Hypervolemia in Dialysis Patients Impairs STAT3 Signaling and Upregulates miR-142-3p: Effects on IL-10 and IL-6
Abstract
1. Introduction
2. Results
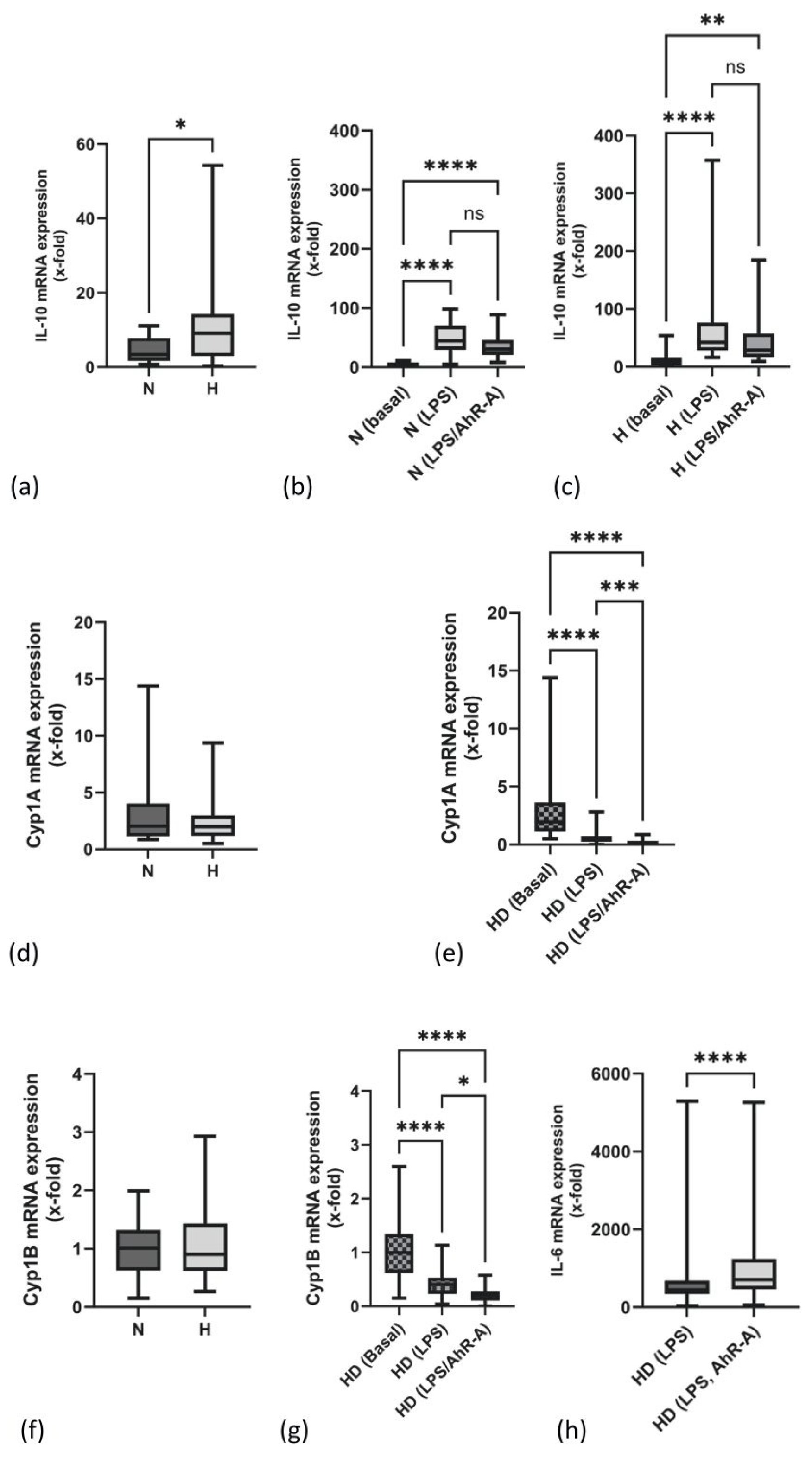
2.1. mRNA Expression of IL-10 Is Elevated in PBMCs of H Patients
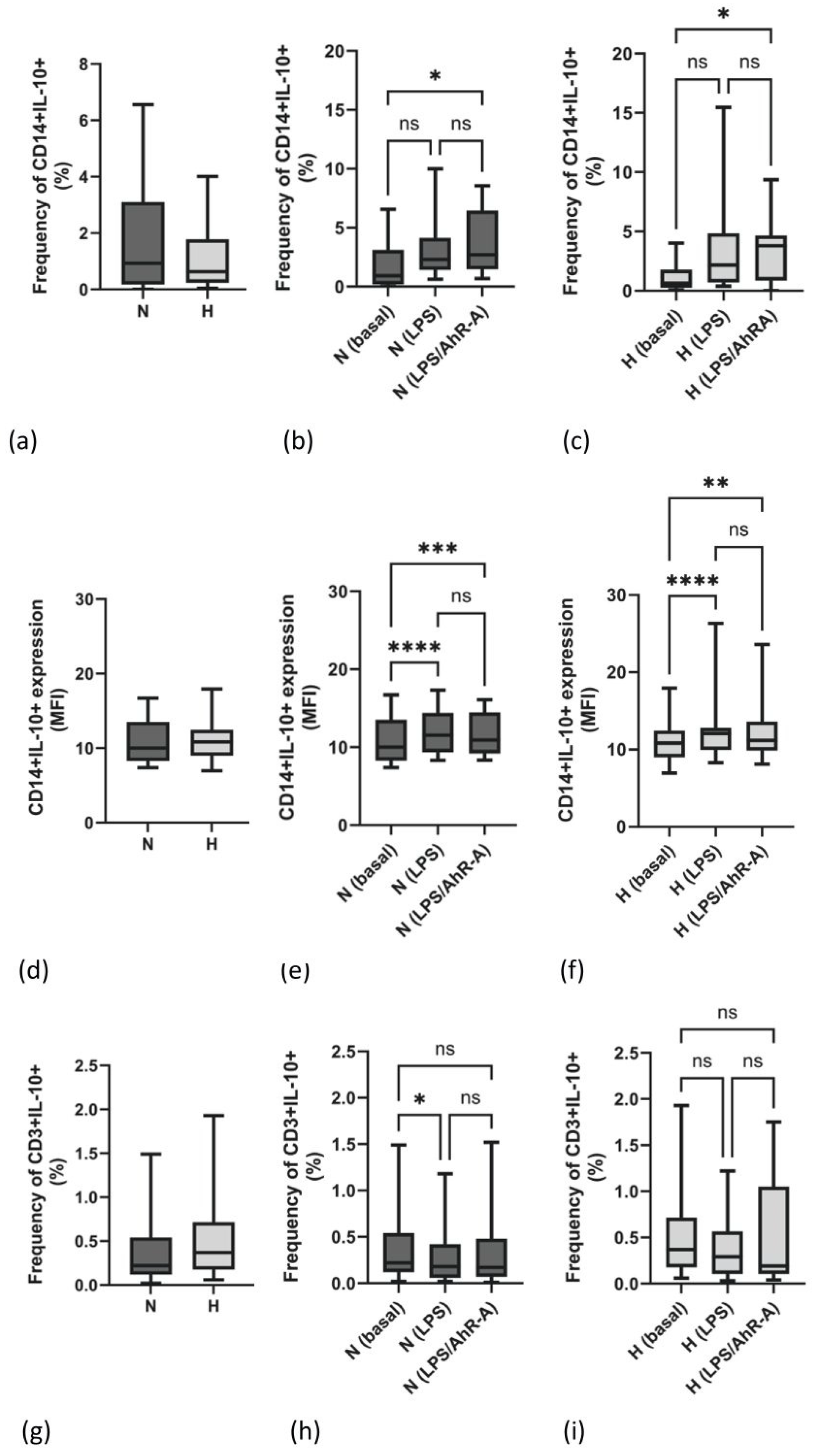
2.2. Frequency and Density Expression of IL-10 Are Not Different between N and H Patients
2.3. The Frequency of p4E-PB1-Producing Immune Cells Is Not Different in N and H Patients
2.4. Lower Monocytic STAT3 (pS727) Activation in Hypervolemia
2.5. Hypervolemia Induces SOCS3 but Not SOCS1 mRNA Expression
2.6. miR-142-3p Expression Is Elevated in H Patients
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Study Population
4.2. PBMC Isolation
4.3. RNA/cDNA/qPCR
4.4. Analysis of IL-10 Secretion by Flow Cytometry
4.5. Analysis of STAT3 (pY705) and STAT3 (pS727) by Phospho-Flow Cytometry
4.6. Analysis of Phospho-4E-BP1 by Phospho-Flow Cytometry
4.7. miR-142-3p/5p Analysis
4.8. Cytokine Analysis
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dekker, M.J.E.; Marcelli, D.; Canaud, B.; Konings, C.J.A.M.; Leunissen, K.M.; Levin, N.W.; Carioni, P.; Maheshwari, V.; Raimann, J.G.; van der Sande, F.M.; et al. Unraveling the relationship between mortality, hyponatremia, inflammation and malnutrition in hemodialysis patients: Results from the international MONDO initiative. Eur. J. Clin. Nutr. 2016, 70, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Bahamonde, J.; Raimann, J.G.; Thijssen, S.; Levin, N.W.; Kotanko, P. Fluid overload and inflammation—A vicious cycle. Semin. Dial. 2013, 26, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.J.E.; Konings, C.; Canaud, B.; van der Sande, F.M.; Stuard, S.; Raimann, J.G.; Öztürk, E.; Usvyat, L.; Kotanko, P.; Kooman, J.P. Interactions between Malnutrition, Inflammation, and Fluid Overload and Their Associations with Survival in Prevalent Hemodialysis Patients. J. Ren. Nutr. 2018, 28, 435–444. [Google Scholar] [CrossRef]
- Ulrich, C.; Wilke, A.; Schleicher, N.; Girndt, M.; Fiedler, R. Hypervolemia-Induced Immune Disturbances Do Not Involve IL-1ß but IL-6 and IL-10 Activation in Haemodialysis Patients. Toxins 2020, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Batra, G.; Ghukasyan Lakic, T.; Lindbäck, J.; Held, C.; White, H.D.; Stewart, R.A.H.; Koenig, W.; Cannon, C.P.; Budaj, A.; Hagström, E.; et al. Interleukin 6 and Cardiovascular Outcomes in Patients With Chronic Kidney Disease and Chronic Coronary Syndrome. JAMA Cardiol. 2021, 6, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.O.; Duarte, R.; Dix-Peek, T.; Vachiat, A.; Dickens, C.; Grinter, S.; Naidoo, S.; Manga, P.; Naicker, S. Volume overload and its risk factors in South African chronic kidney disease patients: An appraisal of bioimpedance spectroscopy and inferior vena cava measurements. Clin. Nephrol. 2016, 86, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, W.; Yu, H.; Zhang, Y.; Dai, Y.; Ning, C.; Tao, L.; Sun, H.; Kellems, R.E.; Blackburn, M.R.; et al. Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension 2012, 59, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Iturbe, B.; Pons, H.; Johnson, R.J. Role of the Immune System in Hypertension. Physiol. Rev. 2017, 97, 1127–1164. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, H.; Wang, P.; Wang, J.; Zou, L. The roles of SOCS3 and STAT3 in bacterial infection and inflammatory diseases. Scand. J. Immunol. 2018, 88, e12727. [Google Scholar] [CrossRef]
- Wen, Z.; Zhong, Z.; Darnell, J.E. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 1995, 82, 241–250. [Google Scholar] [CrossRef]
- Dimri, S.; Malhotra, R.; Shet, T.; Mokal, S.; Gupta, S.; De, A. Noncanonical pS727 post translational modification dictates major STAT3 activation and downstream functions in breast cancer. Exp. Cell Res. 2020, 396, 112313. [Google Scholar] [CrossRef]
- Balic, J.J.; White, C.L.; Dawson, R.; Gough, D.; McCormack, M.P.; Jenkins, B.J. STAT3-driven hematopoiesis and lymphopoiesis abnormalities are dependent on serine phosphorylation. Cytokine 2020, 130, 155059. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chen, D.; Tarcsafalvi, A.; Su, R.; Qin, L.; Bromberg, J.S. Suppressor of cytokine signaling 1 inhibits IL-10-mediated immune responses. J. Immunol. 2003, 170, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Poitevin, S.; Sallée, M.; Addi, T.; Gondouin, B.; McKay, N.; Denison, M.S.; Jourde-Chiche, N.; Duval-Sabatier, A.; Cerini, C.; et al. Aryl hydrocarbon receptor is activated in patients and mice with chronic kidney disease. Kidney Int. 2018, 93, 986–999. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Luo, L.; Tian, L.; Yin, S.; Ma, X.; Cheng, S.; Tang, W.; Yu, J.; Ma, W.; Zhou, X.; et al. Aryl Hydrocarbon Receptor Promotes IL-10 Expression in Inflammatory Macrophages Through Src-STAT3 Signaling Pathway. Front. Immunol. 2018, 9, 2033. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.J.; Hellen, C.U.T.; Pestova, T.V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 2010, 11, 113–127. [Google Scholar] [CrossRef]
- William, M.; Leroux, L.-P.; Chaparro, V.; Lorent, J.; Graber, T.E.; M’Boutchou, M.-N.; Charpentier, T.; Fabié, A.; Dozois, C.M.; Stäger, S.; et al. eIF4E-Binding Proteins 1 and 2 Limit Macrophage Anti-Inflammatory Responses through Translational Repression of IL-10 and Cyclooxygenase-2. J. Immunol. 2018, 200, 4102–4116. [Google Scholar] [CrossRef]
- Sun, Y.; Varambally, S.; Maher, C.A.; Cao, Q.; Chockley, P.; Toubai, T.; Malter, C.; Nieves, E.; Tawara, I.; Wang, Y.; et al. Targeting of microRNA-142-3p in dendritic cells regulates endotoxin-induced mortality. Blood 2011, 117, 6172–6183. [Google Scholar] [CrossRef]
- Ding, S.; Liang, Y.; Zhao, M.; Liang, G.; Long, H.; Zhao, S.; Wang, Y.; Yin, H.; Zhang, P.; Zhang, Q.; et al. Decreased microRNA-142-3p/5p expression causes CD4+ T cell activation and B cell hyperstimulation in systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2953–2963. [Google Scholar] [CrossRef]
- Ulrich, C.; Canim, Z.; Herberger, E.; Girndt, M.; Fiedler, R. Inflammation in Hypervolemic Hemodialysis Patients: The Roles of RelB and Caspase-4. Int. J. Mol. Sci. 2023, 24, 17550. [Google Scholar] [CrossRef] [PubMed]
- Mayne, K.J.; Lees, J.S.; Herrington, W.G. Bioimpedance in CKD: An untapped resource? Nephrol. Dial. Transplant. 2023, 38, 583–585. [Google Scholar] [CrossRef] [PubMed]
- Antlanger, M.; Josten, P.; Kammer, M.; Exner, I.; Lorenz-Turnheim, K.; Eigner, M.; Paul, G.; Klauser-Braun, R.; Sunder-Plassmann, G.; Säemann, M.D.; et al. Blood volume-monitored regulation of ultrafiltration to decrease the dry weight in fluid-overloaded hemodialysis patients: A randomized controlled trial. BMC Nephrol. 2017, 18, 238. [Google Scholar] [CrossRef] [PubMed]
- Beberashvili, I.; Yermolayeva, T.; Katkov, A.; Garra, N.; Feldman, L.; Gorelik, O.; Stav, K.; Efrati, S. Estimating of Residual Kidney Function by Multi-Frequency Bioelectrical Impedance Analysis in Hemodialysis Patients Without Urine Collection. Kidney Blood Press. Res. 2018, 43, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, Z. Effect of bioimpedance-defined overhydration parameters on mortality and cardiovascular events in patients undergoing dialysis: A systematic review and meta-analysis. J. Int. Med. Res. 2021, 49, 3000605211031063. [Google Scholar] [CrossRef] [PubMed]
- Risović, I.; Vlatković, V.; Popović-Pejičić, S.; Trbojević-Stanković, J. Relationship Between Leptin Level, Inflammation, and Volume Status in Maintenance Hemodialysis Patients. Ther. Apher. Dial. 2019, 23, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kerkvliet, N.I. AHR-mediated immunomodulation: The role of altered gene transcription. Biochem. Pharmacol. 2009, 77, 746–760. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Lin, C.-H.; Hsu, P.-C.; Sun, Y.-Y.; Huang, Y.-J.; Zhuo, J.-H.; Wang, C.-Y.; Gan, Y.-L.; Hung, C.-C.; Kuan, C.-Y.; et al. Aryl hydrocarbon receptor mediates both proinflammatory and anti-inflammatory effects in lipopolysaccharide-activated microglia. Glia 2015, 63, 1138–1154. [Google Scholar] [CrossRef]
- Ke, S.; Rabson, A.B.; Germino, J.F.; Gallo, M.A.; Tian, Y. Mechanism of suppression of cytochrome P-450 1A1 expression by tumor necrosis factor-alpha and lipopolysaccharide. J. Biol. Chem. 2001, 276, 39638–39644. [Google Scholar] [CrossRef]
- Nicholson, T.E.; Renton, K.W. Role of cytokines in the lipopolysaccharide-evoked depression of cytochrome P450 in the brain and liver. Biochem. Pharmacol. 2001, 62, 1709–1717. [Google Scholar] [CrossRef]
- Murray, P.J. STAT3-mediated anti-inflammatory signalling. Biochem. Soc. Trans. 2006, 34, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Hillmer, E.J.; Zhang, H.; Li, H.S.; Watowich, S.S. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 2016, 31, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Suzuki, M.; Sakaguchi, R.; Hanada, T.; Yasukawa, H. SOCS, Inflammation, and Autoimmunity. Front. Immunol. 2012, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Niemand, C.; Nimmesgern, A.; Haan, S.; Fischer, P.; Schaper, F.; Rossaint, R.; Heinrich, P.C.; Müller-Newen, G. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J. Immunol. 2003, 170, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Carow, B.; Rottenberg, M.E. SOCS3, a Major Regulator of Infection and Inflammation. Front. Immunol. 2014, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, H.; Ohishi, M.; Mori, H.; Murakami, M.; Chinen, T.; Aki, D.; Hanada, T.; Takeda, K.; Akira, S.; Hoshijima, M.; et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat. Immunol. 2003, 4, 551–556. [Google Scholar] [CrossRef]
- Quinn, S.R.; O’Neill, L.A. The role of microRNAs in the control and mechanism of action of IL-10. Curr. Top. Microbiol. Immunol. 2014, 380, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gu, J.; Pan, X.; Gan, X.; Ju, Z.; Zhang, S.; Xia, Y.; Lu, L.; Wang, X. Blockade of miR-142-3p promotes anti-apoptotic and suppressive function by inducing KDM6A-mediated H3K27me3 demethylation in induced regulatory T cells. Cell Death Dis. 2019, 10, 332. [Google Scholar] [CrossRef]
- Sun, Y.; Oravecz-Wilson, K.; Bridges, S.; McEachin, R.; Wu, J.; Kim, S.H.; Taylor, A.; Zajac, C.; Fujiwara, H.; Peltier, D.C.; et al. miR-142 controls metabolic reprogramming that regulates dendritic cell activation. J. Clin. Investig. 2019, 129, 2029–2042. [Google Scholar] [CrossRef]
- Fordham, J.B.; Naqvi, A.R.; Nares, S. Regulation of miR-24, miR-30b, and miR-142-3p during macrophage and dendritic cell differentiation potentiates innate immunity. J. Leukoc. Biol. 2015, 98, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Zhang, Y.; Peng, Y.; He, G.; Liu, A.; Liu, H. Mir-142-3p Regulates Inflammatory Response by Contributing to Increased TNF-α in Chronic Rhinosinusitis with Nasal Polyposis. Ear Nose Throat J. 2021, 100, NP50–NP56. [Google Scholar] [CrossRef] [PubMed]
- Koratala, A.; Ronco, C.; Kazory, A. Diagnosis of Fluid Overload: From Conventional to Contemporary Concepts. Cardiorenal Med. 2022, 12, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A. Estimation of fluid volumes in hemodialysis patients: Comparing bioimpedance with isotopic and dilution methods. Kidney Int. 2014, 85, 738–741. [Google Scholar] [CrossRef] [PubMed]


| %CD86+ p4E-PB1 (Basal) | %CD86+ p4E-PB1 (LPS) | %CD86+ p4E-PB1 (LPS/AhR) | |
|---|---|---|---|
| N | 2.0 [0.7–13.2] | 13.4 [3.3–65.6] | 13.2 [4.3–65.6] |
| H | 3.1 [0.4–24.2] | 9.6 [2.4–88.9] | 13.8 [3.1–80.7] |
| p value | p = 0.125 | p = 0.823 | p = 0.730 |
| %CD4+ p4E-PB1 (Basal) | %CD4+ p4E-PB1 (LPS) | %CD4+ p4E-PB1 (LPS/AhR) | |
|---|---|---|---|
| N | 1.7 [0.4–83.5] | 2.4 [0.5–92.2] | 2.3 [0.7–90.4] |
| H | 3.2 [0.04–94.0] | 3.9 [0.3–97.5] | 3.7 [0.2–97.1] |
| p value | p = 0.261 | p = 0.569 | p = 0.628 |
| SOCS1 mRNA Expression | SOCS3 mRNA Expression | |
|---|---|---|
| N | 1.7 [0.4–5.4] | 2.2 [1.0–4.8] |
| H | 2.0 [0.4–3.5] | 3.3 [0.6–9.4] |
| p-value | 0.575 | 0.040 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulrich, C.; Fiedler, R.; Herberger, E.; Canim, Z.; Markau, S.; Girndt, M. Hypervolemia in Dialysis Patients Impairs STAT3 Signaling and Upregulates miR-142-3p: Effects on IL-10 and IL-6. Int. J. Mol. Sci. 2024, 25, 3719. https://doi.org/10.3390/ijms25073719
Ulrich C, Fiedler R, Herberger E, Canim Z, Markau S, Girndt M. Hypervolemia in Dialysis Patients Impairs STAT3 Signaling and Upregulates miR-142-3p: Effects on IL-10 and IL-6. International Journal of Molecular Sciences. 2024; 25(7):3719. https://doi.org/10.3390/ijms25073719
Chicago/Turabian StyleUlrich, Christof, Roman Fiedler, Eva Herberger, Zeynep Canim, Silke Markau, and Matthias Girndt. 2024. "Hypervolemia in Dialysis Patients Impairs STAT3 Signaling and Upregulates miR-142-3p: Effects on IL-10 and IL-6" International Journal of Molecular Sciences 25, no. 7: 3719. https://doi.org/10.3390/ijms25073719
APA StyleUlrich, C., Fiedler, R., Herberger, E., Canim, Z., Markau, S., & Girndt, M. (2024). Hypervolemia in Dialysis Patients Impairs STAT3 Signaling and Upregulates miR-142-3p: Effects on IL-10 and IL-6. International Journal of Molecular Sciences, 25(7), 3719. https://doi.org/10.3390/ijms25073719





