Interaction between Oligodendrocytes and Interneurons in Brain Development and Related Neuropsychiatric Disorders
Abstract
1. Introduction
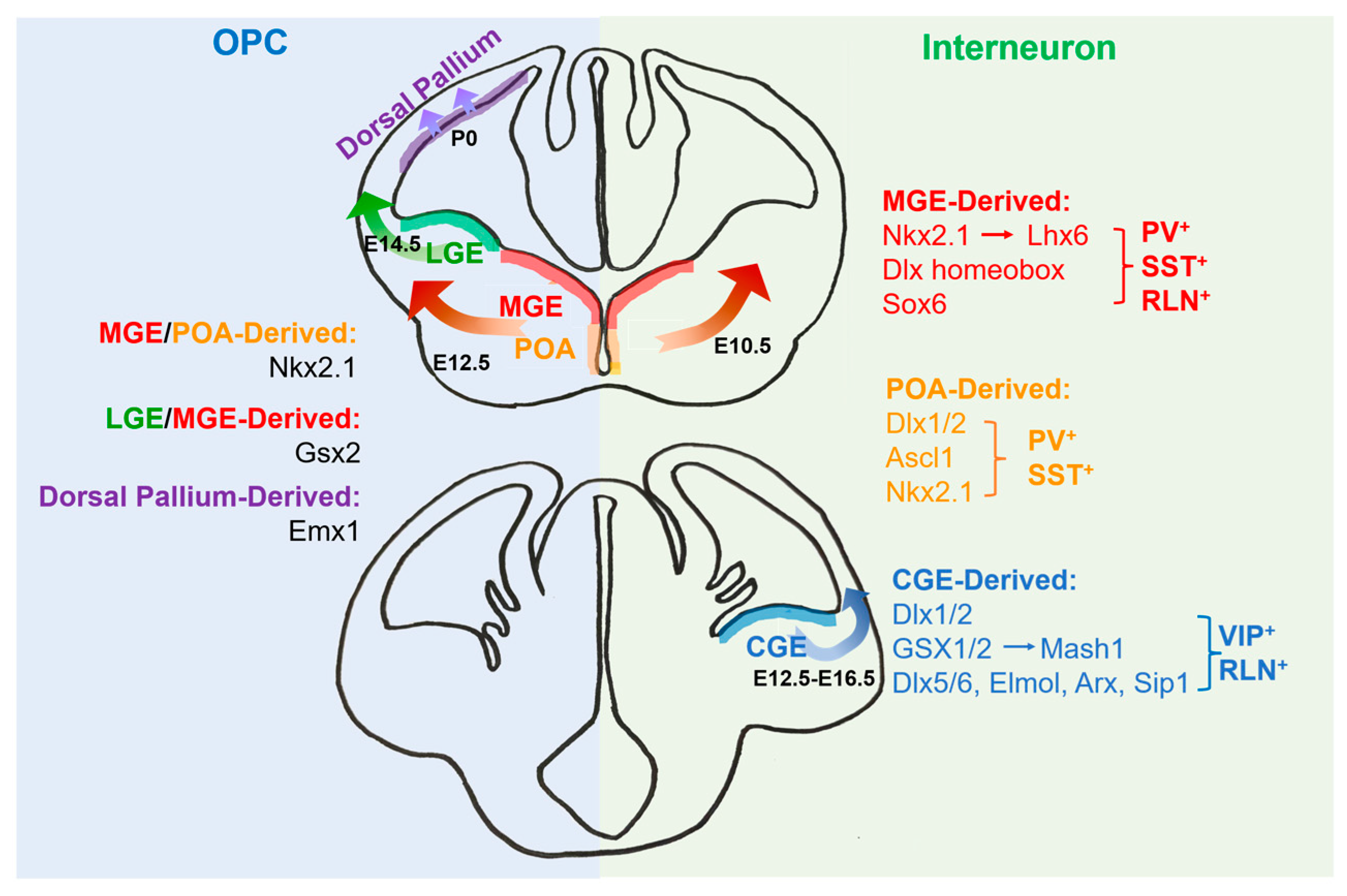
2. The Origins of Oligodendrocytes
3. The Origins of Interneurons
4. Transcriptional Regulation for the Development of Oligodendrocytes and Interneurons
5. The Interaction between Interneurons and Oligodendrocytes
5.1. The Synaptic Contact between Interneurons and Oligodendrocytes
5.2. Effects of GABA Signal in Differentiation and Myelination of OPCs
5.3. Density and Myelination of GABA+ Interneurons
6. Neuropsychiatric Disorders with Interneuron and Oligodendrocyte Involvement
6.1. Schizophrenia
6.2. Depression
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, B.; Zhu, Z.; Ransom, B.R.; Tong, X. Oligodendrocyte lineage cells and depression. Mol. Psychiatry 2020, 26, 103–117. [Google Scholar] [CrossRef]
- Taylor, S.F.; Grove, T.B.; Ellingrod, V.L.; Tso, I.F. The Fragile Brain: Stress Vulnerability, Negative Affect and GABAergic Neurocircuits in Psychosis. Schizophr. Bull. 2019, 45, 1170–1183. [Google Scholar] [CrossRef]
- Bergles, D.E.; Richardson, W.D. Oligodendrocyte Development and Plasticity. Cold Spring Harb. Perspect. Biol. 2015, 8, a020453. [Google Scholar] [CrossRef]
- Orduz, D.; Benamer, N.; Ortolani, D.; Coppola, E.; Vigier, L.; Pierani, A.; Angulo, M.C. Developmental cell death regulates lineage-related interneuron-oligodendroglia functional clusters and oligodendrocyte homeostasis. Nat. Commun. 2019, 10, 4249. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Bhaduri, A.; Velmeshev, D.; Wang, S.; Wang, L.; Rottkamp, C.A.; Alvarez-Buylla, A.; Rowitch, D.H.; Kriegstein, A.R. Origins and Proliferative States of Human Oligodendrocyte Precursor Cells. Cell 2020, 182, 594–608.e11. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, T.J.; Bhaduri, A.; Pollen, A.A.; Alvarado, B.; Mostajo-Radji, M.A.; Di Lullo, E.; Haeussler, M.; Sandoval-Espinosa, C.; Liu, S.J.; Velmeshev, D.; et al. Spatiotemporal gene expression trajectories reveal developmental hierarchies of the human cortex. Science 2017, 358, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Pollen, A.A.; Nowakowski, T.J.; Chen, J.; Retallack, H.; Sandoval-Espinosa, C.; Nicholas, C.R.; Shuga, J.; Liu, S.J.; Oldham, M.C.; Diaz, A.; et al. Molecular identity of human outer radial glia during cortical development. Cell 2015, 163, 55–67. [Google Scholar] [CrossRef]
- Wonders, C.P.; Anderson, S.A. The origin and specification of cortical interneurons. Nature reviews. Neuroscience 2006, 7, 687–696. [Google Scholar]
- Corbin, J.G.; Nery, S.; Fishell, G. Telencephalic cells take a tangent: Non-radial migration in the mammalian forebrain. Nat. Neurosci. 2001, 4, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zeng, Z.; Xie, D.; Chen, R.; Sha, Y.; Huang, S.; Cai, W.; Chen, W.; Li, W.; Ke, R.; et al. Interneuron origin and molecular diversity in the human fetal brain. Nat. Neurosci. 2021, 24, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Cobos, I.; De La Cruz, E.; Rubenstein, J.L.; Anderson, S.A. Origins of cortical interneuron subtypes. J. Neurosci. 2004, 24, 2612–2622. [Google Scholar] [CrossRef]
- Kepecs, A.; Fishell, G. Interneuron cell types are fit to function. Nature 2014, 505, 318–326. [Google Scholar] [CrossRef]
- Emery, B.; Lu, Q.R. Transcriptional and Epigenetic Regulation of Oligodendrocyte Development and Myelination in the Central Nervous System. Cold Spring Harb. Perspect. Biol. 2015, 7, a020461. [Google Scholar] [CrossRef]
- Nakatani, H.; Martin, E.; Hassani, H.; Clavairoly, A.; Maire, C.L.; Viadieu, A.; Kerninon, C.; Delmasure, A.; Frah, M.; Weber, M.; et al. Ascl1/Mash1 promotes brain oligodendrogenesis during myelination and remyelination. J. Neurosci. 2013, 33, 9752–9768. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, Q.; Zheng, K.; Li, H.; Qi, Y.; Cao, Q.; Qiu, M. Co-localization of Nkx6.2 and Nkx2.2 homeodomain proteins in differentiated myelinating oligodendrocytes. Glia 2010, 58, 458–468. [Google Scholar] [CrossRef]
- Zuo, H.; Wood, W.M.; Sherafat, A.; Hill, R.A.; Lu, Q.R.; Nishiyama, A. Age-Dependent Decline in Fate Switch from NG2 Cells to Astrocytes After Olig2 Deletion. J. Neurosci. 2018, 38, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Bylund, M.; Andersson, E.; Novitch, B.G.; Muhr, J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat. Neurosci. 2003, 6, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Küspert, M.; Hammer, A.; Bösl, M.R.; Wegner, M. Olig2 regulates Sox10 expression in oligodendrocyte precursors through an evolutionary conserved distal enhancer. Nucleic Acids Res. 2011, 39, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, M.; Ye, Z.; Zhou, C.; Bi, H.; Wang, L.; Zhang, C.; Fu, H.; Shen, Y.; Yang, J.J.; et al. Akt Regulates Sox10 Expression to Control Oligodendrocyte Differentiation via Phosphorylating FoxO1. J. Neurosci. 2021, 41, 8163–8180. [Google Scholar] [CrossRef] [PubMed]
- Weider, M.; Wegner, M. SoxE factors: Transcriptional regulators of neural differentiation and nervous system development. Semin. Cell Dev. Biol. 2017, 63, 35–42. [Google Scholar] [CrossRef]
- Aprato, J.; Sock, E.; Weider, M.; Elsesser, O.; Fröb, F.; Wegner, M. Myrf guides target gene selection of transcription factor Sox10 during oligodendroglial development. Nucleic Acids Res. 2020, 48, 1254–1270. [Google Scholar] [CrossRef]
- Zolova, O.E.; Wight, P.A. YY1 negatively regulates mouse myelin proteolipid protein (Plp1) gene expression in oligodendroglial cells. ASN Neuro 2011, 3, e00067. [Google Scholar] [CrossRef]
- Wang, Y.; Dye, C.A.; Sohal, V.; Long, J.E.; Estrada, R.C.; Roztocil, T.; Lufkin, T.; Deisseroth, K.; Baraban, S.C.; Rubenstein, J.L. Dlx5 and Dlx6 regulate the development of parvalbumin-expressing cortical interneurons. J. Neurosci. 2010, 30, 5334–5345. [Google Scholar] [CrossRef] [PubMed]
- Azim, E.; Jabaudon, D.; Fame, R.M.; Macklis, J.D. SOX6 controls dorsal progenitor identity and interneuron diversity during neocortical development. Nat. Neurosci. 2009, 12, 1238–1247. [Google Scholar] [CrossRef]
- Liodis, P.; Denaxa, M.; Grigoriou, M.; Akufo-Addo, C.; Yanagawa, Y.; Pachnis, V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J. Neurosci. 2007, 27, 3078–3089. [Google Scholar] [CrossRef] [PubMed]
- Pla, R.; Stanco, A.; Howard, M.A.; Rubin, A.N.; Vogt, D.; Mortimer, N.; Cobos, I.; Potter, G.B.; Lindtner, S.; Price, J.D.; et al. Dlx1 and Dlx2 Promote Interneuron GABA Synthesis, Synaptogenesis, and Dendritogenesis. Cereb. Cortex 2018, 28, 3797–3815. [Google Scholar] [CrossRef] [PubMed]
- Cobos, I.; Borello, U.; Rubenstein, J.L. Dlx transcription factors promote migration through repression of axon and dendrite growth. Neuron 2007, 54, 873–888. [Google Scholar] [CrossRef]
- Roybon, L.; Mastracci, T.L.; Ribeiro, D.; Sussel, L.; Brundin, P.; Li, J.Y. GABAergic differentiation induced by Mash1 is compromised by the bHLH proteins Neurogenin2, NeuroD1, and NeuroD2. Cereb. Cortex 2010, 20, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Gelman, D.; Griveau, A.; Dehorter, N.; Teissier, A.; Varela, C.; Pla, R.; Pierani, A.; Marín, O. A wide diversity of cortical GABAergic interneurons derives from the embryonic preoptic area. J. Neurosci. 2011, 31, 16570–16580. [Google Scholar] [CrossRef]
- Long, J.E.; Cobos, I.; Potter, G.B.; Rubenstein, J.L. Dlx1&2 and Mash1 transcription factors control MGE and CGE patterning and differentiation through parallel and overlapping pathways. Cereb. Cortex 2009, 19 (Suppl. S1), i96–i106. [Google Scholar]
- Grande, A.; Sumiyoshi, K.; López-Juárez, A.; Howard, J.; Sakthivel, B.; Aronow, B.; Campbell, K.; Nakafuku, M. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat. Commun. 2013, 4, 2373. [Google Scholar] [CrossRef]
- Boshans, L.L.; Soh, H.; Wood, W.M.; Nolan, T.M.; Mandoiu, I.I.; Yanagawa, Y.; Tzingounis, A.V.; Nishiyama, A. Direct reprogramming of oligodendrocyte precursor cells into GABAergic inhibitory neurons by a single homeodomain transcription factor Dlx2. Sci. Rep. 2021, 11, 3552. [Google Scholar] [CrossRef]
- Benamer, N.; Vidal, M.; Angulo, M.C. The cerebral cortex is a substrate of multiple interactions between GABAergic interneurons and oligodendrocyte lineage cells. Neurosci. Lett. 2020, 715, 134615. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.C.; Yabut, O.R.; Fregoso, S.P.; Gomez, H.G.; Dwyer, B.E.; Pleasure, S.J.; Franco, S.J. The Dorsal Wave of Neocortical Oligodendrogenesis Begins Embryonically and Requires Multiple Sources of Sonic Hedgehog. J. Neurosci. 2018, 38, 5237–5250. [Google Scholar] [CrossRef] [PubMed]
- Zonouzi, M.; Scafidi, J.; Li, P.; McEllin, B.; Edwards, J.; Dupree, J.L.; Harvey, L.; Sun, D.; Hubner, C.A.; Cull-Candy, S.G.; et al. GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nat. Neurosci. 2015, 18, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, P.P.; Velez-Fort, M.; Angulo, M.C. Is neuronal communication with NG2 cells synaptic or extrasynaptic? J. Anat. 2011, 219, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Bergles, D.E. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci. 2004, 7, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Orduz, D.; Maldonado, P.P.; Balia, M.; Velez-Fort, M.; de Sars, V.; Yanagawa, Y.; Emiliani, V.; Angulo, M.C. Interneurons and oligodendrocyte progenitors form a structured synaptic network in the developing neocortex. eLife 2015, 4, e06953. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Kirchhoff, F.; Scheller, A. Oligodendroglial GABAergic Signaling: More Than Inhibition! Neurosci. Bull. 2021, 37, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Turner, R. Myelin and Modeling: Bootstrapping Cortical Microcircuits. Front. Neural Circuits 2019, 13, 34. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [PubMed]
- Passlick, S.; Grauer, M.; Schäfer, C.; Jabs, R.; Seifert, G.; Steinhäuser, C. Expression of the γ2-subunit distinguishes synaptic and extrasynaptic GABAA receptors in NG2 cells of the hippocam pus. J. Neurosci. 2013, 33, 12030–12040. [Google Scholar] [CrossRef]
- Balia, M.; Vélez-Fort, M.; Passlick, S.; Schäfer, C.; Audinat, E.; Steinhäuser, C.; Seifert, G.; Angulo, M.C. Postnatal down-regulation of the GABAA receptor γ2 subunit in neocortical NG2 cells accompanies synaptic-to-extrasynaptic switch in the GABAergic transmission mode. Cereb. Cortex 2015, 25, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Regal, M.P.; Luengas-Escuza, I.; Bayon-Cordero, L.; Ibarra-Aizpurua, N.; Alberdi, E.; Perez-Samartin, A.; Matute, C.; Sanchez-Gomez, M.V. Oligodendrocyte Differentiation and Myelination Is Potentiated via GABA(B) Receptor Activation. Neuroscience 2020, 439, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, N.B.; Clarke, L.E.; Arancibia-Carcamo, I.L.; Kougioumtzidou, E.; Matthey, M.; Káradóttir, R.; Whiteley, L.; Bergersen, L.H.; Richardson, W.D.; Attwell, D. Endogenous GABA controls oligodendrocyte lineage cell number, myelination, and CNS internode length. Glia 2017, 65, 309–321. [Google Scholar] [CrossRef]
- Phillis, J.W.; Smith-Barbour, M.; Perkins, L.M.; O’Regan, M.H. Characterization of glutamate, aspartate, and GABA release from ischemic rat cerebral cortex. Brain Res. Bull. 1994, 34, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Tozuka, Y.; Takata, T.; Shimazu, N.; Matsumura, N.; Ohta, A.; Hisatsune, T. Excitatory GABAergic activation of cortical dividing glial cells. Cereb. Cortex 2009, 19, 2181–2195. [Google Scholar] [CrossRef]
- Van’t Veer, A.; Du, Y.; Fischer, T.Z.; Boetig, D.R.; Wood, M.R.; Dreyfus, C.F. Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. J. Neurosci. Res. 2009, 87, 69–78. [Google Scholar] [CrossRef]
- Vercellino, M.; Marasciulo, S.; Grifoni, S.; Vallino-Costassa, E.; Bosa, C.; Pasanisi, M.B.; Crociara, P.; Casalone, C.; Chiò, A.; Giordana, M.T.; et al. Acute and chronic synaptic pathology in multiple sclerosis gray matter. Mult. Scler. 2022, 28, 369–382. [Google Scholar] [CrossRef]
- Falcão, A.M.; van Bruggen, D.; Marques, S.; Meijer, M.; Jäkel, S.; Agirre, E.; Samudyata, N.; Floriddia, E.M.; Vanichkina, D.P.; Ffrench-Constant, C.; et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med. 2018, 24, 1837–1844. [Google Scholar] [CrossRef]
- Mazuir, E.; Fricker, D.; Sol-Foulon, N. Neuron-Oligodendrocyte Communication in Myelination of Cortical GABAergic Cells. Life 2021, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.P.; Zhao, N.; Caudal, L.C.; Chang, H.F.; Zhao, R.; Lin, C.H.; Hainz, N.; Meier, C.; Bettler, B.; Huang, W.; et al. Impaired bidirectional communication between interneurons and oligodendrocyte precursor cells affects social cognitive behavior. Nat. Commun. 2022, 13, 1394. [Google Scholar] [CrossRef] [PubMed]
- Micheva, K.D.; Wolman, D.; Mensh, B.D.; Pax, E.; Buchanan, J.; Smith, S.J.; Bock, D.D. A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. eLife 2016, 5, e15784. [Google Scholar] [CrossRef] [PubMed]
- Benamer, N.; Vidal, M.; Balia, M.; Angulo, M.C. Myelination of parvalbumin interneurons shapes the function of cortical sensory inhibitory circuits. Nat. Commun. 2020, 11, 5151. [Google Scholar] [CrossRef]
- Takahashi, N.; Sakurai, T.; Davis, K.L.; Buxbaum, J.D. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog. Neurobiol. 2011, 93, 13–24. [Google Scholar] [CrossRef]
- Maas, D.A.; Eijsink, V.D.; Spoelder, M.; van Hulten, J.A.; De Weerd, P.; Homberg, J.R.; Valles, A.; Nait-Oumesmar, B.; Martens, G.J.M. Interneuron hypomyelination is associated with cognitive inflexibility in a rat model of schizophrenia. Nat. Commun. 2020, 11, 2329. [Google Scholar] [CrossRef]
- Cassoli, J.S.; Guest, P.C.; Malchow, B.; Schmitt, A.; Falkai, P.; Martins-de-Souza, D. Disturbed macro-connectivity in schizophrenia linked to oligodendrocyte dysfunction: From structural findings to molecules. NPJ Schizophr. 2015, 1, 15034. [Google Scholar] [CrossRef]
- Franklin, R.J.M.; Bullmore, E.T. Do Not Adjust Your Mind: The Fault Is in Your Glia. Cell Stem Cell. 2017, 21, 155–156. [Google Scholar] [CrossRef]
- Farzan, F.; Barr, M.S.; Levinson, A.J.; Chen, R.; Wong, W.; Fitzgerald, P.B.; Daskalakis, Z.J. Evidence for gamma inhibition deficits in the dorsolateral prefrontal cortex of patients with schizophrenia. Brain 2010, 133 Pt 5, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Marin, O. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 2012, 13, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, J.; Zhang, K.; Lu, G.; Liu, Y.; Ren, K.; Wang, W.; Xin, D.; Xu, L.; Mao, H.; et al. Ten-eleven translocation 1 mediated-DNA hydroxymethylation is required for myelination and remyelination in the mouse brain. Nat. Commun. 2021, 12, 5091. [Google Scholar] [CrossRef]
- Hashimoto, T.; Volk, D.W.; Eggan, S.M.; Mirnics, K.; Pierri, J.N.; Sun, Z.; Sampson, A.R.; Lewis, D.A. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J. Neurosci. 2003, 23, 6315–6326. [Google Scholar] [CrossRef]
- Woo, T.U.; Whitehead, R.E.; Melchitzky, D.S.; Lewis, D.A. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc. Natl. Acad. Sci. USA 1998, 95, 5341–5346. [Google Scholar] [CrossRef]
- Nakazawa, K.; Zsiros, V.; Jiang, Z.; Nakao, K.; Kolata, S.; Zhang, S.; Belforte, J.E. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology 2012, 62, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Magri, C.; Giacopuzzi, E.; La Via, L.; Bonini, D.; Ravasio, V.; Elhussiny, M.E.A.; Orizio, F.; Gangemi, F.; Valsecchi, P.; Bresciani, R.; et al. A novel homozygous mutation in GAD1 gene described in a schizophrenic patient impairs activity and dimerization of GAD67 enzyme. Sci. Rep. 2018, 8, 15470. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dietz, K.; DeLoyht, J.M.; Pedre, X.; Kelkar, D.; Kaur, J.; Vialou, V.; Lobo, M.K.; Dietz, D.M.; Nestler, E.J.; et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 2012, 15, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.L.; Weigel, T.K.; Elkahloun, A.G.; Herkenham, M. Chronic social defeat reduces myelination in the mouse medial prefrontal cortex. Sci. Rep. 2017, 7, 46548. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, X.; Adebiyi, O.; Wang, J.; Mooshekhian, A.; Cohen, J.; Wei, Z.; Wang, F.; Li, X.M. Venlafaxine Improves the Cognitive Impairment and Depression-Like Behaviors in a Cuprizone Mouse Model by Alleviating Demyelination and Neuroinflammation in the Brain. Front. Pharmacol. 2019, 10, 332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhi, N.; Feng, J.; Liu, Y.; Zhang, M.; Liu, D.; Yuan, J.; Dong, Y.; Jiang, S.; Ge, J.; et al. ITPR2 Mediated Calcium Homeostasis in Oligodendrocytes is Essential for Myelination and Involved in Depressive-Like Behavior in Adolescent Mice. Adv. Sci. 2024, 13, e2306498. [Google Scholar] [CrossRef] [PubMed]
- Cathomas, F.; Azzinnari, D.; Bergamini, G.; Sigrist, H.; Buerge, M.; Hoop, V.; Wicki, B.; Goetze, L.; Soares, S.; Kukelova, D.; et al. Oligodendrocyte gene expression is reduced by and influences effects of chronic social stress in mice. Genes Brain Behav. 2019, 18, e12475. [Google Scholar] [CrossRef] [PubMed]
- Hagemeyer, N.; Goebbels, S.; Papiol, S.; Kästner, A.; Hofer, S.; Begemann, M.; Gerwig, U.C.; Boretius, S.; Wieser, G.L.; Ronnenberg, A.; et al. A myelin gene causative of a catatoniadepression syndrome upon aging. EMBO Mol. Med. 2012, 4, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Hong, Y.; Tao, M.; Shen, L.; Zheng, Z.; Fang, K.; Yuan, F.; Xu, M.; Wang, C.; Zhu, D.; et al. Depressive patient-derived GABA interneurons reveal abnormal neural activity associated with HTR2C. EMBO Mol. Med. 2023, 15, e16364. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, T.A.; Chen, Q.; Justice, M.J.; Artzt, K. The quaking gene product necessary in embryogenesis and myelination combines features of RNA binding and signal transduction proteins. Nat. Genet. 1996, 12, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Lacroix, G.; Haines, J.; Doukhanine, E.; Almazan, G.; Richard, S. The QKI-6 RNA binding protein localizes with the mbp mRNAs in stress granules of glial cells. PLoS ONE 2010, 5, e12824. [Google Scholar] [CrossRef] [PubMed]
- Klempan, T.A.; Ernst, C.; Deleva, V.; Labonte, B.; Turecki, G. Characterization of QKI gene expression, genetics, and epigenetics in suicide victims with major depressive disorder. Biol. Psychiatry 2009, 66, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Pantazatos, S.P.; Huang, Y.Y.; Rosoklija, G.B.; Dwork, A.J.; Arango, V.; Mann, J.J. Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: Evidence for altered glial, endothelial and ATPase activity. Mol. Psychiatry 2017, 22, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Aston, C.; Jiang, L.; Sokolov, B.P. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol. Psychiatry 2005, 10, 309–322. [Google Scholar] [CrossRef]
- Chagraoui, A.; Thibaut, F.; Skiba, M.; Thuillez, C.; Bourin, M. 5-HT2C receptors in psychiatric disorders: A review. Progress. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 66, 120–135. [Google Scholar] [CrossRef]
- Del Águila, Á.; Adam, M.; Ullom, K.; Shaw, N.; Qin, S.; Ehrman, J.; Nardini, D.; Salomone, J.; Gebelein, B.; Lu, Q.R.; et al. Olig2 defines a subset of neural stem cells that produce specific olfactory bulb interneuron subtypes in the subventricular zone of adult mice. Development 2022, 149, dev200028. [Google Scholar] [CrossRef]

| Neuropsychiatric Disorders | Evidence of Interneuron or Oligodendrocyte Involvement | Reference |
|---|---|---|
| Schizophrenia | Dysmyelination during adolescent PFC development in mice model | [55,57,58,61] |
| Decreased expression of GAD67 in PV+ interneurons of SCZ patients | [62] | |
| Conditional knockout of Gababr in OPCs interrupting the impaired bidirectional OPC–interneuron signaling and induing defective social cognitive behavior in the mice | [52] | |
| Impaired electroencephalogram synchronization in the gamma range, originated mainly from PV+ interneurons, in SCZ patients | [59] | |
| Fewer inhibitory synapses from interneurons onto cortical pyramidal cells in SCZ individuals | [63] | |
| Defects in potent excitatory drives from pyramidal cells, causing the failure in recruiting PV+ interneurons in mice models | [65] | |
| Impaired excitation of interneurons in both mouse models and SCZ patients | [65] | |
| Decreased excitatory drive to PV+ interneurons, leading to a reduced expression of GAD67 mRNA | [66] | |
| Depression | Decreased myelin gene expression and impaired myelin formation in protracted social-isolation mice | [66] |
| Myelin genes are the most significantly downregulated in stress-induced depressive mice | [67] | |
| Cuprizone-induced demyelinating mice develop depression-like behaviors | [68] | |
| Calcium homeostasis in OLs is essential for myelination and causes anxiety/depressive like behaviors once interrupted in OLs | [69] | |
| Animal models of chronic unpredictable mild stress show a decreased expression of OL-associated genes | [70] | |
| Myelin gene mutation might be a causative of catatonia-depression syndrome in patients | [71] | |
| Interneurons exhibit abnormal morphology and function in sMDD patients | [72] | |
| Targeting the 5-HT2C receptor can restore neuronal activity deficits in sMDD GABAergic interneurons | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yuan, J.; Dong, Y.; Jiang, S.; Zhang, M.; Zhao, X. Interaction between Oligodendrocytes and Interneurons in Brain Development and Related Neuropsychiatric Disorders. Int. J. Mol. Sci. 2024, 25, 3620. https://doi.org/10.3390/ijms25073620
Liu Y, Yuan J, Dong Y, Jiang S, Zhang M, Zhao X. Interaction between Oligodendrocytes and Interneurons in Brain Development and Related Neuropsychiatric Disorders. International Journal of Molecular Sciences. 2024; 25(7):3620. https://doi.org/10.3390/ijms25073620
Chicago/Turabian StyleLiu, Yingqi, Jie Yuan, Yuhao Dong, Sufang Jiang, Ming Zhang, and Xianghui Zhao. 2024. "Interaction between Oligodendrocytes and Interneurons in Brain Development and Related Neuropsychiatric Disorders" International Journal of Molecular Sciences 25, no. 7: 3620. https://doi.org/10.3390/ijms25073620
APA StyleLiu, Y., Yuan, J., Dong, Y., Jiang, S., Zhang, M., & Zhao, X. (2024). Interaction between Oligodendrocytes and Interneurons in Brain Development and Related Neuropsychiatric Disorders. International Journal of Molecular Sciences, 25(7), 3620. https://doi.org/10.3390/ijms25073620







