Circulating Cancer-Associated Macrophage-like Cells as a Blood-Based Biomarker of Response to Immune Checkpoint Inhibitors
Abstract
1. Introduction
2. Results
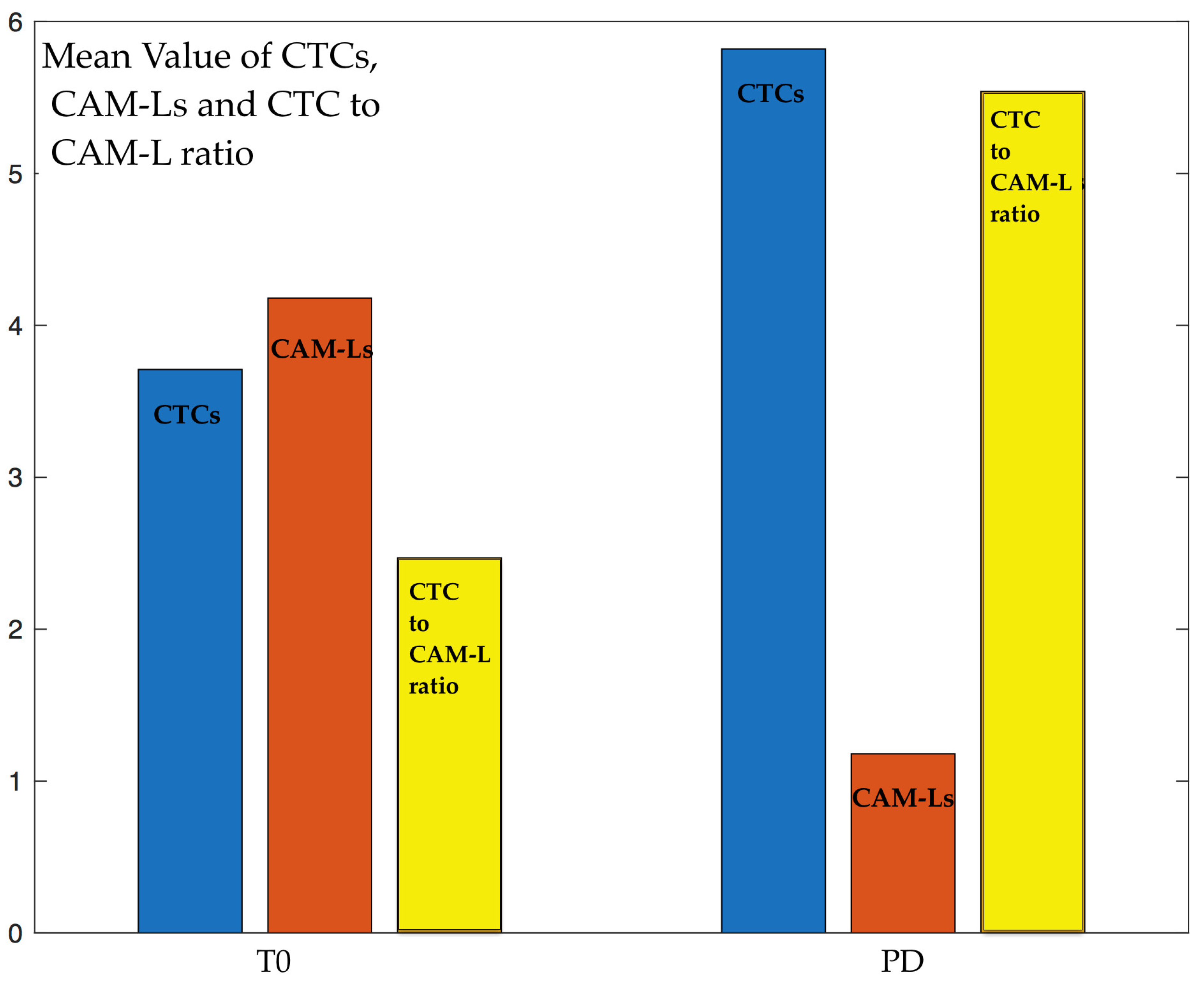
2.1. Serial Analysis of CTCs and CAM-Ls
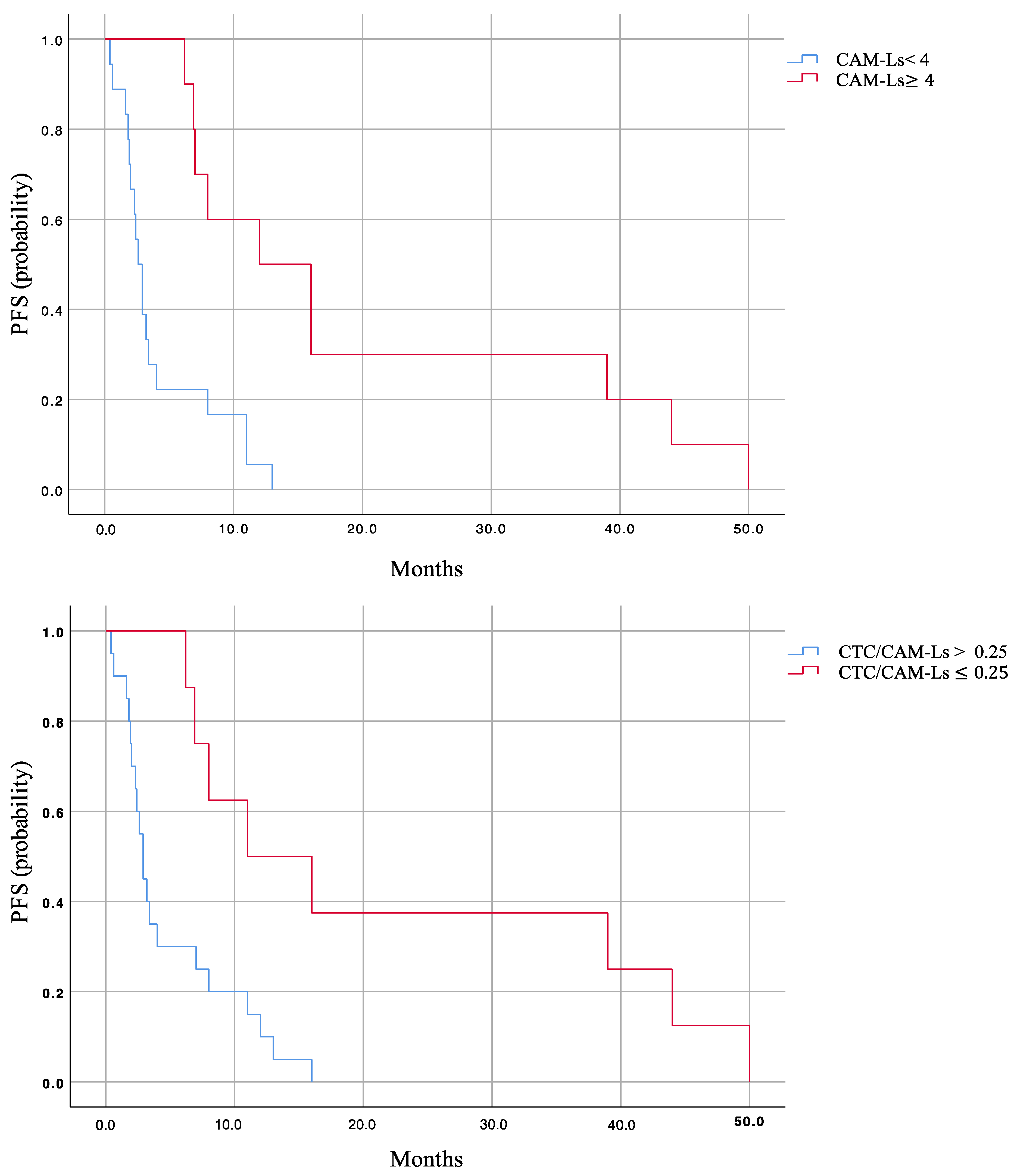
2.2. Prognostic Role of CTCs, CAM-Ls, and CTC/CAM-L Ratio
2.3. Predictive Role of CTCs, CAM-Ls, and CTC/CAM-L Ratio
3. Discussion
4. Materials and Methods
4.1. Patient Population
4.2. Tumor Response
4.3. Enumeration of CAM-Ls and Standard CTCs
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borgeaud, M.; Sandoval, J.; Obeid, M.; Banna, G.; Michielin, O.; Addeo, A.; Friedlaender, A. Novel targets for immune-checkpoint inhibition in cancer. Cancer Treat. Rev. 2023, 120, 102614. [Google Scholar] [CrossRef]
- Mohammed, N.; Xiao E hua Mohsen, S. PD-1/PD-L1 inhibitor treatment and its impact on clinical imaging in non-small cell lung cancer: A systematic review and meta-analysis of immune-related adverse events. Front. Oncol. 2023, 13, 1191681. [Google Scholar] [CrossRef]
- Ratajczak, K.; Grel, H.; Olejnik, P.; Jakiela, S.; Stobiecka, M. Biosensors and Bioelectronics Current progress, strategy, and prospects of PD-1/PDL-1 immune checkpoint biosensing platforms for cancer diagnostics, therapy monitoring, and drug screening. Biosens. Bioelectron. 2023, 240, 115644. [Google Scholar] [CrossRef]
- Lin, X. Progresses in biomarkers for cancer immunotherapy. Med. Comm. 2023, 4, e387. [Google Scholar] [CrossRef]
- Carr David, J.; Welch, G. Assessing the Clinical Utility of Liquid Biopsies Across 5 Potential Indications from Therapy Selection to Population Screening: A Review. JAMA Intern. Med. 2023, 183, 1144–1151. [Google Scholar] [CrossRef]
- Dao, J.; Conway, P.J.; Subramani, B.; Meyyappan, D.; Russell, S.; Mahadevan, D. Using cfDNA and ctDNA as Oncologic Markers: A Path to Clinical Validation. Int. J. Mol. Sci. 2023, 24, 13219. [Google Scholar] [CrossRef]
- Noor, J.; Chaudhry, A.; Noor, R.; Batool, S. Advancements and Applications of Liquid Biopsies in Oncology: A Narrative Review. Cureus 2023, 15, e42731. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef]
- Xiao, J.; Pohlmann, P.R.; Isaacs, C.; Weinberg, B.A.; He, A.R.; Schlegel, R.; Agarwal, S. Circulating Tumor Cells: Technologies and Their Clinical Potential in Cancer Metastasis. Biomedicines 2021, 9, 1111. [Google Scholar] [CrossRef]
- Lindsay, C.R.; Blackhall, F.H.; Carmel, A.; Fernandez-Gutierrez, F.; Gazzaniga, P.; Groen, H.J.M.; Hiltermann, T.J.N.; Krebs, M.G.; Loges, S.; López-López, R.; et al. ScienceDirect EPAC-lung: Pooled analysis of circulating tumour cells in advanced non-small cell lung cancer. Eur. J. Cancer 2019, 117, 60–68. [Google Scholar] [CrossRef]
- Andree, K.C.; Dalum GVan Terstappen, L.W.M.M. ScienceDirect Challenges in circulating tumor cell detection by the CellSearch system 5. Mol. Oncol. 2015, 10, 395–407. [Google Scholar] [CrossRef]
- Raimondi, C.; Gradilone, A.; Naso, G.; Cortesi, E.; Gazzaniga, P. Clinical utility of circulating tumor cell counting through CellSearch®: The dilemma of a concept suspended in Limbo. OncoTarget Ther. 2014, 7, 619–625. [Google Scholar]
- de Wit, S.; Zeune, L.L.; Hiltermann, J.T.N.; Groen, H.J.M.; Dalum GVan Terstappen, L.W.M.M. Classification of Cells in CTC-Enriched Samples by Advanced Image Analysis. Cancers 2018, 10, 377. [Google Scholar] [CrossRef]
- Oeyen, S.; Liégeois, V.; De Laere, B.; Buys, A.; Strijbos, M.; Dirix, P.; Meijnders, P.; Vermeulen, P.; Van Laere, S.; Dirix, L. Automated enumeration and phenotypic characterization of CTCs and tdEVs in patients with metastatic castration resistant prostate cancer. Prostate Cancer Prostat. Dis. 2021, 24, 499–506. [Google Scholar] [CrossRef]
- Reduzzi, C.; Vismara, M.; Gerratana, L.; Silvestri, M.; Braud, F.; De Raspagliesi, F.; Raspagliesi, F.; Verzoni, E.; Di Cosimo, S.; Locati, L.D.; et al. Seminars in Cancer Biology The curious phenomenon of dual-positive circulating cells: Longtime overlooked tumor cells. Semin. Cancer Biol. 2019, 60, 344–350. [Google Scholar] [CrossRef]
- Kaigorodova, E.V.; Kozik, A.V.; Zavaruev, I.S.; Grishchenko, M.Y. Hybrid/Atypical Forms of Circulating Tumor Cells: Current State of the Art. Biochemistry 2022, 87, 380–390. [Google Scholar] [CrossRef]
- Sutton, T.L.; Patel, R.K.; Anderson, A.N.; Bowden, S.G.; Whalen, R.; Giske, N.R.; Wong, M.H. Circulating Cells with Macrophage-like Characteristics in Cancer: The Importance of Circulating Neoplastic-Immune Hybrid Cells in Cancer. Cancers 2022, 14, 3871. [Google Scholar] [CrossRef]
- Nitschke, C.; Markmann, B.; Konczalla, L.; Kropidlowski, J.; Pereira-Veiga, T.; Scognamiglio, P.; Schönrock, M.; Sinn, M.; Tölle, M.; Izbicki, J.; et al. Circulating Cancer Associated Macrophage-like Cells as a Potential New Prognostic Marker in Pancreatic Ductal Adenocarcinoma. Biomedicines 2022, 10, 2955. [Google Scholar] [CrossRef]
- Adams, D.L.; Martin, S.S.; Alpaugh, R.K.; Charpentier, M.; Tsai, S.; Bergan, R.C.; Ogden, I.M.; Catalona, W.; Chumsri, S.; Tang, C.-M.; et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proc. Natl. Acad. Sci. USA 2014, 111, 3514–3519. [Google Scholar] [CrossRef] [PubMed]
- Dotse, E.; Lim, K.H.; Wang, M.; Wijanarko, K.J. An Immunological Perspective of Circulating Tumor Cells as Diagnostic Biomarkers and Therapeutic Targets. Life 2022, 12, 323. [Google Scholar] [CrossRef]
- Wei, C.; Yang, C.; Wang, S.; Shi, D.; Zhang, C.; Lin, X. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell- mediated colorectal cancer metastasis. Mol. Cancer 2019, 18, 64. [Google Scholar] [CrossRef]
- Huang, J.; Wang, K.; Xu, J.; Huang, J.; Zhang, T. Prognostic Significance of Circulating Tumor Cells in Non-Small-Cell Lung Cancer Patients: A Meta-Analysis. PLoS ONE 2013, 8, e78070. [Google Scholar] [CrossRef]
- Gast, C.E.; Silk, A.D.; Zarour, L.; Riegler, L.; Burkhart, J.G.; Gustafson, K.T.; Parappilly, M.S.; Roh-Johnson, M.; Goodman, J.R.; Olson, B.; et al. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci. Adv. 2018, 4, eaat7828. [Google Scholar] [CrossRef]
- Walker, B.S.; Sutton, T.L.; Zarour, L.; Hunter, J.G.; Wood, S.G.; Tsikitis, V.L.; Herzig, D.O.; Lopez, C.D.; Chen, E.Y.; Mayo, S.C.; et al. Circulating Hybrid Cells: A Novel Liquid Biomarker of Treatment Response in Gastrointestinal Cancers. Ann. Surg. Oncol. 2021, 28, 8567–8578. [Google Scholar] [CrossRef]
- Parappilly, M.S.; Chin, Y.; Whalen, R.M.; Anderson, A.N.; Robinson, T.S.; Strgar, L.; Sutton, T.L.; Conley, P.; Klocke, C.; Gibbs, S.L.; et al. Circulating Neoplastic-Immune Hybrid Cells Predict Metastatic Progression in Uveal Melanoma. Cancers 2022, 14, 4617. [Google Scholar] [CrossRef]
- Dietz, M.S.; Sutton, T.L.; Walker, B.S.; Gast, C.E.; Zarour, L.; Sengupta, S.K.; Swain, J.R.; Eng, J.; Parappilly, M.; Limbach, K.; et al. Relevance of circulating hybrid cells as a non-invasive biomarker for myriad solid tumors. Sci. Rep. 2021, 11, 13630. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef]
- Pore, A.A.; Dhanasekara, C.S.; Navaid HBin Vanapalli, S.A. Comprehensive Profiling of Cancer-Associated Cells in the Blood of Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy to Predict Pathological Complete Response. Bioegineering 2023, 10, 485. [Google Scholar] [CrossRef]
- Mu, Z.; Wang, C.; Ye, Z.; Rossi, G.; Sun, C. Prognostic values of cancer associated macrophage-like cells (CAML) enumeration in metastatic breast cancer. Breast Cancer Res. Treat. 2017, 165, 733–741. [Google Scholar] [CrossRef]
- Sun, N.Y.; Chen, Y.L.; Wu, W.Y.; Lin, H.W.; Chiang, Y.C.; Chang, C.F.; Tai, Y.-J.; Hsu, H.-C.; Chen, C.-A.; Sun, W.-Z.; et al. Blockade of PD-L1 Enhances Cancer Immunotherapy by Regulating Dendritic Cell Maturation and Macrophage Polarization. Cancers 2019, 11, 1400. [Google Scholar] [CrossRef]
- Rostam, H.M.; Reynolds, P.M.; Alexander, M.R.; Gadegaard, N. Image based Machine Learning for identification of macrophage subsets. Sci. Rep. 2017, 7, 3521. [Google Scholar] [CrossRef] [PubMed]
- Nicolazzo, C.; Raimondi, C.; Mancini, M.; Caponnetto, S.; Gradilone, A.; Gandini, O.; Mastromartino, M.; del Bene, G.; Prete, A.; Longo, F.; et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci. Rep. 2016, 6, 31726. [Google Scholar] [CrossRef]
- Rossi, E.; Basso, U.; Celadin, R.; Zilio, F.; Pucciarelli, S.; Aieta, M.; Barile, C.; Sava, T.; Bonciarelli, G.; Tumolo, S.; et al. M30 Neoepitope Expression in Epithelial Cancer: Quantification of Apoptosis in Circulating Tumor Cells by CellSearch Analysis. Clin. Cancer Res. 2010, 16, 5233–5243. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.; Piairo, P.; Chícharo, A.; Abalde-Cela, S.; Pires, L.R.; Corredeira, P.; Alves, P.; Muinelo-Romay, L.; Costa, L.; Diéguez, L. HER2 Expression in Circulating Tumour Cells Isolated from Metastatic Breast Cancer Patients Using a Size-Based Microfluidic Device. Cancers 2021, 13, 4446. [Google Scholar] [CrossRef]
- Tang, C.M.; Adams, D.L. Clinical Applications of Cancer-Associated Cells Present in the Blood of Cancer Patients. Biomedicines 2022, 10, 587. [Google Scholar] [CrossRef] [PubMed]

| Baseline (T0) (n = 28) | 3 Months (T1) (n = 13) | 6 Months (T2) (n = 11) | Progression of Disease (n = 28) | |
|---|---|---|---|---|
| CTCs | 3.71 ± 3.31 | 1.53 ± 1.56 | 0.81 ± 0.98 | 5.82 ± 4.19 |
| CAM-Ls | 4.18 ± 4.21 | 9.38 ± 5.02 | 8.81 ± 4.37 | 1.18 ± 0.46 |
| CTC/CAM-L | 2.47 ± 3.05 | 0.39 ± 0.79 | 1.07 ± 1.08 | 5.54 ± 3.72 |
| CAM-Ls < 4 | CAM-Ls ≥ 4 | CTCs/CAM-Ls > 0.25 | CTCs/CAM-Ls ≤ 0.25 | |
|---|---|---|---|---|
| PFS | 2.6 (1.99–3.21) | 13 (6.52–19.47) | 2.6 (1.91–3.29) | 12 (3.73–20.3) |
| p-value | <0.001 | <0.001 | ||
| Baseline (T0) (n = 28) | 3 Months (T1) (n = 13) | 6 Months (T2) (n = 11) | Progression Disease (PD) (n = 28) | |||||
|---|---|---|---|---|---|---|---|---|
| PFS | PFS | PFS | PFS | |||||
| r | p | r | p | r | p | r | p | |
| CTCs | −0.43 | 0.02 | −0.38 | 0.19 | −0.66 | 0.03 | −0.42 | 0.02 |
| CAM-Ls | 0.73 | <0.001 | 0.62 | 0.02 | 0.75 | 0.01 | 0.17 | 0.37 |
| CTCs/CAM-Ls | −0.47 | 0.01 | −0.27 | 0.36 | −0.5 | 0.12 | −0.44 | 0.01 |
| All Patients (n = 28) | Early Progressors (n = 11) | Long Responders (n = 11) | p-Value | |
|---|---|---|---|---|
| PFS | 4 (0–8.8) | 2.4 (1.64–3.17) | 13 (7.8–18.2) | <0.001 |
| CTCs (n = 28) | 3.71 ± 3.31 | 5.2 ± 3.80 | 1.73 ± 1.27 | 0.024 |
| CAM-Ls (n = 28) | 4.18 ± 4.21 | 1.4 ± 0.82 | 6.91 ± 4.12 | <0.001 |
| CTC/CAM-L (n = 28) | 2.47 ± 3.05 | 4.28 ± 3.21 | 0.42 ± 0.47 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magri, V.; De Renzi, G.; Marino, L.; De Meo, M.; Siringo, M.; Gelibter, A.; Gareri, R.; Cataldi, C.; Giannini, G.; Santini, D.; et al. Circulating Cancer-Associated Macrophage-like Cells as a Blood-Based Biomarker of Response to Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2024, 25, 3752. https://doi.org/10.3390/ijms25073752
Magri V, De Renzi G, Marino L, De Meo M, Siringo M, Gelibter A, Gareri R, Cataldi C, Giannini G, Santini D, et al. Circulating Cancer-Associated Macrophage-like Cells as a Blood-Based Biomarker of Response to Immune Checkpoint Inhibitors. International Journal of Molecular Sciences. 2024; 25(7):3752. https://doi.org/10.3390/ijms25073752
Chicago/Turabian StyleMagri, Valentina, Gianluigi De Renzi, Luca Marino, Michela De Meo, Marco Siringo, Alain Gelibter, Roberta Gareri, Chiara Cataldi, Giuseppe Giannini, Daniele Santini, and et al. 2024. "Circulating Cancer-Associated Macrophage-like Cells as a Blood-Based Biomarker of Response to Immune Checkpoint Inhibitors" International Journal of Molecular Sciences 25, no. 7: 3752. https://doi.org/10.3390/ijms25073752
APA StyleMagri, V., De Renzi, G., Marino, L., De Meo, M., Siringo, M., Gelibter, A., Gareri, R., Cataldi, C., Giannini, G., Santini, D., Nicolazzo, C., & Gazzaniga, P. (2024). Circulating Cancer-Associated Macrophage-like Cells as a Blood-Based Biomarker of Response to Immune Checkpoint Inhibitors. International Journal of Molecular Sciences, 25(7), 3752. https://doi.org/10.3390/ijms25073752









