Decoding the Versatile Landscape of Autophagic Protein VMP1 in Cancer: A Comprehensive Review across Tissue Types and Regulatory Mechanisms
Abstract
1. Introduction
2. Role of Autophagy in Cancer
3. Role of VMP1 in Autophagy
4. Role of VMP1 in Cancer
4.1. Relation between VMP1 and Malignancy
4.1.1. Pancreatic Cancer
4.1.2. Breast Cancer
4.1.3. Colon Cancer
4.1.4. Hepatic Cancer
4.1.5. Gastric and Esophageal Cancer
4.1.6. Ovarian Cancer
4.1.7. Glioma
| Type of Cancer | VMP1 Expression [Reference] | Effect of VMP1 in Malignancy | Pathway Involved | Reference |
|---|---|---|---|---|
| Pancreatic | Induction of Autophagy | G12D KRAS/PI3K/AKT1/GLI3/p300 | [5] | |
| Overexpressed [7,42] | Induction by chemotherapy agents | E2F1/p300 | [6] | |
| Resistance to chemotherapy agents | [7] | |||
| Promotion | [43] | |||
| Breast | Overexpressed [45] | Bad prognosis | [46] | |
| Colon | Downregulated [50] | Resistance to chemotherapy agents | [47] | |
| Exosomes/ABCC1/Bcl-2 | [49] | |||
| Resistance to photodynamic therapy | HIF-1α | [48] | ||
| Better prognosis | [50] | |||
| Less invasion | PI3K/Akt/ZO-1/E-cadherin | |||
| Less proliferation | ||||
| Less migration and invasion in CAFs | HIF-1α | [51] | ||
| Hepatic | Downregulated [53] | Less metastasis | miR-210 | [52] |
| Less growth | [53] | |||
| Less invasion | ||||
| Less metastasis | ||||
| Better prognosis | ||||
| Gastric | Overexpressed [55] | Favors metastasis | [54] | |
| Esophagic | Promotion | RPS6KB1/VMP1 fusion | [56] | |
| Ovarian | Overexpressed [57] | Cell proliferation | [57] | |
| Invasion | ||||
| Less cell migration | VHL/HIF-1α/miR210 | [58] | ||
| Glioma | Overexpressed [59] | Bad Prognosis | [59] | |
| Cell Proliferation | ||||
| Less apoptosis | ||||
| Chemotherapy resistance | ||||
| Radiotherapy resistance |
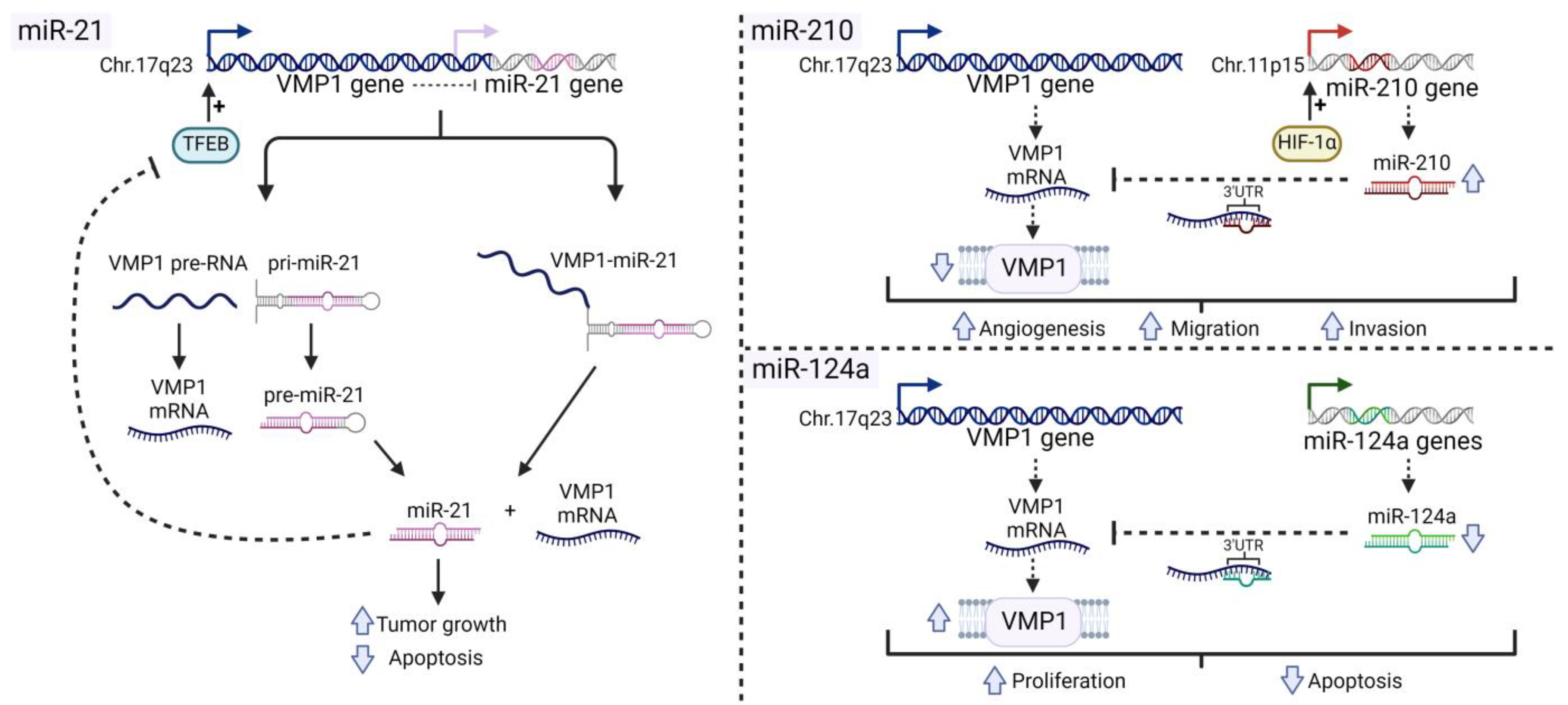
4.2. Relation between VMP1 and MiRNAs in Cancer
4.2.1. MiR-21
4.2.2. MiR-210
4.2.3. MiR-124
4.3. VMP1 Fused with Other Genes in Cancer
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins BASIC PATHOLOGY. In Robbins Basic Pathology; OOO «GEOTAR-Media» Publishing Group: Moscow, Russia, 2022; pp. 1–1136. [Google Scholar]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and Autophagy-Related Pathways in Cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Dusetti, N.J.; Jiang, Y.; Vaccaro, M.I.; Tomasini, R.; Azizi Samir, A.; Calvo, E.L.; Ropolo, A.; Fiedler, F.; Mallo, G.V.; Dagorn, J.-C.; et al. Cloning and Expression of the Rat Vacuole Membrane Protein 1 (VMP1), a New Gene Activated in Pancreas with Acute Pancreatitis, Which Promotes Vacuole Formation. Biochem. Biophys. Res. Commun. 2002, 290, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Ropolo, A.; Grasso, D.; Pardo, R.; Sacchetti, M.L.; Archange, C.; Lo Re, A.; Seux, M.; Nowak, J.; Gonzalez, C.D.; Iovanna, J.L.; et al. The Pancreatitis-Induced Vacuole Membrane Protein 1 Triggers Autophagy in Mammalian Cells. J. Biol. Chem. 2007, 282, 37124–37133. [Google Scholar] [CrossRef] [PubMed]
- Lo Ré, A.E.; Fernández-Barrena, M.G.; Almada, L.L.; Mills, L.D.; Elsawa, S.F.; Lund, G.; Ropolo, A.; Molejon, M.I.; Vaccaro, M.I.; Fernandez-Zapico, M.E. Novel AKT1-GLI3-VMP1 Pathway Mediates KRAS Oncogene-Induced Autophagy in Cancer Cells. J. Biol. Chem. 2012, 287, 25325–25334. [Google Scholar] [CrossRef] [PubMed]
- Ropolo, A.; Catrinacio, C.; Renna, F.J.; Boggio, V.; Orquera, T.; Gonzalez, C.D.; Vaccaro, M.I. A Novel E2F1-EP300-VMP1 Pathway Mediates Gemcitabine-Induced Autophagy in Pancreatic Cancer Cells Carrying Oncogenic KRAS. Front. Endocrinol. 2020, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Gilabert, M.; Vaccaro, M.I.; Fernandez-Zapico, M.E.; Calvo, E.L.; Turrini, O.; Secq, V.; Garcia, S.; Moutardier, V.; Lomberk, G.; Dusetti, N.; et al. Novel Role of VMP1 as Modifier of the Pancreatic Tumor Cell Response to Chemotherapeutic Drugs. J. Cell. Physiol. 2013, 228, 1834–1843. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of Autophagy and Inhibition of Tumorigenesis by Beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef]
- Aita, V.M.; Liang, X.H.; Murty, V.V.; Pincus, D.L.; Yu, W.; Cayanis, E.; Kalachikov, S.; Gilliam, T.C.; Levine, B. Cloning and Genomic Organization of Beclin 1, a Candidate Tumor Suppressor Gene on Chromosome 17q21. Genomics 1999, 59, 59–65. [Google Scholar] [CrossRef]
- Yue, Z.; Jin, S.; Yang, C.; Levine, A.J.; Heintz, N. Beclin 1, an Autophagy Gene Essential for Early Embryonic Development, Is a Haploinsufficient Tumor Suppressor. Proc. Natl. Acad. Sci. USA 2003, 100, 15077–15082. [Google Scholar] [CrossRef]
- Takamura, A.; Komatsu, M.; Hara, T.; Sakamoto, A.; Kishi, C.; Waguri, S.; Eishi, Y.; Hino, O.; Tanaka, K.; Mizushima, N. Autophagy-Deficient Mice Develop Multiple Liver Tumors. Genes Dev. 2011, 25, 795–800. [Google Scholar] [CrossRef]
- Mathew, R.; White, E. Autophagy, Stress, and Cancer Metabolism: What Doesn’t Kill You Makes You Stronger. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.P.; Macleod, K.F. Mitophagy in Tumorigenesis and Metastasis. Cell. Mol. Life Sci. 2021, 78, 3817–3851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tripathi, D.N.; Jing, J.; Alexander, A.; Kim, J.; Powell, R.T.; Dere, R.; Tait-Mulder, J.; Lee, J.-H.; Paull, T.T.; et al. ATM Functions at the Peroxisome to Induce Pexophagy in Response to ROS. Nat. Cell Biol. 2015, 17, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Karsli-Uzunbas, G.; Poillet-Perez, L.; Sawant, A.; Hu, Z.S.; Zhao, Y.; Moore, D.; Hu, W.; White, E. Autophagy Promotes Mammalian Survival by Suppressing Oxidative Stress and P53. Genes Dev. 2020, 34, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Vakifahmetoglu-Norberg, H.; Kim, M.; Xia, H.-G.; Iwanicki, M.P.; Ofengeim, D.; Coloff, J.L.; Pan, L.; Ince, T.A.; Kroemer, G.; Brugge, J.S.; et al. Chaperone-Mediated Autophagy Degrades Mutant P53. Genes Dev. 2013, 27, 1718–1730. [Google Scholar] [CrossRef]
- Fujii, S.; Mitsunaga, S.; Yamazaki, M.; Hasebe, T.; Ishii, G.; Kojima, M.; Kinoshita, T.; Ueno, T.; Esumi, H.; Ochiai, A. Autophagy Is Activated in Pancreatic Cancer Cells and Correlates with Poor Patient Outcome. Cancer Sci. 2008, 99, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Debnath, J. The Evolving, Multifaceted Roles of Autophagy in Cancer. Adv. Cancer Res. 2016, 130, 1–53. [Google Scholar] [PubMed]
- Guo, J.Y.; Chen, H.-Y.; Mathew, R.; Fan, J.; Strohecker, A.M.; Karsli-Uzunbas, G.; Kamphorst, J.J.; Chen, G.; Lemons, J.M.S.; Karantza, V.; et al. Activated Ras Requires Autophagy to Maintain Oxidative Metabolism and Tumorigenesis. Genes Dev. 2011, 25, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, M.T.; O’Prey, J.; Flossbach, L.; Nixon, C.; Morton, J.P.; Sansom, O.J.; Ryan, K.M. PTEN Deficiency Permits the Formation of Pancreatic Cancer in the Absence of Autophagy. Cell Death Differ. 2017, 24, 1303–1304. [Google Scholar] [CrossRef]
- Rosenfeldt, M.T.; O’Prey, J.; Morton, J.P.; Nixon, C.; MacKay, G.; Mrowinska, A.; Au, A.; Rai, T.S.; Zheng, L.; Ridgway, R.; et al. P53 Status Determines the Role of Autophagy in Pancreatic Tumour Development. Nature 2013, 504, 296–300. [Google Scholar] [CrossRef]
- Bhatt, V.; Khayati, K.; Hu, Z.S.; Lee, A.; Kamran, W.; Su, X.; Guo, J.Y. Autophagy Modulates Lipid Metabolism to Maintain Metabolic Flexibility for Lkb1-Deficient Kras-Driven Lung Tumorigenesis. Genes Dev. 2019, 33, 150–165. [Google Scholar] [CrossRef]
- La Belle Flynn, A.; Calhoun, B.C.; Sharma, A.; Chang, J.C.; Almasan, A.; Schiemann, W.P. Autophagy Inhibition Elicits Emergence from Metastatic Dormancy by Inducing and Stabilizing Pfkfb3 Expression. Nat. Commun. 2019, 10, 3668. [Google Scholar] [CrossRef]
- Aqbi, H.F.; Tyutyunyk-Massey, L.; Keim, R.C.; Butler, S.E.; Thekkudan, T.; Joshi, S.; Smith, T.M.; Bandyopadhyay, D.; Idowu, M.O.; Bear, H.D.; et al. Autophagy-Deficient Breast Cancer Shows Early Tumor Recurrence and Escape from Dormancy. Oncotarget 2018, 9, 22113–22122. [Google Scholar] [CrossRef]
- Qiang, L.; Zhao, B.; Ming, M.; Wang, N.; He, T.-C.; Hwang, S.; Thorburn, A.; He, Y.-Y. Regulation of Cell Proliferation and Migration by P62 through Stabilization of Twist1. Proc. Natl. Acad. Sci. USA 2014, 111, 9241–9246. [Google Scholar] [CrossRef]
- Marsh, T.; Kenific, C.M.; Suresh, D.; Gonzalez, H.; Shamir, E.R.; Mei, W.; Tankka, A.; Leidal, A.M.; Kalavacherla, S.; Woo, K.; et al. Autophagic Degradation of NBR1 Restricts Metastatic Outgrowth during Mammary Tumor Progression. Dev. Cell 2020, 52, 591–604.e6. [Google Scholar] [CrossRef] [PubMed]
- Karsli-Uzunbas, G.; Guo, J.Y.; Price, S.; Teng, X.; Laddha, S.V.; Khor, S.; Kalaany, N.Y.; Jacks, T.; Chan, C.S.; Rabinowitz, J.D.; et al. Autophagy Is Required for Glucose Homeostasis and Lung Tumor Maintenance. Cancer Discov. 2014, 4, 914–927. [Google Scholar] [CrossRef] [PubMed]
- Poillet-Perez, L.; Xie, X.; Zhan, L.; Yang, Y.; Sharp, D.W.; Hu, Z.S.; Su, X.; Maganti, A.; Jiang, C.; Lu, W.; et al. Autophagy Maintains Tumour Growth through Circulating Arginine. Nature 2018, 563, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.M.; Biancur, D.E.; Wang, X.; Halbrook, C.J.; Sherman, M.H.; Zhang, L.; Kremer, D.; Hwang, R.F.; Witkiewicz, A.K.; Ying, H.; et al. Pancreatic Stellate Cells Support Tumour Metabolism through Autophagic Alanine Secretion. Nature 2016, 536, 479–483. [Google Scholar] [CrossRef]
- Delage, B.; Fennell, D.A.; Nicholson, L.; McNeish, I.; Lemoine, N.R.; Crook, T.; Szlosarek, P.W. Arginine Deprivation and Argininosuccinate Synthetase Expression in the Treatment of Cancer. Int. J. Cancer 2010, 126, 2762–2772. [Google Scholar] [CrossRef]
- New, J.; Arnold, L.; Ananth, M.; Alvi, S.; Thornton, M.; Werner, L.; Tawfik, O.; Dai, H.; Shnayder, Y.; Kakarala, K.; et al. Secretory Autophagy in Cancer-Associated Fibroblasts Promotes Head and Neck Cancer Progression and Offers a Novel Therapeutic Target. Cancer Res. 2017, 77, 6679–6691. [Google Scholar] [CrossRef]
- Clancy, J.W.; D’Souza-Schorey, C. Tumor-Derived Extracellular Vesicles: Multifunctional Entities in the Tumor Microenvironment. Annu. Rev. Pathol. 2023, 18, 205–229. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Green, D.R.; Zou, W. Autophagy in Tumour Immunity and Therapy. Nat. Rev. Cancer 2021, 21, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Molejon, M.I.; Ropolo, A.; Re, A.L.; Boggio, V.; Vaccaro, M.I. The VMP1-Beclin 1 Interaction Regulates Autophagy Induction. Sci. Rep. 2013, 3, 1055. [Google Scholar] [CrossRef] [PubMed]
- Nascimbeni, A.C.; Giordano, F.; Dupont, N.; Grasso, D.; Vaccaro, M.I.; Codogno, P.; Morel, E. ER –Plasma Membrane Contact Sites Contribute to Autophagosome Biogenesis by Regulation of Local PI 3P Synthesis. EMBO J. 2017, 36, 2018–2033. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.G.; Chen, Y.; Miao, G.; Zhao, H.; Qu, W.; Li, D.; Wang, Z.; Liu, N.; Li, L.; Chen, S.; et al. The ER-Localized Transmembrane Protein EPG-3/VMP1 Regulates SERCA Activity to Control ER-Isolation Membrane Contacts for Autophagosome Formation. Mol. Cell 2017, 67, 974–989.e6. [Google Scholar] [CrossRef] [PubMed]
- Grasso, D.; Ropolo, A.; Lo Ré, A.; Boggio, V.; Molejón, M.I.; Iovanna, J.L.; Gonzalez, C.D.; Urrutia, R.; Vaccaro, M.I. Zymophagy, a Novel Selective Autophagy Pathway Mediated by VMP1-USP9x-P62, Prevents Pancreatic Cell Death. J. Biol. Chem. 2011, 286, 8308–8324. [Google Scholar] [CrossRef] [PubMed]
- Vanasco, V.; Ropolo, A.; Grasso, D.; Ojeda, D.S.; García, M.N.; Vico, T.A.; Orquera, T.; Quarleri, J.; Alvarez, S.; Vaccaro, M.I. Mitochondrial Dynamics and VMP1-Related Selective Mitophagy in Experimental Acute Pancreatitis. Front. Cell Dev. Biol. 2021, 9, 640094. [Google Scholar] [CrossRef] [PubMed]
- Renna, F.J.; Enriqué Steinberg, J.H.; Gonzalez, C.D.; Manifava, M.; Tadic, M.S.; Orquera, T.; Vecino, C.V.; Ropolo, A.; Guardavaccaro, D.; Rossi, M.; et al. Ubiquitination Is a Novel Post-Translational Modification of VMP1 in Autophagy of Human Tumor Cells. Int. J. Mol. Sci. 2023, 24, 12981. [Google Scholar] [CrossRef]
- Ghanbarpour, A.; Valverde, D.P.; Melia, T.J.; Reinisch, K.M. A Model for a Partnership of Lipid Transfer Proteins and Scramblases in Membrane Expansion and Organelle Biogenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2101562118. [Google Scholar] [CrossRef]
- Li, Y.E.; Wang, Y.; Du, X.; Zhang, T.; Mak, H.Y.; Hancock, S.E.; McEwen, H.; Pandzic, E.; Whan, R.M.; Aw, Y.C.; et al. TMEM41B and VMP1 Are Scramblases and Regulate the Distribution of Cholesterol and Phosphatidylserine. J. Cell Biol. 2021, 220, e202103105. [Google Scholar] [CrossRef]
- Bai, R.; Rebelo, A.; Kleeff, J.; Sunami, Y. Identification of Prognostic Lipid Droplet-Associated Genes in Pancreatic Cancer Patients via Bioinformatics Analysis. Lipids Health Dis. 2021, 20, 58. [Google Scholar] [CrossRef]
- Loncle, C.; Molejon, M.I.; Lac, S.; Tellechea, J.I.; Lomberk, G.; Gramatica, L.; Fernandez Zapico, M.F.; Dusetti, N.; Urrutia, R.; Iovanna, J.L. The Pancreatitis-Associated Protein VMP1, a Key Regulator of Inducible Autophagy, Promotes KrasG12D-Mediated Pancreatic Cancer Initiation. Cell Death Dis. 2016, 7, e2295. [Google Scholar] [CrossRef] [PubMed]
- Pardo, R.; Lo Ré, A.; Archange, C.; Ropolo, A.; Papademetrio, D.L.; Gonzalez, C.D.; Alvarez, E.M.; Iovanna, J.L.; Vaccaro, M.I. Gemcitabine Induces the VMP1-Mediated Autophagy Pathway to Promote Apoptotic Death in Human Pancreatic Cancer Cells. Pancreatology 2010, 10, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Park, A.Y.; Han, M.-R.; Seo, B.K.; Ju, H.-Y.; Son, G.S.; Lee, H.Y.; Chang, Y.W.; Choi, J.; Cho, K.R.; Song, S.E.; et al. MRI-Based Breast Cancer Radiogenomics Using RNA Profiling: Association with Subtypes in a Single-Center Prospective Study. Breast Cancer Res. 2023, 25, 79. [Google Scholar] [CrossRef] [PubMed]
- Amirfallah, A.; Arason, A.; Einarsson, H.; Gudmundsdottir, E.T.; Freysteinsdottir, E.S.; Olafsdottir, K.A.; Johannsson, O.T.; Agnarsson, B.A.; Barkardottir, R.B.; Reynisdottir, I. High Expression of the Vacuole Membrane Protein 1 (VMP1) Is a Potential Marker of Poor Prognosis in HER2 Positive Breast Cancer. PLoS ONE 2019, 14, e0221413. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Zhou, H.; Chen, Y.; Shen, C.; He, S.; Zhao, H.; Wang, L.; Wan, D.; Gu, W. VMP1 Related Autophagy and Apoptosis in Colorectal Cancer Cells: VMP1 Regulates Cell Death. Biochem. Biophys. Res. Commun. 2014, 443, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.E.; Catrinacio, C.; Ropolo, A.; Rivarola, V.A.; Vaccaro, M.I. A Novel HIF-1α/VMP1-Autophagic Pathway Induces Resistance to Photodynamic Therapy in Colon Cancer Cells. Photochem. Photobiol. Sci. 2017, 16, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yang, Z.; Chen, G.; Huang, J. VMP1 Promotes Exosome Secretion and Enhances 5-FU Resistance in Colon Cancer Cells. Tissue Cell 2022, 77, 101851. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-Z.; Ye, X.-L.; Xiao, W.-Z.; Wei, X.-N.; You, Q.-H.; Che, X.-H.; Cai, Y.-J.; Chen, F.; Yuan, H.; Liu, X.-J.; et al. Downregulation of VMP1 Confers Aggressive Properties to Colorectal Cancer. Oncol. Rep. 2015, 34, 2557–2566. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Gu, J.; Li, X.; Xue, C.; Ba, L.; Gao, Y.; Zhou, J.; Bai, C.; Sun, Z.; Zhao, R.C. HIF-1α Promotes the Migration and Invasion of Cancer-Associated Fibroblasts by MiR-210. Aging Dis. 2021, 12, 1794–1807. [Google Scholar] [CrossRef]
- Ying, Q.; Liang, L.; Guo, W.; Zha, R.; Tian, Q.; Huang, S.; Yao, J.; Ding, J.; Bao, M.; Ge, C.; et al. Hypoxia-Inducible MicroRNA-210 Augments the Metastatic Potential of Tumor Cells by Targeting Vacuole Membrane Protein 1 in Hepatocellular Carcinoma. Hepatology 2011, 54, 2064–2075. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, L.-Y.; Fan, C.; Chen, G.-D.; Wu, F. Novel Roles of Vmp1: Inhibition Metastasis and Proliferation of Hepatocellular Carcinoma. Cancer Sci. 2012, 103, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jing, J.; Li, H.; Liu, J.; Yuan, Y.; Sun, L. The Expression Characteristics and Prognostic Roles of Autophagy-Related Genes in Gastric Cancer. PeerJ 2021, 9, e10814. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, W.; Zhang, K.; Fan, M.; Lin, R. Epigenetic and Tumor Microenvironment for Prognosis of Patients with Gastric Cancer. Biomolecules 2023, 13, 736. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.E.; Venkitachalam, S.; Guo, Y.; Kieber-Emmons, A.M.; Ravi, L.; Chandar, A.K.; Iyer, P.G.; Canto, M.I.; Wang, J.S.; Shaheen, N.J.; et al. RNA Sequencing Identifies Transcriptionally Viable Gene Fusions in Esophageal Adenocarcinomas. Cancer Res. 2016, 76, 5628–5633. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Chen, L.; Zhang, X.; Zhan, J.; Chen, J. TMEM49-Related Apoptosis and Metastasis in Ovarian Cancer and Regulated Cell Death. Mol. Cell. Biochem. 2016, 416, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhao, L.; Chen, W.; Li, Z.; Hou, H.; Ding, L.; Li, X. Inactivation of von Hippel-Lindau Increases Ovarian Cancer Cell Aggressiveness through the HIF1α/MiR-210/VMP1 Signaling Pathway. Int. J. Mol. Med. 2014, 33, 1236–1242. [Google Scholar] [CrossRef]
- Lin, W.; Sun, Y.; Qiu, X.; Huang, Q.; Kong, L.; Lu, J.J. VMP1, a Novel Prognostic Biomarker, Contributes to Glioma Development by Regulating Autophagy. J. Neuroinflammation 2021, 18, 165. [Google Scholar] [CrossRef] [PubMed]
- Volinia, S.; Calin, G.A.; Liu, C.-G.; Ambs, S.; Cimmino, A.; Petrocca, F.; Visone, R.; Iorio, M.; Roldo, C.; Ferracin, M.; et al. A MicroRNA Expression Signature of Human Solid Tumors Defines Cancer Gene Targets. Proc. Natl. Acad. Sci. USA 2006, 103, 2257–2261. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, V.N. Processing of Intronic MicroRNAs. EMBO J. 2007, 26, 775–783. [Google Scholar] [CrossRef]
- Cai, X.; Hagedorn, C.H.; Cullen, B.R. Human MicroRNAs Are Processed from Capped, Polyadenylated Transcripts That Can Also Function as MRNAs. RNA 2004, 10, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Löffler, D.; Brocke-Heidrich, K.; Pfeifer, G.; Stocsits, C.; Hackermüller, J.; Kretzschmar, A.K.; Burger, R.; Gramatzki, M.; Blumert, C.; Bauer, K.; et al. Interleukin-6 Dependent Survival of Multiple Myeloma Cells Involves the Stat3-Mediated Induction of MicroRNA-21 through a Highly Conserved Enhancer. Blood 2007, 110, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Ozsolak, F.; Poling, L.L.; Wang, Z.; Liu, H.; Liu, X.S.; Roeder, R.G.; Zhang, X.; Song, J.S.; Fisher, D.E. Chromatin Structure Analyses Identify MiRNA Promoters. Genes Dev. 2008, 22, 3172–3183. [Google Scholar] [CrossRef] [PubMed]
- Ribas, J.; Ni, X.; Castanares, M.; Liu, M.M.; Esopi, D.; Yegnasubramanian, S.; Rodriguez, R.; Mendell, J.T.; Lupold, S.E. A Novel Source for MiR-21 Expression through the Alternative Polyadenylation of VMP1 Gene Transcripts. Nucleic Acids Res. 2012, 40, 6821–6833. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Sinclair, C.; Hinson, S.; Ingle, J.N.; Roche, P.C.; Couch, F.J. Structural Analysis of the 17q22-23 Amplicon Identifies Several Independent Targets of Amplification in Breast Cancer Cell Lines and Tumors. Cancer Res. 2001, 61, 4951–4955. [Google Scholar] [PubMed]
- Fujita, S.; Ito, T.; Mizutani, T.; Minoguchi, S.; Yamamichi, N.; Sakurai, K.; Iba, H. MiR-21 Gene Expression Triggered by AP-1 Is Sustained through a Double-Negative Feedback Mechanism. J. Mol. Biol. 2008, 378, 492–504. [Google Scholar] [CrossRef]
- Ribas, J.; Lupold, S.E. The Transcriptional Regulation of MiR-21, Its Multiple Transcripts, and Their Implication in Prostate Cancer. Cell Cycle 2010, 9, 923–929. [Google Scholar] [CrossRef]
- Ribas, J.; Ni, X.; Haffner, M.; Wentzel, E.A.; Salmasi, A.H.; Chowdhury, W.H.; Kudrolli, T.A.; Yegnasubramanian, S.; Luo, J.; Rodriguez, R.; et al. MiR-21: An Androgen Receptor-Regulated MicroRNA That Promotes Hormone-Dependent and Hormone-Independent Prostate Cancer Growth. Cancer Res. 2009, 69, 7165–7169. [Google Scholar] [CrossRef]
- Bhat-Nakshatri, P.; Wang, G.; Collins, N.R.; Thomson, M.J.; Geistlinger, T.R.; Carroll, J.S.; Brown, M.; Hammond, S.; Srour, E.F.; Liu, Y.; et al. Estradiol-Regulated MicroRNAs Control Estradiol Response in Breast Cancer Cells. Nucleic Acids Res. 2009, 37, 4850–4861. [Google Scholar] [CrossRef]
- Medina, P.P.; Nolde, M.; Slack, F.J. OncomiR Addiction in an in Vivo Model of MicroRNA-21-Induced Pre-B-Cell Lymphoma. Nature 2010, 467, 86–90. [Google Scholar] [CrossRef]
- Hatley, M.E.; Patrick, D.M.; Garcia, M.R.; Richardson, J.A.; Bassel-Duby, R.; van Rooij, E.; Olson, E.N. Modulation of K-Ras-Dependent Lung Tumorigenesis by MicroRNA-21. Cancer Cell 2010, 18, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kumar, M.; Choudhury, S.N.; Becker Buscaglia, L.E.; Barker, J.R.; Kanakamedala, K.; Liu, M.-F.; Li, Y. Loss of the MiR-21 Allele Elevates the Expression of Its Target Genes and Reduces Tumorigenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 10144–10149. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Peng, R.; Zeng, M.; Zhang, Z.; Liu, S.; Jiang, D.; Lu, Y.; Zou, F. An Autoregulatory Feedback Loop of MiR-21/VMP1 Is Responsible for the Abnormal Expression of MiR-21 in Colorectal Cancer Cells. Cell Death Dis. 2020, 11, 1067. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Seubert, B.; Stahl, E.; Dietz, H.; Reuning, U.; Moreno-Leon, L.; Ilie, M.; Hofman, P.; Nagase, H.; Mari, B.; et al. Tissue Inhibitor of Metalloproteinases-1 Induces a pro-Tumourigenic Increase of MiR-210 in Lung Adenocarcinoma Cells and Their Exosomes. Oncogene 2015, 34, 3640–3650. [Google Scholar] [CrossRef] [PubMed]
- Qu, A.; Du, L.; Yang, Y.; Liu, H.; Li, J.; Wang, L.; Liu, Y.; Dong, Z.; Zhang, X.; Jiang, X.; et al. Hypoxia-Inducible MiR-210 Is an Independent Prognostic Factor and Contributes to Metastasis in Colorectal Cancer. PLoS ONE 2014, 9, e90952. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-N.; Tang, Y.-L.; Ke, Z.-Y.; Chen, Y.-Q.; Luo, X.-Q.; Zhang, H.; Huang, L.-B. MiR-124 Contributes to Glucocorticoid Resistance in Acute Lymphoblastic Leukemia by Promoting Proliferation, Inhibiting Apoptosis and Targeting the Glucocorticoid Receptor. J. Steroid Biochem. Mol. Biol. 2017, 172, 62–68. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Y.; Liu, L.; Yang, W.; Zhang, Q. HNF1A-AS1 Regulates Cell Migration, Invasion and Glycolysis via Modulating MiR-124/MYO6 in Colorectal Cancer Cells. OncoTargets Ther. 2020, 13, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhuo, H.; Xu, M.; Wang, L.; Xu, H.; Peng, J.; Hou, J.; Lin, L.; Cai, J. Regulatory Network of CircRNA–MiRNA–MRNA Contributes to the Histological Classification and Disease Progression in Gastric Cancer. J. Transl. Med. 2018, 16, 216. [Google Scholar] [CrossRef]
- Long, H.-D.; Ma, Y.-S.; Yang, H.-Q.; Xue, S.-B.; Liu, J.-B.; Yu, F.; Lv, Z.-W.; Li, J.-Y.; Xie, R.-T.; Chang, Z.-Y.; et al. Reduced Hsa-MiR-124-3p Levels Are Associated with the Poor Survival of Patients with Hepatocellular Carcinoma. Mol. Biol. Rep. 2018, 45, 2615–2623. [Google Scholar] [CrossRef]
- Pang, Y.; Wu, J.; Li, X.; Wang, C.; Wang, M.; Liu, J.; Yang, G. NEAT1/MiR-124/STAT3 Feedback Loop Promotes Breast Cancer Progression. Int. J. Oncol. 2019, 55, 745–754. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, W.K.; Lee, E.B.; Son, J.W.; Kim, D.S.; Park, J.Y. Combined Effect of Metastasis-Related MicroRNA, MiR-34 and MiR-124 Family, Methylation on Prognosis of Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2017, 18, e13–e20. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Z.; Li, K.; Wang, P.; Chen, Y.; Deng, S.; Li, W.; Yu, K.; Wang, K. VMP1 Regulated by Chi-MiR-124a Effects Goat Myoblast Proliferation, Autophagy, and Apoptosis through the PI3K/ULK1/MTOR Signaling Pathway. Cells 2022, 11, 2227. [Google Scholar] [CrossRef] [PubMed]
- Mertens, F.; Johansson, B.; Fioretos, T.; Mitelman, F. The Emerging Complexity of Gene Fusions in Cancer. Nat. Rev. Cancer 2015, 15, 371–381. [Google Scholar] [CrossRef]
- Inaki, K.; Hillmer, A.M.; Ukil, L.; Yao, F.; Woo, X.Y.; Vardy, L.A.; Zawack, K.F.B.; Lee, C.W.H.; Ariyaratne, P.N.; Chan, Y.S.; et al. Transcriptional Consequences of Genomic Structural Aberrations in Breast Cancer. Genome Res. 2011, 21, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Nik-Zainal, S.; Davies, H.; Staaf, J.; Ramakrishna, M.; Glodzik, D.; Zou, X.; Martincorena, I.; Alexandrov, L.B.; Martin, S.; Wedge, D.C.; et al. Landscape of Somatic Mutations in 560 Breast Cancer Whole-Genome Sequences. Nature 2016, 534, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Shah, S.P.; Chin, S.-F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The Genomic and Transcriptomic Architecture of 2000 Breast Tumours Reveals Novel Subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef]
- Rueda, O.M.; Sammut, S.-J.; Seoane, J.A.; Chin, S.-F.; Caswell-Jin, J.L.; Callari, M.; Batra, R.; Pereira, B.; Bruna, A.; Ali, H.R.; et al. Dynamics of Breast-Cancer Relapse Reveal Late-Recurring ER-Positive Genomic Subgroups. Nature 2019, 567, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, C.P.; Sun, S.; Varma, S.; Shain, A.H.; Giacomini, M.M.; Balagtas, J.; Sweeney, R.T.; Lai, E.; Del Vecchio, C.A.; Forster, A.D.; et al. Breakpoint Analysis of Transcriptional and Genomic Profiles Uncovers Novel Gene Fusions Spanning Multiple Human Cancer Types. PLoS Genet. 2013, 9, e1003464. [Google Scholar] [CrossRef] [PubMed]
- Kangaspeska, S.; Hultsch, S.; Edgren, H.; Nicorici, D.; Murumägi, A.; Kallioniemi, O. Reanalysis of RNA-Sequencing Data Reveals Several Additional Fusion Genes with Multiple Isoforms. PLoS ONE 2012, 7, e48745. [Google Scholar] [CrossRef]
- Ali, N.M.; Niada, S.; Brini, A.T.; Morris, M.R.; Kurusamy, S.; Alholle, A.; Huen, D.; Antonescu, C.R.; Tirode, F.; Sumathi, V.; et al. Genomic and Transcriptomic Characterisation of Undifferentiated Pleomorphic Sarcoma of Bone. J. Pathol. 2019, 247, 166–176. [Google Scholar] [CrossRef]
- Persson, H.; Søkilde, R.; Häkkinen, J.; Pirona, A.C.; Vallon-Christersson, J.; Kvist, A.; Mertens, F.; Borg, Å.; Mitelman, F.; Höglund, M.; et al. Frequent MiRNA-Convergent Fusion Gene Events in Breast Cancer. Nat. Commun. 2017, 8, 788. [Google Scholar] [CrossRef] [PubMed]
- Khalilian, S.; Bijanvand, A.; Abedinlou, H.; Ghafouri-Fard, S. A Review on the Role of MiR-210 in Human Disorders. Pathol. Res. Pract. 2023, 241, 154244. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Liu, X.; Wang, X.; Li, Z.; Zhou, L.; Li, H. Palmitoylation of Vacuole Membrane Protein 1 Promotes Small Extracellular Vesicle Secretion via Interaction with ALIX and Influences Intercellular Communication. Cell Commun. Signal. 2024, 22, 150. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renna, F.J.; Gonzalez, C.D.; Vaccaro, M.I. Decoding the Versatile Landscape of Autophagic Protein VMP1 in Cancer: A Comprehensive Review across Tissue Types and Regulatory Mechanisms. Int. J. Mol. Sci. 2024, 25, 3758. https://doi.org/10.3390/ijms25073758
Renna FJ, Gonzalez CD, Vaccaro MI. Decoding the Versatile Landscape of Autophagic Protein VMP1 in Cancer: A Comprehensive Review across Tissue Types and Regulatory Mechanisms. International Journal of Molecular Sciences. 2024; 25(7):3758. https://doi.org/10.3390/ijms25073758
Chicago/Turabian StyleRenna, Felipe J., Claudio D. Gonzalez, and Maria I. Vaccaro. 2024. "Decoding the Versatile Landscape of Autophagic Protein VMP1 in Cancer: A Comprehensive Review across Tissue Types and Regulatory Mechanisms" International Journal of Molecular Sciences 25, no. 7: 3758. https://doi.org/10.3390/ijms25073758
APA StyleRenna, F. J., Gonzalez, C. D., & Vaccaro, M. I. (2024). Decoding the Versatile Landscape of Autophagic Protein VMP1 in Cancer: A Comprehensive Review across Tissue Types and Regulatory Mechanisms. International Journal of Molecular Sciences, 25(7), 3758. https://doi.org/10.3390/ijms25073758






