Specific Binding of Alzheimer’s Aβ Peptides to Extracellular Vesicles
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, B.; Kamaleddin, M.A.; Aalishah, K. A Comprehensive Review on Exosomes and Microvesicles as Epigenetic Factors. Curr. Stem Cell Res. Ther. 2017, 12, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Simpson, R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009, 9, 4997–5000. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Desai, N.N.; Qureshi, M.Z.; Librelotto, D.R.N.; Gasparri, M.L.; Bishayee, A.; Nabavi, S.M.; Curti, V.; Daglia, M. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol. Adv. 2018, 36, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Elrashdy, F.; Aljaddawi, A.A.; Redwan, E.M.; Uversky, V.N. On the potential role of exosomes in the COVID-19 reinfection/reactivation opportunity. J. Biomol. Struct. Dyn. 2021, 39, 5831–5842. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Schwartz, J.B.; Abner, E.L.; Jicha, G.A.; Kapogiannis, D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann. Neurol. 2018, 83, 544–552. [Google Scholar] [CrossRef]
- Jiang, L.; Dong, H.; Cao, H.; Ji, X.; Luan, S.; Liu, J. Exosomes in Pathogenesis, Diagnosis, and Treatment of Alzheimer’s Disease. Med. Sci. Monit. 2019, 25, 3329–3335. [Google Scholar] [CrossRef]
- Kaur, S.; Verma, H.; Dhiman, M.; Tell, G.; Gigli, G.L.; Janes, F.; Mantha, A.K. Brain Exosomes: Friend or Foe in Alzheimer’s Disease? Mol. Neurobiol. 2021, 58, 6610–6624. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dhar, R.; Kumar, L.; Shivji, G.G.; Jayaraj, R.; Devi, A. Theranostic signature of tumor-derived exosomes in cancer. Med. Oncol. 2023, 40, 321. [Google Scholar] [CrossRef] [PubMed]
- Lan, B.; Zeng, S.; Grutzmann, R.; Pilarsky, C. The Role of Exosomes in Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 4332. [Google Scholar] [CrossRef]
- Liang, T.; Wu, Z.; Li, J.; Wu, S.; Shi, W.; Wang, L. The emerging double-edged sword role of exosomes in Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1209115. [Google Scholar] [CrossRef] [PubMed]
- Malm, T.; Loppi, S.; Kanninen, K.M. Exosomes in Alzheimer’s disease. Neurochem. Int. 2016, 97, 193–199. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, E.; Bilousova, T.; Melnik, M.; Fakhrutdinov, D.; Poon, W.W.; Vinters, H.V.; Miller, C.A.; Corrada, M.; Kawas, C.; Bohannan, R.; et al. Exosomal tau with seeding activity is released from Alzheimer’s disease synapses, and seeding potential is associated with amyloid beta. Lab. Investig. 2021, 101, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Sardar Sinha, M.; Ansell-Schultz, A.; Civitelli, L.; Hildesjo, C.; Larsson, M.; Lannfelt, L.; Ingelsson, M.; Hallbeck, M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018, 136, 41–56. [Google Scholar] [CrossRef]
- Sur, S.; Khatun, M.; Steele, R.; Isbell, T.S.; Ray, R.; Ray, R.B. Exosomes from COVID-19 Patients Carry Tenascin-C and Fibrinogen-β in Triggering Inflammatory Signals in Cells of Distant Organ. Int. J. Mol. Sci. 2021, 22, 3184. [Google Scholar] [CrossRef]
- Ung, T.H.; Madsen, H.J.; Hellwinkel, J.E.; Lencioni, A.M.; Graner, M.W. Exosome proteomics reveals transcriptional regulator proteins with potential to mediate downstream pathways. Cancer Sci. 2014, 105, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Vandendriessche, C.; Bruggeman, A.; Van Cauwenberghe, C.; Vandenbroucke, R.E. Extracellular Vesicles in Alzheimer’s and Parkinson’s Disease: Small Entities with Large Consequences. Cells 2020, 9, 2485. [Google Scholar] [CrossRef]
- Xiao, T.; Zhang, W.; Jiao, B.; Pan, C.Z.; Liu, X.; Shen, L. The role of exosomes in the pathogenesis of Alzheimer’ disease. Transl. Neurodegener. 2017, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, K.; Igarashi, Y. Exosomes as Carriers of Alzheimer’s Amyloid-ss. Front. Neurosci. 2017, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Tan, L.; Sun, Y.; Qiu, X.; Liao, Y.; Song, C.; Liu, W.; Nair, V.; Ding, C. Exosomes Carry microRNAs into Neighboring Cells to Promote Diffusive Infection of Newcastle Disease Virus. Viruses 2019, 11, 527. [Google Scholar] [CrossRef]
- Zhou, W.; Lovasz, D.; Zizzo, Z.; He, Q.; Coughlan, C.; Kowalski, R.G.; Kennedy, P.G.E.; Graner, A.N.; Lillehei, K.O.; Ormond, D.R.; et al. Phenotype and Neuronal Cytotoxic Function of Glioblastoma Extracellular Vesicles. Biomedicines 2022, 10, 2718. [Google Scholar] [CrossRef]
- Stoorvogel, W.; Strous, G.J.; Geuze, H.J.; Oorschot, V.; Schwartz, A.L. Late endosomes derive from early endosomes by maturation. Cell 1991, 65, 417–427. [Google Scholar] [CrossRef]
- Emanueli, C.; Shearn, A.I.; Angelini, G.D.; Sahoo, S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vasc. Pharmacol. 2015, 71, 24–30. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, A.J.; Woffindale, C.A.; Wood, M.J. Exosomes and the emerging field of exosome-based gene therapy. Curr. Gene Ther. 2012, 12, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Alenquer, M.; Amorim, M.J. Exosome Biogenesis, Regulation, and Function in Viral Infection. Viruses 2015, 7, 5066–5083. [Google Scholar] [CrossRef] [PubMed]
- Larios, J.; Mercier, V.; Roux, A.; Gruenberg, J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J. Cell Biol. 2020, 219, e201904113. [Google Scholar] [CrossRef] [PubMed]
- Bubak, A.N.; Coughlan, C.; Posey, J.; Saviola, A.J.; Niemeyer, C.S.; Lewis, S.W.R.; Lopez, S.B.; Solano, A.; Tyring, S.K.; Delaney, C.; et al. Zoster-Associated Prothrombotic Plasma Exosomes and Increased Stroke Risk. J. Infect. Dis. 2023, 227, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Flores-Bellver, M.; Mighty, J.; Aparicio-Domingo, S.; Li, K.V.; Shi, C.; Zhou, J.; Cobb, H.; McGrath, P.; Michelis, G.; Lenhart, P.; et al. Extracellular vesicles released by human retinal pigment epithelium mediate increased polarised secretion of drusen proteins in response to AMD stressors. J. Extracell. Vesicles 2021, 10, e12165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ma, S.; Lv, J.; Wang, X.; Afewerky, H.K.; Li, H.; Lu, Y. The emerging role of exosomes in Alzheimer’s disease. Ageing Res. Rev. 2021, 68, 101321. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tao, J.; Li, Y.; Feng, Y.; Ju, H.; Wang, Z.; Ding, L. Quantitative Localized Analysis Reveals Distinct Exosomal Protein-Specific Glycosignatures: Implications in Cancer Cell Subtyping, Exosome Biogenesis, and Function. J. Am. Chem. Soc. 2020, 142, 7404–7412. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Gazouli, M.; Theodoropoulos, C.; Zografos, E.; Zografos, G.C.; Michalopoulos, N.V. Exosomes in breast cancer management: Where do we stand? A literature review. Biol. Cell 2022, 114, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.C.; Li, C.; Zhang, W.; Pi, W.; Han, N. Potential Effects of Exosomes and their MicroRNA Carrier on Osteoporosis. Curr. Pharm. Des. 2022, 28, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, S.; Jing, D.; Li, X.; Zhang, B.; Bie, Q. Signal transduction mechanism of exosomes in diabetic complications (Review). Exp. Ther. Med. 2022, 23, 155. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tan, W.F.; Yang, M.Q.; Li, J.Y.; Geller, D.A. The therapeutic potential of exosomes derived from different cell sources in liver diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G397–G404. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.Y.; Javid, M.A.; Nazir, M.M.; Niaz, N.; Nisar, M.F.; Manzoor, Z.; Bhatti, S.A.; Hameed, S.; Khaliq, M.H. Extracellular vesicles and exosome: Insight from physiological regulatory perspectives. J. Physiol. Biochem. 2022, 78, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Hellwinkel, J.E.; Redzic, J.S.; Harland, T.A.; Gunaydin, D.; Anchordoquy, T.J.; Graner, M.W. Glioma-derived extracellular vesicles selectively suppress immune responses. Neuro Oncol. 2016, 18, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Lee, V.M.; Otvos, L., Jr.; Greenberg, B.D.; Lowery, D.E.; Sharma, S.K.; Schmidt, M.L.; Trojanowski, J.Q. Defined neurofilament, tau, and beta-amyloid precursor protein epitopes distinguish Alzheimer from non-Alzheimer senile plaques. Proc. Natl. Acad. Sci. USA 1990, 87, 2249–2253. [Google Scholar] [CrossRef] [PubMed]
- Roher, A.E.; Chaney, M.O.; Kuo, Y.M.; Webster, S.D.; Stine, W.B.; Haverkamp, L.J.; Woods, A.S.; Cotter, R.J.; Tuohy, J.M.; Krafft, G.A.; et al. Morphology and toxicity of Abeta-(1–42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer’s disease. J. Biol. Chem. 1996, 271, 20631–20635. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Cummings, J.; Blennow, K.; Gao, P.; Jack, C.R., Jr.; Vergallo, A. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat. Rev. Neurol. 2021, 17, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-beta Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Dore, V.; Fowler, C.; Li, Q.X.; Martins, R.; Rowe, C.; et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef]
- Peng, C.; Trojanowski, J.Q.; Lee, V.M. Protein transmission in neurodegenerative disease. Nat. Rev. Neurol. 2020, 16, 199–212. [Google Scholar] [CrossRef]
- Giau, V.V.; Bagyinszky, E.; An, S.S.A. Potential Fluid Biomarkers for the Diagnosis of Mild Cognitive Impairment. Int. J. Mol. Sci. 2019, 20, 4149. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, I.; Pardossi-Piquard, R.; Bauer, C.; Brigham, E.; Abraham, J.D.; Ranaldi, S.; Fraser, P.; St-George-Hyslop, P.; Le Thuc, O.; Espin, V.; et al. The beta-secretase-derived C-terminal fragment of betaAPP, C99, but not Abeta, is a key contributor to early intraneuronal lesions in triple-transgenic mouse hippocampus. J. Neurosci. 2012, 32, 16243–16255. [Google Scholar] [CrossRef]
- Guix, F.X.; Ill-Raga, G.; Bravo, R.; Nakaya, T.; de Fabritiis, G.; Coma, M.; Miscione, G.P.; Villa-Freixa, J.; Suzuki, T.; Fernandez-Busquets, X.; et al. Amyloid-dependent triosephosphate isomerase nitrotyrosination induces glycation and tau fibrillation. Brain 2009, 132 Pt 5, 1335–1345. [Google Scholar] [CrossRef]
- Guix, F.X.; Wahle, T.; Vennekens, K.; Snellinx, A.; Chavez-Gutierrez, L.; Ill-Raga, G.; Ramos-Fernandez, E.; Guardia-Laguarta, C.; Lleo, A.; Arimon, M.; et al. Modification of gamma-secretase by nitrosative stress links neuronal ageing to sporadic Alzheimer’s disease. EMBO Mol. Med. 2012, 4, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Shin, K.Y.; Chang, K.A. Brain-Derived Exosomal Proteins as Effective Biomarkers for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Biomolecules 2021, 11, 980. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Guerra, F.; Calvani, R.; Coelho-Junior, H.J.; Bucci, C.; Marzetti, E. Circulating extracellular vesicles: Friends and foes in neurodegeneration. Neural Regen. Res. 2022, 17, 534–542. [Google Scholar] [CrossRef]
- Cai, H.; Pang, Y.; Wang, Q.; Qin, W.; Wei, C.; Li, Y.; Li, T.; Li, F.; Wang, Q.; Li, Y.; et al. Proteomic profiling of circulating plasma exosomes reveals novel biomarkers of Alzheimer’s disease. Alzheimers Res. Ther. 2022, 14, 181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gu, Y.; Zhang, Q.; Liu, H.; Liu, Y. The Potential Roles of Exosomes Carrying APP and Tau Cleavage Products in Alzheimer’s Disease. J. Clin. Med. 2023, 12, 1883. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Esteban-de-Antonio, E.; Bernuz, M.; Puerta, R.; Garcia-Gonzalez, P.; de Rojas, I.; Olive, C.; Perez-Cordon, A.; Montrreal, L.; Nunez-Llaves, R.; et al. Plasma extracellular vesicles reveal early molecular differences in amyloid positive patients with early-onset mild cognitive impairment. J. Nanobiotechnol. 2023, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, C.; Bruce, K.D.; Burgy, O.; Boyd, T.D.; Michel, C.R.; Garcia-Perez, J.E.; Adame, V.; Anton, P.; Bettcher, B.M.; Chial, H.J.; et al. Exosome Isolation by Ultracentrifugation and Precipitation and Techniques for Downstream Analyses. Curr. Protoc. Cell Biol. 2020, 88, e110. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Mun, J.Y. Sample Preparation and Imaging of Exosomes by Transmission Electron Microscopy. J. Vis. Exp. 2018, 131, e56482. [Google Scholar] [CrossRef]
- Abdelrasoul, M.; Yuyama, K.; Swamy, M.M.M.; Murai, Y.; Monde, K. Stereochemistry-activity relationship of ceramide-induced exosome production to clear amyloid-beta in Alzheimer’s disease. Chirality 2023, 35, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Takase, H.; Nunome, M.; Enomoto, H.; Ito, J.; Gong, J.S.; Michikawa, M. Amyloid-beta Reduces Exosome Release from Astrocytes by Enhancing JNK Phosphorylation. J. Alzheimers Dis. 2016, 53, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Feng, Z.; Li, Y.; Lv, H.; Liu, S.; Luo, Y.; Hao, N.; Zhao, L.; Liu, J. Mesenchymal stem cell-derived extracellular vesicles: A novel promising neuroprotective agent for Alzheimer’s disease. Biochem. Pharmacol. 2024, 222, 116064. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, J.; Xiao, Y.; Li, F.; Niu, L.; Liu, X.; Meng, L.; Zheng, H. Ultrasound-mediated augmented exosome release from astrocytes alleviates amyloid-beta-induced neurotoxicity. Theranostics 2021, 11, 4351–4362. [Google Scholar] [CrossRef]
- Dinkins, M.B.; Dasgupta, S.; Wang, G.; Zhu, G.; Bieberich, E. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 1792–1800. [Google Scholar] [CrossRef]
- Huang, S.; Liao, X.; Wu, J.; Zhang, X.; Li, Y.; Xiang, D.; Luo, S. The Microglial membrane receptor TREM2 mediates exosome secretion to promote phagocytosis of amyloid-beta by microglia. FEBS Lett. 2022, 596, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Janas, T.; Sapon, K.; Stowell, M.H.B.; Janas, T. Selection of Membrane RNA Aptamers to Amyloid Beta Peptide: Implications for Exosome-Based Antioxidant Strategies. Int. J. Mol. Sci. 2019, 20, 299. [Google Scholar] [CrossRef]
- Khursheed, A.; Viles, J.H. Impact of Membrane Phospholipids and Exosomes on the Kinetics of Amyloid-beta Fibril Assembly. J. Mol. Biol. 2024, 436, 168464. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.Z.J.; Zhang, Y.; Chen, Y.; Zhao, H.; Stephenson, M.C.; Ho, N.R.Y.; Chen, Y.; Chung, J.; Reilhac, A.; Loh, T.P.; et al. Subtyping of circulating exosome-bound amyloid beta reflects brain plaque deposition. Nat. Commun. 2019, 10, 1144. [Google Scholar] [CrossRef] [PubMed]
- Mantellatto Grigoli, M.; Pelegrini, L.N.C.; Whelan, R.; Cominetti, M.R. Present and Future of Blood-Based Biomarkers of Alzheimer’s Disease: Beyond the Classics. Brain Res. 2024, 1830, 148812. [Google Scholar] [CrossRef]
- Perez-Gonzalez, R.; Gauthier, S.A.; Kumar, A.; Levy, E. The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J. Biol. Chem. 2012, 287, 43108–43115. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Honsho, M.; Zahn, T.R.; Keller, P.; Geiger, K.D.; Verkade, P.; Simons, K. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. USA 2006, 103, 11172–11177. [Google Scholar] [CrossRef] [PubMed]
- Sproviero, D.; Gagliardi, S.; Zucca, S.; Arigoni, M.; Giannini, M.; Garofalo, M.; Fantini, V.; Pansarasa, O.; Avenali, M.; Ramusino, M.C.; et al. Extracellular Vesicles Derived from Plasma of Patients with Neurodegenerative Disease Have Common Transcriptomic Profiling. Front. Aging Neurosci. 2022, 14, 785741. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Tripathi, P.N.; Sharma, P.; Rai, S.N.; Singh, S.P.; Srivastava, R.K.; Shankar, S.; Shrivastava, S.K. Design and development of some phenyl benzoxazole derivatives as a potent acetylcholinesterase inhibitor with antioxidant property to enhance learning and memory. Eur. J. Med. Chem. 2019, 163, 116–135. [Google Scholar] [CrossRef]
- Tamboli, I.Y.; Barth, E.; Christian, L.; Siepmann, M.; Kumar, S.; Singh, S.; Tolksdorf, K.; Heneka, M.T.; Lutjohann, D.; Wunderlich, P.; et al. Statins promote the degradation of extracellular amyloid beta-peptide by microglia via stimulation of exosome-associated insulin-degrading enzyme (IDE) secretion. J. Biol. Chem. 2010, 285, 37405–37414. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.N.; Srivastava, P.; Sharma, P.; Tripathi, M.K.; Seth, A.; Tripathi, A.; Rai, S.N.; Singh, S.P.; Shrivastava, S.K. Biphenyl-3-oxo-1,2,4-triazine linked piperazine derivatives as potential cholinesterase inhibitors with anti-oxidant property to improve the learning and memory. Bioorg. Chem. 2019, 85, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, K.; Sun, H.; Mitsutake, S.; Igarashi, Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J. Biol. Chem. 2012, 287, 10977–10989. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, K.; Yamamoto, N.; Yanagisawa, K. Accelerated release of exosome-associated GM1 ganglioside (GM1) by endocytic pathway abnormality: Another putative pathway for GM1-induced amyloid fibril formation. J. Neurochem. 2008, 105, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Drabik, D.; Chodaczek, G.; Kraszewski, S. Effect of Amyloid-beta Monomers on Lipid Membrane Mechanical Parameters-Potential Implications for Mechanically Driven Neurodegeneration in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 22, 18. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Merino, C. Brain Hydrophobic Peptides Antagonists of Neurotoxic Amyloid beta Peptide Monomers/Oligomers-Protein Interactions. Int. J. Mol. Sci. 2023, 24, 3846. [Google Scholar] [CrossRef] [PubMed]
- Kravenska, Y.; Nieznanska, H.; Nieznanski, K.; Lukyanetz, E.; Szewczyk, A.; Koprowski, P. The monomers, oligomers, and fibrils of amyloid-beta inhibit the activity of mitoBK(Ca) channels by a membrane-mediated mechanism. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183337. [Google Scholar] [CrossRef] [PubMed]
- Orjuela, A.; Lakey-Beitia, J.; Mojica-Flores, R.; Hegde, M.L.; Lans, I.; Ali-Torres, J.; Rao, K.S. Computational Evaluation of Interaction between Curcumin Derivatives and Amyloid-beta Monomers and Fibrils: Relevance to Alzheimer’s Disease. J. Alzheimers Dis. 2021, 82, S321–S333. [Google Scholar] [CrossRef]
- Santangelo, R.; Giuffrida, M.L.; Satriano, C.; Tomasello, M.F.; Zimbone, S.; Copani, A. beta-amyloid monomers drive up neuronal aerobic glycolysis in response to energy stressors. Aging 2021, 13, 18033–18050. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, T.G.; Schreiner, O.D.; Adam, M.; Popescu, B.O. The Roles of the Amyloid Beta Monomers in Physiological and Pathological Conditions. Biomedicines 2023, 11, 1411. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Novo, M.; Perez-Gonzalez, C.; Freire, S.; Al-Soufi, W. Early Aggregation of Amyloid-beta(1–42) Studied by Fluorescence Correlation Spectroscopy. Methods Mol. Biol. 2023, 2551, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Schrader, J.M.; Irizarry, B.A.; Smith, S.O.; Van Nostrand, W.E. Impact of Abeta40 and Abeta42 Fibrils on the Transcriptome of Primary Astrocytes and Microglia. Biomedicines 2022, 10, 2982. [Google Scholar] [CrossRef] [PubMed]
- Radeghieri, A.; Bergese, P. The biomolecular corona of extracellular nanoparticles holds new promises for advancing clinical molecular diagnostics. Expert Rev. Mol. Diagn. 2023, 23, 471–474. [Google Scholar] [CrossRef]
- Musico, A.; Zenatelli, R.; Romano, M.; Zendrini, A.; Alacqua, S.; Tassoni, S.; Paolini, L.; Urbinati, C.; Rusnati, M.; Bergese, P.; et al. Surface functionalization of extracellular vesicle nanoparticles with antibodies: A first study on the protein corona “variable”. Nanoscale Adv. 2023, 5, 4703–4717. [Google Scholar] [CrossRef] [PubMed]
- Yunusova, N.V.; Popova, N.O.; Udintseva, I.N.; Klyushina, T.S.; Kazantseva, D.V.; Smirnova, L.P. The Role of Intravesicular Proteins and the Protein Corona of Extracellular Vesicles in the Development of Drug-Induced Polyneuropathy. Curr. Issues Mol. Biol. 2023, 45, 3302–3314. [Google Scholar] [CrossRef]
- Potter, H.; Woodcock, J.H.; Boyd, T.D.; Coughlan, C.M.; O’Shaughnessy, J.R.; Borges, M.T.; Thaker, A.A.; Raj, B.A.; Adamszuk, K.; Scott, D.; et al. Safety and efficacy of sargramostim (GM-CSF) in the treatment of Alzheimer’s disease. Alzheimers Dement. 2021, 7, e12158. [Google Scholar] [CrossRef] [PubMed]
- Bettcher, B.M.; Olson, K.E.; Carlson, N.E.; McConnell, B.V.; Boyd, T.; Adame, V.; Solano, D.A.; Anton, P.; Markham, N.; Thaker, A.A.; et al. Astrogliosis and episodic memory in late life: Higher GFAP is related to worse memory and white matter microstructure in healthy aging and Alzheimer’s disease. Neurobiol. Aging 2021, 103, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Gorai, S.; Bagdi, P.R.; Borah, R.; Paul, D.; Santra, M.K.; Khan, A.T.; Manna, D. Insights into the inhibitory mechanism of triazole-based small molecules on phosphatidylinositol-4,5-bisphosphate binding pleckstrin homology domain. Biochem. Biophys. Rep. 2015, 2, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, P.; Beck, G.; Esselmann, H.; Bruckmoser, R.; Zimmermann, R.; Fiszer, M.; Bibl, M.; Maler, J.M.; Kornhuber, J.; Wiltfang, J. Effect of sample collection tubes on cerebrospinal fluid concentrations of tau proteins and amyloid beta peptides. Clin. Chem. 2006, 52, 332–334. [Google Scholar] [CrossRef] [PubMed]
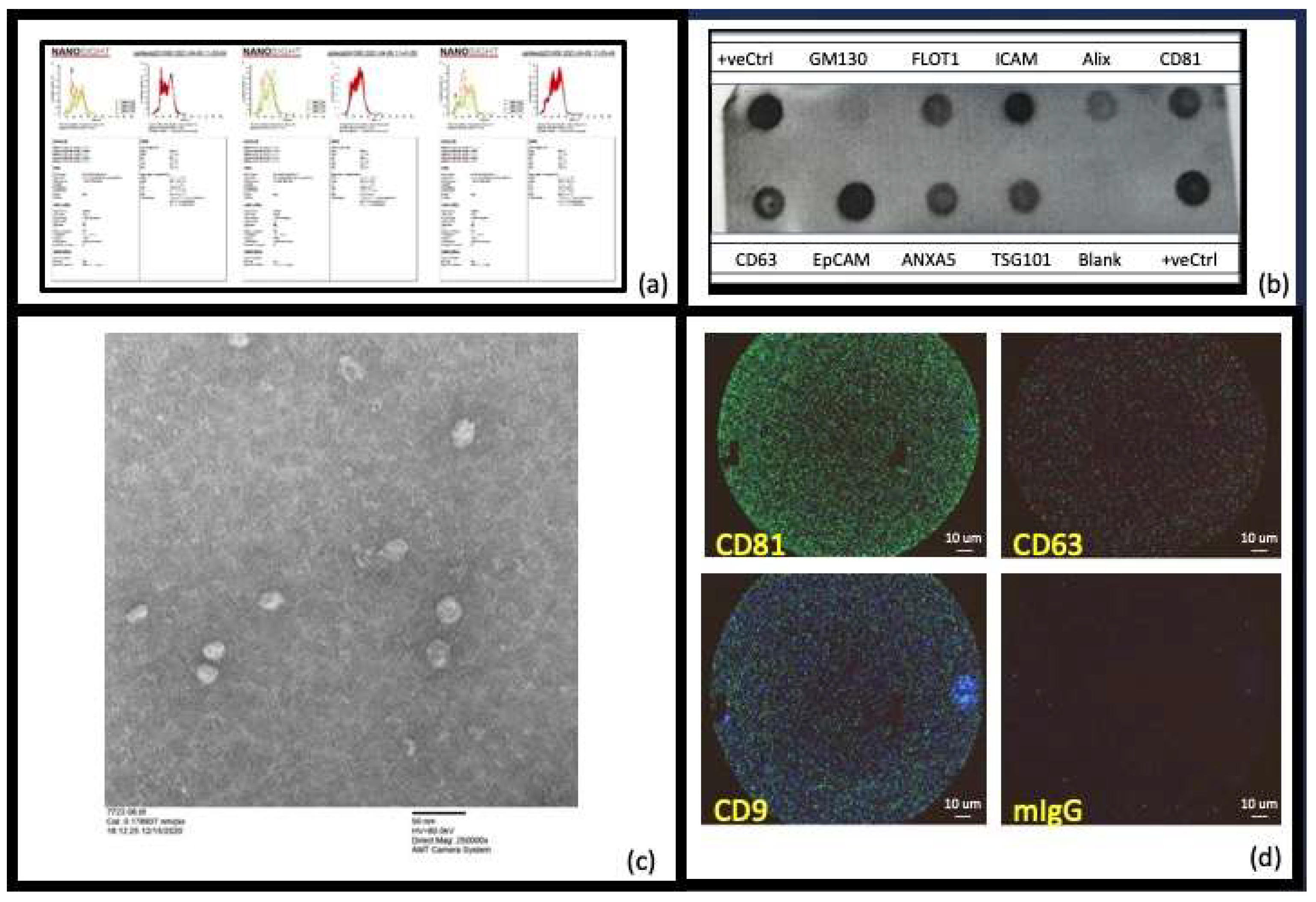
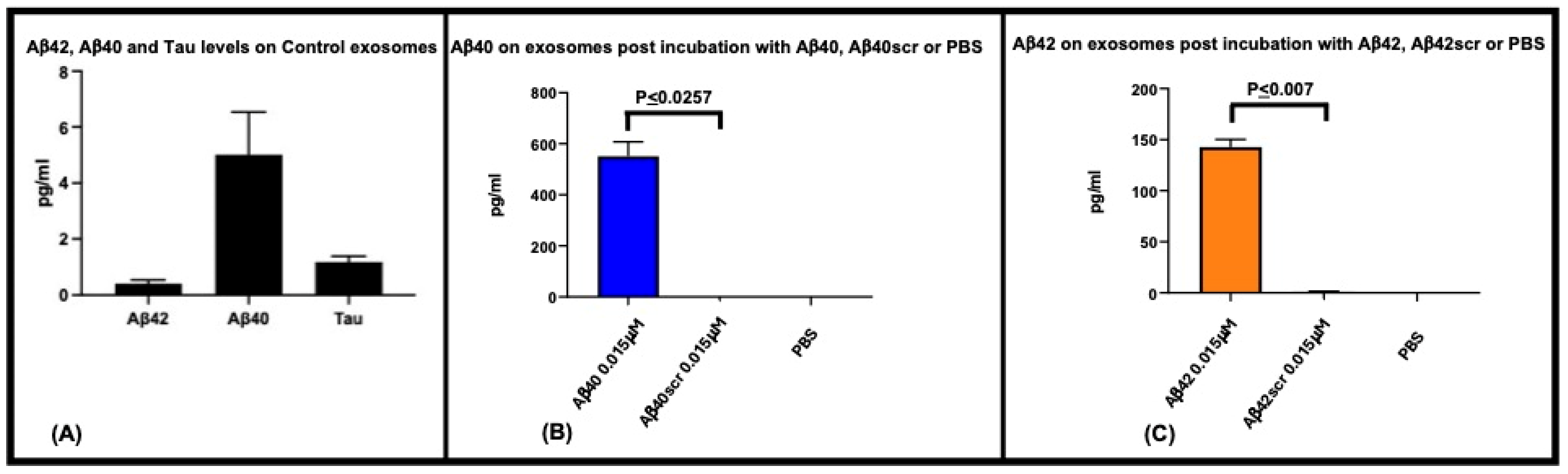
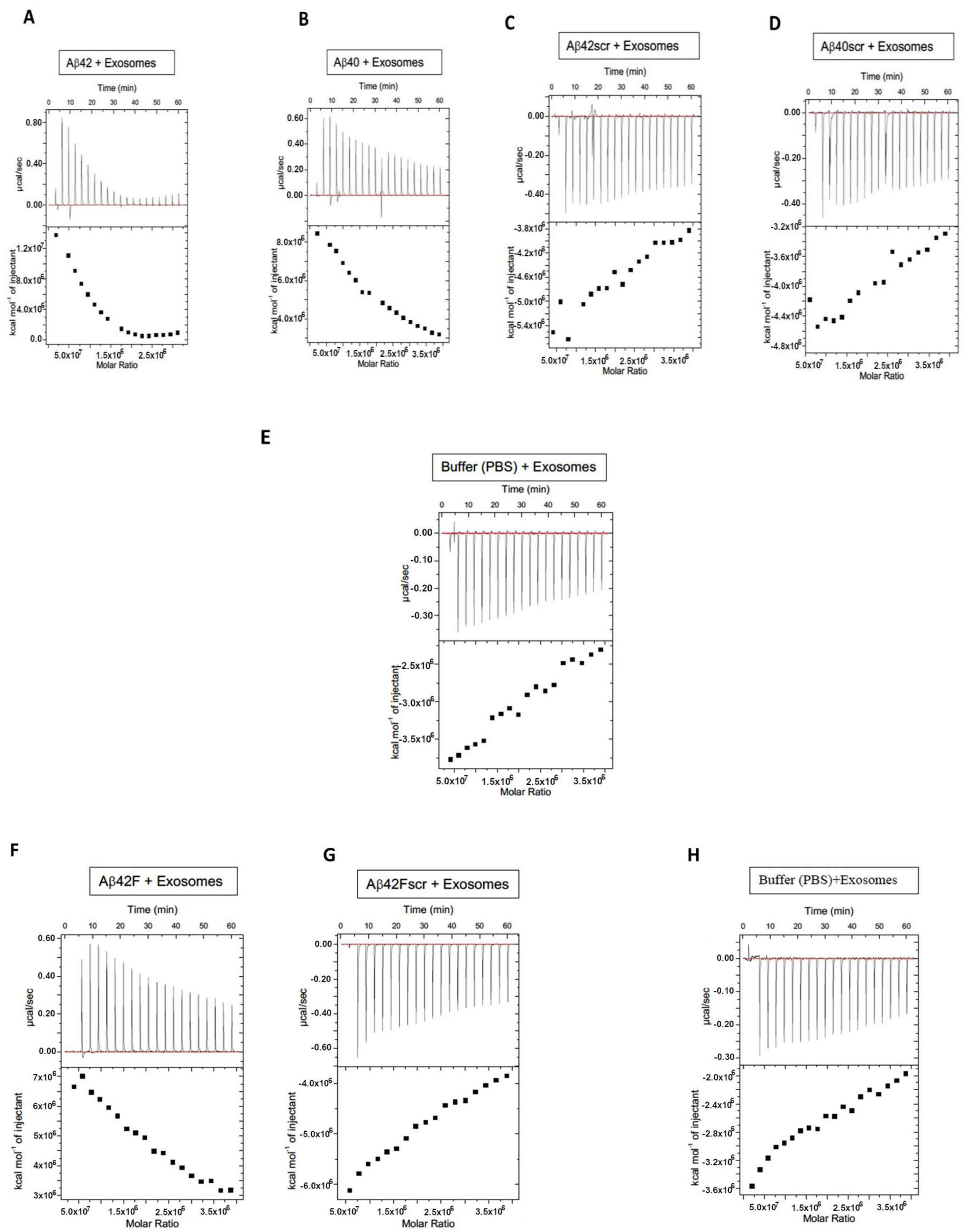

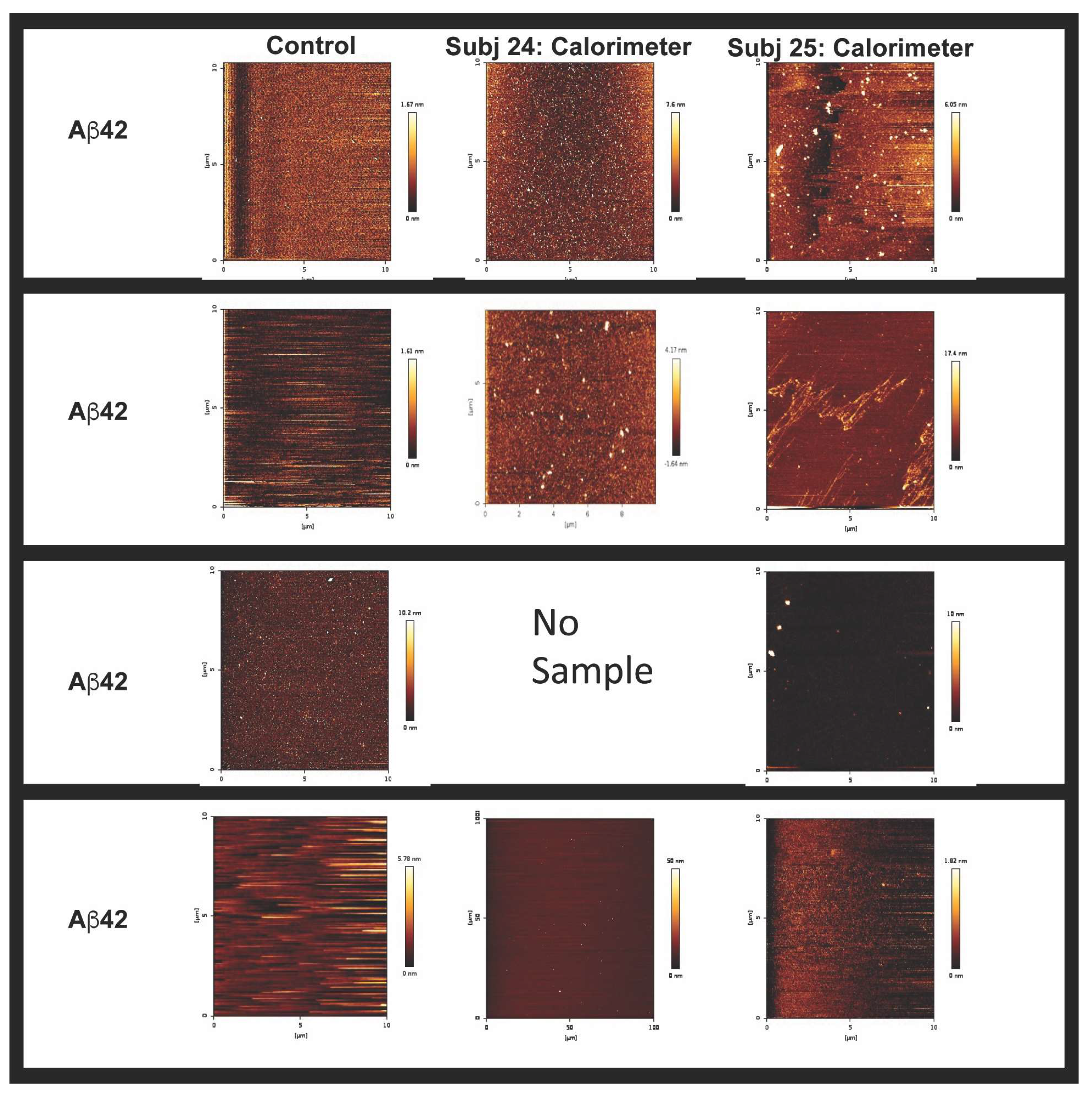
| Sample | Stock EVs (nM) | Concn EVs/mL | EVs First Injection (0.4 mL) | EVs Per Injection (19 × 2 mL) | Total EVs in ITC Assay (38.4 mL) |
|---|---|---|---|---|---|
| EVs Figure 2 | 3.85 | 0.231 × 1012 | 0.092 × 109 | 0.46 × 109 | 0.887 × 1010 |
| Ppt Subj 23 | 18.9 | 1.14 × 1012 | 0.46 × 109 | 2.3 × 109 | 4.38 × 1010 |
| Ppt Subj 24 | 18.3 | 1.1 × 1012 | 0.44 × 109 | 2.2 × 109 | 4.22 × 1010 |
| Ppt Subj 25 | 21.1 | 1.27 × 1012 | 0.51 × 109 | 2.55 × 109 | 4.88 × 1010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coughlan, C.; Lindenberger, J.; Jacot, J.G.; Johnson, N.R.; Anton, P.; Bevers, S.; Welty, R.; Graner, M.W.; Potter, H. Specific Binding of Alzheimer’s Aβ Peptides to Extracellular Vesicles. Int. J. Mol. Sci. 2024, 25, 3703. https://doi.org/10.3390/ijms25073703
Coughlan C, Lindenberger J, Jacot JG, Johnson NR, Anton P, Bevers S, Welty R, Graner MW, Potter H. Specific Binding of Alzheimer’s Aβ Peptides to Extracellular Vesicles. International Journal of Molecular Sciences. 2024; 25(7):3703. https://doi.org/10.3390/ijms25073703
Chicago/Turabian StyleCoughlan, Christina, Jared Lindenberger, Jeffrey G. Jacot, Noah R. Johnson, Paige Anton, Shaun Bevers, Robb Welty, Michael W. Graner, and Huntington Potter. 2024. "Specific Binding of Alzheimer’s Aβ Peptides to Extracellular Vesicles" International Journal of Molecular Sciences 25, no. 7: 3703. https://doi.org/10.3390/ijms25073703
APA StyleCoughlan, C., Lindenberger, J., Jacot, J. G., Johnson, N. R., Anton, P., Bevers, S., Welty, R., Graner, M. W., & Potter, H. (2024). Specific Binding of Alzheimer’s Aβ Peptides to Extracellular Vesicles. International Journal of Molecular Sciences, 25(7), 3703. https://doi.org/10.3390/ijms25073703







