Targeting Vascular Impairment, Neuroinflammation, and Oxidative Stress Dynamics with Whole-Body Cryotherapy in Multiple Sclerosis Treatment
Abstract
:1. Introduction
2. Methods
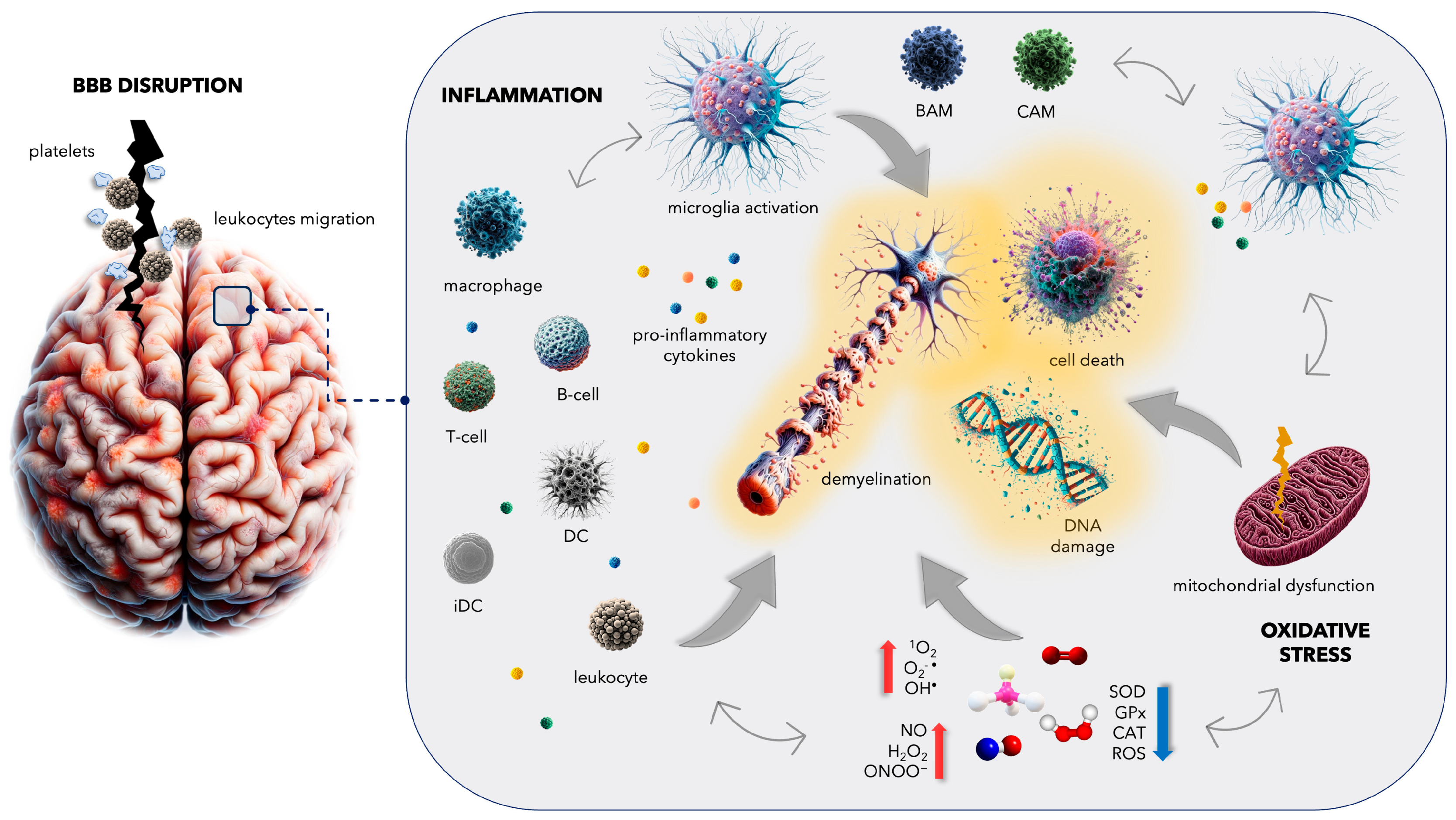
3. Complications in Multiple Sclerosis: From Blood–Brain Barrier Disruption to Increased Cardiovascular Disease Risk
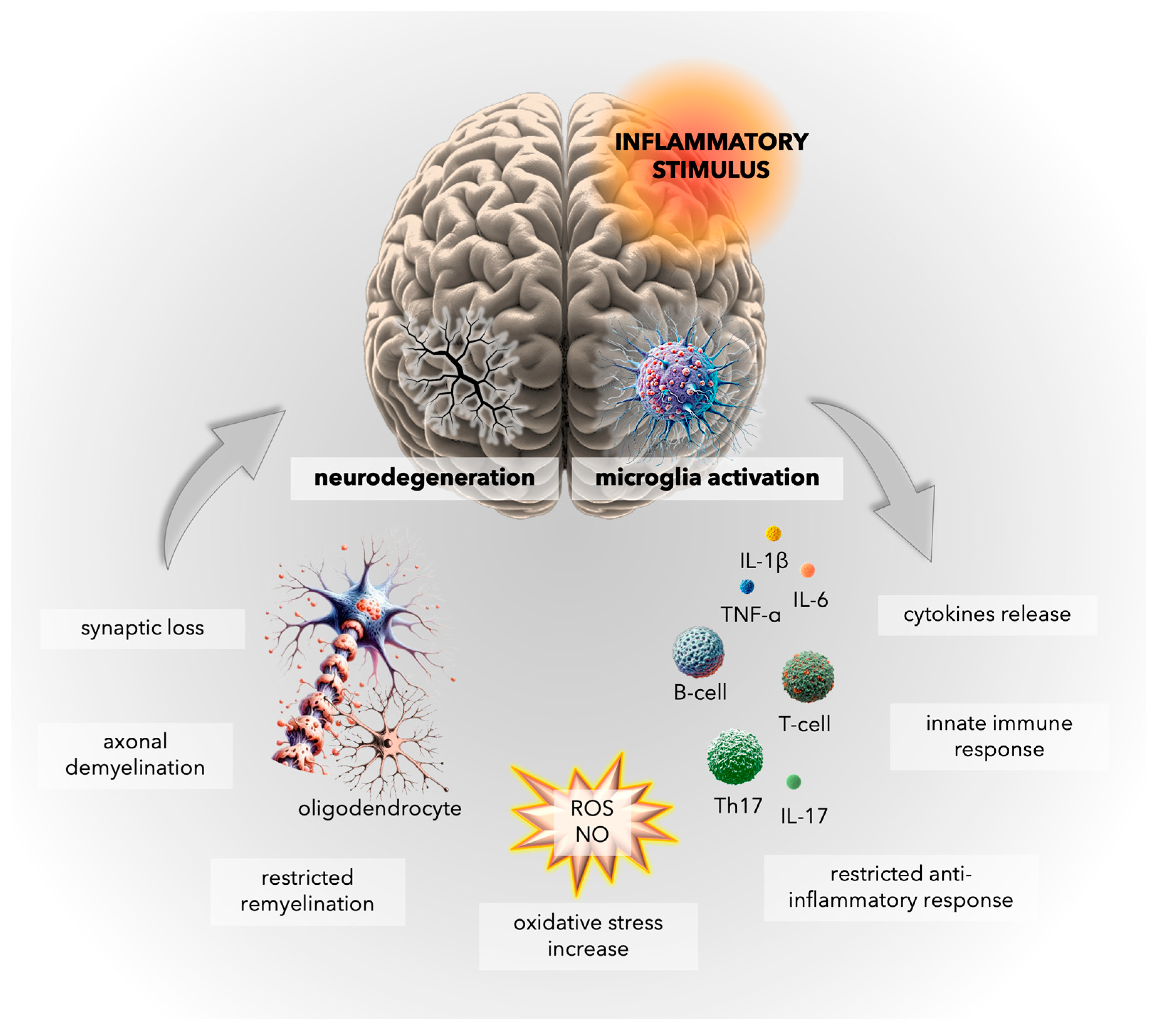
4. Inflammatory Mechanisms in Multiple Sclerosis: Insights into Microglial Activation and CNS Immune Responses
5. Oxidative Stress in Multiple Sclerosis: Unraveling the Complex Interplay
5.1. General Facts about Oxidative Damage in MS
5.2. Mitochondrial Dysfunction in MS
5.3. Genetic Factors Potentially Responsible for Oxidative Stress in MS
6. Whole-Body Cryotherapy (WBCT): Antioxidant Effects and Clinical Implications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, P.; Murray, S.; Hayes, S.M. Clinical decision-making in multiple sclerosis: Challenges reported internationally with emerging treatment complexity. Mult. Scler. Relat. Disord. 2015, 4, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Primers 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, T.; Kern, R.; Thomas, K. Multiple sclerosis: Clinical profiling and data collection as prerequisite for personalized medicine approach. BMC Neurol. 2016, 16, 124. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.D.; Reingold, S.C. Defining the clinical course of multiple sclerosis: Results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996, 46, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H. Multiple Sclerosis Pathology. Cold Spring Harb. Perspect. Med. 2018, 8, a028936. [Google Scholar] [CrossRef] [PubMed]
- Ontaneda, D.; Fox, R.J. Progressive multiple sclerosis. Curr. Opin. Neurol. 2015, 28, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Kutzelnigg, A.; Lassmann, H. Pathology of multiple sclerosis and related inflammatory demyelinating diseases. Handb. Clin. Neurol. 2014, 122, 15–58. [Google Scholar] [CrossRef] [PubMed]
- Palladino, R.; Marrie, R.A.; Majeed, A.; Chataway, J. Evaluating the Risk of Macrovascular Events and Mortality among People with Multiple Sclerosis in England. JAMA Neurol. 2020, 77, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Cashion, J.M.; Young, K.M.; Sutherland, B.A. How does neurovascular unit dysfunction contribute to multiple sclerosis? Neurobiol. Dis. 2023, 178, 106028. [Google Scholar] [CrossRef]
- Papiri, G.; D’Andreamatteo, G.; Cacchiò, G.; Alia, S.; Silvestrini, M.; Paci, C.; Luzzi, S.; Vignini, A. Multiple Sclerosis: Inflammatory and Neuroglial Aspects. Curr. Issues Mol. Biol. 2023, 45, 94. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Pegoretti, V.; Swanson, K.A.; Bethea, J.R.; Probert, L.; Eisel, U.L.M.; Fischer, R. Inflammation and Oxidative Stress in Multiple Sclerosis: Consequences for Therapy Development. Oxidative Med. Cell. Longev. 2020, 2020, 7191080. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Kostka, J.; Włodarczyk, T.; Dugué, B. Whole-body cryostimulation (cryotherapy) provides benefits for fatigue and functional status in multiple sclerosis patients. A case–control study. Acta Neurol. Scand. 2016, 134, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Alva, N.; Palomeque, J.; Carbonell, T. Oxidative stress and antioxidant activity in hypothermia and rewarming: Can RONS modulate the beneficial effects of therapeutic hypothermia? Oxid. Med. Cell Longev. 2013, 2013, 957054. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Melegati, G.; Barassi, A.; Dogliotti, G.; Melzi d’Eril, G.; Dugué, B.; Corsi, M.M. Effects of whole-body cryotherapy on serum mediators of inflammation and serum muscle enzymes in athletes. J. Therm. Biol. 2009, 34, 55–59. [Google Scholar] [CrossRef]
- Schreiner, T.G.; Romanescu, C.; Popescu, B.O. The Blood-Brain Barrier-A Key Player in Multiple Sclerosis Disease Mechanisms. Biomolecules 2022, 12, 538. [Google Scholar] [CrossRef] [PubMed]
- Jain, S. Role of interleukin-17 signaling pathway in the interaction between multiple sclerosis and acute myocardial infarction. Mult. Scler. Relat. Disord. 2022, 58, 103515. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hu, T.; He, K.; Ying, J.; Cui, H. Multiple Sclerosis and the Risk of Cardiovascular Diseases: A Mendelian Randomization Study. Front. Immunol. 2022, 13, 861885. [Google Scholar] [CrossRef] [PubMed]
- Ghoshouni, H.; Shafaei, B.; Farzan, M.; Hashemi, S.M.; Afshari-Safavi, A.; Ghaffary, E.M.; Mohammadzamani, M.; Shaygannejad, V.; Shamloo, A.S.; Mirmosayyeb, O. Multiple sclerosis and the incidence of venous thromboembolism: A systematic review and meta-analysis. J. Thromb. Thrombolysis 2023, 56, 463–473. [Google Scholar] [CrossRef]
- Setyawan, J.; Mu, F.; Yarur, A.; Zichlin, M.L.; Yang, H.; Fernan, C.; Billmyer, E.; Downes, N.; Azimi, N.; Strand, V. Risk of Thromboembolic Events and Associated Risk Factors, Including Treatments, in Patients with Immune-mediated Diseases. Clin. Ther. 2021, 43, 1392–1407.e1391. [Google Scholar] [CrossRef]
- Brønnum-Hansen, H.; Koch-Henriksen, N.; Stenager, E. Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain 2004, 127, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.F.; Christensen, S.; Farkas, D.K.; Miret, M.; Sørensen, H.T.; Pedersen, L. Risk of arterial cardiovascular diseases in patients with multiple sclerosis: A population-based cohort study. Neuroepidemiology 2010, 35, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.; Farkas, D.K.; Pedersen, L.; Miret, M.; Christiansen, C.F.; Sorensen, H.T. Multiple sclerosis and risk of venous thromboembolism: A population-based cohort study. Neuroepidemiology 2012, 38, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Jadidi, E.; Mohammadi, M.; Moradi, T. High risk of cardiovascular diseases after diagnosis of multiple sclerosis. Mult. Scler. 2013, 19, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Koch-Henriksen, N.; Brønnum-Hansen, H.; Stenager, E. Underlying cause of death in Danish patients with multiple sclerosis: Results from the Danish Multiple Sclerosis Registry. J. Neurol. Neurosurg. Psychiatry 1998, 65, 56–59. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, X.; Liu, Y.; Wang, C.; Li, J.; Tian, L.; Teng, W. Sedentary behavior and the risk of stroke: A systematic review and dose-response meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, G.; Bavera, P.M.; Caputo, D.; Mendozzi, L.; Cavarretta, R.; Agus, G.B.; Milani, M.; Ippolito, E.; Cimminiello, C. Risk of deep venous thrombosis (DVT) in bedridden or wheelchair-bound multiple sclerosis patients: A prospective study. Thromb. Res. 2010, 125, 315–317. [Google Scholar] [CrossRef]
- Sternberg, Z.; Leung, C.; Sternberg, D.; Li, F.; Karmon, Y.; Chadha, K.; Levy, E. The prevalence of the classical and non-classical cardiovascular risk factors in multiple sclerosis patients. CNS Neurol. Disord. Drug Targets 2013, 12, 104–111. [Google Scholar] [CrossRef]
- Saluk-Bijak, J.; Dziedzic, A.; Bijak, M. Pro-Thrombotic Activity of Blood Platelets in Multiple Sclerosis. Cells 2019, 8, 110. [Google Scholar] [CrossRef]
- Stojkovic, S.; Wadowski, P.P.; Haider, P.; Weikert, C.; Pultar, J.; Lee, S.; Eichelberger, B.; Hengstenberg, C.; Wojta, J.; Panzer, S.; et al. Circulating MicroRNAs and Monocyte-Platelet Aggregate Formation in Acute Coronary Syndrome. Thromb. Haemost. 2021, 121, 913–922. [Google Scholar] [CrossRef]
- Bacigaluppi, M.; Semerano, A.; Gullotta, G.S.; Strambo, D. Insights from thrombi retrieved in stroke due to large vessel occlusion. J. Cereb. Blood Flow. Metab. 2019, 39, 1433–1451. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, A.M.; Hemker, H.C.; Spronk, H.M.H.; Henskens, Y.M.C.; Ten Cate, H. Thrombin-Fibrin(ogen) Interactions, Host Defense and Risk of Thrombosis. Int. J. Mol. Sci. 2021, 22, 2590. [Google Scholar] [CrossRef] [PubMed]
- Muravlev, I.A.; Dobrovolsky, A.B.; Antonova, O.A.; Khaspekova, S.G.; Alieva, A.K.; Pevzner, D.V.; Mazurov, A.V. Effects of Antiplatelet Drugs on Platelet-Dependent Coagulation Reactions. Biomolecules 2023, 13, 1124. [Google Scholar] [CrossRef] [PubMed]
- Orian, J.M.; D’Souza, C.S.; Kocovski, P.; Krippner, G.; Hale, M.W.; Wang, X.; Peter, K. Platelets in Multiple Sclerosis: Early and Central Mediators of Inflammation and Neurodegeneration and Attractive Targets for Molecular Imaging and Site-Directed Therapy. Front. Immunol. 2021, 12, 620963. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, A.; Morel, A.; Miller, E.; Bijak, M.; Sliwinski, T.; Synowiec, E.; Ceremuga, M.; Saluk-Bijak, J. Oxidative Damage of Blood Platelets Correlates with the Degree of Psychophysical Disability in Secondary Progressive Multiple Sclerosis. Oxidative Med. Cell. Longev. 2020, 2020, 2868014. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Olejnik, A.; Rokita, B.; Morel, A.; Dziedzic, A.; Miller, E.; Saluk-Bijak, J. Increased level of fibrinogen chains in the proteome of blood platelets in secondary progressive multiple sclerosis patients. J. Cell Mol. Med. 2019, 23, 3476–3482. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, A.; Miller, E.; Saluk-Bijak, J.; Niwald, M.; Bijak, M. The Molecular Aspects of Disturbed Platelet Activation through ADP/P2Y(12) Pathway in Multiple Sclerosis. Int. J. Mol. Sci. 2021, 22, 6572. [Google Scholar] [CrossRef]
- Sen, U.; Tyagi, N.; Patibandla, P.K.; Dean, W.L.; Tyagi, S.C.; Roberts, A.M.; Lominadze, D. Fibrinogen-induced endothelin-1 production from endothelial cells. Am. J. Physiol. Cell Physiol. 2009, 296, C840–C847. [Google Scholar] [CrossRef] [PubMed]
- Jennewein, C.; Tran, N.; Paulus, P.; Ellinghaus, P.; Eble, J.A.; Zacharowski, K. Novel aspects of fibrin(ogen) fragments during inflammation. Mol. Med. 2011, 17, 568–573. [Google Scholar] [CrossRef]
- Davalos, D.; Kyu Ryu, J.; Merlini, M.; Baeten, K.M.; Le Moan, N.; Petersen, M.A.; Deerinck, T.J.; Smirnoff, D.S.; Bedard, C.; Hakozaki, H.; et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat. Commun. 2012, 3, 1227. [Google Scholar] [CrossRef]
- Ptaszek, B.; Teległów, A.; Adamiak, J.; Głodzik, J.; Podsiadło, S.; Mucha, D.; Marchewka, J.; Halski, T. Effect of Whole-Body Cryotherapy on Morphological, Rheological and Biochemical Indices of Blood in People with Multiple Sclerosis. J. Clin. Med. 2021, 10, 2833. [Google Scholar] [CrossRef] [PubMed]
- Rawish, E.; Nording, H.; Münte, T.; Langer, H.F. Platelets as Mediators of Neuroinflammation and Thrombosis. Front. Immunol. 2020, 11, 2560. [Google Scholar] [CrossRef] [PubMed]
- Magliozzi, R.; Hametner, S.; Facchiano, F.; Marastoni, D.; Rossi, S.; Castellaro, M.; Poli, A.; Lattanzi, F.; Visconti, A.; Nicholas, R.; et al. Iron homeostasis, complement, and coagulation cascade as CSF signature of cortical lesions in early multiple sclerosis. Ann. Clin. Transl. Neurol. 2019, 6, 2150–2163. [Google Scholar] [CrossRef] [PubMed]
- Langer, H.F.; Chavakis, T. Platelets and neurovascular inflammation. Thromb. Haemost. 2013, 110, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Langer, H.F.; Choi, E.Y.; Zhou, H.; Schleicher, R.; Chung, K.J.; Tang, Z.; Göbel, K.; Bdeir, K.; Chatzigeorgiou, A.; Wong, C.; et al. Platelets contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Circ. Res. 2012, 110, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.; Bijak, M.; Miller, E.; Rywaniak, J.; Miller, S.; Saluk, J. Relationship between the Increased Haemostatic Properties of Blood Platelets and Oxidative Stress Level in Multiple Sclerosis Patients with the Secondary Progressive Stage. Oxidative Med. Cell. Longev. 2015, 2015, 240918. [Google Scholar] [CrossRef] [PubMed]
- Cognasse, F.; Duchez, A.C.; Audoux, E.; Ebermeyer, T.; Arthaud, C.A.; Prier, A.; Eyraud, M.A.; Mismetti, P.; Garraud, O.; Bertoletti, L.; et al. Platelets as Key Factors in Inflammation: Focus on CD40L/CD40. Front. Immunol. 2022, 13, 825892. [Google Scholar] [CrossRef]
- Dehghani, T.; Panitch, A. Endothelial cells, neutrophils and platelets: Getting to the bottom of an inflammatory triangle. Open Biol. 2020, 10, 200161. [Google Scholar] [CrossRef] [PubMed]
- Midgley, A.; Barakat, D.; Braitch, M.; Nichols, C.; Nebozhyn, M.; Edwards, L.J.; Fox, S.C.; Gran, B.; Robins, R.A.; Showe, L.C.; et al. PAF-R on activated T cells: Role in the IL-23/Th17 pathway and relevance to multiple sclerosis. Immunobiology 2021, 226, 152023. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef]
- Gresele, P.; Falcinelli, E.; Momi, S.; Petito, E.; Sebastiano, M. Platelets and Matrix Metalloproteinases: A Bidirectional Interaction with Multiple Pathophysiologic Implications. Hamostaseologie 2021, 41, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Kassassir, H.; Papiewska-Pająk, I.; Kryczka, J.; Boncela, J.; Kowalska, M.A. Platelet-derived microparticles stimulate the invasiveness of colorectal cancer cells via the p38MAPK-MMP-2/MMP-9 axis. Cell Commun. Signal 2023, 21, 51. [Google Scholar] [CrossRef]
- Masuda, H.; Mori, M.; Uchida, T.; Uzawa, A.; Ohtani, R.; Kuwabara, S. Soluble CD40 ligand contributes to blood-brain barrier breakdown and central nervous system inflammation in multiple sclerosis and neuromyelitis optica spectrum disorder. J. Neuroimmunol. 2017, 305, 102–107. [Google Scholar] [CrossRef]
- Marcos-Ramiro, B.; Oliva Nacarino, P.; Serrano-Pertierra, E.; Blanco-Gelaz, M.A.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; Tuñón, A.; López-Larrea, C.; Millán, J.; et al. Microparticles in multiple sclerosis and clinically isolated syndrome: Effect on endothelial barrier function. BMC Neurosci. 2014, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.D.; DaSilva, A.; Aronovich, E.; Nguyen, A.; Nguyen, J.; Hargis, G.; Reynolds, D.; Vercellotti, G.M.; Betts, B.; Wood, D.K. JAK-STAT inhibition reduces endothelial prothrombotic activation and leukocyte-endothelial proadhesive interactions. J. Thromb. Haemost. 2023, 21, 1366–1380. [Google Scholar] [CrossRef]
- Balasa, R.; Barcutean, L.; Mosora, O.; Manu, D. Reviewing the Significance of Blood-Brain Barrier Disruption in Multiple Sclerosis Pathology and Treatment. Int. J. Mol. Sci. 2021, 22, 8370. [Google Scholar] [CrossRef]
- Engelhardt, B.; Ransohoff, R.M. The ins and outs of T-lymphocyte trafficking to the CNS: Anatomical sites and molecular mechanisms. Trends Immunol. 2005, 26, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, I.; Tietz, S.; Nishihara, H.; Deutsch, U.; Sallusto, F.; Gosselet, F.; Lyck, R.; Muller, W.A.; Lassmann, H.; Engelhardt, B. PECAM-1 Stabilizes Blood-Brain Barrier Integrity and Favors Paracellular T-Cell Diapedesis Across the Blood-Brain Barrier during Neuroinflammation. Front. Immunol. 2019, 10, 711. [Google Scholar] [CrossRef] [PubMed]
- Kuznik, B.I.; Vitkovsky, Y.A.; Gvozdeva, O.V.; Solpov, A.V.; Magen, E. Lymphocyte-platelet crosstalk in Graves’ disease. Am. J. Med. Sci. 2014, 347, 206–210. [Google Scholar] [CrossRef]
- Dong, Y.; Yong, V.W. When encephalitogenic T cells collaborate with microglia in multiple sclerosis. Nat. Rev. Neurol. 2019, 15, 704–717. [Google Scholar] [CrossRef]
- Lassmann, H. Pathogenic Mechanisms Associated with Different Clinical Courses of Multiple Sclerosis. Front. Immunol. 2018, 9, 3116. [Google Scholar] [CrossRef] [PubMed]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and non-immune functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.Y.; Vereyken, E.J.; Glim, J.E.; Heijnen, P.D.; Moeton, M.; van der Valk, P.; Amor, S.; Teunissen, C.E.; van Horssen, J.; Dijkstra, C.D. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J. Neuroinflammation 2013, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D.; Peterson, J.; Ransohoff, R.M.; Rudick, R.; Mörk, S.; Bö, L. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 1998, 338, 278–285. [Google Scholar] [CrossRef]
- Sosa, R.A.; Murphey, C.; Ji, N.; Cardona, A.E.; Forsthuber, T.G. The kinetics of myelin antigen uptake by myeloid cells in the central nervous system during experimental autoimmune encephalomyelitis. J. Immunol. 2013, 191, 5848–5857. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Park, T.I.; Aalderink, M.; Oldfield, R.L.; Bergin, P.S.; Mee, E.W.; Faull, R.L.M.; Dragunow, M. Distinct characteristics of microglia from neurogenic and non-neurogenic regions of the human brain in patients with Mesial Temporal Lobe Epilepsy. Front. Cell Neurosci. 2022, 16, 1047928. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, A.; Liu, K.; Dorovini-Zis, K. Dendritic cell adhesion to cerebral endothelium: Role of endothelial cell adhesion molecules and their ligands. J. Neuropathol. Exp. Neurol. 2009, 68, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Mrdjen, D.; Pavlovic, A.; Hartmann, F.J.; Schreiner, B.; Utz, S.G.; Leung, B.P.; Lelios, I.; Heppner, F.L.; Kipnis, J.; Merkler, D.; et al. High-Dimensional Single-Cell Mapping of Central Nervous System Immune Cells Reveals Distinct Myeloid Subsets in Health, Aging, and Disease. Immunity 2018, 48, 380–395.e386. [Google Scholar] [CrossRef]
- Goldmann, T.; Wieghofer, P.; Jordão, M.J.; Prutek, F.; Hagemeyer, N.; Frenzel, K.; Amann, L.; Staszewski, O.; Kierdorf, K.; Krueger, M.; et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 2016, 17, 797–805. [Google Scholar] [CrossRef]
- Utz, S.G.; See, P.; Mildenberger, W.; Thion, M.S.; Silvin, A.; Lutz, M.; Ingelfinger, F.; Rayan, N.A.; Lelios, I.; Buttgereit, A.; et al. Early Fate Defines Microglia and Non-Parenchymal Brain Macrophage Development. Cell 2020, 181, 557–573.e518. [Google Scholar] [CrossRef] [PubMed]
- Dermitzakis, I.; Theotokis, P.; Evangelidis, P.; Delilampou, E.; Evangelidis, N.; Chatzisavvidou, A.; Avramidou, E.; Manthou, M.E. CNS Border-Associated Macrophages: Ontogeny and Potential Implication in Disease. Curr. Issues Mol. Biol. 2023, 45, 272. [Google Scholar] [CrossRef] [PubMed]
- Sathiyanadan, K.; Coisne, C.; Enzmann, G.; Deutsch, U.; Engelhardt, B. PSGL-1 and E/P-selectins are essential for T-cell rolling in inflamed CNS microvessels but dispensable for initiation of EAE. Eur. J. Immunol. 2014, 44, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Döring, A.; Wild, M.; Vestweber, D.; Deutsch, U.; Engelhardt, B. E- and P-Selectin Are Not Required for the Development of Experimental Autoimmune Encephalomyelitis in C57BL/6 and SJL Mice. J. Immunol. 2007, 179, 8470. [Google Scholar] [CrossRef]
- Kerfoot, S.M.; Kubes, P. Overlapping roles of P-selectin and alpha 4 integrin to recruit leukocytes to the central nervous system in experimental autoimmune encephalomyelitis. J. Immunol. 2002, 169, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, B.; Weng, Q.; He, Q. Targeting Microglia and Macrophages: A Potential Treatment Strategy for Multiple Sclerosis. Front. Pharmacol. 2019, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. The role of oxidative stress in the pathogenesis of multiple sclerosis: The need for effective antioxidant therapy. J. Neurol. 2004, 251, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Bryczkowska, I.; Radecka, A.; Knyszyńska, A.; Łuczak, J.; Lubkowska, A. Effect of whole body cryotherapy treatments on antioxidant enzyme activity and biochemical parameters in patients with multiple sclerosis. Fam. Med. Prim. Care Rev. 2018, 20, 214–217. [Google Scholar] [CrossRef]
- Dugué, B.; Smolander, J.; Westerlund, T.; Oksa, J.; Nieminen, R.; Moilanen, E.; Mikkelsson, M. Acute and long-term effects of winter swimming and whole-body cryotherapy on plasma antioxidative capacity in healthy women. Scand. J. Clin. Lab. Invest. 2005, 65, 395–402. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Correale, J.; Marrodan, M.; Ysrraelit, M.C. Mechanisms of Neurodegeneration and Axonal Dysfunction in Progressive Multiple Sclerosis. Biomedicines 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Jové, M.; Mota-Martorell, N.; Obis, È.; Sol, J.; Martín-Garí, M.; Ferrer, I.; Portero-Otín, M.; Pamplona, R. Lipid Adaptations against Oxidative Challenge in the Healthy Adult Human Brain. Antioxidants 2023, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Tobore, T.O. Oxidative/Nitroxidative Stress and Multiple Sclerosis. J. Mol. Neurosci. 2021, 71, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive Oxygen Species: Physiological and Physiopathological Effects on Synaptic Plasticity. J. Exp. Neurosci. 2016, 10, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Abdel Naseer, M.; Rabah, A.M.; Rashed, L.A.; Hassan, A.; Fouad, A.M. Glutamate and Nitric Oxide as biomarkers for disease activity in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 38, 101873. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- D’Aiuto, N.; Hochmann, J.; Millán, M.; Di Paolo, A.; Bologna-Molina, R.; Sotelo Silveira, J.; Arocena, M. Hypoxia, acidification and oxidative stress in cells cultured at large distances from an oxygen source. Sci. Rep. 2022, 12, 21699. [Google Scholar] [CrossRef] [PubMed]
- Siotto, M.; Filippi, M.M.; Simonelli, I.; Landi, D.; Ghazaryan, A.; Vollaro, S.; Ventriglia, M.; Pasqualetti, P.; Rongioletti, M.C.A.; Squitti, R.; et al. Oxidative Stress Related to Iron Metabolism in Relapsing Remitting Multiple Sclerosis Patients with Low Disability. Front. Neurosci. 2019, 13, 86. [Google Scholar] [CrossRef]
- Tasset, I.; Agüera, E.; Sánchez-López, F.; Feijóo, M.; Giraldo, A.; Cruz, A.; Luna, F.; Túnez, I. Peripheral oxidative stress in relapsing-remitting multiple sclerosis. Clin. Biochem. 2012, 45, 440–444. [Google Scholar] [CrossRef]
- Gironi, M.; Borgiani, B.; Mariani, E.; Cursano, C.; Mendozzi, L.; Cavarretta, R.; Saresella, M.; Clerici, M.; Comi, G.; Rovaris, M.; et al. Oxidative Stress Is Differentially Present in Multiple Sclerosis Courses, Early Evident, and Unrelated to Treatment. J. Immunol. Res. 2014, 2014, 961863. [Google Scholar] [CrossRef] [PubMed]
- Leurs, C.E.; Podlesniy, P.; Trullas, R.; Balk, L.; Steenwijk, M.D.; Malekzadeh, A.; Piehl, F.; Uitdehaag, B.M.; Killestein, J.; van Horssen, J.; et al. Cerebrospinal fluid mtDNA concentration is elevated in multiple sclerosis disease and responds to treatment. Mult. Scler. 2018, 24, 472–480. [Google Scholar] [CrossRef]
- Nakajima, H.; Amano, W.; Kubo, T.; Fukuhara, A.; Ihara, H.; Azuma, Y.-T.; Tajima, H.; Inui, T.; Sawa, A.; Takeuchi, T. Glyceraldehyde-3-phosphate dehydrogenase aggregate formation participates in oxidative stress-induced cell death. J. Biol. Chem. 2009, 284, 34331–34341. [Google Scholar] [CrossRef]
- Sadeghian, M.; Mastrolia, V.; Rezaei Haddad, A.; Mosley, A.; Mullali, G.; Schiza, D.; Sajic, M.; Hargreaves, I.; Heales, S.; Duchen, M.R.; et al. Mitochondrial dysfunction is an important cause of neurological deficits in an inflammatory model of multiple sclerosis. Sci. Rep. 2016, 6, 33249. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Ramos-Campo, D.J.; Belinchón-deMiguel, P.; Martinez-Guardado, I.; Dalamitros, A.A.; Yáñez-Sepúlveda, R.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Mitochondria and Brain Disease: A Comprehensive Review of Pathological Mechanisms and Therapeutic Opportunities. Biomedicines 2023, 11, 2488. [Google Scholar] [CrossRef]
- Barcelos, I.P.d.; Troxell, R.M.; Graves, J.S. Mitochondrial Dysfunction and Multiple Sclerosis. Biology 2019, 8, 37. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef]
- Witte, M.E.; Bø, L.; Rodenburg, R.J.; Belien, J.A.; Musters, R.; Hazes, T.; Wintjes, L.T.; Smeitink, J.A.; Geurts, J.J.; De Vries, H.E.; et al. Enhanced number and activity of mitochondria in multiple sclerosis lesions. J. Pathol. 2009, 219, 193–204. [Google Scholar] [CrossRef]
- Pandit, A.; Vadnal, J.; Houston, S.; Freeman, E.; McDonough, J. Impaired regulation of electron transport chain subunit genes by nuclear respiratory factor 2 in multiple sclerosis. J. Neurol. Sci. 2009, 279, 14–20. [Google Scholar] [CrossRef]
- Dutta, R.; McDonough, J.; Yin, X.; Peterson, J.; Chang, A.; Torres, T.; Gudz, T.; Macklin, W.B.; Lewis, D.A.; Fox, R.J.; et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann. Neurol. 2006, 59, 478–489. [Google Scholar] [CrossRef]
- Campbell, G.R.; Ziabreva, I.; Reeve, A.K.; Krishnan, K.J.; Reynolds, R.; Howell, O.; Lassmann, H.; Turnbull, D.M.; Mahad, D.J. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann. Neurol. 2011, 69, 481–492. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Accetta, R.; Damiano, S.; Morano, A.; Mondola, P.; Paternò, R.; Avvedimento, E.V.; Santillo, M. Reactive Oxygen Species Derived from NOX3 and NOX5 Drive Differentiation of Human Oligodendrocytes. Front. Cell Neurosci. 2016, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Carlström, K.E.; Ewing, E.; Granqvist, M.; Gyllenberg, A.; Aeinehband, S.; Enoksson, S.L.; Checa, A.; Badam, T.V.S.; Huang, J.; Gomez-Cabrero, D.; et al. Therapeutic efficacy of dimethyl fumarate in relapsing-remitting multiple sclerosis associates with ROS pathway in monocytes. Nat. Commun. 2019, 10, 3081. [Google Scholar] [CrossRef]
- Singhal, S.S.; Singh, S.P.; Singhal, P.; Horne, D.; Singhal, J.; Awasthi, S. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol. Appl. Pharmacol. 2015, 289, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Alexoudi, A.; Zachaki, S.; Stavropoulou, C.; Gavrili, S.; Spiliopoulou, C.; Papadodima, S.; Karageorgiou, C.E.; Sambani, C. Possible Implication of GSTP1 and NQO1 Polymorphisms on Natalizumab Response in Multiple Sclerosis. Ann. Clin. Lab. Sci. 2016, 46, 586–591. [Google Scholar] [PubMed]
- Pey, A.L.; Megarity, C.F.; Timson, D.J. NAD(P)H quinone oxidoreductase (NQO1): An enzyme which needs just enough mobility, in just the right places. Biosci. Rep. 2019, 39, BSR20180459. [Google Scholar] [CrossRef] [PubMed]
- Louis, J.; Theurot, D.; Filliard, J.R.; Volondat, M.; Dugué, B.; Dupuy, O. The use of whole-body cryotherapy: Time- and dose-response investigation on circulating blood catecholamines and heart rate variability. Eur. J. Appl. Physiol. 2020, 120, 1733–1743. [Google Scholar] [CrossRef]
- Capodaglio, P.; Cremascoli, R.; Piterà, P.; Fontana, J.M. Whole-Body Cryostimulation: A Rehabilitation Booster. J. Rehabil. Med. Clin. Commun. 2022, 5, 2810. [Google Scholar] [CrossRef] [PubMed]
- Ghaidar, D.; Sippel, A.; Riemann-Lorenz, K.; Kofahl, C.; Morrison, R.; Kleiter, I.; Schmidt, S.; Dettmers, C.; Schulz, H.; Heesen, C. Experiences of persons with multiple sclerosis with rehabilitation—A qualitative interview study. BMC Health Serv. Res. 2022, 22, 770. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Selak, M.; O’Connor, J.; Croul, S.; Lorenzana, C.; Butunoi, C.; Kalman, B. Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J. Neurol. Sci. 2000, 177, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Mrowicka, M.; Malinowska, K.; Mrowicki, J.; Saluk-Juszczak, J.; Kędziora, J. Effects of whole-body cryotherapy on a total antioxidative status and activities of antioxidative enzymes in blood of depressive multiple sclerosis patients. World J. Biol. Psychiatry 2011, 12, 223–227. [Google Scholar] [CrossRef]
- Lubkowska, A.; Radecka, A.; Knyszyńska, A.; Łuczak, J. Effect of whole-body cryotherapy treatments on the functional state of patients with MS (multiple sclerosis) in a Timed 25-Foot Walk Test and Hand Grip Strength Test. Pomeranian J. Life Sci. 2019, 65, 46–49. [Google Scholar] [CrossRef]
- Siqueira, A.F.; Vieira, A.; Ramos, G.V.; Marqueti, R.C.; Salvini, T.F.; Puntel, G.O.; Durigan, J.L.Q. Multiple cryotherapy applications attenuate oxidative stress following skeletal muscle injury. Redox Rep. 2017, 22, 323–329. [Google Scholar] [CrossRef]
- Siems, W.G.; van Kuijk, F.J.; Maass, R.; Brenke, R. Uric acid and glutathione levels during short-term whole body cold exposure. Free Radic. Biol. Med. 1994, 16, 299–305. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziedzic, A.; Maciak, K.; Miller, E.D.; Starosta, M.; Saluk, J. Targeting Vascular Impairment, Neuroinflammation, and Oxidative Stress Dynamics with Whole-Body Cryotherapy in Multiple Sclerosis Treatment. Int. J. Mol. Sci. 2024, 25, 3858. https://doi.org/10.3390/ijms25073858
Dziedzic A, Maciak K, Miller ED, Starosta M, Saluk J. Targeting Vascular Impairment, Neuroinflammation, and Oxidative Stress Dynamics with Whole-Body Cryotherapy in Multiple Sclerosis Treatment. International Journal of Molecular Sciences. 2024; 25(7):3858. https://doi.org/10.3390/ijms25073858
Chicago/Turabian StyleDziedzic, Angela, Karina Maciak, Elżbieta Dorota Miller, Michał Starosta, and Joanna Saluk. 2024. "Targeting Vascular Impairment, Neuroinflammation, and Oxidative Stress Dynamics with Whole-Body Cryotherapy in Multiple Sclerosis Treatment" International Journal of Molecular Sciences 25, no. 7: 3858. https://doi.org/10.3390/ijms25073858




