–D and limonoids such as rohituka-3 and -7; nymania-1; and rubrin G [41]. Second, the product can be found in other parts of the plant, including the seeds and fruits, thus providing a renewable source of crude materials [46]. Third, A. polystachya is abundant, notably in Bangladesh, where the seed oil (known as pithraj seed oil) is exploited for the production of biodiesel [47,48,49]. Therefore, a phytochemical supply chain could be developed, but a comparison to other sources should also be considered.
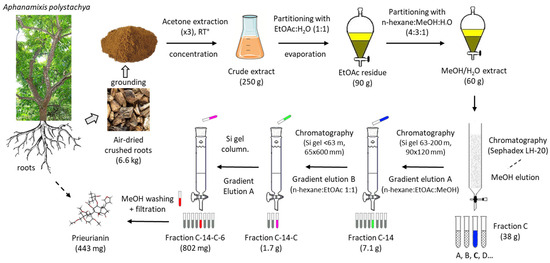
Figure 4.
Prieurianin isolation process from the plant Aphanamixis polystachya (Wall.) R. Parker, as originally reported in [41]. The successive steps are schematized to show the full extraction/chromatography process, which afforded 443 mg of purified prieurianin starting with 6.6 kg of the dried roots.
The plant Aphanamixis polystachya is a prominent source of tetranortriterpenes and limonoids. Several key compounds have been isolated from these plant roots, including prieurianin (5) and nymania 1 (9) but also the related limonoids rubrin G (10) and rohituka-7 (11) [41] (Figure 1). Rubrins A-G are degraded limonoids originally from the roots of the tree Trichilia rubra C.DC., native to tropical South America [50]. A. polystachya contains other prieurianin-type limonoids, such as the insecticidal compounds designated as aphapolynins and aphanamixoids, isolated, respectively, from the fruits and leaves of the plant [46,51]. Recently, other complex compounds, called aphanaonoids, with an oxygen-bridged scaffold, were identified from A. polystachya, but no bioactivity was reported [52]. Several other prieurianin-type limonoids have been isolated from the roots, aerial parts, fruits and seeds of various Trichilia and Munronia species (Table 1). Limonoids are abundant in Trichilia species, but prieurianin-type limonoids are not so frequent [53,54]. An interesting series is that of the compounds called zaphaprinins A–Y from Aphanamixis grandifolia, with potent insecticidal agents such as zaphaprinins I (12), for example [27,55].

Table 1.
Other prieurianin-type limonoids found in Meliaceae and their properties.
Rohitukin (6) and rohituka compounds correspond to a small group of prieurianin-type limonoids found in some Meliaceae, notably in neem (Azadirachta indica A. Juss.), a versatile medicinal plant that also contains the classical limonoid nimbolide [74,75,76]. Both prieurianin (5) and rohitukin (6) have been found in Turraea obtusifolia [39]. Rohitukin (6) has also been isolated from Aphanamixis polystacha, together with dregeanin (7) [77,78,79]. The limonoid rohitukin is less active than prieurianin as an insecticidal agent [39]. There is a complete series of related limonoids, designated rohituka-#, such as rohituka-3, -5, -7, -14 and -15, isolated from the seeds of A. polystacha [80,81]. They are insecticidal A,B-seco limonoids that are generally much less active than prieurianin [82]. The same observation can be made for compounds related to dregeanin and designated dregeana-#, such as dregeana-1 to dregeana-5 [31]. The limonoid dregeanin has also been isolated from the roots of Turreanthus africanus and found to be poorly active as an antibacterial agent [83]. There is also a related compound designated dregeanin DM4 (13) from the West African species Trichilia welwitschia, characterized as a modest inhibitor of acetylcholinesterase (AChE), a little less active than rohituka-3 (14) [84,85] (Figure 5).
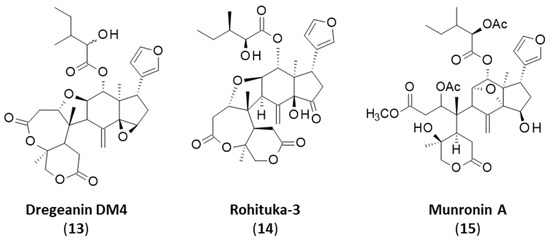
Figure 5.
Prieurianin-type limonoids 13–15.
Among the 70 or so prieurianin-type limonoids mentioned in Table 1, only a few have shown interesting biological properties, such as the antiproliferative agent munronin A (15), which is active against SW480 colon cancer cells [65]. The objective of the present study is not to detail these 70 compounds; there are recent comprehensive reviews for that [54,86,87]. Our analysis mainly focuses on the pharmacological properties of the lead compound prieurianin (5), with a new proposal for a drug target. Most studies on these prieurianin-type limonoids are concerned with the structural characterization of new complex molecular entities, with only preliminary biological tests (generally one or two specific cellular or biological assays). The pharmacological potential of these natural products remains little known. However, a recent discovery made with fraxinellone (4) led us to propose a new mechanistic option for prieurianin (5).
3. Bioactivities of Prieurianin and Analogs
Prieurianin exerts marked insecticidal action. The compound has been shown to antagonize molting steroid hormone 20-hydroxyecdysone activity in Drosophila cells. Prieurianin is significantly more potent than rohitukin as an antagonist of 20-hydroxyecdysone action in Drosophila melanogaster BII cells (ED50 = 10 µM and 125 µM) [39]. Moreover, antifeedant activity has been reported when using the pod borer Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). Prieurianin and its epoxy derivative reduce the feeding of larvae without inducing cytotoxic effects [42,51].
In addition, prieurianin has revealed both antiadipogenic and anorexigenic effects in mice. The compound inhibits the proliferation and differentiation of preadipocytes into adipocytes and modifies mature adipocytes, inducing their dedifferentiation or delipidation. These effects lead to significant weight loss by reducing energy intake in obese mice [88]. In one study, when mice under a high-fat diet received prieurianin at 1–3 mg/kg for 3 weeks (intraperitoneally), a dose-dependent loss of weight was observed. The mice recovered a normal weight at the end of the treatment period, and the weight loss was accompanied by a major (70–80%) decrease in food consumption. Prieurianin exhibited marked anti-obesity properties [88]. Surprisingly, this interesting activity has not been investigated further, perhaps due to the difficulty in accessing the compound. However, similar effects have been reported with a few other limonoids, notably with nimbolide, which suppressed high-fat-diet-induced obesity in rats in [89]. Nomilin also displays anti-obesity effects [90,91]. The antiadipogenic and anorexigenic properties of prieurianin merit further studies. There is a constant need for efficient and safe products to treat patients with overweight or obesity.
Antiproliferative activity has been reported in prieurianin. The compound has been shown to inhibit the proliferation of KB3-1 human cervix carcinoma cells (IC50 = 1.47 μM). This effect has been attributed to the potential binding of the compound to molecular targets such as α,β-tubulin dimer, DNA-topoisomerase I and human neutrophil collagenase (MMP-8), but these predicted interactions (based on molecular modeling) have not yet been validated experimentally [43]. Moreover, the marked antiproliferative activity reported with KB3-1 cells is a little surprising because another study concluded that prieurianin exerts no cytotoxic action against Hep-G2 (human hepatocellular carcinoma), A549 (human lung carcinoma) or MCF-7 (human breast carcinoma) [IC50 > 40 µg/mL) and modest activity against HEp-2 cells (laryngeal cancer cells) (IC50 = 16.8 µg/mL (22 μM)) [41]. It is likely that the mechanism is multi-factorial and dependent on the cell species (cancer cells or insects) and the histological type. More work is needed to clarify this cytotoxicity aspect, including integrative approaches and biology-based screening methods to further delineate the potency and mechanism of action of prieurianin, as achieved with other limonoids [92,93].
4. Potential Molecular Targets of Prieurianin and Analogs
Image-based screening for chemicals capable of modulating the trafficking of proteins to the plasma membrane via endosomes in plants has led to the identification of a compound called endosidin 1. This compound was found to block the endocytosis of auxin transporter proteins in the roots of the plant Arabidopsis thaliana (L.) Heynh. Endosidin 1 affects endosome trafficking at the stage of early endosome formation. The compound changes the distribution of markers residing in the trans-Golgi network/early endosomes [94]. A subsequent study revealed that endosidin 1 is in fact prieurianin and functions as a modulator of actin cytoskeleton dynamics. Moreover, prieurianin was identified as an effector of the circadian clock in A. thaliana, causing a shortening of circadian period lengths. The authors stated that prieurianin affects the actin cytoskeleton through a mode of action distinct from that of previously described inhibitors of actin dynamics. It does not function as a stabilizer or destabilizer of actin filaments but most likely targets an actin-associated protein implicated in cytoskeleton formation and vesicle trafficking [95]. The compound impairs actin dynamics via the indirect stabilization of the actin cytoskeleton (Figure 6). The effects of the natural products have been evidenced using both plant cells (hypocotyl cells from Arabidopsis thaliana) and mammalian cells (BSC-1 monkey epithelial fibroblasts). Prieurianin severely alters the architecture of the actin network, reducing filament flexibility and shrinkage and decreasing the number of breaks per filament, but the filament growth rate is not affected. The compound has shown an atypical effect on the dynamics of the actin cytoskeleton, reducing actin rearrangements [96]. As such, the mechanism of action of prieurianin seems to be distinct from that of azadirachtin A, the major limonoid found in neem (Azadirachta indica), which induces depolymerization of actin [96]. Computational analyses have helped to define the actin-binding site of azadirachtin A, located in subdomain 4 of a subunit (n + 2) of actin [97,98]. Prieurianin functions differently, altering membrane trafficking from the trans-Golgi network via actin-binding proteins implicated in transport processes from the trans-Golgi network, such as Rab GTPases and, in particular, a member of the Rab-A1 subclass called Rab-A1c [99,100]. These Rab proteins are key regulators of membrane transport in eukaryotes [101].
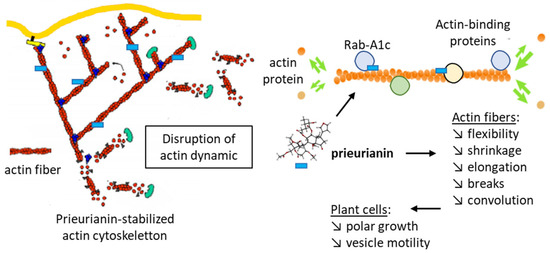
Figure 6.
Effects of prieurianin on actin dynamics. Prieurianin stabilizes the actin cytoskeleton, reducing actin fiber flexibility and shrinkage and causing changes in vesicle trafficking. The drug action implicates actin-binding proteins and the modulation of endosome trafficking [95]. The action of prieurianin (blue rectangle) is schematized to illustrate binding to actin-binding proteins (green and yellow circles), including Rab-A1c GTPase (blue circles) and the resulting effects in actin fibers and plant cells.
A recent study performed with the degraded limonoid fraxinellone (4) brought other key information to help understand the mechanism of action of these limonoids. Fraxinellone is an insecticidal and anticancer limonoid isolated from the root bark of Dictamnus dasycarpus [22]. The compound has been shown to interact with heat shock protein 47 (Hsp47, also known as Serpin H1), which is implicated in the development of intestinal fibrosis. A direct binding of (4) to the purified protein has been evidenced by surface plasmon resonance (Kd = 3.542 × 10−5 M), and a modeling analysis helped to define the location of the binding site with the implication of some key amino acids in the binding process, notably residues Tyr383 and Asp385. This drug interaction inhibits and destroys the complex between the chaperone Hsp47 and collagen, thereby perturbating procollagen folding and collagen processing [102]. The interaction with Hsp47 directly implicates the furan unit of fraxinellone. Fraxinellone can bind reasonably well to Hsp47, forming stable protein complexes at the interface of the Hsp47-collagen region, as represented in Figure 7a–c. There is a protein cavity at the junction between the three collagen fibers and the protein, accessible for drug binding.
The regulation of Hsp47-collagen’s interaction with fraxinellone accounts for the antifibrotic action of the limonoid [102], and it may also contribute to its antimetastatic activity because Hsp47 is a known stimulator of metastasis in solid tumors, notably in breast cancer [103]. These observations prompted us to consider that, by analogy, prieurianin could bind to Hsp47 via its fraxinellone-like moiety. The tetrahydrobenzofuranone unit with the appended furanyl group in fraxinellone is similar to the core of prieurianin, with different substitutions. Prieurianin is significantly larger than fraxinellone, with an appended 7-oxo-oxepanyl ring, but its binding to Hsp47 is apparently conceivable, as inferred from a preliminary molecular modeling analysis (Figure 7d–f). The docking analysis suggested that prieurianin can form much stabler complexes with Hsp47 compared with fraxinellone. Both the calculated empirical energy of interaction (ΔE) and free energy of hydration (ΔG) are much more favorable with prieurianin than with fraxinellone. The ΔE value calculated with prieurianin (5) is 2.5-fold more negative than that measured with fraxinellone (4) (−41.7 kcal/mol vs. −106.5 kcal/mol for 4 and 5, respectively) (Table 2). The difference is considerable and suggests that prieurianin exhibits a high affinity for this binding site on Hsp47. Multiple drug–protein contacts stabilize the complex, including H-bonds with the key residue Tyr383 and with residue Lys380 in contact with the oxepanyl ring of prieurianin, together with multiple weaker hydrophobic interactions (Figure 7f). Interestingly, the same trend was observed with the related products dregeanin and rohitukin. The ΔE values for the three compounds rank in the order prieurianin < dregeanin < rohitukin (Table 2). The modeling analysis strongly supports the potential binding of prieurianin to the same site of Hsp47 as the fragmented limonoid fraxinellone. For the time being, this is only a computer-based prediction, with the inherent inaccuracies of molecular docking [104]. The limited reliability of scoring functions is known [105], but the hypothesis is entirely plausible considering the capacity of other limonoids to function as heat shock protein inhibitors, such as gedunin and chisomicine D [106,107,108].
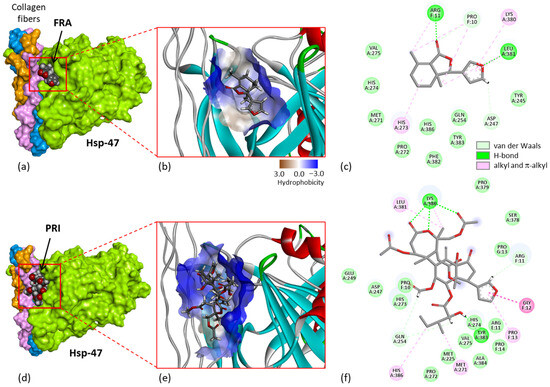

Figure 7.
Molecular models of fraxinellone (a–c) and prieurianin (d–f) bound to Hsp47 (protein data bank (PDB) code 3ZHA). (a) Surface model with a close-up view of the binding cavity that accommodates the compound (4). (b) A view of the fraxinellone-binding site, with the hydrophobicity surface surrounding the drug-binding zone (color code indicated). (c) Binding map contacts for (4) bound to Hsp47 (color code indicated). Same models in panels (d–f) for compound (5). The modeling analysis was performed as previously described in [109,110].

Table 2.
Calculated potential energy of interaction (ΔE) and free energy of hydration (ΔG) for the interaction of selected limonoids with heat shock protein 47 (Hsp47) 1.
The interaction of prieurianin with Hsp47 would be entirely compatible with its insecticidal effects. Different chaperone proteins have been shown to play a role in the signaling of the ecdysone receptor, which is a major steroid receptor in insects [112,113,114]. Heat shock proteins play important roles in the nucleocytoplasmic shuttle of the ecdysone receptor. Notably, 20-hydroxyecdysone regulates the expression of the chaperone Hsp70 and other small Hsp proteins [115]. The expression of heat shock proteins and the development of the smooth endoplasmic reticulum have been observed in some insects, such as the armyworm Spodoptera eridania and the predator Ceraeochrysa claveri, in response to cellular damage [116]. Limonoids could block the expression and/or function of Hsp. For example, Hsp23 is considered to be a potential target of the neem limonoid azadirachtin in Drosophila melanogaster larvae [117]. The two andirobin-type limonoids moluccensin-N and moluccensin-O have been shown to interact with Hsp90 [118]. It is known that Hsp proteins play a role in insect metamorphosis and that stress induces the expression of heat shock protein genes [119,120].
5. Conclusions and Perspectives
This work shed light on a group of degraded limonoids not frequently studied. Prieurianin is the leader molecule in a series that includes about 70 compounds (Table 1). Most of these compounds have been structurally described, together with corresponding isolation processes from different plant species. However, their pharmacological activities and mechanisms have rarely been investigated. Priority is generally given to the most abundant polycyclic limonoids in plants, in particular, the classical intact limonoids, such as limonin and nomilin, and to smaller fragmented limonoids with easier synthetic access. The field of limonoids is very active, with more than 1600 compounds characterized over the past 15 years [2] and a profusion of synthetic derivatives (>800) elaborated during roughly the same period [121]. But in this vast molecular armamentarium, the use of degraded limonoids of the prieurianin type has been little considered, either because the molecules are too complex structurally or not well known and hardly available or because of the limited information concerning their bioactivities and mechanism of action. It is time to highlight the potential benefits of prieurianin, which is an insecticidal agent with an atypical capacity to modulate the dynamics of the actin cytoskeleton in cells. In recent years, novel bioactive prieurianin-type limonoids have been identified, such as trichilianones A-E [72] and munronins T-U [68]. The latter example is appealing because the mode of action invoked for these two compounds is an inhibition of the expression of the two Hsp genes NtHsp70–1 and Nthsp70–261. There is probably a strong link between the impact of the compounds on the Hsp machinery and their antiviral effects. Further investigation into the mechanism of action of prieurianin and related compounds is warranted. Prieurianin is a convenient tool for studying interference with endocytosis and vesicular recycling in plant cells [122,123]. This natural product warrants better consideration as an insecticidal agent and as an antiadipogenic molecule. The hypothesis that prieurianin binds well to the collagen-binding protein Hsp47 is attractive and opens new perspectives for the design of other compounds with antifibrotic properties because this protein is a major recognized target to combat pulmonary fibrosis and other fibrotic diseases [124,125,126]. Hsp47 inhibitors mediate antifibrotic effects by suppressing the overexpression of collagen and inhibiting the viability and migration of fibroblasts [127]. Both fraxinellone and prieurianin may provide a molecular basis for the development of novel antifibrotic therapeutics.
Author Contributions
Credit roles: G.V.: visualization, software, computations, molecular modeling; C.B.: conceptualization, investigation, visualization, writing—original draft, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Roy, A.; Saraf, S. Limonoids: Overview of significant bioactive triterpenes distributed in plants kingdom. Biol. Pharm. Bull. 2006, 29, 191–201. [Google Scholar] [CrossRef]
- Luo, J.; Sun, Y.; Li, Q.; Kong, L. Research progress of meliaceous limonoids from 2011 to 2021. Nat. Prod. Rep. 2022, 39, 1325–1365. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Qin, D. Classification of Diverse Novel Limonoids. In Novel Plant Natural Product Skeletons; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Gualdani, R.; Cavalluzzi, M.M.; Lentini, G.; Habtemariam, S. The Chemistry and Pharmacology of Citrus Limonoids. Molecules 2016, 21, 1530. [Google Scholar] [CrossRef]
- Arora, S.; Mohanpuria, P.; Sidhu, G.S. Citrus limonoids: Mechanism, function and its metabolic engineering for human health. Fruits 2018, 73, 158–173. [Google Scholar] [CrossRef]
- Shi, Y.S.; Zhang, Y.; Li, H.T.; Wu, C.H.; El-Seedi, H.R.; Ye, W.K.; Wang, Z.W.; Li, C.B.; Zhang, X.F.; Kai, G.Y. Limonoids from Citrus: Chemistry, anti-tumor potential, and other Bioactivities. J. Function. Food 2020, 75, 104213. [Google Scholar] [CrossRef]
- Hilmayanti, E.; Nurlelasari Supratman, U.; Kabayama, K.; Shimoyama, A.; Fukase, K. Limonoids with anti-inflammatory activity: A review. Phytochemistry 2022, 204, 113469. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, S.; Chen, X. The pharmacological and pharmacokinetic properties of obacunone from citrus fruits: A comprehensive narrative review. Fitoterapia 2023, 169, 105569. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, Y.; Li, H.; Feng, Y.; Huang, C.; Fan, S. Nomilin and Its Analogues in Citrus Fruits: A Review of Its Health Promotion Effects and Potential Application in Medicine. Molecules 2022, 29, 269. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, L.; Hu, M.; Yang, Y.; Ma, Q.; Chen, J. Network Pharmacology to Reveal the Molecular Mechanisms of Rutaceous Plant-derived Limonin Ameliorating Non-alcoholic Steatohepatitis. Crit. Rev. Immunol. 2023, 43, 11–23. [Google Scholar] [CrossRef]
- Liang, H.; Liu, G.; Fan, Q.; Nie, Z.; Xie, S.; Zhang, R. Limonin, a novel AMPK activator, protects against LPS-induced acute lung injury. Int. Immunopharmacol. 2023, 122, 110678. [Google Scholar] [CrossRef]
- Zhou, X.; Xiang, Y.; Li, D.; Zhong, M.; Hong, X.; Gui, Y.; Min, W.; Chen, Y.; Zeng, X.; Zhu, H.; et al. Limonin, a natural ERK2 agonist, protects against ischemic acute kidney injury. Int. J. Biol. Sci. 2023, 19, 2860–2878. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, C.; Luo, T.; Wang, J.; Tang, Y.; Chen, Z.; Yu, L. Limonin: A Review of Its Pharmacology, Toxicity, and Pharmacokinetics. Molecules 2019, 24, 3679. [Google Scholar] [CrossRef]
- Furiassi, L.; Tonogai, E.J.; Hergenrother, P.J. Limonin as a Starting Point for the Construction of Compounds with High Scaffold Diversity. Angew. Chem. Int. Ed. Engl. 2021, 60, 16119–16128. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, H.; De La Peña, R.; Stephenson, M.J.; Thimmappa, R.; Vincent, J.L.; Sattely, E.S.; Osbourn, A. Identification of key enzymes responsible for protolimonoid biosynthesis in plants: Opening the door to azadirachtin production. Proc. Natl. Acad. Sci. USA 2019, 116, 17096–17104. [Google Scholar] [CrossRef] [PubMed]
- Aarthy, T.; Mulani, F.A.; Pandreka, A.; Kumar, A.; Nandikol, S.S.; Haldar, S.; Thulasiram, H.V. Tracing the biosynthetic origin of limonoids and their functional groups through stable isotope labeling and inhibition in neem tree (Azadirachta indica) cell suspension. BMC Plant Biol. 2018, 18, 230. [Google Scholar] [CrossRef]
- De La Peña, R.; Hodgson, H.; Liu, J.C.; Stephenson, M.J.; Martin, A.C.; Owen, C.; Harkess, A.; Leebens-Mack, J.; Jimenez, L.E.; Osbourn, A.; et al. Complex scaffold remodeling in plant triterpene biosynthesis. Science 2023, 379, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.G.; Luo, X.D. Meliaceous Limonoids: Chemistry and Biological Activities. Chem. Rev. 2011, 111, 7437–7522. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H. Recent progress in the chemistry and biology of limonoids. RSC Adv. 2017, 7, 35191. [Google Scholar] [CrossRef]
- Durán-Peña, M.J.; Botubol-Ares, J.M.; Collado, I.G.; Hernandez-Galan, R. Degraded limonoids: Biologically active limonoid fragments re-enhancing interest in Meliaceae and Rutaceae sources. Phytochem. Rev. 2023, 22, 695–741. [Google Scholar] [CrossRef]
- Ferrera-Suanzes, M.; Prieto, V.; Medina-Olivera, A.J.; Botubol-Ares, J.M.; Galán-Sánchez, F.; Rodríguez-Iglesias, M.A.; Hernández-Galán, R.; Durán-Peña, M.J. Synthesis of Degraded Limonoid Analogs as New Antibacterial Scaffolds against Staphylococcus aureus. Antibiotics 2020, 9, 488. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Fraxinellone: From pesticidal control to cancer treatment. Pestic. Biochem. Physiol. 2020, 168, 104624. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, R.; Xu, H. New Insecticidal Agents from Halogenation/Acylation of the Furyl-Ring of Fraxinellone. Sci. Rep. 2016, 6, 35321. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Fan, J.; Zhang, Q.; Bao, C.; Liu, Z.; Yang, R. Turning natural products into insecticide candidates: Design and semisynthesis of novel fraxinellone-based N-(1,3-thiazol-2-yl)carboxamides against two crop-threatening insect pests. Bioorg. Med. Chem. Lett. 2019, 29, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Dong, Q.M.; Wang, M.R.; Tang, J.J. Semi-Synthesis of C-Ring Cyclopropyl Analogues of Fraxinellone and Their Insecticidal Activity Against Mythimna separata Walker. Molecules 2020, 25, 1109. [Google Scholar] [CrossRef] [PubMed]
- Happi, G.M.; Nangmo, P.K.; Dzouemo, L.C.; Kache, S.F.; Kouam, A.D.K.; Wansi, J.D. Contribution of Meliaceous plants in furnishing lead compounds for antiplasmodial and insecticidal drug development. J. Ethnopharmacol. 2022, 285, 114906. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Yang, S.; Huang, J.; Zhou, L. Insecticidal Triterpenes in Meliaceae: Plant Species, Molecules and Activities: Part I (Aphanamixis-Chukrasia). Int. J. Mol. Sci. 2021, 22, 13262. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Bi, X.; Zhou, L.; Huang, J. Insecticidal Triterpenes in Meliaceae: Plant Species, Molecules, and Activities: Part II (Cipadessa, Melia). Int. J. Mol. Sci. 2022, 23, 5329. [Google Scholar] [CrossRef]
- Bevan, C.W.L.; Ekong, D.E.U.; Taylor, D.A.H. Extractives from West African Members of the Family Meliaceae. Nature 1965, 206, 1323–1325. [Google Scholar] [CrossRef]
- Trichilia prieuriana. Available online: https://tropical.theferns.info/viewtropical.php?id=Trichilia+prieuriana (accessed on 18 February 2024).
- Oyedeji-Amusa, M.O.; Sadgrove, N.J.; Van Wyk, B.E. The Ethnobotany and Chemistry of South African Meliaceae: A Review. Plants 2021, 10, 1796. [Google Scholar] [CrossRef]
- Kangbéto Bidossessi, R.; Attakpa Sèlidji, E.; Guinnin, F.; Sénou, M.; Lagnika, L. Toxicological assessment of ethanolic extracts of Annona senegalensis and Trichilia prieureana in the treatment of type 2 diabetes in Benin. J. Physiol. Pathophysiol. 2022, 13, 27–35. [Google Scholar] [CrossRef]
- Gullo, V.P.; Miura, I.; Akanishi, K. Structure of Prieurianin, a Complex Tetranortriterpenoid; Nuclear Magnetic Resonance Analysis at Nonambient Temperatures and X-Ray Structure Determination. J. Chem. Soc. Chem. Commun. 1975, 9, 345–346. [Google Scholar] [CrossRef]
- Pagna, J.I.M.; Mbekou, I.M.K.; Tsamo, A.T.; Mkounga, P.; Frese, M.; Stammler, H.G.; Fekam, F.B.; Lenta, B.N.; Sewald, N.; Nkengfack, A.E. Antibacterial activity of some chemical constituents from Trichilia prieuriana (Meliaceae). Z. Naturforschung B 2021, 76, 439–446. [Google Scholar] [CrossRef]
- Resetar, M.; Tietcheu Galani, B.R.; Tsamo, A.T.; Chen, Y.; Schachner, D.; Stolzlechner, S.; Mawouma Pagna, J.I.; Beniddir, M.A.; Kirchmair, J.; Dirsch, V.M. Flindissone, a Limonoid Isolated from Trichilia prieuriana, Is an LXR Agonist. J. Nat. Prod. 2023, 86, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Olugbade, T.A. Tetracyclic triterpenoids from Trichilia prieuriana leaves. Phytochemistry 1991, 30, 698–700. [Google Scholar] [CrossRef]
- Olugbade, T.A.; Adesanya, S.A. Prieurianoside, a protolimonoid glucoside from the leaves of Trichilia prieuriana. Phytochemistry 2000, 54, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Lukacova, V.; Polonsky, J.; Moretti, C. Isolation and structure of 14,15β-epoxyprieurianin from the South American tree Guarea guidona. J. Nat. Prod. 1982, 45, 288–294. [Google Scholar] [CrossRef]
- Sarker, S.D.; Savchenko, T.; Whiting, P.; Sik, V.; Dinan, L. Two limonoids from Turraea obtusifolia (Meliaceae), prieurianin and rohitukin, antagonise 20-hydroxyecdysone action in a Drosophila cell line. Arch. Insect. Biochem. Physiol. 1997, 35, 211–217. [Google Scholar] [CrossRef]
- MacLachlan, L.K.; Taylor, D.A.H. Limonoids from Nymania capensis. Phytochemistry 1982, 21, 1701–1703. [Google Scholar] [CrossRef]
- Lin, C.J.; Lo, I.W.; Lin, Y.C.; Chen, S.Y.; Chien, C.T.; Kuo, Y.H.; Hwang, T.L.; Liou, S.S.; Shen, Y.C. Tetranortriterpenes and Limonoids from the Roots of Aphanamixis polystachya. Molecules 2016, 21, 1167. [Google Scholar] [CrossRef]
- Koul, O.; Daniewski, W.M.; Multani, J.S.; Gumulka, M.; Singh, G. Antifeedant effects of the limonoids from Entandrophragma candolei (Meliaceae) on the gram pod borer, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Agric. Food Chem. 2003, 51, 7271–7275. [Google Scholar] [CrossRef]
- Happi, G.M.; Mouthe Kemayou, G.P.; Stammler, H.G.; Neumann, B.; Ismail, M.; Kouam, S.F.; Wansi, J.D.; Tchouankeu, J.C.; Frese, M.; Lenta, B.N.; et al. Three phragmalin-type limonoids orthoesters and the structure of odoratone isolated from the bark of Entandrophragma candollei (Meliaceae). Phytochemistry 2021, 181, 112537. [Google Scholar]
- Mutombo Mianda, S.; Moyo, P.; Maboane, S.; Birkholtz, L.M.; Maharaj, V.J. Phytoconstituents from Turraea obtusifolia and their antiplasmodial activity. Nat. Prod. Res. 2023, 1–13. [Google Scholar] [CrossRef]
- Brown, D.A.; Taylor, D.A.H. Limonoid extractives from Aphanamixis polystachia. Phytochemistry 1978, 17, 1995–1999. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.S.; Wang, X.B.; Gu, Y.C.; Wei, D.D.; Guo, C.; Yang, M.H.; Kong, L.Y. Limonoids from the fruits of Aphanamixis polystachya (Meliaceae) and their biological activities. J. Agric. Food Chem. 2013, 61, 2171–2182. [Google Scholar] [CrossRef]
- Fattah, I.M.T.R. Biodiesel production, characterization, diesel engine performance, and emission characteristics of methyl esters from Aphanamixis polystachya oil of Bangladesh. Energy Convers. Manag. 2015, 91, 149–157. [Google Scholar]
- Ifteqar, S.; Sultana, R.; Banik, S.; Rahman, A.F.M.M. Production and Characterization of Biodiesel from Aphanamixis polystachya Seed Oil. Dhaka Univ. J. Sci. 2020, 68, 129–136. [Google Scholar] [CrossRef]
- Ahmmed, R.; Hasan, I.; Mortuza, G.; Ismail, M. Preparation and physico-chemcial properties evaluation of biodiesel from Pithraj (Aphanamixis polystachya) seeds available in Bangladesh. J. Chem. Engineer. Res. Bull. 2020, 22, 43–48. [Google Scholar]
- Musza, L.L.; Killar, L.M.; Speight, P.; McElhiney, S.; Barrow, C.J.; Gillum, A.M.; Cooper, R. Potent New Cell Adhesion Inhibitory Compounds from the Root of Trichilia rubra. Tetrahedron 1994, 50, 11369–11378. [Google Scholar] [CrossRef]
- Cai, J.Y.; Chen, D.Z.; Luo, S.H.; Kong, N.C.; Zhang, Y.; Di, Y.T.; Zhang, Q.; Hua, J.; Jing, S.X.; Li, S.L.; et al. Limonoids from Aphanamixis polystachya and their antifeedant activity. J. Nat. Prod. 2014, 77, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xue, S.; Huang, W.; Wang, C.C.; Cui, Z.; Luo, J.; Kong, L. Diverse prieurianin-type limonoids with oxygen-bridged caged skeletons from two Aphanamixis species: Discovery and biomimetic conversion. Org. Chem. Front. 2021, 8, 566–571. [Google Scholar] [CrossRef]
- Xie, Y.S.; Isman, M.B.; Gunning, P.; Mackinnon, S.; Arnason, J.T.; Taylor, D.R.; Sanchez, P.; Hasbun, C.; Towers, G.H.N. Biological Activity of Extracts of Trichilia Species and the Limonoid Hirtin Against Lepidopteran Larvae. Biochem. System. Ecol. 1994, 22, 129–136. [Google Scholar] [CrossRef]
- Passos, M.; Nogueira, T.S.R.; Azevedo, O.; Vieira, M.G.C.; da silva Terra, W.; Braz-Filho, R.; Vieira, I.J.C. Limonoids from the genus Trichilia and biological activities: Review. Phytochem. Rev. 2021, 20, 1055–1086. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.S.; Gu, Y.C.; Wang, X.B.; Kong, L.Y. Diverse prieurianin-type limonoid derivatives from the fruits of Aphanamixis grandifolia and their absolute configuration Determination. Tetrahedron 2014, 70, 6594–6606. [Google Scholar] [CrossRef]
- Chatterjee, A.; Kundu, A.B.; Chakrabortty, T.; Chandrasekharan, S. Extractives of Aphanamixis polystachya Wall (Parker). The structures and stereochemistry of aphanamixin and aphanamixinin. Tetrahedron 1970, 26, 1859–1867. [Google Scholar] [CrossRef]
- Agnihotri, V.K.; Srivastava, S.D.; Srivastava, S.K. A New Limonoid, Amoorinin, from the Stem Bark of Amoora rohituka. Planta Med. 1987, 53, 298–299. [Google Scholar] [CrossRef]
- Cai, J.Y.; Zhang, Y.; Luo, S.H.; Chen, D.Z.; Tang, G.H.; Yuan, C.M.; Di, Y.T.; Li, S.H.; Hao, X.J.; He, H.P. Aphanamixoid A, a potent defensive limonoid, with a new carbon skeleton from Aphanamixis polystachya. Org. Lett. 2012, 14, 2524–7252. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.B.; Wei, D.D.; Luo, J.G.; Kuo, J.; Yang, M.H.; Kong, L.Y. Aphapolynins A and B, two new limonoids from the fruits of Aphanamixis polystachya. Tetrahedron Lett. 2011, 52, 2590–2593. [Google Scholar] [CrossRef]
- Yu, J.H.; Wang, G.C.; Han, Y.S.; Wu, Y.; Wainberg, M.A.; Yue, J.M. Limonoids with Anti-HIV Activity from Cipadessa cinerascens. J. Nat. Prod. 2015, 78, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Han, M.L.; Zhao, J.X.; Liu, H.C.; Ni, G.; Ding, J.; Yang, S.P.; Yue, J.M. Limonoids and Triterpenoids from Dysoxylum mollissimum var. glaberrimum. J. Nat. Prod. 2015, 78, 754–761. [Google Scholar] [CrossRef]
- Luo, X.D.; Wu, S.H.; Ma, Y.B.; Wu, D.G. Prieurianin-type tetranortriterpenoids from the bark of Dysoxylum hainanense. Heterocycles 2000, 53, 2225–2232. [Google Scholar] [CrossRef]
- Adul, G.O.; Bentley, M.D.; Benson, B.W.; Huang, F.Y.; Gelbaum, L.; Hassanali, A. Two new prieurianin-class limonoids from Turraea mombasana. J. Nat. Prod. 1993, 56, 1414–1417. [Google Scholar] [CrossRef]
- Ge, H.Y.; Liu, K.X.; Zhang, J.X.; Mu, S.Z.; Hao, X.J. The Limonoids and Their Antitobacco Mosaic Virus (TMV) Activities from Munronia unifoliolata Oliv. J Agric Food Chem. 2012, 60, 4289–4295. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.X.; Huang, T.; Mao, X.Y.; Gu, W.; He, H.P.; Di, Y.T.; Li, S.L.; Chen, D.Z.; Zhang, Y.; et al. Bioactive Limonoid Constituents of Munronia henryi. J. Nat. Prod. 2015, 78, 811–821. [Google Scholar] [CrossRef]
- Yan, Y.; Yuan, C.M.; Di, Y.T.; Huang, T.; Fan, Y.M.; Ma, Y.; Zhang, J.X.; Hao, X.J. Limonoids from Munronia henryi and their anti-tobacco mosaic virus activity. Fitoterapia 2015, 107, 29–35. [Google Scholar] [CrossRef]
- Yan, Y.; Tang, L.; Hu, J.; Wang, J.; Adelakun, T.A.; Yang, D.; Di, Y.; Zhang, Y.; Hao, X. Munronin O, a potential activator for plant resistance. Pestic. Biochem. Physiol. 2018, 146, 13–18. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, D.; Zhang, X.; Peng, M.; Yan, X.; Guo, Y.; Jia, M.; Zhou, J.; Tang, L.; Hao, X. Anti-TMV activity and effects of three prieurianin-type limonoids from Munronia henryi. Pestic. Biochem. Physiol. 2022, 184, 105108. [Google Scholar] [CrossRef]
- Yang, X.R.; Tanaka, N.; Tsuji, D.; Lu, F.L.; Yan, X.J.; Itoh, K.; Li, D.P.; Kashiwada, Y. Limonoids from the aerial parts of Munronia pinnata. Tetrahedron 2019, 75, 130779. [Google Scholar] [CrossRef]
- Rodriguez-Hahn, L.; Cardenas, J.; Arenas, C. Trichavensin, a prieurianin derivative from Trichilia havannensis. Phytochemistry 1996, 43, 457–459. [Google Scholar] [CrossRef]
- Limachi, I.; Gonzalez-Ramirez, M.; Manner, S.; Ticona, J.C.; Salamanca, E.; Gimenez, A.; Sterner, O. Trichilianones A-D, Novel Cyclopropane-Type Limonoids from Trichilia adolfi. Molecules 2021, 26, 1019. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ramirez, M.; Limachi, I.; Manner, S.; Ticona, J.C.; Salamanca, E.; Gimenez, A.; Sterner, O. Trichilones A-E: New Limonoids from Trichilia adolfi. Molecules 2021, 26, 3070. [Google Scholar] [CrossRef] [PubMed]
- Tsopgni, W.D.T.; Happi, G.M.; Stammler, H.-G.; Neumann, B.; Mbobda, A.S.W.; Kouam, S.F.; Frese, M.; Azébazé, A.G.B.; Lenta, B.N.; Sewald, N. Chemical constituents from the bark of the Cameroonian mahogany Trichilia emetica Vahl (Meliaceae). Phytochem. Lett. 2019, 33, 49–54. [Google Scholar] [CrossRef]
- Ogbuewu, I.P.; Odoemenam, V.U.; Obikaonu, H.O.; Opara, M.N.; Emenalom, O.O.; Uchegbu, M.C.; Okoli, I.C.; Esonu, B.O.; Iloeje, M.U. The growing importance of neen (Azadirachta indica A. Juss) in agriculture, industry, medicine and environment: A review. Res. J. Med. Plant 2011, 5, 230–245. [Google Scholar]
- Sarkar, S.; Singh, R.P.; Bhattacharya, G. Exploring the role of Azadirachta indica (neem) and its active compounds in the regulation of biological pathways: An update on molecular approach. 3 Biotech 2021, 11, 178. [Google Scholar] [CrossRef]
- Nagini, S.; Palrasu, M.; Bishayee, A. Limonoids from neem (Azadirachta indica A. Juss.) are potential anticancer drug candidates. Med. Res. Rev. 2024, 44, 457–496. [Google Scholar] [CrossRef]
- Connolly, J.D.; Okorie, D.A.; de Wit, L.D.; Taylor, D.A.H. Structure of dregeanin and rohitukin, limonoids from the subfamily Melioideae of the family Meliaceae. An unusually high absorption frequency for a six-membered lactone ring. J. Chem. Soc. Chem. Commun. 1976, 22, 909. [Google Scholar] [CrossRef]
- Connolly, J.D.; Labbé, C.; Rycroft, D.S.; Okorie, D.A.; Taylor, D.A.H. Tetranortriterpenoids and related compounds. Part 23. Complex tetranortriterpenoids from Trichilia prieuriana and Guarea thompsonii (Meliaceae), and the hydrolysis products of dregeanin, prieurianin, and related compounds. J. Chem. Res. 1979, 8, 256–257. [Google Scholar]
- Polonsky, J.; Varon, Z.; Marazano, C.; Arnoux, B.; Pettit, G.R.; Schmid, J.M.; Ochi, M.; Kotsuki, H. The structure of amoorastatone and the cytotoxic limonoid 12-hydroxyamoorastatin. Experientia 1979, 35, 987–989. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, D.A.; Naidoo, N. Limonoids from Aphanamixis polystacha. Phytochemistry 1999, 51, 927–930. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, F.; Wu, D.; Chen, Q. Complete assignments of 1H and 13C NMR data for rings A,B-seco limonoids from the seed of Aphanamixis polystachya. Magn. Reson. Chem. 2007, 45, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Lidert, Z.; Taylor, D.A.H.; Thirugnanam, M. Insect antifeedant activity of four prieurianin-type limonoids. J. Nat. Prod. 1985, 48, 843–845. [Google Scholar] [CrossRef]
- Nganso Ditchou, Y.O.; Kahouo Doutsing, A.; Simo Nemg, F.B.; Nyasse, B. Chemical Constituents of Root from Turraeanthus africanus (Meliaceae) and in Vitro Antimicrobial Activity. Int. J. Innov. Stud. Sci. Engineer. Technol. 2019, 5, 36–44. [Google Scholar]
- Tsamo, A.; Langat, M.K.; Nkounga, P.; Kamden Waffo, A.F.; Nkengfack, A.E.; Mulholland, D.A. Limonoids from the West African Trichilia welwitschia (Meliaceae). Biochem. System. Ecol. 2013, 50, 368–370. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; Tsamo, A.T.; Melong, R.; Mkounga, P.; Nkengfack, A.E.; McGaw, L.J.; Eloff, J.N. Cytotoxicity, nitric oxide and acetylcholinesterase inhibitory activity of three limonoids isolated from Trichilia welwitschii (Meliaceae). Biol. Res. 2015, 48, 57. [Google Scholar] [CrossRef]
- Fan, W.; Fan, L.; Wang, Z.; Yang, L. Limonoids from the Genus Melia (Meliaceae): Phytochemistry, Synthesis, Bioactivities, Pharmacokinetics, and Toxicology. Front. Pharmacol. 2022, 12, 795565. [Google Scholar] [CrossRef]
- Mulani, F.A.; Nandikol, S.S.; Thulasiram, H.V. Chemistry and Biology of Novel Meliaceae Limonoids. ChemRXiv 2022. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiAgOa7xcaDAxVnVqQEHZFIBtsQFnoECBoQAQ&url=https%3A%2F%2Fchemrxiv.org%2Fengage%2Fapi-gateway%2Fchemrxiv%2Fassets%2Forp%2Fresource%2Fitem%2F629aedbb80f81c39c69bcff3%2Foriginal%2Fchemistry-and-biology-of-novel-meliaceae-limonoids.pdf&usg=AOvVaw1QSsMnVYlkA4_4CKybEiPQ&opi=89978449 (accessed on 18 February 2024).
- Kablan, A.; Saunders, R.A.; Szkudlarek-Mikho, M.; Chin, A.J.; Bosio, R.M.; Fujii, K.; Shapiro, J.; Chin, K.V. Prieurianin Causes Weight Loss in Diet-Induced Obese Mice and Inhibits Adipogenesis in Cultured Preadipocytes. J. Diabetes Metab. 2010, 1, 1000101. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Sun, D.; Bai, F. Protective Effect of Nimbolide against High Fat Diet-induced Obesity in Rats via Nrf2/HO-1 Pathway. J. Oleo. Sci. 2022, 71, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Inoue, J.; Hashidume, T.; Shimizu, M.; Sato, R. Anti-obesity and anti-hyperglycemic effects of the dietary citrus limonoid nomilin in mice fed a high-fat diet. Biochem. Biophys. Res. Commun. 2011, 410, 677–681. [Google Scholar] [CrossRef]
- Sato, R. Nomilin as an anti-obesity and anti-hyperglycemic agent. Vitam. Horm. 2013, 91, 425–439. [Google Scholar]
- Shen, Y.; Hao, X. Natural product sciences: An integrative approach to the innovations of plant natural products. Sci. China Life Sci. 2020, 63, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef]
- Robert, S.; Chary, S.N.; Drakakaki, G.; Li, S.; Yang, Z.; Raikhel, N.V.; Hicks, G.R. Endosidin1 defines a compartment involved in endocytosis of the brassinosteroid receptor BRI1 and the auxin transporters PIN2 and AUX1. Proc. Natl. Acad. Sci. USA 2008, 105, 8464–8469. [Google Scholar] [CrossRef]
- Tóth, R.; Gerding-Reimers, C.; Deeks, M.J.; Menninger, S.; Gallegos, R.M.; Tonaco, I.A.; Hübel, K.; Hussey, P.J.; Waldmann, H.; Coupland, G. Prieurianin/endosidin 1 is an actin-stabilizing small molecule identified from a chemical genetic screen for circadian clock effectors in Arabidopsis thaliana. Plant J. 2012, 71, 338–352. [Google Scholar] [CrossRef]
- Anuradha, A.; Annadurai, R.S.; Shashidhara, L.S. Actin cytoskeleton as a putative target of the neem limonoid Azadirachtin A. Insect. Biochem. Mol. Biol. 2007, 37, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Pravin Kumar, R.; Manoj, M.N.; Kush, A.; Annadurai, R.S. In silico approach of azadirachtin binding with actins. Insect. Biochem. Mol. Biol. 2007, 37, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Pravin Kumar, R.; Roopa, L.; Sudheer Mohammed, M.M.; Kulkarni, N. Azadirachtin(A) distinctively modulates subdomain 2 of actin—Novel mechanism to induce depolymerization revealed by molecular dynamics study. J. Biomol. Struct. Dyn. 2016, 34, 2698–2710. [Google Scholar]
- Woollard, A.A.; Moore, I. The functions of Rab GTPases in plant membrane traffic. Curr. Opin. Plant Biol. 2008, 11, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zheng, H. Rab-A1c GTPase defines a population of the trans-Golgi network that is sensitive to endosidin1 during cytokinesis in Arabidopsis. Mol. Plant 2013, 6, 847–859. [Google Scholar] [CrossRef]
- Qi, X.; Zheng, H. Functional analysis of small Rab GTPases in cytokinesis in Arabidopsis thaliana. Methods Mol. Biol. 2013, 1043, 103–112. [Google Scholar]
- Wang, J.; Bai, M.; Zhang, C.; An, N.; Wan, L.; Wang, X.N.; Du, R.H.; Shen, Y.; Yuan, Z.Y.; Wu, X.D.; et al. Natural compound fraxinellone ameliorates intestinal fibrosis in mice via direct intervention of HSP47-collagen interaction in the epithelium. Acta Pharmacol. Sin. 2023, 44, 2469–2478. [Google Scholar] [CrossRef]
- Yoneda, A.; Minomi, K.; Tamura, Y. HSP47 promotes metastasis of breast cancer by interacting with myosin IIA via the unfolded protein response transducer IRE1α. Oncogene 2020, 39, 4519–4537. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Smieško, M.; Sellner, M.; Lill, M.A. Decision Making in Structure-Based Drug Discovery: Visual Inspection of Docking Results. J. Med. Chem. 2021, 64, 2489–2500. [Google Scholar] [CrossRef] [PubMed]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef] [PubMed]
- Bellone, M.L.; Muñoz Camero, C.; Chini, M.G.; Dal Piaz, F.; Hernandez, V.; Bifulco, G.; De Tommasi, N.; Braca, A. Limonoids from Guarea guidonia and Cedrela odorata: Heat Shock Protein 90 (Hsp90) Modulator Properties of Chisomicine D. J. Nat. Prod. 2021, 84, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.M.; Rocha, L.; Chung, T.Y.; Oliveira, R.F.; Pinho, C.; Oliveira, A.I.; Morgado, J.; Cruz, A. Biological Activities of Gedunin-A Limonoid from the Meliaceae Family. Molecules 2020, 25, 493. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Haque, E.; Hameed, R.; Maier, P.N.; Irfan, S.; Kamil, M.; Nazir, A.; Mir, S.S. Hsp90 inhibitor gedunin causes apoptosis in A549 lung cancer cells by disrupting Hsp90:Beclin-1:Bcl-2 interaction and downregulating autophagy. Life Sci. 2020, 256, 118000. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Vergoten, G. Interaction of Camptothecin Anticancer Drugs with Ribosomal Proteins L15 and L11: A Molecular Docking Study. Molecules 2023, 28, 1828. [Google Scholar] [CrossRef]
- Vergoten, G.; Bailly, C. Molecular Docking of Cryptoconcatones to α-Tubulin and Related Pironetin Analogues. Plants 2023, 12, 296. [Google Scholar] [CrossRef]
- Widmer, C.; Gebauer, J.M.; Brunstein, E.; Rosenbaum, S.; Zaucke, F.; Drögemüller, C.; Leeb, T.; Baumann, U. Molecular basis for the action of the collagen-specific chaperone Hsp47/SERPINH1 and its structure-specific client recognition. Proc. Natl. Acad. Sci. USA 2012, 109, 13243–13247. [Google Scholar] [CrossRef]
- Zheng, W.W.; Yang, D.T.; Wang, J.X.; Song, Q.S.; Gilbert, L.I.; Zhao, X.F. Hsc70 binds to ultraspiracle resulting in the upregulation of 20-hydroxyecdsone-responsive genes in Helicoverpa armigera. Mol. Cell. Endocrinol. 2010, 315, 282–291. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, F.X.; Cai, M.J.; Zhao, W.L.; Li, X.R.; Wang, J.X.; Zhao, X.F. The hormone-dependent function of Hsp90 in the crosstalk between 20-hydroxyecdysone and juvenile hormone signaling pathways in insects is determined by differential phosphorylation and protein interactions. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 5184–5192. [Google Scholar] [CrossRef] [PubMed]
- Nojima, Y. Characterization of Heat Shock Protein 60 as an Interacting Partner of Superoxide Dismutase 2 in the Silkworm, Bombyx mori, and Its Response to the Molting Hormone, 20-Hydroxyecdysone. Antioxidants 2021, 10, 1385. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Ye, Y.; Zheng, Z.W.; Luo, W.; Gong, Y.J.; Feng, Q.L.; Li, S.; Huang, L.H. Cytoplasmic Hsp70s promote EcR transport into the nucleus by responding to various stimuli. Insect Biochem. Mol. Biol. 2023, 157, 103964. [Google Scholar] [CrossRef] [PubMed]
- Scudeler, E.L.; Garcia, A.S.G.; Padovani, C.R.; Dos Santos, D.C. Pest and natural enemy: How the fat bodies of both the southern armyworm Spodoptera eridania and the predator Ceraeochrysa claveri react to azadirachtin exposure. Protoplasma 2019, 256, 839–856. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lai, D.; Yuan, M.; Xu, H. Growth inhibition and differences in protein profiles in azadirachtin-treated Drosophila melanogaster larvae. Electrophoresis 2014, 35, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Camero, C.M.; Vassallo, A.; De Leo, M.; Temraz, A.; De Tommasi, N.; Braca, A. Limonoids from Aphanamixis polystachya Leaves and Their Interaction with Hsp90. Planta Med. 2018, 8, 964–970. [Google Scholar] [CrossRef]
- Su, Y.; Li, X.; Zhang, W.; Zhang, B.; Ma, C.S. Dynamics of heat shock protein responses to thermal stress changes after metamorphosis in a lepidopteran insect. Arch. Insect Biochem. Physiol. 2021, 107, e21791. [Google Scholar] [CrossRef]
- King, A.M.; MacRae, T.H. Insect heat shock proteins during stress and diapause. Annu. Rev. Entomol. 2015, 60, 59–75. [Google Scholar] [CrossRef]
- Wang, S.; Kuperman, L.L.; Song, Z.; Chen, Y.; Liu, K.; Xia, Z.; Xu, Y.; Yu, Q. An overview of limonoid synthetic derivatives as promising bioactive molecules. Eur. J. Med. Chem. 2023, 259, 115704. [Google Scholar] [CrossRef] [PubMed]
- Berson, T.; von Wangenheim, D.; Takáč, T.; Šamajová, O.; Rosero, A.; Ovečka, M.; Komis, G.; Stelzer, E.H.; Šamaj, J. Trans-Golgi network localized small GTPase RabA1d is involved in cell plate formation and oscillatory root hair growth. BMC Plant Biol. 2014, 14, 252. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Zhou, J.; Faulkner, C.; MacLean, D.; Robatzek, S. Spatio-temporal cellular dynamics of the Arabidopsis flagellin receptor reveal activation status-dependent endosomal sorting. Plant Cell. 2012, 24, 4205–4219. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Okuno, D.; Tokito, T.; Yura, H.; Kido, T.; Ishimoto, H.; Tanaka, Y.; Mukae, H. HSP47: A Therapeutic Target in Pulmonary Fibrosis. Biomedicines 2023, 11, 2387. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Fattah, E.E.; Zakaria, A.Y. Targeting HSP47 and HSP70: Promising therapeutic approaches in liver fibrosis management. J. Transl. Med. 2022, 20, 544. [Google Scholar] [CrossRef] [PubMed]
- Bellaye, P.S.; Burgy, O.; Bonniaud, P.; Kolb, M. HSP47: A potential target for fibrotic diseases and implications for therapy. Expert Opin. Ther. Targets 2021, 25, 49–62. [Google Scholar] [CrossRef]
- Miyamura, T.; Sakamoto, N.; Kakugawa, T.; Taniguchi, H.; Akiyama, Y.; Okuno, D.; Moriyama, S.; Hara, A.; Kido, T.; Ishimoto, H.; et al. Small molecule inhibitor of HSP47 prevents pro-fibrotic mechanisms of fibroblasts in vitro. Biochem. Biophys. Res. Commun. 2020, 530, 561–565. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).