Comparative Transcriptomic Analysis Revealing the Potential Mechanisms of Erythritol-Caused Mortality and Oviposition Inhibition in Drosophila melanogaster
Abstract
1. Introduction
2. Results
2.1. Effect of Erythritol on Fly Survival and Egg Production
2.2. Effect of Erythritol on Gene Expression
2.3. GO Enrichment Results of Differentially Expressed Genes
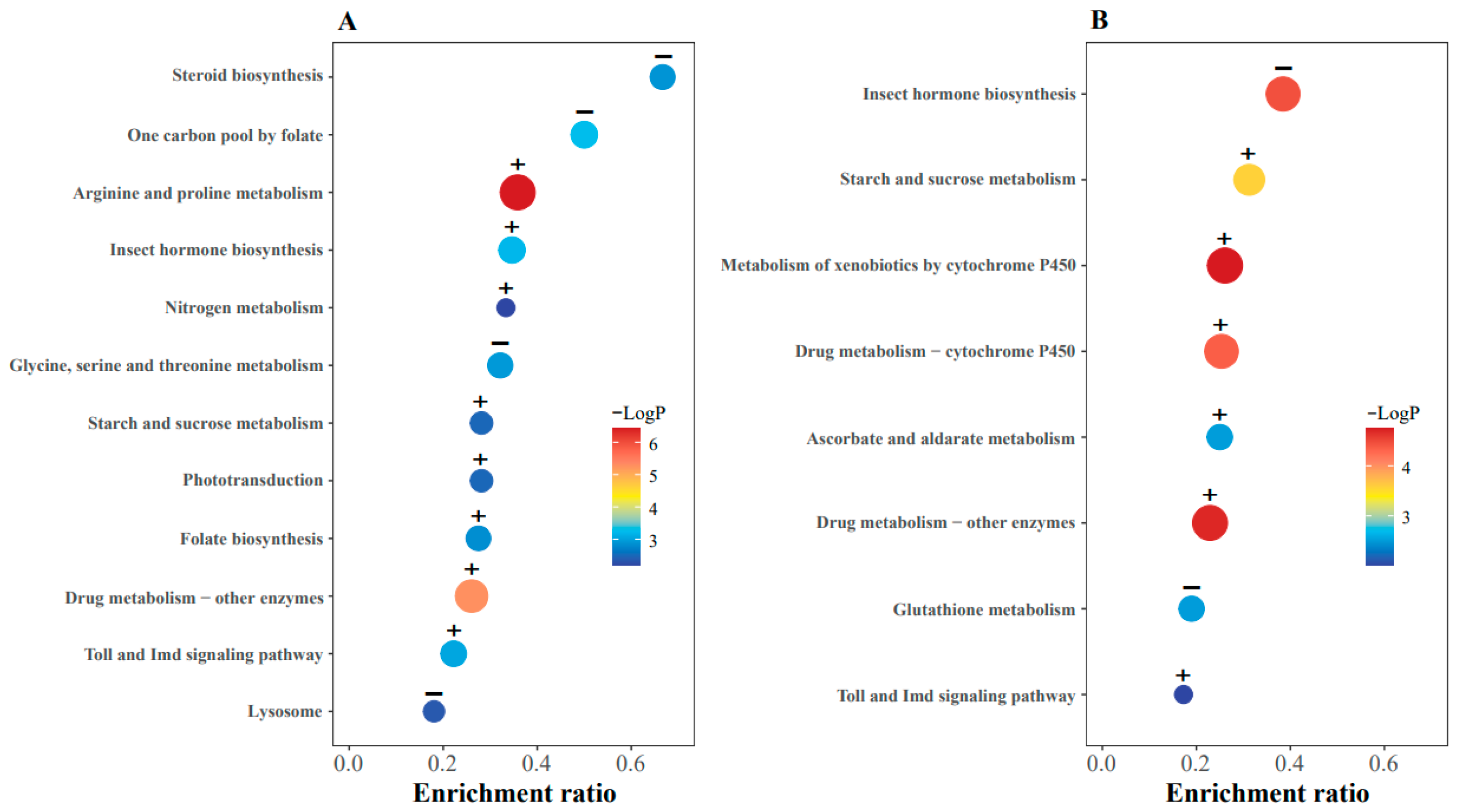
2.4. Enrichment Results of KEGG Pathways for Differentially Expressed Genes
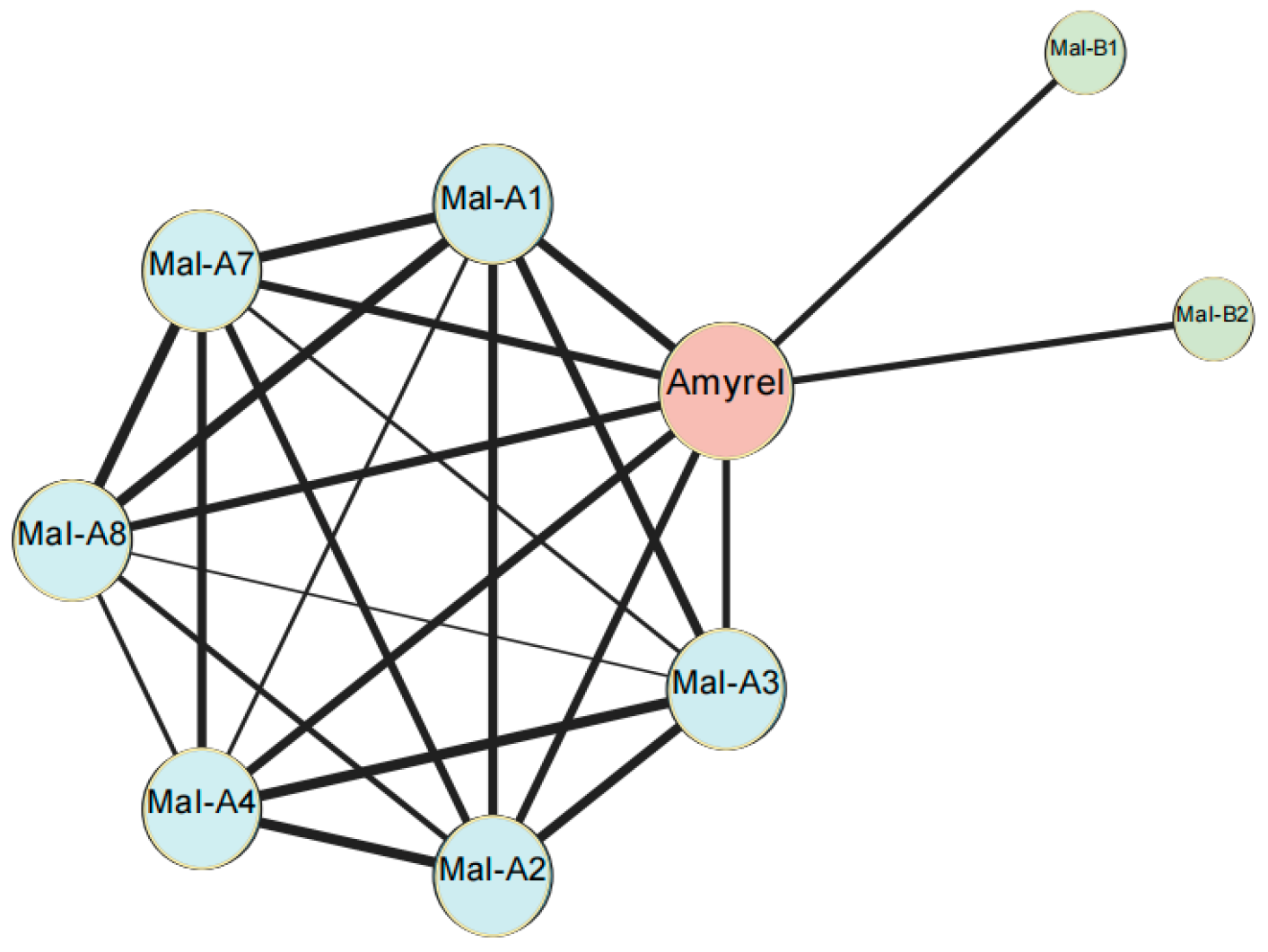
2.5. Key Genes Associated with Carbohydrate Metabolism
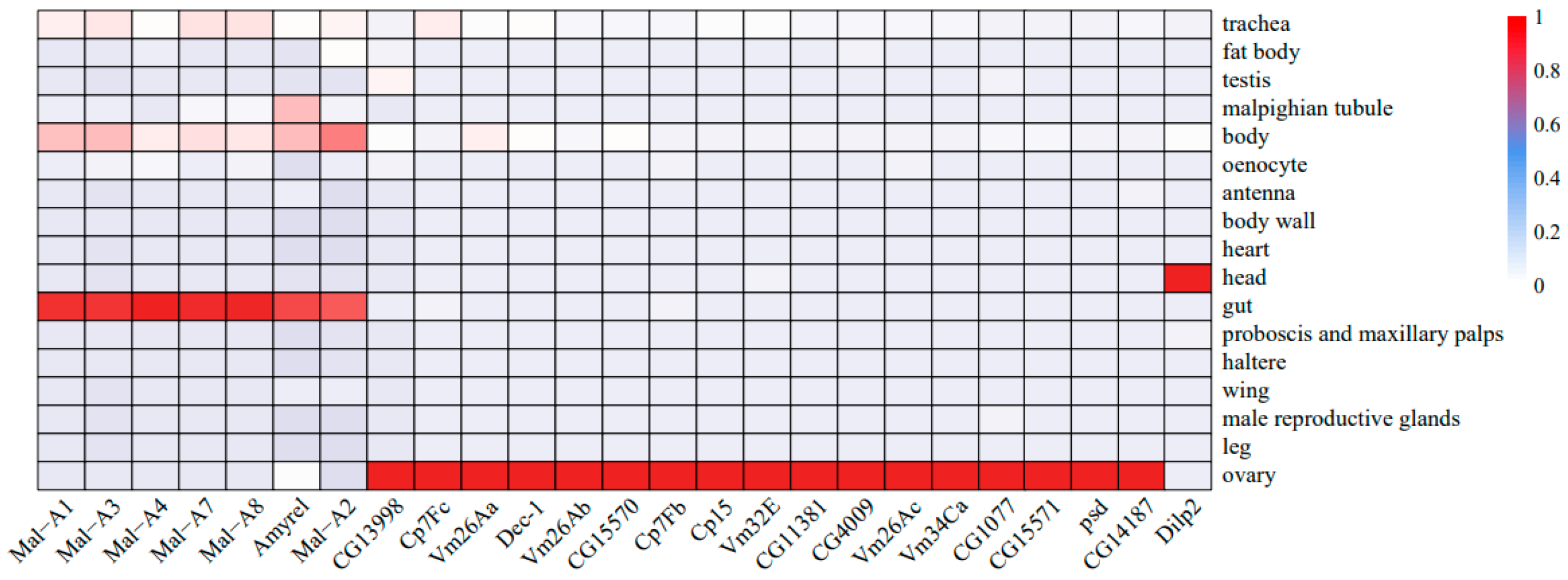
2.6. Tissue-Specific Enrichment Results of Key Genes
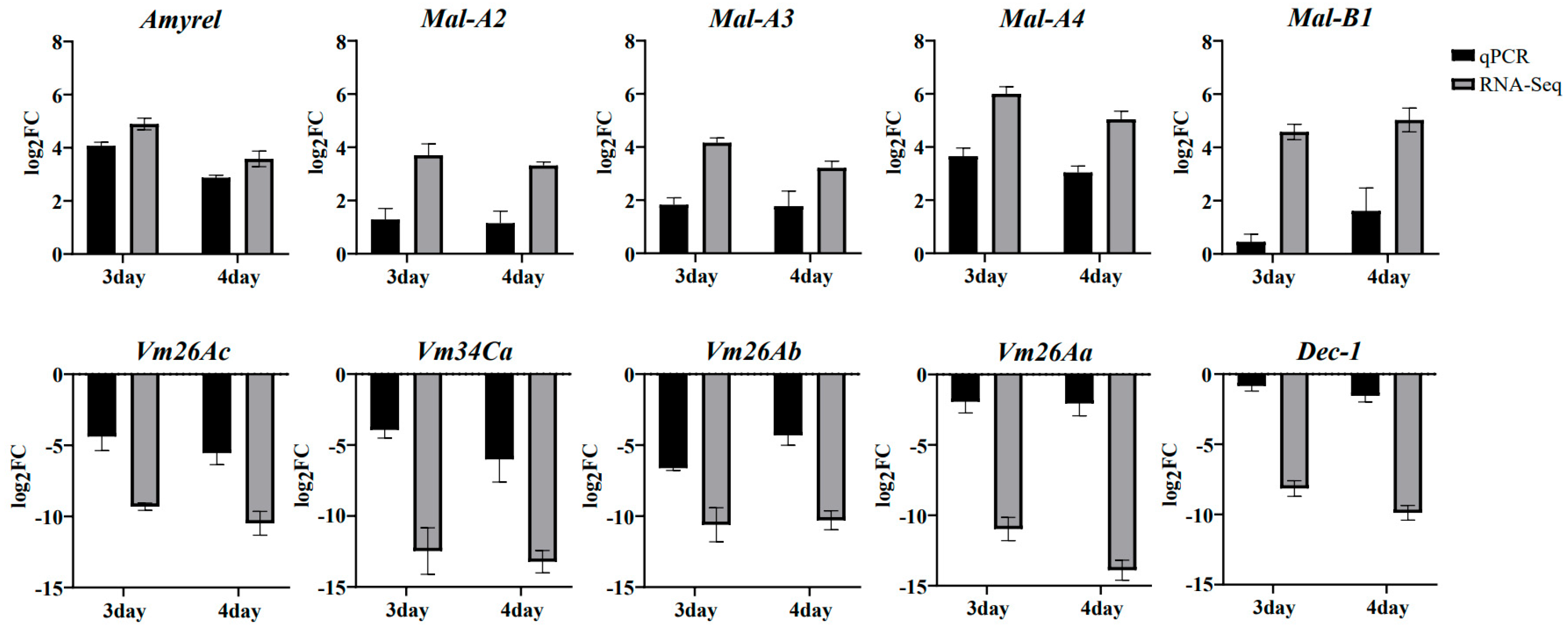
2.7. qRT-PCR Validation
3. Discussion
3.1. Variation of Hemolymph Osmolality Is Not Necessary for Fly Death
3.2. Energy Deficiency Caused by Erythritol
3.3. Mortality Caused by Energy Deficiency
3.4. Molecular Mechanism of Non-Oviposition Caused by Erythritol
4. Materials and Methods
4.1. Fly Rearing and Treatment
4.2. RNA Extraction, Library Construction and Sequencing
4.3. Data Quality Control and Reference Genome Alignment
4.4. Bioinformatic Analysis
4.4.1. Identification of Differentially Expressed Genes and Enrichment of GO and KEGG
4.4.2. Hub Genes Screening of KEGG Pathways
4.4.3. Tissue-Specific Enrichment Analysis of Key Gene
4.5. Validation of the DEGs by Quantitative Real-Time PCR (qRT-PCR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, M.F.F.; Pazin, M.; Pereira, L.C.; Dorta, D.J. Impact of pesticides on environmental and human health. In Toxicology Studies-Cells, Drugs and Environment; InTech: London, UK, 2015; pp. 195–233. [Google Scholar]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotox. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.F.; Gil, F.; Lacasaña, M.; Rodríguez-Barranco, M.; Tsatsakis, A.M.; Requena, M.; Parrón, T.; Alarcón, R. Pesticide exposure and genetic variation in xenobiotic-metabolizing enzymes interact to induce biochemical liver damage. Food Chem Toxicol. 2013, 61, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Regnat, K.; Mach, R.; Mach-Aigner, A. Erythritol as sweetener—Wherefrom and whereto? Appl. Microbiol. Biotechnol. 2018, 102, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-Y.; Lucas, H.; Sagili, R.; Cha, D.H.; Lee, J.C. Effect of erythritol on Drosophila suzukii (Diptera: Drosophilidae) in the presence of naturally-occurring sugar sources, and on the survival of Apis mellifera (Hymenoptera: Apidae). J. Econ. Entomol. 2019, 112, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Caponera, V.; Barrett, M.; Marenda, D.R.; O’donnell, S. Erythritol ingestion causes concentration-dependent mortality in eastern subterranean termites (Blattodea: Rhinotermitidae). J. Econ. Entomol. 2020, 113, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Burgess, E.R., IV; Geden, C.J. Larvicidal potential of the polyol sweeteners erythritol and xylitol in two filth fly species. J. Vector Ecol. 2019, 44, 11–17. [Google Scholar] [CrossRef]
- Wentz, K.; Cooper, W.R.; Horton, D.R.; Kao, R.; Nottingham, L.B. The artificial sweetener, erythritol, has insecticidal properties against pear psylla (Hemiptera: Psyllidae). J. Econ. Entomol. 2020, 113, 2293–2299. [Google Scholar] [CrossRef]
- Sharma, A.; Reyes, J.; Borgmeyer, D.; Ayala-Chavez, C.; Snow, K.; Arshad, F.; Nuss, A.; Gulia-Nuss, M. The sugar substitute Stevia shortens the lifespan of Aedes aegypti potentially by N-linked protein glycosylation. J. Econ. Entomol. 2020, 10, 6195. [Google Scholar] [CrossRef]
- Barrett, M.; Caponera, V.; McNair, C.; O’Donnell, S.; Marenda, D.R. Potential for use of erythritol as a socially transferrable ingested insecticide for ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2020, 113, 1382–1388. [Google Scholar] [CrossRef]
- Baudier, K.M.; Kaschock-Marenda, S.D.; Patel, N.; Diangelus, K.L.; O’Donnell, S.; Marenda, D.R. Erythritol, a non-nutritive sugar alcohol sweetener and the main component of Truvia®, is a palatable ingested insecticide. PLoS ONE 2014, 9, e98949. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, S.; Baudier, K.; Fiocca, K.; Marenda, D. Erythritol ingestion impairs adult reproduction and causes larval mortality in Drosophila melanogaster fruit flies (Diptera: Drosophilidae). J. Appl. Entomol. 2018, 142, 37–42. [Google Scholar] [CrossRef]
- Tang, S.B.; Lee, J.C.; Jung, J.K.; Choi, M.-Y. Effect of erythritol formulation on the mortality, fecundity and physiological excretion in Drosophila suzukii. J. Insect Physiol. 2017, 101, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zeng, L.; Xu, Y. Effect of sweeteners on the survival and behaviour of Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Pest Manag. Sci. 2016, 72, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.C.; Stoffolano, J.G., Jr.; Chiu, K.; Colletti, K.; Dixon, Z.; Salemme, V.; Crnjar, R.; Sollai, G. Effects of chitosan and erythritol on labellar taste neuron activity, proboscis extension reflex, daily food intake, and mortality of male and female spotted-winged drosophila, Drosophila suzukii. J. Insect Physiol. 2021, 131, 104240. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-Y.; Tang, S.B.; Ahn, S.-J.; Amarasekare, K.G.; Shearer, P.; Lee, J.C. Effect of non-nutritive sugars to decrease the survivorship of spotted wing drosophila, Drosophila suzukii. J. Insect Physiol. 2017, 99, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.L.; Fowler, F.E.; Denning, S.S.; Watson, D.W. Survival of the house fly (Diptera: Muscidae) on Truvia and other sweeteners. J. Med. Entomol. 2017, 54, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Albers, M.A.; Bradley, T.J. Osmotic regulation in adult Drosophila melanogaster during dehydration and rehydration. J. Exp. Biol. 2004, 207, 2313–2321. [Google Scholar] [CrossRef]
- Cannell, E.; Dornan, A.J.; Halberg, K.A.; Terhzaz, S.; Dow, J.A.; Davies, S.-A. The corticotropin-releasing factor-like diuretic hormone 44 (DH44) and kinin neuropeptides modulate desiccation and starvation tolerance in Drosophila melanogaster. Peptides 2016, 80, 96–107. [Google Scholar] [CrossRef]
- Paluzzi, J.-P.V. Anti-diuretic factors in insects: The role of CAPA peptides. Gen. Comp. Endocr. 2012, 176, 300–308. [Google Scholar] [CrossRef]
- Spring, J.H.; Robichaux, S.R.; Hamlin, J.A. The role of aquaporins in excretion in insects. J. Exp. Biol. 2009, 212, 358–362. [Google Scholar] [CrossRef]
- Gabriško, M.; Janeček, Š. Characterization of maltase clusters in the genus Drosophila. J. Mol. Evol. 2011, 72, 104–118. [Google Scholar] [CrossRef]
- Inomata, N.; Takahasi, K.R.; Koga, N. Association between duplicated maltase genes and the transcriptional regulation for the carbohydrate changes in Drosophila melanogaster. Gene 2019, 686, 141–145. [Google Scholar] [CrossRef]
- Arrese, E.L.; Patel, R.T.; Soulages, J.L. The main triglyceride-lipase from the insect fat body is an active phospholipase A1: Identification and characterization. J. Lipid Res. 2006, 47, 2656–2667. [Google Scholar] [CrossRef]
- Grönke, S.; Mildner, A.; Fellert, S.; Tennagels, N.; Petry, S.; Müller, G.; Jäckle, H.; Kühnlein, R.P. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab. 2005, 1, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Staubli, F.; Jørgensen, T.J.; Cazzamali, G.; Williamson, M.; Lenz, C.; Søndergaard, L.; Roepstorff, P.; Grimmelikhuijzen, C.J. Molecular identification of the insect adipokinetic hormone receptors. Proc. Natl. Acad. Sci. USA 2002, 99, 3446–3451. [Google Scholar] [CrossRef] [PubMed]
- Grönke, S.; Müller, G.; Hirsch, J.; Fellert, S.; Andreou, A.; Haase, T.; Jäckle, H.; Kühnlein, R.P. Dual lipolytic control of body fat storage and mobilization in Drosophila. PLoS Biol. 2007, 5, e137. [Google Scholar] [CrossRef] [PubMed]
- Nässel, D.R.; Liu, Y.; Luo, J. Insulin/IGF signaling and its regulation in Drosophila. Gen. Comp. Endocr. 2015, 221, 255–266. [Google Scholar] [CrossRef]
- Teleman, A.A. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem. J. 2010, 425, 13–26. [Google Scholar] [CrossRef]
- Okamoto, N.; Yamanaka, N. Nutrition-dependent control of insect development by insulin-like peptides. Curr. Opin. Insect Sci. 2015, 11, 21–30. [Google Scholar] [CrossRef]
- Nässel, D.R.; Broeck, J.V. Insulin/IGF signaling in Drosophila and other insects: Factors that regulate production, release and post-release action of the insulin-like peptides. Cell Mol. Life Sci. 2016, 73, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Neufeld, T.P. Dietary sugar promotes systemic TOR activation in Drosophila through AKH-dependent selective secretion of Dilp3. Nat. Commun. 2015, 6, 6846. [Google Scholar] [CrossRef] [PubMed]
- Van der Horst, D.J. Insect adipokinetic hormones: Release and integration of flight energy metabolism. Comp. Biochem. Phys. B 2003, 136, 217–226. [Google Scholar] [CrossRef]
- Waring, G.L.; Hawley, R.J.; Schoenfeld, T. Multiple proteins are produced from the dec-1 eggshell gene in Drosophila by alternative RNA splicing and proteolytic cleavage events. Dev. Biol. 1990, 142, 1–12. [Google Scholar] [CrossRef]
- Noguerón, M.I.; Waring, G.L. Regulated processing of dec-1 eggshell proteins in Drosophila. Dev. Biol. 1995, 172, 272–279. [Google Scholar] [CrossRef]
- Mauzy-Melitz, D.; Waring, G.L. fc177, a minor dec-1 proprotein, is necessary to prevent ectopic aggregation of the endochorion during eggshell assembly in Drosophila. Dev. Biol. 2003, 255, 193–205. [Google Scholar] [CrossRef]
- Short, S.M.; Lazzaro, B.P. Reproductive status alters transcriptomic response to infection in female Drosophila melanogaster. G3—Genes. Genom. Genet. 2013, 3, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Manogaran, A.; Waring, G.L. The N-terminal prodomain of sV23 is essential for the assembly of a functional vitelline membrane network in Drosophila. Dev. Biol. 2004, 270, 261–271. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Velentzas, A.D.; Velentzas, P.D.; Katarachia, S.A.; Anagnostopoulos, A.K.; Sagioglou, N.E.; Thanou, E.V.; Tsioka, M.M.; Mpakou, V.E.; Kollia, Z.; Gavriil, V.E. The indispensable contribution of s38 protein to ovarian-eggshell morphogenesis in Drosophila melanogaster. Sci. Rep. 2018, 8, 16103. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Margaritis, L.H. Immunolocalization of the temporally “early” secreted major structural chorion proteins, Dvs38 and Dvs36, in the eggshell layers and regions of Drosophila virilis. J. Struct. Biol. 1998, 123, 111–123. [Google Scholar] [CrossRef]
- Bownes, M. The regulation of the yolk protein genes, a family of sex differentiation genes in Drosophila melanogaster. Bioessays 1994, 16, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Cernilogar, F.M.; Fabbri, F.; Andrenacci, D.; Taddei, C.; Gargiulo, G. Drosophila vitelline membrane cross-linking requires the fs(1)Nasrat, fs(1)polehole and chorion genes activities. Dev. Genes Evol. 2001, 211, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Elalayli, M.; Hall, J.D.; Fakhouri, M.; Neiswender, H.; Ellison, T.T.; Han, Z.; Roon, P.; LeMosy, E.K. Palisade is required in the Drosophila ovary for assembly and function of the protective vitelline membrane. Dev. Biol. 2008, 319, 359–369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fakhouri, M.; Elalayli, M.; Sherling, D.; Hall, J.D.; Miller, E.; Sun, X.; Wells, L.; LeMosy, E.K. Minor proteins and enzymes of the Drosophila eggshell matrix. Dev. Biol. 2006, 293, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Scardoni, G.; Petterlini, M.; Laudanna, C. Analyzing biological network parameters with CentiScaPe. Bioinformatics 2009, 25, 2857–2859. [Google Scholar] [CrossRef]
- Li, H.; Janssens, J.; De Waegeneer, M.; Kolluru, S.S.; Davie, K.; Gardeux, V.; Saelens, W.; David, F.P.; Brbić, M.; Spanier, K. Fly Cell Atlas: A single-nucleus transcriptomic atlas of the adult fruit fly. Science 2022, 375, eabk2432. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M.; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M. Integrated analysis of multimodal single-cell data. Cell 2021, 184, 3573–3587.E29. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Wang, Z.; Pe’er, D.; Danko, C.G. Cell type and gene expression deconvolution with BayesPrism enables Bayesian integrative analysis across bulk and single-cell RNA sequencing in oncology. Nat. Cancer 2022, 3, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Ponton, F.; Chapuis, M.-P.; Pernice, M.; Sword, G.A.; Simpson, S.J. Evaluation of potential reference genes for reverse transcription-qPCR studies of physiological responses in Drosophila melanogaster. J. Insect Physiol. 2011, 57, 840–850. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]

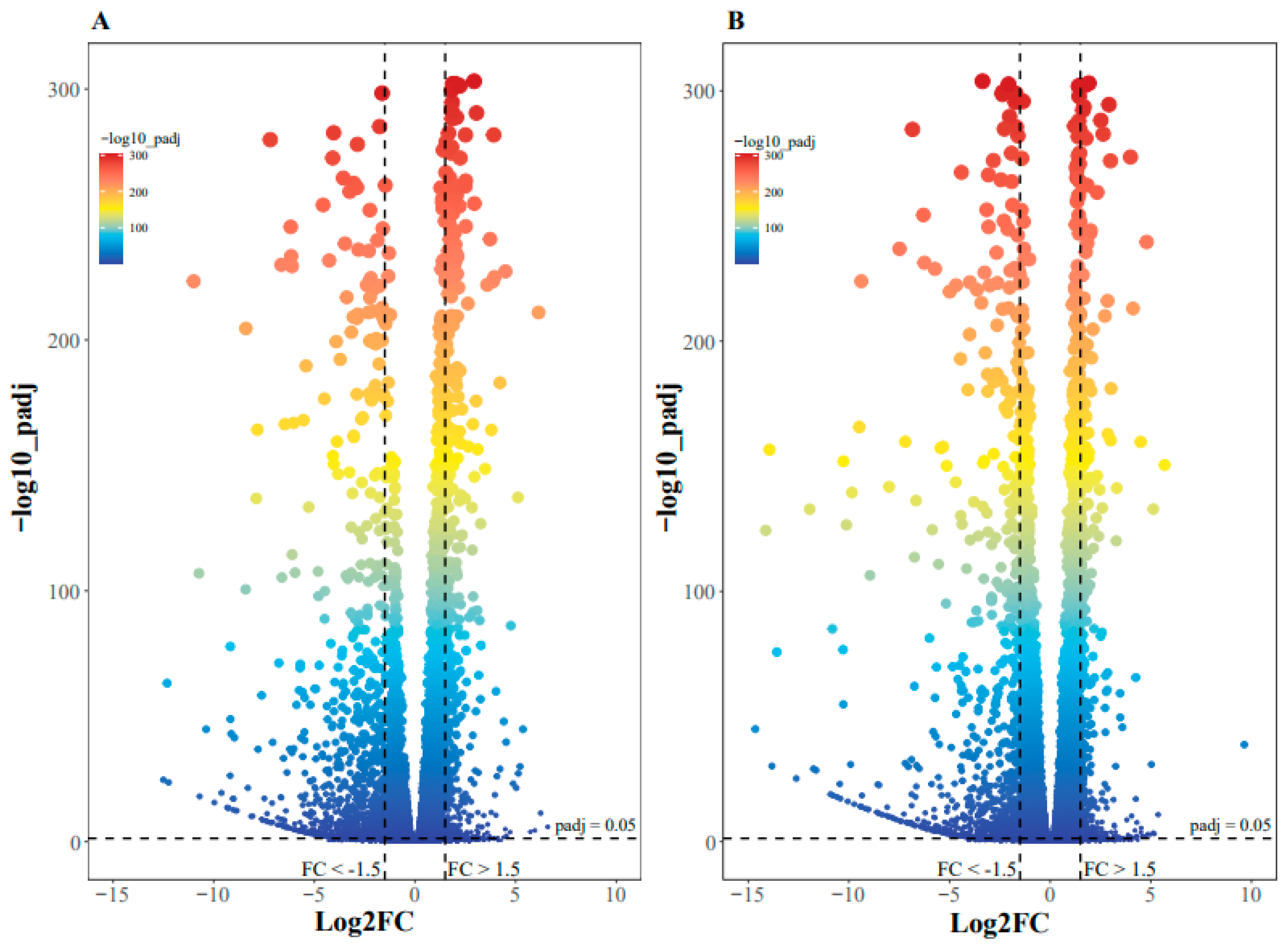

 : up-regulated genes.
: up-regulated genes.  : down-regulated genes.
: down-regulated genes.
 : up-regulated genes.
: up-regulated genes.  : down-regulated genes.
: down-regulated genes.
| Gene | Enriched Tissue | Category | Functions | Log2FC |
|---|---|---|---|---|
| Dilp2 | head | insulin-like peptide | carbohydrates/lipids metabolism | −2.4↓ |
| Dilp3 | −1.8↓ | |||
| Dilp5 | −2.6↓ | |||
| Akh | body | adipokinetic hormone | carbohydrates/lipids metabolism | 1.6↑ |
| Mal-B1 | trachea | α-glucosidase | carbohydrate metabolism | 4.6↑ |
| Mal-B2 | fat body | α-glucosidase | carbohydrate metabolism | 2.1↑ |
| Bmm | triglyceride lipase | lipid/triglyceride metabolism | 2.8↑ | |
| Amyrel | gut | α-amylase | carbohydrate metabolism | 4.9↑ |
| Mal-A1 | α-glucosidase | 3.0↑ | ||
| Mal-A2 | 3.7↑ | |||
| Mal-A3 | 4.3↑ | |||
| Mal-A4 | 6.0↑ | |||
| Mal-A6 | 2.7↑ | |||
| Mal-A7 | 2.9↑ | |||
| Mal-A8 | 2.3↑ | |||
| Dec-1 | ovary | defective chorion | eggshell assembly | −8.21↓ |
| Vm26Ab | vitelline membrane | major early eggshell protein | −10.7↓ | |
| Vm26Ac | −9.3↓ | |||
| Vm26Aa | −11.0↓ | |||
| Vm34Ca | −12.3↓ | |||
| Vm32E | −12.2↓ | |||
| Psd | act with the vitelline of eggshell | −9.0↓ | ||
| Cp7Fb | chorion protein | egg protection | −6.5↓ | |
| Cp7Fc | −6.6↓ | |||
| Cp15 | −4.9↓ | |||
| Cp36 | −4.0↓ | |||
| fs(1)M3 | −2.3↓ | |||
| fs(1)N | −2.2↓ | |||
| CG1077 | chorion containing eggshell formation | −5.0↓ | ||
| CG4009 | −5.5↓ | |||
| CG14187 | −6.4↓ | |||
| CG13998 | −5.5↓ | |||
| CG11381 | egg chorion | −6.1↓ | ||
| CG15571 | −2.5↓ | |||
| CG15570 | −5.4↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Duo, H.; Zhang, X.; Gong, H.; Li, B.; Hao, Y. Comparative Transcriptomic Analysis Revealing the Potential Mechanisms of Erythritol-Caused Mortality and Oviposition Inhibition in Drosophila melanogaster. Int. J. Mol. Sci. 2024, 25, 3738. https://doi.org/10.3390/ijms25073738
Li L, Duo H, Zhang X, Gong H, Li B, Hao Y. Comparative Transcriptomic Analysis Revealing the Potential Mechanisms of Erythritol-Caused Mortality and Oviposition Inhibition in Drosophila melanogaster. International Journal of Molecular Sciences. 2024; 25(7):3738. https://doi.org/10.3390/ijms25073738
Chicago/Turabian StyleLi, Lei, Hongrui Duo, Xiaoxi Zhang, Huiming Gong, Bo Li, and Youjin Hao. 2024. "Comparative Transcriptomic Analysis Revealing the Potential Mechanisms of Erythritol-Caused Mortality and Oviposition Inhibition in Drosophila melanogaster" International Journal of Molecular Sciences 25, no. 7: 3738. https://doi.org/10.3390/ijms25073738
APA StyleLi, L., Duo, H., Zhang, X., Gong, H., Li, B., & Hao, Y. (2024). Comparative Transcriptomic Analysis Revealing the Potential Mechanisms of Erythritol-Caused Mortality and Oviposition Inhibition in Drosophila melanogaster. International Journal of Molecular Sciences, 25(7), 3738. https://doi.org/10.3390/ijms25073738






