Abstract
Chagas disease (CD) is a vector-borne Neglected Zoonotic Disease (NZD) caused by a flagellate protozoan, Trypanosoma cruzi, that affects various mammalian species across America, including humans and domestic animals. However, due to an increase in population movements and new routes of transmission, T. cruzi infection is presently considered a worldwide health concern, no longer restricted to endemic countries. Dogs play a major role in the domestic cycle by acting very efficiently as reservoirs and allowing the perpetuation of parasite transmission in endemic areas. Despite the significant progress made in recent years, still there is no vaccine against human and animal disease, there are few drugs available for the treatment of human CD, and there is no standard protocol for the treatment of canine CD. In this review, we highlight human and canine Chagas Disease in its different dimensions and interconnections. Dogs, which are considered to be the most important peridomestic reservoir and sentinel for the transmission of T. cruzi infection in a community, develop CD that is clinically similar to human CD. Therefore, an integrative approach, based on the One Health concept, bringing together the advances in genomics, immunology, and epidemiology can lead to the effective development of vaccines, new treatments, and innovative control strategies to tackle CD.
1. Introduction
Chagas disease (CD) or American trypanosomiasis, caused by a flagellated protozoan, Trypanosoma cruzi, is one of the most Neglected Zoonotic Diseases (NZD). According to recent data, CD has an annual incidence of 30,000 new cases in 21 Latin American countries, affecting nearly 6 million people, and causing on average 12,000 deaths annually. Furthermore, an estimated 8600 newborns become infected during gestation [1]. The efforts of the last decades have resulted in vectorial control in Central America. However, while the prevalence has been reduced in endemic areas, a significant increase in non-endemic countries has been observed due to the massive influx of Latin American migrants to Asia, North America, Oceania, and Europe, especially to Spain, Portugal, and Italy [2,3], making the disease a global health issue. In non-endemic countries, blood transfusion, organ transplantation, or vertical transmission from mother to child are the main forms of transmission of infection [4]. Addressing CD is also challenging due to the heterogeneity of healthcare systems and a substantial number of underdiagnosed and undertreated individuals in non-endemic areas [3]. The diagnosis depends on the disease’s stage and, classically, two clinical phases are defined—acute and chronic [5]. The first is difficult to characterize clinically as it is usually asymptomatic and, if there are manifestations, they are transient and non-specific, so tend to be overlooked/disregarded [6]. The chronic phase is usually associated with permanent alterations in the nervous and digestive systems as well as severe cardiac modifications [6,7] and may last the patient’s entire life [8].
T. cruzi infections affect a diverse variety of hosts including humans and domestic animals, such as horses, pigs, cats, and dogs. Moreover, the multifaceted nature of human–animal relationships is constantly evolving, influenced by climate changes, anthropogenic impacts and natural factors. The increase in travel and tourism and the international trade of live animals as pets or as part of breeding programs for endangered wildlife species constitute major factors impacting the epidemiology of CD and posing important challenges to veterinary sciences [9]. The peridomestic cycle of the parasite plays an important role since infection of those species can indicate the presence of an active T. cruzi transmission cycle and represent an increased risk for human infection, as observed in Latin America and the United States of America [10,11]. Also, in recent years, T. cruzi, traditionally considered a vector-borne disease (VBD), has been found to be able to infect humans through the ingestion of contaminated food/drink, elevating CD to a global challenge due to large-scale food production, processing, and worldwide distribution [12]. Research on T. cruzi has been focused on developing new treatment and prophylaxis strategies, understanding the biology and genetics of the parasite, and investigating the transmission dynamics of CD. Still, there are few treatment options and no commercially available diagnostic tests to detect T. cruzi infections in dogs [13]. Besides that, at present time, there is no prophylactic or therapeutical vaccine against human or canine CD.
2. The Dog’s Role in T. cruzi Complex Life
The protozoan T. cruzi, the causative agent of CD, has a complex life cycle not only due to the parasite’s ability to infect a wide variety of mammals but also to the multiple ways of transmission (Figure 1). Extensive environmental changes, such as urbanization and deforestation in CD endemic areas, increased the likelihood of outbreaks [14]. The most studied form of CD transmission is vectorial, by the triatomine, also known as the ‘kissing bug’. The triatomine can effectively infect more than 180 species of mammals [2,15], Triatoma dimidiata, Triatoma infestans and Rhodnius prolixus being the most medically concerning species in Central and South America [16,17,18,19]. These hematophagous and nocturnal insects feed on humans, domestic animals such as dogs and cats, or wild animals, including armadillos, raccoons, and rats [20,21,22].
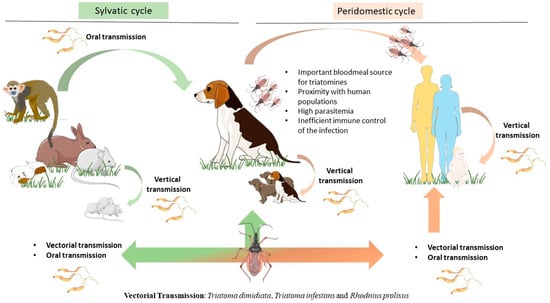
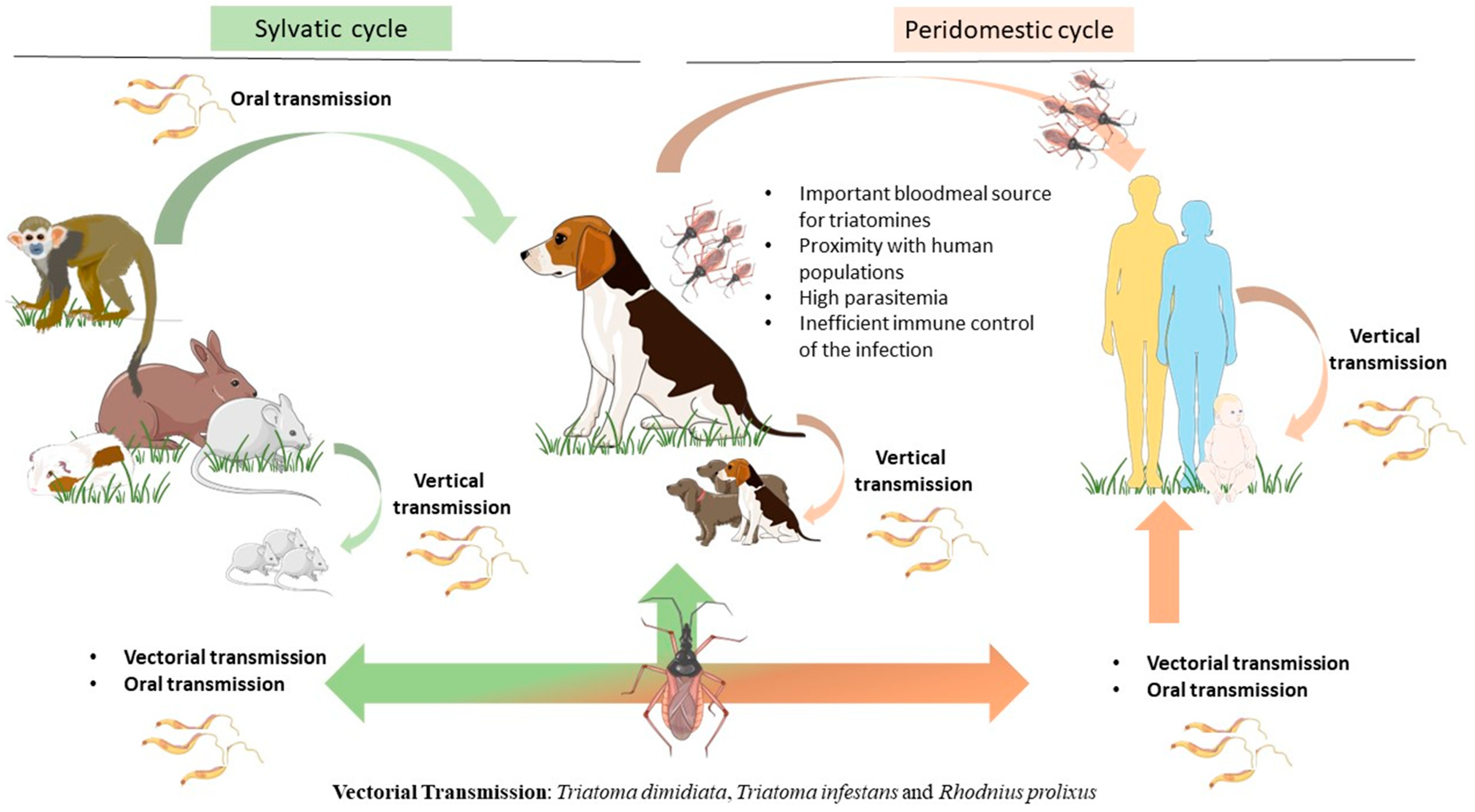
Figure 1.
Epidemiological importance of the dog as the main peridomestic reservoir of T. cruzi parasites. Dogs can be infected by T. cruzi directly by vectorial transmission. However, triatomines can feed on a huge variety of mammals, including humans and dogs as well as sylvatic animals such as rats, guinea pigs, monkeys, raccoons, and armadillos. Infected mammals can transmit T. cruzi by vertical transmission, perpetuating the parasite cycle. In more recent years, a new transmission route by ingestion of infected animal tissue or vector-contaminated food or drinks has emerged as a major transmission route for T. cruzi. Dogs play a key role as peridomestic reservoirs of T. cruzi parasites, as they live close to humans, constitute an important bloodmeal source for triatomines, and also are susceptible hosts to T. cruzi infection. The figure was partly drawn using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 4.0 unported license (https://creativecommons.org/licenses/by/4.0/).
The vectorial transmission is initiated when triatomine (either male or female) feeds on an infected host and ingests blood trypomastigotes (BTs). In the insect’s midgut, BTs differentiate into highly replicative epimastigote forms. The differentiation from epimastigotes into infective forms (metacyclic trypomastigotes, MTs, metacyclogenesis) is accomplished only in the rectal ampulla, from where MTs are excreted within the feces. When this infected triatomine feeds again from another mammal, it deposits its MT-contaminated feces near the feed-borne wound [23,24]. By defecating while feeding, the triatomine creates in the host an entry point for the infectious parasite, potentiating the chance of infection (stercorarian transmission) [25]. Once in the host, MTs enter the mammal’s blood, invade host cells (macrophages, smooth and striated muscle cells), and begin their intracellular progression from MTs into amastigotes (AMs) [26,27]. AMs multiply by binary fission in the cell’s cytoplasm and differentiate into BTs. Such multiplication causes cell rupture, freeing these forms into the mammal’s bloodstream and allowing them to infect new cells [28]. AMs can establish themselves in muscle tissue, causing several clinical signs associated with the chronic phase of the disease [23].
When it comes to dogs, it is not clear that vectorial transmission is the most common route on the peridomestic cycle. Many authors suspect that oral transmission, either by directly eating the infected vectors, ingesting meat from infected mammals, or feeding on a parasite-infected lactating mother, might be the most frequent [29,30]. The oral-transmitted cycle begins with the intake of elements containing either macerated triatomines or contaminated secretions [31]. Several regions have reported cases of T. cruzi infection outbreaks in human populations linked to the ingestion of drinks and food contaminated with infected mammals’ secretions or triatomine feces [32,33,34]. Once in the mammal’s mouth or nose cavity, the parasites’ transalidases can adhere to the sialic acids in the palate or go through the digestive tract. If they stick to the palate, they efficiently replicate in the nasal cavity, enter the bloodstream or nasal nerves, and have the direct possibility to invade and establish into the muscle tissue (cardiomyocytes, for example) as AMs. If they reach the stomach, MTs adhere to the gastric mucosal epithelium and make their way to other organs. T. cruzi can now invade surrounding cells, transform into AMs, multiply, and generate permanent pseudocysts or differentiate again and infect new cells [33,35,36]. Both pathways most certainly lead to chronic CD once the AMs form enough pseudocysts, causing many life-threatening manifestations such as organomegaly and cardiac dysfunctions, depending on which tissues are parasitized [29,37,38].
Within the realm of animal sentinel systems, particularly dogs, there is a prominent role in using them for assessing human exposure and environmental risks and monitoring complex ecosystems. The suitability of an animal species as a sentinel hinges on various critical characteristics. Ideally, an effective sentinel should exhibit susceptibility to the pathogen and its responses should be quantifiable. Additionally, it should occupy a territorial domain or “home range” that aligns with the target monitoring area. The sentinel species should be readily accessible, easily countable, and capture-friendly. Moreover, a significant population size or density is essential to enable the collection of representative samples. These criteria underscore the importance of selecting the most appropriate sentinel species for successful environmental surveillance and risk assessment endeavors, ultimately improving both human and dog living conditions [39]. In this context, dogs are considered simultaneously the most important peridomestic reservoir and sentinel for the transmission of T. cruzi infection. They live in close proximity to humans, constituting an important bloodmeal source for triatomine bugs, and they are also highly susceptible to T. cruzi infection [40]. Additionally, exhibits high parasitemia in the acute phase of the disease [41], mostly associated with a deficient innate immune response. Due to prolonged outdoor staying, where vectors could be present, shelter and stray dogs are more likely to encounter T. cruzi. Moreover, wildlife species involved in T. cruzi transmission often inhabit areas around human dwellings, where dogs may come into contact with infected vectors or even preying on infected mammals [42,43]. Interestingly, T. cruzi infections do not show strong breed associations, indicating that any exposed dog is susceptible to infection [44]. Thus, the reservoir competence of dogs for T. cruzi seems to be related not only to their living conditions but also to age and poor external clinical aspect (ECA) based on nutritional condition parameters, such as the degree of muscle development, external evidence of bone structures, state of the animal hair, and existence of fatty deposits. According to Petersen and colleagues [45], dogs with a bad ECA had a 2.6 to 6.3 times greater probability of infecting T. infestans after a bloodmeal, which strongly contributes to increasing the transmission risk inside human dwellings [46,47,48].
3. T. cruzi: A Parasite with Multiple Identities?
Considered by many to be a very taxonomically diverse species, incorporating a vast group of strains, the CD causative agent has been classified as a protozoan belonging to the Kinetoplastea class, Trypanosomatida order, Tripanosomatidae family, Trypanosoma genus and Trypanosoma cruzi species [49]. Antigenic variation is one of the abilities demonstrated by this parasite to evade the mammal’s host immune system. This mechanism occurs through the alteration of its surface glycoproteins, using plenty of molecules such as mucins, gp63 peptidases, mucin-associated surface proteins (MASPs), dispersed gene family 1 (DGF-1), and trans-sialidases enzymes (TS). TS is considered the most important molecule in this process. More specifically, by acquiring sialic residues from host glycoconjugates and installing them in the form of a coat of sialylated molecules, the parasite mimics the host’s glycocalyx. This maneuver constitutes a crucial parasite survival strategy and demonstrates its close adaptation to the mammal host. The genetic diversity of T. cruzi is compiled into seven discrete typing units (DTUs), from TcI to TcVI and TcBat, each one of these being defined by Veláquez-Ortiz and Ramírez (2020) [33] as “a group of strains that share genetic features and can be identified using a set of specific genetic markers”.
The regulation of TS enzymes’ expression has been proven to be different across the known DTUs. This regulation occurs both by limiting the amount of mRNA that is being produced and by post-transcriptional processes. Some DTUs present elevated TS expression, for example, TcI and TcII. Meanwhile, TcIII and TcIV appear to have lower expression of TS [50,51]. Quantitative differences in TS expression patterns across the seven DTUs appear to be at the center of antigenic variation and CD clinical manifestations [50] Thus, it is proposed by some authors that the differences in clinical manifestations caused by each DTU are probably due to the variation in TS expression [52,53,54].
Currently, in endemic countries, the genotypes TcI, TcII, TcV, and TcVI are the most commonly found causing American human trypanosomiasis [55,56]. Meanwhile, when it comes to canine trypanosomiasis, the genotypes TcI and TcIV, shared with humans are the ones found to be the cause of the disease [48,57]. As demonstrated in Table 1, all T. cruzi DTUs identified in the sylvatic reservoirs are already found in the canine and in the human population. As such, T. cruzi identification in dogs is considered as an indicator of the presence of Chagas disease across diverse biotopes and regions. However, the true potential of dogs as sentinels for T. cruzi, could be more explored, which is essential for mapping T.cruzi DTUss natural distribution across different regions and anticipating the introduction of new DTU in the community.

Table 1.
Trypanosoma cruzi main host species and respective discrete typing units (DTUs) and geographic localization on the American continent. Virtually all mammals are susceptible to T. cruzi parasitic infection, although dogs have been playing a critical role in the transition from the sylvatic to peridomestic parasite life cycle.
As discussed before, CD presents complex epidemiological variables regarding the transmission and a few examples of field studies performed throughout the Americas illustrate the epidemiological situation regarding canine CD. Several studies in the Southwestern United States demonstrated that T. cruzi actively circulates through vector, wildlife, and domestic dog populations [65,66,67]. Research conducted in Texas, Oklahoma, and Louisiana has revealed prevalence rates of dog infection ranging from 3.6% to 22.1%, and reaching as high as 71.0% in certain multi-dog kennels, underscoring the significant burden of Chagas disease among domestic dogs [42,65,66,67,68,69,70]. Moreover, in Texas, 54.4% of triatomines were infected with T. cruzi, the prevalence of infection being higher in adults and males (58.7%) than in nymphs (11.3%). Additionally, triatomines were infected with discrete typing units TcI and/or TcIV [71].
However, countries such as Argentina, Colombia, Panamá, and Venezuela have predominantly an enzootic cycle, in which dogs are pivotal for the transmission dynamics and the emergence of new epidemiological scenarios [46,48,59,62]. In Brazil, the epidemiological relevance of the dog population needs to be deepened, since, according to the state, the T. cruzi seroprevalence ranges from not reported to 53% as recently reviewed by Freitas and colleagues (2022) [13]. Thus, the development of diagnostic tools to identify T. cruzi infection in dogs and other domestic animals is urgently needed. It is also worth noting that the migration of dogs from endemic to non-endemic regions may introduce a veterinary health challenge, as infected dogs may exhibit clinical symptoms in areas where veterinarians are less experienced in recognizing signs of Chagas disease.
4. Immunopathogenesis and Clinical Manifestations Chagas Disease
Infection by T. cruzi can cause a wide range of clinical signs that share several similarities among the different species affected. In general, the clinical presentation of CD has two phases—acute and chronic [29,72,73] (Figure 2) (p. 6). Nevertheless, in any stage of CD, clinical signs may vary from absent to severe and life-threatening.
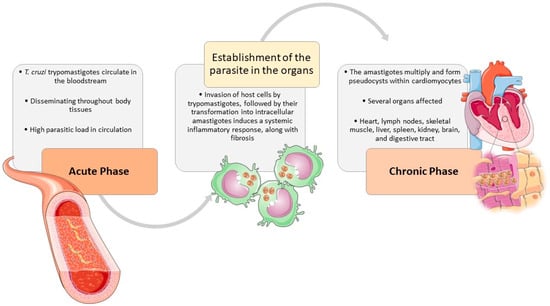
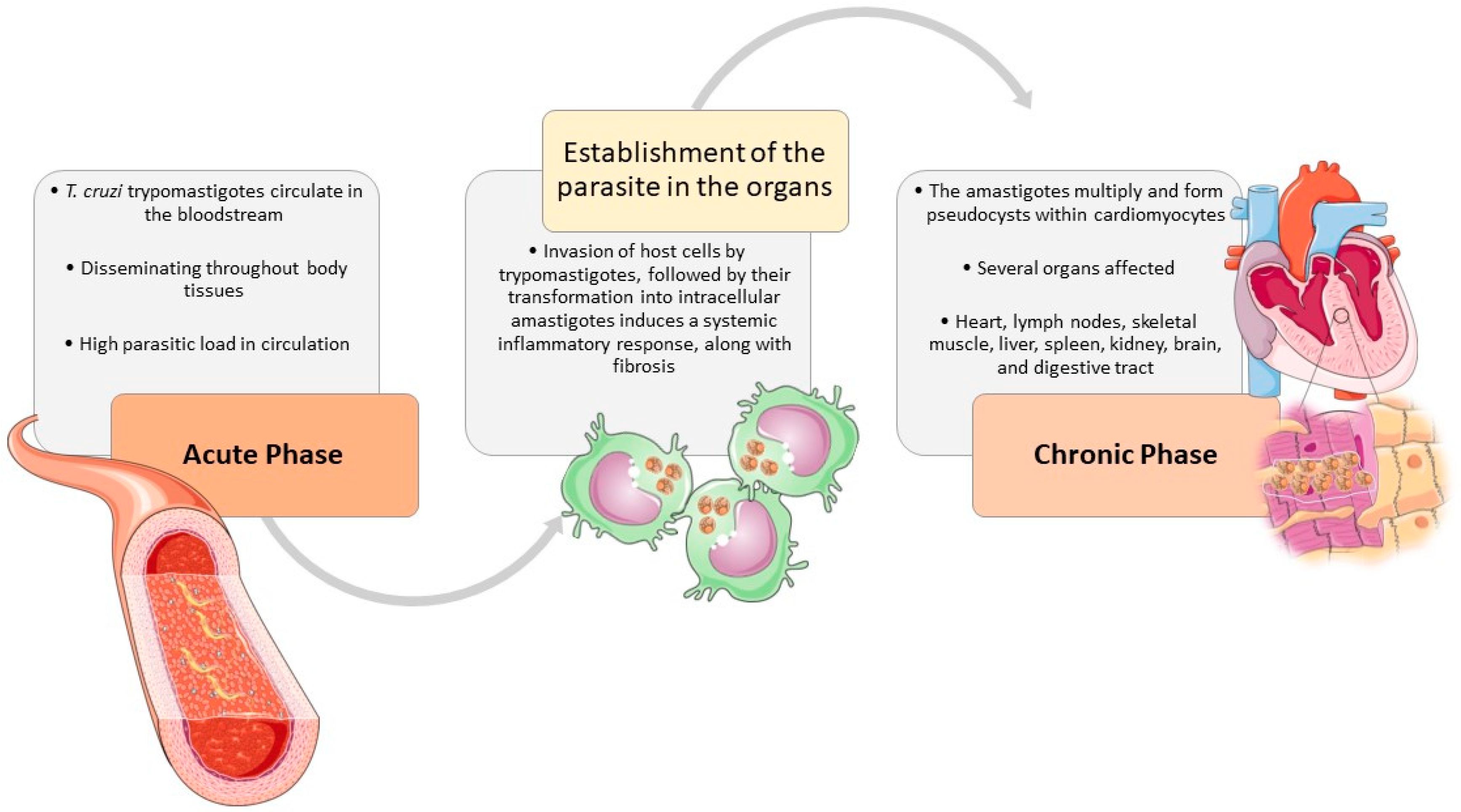
Figure 2.
The natural course of T. cruzi infection in the host. The clinical signs, associated with T. cruzi infection, are shared by different affected species, especially humans and dogs. During the acute phase, T. cruzi trypomastigotes circulate in the bloodstream and can enter macrophages, disseminating throughout most body tissues The invasion of host cells, triggers trypomastigotes transformation into intracellular amastigotes and induces a systemic inflammatory response in the host, along with fibrosis. In the chronic phase, the heart is usually the most affected organ, where the amastigotes multiply and form pseudocysts within cardiomyocytes, causing an inflammatory response resulting in cardiac damage. But many other tissues may also be implied, such as lymph nodes, skeletal muscle, liver, spleen, kidney, brain, and digestive tract. The figure was partly designed using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 4.0 unported license (https://creativecommons.org/licenses/by/4.0/).
During the acute phase of infection, T. cruzi trypomastigotes circulate within the bloodstream and can enter macrophages, disseminating throughout most body tissues The invasion of host cells by trypomastigotes, followed by their transformation into intracellular amastigotes induces a systemic inflammatory response, along with fibrosis. While inflammation is more pronounced in the acute stage, fibrosis predominates during the chronic phase [29,73,74,75,76,77]. The heart is usually the most affected organ [29,75,77,78,79,80,81], but many other tissues may also be implied, such as lymph nodes, skeletal muscle, liver, spleen, kidney, brain, and digestive tract [29,76]. The amastigotes multiply and form pseudocysts within cardiomyocytes, causing an inflammatory response resulting in cardiac damage [77,79,82]. Besides the parasite-induced lesions the innate and immune responses contribute to sustained inflammation, fibrosis, and oxidative stress lesions, causing myocyte necrosis, autonomic dysfunction, microvascular dysfunction, cardiac hypertrophy, and fibrosis, which may culminate in heart failure [13,29,75,76,77].
The CD acute phase is observed in around 1–2% of infected people and occurs within the first few weeks after exposure. It is characterized by a high parasitic load in the bloodstream and lasts a few weeks or months [73,76]. Human patients usually have absent to mild symptoms, such as lymphadenopathy or self-limiting fever [6,78]. Other clinical signs may include body aches (e.g., headache, muscle, abdominal, or chest pain), weakness/fatigue, loss of appetite, diarrhea, vomiting, rash, pallor, respiratory difficulties, swelling of the face or limbs, hepatomegaly, splenomegaly, and tachycardia [6,73,78]. The so-called “chagoma” is a typical sign of CD and is a swelling area near the site of infection. When it is in the eyelid(s) is called “Romaña’s sign” [1,72]. Despite being characteristic, these clinical signs appear in less than 50% of T. cruzi-infected people [72,73].
In dogs, clinical signs of acute disease vary largely with the age of diagnosis. In adult dogs diagnosed with CD, survival time is higher and prognosis is more favorable than when the diagnosis occurs in puppies [29,67,83,84]. Puppies are more likely to develop severe signs, such as lethargy, generalized lymphadenopathy, slow capillary refill time with pale mucous membranes, and signs of acute myocarditis with heart failure as well as ascites, weak pulse, hepatomegaly, splenomegaly, and sudden death, while adults mostly present mild signs such as slight depression or low-rising parasitemia [22,67,79,80,82,83,84,85,86,87].
These conclusions align with the long-dated concept of dogs as sentinels for Chagas disease, since they develop similar clinical signs to those found in humans and for many years now have been considered models for scientific purposes [40,87,88].
In rare occasions (<5–10%), acute CD may progress to severe cardiomyopathy [75,89,90,91,92] or meningoencephalitis [93,94,95], generally leading to death [62,78]. These alterations are rare and affect mostly young children or immunocompromised people, such as those taking immunosuppressant therapies or coinfected with HIV [73,95]. The other 90% of infected people resolve acute disease manifestations spontaneously, from which 60–70% remain asymptomatic (the so-called “indeterminate form” [IF]), while 30–40% convert into the symptomatic chronic form, approximately 10–30 years after the initial infection [94]. In dogs, these proportions are not so well established, given their high variability with age and shorter lifetime. However, Nogueira-Paiva and collaborators (2014) [96] and Barr et al. (2009) [29] considered that infected dogs that are more than 6 months old show no signs of acute disease and enter the chronic phase about 30 days after infection. Similar to humans, infected dogs surviving the acute phase will also enter the chronic phase, either asymptomatic (IF) or symptomatic, and clinical signs share several similarities and vary from absent to severe [29,81,97,98].
The chronic phase can last up to the entire life of the host [62,73]. During this phase, parasitemia diminishes since parasites are mainly hidden in the heart and digestive muscles [75,99]. The IF is considered a benign clinical condition, characterized by positive serological and/or parasitological results for T. cruzi infection, but absence of clinical manifestations of disease. In humans, there is a consensus that IF patients must have normal electrocardiogram (ECG) readings and normal radiological findings in the heart, esophagus, and colon [13,99,100]. In dogs, IF is not so extensively characterized and is generally attributed to dogs that are seropositive for T. cruzi infection and clinically asymptomatic [13,29,81]. In humans, the IF affects approximately 70% of chronic patients, although around 6.9% of IF patients may convert into symptomatic [62,73,101]. The prognosis of human IF is similar to that of healthy individuals with normal ECG readings [101,102].
The symptomatic form of chronic CD may be manifested by cardiac, digestive neurological, and mixed forms [62,73,96,103], depending on the main clinical manifestations shown. Cardiomyopathy is the most frequent and serious (high morbidity and mortality rates) clinical presentation of CD in humans [6,76], being the main cause of human infectious myocarditis worldwide, leading to a substantial public health burden [76,78,104]. Cardiac disease is also the most severe and life threating clinical presentation of CD [22,29,81]. The clinical signs derived from chronic myocarditis include altered heart rate, arrhythmias, cardiomegaly, congestive heart failure, thromboembolism, and cardiac arrest with sudden death. The ECG abnormalities are frequently seen and associated with T. cruzi infection, demonstrating cardiac conduction abnormalities such as ventricular arrythmias and atrioventricular block in humans [76,99,104] and dogs [86,105,106,107,108,109]. In dogs, cardiac disease has been the most extensively studied clinical presentation of CD [22,29,81]. In a study with 537 confirmed cases of canine CD in Texas, Kjos et al. (2008) reported “enlarged heart” as the most common clinical observation (33.6%) in dogs diagnosed with CD, followed by lethargy (28.7%), anorexia (23.0%) ascites (22.1%), cardiac conduction disturbances (21.3%), among other abnormalities with lower proportions each [67]. However, there is no compelling research defining the cardiac form as the most prevalent in canine CD, compared with digestive, neurological or other clinical signs. Cardiac alterations generally begin in the right side of the heart, then may progress to the left [29,82,83,110]. Another marker of cardiac disease which has been increasingly studied is troponin I. Studies conducted in dogs [81,82,84] and humans [111,112] with CD have associated increased levels of serum troponin I with heart disease, suggesting that it could be used as biomarker and predictor of disease outcome in such patients. In dogs with CD, increased troponin I has been associated with the presence of heart disease [81,82,84]. In dogs, sensibility of ECG as a diagnostic tool varies among studies. On the one hand, some studies report that ECG abnormalities may be detected in over 76% of seropositive patients [22,80,83]. On the other hand, a case report by Vitt et al. (2016) suggested that sensibility of ECG and also serology may be reduced in acute CD, compared with other markers such as troponin I [82]; furthermore, Kjos et al. (2008) reported only 22.1% of cardiac conduction disturbances detected among 537 dogs confirmed for T. cruzi infection by serology (n = 444), histopathology [86] and the combination of both methods (n = 7) [67]. These differences may also be reflecting a different host immune response. Oral transmission is more likely in dogs due to ingesting infected bugs [29,69,86] and thus being exposed to a higher density of parasites [113]. Carvalho et al. (2019) [107] estimated that 28% of 78 dogs experimentally infected with T. cruzi were affected by chronic cardiac signs, 6–9 months post-infection, a percentage that is similar to that reported in humans.
In humans, the digestive, neurological, and mixed forms affect approximately 10% of individuals with chronic CD [81]. The digestive form generally manifests as peristalsis dysfunction and progressive enlargement of the esophagus (megaesophagus), colon (megacolon), or other parts of the intestine [76,78,114], affecting normal digestive function. In humans, difficulties in eating (dysphagia) or defecating (constipation) have been described in CD patients [76,99]. These signs have been associated with chronic inflammation and lesion/degeneration of parasympathetic enteric neurons [76,78,96]. In dogs, there is scarce information concerning digestive signs of CD, which may include decreased appetite and diarrhea [80,82]. Nogueira-Paiva and colleagues (2014) [96] noticed that two different strains of T. cruzi that were inoculated in dogs were detected in the esophagus and colon, along with lesions of inflammation and myenteric denervation 30 days post-infection. However, lesions caused by the Be-78 strain persisted until 720 days post-infection and specific lesions of megaesophagus or megacolon were not reported.
Neurological signs in chronic CD derive from the damage of the nervous system, including inflammation, infiltration, and demyelination lesions in the nerves, which affect sensory and motor capabilities [46,76,96], and digestive signs are one of the clinical manifestations of this phenomenon. Sporadically, the central nervous system may be affected, causing dementia, confusion, chronic encephalopathy, and sensory/motor deficits [46,76]. In dogs, neurologic signs are also rare, being associated with multifocal encephalitis associated with parasitic invasion of the neurologic system (pseudocysts). Clinical manifestations include weakness, pelvic limb ataxia, and hyperreflexive spinal reflexes that resemble distemper [29,67,82,115]. Laboratory abnormalities may include anemia, lymphocytosis, hyperproteinemia with hypoalbuminemia and hyperglobulinemia, hypoglycemia, and increased levels of lactate dehydrogenase (LDH), aspartate transferase (AST), ALT, BUN, creatine kinase (CK), creatine kinase myocardial band (CK-MB) and serum troponin I [82,85,88,113,116,117]. Table 2 presents a summary of the similarities and differences between human and canine CD detailed in this section.

Table 2.
Clinical signs of acute and chronic CD in humans and dogs.
5. Diagnosis: A Key Step in Tackling Chagas Disease
The large spectrum of different and non-specific clinical signs, considering that the vast majority of infections are asymptomatic, hampers the diagnosis of infection by T. cruzi as their etiological cause [1,72,73]. Diagnosis of CD is complex and should integrate information about the epidemiological context, clinical history, clinical signs and/or laboratory abnormalities compatible with the disease and further confirmation of parasite infection through one or more complementary diagnostic methods [13,78,117].
Tests to detect T. cruzi infection include direct techniques, which confirm the presence of the parasite or its components, such as parasitological and molecular assays, and indirect techniques that assess the host’s immune response to the parasite by detecting specific humoral immune response [11,73].
Direct quantification of CD incidence in dogs is rare, most likely due to the challenges of collecting longitudinal data. However, a recent study conducted in Texas evaluated T. cruzi serology and DNA of 64 dogs at three time points over a year, recording an incidence rate of 30.7 new infections per 100 dogs per year [11]. In contrast, a previous serological survey carried out in the same state by Garcia and colleagues (2016) [118] estimated an overall serological incidence of 3.8% (8 out of 209 samples) in dogs in addition to high infection rates (51–82%) in triatomine vectors. Despite these studies, the epidemiology and seroprevalence of T. cruzi infection in companion animals is largely unknown, and canine CD is likely to be underdetected and underreported.
The serological diagnosis allows the detection of parasite antibodies and is considered the primary choice (gold standard) for CD diagnosis in both humans [117,119] and dogs [29,81,82,86] (Table 3). Serological methods include qualitative immunochromatographic tests (ICT), and quantitative tests such as enzyme-linked immunosorbent assay (ELISA), chemiluminescent microparticle immunoassay (CMIA), indirect immunofluorescence (IIF), or hemagglutination inhibition assay (HAI). In dogs and humans, the sensitivity and specificity of serological assays are greater than 90% and increase over infection time [13,76]. Positive serology indicates previous exposure to the parasite, but also a probable current infection as a parasitological cure is considered highly unlikely [29,84,119]. However, sensitivity varies within different serological tests and T. cruzi strains being screened and is lower in acute infection [13,82,117,119]. Moreover, the specificity of serological tests can be affected by cross-reactivity with other trypanosomatids, such as Leishmania spp., in both humans [120,121] and dogs [113,122,123]. The WHO (2012) [76] stressed the need to develop less invasive and more accurate diagnostic techniques, especially point-of-care tools to improve screening and allow rapid, cost-effective action and treatment. Rapid diagnostic tests have been tested in both humans [124] and dogs [84,125,126], but are currently available for humans only. Nevertheless, they have been used off-label in animal studies and some have shown good accuracy [81,125,126,127]. Additionally, advances in DNA recombination technology have allowed the use of recombinant proteins in immunoassays. IBMP antigens are chimeric recombinant T. cruzi antigens, which have shown good diagnostic performance in both humans [128] and dogs [127], including negligible cross-reaction with Leishmania spp. [13,128]. In veterinary medicine, the use of serological tests for T. cruzi infection is still limited by the species-specific test kits available for most animal species, with few laboratories offering options for dogs [74].
Parasitological diagnosis consists of the direct microscopic observation of T. cruzi parasites through cytology, histology, immunohistochemistry, xenodiagnoses, and culture of biological samples, such as blood, host tissues, or even triatomine feces [78,119,129,130]. Parasitology enables confirmation of infection, with a specificity of about 100%, and can be helpful when antibody levels are low, such as in recently infected cases [13,74,76]. In contrast, sensitivity is generally low and decreases in CD’s chronic phase [76,82,87]. Concentration techniques may be used to increase sensitivity, such as the microhaematocrit concentration method (MH), the Strout concentration method as well as the examination of the buffy coat or the pellet (red and white blood cells) obtained after plasma centrifugation [76]. Culture/hemoculture and xenodiagnosis allow amplification of the parasite, but sensitivity remains low (22% in hemoculture and 11% in xenodiagnosis, and are not practical for clinical settings, mostly being performed in research scenarios [131].

Table 3.
Diagnosis methods for CD. Available tests to identify T. cruzi infection include direct techniques, and may be useful to confirm the parasite or its cellular components by parasitological and molecular methods (PCR or qPCR: quantitative polymerase chain reaction of T. cruzi nuclear satellite DNA [nDNA] and minicircle kinetoplast DNA [kDNA] and loop-mediated isothermal amplification [LAMP]). Indirect methods can be used based on evaluating the host’s humoral immune response against the parasite (serological methods) such as: (i) enzyme-linked immunosorbent assay (ELISA); (ii) immunochromatographic test (ICT); (iii) chemiluminescent microparticle immunoassay (CMIA); (iv) hemagglutination inhibition assay (HAI) and (v) indirect antibody immunofluorescence reaction test (IFAT).
Table 3.
Diagnosis methods for CD. Available tests to identify T. cruzi infection include direct techniques, and may be useful to confirm the parasite or its cellular components by parasitological and molecular methods (PCR or qPCR: quantitative polymerase chain reaction of T. cruzi nuclear satellite DNA [nDNA] and minicircle kinetoplast DNA [kDNA] and loop-mediated isothermal amplification [LAMP]). Indirect methods can be used based on evaluating the host’s humoral immune response against the parasite (serological methods) such as: (i) enzyme-linked immunosorbent assay (ELISA); (ii) immunochromatographic test (ICT); (iii) chemiluminescent microparticle immunoassay (CMIA); (iv) hemagglutination inhibition assay (HAI) and (v) indirect antibody immunofluorescence reaction test (IFAT).
| Diagnosis | Humans | Dog | Limitation | |||
|---|---|---|---|---|---|---|
| Acute | Chronic | Acute | Chronic | |||
| Serological | + ELISA | Yes | Yes | Yes | Yes | Sensitivity varies within different serological tests and T. cruzi genetical variability and is lower in acute infection. In acute phase most of humans and dogs are asymptomatic. Cross-reactivity with other trypanosomatids, such as Leishmania spp. in endemic overlap area. Serological tests present variability in the different kits. Besides, a high cost of commercially assay available at such as ICT and CMIA. |
| + ICT | Yes | Yes | # NA | # NA | ||
| * CMIA | Yes | Yes | NA | NA | ||
| ++ ELISA + HAI ++ ELISA + IFAT | Yes | Yes | Yes | + Yes | ||
| Parasitological | Microscopy: Direct observations (stained or fresh blood preparation sample) | Yes | Yes | Parasitemia depends on the phase of the infection. Detection of parasites is primarily applicable during the acute phase of infection. During chronic phase, parasitemia tends to be low and intermittent reducing sensitivity in detection. Parasitemia in domestic dogs vary according to region. | ||
| Artificial xenodiagnosis | Yes | No | ||||
| Haemoculture | Yes | * Yes | ||||
| Molecular | PCR, qPCR (nDNA- and kDNA-based qualitative) | Yes | + Yes | + Yes | + Yes | T. cruzi DNA detection is solely applicable in acute phase. Parasitemia generally low and intermittent in the chronic phase. Thus, reduce the sensitivity. |
| LAMP (Trypanosoma cruzi Loopamp kit) | NA | NA | ||||
+ Recommended in sero-epidemiological surveys and follow-up. Although, ICT is not recommended in patients screened for Chagas disease (chronic infection) in hemotherapy services (PAHO, 2019). ++ Diagnostic gold standard diagnosis, i.e., the combining of two positive serological tests and potentially a third test if the results are conflicting. * Recommended to screen Chagas disease in hemotherapy. NA: Not approved for human and veterinary clinical practice. # NA: Not approved in clinical veterinary practice. Rapid tests (ICTs) that detect antibodies to T. cruzi for the diagnosis of Chagas disease in humans are not currently approved for clinical use in animals. However, few studies were testing Chagas/Bio-Manguinhos Lateral Flow Immunochromatographic Rapid Test (Chagas-LFRT); Trypanosoma Detect™ InBios and CHAGAS STAT-PAK™ in dogs [132].
Molecular methods enable the detection of T. cruzi DNA from various biological samples, including triatomine vectors. Similarly, with parasitological methods, it may be important to confirm an active infection and differentiate it from an exposed (seropositive), but non-actively infected animal. Molecular techniques based on PCR are highly specific, but according to the biological sample, sensitivity can be relatively low [13,29,76,131]. Since it can be performed in all tissue samples, it may be more sensitive than histopathology [87], and can provide information on the genetic strain of the parasite, which is useful for research. Eloy and Lucheis [133] have concluded that PCR using TCZ1/TCZ2 primers constitutes a suitable tool for parasite detection in cat and dog hemocultures, and could be used as an enabler for diagnosis. Also, molecular techniques may help to confirm infection in seronegative (recently infected) patients [78].
The development of new molecular-based diagnosis for use in resource-limited areas, such as the isothermal amplification of nucleic acids (LAMP) has the potential to be applied in the detection of T. cruzi and Leishmania sp. infections. Besuschio and colleagues [134] evaluated LAMP’s capacity to accurately diagnose acute human CD in different epidemiological and clinical scenarios. Their conclusion suggested that the T. cruzi Loopamp kit shows promise for the swift detection of T. cruzi infection in cases of congenital transmission, acute infection, and Chagas disease reactivation associated with HIV infection. According to the most recent guidelines for CD diagnosis [119], the diagnostic tests recommended varied according to the clinical context/scenario. The guidelines evaluated four main diagnostic methods: ELISA, ICT, CMIA and the diagnostic gold standard method, which is the combination of two positive serological tests (ELISA, HAI, or IIF), and a third test could be added in case of contradictory results. In summary, the guidelines recommend the use of the diagnostic gold standard (rather than ELISA, ICT, or CMIA as single isolated tests) to obtain a definitive diagnosis in patients with suspected chronic T. cruzi infection. However, to screen CD in populations, the recommendation is to perform the ELISA or ICT tests as single tests since these assays are easier to implement. However, when the aim is to screen CD in hemotherapy services, the guidelines advise the use of ELISA (highly sensitive kits) or CMIA.
Complementary exams such as ECG and echocardiographic examination are important and have been recommended to screen for cardiac disease in T. cruzi-infected patients [22,29,78,87,106,107,108], and serum cardiac troponin I (cTnI) has been increasingly studied as an early biomarker of parasitic myocarditis in dogs with CD [81,82,113]. In dogs, echocardiographic and ECG evaluations are not as sensitive, lacking significant changes in dogs with acute CD [82]. Although not confirming the presence of the parasite, ECG, echocardiography and cTnI may provide helpful information for a more robust diagnosis and characterization of CD [81,82,86,87,106,107,113], and researchers have recommended its use in periodic screening of patients serological positive for T. cruzi antibodies, either symptomatic or not [81,82]. Some dog breeds seem to have a genetic predisposition to heart diseases, as is the case with German Shepards, Bulldogs, or Boxers, and in these breeds, the identification of the etiological agent causing cardiac abnormalities should be carefully investigated [29,84].
Therefore, the diagnosis of human and canine CD faces several challenges. For human CD, most diagnostic tests require invasive sampling and accuracy is highly variable according to the clinical phase of infection [70]. In veterinary medicine, the situation worsens since there are few diagnostic options available and standardized for animals, and tests are often discordant [13,44,125]. Additionally, clinical information concerning animals is often less solid or inaccessible, namely in field or wildlife studies [74,78]. However, according to the London Declaration on Neglected Tropical Diseases initiative, any interventions to reduce infection in dogs and improve their overall health may contribute to decreasing the risk of locally acquired human disease [74,135]. Therefore, veterinary CD diagnostics should not be overlooked.
6. Tackling CD Control: Current Treatment and Prophylaxis Strategies
Treatment of T. cruzi infection is unsatisfactory and challenging. Only two therapeutic options with proven efficacy are available: Benznidazole (BNZ) and Nifurtimox (NFX). Widely used in Latin America, BNZ is a nitroimidazole derivative that induces oxidative stress generating nitrate and oxygen-reactive species, which can cause damage to parasite cellular machinery. Similarly, the nitrofuran NFX generates oxidative metabolites, such as oxygen peroxide free radicals, capable of inactivating the parasite [136,137]. The knowledge of the mechanism of action of a drug is essential for optimizing its therapeutic benefits, minimizing adverse effects, and advancing drug development towards more effective and eventually even T. cruzi DTU-specific treatments. The analysis of DTUs has gained traction in recent years, with several published studies examining the DTU impact on patient outcomes [138,139,140]. However, it is important to note that the DTU system alone cannot reliably predict disease outcomes or responses to therapy, and so far, there is no singular outcome associated with any specific DTU.
Although their safety and efficacy profiles are far from ideal, both drugs have been the first-line treatment for about 50 years. BNZ and NFX have beneficial effects in the acute phase of CD, achieving resolution in up to 80% of treated patients, especially children under 14 years old [5]. In contrast, treatment effectiveness in chronic patients continues to be highly debated. In a systematic review, trypanocide therapy was considered optional for adults older than 50 years without advanced cardiomyopathy and for patients with gastrointestinal disease but without advanced cardiomyopathy due to an unclear risk–benefit balance [136,141]. In the chronic phase, NFX achieves resolution rates between 7 and 10% [110,142,143] and BNZ ranges between 2 and 40% [5,144,145]. However, the adverse side-effect profiles have led Brazil and other South American countries to discontinue the production and the clinical application of NFX [145]. Standard doses of BNZ also can cause adverse reactions, such as manifestations of hypersensitivity, generalized edema, fever, muscle pain, bone marrow depression, neurological and sleep disorders, weight loss, nausea, and vomiting, among others [146,147]. Consequently, the rate of suspension or abdication of treatment ranges from 15% to 20% of patients [148,149]. Adding to poor drug tolerability in adult populations with chronic disease, there is also a gap in the definition of adequate cure criteria. The issue lies in the fact that the widely accepted criterion for cure is seroconversion by presenting two negative results from two different conventional serology tests. However, antibodies may persist in the host and existing serological techniques continue to yield positive results for years post-treatment. This challenge persists because, as an intracellular parasite, the presence of parasitemia does not serve as a significant indicator of cure.
Treatment of indeterminate chronic infection is insufficient and does not seem to have a significant impact on the clinical course of the disease [150]. Currently, the international clinical guidelines recommend that anti-parasitic treatment should be offered to adults aged 19 to 50 years who are in the chronic indeterminate stage or have mild to moderate cardiomyopathy, children with congenital or acquired acute disease, immunosuppressed hosts with acute or reactivation of chronic disease, and women of childbearing age to prevent congenital transmissions [5,6,136]. In August 2017, the United States Food and Drug Administration (FDA) approved the first BNZ treatment for CD in children aged 2 to 12 years, while in Europe, it is not yet formally approved [151].
As for canine CD, there is no established protocol for the use of BNZ and NFX. There are few studies that evaluated the effects of BNZ in experimentally induced chronic Chagas disease [152,153]. Preclinical results demonstrated the beneficial effect of etiological treatment in reducing tissue damage and transient parasite burden [153]. However, BNZ therapy did not prevent echocardiographic abnormalities associated with cardiomegaly and instead showed an increase in ventricular size similar between infected, treated, and untreated animals. Altogether, treatment with BNZ was unable to prevent the development and progression of chagasic cardiomyopathy. Therefore, the investment in early diagnosis for human and canine CD is essential to ensure the best therapeutic outcome.
Alternately, specialists in CD chemotherapy recommended the evaluation of drug combinations to explore alternative strategies and reproposing of medical treatment options. However, a study combining the two antiparasitic drugs itraconazole and BNZ failed to reduce parasite-induced lesions in dogs. Furthermore, regarding resistant T. cruzi strains, the BNZ–intraconazole formulation was not effective in inducing parasitological cure or sustained reduction in the parasite load in the blood and infected organs during the acute CD phase [154]. Madigan and colleagues (2019) [155] evaluated the clinical, serologic, parasitological, and histologic outcomes of naturally infected dogs treated with a combination of amiodarone and itraconazole for 12 months. The results have demonstrated the improvement of clinical signs, increased survival time, and negative PCR for T. cruzi DNA. Despite these results, a recent study reported the sudden death of two dogs with symptomatic chagasic cardiomyopathy after receiving amiodarone and itraconazole in addition to cardiac therapy [156]. The development of new therapeutic options has also been addressed by the scientific community. Some pharmacological classes are especially promising to treat CD such as nitroheterocyclic compounds, inhibitors of sterol biosynthesis, cruzipain inhibitors, aromatic amides, trypanothione reductase inhibitors, ruthenium complexes carrying trypanocidal molecules, oxaboroles and nucleoside derivatives, as detailed in a review by Mazzeti and colleagues [157]. Alternative drugs, such as posaconazole, have demonstrated superior results in the treatment of acute disease compared with BNZ; however, only BNZ had efficacy in the chronic mouse CD model [158]. In a double-blind, randomized, placebo-controlled, dose-finding, proof-of-concept study conducted in Bolivia, the nitroimidazole fexinidazole demonstrated high efficacy in chronic T. cruzi infection, even at the lowest tested dose, and at less than 3 days of treatment [159]. Making this a very promising drug alternative for further studies. Likewise, the generation of new regimens of BNZ application in combination with fosravuconazole has been demonstrated to be promising in preclinical studies [160]. However, fosravuconazole monotherapy resulted in only a transient response and no sustained effect in a phase 2 study [161]. The effectiveness of a drug’s trypanocidal activity can be dependent on internal factors of the host (such as genetics, immunocompetence, metabolism, and other chronic conditions) associated with parasite genetic heterogeneity. Consequently, further studies should use a target-based or phenotype-based approach to improve the efficacy of compounds for the treatment of human and canine CD. Undoubtedly, there is an urgent need for effective therapeutic options directed at chronic CD. However, for the time being, and due to the few therapeutic options, preventive measures related to vector ecology for transmission control such as insecticide-treated bed nets or netting and the use of insecticide-treated dog collars still play a crucial role in controlling CD.
7. Vaccines for CD: Challenges and Opportunities
Recent advances in the search for control and cure of Chagas disease have been focused on the development of prophylactic and therapeutic vaccines that can integrate an effective strategy for prevention and control of T. cruzi transmission by modulating the host’s immune effector mechanisms, culminating in parasite clearance, infection control, and pathogenesis prevention, long-term protection. The accumulated knowledge of the biology and genetics of T. cruzi, together with an increased understanding of the host immune response, has led to the development of several vaccine candidates against this parasite.
Several vaccine candidates are being developed using different strategies and tested in animal models, such as live attenuated vaccines, recombinant proteins vaccines, replicating recombinant vector vaccines, DNA, and mRNA vaccines [162,163,164,165,166]. Therapeutic DNA vaccines with plasmid DNA encoding T. cruzi antigens of the parasite surface trans-sialidase family (TSA-1, Tc52, or Tc24), which seems to be crucial for parasite evade host immune response have been tested in acute and chronically infected mice with different outcomes. Tc24, TSA-1, and Tc52 reduced parasitemia, controlled myocarditis, and decreased mortality [167,168,169] associated with a rapid expansion of CD4+ and CD8+ T cell populations [170]. Other studies have addressed the immune response generated by Tc24 and TSA-1 [168,171,172,173,174,175]. Both recombinant proteins induced a strong humoral and cellular immune response in preclinical trials, but these same candidates did not halt cardiomyopathy in infected mice or dogs. Barry et al. (2016) [176] explored the potential for a therapeutic nanoparticle vaccine by encapsulating Tc24 protein in poly (lactic-co-glycolic acid) nanoparticles. Mice infected with a highly lethal H1 strain of T. cruzi and then immunized with Tc24-nanoparticles exhibited antigenic specific proliferative cytotoxic (CD8+) T cells and Th1 immune response associated with increased production of antigen-specific interferon (IFN)-γ by splenocytes and high IgG2a titers. There was also parasitemia reduction, low inflammatory cell infiltrate, and a decrease in the parasite burden of cardiac tissue. In another study, the same group used the recombinant Tc24 protein adjuvanted by the Toll-like receptor 4 agonist E6020 to immunize mice chronically infected with the H1 strain of T. cruzi and showed that 60% of therapeutically vaccinated mice had untraceable parasites accompanied by a decrease in cardiac fibrosis [177].
Due to CD complexity, the use of bioinformatic resources to screen parasite genomes aiming to identify potential vaccine candidates has been carried out by several researchers. Potential candidate antigens selected by screening the T. cruzi genome sequence database [178,179] were then analyzed in vitro considering biological parameters. Of the in silico selected antigens, three intracellular candidates (TcG1, TcG2, and TcG4) phylogenetically conserved within the parasite, recognized by host IgG antibody and able to induce proinflammatory CD8+ T cell response were used for vaccine development [178,179,180]. It was further confirmed that these antigens were recognized by antibodies and CD8+ T cells of a variety of T. cruzi-infected hosts [181]. Furthermore, when administered individually as a DNA prime/boost vaccine in mice, these antigens induced trypanolytic activity, a characteristic that has been associated with a protective immune response against T. cruzi [178].
The protective efficacy of a T. rangeli booster vaccine with primed/inactivated DNA (TcVac4) against T. cruzi infection and Chagas disease in a canine model has also been addressed. The use of heterologous DNA priming vaccine/inactivated microorganism booster [181] or DNA booster vaccine/inactivated microorganism priming [174] has been previously reported with promising results. In this scientific approach, inactivated T. rangeli was used as a booster dose of the vaccine for several reasons: (i) T. cruzi lysates were first tested and demonstrated to provide limited or no protection; (ii) it was considered that T. rangeli exhibits significant homology (>60%) with the T. cruzi proteome [182,183] but is not pathogenic for mammals [184,185] and (iii) mice immunized with T. rangeli fixed in glutaraldehyde elicited B and T responses that recognized T. cruzi antigens [186,187]. Mice immunized with T. rangeli were able to control T. cruzi challenge, showing a significant reduction in parasitemia, the absence of histopathological lesions, and low mortality [186,187]. The T. rangeli-based vaccine has also been tested in dogs with positive results. Dogs immunized with glutaraldehyde-inactivated T. rangeli epimastigotes exhibited reduced parasitemia following T. cruzi challenge and were less infective to triatomines when compared to unvaccinated controls [188].
Co-delivery of parasite antigens as a DNA vaccine induced additive immunity and a greater degree of protection against T. cruzi infection in mice [179]. When tested in dogs, TcVac1, which is constituted by antigen-encoding plasmids (pCDNA3.TcG1, pCDNA3.TcG2, and pCDNA3.TcG4) and IL-12 and GMCSF expression plasmids, induced a parasite-specific IgM and IgG response, but phagocyte activity was suppressed, resulting in parasite escape and dissemination into tissues that lead to cardiac histopathological abnormalities, remained infective to triatomines [180]. A further similar approach with TcVac4 (DNA-prime/T. rangeli-boost) vaccine in dogs provided control of cardiac pathology, resistance to disease progression, and decreased parasite transmission to triatomines [189]. However, it was not possible in any of the studies carried out to achieve sterile immunity against T. cruzi by vaccination.
Recently, immunization of mice with heterologous mRNA Tc24 protein [190]. This new approach to RNA vaccines is based on a new generation of RNA-based vaccines that have demonstrated the ability to induce protective immunity, inducing strong antigen-specific CD8+ T cell responses and effective responses of CD4+ T cells [191,192]. In this study, it was possible to verify that heterologous mRNA protein vaccination with Tc24 mRNA to prime and Tc24-C4 protein (a genetically engineered polypeptide construct free of cysteines) to boost promotes a cellular immune response against T. cruzi, mainly characterized by an increased level of polyfunctional CD8+ T cells.
Although the results of these vaccine candidates are encouraging, to date, no anti-T. cruzi vaccine has achieved the expected results of producing sterile immunity in dogs or mice. Despite the different strategies that are currently being pursued by researchers, the challenges of developing a therapeutic and/or prophylactic vaccine for human or canine CD are immense. In the vast majority of the studies conducted, significant control of the infection is achieved, with induction of protective immunity and parasitemia reduction. However, blocking the development of cardiac fibrosis and cardiomyopathies and complete parasite clearance remain major challenges to be addressed.
8. Final Considerations and Future Perspectives
Trypanosoma cruzi is a genetically and ecologically heterogeneous parasite associated with different geographic regions and zoonotic transmission cycles throughout the Americas, presenting different epidemiologic importance and diverse clinical outcomes [139,193]. Despite recent efforts to control CD, much remains unknown and further studies that take into account the complexity of the disease and the current knowledge of parasite–host interactions are needed to evaluate potential new immunotherapies. Moreover, little is known about the factors influencing the disease progression and the role played by an immune response in parasite reactivation and further research should be conducted. Recently, Gil-Jaramillo and colleagues (2021) [194] carried out a comparative RNA-sequencing-based transcriptome analysis of infected human monocyte-derived dendritic cells and demonstrated a new and unexplored pathway process during the first hours of T. cruzi–host interaction, similar to anti-viral immune response. These discoveries highlight the close evolutionary relationship between T. cruzi and its host’s immune system in order to successfully invade and survive in the host, completing their life cycle. Further, the immunomodulatory functions played by extracellular vesicles produced by the parasite and their potential to contribute to the development of new prophylactic or therapeutic tools against trypanosomatids have been attracting the attention of the scientific community [195,196,197] and should be taken into consideration as a possible innovative control strategy. Currently, the therapeutic approach is focused only on the control of the parasite load and is not sufficient to prevent the progression of the disease to the chronic phase. As there is no defined treatment for CD in dogs or prevention strategies, an immune-precision therapy against the parasite to prevent severe disease should be the focus of future research. In 2021, Mazzeti et al. [157] reviewed, in detail, some studies using innovative experimental treatment strategies, testing new drug candidates and innovative drug associations, and even designing new drug delivery systems to improve drug stability. Although some studies show promising results, still, the real effectiveness of new therapies in humans or animals is not yet established.
The present review highlights canine Chagas disease from different perspectives. The epidemiological role of the dog in CD has been strengthened in recent years, as dogs are likely to be the predominant animal reservoir of T. cruzi for human populations and can act as sentinels, since dogs are highly susceptible to T. cruzi infection and can develop high parasitemia, facilitating the parasite transmission to the vector. Generally, dogs present the same infection pattern as humans. However, the experimental infection of dogs with strains from South and Central America has revealed some differences in disease outcomes related to T. cruzi strain types, including intensity and timing of peak parasitemia, as well as cardiac pathology, thus revealing a complex and dynamic. A One Health perspective recognizes the critical need to protect wildlife, pets/companion animals and human populations from infectious diseases. By improving overall health, the benefit of reducing the risk of locally acquired CD disease will improve public health. This will undoubtedly have a socio-economic impact on the populations affected by CD.
Author Contributions
Conceptualization, J.D.-O. and J.P.-M.; Bibliographic research, J.D.-O., J.P.-M., C.M., A.R. and M.M.; Figures, A.R.; Tables, A.R., C.M. and M.M.; Writing—original draft preparation, J.D.-O., C.M. and M.M.; Review and editing, A.R. and G.S.-G.; supervision, G.S.-G.; funding acquisition, A.R., G.A.-P., I.P.d.F. and G.S.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by FCT—Foundation for Science and Technology, I.P., through research grants EXPL/CVT-CVT/0175/2021 (DOI 10.54499/EXPL/CVT-CVT/0175/2021) and PTDC/CVT-CVT/0228/2020 (DOI 10.54499/PTDC/CVT-CVT/0228/2020) and by national funds within the scope of Centro de Investigação Interdisciplinar em Sanidade Animal (CIISA, UIDB/00276/2020), Al4AnimalS (LA/P/0059/2020), Global Health and Tropical Medicine (GHTM, UID/04413/2020) and LA-REAL (LA/P/0117/2020). Joana Palma Marques and Marta Monteiro have Ph.D. scholarship references 2021.05579BD and UI/BD/152819/2022, respectively. A. Rodrigues awards a CEECIND/CP1725/CT0023 (10.54499/2022.00499.CEECIND/CP1725/CT0023).
Conflicts of Interest
The authors declare no competing personal or financial interests.
References
- Pan American Health Organization. Factsheet: Chagas Disease in the Americas for Public Health Workers-PAHO/WHO. 2022. Available online: https://www.paho.org/en/documents/factsheet-chagas-disease-americas-public-health-workers (accessed on 21 March 2024).
- Wozniak, E.J.; Lawrence, G.; Gorchakov, R.; Murray, K.O.; Alamgir, A.H.; Dotson, E.; Sissel, B.; Sarkar, S. The Biology of the Triatomine Bugs Native to South Central Texas and Assessment of the Risk They Pose for Autochthonous Chagas Disease Exposure. J. Parasitol. 2015, 101, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Reguero, L.; Subirà, C.; Blázquez-Pérez, A.; Requena-Méndez, A. Estimating chagas disease prevalence and number of underdiagnosed, and undertreated individuals in Spain. Travel Med. Infect. Dis. 2022, 47, 102284. [Google Scholar] [CrossRef]
- Requena-Méndez, A.; Albajar-Viñas, P.; Angheben, A.; Chiodini, P.; Gascón, J.; Muñoz, J.; Chagas Disease COHEMI Working Group. Health Policies to Control Chagas Disease Transmission in European Countries. PLoS Neglected Trop. Dis. 2014, 8, e3245. [Google Scholar] [CrossRef]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.d.M.; Da Câmara, A.C.J.; Nunes, D.F.; Guedes, P.M.d.M.; Pereira, W.O.; Chiari, E.; Diniz, R.V.Z.; Galvão, L.M.d.C. Chagas disease: Morbidity profile in an endemic area of Northeastern Brazil. Rev. Soc. Bras. Med. Trop. 2015, 48, 706–715. [Google Scholar] [CrossRef]
- Moncayo, A.; Yanine, M.I. An update on Chagas disease (human American trypanosomiasis). Ann. Trop. Med. Parasitol. 2006, 100, 663–677. [Google Scholar] [CrossRef]
- Cutler, S.J.; Fooks, A.R.; van der Poel, W.H.M. Public Health Threat of New, Reemerging, and Neglected Zoonoses in the Industrialized World. Emerg. Infect. Dis. 2010, 16, 1–7. [Google Scholar] [CrossRef]
- Dario, M.A.; Furtado, C.; Lisboa, C.V.; de Oliveira, F.; Santos, F.M.; D’Andrea, P.S.; Roque, A.L.R.; Xavier, S.C.d.C.; Jansen, A.M. Trypanosomatid Richness among Rats, Opossums, and Dogs in the Caatinga Biome, Northeast Brazil, a Former Endemic Area of Chagas Disease. Front. Cell. Infect. Microbiol. 2022, 12, 851903. [Google Scholar] [CrossRef]
- Busselman, R.E.; Meyers, A.C.; Zecca, I.B.; Auckland, L.D.; Castro, A.H.; Dowd, R.E.; Curtis-Robles, R.; Hodo, C.L.; Saunders, A.B.; Hamer, S.A. High incidence of Trypanosoma cruzi infections in dogs directly detected through longitudinal tracking at 10 multi-dog kennels, Texas, USA. PLoS Neglected Trop. Dis. 2021, 15, e0009935. [Google Scholar] [CrossRef]
- Naicker, P.R. The Impact of Climate Change and Other Factors on Zoonotic Diseases. IMedPub J.-Arch. Clin. Microbiol. 2011, 2, 1–6. [Google Scholar] [CrossRef]
- Freitas, N.E.M.; Habib, F.L.; Santos, E.F.; Silva, Â.A.O.; Fontes, N.D.; Leony, L.M.; Sampaio, D.D.; de Almeida, M.C.; Dantas-Torres, F.; Santos, F.L.N. Technological advances in the serological diagnosis of Chagas disease in dogs and cats: A systematic review. Parasites Vectors 2022, 15, 343. [Google Scholar] [CrossRef]
- Díaz, M.L.; Leal, S.; Mantilla, J.C.; Molina-Berríos, A.; López-Muñoz, R.; Solari, A.; Escobar, P.; Rugeles, C.I. Acute Chagas outbreaks: Molecular and biological features of Trypanosoma cruzi isolates, and clinical aspects of acute cases in Santander, Colombia. Parasites Vectors 2015, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, L.E.; Morillo, C.A. American Trypanosomiasis (Chagas Disease). Infect. Dis. Clin. N. Am. 2019, 33, 119–134. [Google Scholar] [CrossRef]
- Manoel-Caetano, F.d.S.; Silva, A.E. Implications of genetic variability of Trypanosoma cruzi for the pathogenesis of Chagas disease. Cad. Saúde Pública 2007, 23, 2263–2274. [Google Scholar] [CrossRef]
- de Fuentes-Vicente, J.A.; Gutiérrez-Cabrera, A.E.; Flores-Villegas, A.L.; Lowenberger, C.; Benelli, G.; Salazar-Schettino, P.M.; Córdoba-Aguilar, A. What makes an effective Chagas disease vector? Factors underlying Trypanosoma cruzi-triatomine interactions. Acta Trop. 2018, 183, 23–31. [Google Scholar] [CrossRef]
- Alevi, K.; de Oliveira, J.; Garcia, A.; Cristal, D.; Delgado, L.; Bittinelli, I.; dos Reis, Y.; Ravazi, A.; de Oliveira, A.; Galvão, C.; et al. Triatoma rosai sp. nov. (Hemiptera, Triatominae): A new species of Argentinian chagas disease vector described based on integrative taxonomy. Insects 2020, 11, 830. [Google Scholar] [CrossRef]
- Alevi, K.C.; de Oliveira, J.; Rocha, D.; Galvão, C. Trends in taxonomy of chagas disease vectors (Hemiptera, Reduviidae, triatominae): From Linnaean to integrative taxonomy. Pathogens 2021, 10, 1627. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, M.C.; Castro-Vásquez, R.M.; Herrero-Acosta, M.V.; Urbina-Villalobos, A.; Dolz, G. Canine trypanosomiasis in an endemic Costa Rican community: Demonstration of the active infection cycle. Vet. Parasitol. Reg. Stud. Rep. 2019, 17, 100307. [Google Scholar] [CrossRef]
- Jansen, A.M.; Xavier, S.C.d.C.; Roque, A.L.R. Landmarks of the Knowledge and Trypanosoma cruzi Biology in the Wild Environment. Front. Cell. Infect. Microbiol. 2020, 10, 10. [Google Scholar] [CrossRef]
- Meyers, A.C.; Ellis, M.M.; Purnell, J.C.; Auckland, L.D.; Meinders, M.; Saunders, A.B.; Hamer, S.A. Selected cardiac abnormalities in Trypanosoma cruzi serologically positive, discordant, and negative working dogs along the Texas-Mexico border. BMC Vet. Res. 2020, 16, 101. [Google Scholar] [CrossRef]
- Moretti, N.S.; Mortara, R.A.; Schenkman, S. Trypanosoma cruzi. Trends Parasitol. 2020, 36, 404–405. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.R.L.; Gomes, C.; Lozzi, S.P.; Hecht, M.M.; Rosa, A.d.C.; Monteiro, P.S.; Bussacos, A.C.; Nitz, N.; McManus, C. Environment, interactions between Trypanosoma cruzi and its host, and health. Cad. Saúde Pública 2009, 25 (Suppl. S1), S32–S44. [Google Scholar] [CrossRef]
- Osorio, L.; Ríos, I.; Gutiérrez, B.; González, J. Virulence factors of Trypanosoma cruzi: Who is who? Microbes Infect. 2012, 14, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Salassa, B.N.; Romano, P.S. Autophagy: A necessary process during the Trypanosoma cruzi life-cycle. Virulence 2019, 10, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Martín-Escolano, J.; Marín, C.; Rosales, M.J.; Tsaousis, A.D.; Medina-Carmona, E.; Martín-Escolano, R. An Updated View of the Trypanosoma cruzi Life Cycle: Intervention Points for an Effective Treatment. ACS Infect. Dis. 2022, 8, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Herreros-Cabello, A.; Callejas-Hernández, F.; Gironès, N.; Fresno, M. Trypanosoma cruzi genome: Organization, multi-gene families, transcription, and biological implications. Genes 2020, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.C. Canine Chagas’ Disease (American Trypanosomiasis) in North America. Vet. Clin. N. Am.-Small Anim. Pract. 2009, 39, 1055–1064. [Google Scholar] [CrossRef]
- Roellig, D.M.; Ellis, A.E.; Yabsley, M.J. Oral transmission of Trypanosoma cruzi with opposing evidence for the theory of carnivory. J. Parasitol. 2009, 95, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Santana, R.A.G.; Guerra, M.G.V.B.; Sousa, D.R.; Couceiro, K.; Ortiz, J.V.; Oliveira, M.; Ferreira, L.S.; Souza, K.R.; Tavares, I.C.; Morais, R.F.; et al. Oral transmission of Trypanosoma cruzi, Brazilian Amazon. Emerg. Infect. Dis. 2019, 25, 132–135. [Google Scholar] [CrossRef] [PubMed]
- de Noya, B.; Colmenares, C.; Díaz-Bello, Z.; Ruiz-Guevara, R.; Medina, K.; Muñoz-Calderón, A.; Mauriello, L.; Cabrera, E.; Montiel, L.; Losada, S.; et al. Orally-transmitted Chagas disease: Epidemiological, clinical, serological and molecular outcomes of a school microepidemic in Chichiriviche de la Costa, Venezuela. Parasite Epidemiol. Control 2016, 1, 188–198. [Google Scholar] [CrossRef]
- Velásquez-Ortiz, N.; Ramírez, J.D. Understanding the oral transmission of Trypanosoma cruzi as a veterinary and medical foodborne zoonosis. Res. Vet. Sci. 2020, 132, 448–461. [Google Scholar] [CrossRef]
- Pacheco, L.V.; Santana, L.S.; Barreto, B.C.; Santos, E.D.S.; Meira, C.S. Oral transmission of Chagas disease: A literature review. Res. Soc. Dev. 2021, 10, e31910212636. [Google Scholar] [CrossRef]
- Neira, I.; Silva, F.A.; Cortez, M.; Yoshida, N. Involvement of Trypanosoma cruzi metacyclic trypomastigote surface molecule gp82 in adhesion to gastric mucin and invasion of epithelial cells. Infect. Immun. 2003, 71, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Cortez, M.; Silva, M.R.; Neira, I.; Ferreira, D.; Sasso, G.R.S.; Luquetti, A.O.; Rassi, A.; Yoshida, N. Trypanosoma cruzi surface molecule gp90 downregulates invasion of gastric mucosal epithelium in orally infected mice. Microbes Infect. 2006, 8, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.B.; Hamer, S.A. Chagas Disease Trypanosoma cruzi Infection in Dogs. Today’s Vet. Pract. 2020, 10, 16–22. Available online: https://todaysveterinarypractice.com/parasitology/chagas-disease-dogs/ (accessed on 21 March 2024).
- Stoner, C.H.; Saunders, A.B. Cardiac Manifestations of Trypanosoma cruzi Infection in a Domestic Dog. CASE 2020, 4, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Castañera, M.B.; Lauricella, M.A.; Chuit, R.; Gürtler, R.E. Evaluation of dogs as sentinels of the transmission of Trypanosoma cruzi in a rural area of north-western Argentina. Ann. Trop. Med. Parasitol. 1998, 92, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Jaimes-Dueñez, J.; Jiménez-Leaño, Á.P.; Esteban-Mendoza, M.; Moreno-Salcedo, L.A.; Triana-Chávez, O.; Cantillo-Barraza, O. Epidemiological and clinical characteristics of Trypanosoma cruzi infection in dogs (Canis lupus familiaris) from a Chagas Disease-Endemic Urban Area in Colombia. Prev. Vet. Med. 2020, 182, 105093. [Google Scholar] [CrossRef] [PubMed]
- Travi, B.L. Considering Dogs as Complementary Targets of Chagas Disease Control. Vector Borne Zoonotic Dis. 2019, 19, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Elmayan, A.; Tu, W.; Duhon, B.; Marx, P.; Wolfson, W.; Balsamo, G.; Dumonteil, E. High prevalence of Trypanosoma cruzi infection in shelter dogs from southern Louisiana, USA. Parasites Vectors 2019, 12, 322. [Google Scholar] [CrossRef]
- Tenney, T.D.; Curtis-Robles, R.; Snowden, K.F.; Hamer, S.A. Shelter dogs as sentinels for Trypanosoma cruzi transmission across Texas. Emerg. Infect. Dis. 2014, 20, 1323–1326. [Google Scholar] [CrossRef]
- Meyers, A.C.; Meinders, M.; Hamer, S.A. Widespread Trypanosoma cruzi infection in government working dogs along the Texas-Mexico border: Discordant serology, parasite genotyping and associated vectors. PLoS Neglected Trop. Dis. 2017, 11, e0005819. [Google Scholar] [CrossRef]
- Petersen, R.M.; Gürtler, R.E.; Cecere, M.C.; Rubel, D.N.; Lauricella, M.A.; Hansen, D.; Carlomagno, M.A. Association between nutritional indicators and infectivity of dogs seroreactive for Trypanosoma cruzi in a rural area of northwestern Argentina. Parasitol. Res. 2001, 87, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Crisante, G.; Rojas, A.; Teixeira, M.M.G.; Añez, N. Infected dogs as a risk factor in the transmission of human Trypanosoma cruzi infection in western Venezuela. Acta Trop. 2006, 98, 247–254. [Google Scholar] [CrossRef]
- Estrada-Franco, J.G.; Bhatia, V.; Diaz-Albiter, H.; Ochoa-Garcia, L.; Barbabosa, A.; Vazquez-Chagoyan, J.C.; Martinez-Perez, M.A.; Guzman-Bracho, C.; Garg, N. Human Trypanosoma cruzi infection and seropositivity in dogs, Mexico. Emerg. Infect. Dis. 2006, 12, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.D.; Turriago, B.; Tapia-Calle, G.; Guhl, F. Understanding the role of dogs (Canis lupus familiaris) in the transmission dynamics of Trypanosoma cruzi genotypes in Colombia. Vet. Parasitol. 2013, 196, 216–219. [Google Scholar] [CrossRef]
- NCBI. Taxonomy Browser/Full Lineage of T. cruzi/Trypanosoma cruzi. 2023. Available online: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=info&id=5693 (accessed on 13 April 2023).
- Risso, M.G.; Garbarino, G.B.; Mocetti, E.; Campetella, O.; Lez Cappa, S.M.G.; Buscaglia, C.A.; Leguizamón, M.S. Differential Expression of a Virulence Factor, the trans-Sialidase, by the Main Trypanosoma cruzi Phylogenetic Lineages. J. Infect. Dis. 2004, 189, 2250–2259. [Google Scholar] [CrossRef]
- de Pablos, L.M.; Osuna, A. Multigene families in Trypanosoma cruzi and their role in infectivity. Infect. Immun. 2012, 80, 2258–2264. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Falconar, A.K.; Luquetti, A.O.; Costales, J.A.; Grijalva, M.J.; Lewis, M.D.; Messenger, L.A.; Tran, T.T.; Ramirez, J.D.; Guhl, F.; et al. Development of Peptide-Based Lineage-Specific Serology for Chronic Chagas Disease: Geographical and Clinical Distribution of Epitope Recognition. PLoS Neglected Trop. Dis. 2014, 8, e2892. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rincón, G.J.; Gallo-Bernal, S.; Jiménez, P.A.; Cruz-Saavedra, L.; Ramírez, J.D.; Rodríguez, M.J.; Medina-Mur, R.; Díaz-Nassif, G.; Valderrama-Achury, M.D.; Medina, H.M. Molecular and clinical aspects of chronic manifestations in chagas disease: A state-of-the-art review. Pathogens 2021, 10, 1493. [Google Scholar] [CrossRef]
- Macedo, A.M.; Machado, C.R.; Oliveira, R.P.; Pena, S.D. Trypanosoma cruzi: Genetic Structure of Populations and Relevance of Genetic Variability to the Pathogenesis of Chagas Disease. Mem. Inst. Oswaldo Cruz 2004, 99, 1–12. [Google Scholar] [CrossRef]
- De Oliveira, M.T.; Sulleiro, E.; Gimenez, A.S.; de Lana, M.; Zingales, B.; da Silva, J.S.; Marin-Neto, J.A.; Molina, I. Quantification of parasite burden of Trypanosoma cruzi and identification of discrete typing units (Dtus) in blood samples of latin american immigrants residing in Barcelona, Spain. PLoS Neglected Trop. Dis. 2020, 14, e0008311. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Ortiz, N.; Herrera, G.; Hernández, C.; Muñoz, M.; Ramírez, J.D. Discrete typing units of Trypanosoma cruzi: Geographical and biological distribution in the Americas. Sci. Data 2022, 9, 360. [Google Scholar] [CrossRef] [PubMed]
- Dumonteil, E.; Elmayan, A.; Majeau, A.; Tu, W.; Duhon, B.; Marx, P.; Wolfson, W.; Balsamo, G.; Herrera, C. Genetic diversity of Trypanosoma cruzi parasites infecting dogs in southern Louisiana sheds light on parasite transmission cycles and serological diagnostic performance. PLoS Neglected Trop. Dis. 2020, 14, e0008932. [Google Scholar] [CrossRef] [PubMed]
- Lauthier, J.J.; Tomasini, N.; Barnabé, C.; Rumi, M.M.; D’Amato, A.M.; Ragone, P.G.; Yeo, M.; Lewis, M.D.; Llewellyn, M.S.; Basombrío, M.A.; et al. Candidate targets for Multilocus Sequence Typing of Trypanosoma cruzi: Validation using parasite stocks from the Chaco Region and a set of reference strains. Infect. Genet. Evol. 2012, 12, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Cantillo-Barraza, O.; Solis, C.; Zamora, A.; Herazo, R.; Osorio, M.I.; Garcés, E.; Xavier, S.; Mejía-Jaramillo, A.M.; Triana-Chávez, O. Enzootic Trypanosoma cruzi infection by Rhodnius prolixus shows transmission to humans and dogs in Vichada, Colombia. Front. Cell Infect. Microbiol. 2022, 12, 999082. [Google Scholar] [CrossRef] [PubMed]
- Herrera, L.; Morocoima, A.; Lozano-Arias, D.; García-Alzate, R.; Viettri, M.; Lares, M.; Ferrer, E. Infections and Coinfections by Trypanosomatid Parasites in a Rural Community of Venezuela. Acta Parasitol. 2022, 67, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Villa, L.M.; Guhl, F.; Zabala, D.; Ramírez, J.D.; Urrea, D.A.; Hernández, D.C.; Cucunubá, Z.; Montilla, M.; Carranza, J.C.; Rueda, K.; et al. The identification of two Trypanosoma cruzi I genotypes from domestic and sylvatic transmission cycles in Colombia based on a single polymerase chain reaction amplification of the spliced-leader intergenic region. Mem. Inst. Oswaldo Cruz 2013, 108, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Cantillo-Barraza, O.; Bedoya, S.C.; Xavier, S.C.C.; Zuluaga, S.; Salazar, B.; Vélez-Mira, A.; Carrillo, L.M.; Triana-Chávez, O. Trypanosoma cruzi infection in domestic and synanthropic mammals such as potential risk of sylvatic transmission in a rural area from north of Antioquia, Colombia. Parasite Epidemiol. Control 2020, 11, e00171. [Google Scholar] [CrossRef] [PubMed]
- Brandão, E.M.V.; Xavier, S.C.C.; Rocha, F.L.; Lima, C.F.M.; Candeias, Í.Z.; Lemos, F.G.; Azevedo, F.C.; Jansen, A.M.; Roque, A.L.R. Wild and Domestic Canids and Their Interactions in the Transmission Cycles of Trypanosoma Cruzi and Leishmania spp. in an Area of the Brazilian Cerrado. Pathogens 2020, 9, 818. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Lima, L.; de Almeida, L.A.; Monteiro, J.; Moreno, C.J.G.; Nascimento, J.D.; de Araújo, R.F.; Mello, F.; Martins, L.P.A.; Graminha, M.A.S.; et al. Biological and Molecular Characterization of Trypanosoma cruzi Strains from Four States of Brazil. ASTMH 2018, 98, 453–463. [Google Scholar] [CrossRef]
- Chaves, L.F.; Meyers, A.C.; Hodo, C.L.; Sanders, J.P.; Curtis-Robles, R.; Hamer, G.L.; Hamer, S.A. Trypanosoma cruzi infection in dogs along the US-Mexico border: R0 changes with vector species composition. Epidemics 2023, 45, 100723. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; Luna, B.S.; Calderon, O.; Manriquez-Roman, C.; Amezcua-Winter, K.; Cedillo, J.; Garcia-Vazquez, R.; Tejeda, I.A.; Romero, A.; Waldrup, K.; et al. Surveillance of Trypanosoma cruzi infection in Triatomine vectors, feral dogs and cats, and wild animals in and around El Paso county, Texas, and New Mexico. PLoS Negl. Trop. Dis. 2021, 18, e0009147. [Google Scholar] [CrossRef] [PubMed]
- Kjos, S.A.; Snowden, K.F.; Craig, T.M.; Lewis, B.; Ronald, N.; Olson, J.K. Distribution and characterization of canine Chagas disease in Texas. Vet. Parasitol. 2008, 152, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Curtis-Robles, R.; Snowden, K.F.; Dominguez, B.; Dinges, L.; Rodgers, S.; Mays, G.; Hamer, S.A. Epidemiology and Molecular Typing of Trypanosoma cruzi in Naturally-Infected Hound Dogs and Associated Triatomine Vectors in Texas, USA. PLoS Neglected Trop. Dis. 2017, 11, e0005298. [Google Scholar] [CrossRef]
- Bradley, K.K.; Bergman, D.K.; Woods, J.P.; Crutcher, J.M.; Kirchhoff, L.V. Prevalence of American trypanosomiasis (Chagas disease) among dogs in Oklahoma. J. Am. Vet. Med. Assoc. 2000, 217, 1853–1857. [Google Scholar] [CrossRef] [PubMed]
- Beard, C.B.; Pye, G.; Steurer, F.J.; Rodriguez, R.; Campman, R.; Peterson, A.T.; Robinson, L.E. Chagas disease in a domestic transmission cycle, southern Texas, USA. Emerg. Infect. Dis. 2003, 9, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Curtis-Robles, R.; Zecca, I.B.; Roman-Cruz, V.; Carbajal, E.S.; Auckland, L.D.; Flores, I.; Hamer, S.A. Trypanosoma cruzi (agent of Chagas disease) in sympatric human and dog populations in “Colonias” of the Lower Rio Grande Valley of Texas. Am. J. Trop. Med. Hyg. 2017, 96, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (WHO). About Chagas Disease. 2022. Available online: https://www.cdc.gov/parasites/chagas/gen_info/detailed.html (accessed on 16 April 2023).
- World Health Organization (WHO). Chagas Disease (Also Known as American Trypanosomiasis). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 16 May 2023).
- Busselman, R.E.; Hamer, S.A. Chagas Disease Ecology in the United States: Recent Advances in Understanding Trypanosoma cruzi Transmission among Triatomines, Wildlife, and Domestic Animals and a Quantitative Synthesis of Vector-Host Interactions. Annu. Rev. Anim. Biosci. 2022, 10, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Marin-Neto, J.A.; Cunha-Neto, E.; Maciel, B.C.; Simões, M.V. Pathogenesis of chronic Chagas heart disease. Circulation 2007, 115, 1109–1123. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Research Priorities for Chagas Disease, Human African Trypanosomiasis and Leishmaniasis; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Bonney, K.M.; Luthringer, D.J.; Kim, S.A.; Garg, N.J.; Engman, D.M. Pathology and Pathogenesis of Chagas Heart Disease. Annu. Rev. Pathol. 2019, 14, 421–447. [Google Scholar] [CrossRef]
- Dias, J.C.; Ramos, A.N.; Gontijo, E.D.; Luquetti, A.; Shikanai-Yasuda, M.A.; Coura, J.R.; Torres, R.M.; Melo, J.R.D.C.; De Almeida, E.A.; de Oliveira, W., Jr.; et al. 2nd Brazilian Consensus on Chagas Disease. Rev. Soc. Bras. Med. Trop. 2016, 49 (Suppl. S1), 3–60. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.C.; Schmidt, S.P.; Brown, C.C.; Klei, T.R. Pathologic features of dogs inoculated with North American Trypanosoma cruzi isolates. Am. J. Vet. Res. 1991, 52, 2033–2039. [Google Scholar] [CrossRef] [PubMed]
- de Lana, M.; Chiari, E.; Tafuri, W.L. Experimental Chagas’ disease in dogs. Mem. Inst. Oswaldo Cruz 1992, 87, 59–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matthews, D.J.; Saunders, A.B.; Meyers, A.C.; Gordon, S.G.; Hamer, S.A. Cardiac diagnostic test results and outcomes in 44 dogs naturally infected with Trypanosoma cruzi. J. Vet. Intern. Med. 2021, 35, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Vitt, J.P.; Saunders, A.B.; O’Brien, M.T.; Mansell, J.; Ajithdoss, D.K.; Hamer, S.A. Diagnostic Features of Acute Chagas Myocarditis with Sudden Death in a Family of Boxer Dogs. J. Vet. Intern. Med. 2016, 30, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Meurs, K.M.; Anthony, M.A.; Slater, M.; Miller, M.W. Chronic Trypanosoma cruzi infection in dogs: 11 cases (1987–1996). J. Am. Vet. Med. Assoc. 1998, 213, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Meyers, A.C.; Hamer, S.A.; Matthews, D.; Gordon, S.G.; Saunders, A.B. Risk factors and select cardiac characteristics in dogs naturally infected with Trypanosoma cruzi presenting to a teaching hospital in Texas. J. Vet. Intern. Med. 2019, 33, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.C.; Gossett, K.A.; Klei, T.R. Clinical, clinicopathologic, and parasitologic observations of trypanosomiasis in dogs infected with North American Trypanosoma cruzi isolates. Am. J. Vet. Res. 1991, 52, 954–960. [Google Scholar] [CrossRef]
- Montenegro, V.M.; Jimenez, M.; Dias, J.C.; Zeledon, R. Chagas disease in dogs from endemic areas of Costa Rica. Mem. Inst. Oswaldo Cruz 2002, 97, 491–494. [Google Scholar] [CrossRef]
- Pereira, H.d.S.; Scofield, A.; Soares, P.; Júnior, B.; Lira, D.; Santos, D.; Siqueira, J.; Chaves, J.; De, R.; Cardoso, J.; et al. Chagas disease in urban and peri-urban environment in the Amazon: Sentinel hosts, vectors, and the environment. Acta Trop. 2021, 217, 105858. [Google Scholar] [CrossRef]
- de Souza, A.I.; Paulino, D.; Sousa, M.G.; Camacho, A.A. Aspectos clínico-laboratoriais da infecção natural por Trypanosoma cruzi em cães de Mato Grosso do Sul. Ciênc. Rural 2008, 38, 1351–1356. [Google Scholar] [CrossRef]
- Saraiva, R.M.; Mediano, M.F.F.; Mendes, F.S.; da Silva, G.M.S.; Veloso, H.H.; Sangenis, L.H.C.; da Silva, P.S.; Mazzoli-Rocha, F.; Sousa, A.S.; Holanda, M.T.; et al. Chagas heart disease: An overview of diagnosis, manifestations, treatment, and care. World J. Cardiol. 2021, 13, 654–675. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.O.; Miziara, J.L. Aspetos clínicos da cardiopatia chagásica aguda. In Cardiopatia Chagásica; Cançado, J.R., Chuster, M., Eds.; Imprensa Oficial: Belo Horizonte, Brazil, 1985. [Google Scholar]
- Bocchi, E.A.; Bestetti, R.B.; Scanavacca, M.I.; Neto, E.; Issa, V.S. Chronic Chagas Heart Disease Management: From Etiology to Cardiomyopathy Treatment. J. Am. Coll. Cardiol. 2017, 70, 1510–1524. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A., Jr.; Rassi, A.; de Rezende, J.M. American Trypanosomiasis (Chagas Disease). Infect. Dis. Clin N. Am. 2012, 26, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Cordova, E.; Boschi, A.; Ambrosioni, J.; Cudos, C.; Corti, M. Reactivation of Chagas disease with central nervous system involvement in HIV-infected patients in Argentina, 1992–2007. Int. J. Infect. Dis. 2008, 12, 587–592. [Google Scholar] [CrossRef]
- Useche, Y.; Pérez, A.R.; de Meis, J.; Bonomo, A.; Savino, W. Central nervous system commitment in Chagas disease. Front. Immunol. 2022, 13, 975106. [Google Scholar] [CrossRef] [PubMed]
- Córdova, E.; Maiolo, E.; Corti, M.; Orduña, T. Neurological manifestations of Chagas disease. Neurol. Res. 2010, 32, 238244. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-Paiva, N.C.; Fonseca, K.d.S.; Vieira, P.M.; Diniz, L.F.; Caldas, I.S.; Moura, S.A.; Veloso, V.M.; Guedes, P.M.; Tafuri, W.L.; Bahia, M.T.; et al. Myenteric plexus is differentially affected by infection with distinct Trypanosoma cruzi strains in Beagle dogs. Mem. Inst. Oswaldo Cruz 2014, 109, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Barbabosa-Pliego, A.; Díaz-Albiter, H.M.; Ochoa-García, L.; Aparicio-Burgos, E.; López-Heydeck, S.M.; Velásquez-Ordoñez, V.; Vázquez-Chagoyán, J.C. Trypanosoma cruzi circulating in the southern region of the State of Mexico (Zumpahuacan) are pathogenic: A dog model. Am. J. Trop. Med. Hyg. 2009, 81, 390–395. [Google Scholar] [CrossRef]
- Andrade, S.G.; Campos, R.F.; Steindel, M.; Guerreiro, M.L.; Magalhães, J.B.; de Almeida, M.C.; Reis, J.N.; Santos, V.C.; Valadares, H.M.; dos Reis, M.G.; et al. Biological, biochemical and molecular features of Trypanosoma cruzi strains isolated from patients infected through oral transmission during a 2005 outbreak in the state of Santa Catarina, Brazil: Its correspondence with the new T. cruzi Taxonomy Consensus. Mem. Inst. Oswaldo Cruz 2011, 106, 948–956. [Google Scholar] [CrossRef]
- Hasslocher-Moreno, A.M.; Xavier, S.S.; Saraiva, R.M.; Sousa, A.S. Indeterminate form of Chagas disease: Historical, conceptual, clinical, and prognostic aspects. Rev. Soc. Bras. Med. Trop. 2021, 54, e02542021. [Google Scholar] [CrossRef] [PubMed]
- Sociedade Brasileira de Medicina Tropical. I Reunião de Pesquisa Aplicada em Doença de Chagas. Validade do conceito de forma indeterminada de doença de Chagas. Rev. Soc. Bras. Med. Trop. 1985, 18, 46. [Google Scholar] [CrossRef]
- Hasslocher-Moreno, A.M.; Xavier, S.S.; Saraiva, R.M.; Sangenis, L.H.C.; Holanda, M.T.; Veloso, H.H.; da Costa, A.R.; Mendes, F.d.S.N.S.; Brasil, P.E.A.A.D.; da Silva, G.M.S.; et al. Progression Rate from the Indeterminate Form to the Cardiac Form in Patients with Chronic Chagas Disease: Twenty-Two-Year Follow-Up in a Brazilian Urban Cohort. Trop. Med. Infect. Dis. 2020, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.H.; Hoff, R.; Sherlock, I.; Guimarães, A.C.; Sleigh, A.C.; Ramos, N.B.; Mott, K.E.; Weller, T.H. Cardiac morbidity and mortality due to Chagas’ disease: Prospective electrocardiographic study of a Brazilian community. Circulation 1987, 75, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Bahia, M.T.; Tafuri, W.L.; Caliari, M.V.; Veloso, V.M.; Carneiro, C.M.; Coelho, G.L.; Lana, M. Comparison of Trypanosoma cruzi infection in dogs inoculated with blood or metacyclic trypomastigotes of Berenice-62 and Berenice-78 strains via intraperitoneal and conjunctival routes. Rev. Soc. Bras. Med. Trop. 2002, 35, 339–345. [Google Scholar] [CrossRef] [PubMed]
- European Society of Cardiology (ESC). Chagas Cardiomyopathy. Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-14/Chagas-cardiomyopathy (accessed on 20 August 2023).
- Guedes, P.M.; Veloso, V.M.; Caliari, M.V.; Carneiro, C.M.; Souza, S.M.; de Lana, M.; Chiari, E.; Bahia, M.T.; Galvão, L. Trypanosoma cruzi high infectivity in vitro is related to cardiac lesions during long-term infection in Beagle dogs. Mem. Inst. Oswaldo Cruz 2007, 102, 141–147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- González-Vieyra, S.D.; Ramírez-Durán, N.; Sandoval-Trujillo, Á.H.; Vázquez-Chagoyán, J.C.; Monroy-Salazar, H.G.; Barbabosa-Pliego, A. Trypanosoma cruzi in dogs: Electrocardiographic and echocardiographic evaluation, in Malinalco, State of Mexico. Res. Rep. Trop. Med. 2011, 2, 155–161. [Google Scholar] [CrossRef]
- Carvalho, E.B.; Ramos, I.P.R.; Nascimento, A.F.S.; Brasil, G.V.; Mello, D.B.; Oti, M.; Sammeth, M.; Bahia, M.T.; de Carvalho, A.C.C.; Carvalho, A.B. Echocardiographic Measurements in a Preclinical Model of Chronic Chagasic Cardiomyopathy in Dogs: Validation and Reproducibility. Front. Cell Infect. Microbiol. 2019, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Meyers, A.C.; Purnell, J.C.; Ellis, M.M.; Auckland, L.D.; Meinders, M.; Hamer, S.A. Nationwide exposure of U.S. working dogs to the Chagas disease parasite, Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 2020, 102, 1078–1085. [Google Scholar] [CrossRef]
- Zecca, I.B.; Hodo, C.L.; Slack, S.; Auckland, L.; Rodgers, S.; Killets, K.C.; Saunders, A.B.; Hamer, S.A. Prevalence of Trypanosoma cruzi infection and associated histologic findings in domestic cats (Felis catus). Vet. Parasitol. 2020, 278, 109014. [Google Scholar] [CrossRef]
- Andrade, Z.A.; Andrade, S.G. Pathology of experimental Chagas disease in dogs. Mem. Inst. Oswaldo Cruz 1980, 75, 77–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Oliveira Vieira, A.; Nascentes, G.A.N.; de Morais Oliveira, A.C.; Correia, D.; Cabrine-Santos, M. Biomarkers assessment in patients with Chagas disease and systemic arterial hypertension. Parasitol. Res. 2021, 120, 1429–1435. [Google Scholar] [CrossRef]
- Ribeiro, A.L.; Nunes, M.P.; Teixeira, M.M.; Rocha, M.O. Diagnosis and management of Chagas disease and cardiomyopathy. Nat. Rev. Cardiol. 2012, 9, 576–589. [Google Scholar] [CrossRef]
- Meyers, A.C.; Edwards, E.E.; Sanders, J.P.; Saunders, A.B.; Hamer, S.A. Fatal Chagas myocarditis in government working dogs in the southern United States: Cross-reactivity and differential diagnoses in five cases across six months. Veter-Parasitol. Reg. Stud. Rep. 2021, 24, 100545. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, N.M.; Miller, S.M.; Evora, P.R. The chronic gastrointestinal manifestations of Chagas disease. Clinics 2009, 64, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.L.; Palmer, R.H.; Hodges, C.C.; Hall, D.G. Neurologic manifestations of trypanosomiasis in a dog. J. Am. Vet. Med. Assoc. 1991, 198, 132–134. [Google Scholar] [CrossRef]
- Pascon, J.P.E.; Neto, G.B.P.; Sousa, M.G.; Paulino, D.; Camacho, A.A. Clinical characterization of chronic chagasic cardiomyopathy in dogs. Pesqui. Vet. Bras. 2010, 30, 115–120. [Google Scholar] [CrossRef][Green Version]
- Santana, V.L.; Souza, A.P.; Lima, D.A.S.D.; Araujo, A.L.; Justiniano, S.V.; Dantas, R.P.; Guedes, P.M.; Melo, M.A. Caracterizacao clinica e laboratorial de caes naturalmente infectados com Trypanosoma cruzi no semiarido nordestino. Pesqui. Vet. Bras. 2012, 32, 536–541. [Google Scholar] [CrossRef]
- Garcia, M.N.; O’Day, S.; Fisher-Hoch, S.; Gorchakov, R.; Patino, R.; Arroyo, T.P.F.; Laing, S.T.; Lopez, J.E.; Ingber, A.; Jones, K.M.; et al. One Health Interactions of Chagas Disease Vectors, Canid Hosts, and Human Residents along the Texas-Mexico Border. PLoS Neglected Trop. Dis. 2016, 10, e0005074. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for the Diagnosis and Treatment of Chagas Disease; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Garcia, V.S.; Gonzalez, V.D.; Marcipar, I.S.; Gugliotta, L.M. Immunoagglutination test to diagnose Chagas disease: Comparison of different latex-antigen complexes. Trop. Med. Int. Health 2014, 19, 1346–1354. [Google Scholar] [CrossRef]
- Santos, F.L.N.; Celedon, P.A.F.; Zanchin, N.I.T.; Leitolis, A.; Crestani, S.; Foti, L.; de Souza, W.V.; Gomes, Y.M.; Krieger, M.A. Performance Assessment of a Trypanosoma cruzi Chimeric Antigen in Multiplex Liquid Microarray Assays. J. Clin. Microbiol. 2017, 55, 2934–2945. [Google Scholar] [CrossRef] [PubMed]
- Nabity, M.B.; Barnhart, K.; Logan, K.S.; Santos, R.L.; Kessell, A.; Melmed, C.; Snowden, K. An atypical case of Trypanosoma cruzi infection in a young English Mastiff. Vet. Parasitol. 2006, 140, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Hamer, S.A.; Saunders, A.B. Veterinary Chagas Disease (American Trypanosomiasis) in the United States. Vet. Clin. N. Am. Small Anim. Pract. 2022, 52, 1267–1281. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, C.L.; Albajar-Viñas, P.; Wilkins, P.P.; Nieto, J.; Leiby, D.A.; Paris, L.; Scollo, K.; Flórez, C.; Guzmán-Bracho, C.; Luquetti, A.O.; et al. Comparative evaluation of 11 commercialized rapid diagnostic tests for detecting Trypanosoma cruzi antibodies in serum banks in areas of endemicity and nonendemicity. J. Clin. Microbiol. 2014, 52, 2506–2512. [Google Scholar] [CrossRef] [PubMed]
- Nieto, P.D.; Boughton, R.; Dorn, P.L.; Steurer, F.; Raychaudhuri, S.; Esfandiari, J.; Gonçalves, E.; Diaz, J.; Malone, J.B. Comparison of two immunochromatographic assays and the indirect immunofluorescence antibody test for diagnosis of Trypanosoma cruzi infection in dogs in south central Louisiana. Vet. Parasitol. 2009, 165, 241–247. [Google Scholar] [CrossRef]
- McClean, M.C.W.; Bhattacharyya, T.; Mertens, P.; Murphy, N.; Gilleman, Q.; Gustin, Y.; Zeippen, N.; Xavier, S.C.C.; Jansen, A.M.; Miles, M.A. A lineage-specific rapid diagnostic test (Chagas Sero K-SeT) identifies Brazilian Trypanosoma cruzi II/V/VI reservoir hosts among diverse mammalian orders. PLoS ONE 2020, 15, e0227828. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.S.; Santos, G.Q.; da Silva, M.V.; Barros, J.H.S.; Bernardo, A.R.; Diniz, R.L.; Rubim, N.M.; Roque, A.L.R.; Jansen, A.M.; Silva, E.D.; et al. Chagas Immunochromatographic Rapid Test in the Serological Diagnosis of Trypanosoma cruzi Infection in Wild and Domestic Canids. Front. Cell Infect. Microbiol. 2022, 12, 835383. [Google Scholar] [CrossRef] [PubMed]
- Daltro, R.T.; Leony, L.M.; Freitas, N.E.M.; Silva, Â.A.O.; Santos, E.F.; Del-Rei, R.P.; Brito, M.E.F.; Brandão-Filho, S.P.; Gomes, Y.M.; Silva, M.S.; et al. Cross-Reactivity Using Chimeric Trypanosoma cruzi Antigens: Diagnostic Performance in Settings Where Chagas Disease and American Cutaneous or Visceral Leishmaniasis Are Coendemic. J. Clin. Microbiol. 2019, 57, e00762-19. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.J.; Manne-Goehler, J.; Bern, C.; Whitman, J.; Hochberg, N.S.; Edwards, M.; Marcus, R.; Beatty, N.L.; Castro-Sesquen, Y.E.; Coyle, C.; et al. US Chagas Diagnostic Working Group, Recommendations for Screening and Diagnosis of Chagas Disease in the United States. J. Infect. Dis. 2022, 225, 1601–1610. [Google Scholar] [CrossRef]
- Barreto, M.L.; Teixeira, M.G.; Bastos, F.I.; Ximenes, R.A.; Barata, R.B.; Rodrigues, L.C. Successes and failures in the control of infectious diseases in Brazil: Social and environmental context, policies, interventions, and research needs. Lancet 2011, 377, 1877–1889. [Google Scholar] [CrossRef]
- Araújo, F.M.G.; Bahia, M.T.; Magalhães, N.M.; Martins-Filho, O.A.; Veloso, V.M.; Carneiro, C.M.; Tafuri, W.L.; Lana, M. Follow-up of experimental chronic Chagas’ disease in dogs: Use of polymerase chain reaction (PCR) compared with parasitological and serological methods. Acta Trop. 2002, 81, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Rosypal, A.C.; Hill, R.; Lewis, S.; Barr, S.C.; Valadas, S.; Gennari, S.M.; Lindsay, D.S. Evaluation of a rapid immunochromatographic dipstick test for detection of antibodies to Trypanosoma cruzi in dogs experimentally infected with isolates obtained from opossums (Didelphis virginiana), armadillos (Dasypus novemcinctus), and dogs (Canis familiaris) from the United States. J. Parasitol. 2011, 97, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Eloy, L.J.; Lucheis, S.B. Hemoculture and Polymerase Chain Reaction Using Primers TCZ1/TCZ2 for the Diagnosis of Canine and Feline Trypanosomiasis. ISRN Vet. Sci. 2012, 2012, 419378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Besuschio, S.A.; Picado, A.; Muñoz-Calderón, A.; Wehrendt, D.P.; Fernández, M.; Benatar, A.; Diaz-Bello, Z.; Irurtia, C.; Cruz, I.; Ndung’u, J.M.; et al. Trypanosoma cruzi loop-mediated isothermal amplification (Trypanosoma cruzi Loopamp) kit for detection of congenital, acute and Chagas disease reactivation. PLoS Neglected Trop. Dis. 2020, 14, e0008402. [Google Scholar] [CrossRef] [PubMed]
- Tarleton, R.L.; Gürtler, R.E.; Urbina, J.A.; Ramsey, J.; Viotti, R. Chagas Disease and the London Declaration on Neglected Tropical Diseases. PLoS Neglected Trop. Dis. 2014, 8, e3219. [Google Scholar] [CrossRef] [PubMed]
- Lascano, F.; García Bournissen, F.; Altcheh, J. Review of pharmacological options for the treatment of Chagas disease. Br. J. Clin. Pharmacol. 2022, 88, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Garavaglia, P.A.; Laverrière, M.; Cannata, J.J.; García, G.A. Putative Role of the Aldo-Keto Reductase from Trypanosoma cruzi in Benznidazole Metabolism. Antimicrob. Agents Chemother. 2016, 60, 2664–2670. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.T.; de Branquinho, R.T.; Alessio, G.D.; Mello, C.G.C.; Nogueira-de-Paiva, N.C.; Carneiro, C.M.; Toledo, M.J.d.O.; Reis, A.B.; Martins-Filho, O.A.M.; de Lana, M. TcI, TcII and TcVI Trypanosoma cruzi samples from Chagas disease patients with distinct clinical forms and critical analysis of in vitro and in vivo behavior, response to treatment and infection evolution in murine model. Acta Trop. 2017, 167, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B. Trypanosoma cruzi genetic diversity: Something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018, 184, 38–52. [Google Scholar] [CrossRef]
- Santana, R.A.; Magalhães, L.K.; Magalhães, L.K.; Prestes, S.; Maciel, M.; da Silva, G.A.; Monteiro, W.M.; de Brito, F.R.; Coelho, L.I.d.A.R.C.; Barbosa-Ferreira, J.M.; et al. Trypanosoma cruzi strain TcI is associated with chronic Chagas disease in the Brazilian Amazon. Parasites Vectors 2014, 7, 267. [Google Scholar] [CrossRef]
- Bern, C.; Montgomery, S.P.; Herwaldt, B.L.; Rassi, A.; Marin-Neto, J.A.; Dantas, R.O.; Moore, A.C. Evaluation and treatment of Chagas disease in the United States: A systematic review. JAMA 2007, 298, 2171–2181. [Google Scholar] [CrossRef] [PubMed]
- Rodriques Coura, J.; de Castro, S.L. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz 2002, 97, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.A.; deMecca, M.M.; Bartel, L.C. Toxic Side Effects of Drugs Used to Treat Chagas’ Disease (American Trypanosomiasis). Hum. Exp. Toxicol. 2006, 25, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Molina, J.A.; Crespillo-Andújar, C.; Bosch-Nicolau, P.; Molina, I. Trypanocidal treatment of Chagas disease. Enferm. Infecc. Microbiol. Clin. 2021, 39, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Coura, J.R.; Borges-Pereira, J. Chagas disease: What is known and what should be improved: A systemic review. Rev. Soc. Bras. Med. Trop. 2012, 45, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Molina, I.; Salvador, F.; Sánchez-Montalvá, A.; Treviño, B.; Serre, N.; Sao Avilés, A.; Almirante, B. Toxic Profile of Benznidazole in Patients with Chronic Chagas Disease: Risk Factors and Comparison of the Product from Two Different Manufacturers. Antimicrob. Agents Chemother. 2015, 59, 6125–6131. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ayala, A.; Pérez-Molina, J.A.; Norman, F.; Monge-Maillo, B.; Faro, M.V.; López-Vélez, R. Gastro-intestinal Chagas disease in migrants to Spain: Prevalence and methods for early diagnosis. Ann. Trop. Med. Parasitol. 2011, 105, 25–29. [Google Scholar] [CrossRef]
- Crespillo-Andújar, C.; López-Vélez, R.; Trigo, E.; Norman, F.; Díaz-Menéndez, M.; Monge-Maillo, B.; Arsuaga, M.; Pérez-Molina, J.A. Comparison of the toxicity of two treatment schemes with benznidazole for chronic Chagas disease: A prospective cohort study in two Spanish referral centres. Clin. Microbiol. Infect. 2020, 26, e1–e384. [Google Scholar] [CrossRef] [PubMed]
- Kratz, J.M. Drug discovery for Chagas disease: A viewpoint. Acta Trop. 2019, 198, 105107. [Google Scholar] [CrossRef]
- Crespillo-Andújar, C.; Comeche, B.; Hamer, D.H.; Arevalo-Rodriguez, I.; Alvarez-Díaz, N.; Zamora, J.; Pérez-Molina, J.A. Use of benznidazole to treat chronic Chagas disease: An updated systematic review with a meta-analysis. PLoS Neglected Trop. Dis. 2022, 16, e0010386. [Google Scholar] [CrossRef]
- Altcheh, J.; Moscatelli, G.; Caruso, M.; Moroni, S.; Bisio, M.; Miranda, M.R.; Monla, C.; Vaina, M.; Valdez, M.; Moran, L.; et al. Population pharmacokinetics of benznidazole in neonates, infants and children using a new pediatric formulation. PLoS Neglected Trop. Dis. 2023, 17, e0010850. [Google Scholar] [CrossRef]
- Santos, F.M.; Lima, W.G.; Gravel, A.S.; Martins, T.A.F.; Talvani, A.; Torres, R.M.; Bahia, M.T. Cardiomyopathy prognosis after benznidazole treatment in chronic canine Chagas’ disease. J. Antimicrob. Chemother. 2012, 67, 1987–1995. [Google Scholar] [CrossRef][Green Version]
- Santos, F.M.; Mazzeti, A.L.; Caldas, S.; Gonçalves, K.R.; Lima, W.G.; Torres, R.M.; Bahia, M.T. Chagas cardiomyopathy: The potential effect of benznidazole treatment on diastolic dysfunction and cardiac damage in dogs chronically infected with Trypanosoma cruzi. Acta Trop. 2016, 161, 44–54. [Google Scholar] [CrossRef]
- Cunha, E.L.A.; Torchelsen, F.K.V.d.S.; Cunha, L.M.; de Oliveira, M.T.; Fonseca, K.d.S.; Vieira, P.M.A.; Carneiro, C.M.; de Lana, M. Benznidazole, itraconazole and their combination in the treatment of acute experimental chagas disease in dogs. Exp. Parasitol. 2019, 204, 107711. [Google Scholar] [CrossRef] [PubMed]
- Madigan, R.; Majoy, S.; Ritter, K.; Concepción, J.; Márquez, M.E.; Silva, S.C.; Zao, C.-L.; Alvarez, A.; Rodriguez-Morales, A.J. Investigation of a combination of amiodarone and itraconazole for treatment of American trypanosomiasis (Chagas disease) in dogs. J. Am. Vet. Med. Assoc. 2019, 255, 317–329. [Google Scholar] [CrossRef]
- Malcolm, E.L.; Saunders, A.B.; Vitt, J.P.; Boutet, B.G.; Hamer, S.A. Antiparasitic treatment with itraconazole and amiodarone in 2 dogs with severe, symptomatic Chagas cardiomyopathy. J. Vet. Intern. Med. 2022, 36, 1100–1105. [Google Scholar] [CrossRef]
- Mazzeti, A.L.; Capelari-Oliveira, P.; Bahia, M.T.; Mosqueira, V.C.F. Review on Experimental Treatment Strategies against Trypanosoma cruzi. J. Exp. Pharmacol. 2021, 13, 409–432. [Google Scholar] [CrossRef] [PubMed]
- Calvet, C.M.; Silva, T.A.; Thomas, D.; Suzuki, B.; Hirata, K.; Siqueira-Neto, J.L.; McKerrow, J.H. Long term follow-up of Trypanosoma cruzi infection and Chagas disease manifestations in mice treated with benznidazole or posaconazole. PLoS Neglected Trop. Dis. 2020, 14, e0008726. [Google Scholar] [CrossRef] [PubMed]
- Torrico, F.; Gascón, J.; Ortiz, L.; Pinto, J.; Rojas, G.; Palacios, A.; Barreira, F.; Blum, B.; Schijman, A.G.; Vaillant, M.; et al. A Phase 2, Randomized, Multicenter, Placebo-Controlled, Proof-of-Concept Trial of Oral Fexinidazole in Adults with Chronic Indeterminate Chagas Disease. Clin. Infect. Dis. 2023, 76, e1186–e1194. [Google Scholar] [CrossRef]
- Torrico, F.; Gascón, J.; Barreira, F.; Blum, B.; Almeida, I.C.; Alonso-Vega, C.; Barboza, T.; Bilbe, G.; Correia, E.; Garcia, W.; et al. New regimens of benznidazole monotherapy and in combination with fosravuconazole for treatment of Chagas disease (BENDITA): A phase 2, double-blind, randomised trial. Lancet Infect. Dis. 2021, 21, 1129–1140. [Google Scholar] [CrossRef]
- Torrico, F.; Gascon, J.; Ortiz, L.; Alonso-Vega, C.; Pinazo, M.J.; Schijman, A.; Almeida, I.C.; Alves, F.; Strub-Wourgaft, N.; Ribeiro, I.; et al. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: A proof-of-concept, randomised, placebo-controlled trial. Lancet. Infect. Dis. 2018, 18, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Miyahira, Y.; Akiba, H.; Katae, M.; Kubota, K.; Kobayashi, S.; Takeuchi, T.; García-Sastre, A.; Fukuchi, Y.; Okumura, K.; Yagita, H. Cutting edge: A potent adjuvant effect of ligand to receptor activator of NF-kappa B gene for inducing antigen-specific CD8+ T cell response by DNA and viral vector vaccination. J. Immunol. 2003, 171, 6344–6348. [Google Scholar] [CrossRef] [PubMed]
- Miyahira, Y. Trypanosoma cruzi infection from the view of CD8+ T cell immunity-an infection model for developing T cell vaccine. Parasitol. Int. 2008, 57, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Quijano-Hernandez, I.; Dumonteil, E. Advances and challenges towards a vaccine against Chagas disease. Hum. Vaccin. 2011, 7, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Arce-Fonseca, M.; Rios-Castro, M.; Carrillo-Sánchez, S.d.C.; Martínez-Cruz, M.; Rodríguez-Morales, O. Prophylactic and therapeutic DNA vaccines against Chagas disease. Parasites Vectors 2015, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Dzul-Huchim, V.M.; Ramirez-Sierra, M.J.; Martinez-Vega, P.P.; Rosado-Vallado, M.E.; Arana-Argaez, V.E.; Ortega-Lopez, J.; Gusovsky, F.; Dumonteil, E.; Cruz-Chan, J.V.; Hotez, P. Vaccine-linked chemotherapy with a low dose of benznidazole plus a bivalent recombinant protein vaccine prevents the development of cardiac fibrosis caused by Trypanosoma cruzi in chronically infected BALB/c mice. PLoS Neglected Trop. Dis. 2022, 16, e0010258. [Google Scholar] [CrossRef] [PubMed]
- Nardy, A.F.; Freire-de-Lima, C.G.; Pérez, A.R.; Morrot, A. Role of Trypanosoma cruzi Trans-sialidase on the Escape from Host Immune Surveillance. Front. Microbiol. 2016, 7, 348. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Burgos, G.; Mezquita-Vega, R.G.; Escobedo-Ortegon, J.; Ramirez-Sierra, M.J.; Arjona-Torres, A.; Ouaissi, A.; Rodrigues, M.M.; Dumonteil, E. Comparative evaluation of therapeutic DNA vaccines against Trypanosoma cruzi in mice. FEMS Immunol. Med. Microbiol. 2007, 50, 333–341. [Google Scholar] [CrossRef]
- Dumonteil, E.; Escobedo-Ortegon, J.; Reyes-Rodriguez, N.; Arjona-Torres, A.; Ramirez-Sierra, M.J. Immunotherapy of Trypanosoma cruzi infection with DNA vaccines in mice. Infect. Immun. 2004, 72, 46–53. [Google Scholar] [CrossRef]
- Zapata-Estrella, H.; Hummel-Newell, C.; Sanchez-Burgos, G.; Escobedo-Ortegon, J.; Ramirez-Sierra, M.J.; Arjona-Torres, A.; Dumonteil, E. Control of Trypanosoma cruzi infection and changes in T-cell populations induced by a therapeutic DNA vaccine in mice. Immunol. Lett. 2006, 103, 186–191. [Google Scholar] [CrossRef]
- Dumonteil, E.; Herrera, C.; Tu, W.; Goff, K.; Fahlberg, M.; Haupt, E.; Kaur, A.; Marx, P.A.; Ortega-Lopez, J.; Hotez, P.J.; et al. Safety and immunogenicity of a recombinant vaccine against Trypanosoma cruzi in Rhesus macaques. Vaccine 2020, 38, 4584–4591. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Lizama, L.E.; Cruz-Chan, J.V.; Aguilar-Cetina, A.D.C.; Herrera-Sanchez, L.F.; Rodriguez-Perez, J.M.; Rosado-Vallado, M.E.; Ramirez-Sierra, M.J.; Ortega-Lopez, J.; Jones, K.; Hotez, P. Trypanosoma cruzi vaccine candidate antigens Tc24 and TSA-1 recall memory immune response associated with HLA-A and -B supertypes in Chagasic chronic patients from Mexico. PLoS Neglected Trop. Dis. 2018, 12, e0006240. [Google Scholar] [CrossRef] [PubMed]
- Dumonteil, E.; Herrera, C.; Marx, P.A. Safety and preservation of cardiac function following therapeutic vaccination against Trypanosoma cruzi in rhesus macaques. J. Microbiol. Immunol. Infect. 2023, 56, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Quijano-Hernández, I.A.; Castro-Barcena, A.; Vázquez-Chagoyán, J.C.; Bolio-González, M.E.; Ortega-López, J.; Dumonteil, E. Preventive and therapeutic DNA vaccination partially protects dogs against an infectious challenge with Trypanosoma cruzi. Vaccine 2013, 31, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Campos, V.; Martinez-Vega, P.; Ramirez-Sierra, M.J.; Rosado-Vallado, M.; Seid, C.A.; Hudspeth, E.M.; Wei, J.; Liu, Z.; Kwityn, C.; Hammond, M. Expression, purification, immunogenicity, and protective efficacy of a recombinant Tc24 antigen as a vaccine against Trypanosoma cruzi infection in mice. Vaccine 2015, 33, 4505–4512. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.A.; Wang, Q.; Jones, K.M.; Heffernan, M.J.; Buhaya, M.H.; Beaumier, C.M.; Keegan, B.P.; Zhan, B.; Dumonteil, E.; Bottazzi, M.E.; et al. A therapeutic nanoparticle vaccine against Trypanosoma cruzi in a BALB/c mouse model of Chagas disease. Hum. Vaccines Immunother. 2016, 12, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.A.; Versteeg, L.; Wang, Q.; Pollet, J.; Zhan, B.; Gusovsky, F.; Bottazzi, M.E.; Hotez, P.J.; Jones, K.M. A therapeutic vaccine prototype induces protective immunity and reduces cardiac fibrosis in a mouse model of chronic Trypanosoma cruzi infection. PLoS Neglected Trop. Dis. 2019, 13, e0007413. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Sinha, M.; Luxon, B.; Garg, N.J. Utility of Trypanosoma cruzi sequence database for the identification of potential vaccine candidates: In silico and In vitro screening. Infect. Immun. 2004, 72, 6245–6254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhatia, V.; Garg, N.J. Previously unrecognized vaccine candidates control Trypanosoma cruzi infection and immunopathology in mice. Clin. Vaccine Immunol. 2008, 15, 1158–1164. [Google Scholar] [CrossRef]
- Aparicio-Burgos, J.E.; Ochoa-Garcia, L.; Zepeda-Escobar, J.A.; Gupta, S.; Dhiman, M.; Martinez, J.S.; de Oca-Jiménez, R.M.; Arreola, M.V.; Barbabosa-Pliego, A.; Vázquez-Chagoyán, J.C.; et al. Testing the efficacy of a multi-component DNA-prime/DNA-boost vaccine against Trypanosoma cruzi infection in dogs. PLoS Neglected Trop. Dis. 2011, 5, e1050. [Google Scholar] [CrossRef]
- Arce-Fonseca, M.; Ballinas-Verdugo, M.A.; Zenteno, E.R.; Suarez-Flores, D.; Carrillo-Sanchez, S.C.; Alejandre-Aguilar, R.; Rosales-Encina, J.L.; Reyes, P.A.; Rodríguez-Morales, O. Specific humoral and cellular immunity induced by Trypanosoma cruzi DNA immunization in a canine model. Vet. Res. 2013, 44, 15. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, A.; Sousa, O.E. Trypanosoma rangeli: Epimastigote immunogenicity and cross-reaction with Trypanosoma cruzi. J. Parasitol. 1996, 82, 363–366. [Google Scholar] [CrossRef]
- Stevens, J.R.; Teixeira, M.M.; Bingle, L.E.; Gibson, W.C. The taxonomic position and evolutionary relationships of Trypanosoma rangeli. Int. J. Parasitol. 1999, 29, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Grisard, E.C. Salivaria or Stercoraria? The Trypanosoma rangeli dilemma. Kinetoplastid Biol. Dis. 2002, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Guhl, F.; Vallejo, G.A. Trypanosoma (Herpetosoma) rangeli Tejera, 1920: An updated review. Mem. Inst. Oswaldo Cruz 2003, 98, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Basso, B.; Cervetta, L.; Moretti, E.; Carlier, Y.; Truyens, C. Acute Trypanosoma cruzi infection: IL-12, IL-18, TNF, sTNFR and NO in T. rangeli-vaccinated mice. Vaccine 2004, 22, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Basso, B.; Moretti, E.; Fretes, R. Vaccination with epimastigotes of different strains of Trypanosoma rangeli protects mice against Trypanosoma cruzi infection. Mem. Inst. Oswaldo Cruz 2008, 103, 370–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Basso, B.; Castro, I.; Introini, V.; Gil, P.; Truyens, C.; Moretti, E. Vaccination with Trypanosoma rangeli reduces the infectiousness of dogs experimentally infected with Trypanosoma cruzi. Vaccine 2007, 25, 3855–3858. [Google Scholar] [CrossRef]
- Aparicio-Burgos, J.E.; Zepeda-Escobar, J.A.; de Oca-Jimenez, R.M.; Estrada-Franco, J.G.; Barbabosa-Pliego, A.; Ochoa-García, L.; Alejandre-Aguilar, R.; Rivas, N.; Peñuelas-Rivas, G.; Val-Arreola, M.; et al. Immune protection against Trypanosoma cruzi induced by TcVac4 in a canine model. PLoS Neglected Trop. Dis. 2015, 9, e0003625. [Google Scholar] [CrossRef]
- Poveda, C.; Leão, A.C.; Mancino, C.; Taraballi, F.; Chen, Y.L.; Adhikari, R.; Villar, M.J.; Kundu, R.; Nguyen, D.M.; Versteeg, L.; et al. Heterologous mRNA-protein vaccination with Tc24 induces a robust cellular immune response against Trypanosoma cruzi, characterized by an increased level of polyfunctional CD8+ T-cells. Curr. Res. Immunol. 2023, 4, 100066. [Google Scholar] [CrossRef]
- Chahal, J.S.; Fang, T.; Woodham, A.W.; Khan, O.F.; Ling, J.; Anderson, D.G.; Ploegh, H.L. An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Sci. Rep. 2017, 7, 252. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B.; Miles, M.A.; Campbell, D.A.; Tibayrenc, M.; Macedo, A.M.; Teixeira, M.M.G.; Schijman, A.G.; Llewellyn, M.S.; Lages-Silva, E.; Machado, C.R. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect. Genet. Evol. 2012, 12, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Gil-Jaramillo, N.; Rocha, A.P.; Raiol, T.; Motta, F.N.; Favali, C.; Brigido, M.M.; Bastos, I.M.D.; Santana, J.M. The First Contact of Human Dendritic Cells with Trypanosoma cruzi Reveals Response to Virus as an Unexplored Central Pathway. Front. Immunol. 2021, 12, 638020. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, B.C.; Ancarola, M.E.; Volpato-Rossi, I.; Marcilla, A.; Ramirez, M.I.; Rosenzvit, M.C.; Cucher, M.; Poncini, C.V. Extracellular vesicles from Trypanosoma cruzi-dendritic cell interaction show modulatory properties and confer resistance to lethal infection as a cell-free based therapy strategy. Front. Cell Infect. Microbiol. 2022, 12, 980817. [Google Scholar] [CrossRef] [PubMed]
- Cornet-Gomez, A.; Retana Moreira, L.; Kronenberger, T.; Osuna, A. Extracellular vesicles of trypomastigotes of Trypanosoma cruzi induce changes in ubiquitin-related processes, cell-signaling pathways and apoptosis. Sci. Rep. 2023, 13, 7618. [Google Scholar] [CrossRef]
- Rossi, I.V.; de Almeida, R.F.; Sabatke, B.; de Godoy, L.M.F.; Ramirez, M.I. Trypanosoma cruzi interaction with host tissues modulate the composition of large extracellular vesicles. Sci. Rep. 2024, 14, 5000. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).