Beyond Borders of the Cell: How Extracellular Vesicles Shape COVID-19 for People with Cystic Fibrosis
Abstract
:1. Introduction
2. The Origin and Role of Extracellular Vesicles (EVs)
3. The Role of EVs in the Pathogenesis of Inflammation
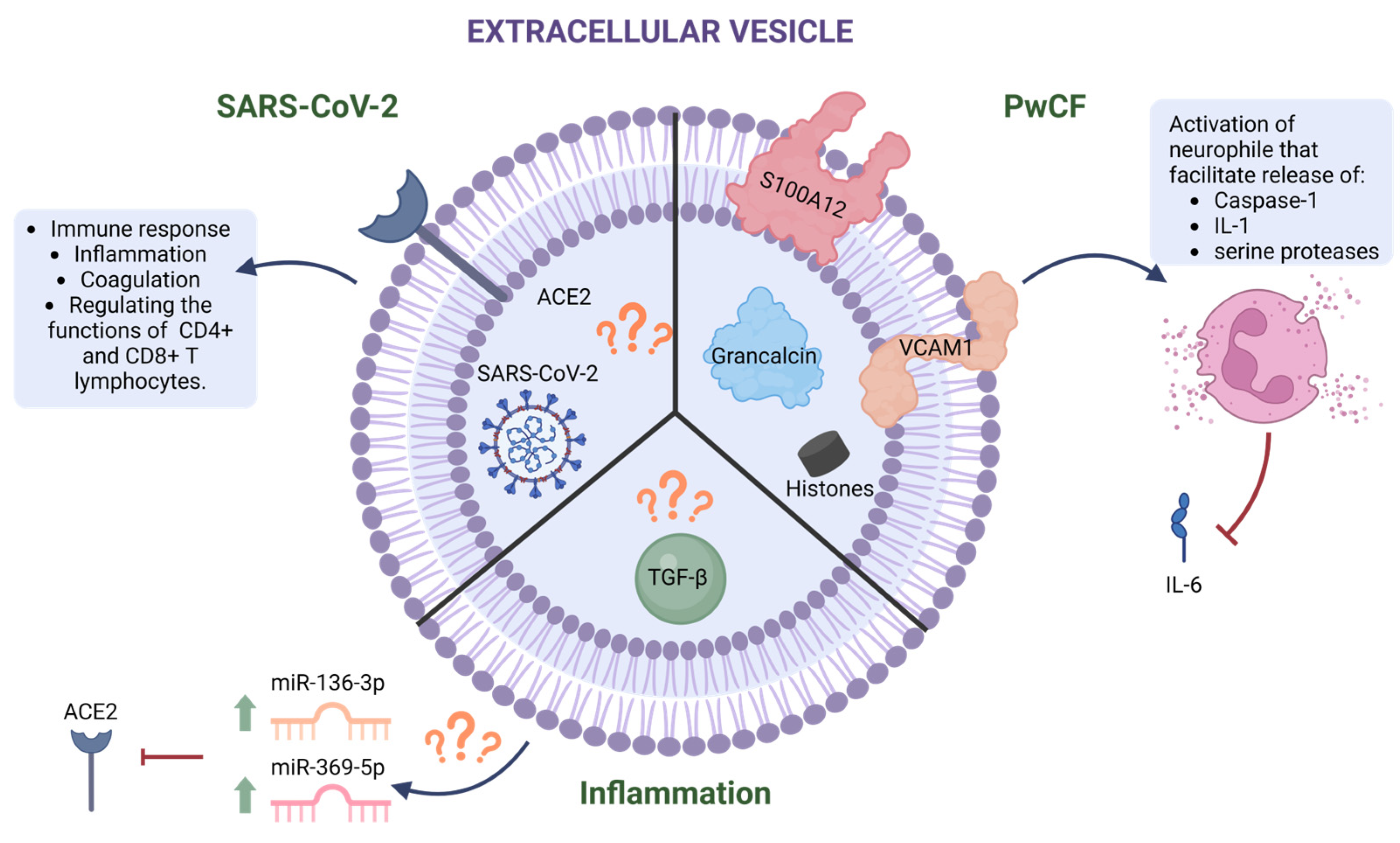
4. The Role of EVs in the Pathogenesis of CF
5. EVs As Mediators of SARS-CoV-2 Infection
6. The Role of EVs in TGF-β Signaling
7. Conclusions
8. Knowledge Gaps and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Number of COVID-19 Cases Reported to WHO. 2023. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 10 January 2023).
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhao, R.; Gao, L.J.; Gao, X.F.; Wang, D.P.; Cao, J.M. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell Infect. Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Iwata-Yoshikawa, N.; Okamura, T.; Shimizu, Y.; Hasegawa, H.; Takeda, M.; Nagata, N. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. J. Virol. 2019, 93, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M. Proteolytic activation of SARS-CoV-2 spike protein. Microbiol. Immunol. 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e1019. [Google Scholar] [CrossRef]
- Brevini, T.; Maes, M.; Webb, G.J.; John, B.V.; Fuchs, C.D.; Buescher, G.; Wang, L.; Griffiths, C.; Brown, M.L.; Scott, W.E.; et al. FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2. Nature 2023, 615, 134–142. [Google Scholar] [CrossRef]
- Hejenkowska, E.D.; Mitash, N.; Donovan, J.E.; Chandra, A.; Bertrand, C.; De Santi, C.; Greene, C.M.; Mu, F.; Swiatecka-Urban, A. TGF-β1 Inhibition of ACE2 Mediated by miRNA Uncovers Novel Mechanism of SARS-CoV-2 Pathogenesis. J. Innate Immun. 2023, 15, 629–646. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Penninger, J.M. Angiotensin-converting enzyme 2 in lung diseases. Curr. Opin. Pharmacol. 2006, 6, 271–276. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Starling, R.C.; Xanthopoulos, A.; Butler, J.; Boudoulas, H. The Counter Regulatory Axis of the Lung Renin-Angiotensin System in Severe COVID-19: Pathophysiology and Clinical Implications. Heart Lung Circ. 2021, 30, 786–794. [Google Scholar] [CrossRef]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef] [PubMed]
- de Dios Caballero, J.; Vida, R.; Cobo, M.; Máiz, L.; Suárez, L.; Galeano, J.; Baquero, F.; Cantón, R.; Del Campo, R. Individual Patterns of Complexity in Cystic Fibrosis Lung Microbiota, Including Predator Bacteria, over a 1-Year Period. mBio 2017, 8, e00959-17. [Google Scholar] [CrossRef] [PubMed]
- Cosgriff, R.; Ahern, S.; Bell, S.C.; Brownlee, K.; Burgel, P.R.; Byrnes, C.; Corvol, H.; Cheng, S.Y.; Elbert, A.; Faro, A.; et al. A multinational report to characterise SARS-CoV-2 infection in people with cystic fibrosis. J. Cyst. Fibros. 2020, 19, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Poli, P.; Timpano, S.; Goffredo, M.; Padoan, R.; Badolato, R. Asymptomatic case of COVID-19 in an infant with cystic fibrosis. J. Cyst. Fibros. 2020, 19, e18. [Google Scholar] [CrossRef] [PubMed]
- Jaudszus, A.; Pavlova, M.; Rasche, M.; Baier, M.; Moeser, A.; Lorenz, M. One year monitoring of SARS-CoV-2 prevalence in a German cohort of patients with cystic fibrosis. BMC Pulm. Med. 2022, 22, 101. [Google Scholar] [CrossRef] [PubMed]
- Bain, R.; Cosgriff, R.; Zampoli, M.; Elbert, A.; Burgel, P.R.; Carr, S.B.; Castaños, C.; Colombo, C.; Corvol, H.; Faro, A.; et al. Clinical characteristics of SARS-CoV-2 infection in children with cystic fibrosis: An international observational study. J. Cyst. Fibros. 2021, 20, 25–30. [Google Scholar] [CrossRef]
- Colombo, C.; Burgel, P.R.; Gartner, S.; van Koningsbruggen-Rietschel, S.; Naehrlich, L.; Sermet-Gaudelus, I.; Southern, K.W. Impact of COVID-19 on people with cystic fibrosis. Lancet Respir. Med. 2020, 8, e35–e36. [Google Scholar] [CrossRef]
- Bezzerri, V.; Lucca, F.; Volpi, S.; Cipolli, M. Does cystic fibrosis constitute an advantage in COVID-19 infection? Ital. J. Pediatr. 2020, 46, 143. [Google Scholar] [CrossRef]
- McGreal, E.P.; Davies, P.L.; Powell, W.; Rose-John, S.; Spiller, O.B.; Doull, I.; Jones, S.A.; Kotecha, S. Inactivation of IL-6 and soluble IL-6 receptor by neutrophil derived serine proteases in cystic fibrosis. Biochim. Biophys. Acta 2010, 1802, 649–658. [Google Scholar] [CrossRef]
- Oldenburg, C.E.; Pinsky, B.A.; Brogdon, J.; Chen, C.; Ruder, K.; Zhong, L.; Nyatigo, F.; Cook, C.A.; Hinterwirth, A.; Lebas, E.; et al. Effect of Oral Azithromycin vs. Placebo on COVID-19 Symptoms in Outpatients with SARS-CoV-2 Infection: A Randomized Clinical Trial. JAMA 2021, 326, 490–498. [Google Scholar] [CrossRef]
- Lotti, V.; Merigo, F.; Lagni, A.; Di Clemente, A.; Ligozzi, M.; Bernardi, P.; Rossini, G.; Concia, E.; Plebani, R.; Romano, M.; et al. CFTR Modulation Reduces SARS-CoV-2 Infection in Human Bronchial Epithelial Cells. Cells 2022, 11, 1347. [Google Scholar] [CrossRef]
- Carr, S.B.; McClenaghan, E.; Elbert, A.; Faro, A.; Cosgriff, R.; Abdrakhmanov, O.; Brownlee, K.; Burgel, P.-R.; Byrnes, C.A.; Cheng, S.Y.; et al. Factors associated with clinical progression to severe COVID-19 in people with cystic fibrosis: A global observational study. J. Cyst. Fibros. 2022, 21, e221–e231. [Google Scholar] [CrossRef]
- Naehrlich, L.; Orenti, A.; Dunlevy, F.; Kasmi, I.; Harutyunyan, S.; Pfleger, A.; Keegan, S.; Daneau, G.; Petrova, G.; Tješić-Drinković, D.; et al. Incidence of SARS-CoV-2 in people with cystic fibrosis in Europe between February and June 2020. J. Cyst. Fibros. 2021, 20, 566–577. [Google Scholar] [CrossRef]
- Marques, L.S.; Boschiero, M.N.; Sansone, N.M.S.; Brienze, L.R.; Marson, F.A.L. Epidemiological Profile of Hospitalized Patients with Cystic Fibrosis in Brazil Due to Severe Acute Respiratory Infection during the COVID-19 Pandemic and a Systematic Review of Worldwide COVID-19 in Those with Cystic Fibrosis. Healthcare 2023, 11, 1936. [Google Scholar] [CrossRef]
- Lagni, A.; Lotti, V.; Diani, E.; Rossini, G.; Concia, E.; Sorio, C.; Gibellini, D. CFTR Inhibitors Display In Vitro Antiviral Activity against SARS-CoV-2. Cells 2023, 12, 776. [Google Scholar] [CrossRef]
- Cui, G.; Khazanov, N.; Stauffer, B.B.; Infield, D.T.; Imhoff, B.R.; Senderowitz, H.; McCarty, N.A. Potentiators exert distinct effects on human, murine, and Xenopus CFTR. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L192–L207. [Google Scholar] [CrossRef]
- Bezzerri, V.; Gentili, V.; Api, M.; Finotti, A.; Papi, C.; Tamanini, A.; Boni, C.; Baldisseri, E.; Olioso, D.; Duca, M.; et al. SARS-CoV-2 viral entry and replication is impaired in Cystic Fibrosis airways due to ACE2 downregulation. Nat. Commun. 2023, 14, 132. [Google Scholar] [CrossRef]
- Chen, L.; Guan, W.-J.; Qiu, Z.-E.; Xu, J.-B.; Bai, X.; Hou, X.-C.; Sun, J.; Qu, S.; Huang, Z.-X.; Lei, T.-L.; et al. SARS-CoV-2 nucleocapsid protein triggers hyperinflammation via protein-protein interaction-mediated intracellular Cl− accumulation in respiratory epithelium. Signal Transduct. Target. Ther. 2022, 7, 255. [Google Scholar] [CrossRef]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.W.; Savani, R.C.; Zaki, H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. eLife 2021, 10, e68563. [Google Scholar] [CrossRef]
- Chen, Q.; Langenbach, S.; Li, M.; Xia, Y.C.; Gao, X.; Gartner, M.J.; Pharo, E.A.; Williams, S.M.; Todd, S.; Clarke, N.; et al. ACE2 Expression in Organotypic Human Airway Epithelial Cultures and Airway Biopsies. Front. Pharmacol. 2022, 13, 813087. [Google Scholar] [CrossRef]
- Hou, Y.J.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H., 3rd; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182, 429–446.e414. [Google Scholar] [CrossRef]
- Stanton, B.A.; Hampton, T.H.; Ashare, A. SARS-CoV-2 (COVID-19) and cystic fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L408–L415. [Google Scholar] [CrossRef]
- Xia, X.; Yuan, P.; Liu, Y.; Wang, Y.; Cao, W.; Zheng, J.C. Emerging roles of extracellular vesicles in COVID-19, a double-edged sword? Immunology 2021, 163, 416–430. [Google Scholar] [CrossRef]
- Useckaite, Z.; Ward, M.P.; Trappe, A.; Reilly, R.; Lennon, J.; Davage, H.; Matallanas, D.; Cassidy, H.; Dillon, E.T.; Brennan, K.; et al. Increased extracellular vesicles mediate inflammatory signalling in cystic fibrosis. Thorax 2020, 75, 449–458. [Google Scholar] [CrossRef]
- Ohayon, L.; Zhang, X.; Dutta, P. The role of extracellular vesicles in regulating local and systemic inflammation in cardiovascular disease. Pharmacol. Res. 2021, 170, 105692. [Google Scholar] [CrossRef]
- Moulin, C.; Crupi, M.J.F.; Ilkow, C.S.; Bell, J.C.; Boulton, S. Extracellular Vesicles and Viruses: Two Intertwined Entities. Int. J. Mol. Sci. 2023, 24, 1036. [Google Scholar] [CrossRef]
- Miura, T.A. Respiratory epithelial cells as master communicators during viral infections. Curr. Clin. Microbiol. Rep. 2019, 6, 10–17. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, L.; Lizarraga, R.; Chen, Y. Human Airway Epithelial Cells Direct Significant Rhinovirus Replication in Monocytic Cells by Enhancing ICAM1 Expression. Am. J. Respir. Cell Mol. Biol. 2017, 57, 216–225. [Google Scholar] [CrossRef]
- Maemura, T.; Fukuyama, S.; Kawaoka, Y. High Levels of miR-483-3p Are Present in Serum Exosomes Upon Infection of Mice With Highly Pathogenic Avian Influenza Virus. Front. Microbiol. 2020, 11, 144. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Stathatos, I.; Koumandou, V.L. Comparative Analysis of Prokaryotic Extracellular Vesicle Proteins and Their Targeting Signals. Microorganisms 2023, 11, 1977. [Google Scholar] [CrossRef]
- Chargaff, E. Cell Structure and the Problem of Blood Coagulation. J. Biol. Chem. 1945, 160, 351–359. [Google Scholar] [CrossRef]
- Chargaff, E.; West, R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [CrossRef]
- Wolf, P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Crawford, N. The Presence of Contractile Proteins in Platelet Microparticles Isolated from Human and Animal Platelet-free Plasma. Br. J. Haematol. 1971, 21, 53–69. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Bianchini, A.; Teng, K. Reticulocyte maturation and exosome release: Transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood 1989, 74, 1844–1851. [Google Scholar] [CrossRef]
- Johnstone, R.M. Maturation of reticulocytes: Formation of exosomes as a mechanism for shedding membrane proteins. Biochem. Cell Biol. 1992, 70, 179–190. [Google Scholar] [CrossRef]
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 538. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Couch, Y.; Buzàs, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C.; et al. A brief history of nearly EV-erything—The rise and rise of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef] [PubMed]
- Asleh, K.; Dery, V.; Taylor, C.; Davey, M.; Djeungoue-Petga, M.-A.; Ouellette, R.J. Extracellular vesicle-based liquid biopsy biomarkers and their application in precision immuno-oncology. Biomark. Res. 2023, 11, 99. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Neven, K.Y.; Nawrot, T.S.; Bollati, V. Extracellular Vesicles: How the External and Internal Environment Can Shape Cell-To-Cell Communication. Curr. Environ. Health Rep. 2017, 4, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Huang, G.; Yi, Z.; Dai, S.; Zhuang, W.; Guo, S. Advances in extracellular vesicle-based combination therapies for spinal cord injury. Neural Regen. Res. 2024, 19, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. BioScience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Théry, C.; Boussac, M.; Véron, P.; Ricciardi-Castagnoli, P.; Raposo, G.A.; Garin, J.M.; Amigorena, S. Proteomic Analysis of Dendritic Cell-Derived Exosomes: A Secreted Subcellular Compartment Distinct from Apoptotic Vesicles1. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef] [PubMed]
- El Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, S.; Daniello, V.; Conese, M. Extracellular Vesicles’ Role in the Pathophysiology and as Biomarkers in Cystic Fibrosis and COPD. Int. J. Mol. Sci. 2022, 24, 228. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.G.; Cao, Y.; Yang, J.; Lee, J.H.; Choi, H.S.; Jin, Y. Lung epithelial cell-derived extracellular vesicles activate macrophage-mediated inflammatory responses via ROCK1 pathway. Cell Death Dis. 2015, 6, e2016. [Google Scholar] [CrossRef] [PubMed]
- Trappe, A.; Donnelly, S.C.; McNally, P.; Coppinger, J.A. Role of extracellular vesicles in chronic lung disease. Thorax 2021, 76, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Zhang, D.; Laskin, D.L.; Jin, Y. Functional Evidence of Pulmonary Extracellular Vesicles in Infectious and Noninfectious Lung Inflammation. J. Immunol. 2018, 201, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Junior, D.M.; Tsirigoti, C.; Lim, S.K.; Heldin, C.-H.; Moustakas, A. Extracellular Vesicles and Transforming Growth Factor β Signaling in Cancer. Front. Cell Dev. Biol. 2022, 10, 849938. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, W.; Ai, X.; Kilic, E.; Hermann, D.M.; Venkataramani, V.; Bähr, M.; Doeppner, T.R. Extracellular vesicles from hypoxia-preconditioned microglia promote angiogenesis and repress apoptosis in stroke mice via the TGF-β/Smad2/3 pathway. Cell Death Dis. 2021, 12, 1068. [Google Scholar] [CrossRef]
- Leyfman, Y.; Gohring, G.; Joshi, M.; Menon, G.P.; Van de Kieft, A.; Rivero, T.D.; Bellio, M.A.; Mitrani, M.I. Extracellular vesicles: A promising therapy against SARS-CoV-2 infection. Mol. Ther. 2023, 31, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Dogrammatzis, C.; Waisner, H.; Kalamvoki, M. Cloaked Viruses and Viral Factors in Cutting Edge Exosome-Based Therapies. Front. Cell Dev. Biol. 2020, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Näslund, T.I.; Paquin-Proulx, D.; Paredes, P.T.; Vallhov, H.; Sandberg, J.K.; Gabrielsson, S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS 2014, 28, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Caobi, A.; Nair, M.; Raymond, A.D. Extracellular Vesicles in the Pathogenesis of Viral Infections in Humans. Viruses 2020, 12, 1200. [Google Scholar] [CrossRef]
- Johnson, B.L.; Midura, E.F.; Prakash, P.S.; Rice, T.C.; Kunz, N.; Kalies, K.; Caldwell, C.C. Neutrophil derived microparticles increase mortality and the counter-inflammatory response in a murine model of sepsis. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017, 1863, 2554–2563. [Google Scholar] [CrossRef] [PubMed]
- Rollet-Cohen, V.; Bourderioux, M.; Lipecka, J.; Chhuon, C.; Jung, V.A.; Mesbahi, M.; Nguyen-Khoa, T.; Guérin-Pfyffer, S.; Schmitt, A.; Edelman, A.; et al. Comparative proteomics of respiratory exosomes in cystic fibrosis, primary ciliary dyskinesia and asthma. J. Proteom. 2018, 185, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Xiang, M.; Xu, M.; Lu, P.; Wang, S.; Zou, Y.; Qiao, K.; Jin, C.; Li, Y.; Lu, M.; et al. VCAM-1-mediated neutrophil infiltration exacerbates ambient fine particle-induced lung injury. Toxicol. Lett. 2019, 302, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Meijer, B.; Gearry, R.B.; Day, A.S. The role of S100A12 as a systemic marker of inflammation. Int. J. Inflam. 2012, 2012, 907078. [Google Scholar] [CrossRef] [PubMed]
- Boyhan, A.; Casimir, C.M.; French, J.K.; Teahan, C.G.; Segal, A.W. Molecular cloning and characterization of grancalcin, a novel EF-hand calcium-binding protein abundant in neutrophils and monocytes. J. Biol. Chem. 1992, 267, 2928–2933. [Google Scholar] [CrossRef]
- Forrest, O.A.; Dobosh, B.; Ingersoll, S.A.; Rao, S.; Rojas, A.; Laval, J.; Alvarez, J.A.; Brown, M.R.; Tangpricha, V.; Tirouvanziam, R. Neutrophil-derived extracellular vesicles promote feed-forward inflammasome signaling in cystic fibrosis airways. J. Leukoc. Biol. 2022, 112, 707–716. [Google Scholar] [CrossRef]
- Shaba, E.; Landi, C.; Carleo, A.; Vantaggiato, L.; Paccagnini, E.; Gentile, M.; Bianchi, L.; Lupetti, P.; Bargagli, E.; Prasse, A.; et al. Proteome Characterization of BALF Extracellular Vesicles in Idiopathic Pulmonary Fibrosis: Unveiling Undercover Molecular Pathways. Int. J. Mol. Sci. 2021, 22, 5696. [Google Scholar] [CrossRef]
- Palviainen, M.; Saari, H.; Kärkkäinen, O.; Pekkinen, J.; Auriola, S.; Yliperttula, M.; Puhka, M.; Hanhineva, K.; Siljander, P.R.M. Metabolic signature of extracellular vesicles depends on the cell culture conditions. J. Extracell. Vesicles 2019, 8, 1596669. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Maremanda, K.P.; Campos, M.; Chand, H.S.; Li, F.; Hirani, N.; Haseeb, M.A.; Li, D.; Rahman, I. Distinct Exosomal miRNA Profiles from BALF and Lung Tissue of COPD and IPF Patients. Int. J. Mol. Sci. 2021, 22, 11830. [Google Scholar] [CrossRef] [PubMed]
- Krishnamachary, B.; Cook, C.; Kumar, A.; Spikes, L.; Chalise, P.; Dhillon, N.K. Extracellular vesicle-mediated endothelial apoptosis and EV-associated proteins correlate with COVID-19 disease severity. J. Extracell. Vesicles 2021, 10, e12117. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Prasad, N.; Kushwaha, R.S.; Patel, M.R.; Bhadauria, D.; Kaul, A. Higher pro-inflammatory cytokines IL-6 and IFN-γ are associated with anti-SARS-CoV-2 spike protein-specific seroconversion in renal allograft recipients. Transplant. Infect. Dis. 2023, 25, e14133. [Google Scholar] [CrossRef] [PubMed]
- Rose, V.D. Mechanisms and markers of airway inflammation in cystic fibrosis. Eur. Respir. J. 2002, 19, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Graterol Torres, F.; Molina, M.; Soler-Majoral, J.; Romero-González, G.; Rodríguez Chitiva, N.; Troya-Saborido, M.; Socias Rullan, G.; Burgos, E.; Paúl Martínez, J.; Urrutia Jou, M.; et al. Evolving Concepts on Inflammatory Biomarkers and Malnutrition in Chronic Kidney Disease. Nutrients 2022, 14, 4297. [Google Scholar] [CrossRef] [PubMed]
- Puhm, F.; Allaeys, I.; Lacasse, E.; Dubuc, I.; Galipeau, Y.; Zaid, Y.; Khalki, L.; Belleannée, C.; Durocher, Y.; Brisson, A.R.; et al. Platelet activation by SARS-CoV-2 implicates the release of active tissue factor by infected cells. Blood Adv. 2022, 6, 3593–3605. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Nukala, S.B.; Srivastava, S.; Miyamoto, H.; Ismail, N.I.; Jousma, J.; Rehman, J.; Ong, S.-B.; Lee, W.H.; Ong, S.-G. Detection of viral RNA fragments in human iPSC cardiomyocytes following treatment with extracellular vesicles from SARS-CoV-2 coding sequence overexpressing lung epithelial cells. Stem Cell Res. Ther. 2020, 11, 514. [Google Scholar] [CrossRef]
- Xia, B.; Pan, X.; Luo, R.-H.; Shen, X.; Li, S.; Wang, Y.; Zuo, X.; Wu, Y.; Guo, Y.; Xiao, G.; et al. Extracellular vesicles mediate antibody-resistant transmission of SARS-CoV-2. Cell Discov. 2023, 9, 2. [Google Scholar] [CrossRef]
- Hernández-Díazcouder, A.; Díaz-Godínez, C.; Carrero, J.C. Extracellular vesicles in COVID-19 prognosis, treatment, and vaccination: An update. Appl. Microbiol. Biotechnol. 2023, 107, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N.; Antoniak, S.; Wolberg, A.S.; Kasthuri, R.; Key, N.S. Coagulation Abnormalities and Thrombosis in Patients Infected With SARS-CoV-2 and Other Pandemic Viruses. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Barberis, E.; Vanella, V.V.; Falasca, M.; Caneapero, V.; Cappellano, G.; Raineri, D.; Ghirimoldi, M.; De Giorgis, V.; Puricelli, C.; Vaschetto, R.; et al. Circulating Exosomes Are Strongly Involved in SARS-CoV-2 Infection. Front. Mol. Biosci. 2021, 8, 632290. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, M.; Bansal, S.; Ravichandran, R.; Sharma, M.; Perincheri, S.; Rodriguez, F.; Hachem, R.; Fisher, C.E.; Limaye, A.P.; Omar, A.; et al. Respiratory viral infection in lung transplantation induces exosomes that trigger chronic rejection. J. Heart Lung Transplant. 2020, 39, 379–388. [Google Scholar] [CrossRef] [PubMed]
- George, M.S.; Sanchez, J.; Rollings, C.; Fear, D.; Irving, P.; Sinclair, L.V.; Schurich, A. Extracellular vesicles in COVID-19 convalescence can regulate T cell metabolism and function. iScience 2023, 26, 107280. [Google Scholar] [CrossRef] [PubMed]
- El-Shennawy, L.; Hoffmann, A.D.; Dashzeveg, N.K.; McAndrews, K.M.; Mehl, P.J.; Cornish, D.; Yu, Z.; Tokars, V.L.; Nicolaescu, V.; Tomatsidou, A.; et al. Circulating ACE2-expressing extracellular vesicles block broad strains of SARS-CoV-2. Nat. Commun. 2022, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Cho, J.; Kim, E.; Kim, J.; Yang, J.-S.; Kim, K.-C.; Lee, J.-Y.; Shin, Y.; Palomera, L.F.; Park, J.; et al. Engineered small extracellular vesicles displaying ACE2 variants on the surface protect against SARS-CoV-2 infection. J. Extracell. Vesicles 2022, 11, e12179. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, S.; Bihl, J. Exosome-Mediated Transfer of ACE2 (Angiotensin-Converting Enzyme 2) from Endothelial Progenitor Cells Promotes Survival and Function of Endothelial Cell. Oxid. Med. Cell Longev. 2020, 2020, 4213541. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Ferreira-Gomes, M.; Kruglov, A.; Durek, P.; Heinrich, F.; Tizian, C.; Heinz, G.A.; Pascual-Reguant, A.; Du, W.; Mothes, R.; Fan, C.; et al. SARS-CoV-2 in severe COVID-19 induces a TGF-β-dominated chronic immune response that does not target itself. Nat. Commun. 2021, 12, 1961. [Google Scholar] [CrossRef]
- Chu, H.W.; Trudeau, J.B.; Balzar, S.; Wenzel, S.E. Peripheral blood and airway tissue expression of transforming growth factor beta by neutrophils in asthmatic subjects and normal control subjects. J. Allergy Clin. Immunol. 2000, 106, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Lienesch, D.W.; Zhou, M.; Bommireddy, R.; Azhar, M.; Doetschman, T.; Singh, R.R. Dual roles of immunoregulatory cytokine TGF-beta in the pathogenesis of autoimmunity-mediated organ damage. J. Immunol. 2008, 180, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Buttgereit, A.; Lelios, I.; Utz, S.G.; Cansever, D.; Becher, B.; Greter, M. The Cytokine TGF-β Promotes the Development and Homeostasis of Alveolar Macrophages. Immunity 2017, 47, 903–912.e904. [Google Scholar] [CrossRef] [PubMed]
- Drumm, M.L.; Konstan, M.W.; Schluchter, M.D.; Handler, A.; Pace, R.; Zou, F.; Zariwala, M.; Fargo, D.; Xu, A.; Dunn, J.M.; et al. Genetic modifiers of lung disease in cystic fibrosis. N. Engl. J. Med. 2005, 353, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Brazova, J.; Sismova, K.; Vavrova, V.; Bartosova, J.; Macek, M., Jr.; Lauschman, H.; Sediva, A. Polymorphisms of TGF-beta1 in cystic fibrosis patients. Clin. Immunol. 2006, 121, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Trojan, T.; Alejandre Alcazar, M.A.; Fink, G.; Thomassen, J.C.; Maessenhausen, M.V.; Rietschel, E.; Schneider, P.M.; van Koningsbruggen-Rietschel, S. The effect of TGF-β1 polymorphisms on pulmonary disease progression in patients with cystic fibrosis. BMC Pulm. Med. 2022, 22, 183. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Fujiwara, K.; Takahashi, K.; Yoshioka, Y.; Ochiya, T.; Podyma-Inoue, K.A.; Watabe, T. Transforming growth factor-β-induced secretion of extracellular vesicles from oral cancer cells evokes endothelial barrier instability via endothelial-mesenchymal transition. Inflamm. Regen. 2022, 42, 38. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Jung, M.Y.; Choudhury, M.; Leof, E.B. Transforming growth factor beta induces fibroblasts to express and release the immunomodulatory protein PD-L1 into extracellular vesicles. FASEB J. 2020, 34, 2213–2226. [Google Scholar] [CrossRef]
- Lotti, V.; Lagni, A.; Diani, E.; Sorio, C.; Gibellini, D. Crosslink between SARS-CoV-2 replication and cystic fibrosis hallmarks. Front. Microbiol. 2023, 14, 1162470. [Google Scholar] [CrossRef]
- Sun, R.; Cai, Y.; Zhou, Y.; Bai, G.; Zhu, A.; Kong, P.; Sun, J.; Li, Y.; Liu, Y.; Liao, W.; et al. Proteomic profiling of single extracellular vesicles reveals colocalization of SARS-CoV-2 with a CD81/integrin-rich EV subpopulation in sputum from COVID-19 severe patients. Front. Immunol. 2023, 14, 1052141. [Google Scholar] [CrossRef]
| Controls | PwCF |
|---|---|
| BALF: HSP70; HSP90 [82] | BALF: HSP70; HSP90 [82] |
| BALF: MHC-I MHC-II [82] | BALF: MHC-I MHC-II [82] |
| Cells: Metabolites [83] BALF: miR-122-5p; miR-423-5p; miR-375-3p; miR-200a-3p; miR-200b-3p; miR-141-3p [82,84] | Cells: VCAM1 [38] Cells: S100A12 [38] BALF: Grancalcin [77] BALF: Histones [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hejenkowska, E.D.; Yavuz, H.; Swiatecka-Urban, A. Beyond Borders of the Cell: How Extracellular Vesicles Shape COVID-19 for People with Cystic Fibrosis. Int. J. Mol. Sci. 2024, 25, 3713. https://doi.org/10.3390/ijms25073713
Hejenkowska ED, Yavuz H, Swiatecka-Urban A. Beyond Borders of the Cell: How Extracellular Vesicles Shape COVID-19 for People with Cystic Fibrosis. International Journal of Molecular Sciences. 2024; 25(7):3713. https://doi.org/10.3390/ijms25073713
Chicago/Turabian StyleHejenkowska, Ewelina D., Hayrettin Yavuz, and Agnieszka Swiatecka-Urban. 2024. "Beyond Borders of the Cell: How Extracellular Vesicles Shape COVID-19 for People with Cystic Fibrosis" International Journal of Molecular Sciences 25, no. 7: 3713. https://doi.org/10.3390/ijms25073713
APA StyleHejenkowska, E. D., Yavuz, H., & Swiatecka-Urban, A. (2024). Beyond Borders of the Cell: How Extracellular Vesicles Shape COVID-19 for People with Cystic Fibrosis. International Journal of Molecular Sciences, 25(7), 3713. https://doi.org/10.3390/ijms25073713







