The Effect of Low-Intensity Pulsed Ultrasound on Bone Regeneration and the Expression of Osterix and Cyclooxygenase-2 during Critical-Size Bone Defect Repair
Abstract
1. Introduction
2. Results
2.1. Histological Analyses
2.2. Histomorphometric Analysis
2.3. Immunohistochemical Analyses
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Experimental Equipment and Treatment
4.3. Surgical Protocol of Performing CSBD
4.4. Bone Tissue Processing
4.5. Histological Staining
4.6. Histomorphometric Analysis
4.7. Immunohistochemical Analyses and the Intensity of the Immunohistochemical Expression
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ter Haar, G. Therapeutic Applications of Ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Smith, N.B.; Bailey, M.R.; Czarnota, G.J.; Hynynen, K.; Makin, I.R.S.; Bioeffects Committee of the American Institute of Ultrasound in Medicine. Overview of Therapeutic Ultrasound Applications and Safety Considerations. J. Ultrasound Med. 2012, 31, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Watson, T. Ultrasound in Contemporary Physiotherapy Practice. Ultrasonics 2008, 48, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Žauhar, G.; Duck, F.A.; Starritt, H.C. Comparison of the Acoustic Streaming in Amniotic Fluid and Water in Medical Ultrasonic Beams. Ultraschall Med. 2006, 27, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Savchenko, O.; Li, Y.; Qi, S.; Yang, T.; Zhang, W.; Chen, J. A Review of Low-Intensity Pulsed Ultrasound for Therapeutic Applications. IEEE Trans. Biomed. Eng. 2019, 66, 2704–2718. [Google Scholar] [CrossRef] [PubMed]
- Leskinen, J.J.; Hynynen, K. Study of Factors Affecting the Magnitude and Nature of Ultrasound Exposure with in Vitro Set-Ups. Ultrasound Med. Biol. 2012, 38, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.A.M.; Duarte, L.R. Ultrasonic Stimulation of Bone Callus: Clinical Applications. Rev. Braz. Orthop. 1983, 18, 73–80. [Google Scholar]
- Heckman, J.D.; Ryaby, J.P.; McCabe, J.; Frey, J.J.; Kilcoyne, R.F. Acceleration of Tibial Fracture-Healing by Non-Invasive, Low-Intensity Pulsed Ultrasound. J. Bone Jt. Surg. Am. 1994, 76, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, T.K.; Ryaby, J.P.; McCabe, J.; Frey, J.J.; Roe, L.R. Accelerated Healing of Distal Radial Fractures with the Use of Specific, Low-Intensity Ultrasound. A Multicenter, Prospective, Randomized, Double-Blind, Placebo-Controlled Study. J. Bone Jt. Surg. Am. 1997, 79, 961–973. [Google Scholar] [CrossRef]
- Nolte, P.A.; van der Krans, A.; Patka, P.; Janssen, I.M.; Ryaby, J.P.; Albers, G.H. Low-Intensity Pulsed Ultrasound in the Treatment of Nonunions. J. Trauma 2001, 51, 693–702; discussion 702–703. [Google Scholar] [CrossRef]
- Gebauer, D.; Mayr, E.; Orthner, E.; Ryaby, J.P. Low-Intensity Pulsed Ultrasound: Effects on Nonunions. Ultrasound Med. Biol. 2005, 31, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, T.; Liu, F.; Qu, J.; Chen, Y.; Fan, S.; Chen, H.; Sun, L.; Zhao, C.; Hu, J.; et al. Effect of Low-Intensity Pulsed Ultrasound After Autologous Adipose-Derived Stromal Cell Transplantation for Bone-Tendon Healing in a Rabbit Model. Am. J. Sports Med. 2019, 47, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.C.; Iglesias, B.C.; Mark, B.J.; Wang, D. Low-Intensity Pulsed Ultrasound Augments Tendon, Ligament, and Bone-Soft Tissue Healing in Preclinical Animal Models: A Systematic Review. Arthroscopy 2021, 37, 2318–2333.e3. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, P.; Hosainzadegan, H.; Brazvan, B.; Roshangar, L.; Shafaei, H.; Salimnejad, R. Treatment of Cartilage Defects by Low-Intensity Pulsed Ultrasound in a Sheep Model. Cell Tissue Bank. 2021, 22, 369–378. [Google Scholar] [CrossRef]
- Hsu, S.K.; Huang, W.T.; Liu, B.S.; Li, S.M.; Chen, H.T.; Chang, C.J. Effects of Near-Field Ultrasound Stimulation on New Bone Formation and Osseointegration of Dental Titanium Implants in Vitro and in Vivo. Ultrasound Med. Biol. 2011, 37, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Padilla, F.; Puts, R.; Vico, L.; Raum, K. Stimulation of Bone Repair with Ultrasound: A Review of the Possible Mechanic Effects. Ultrasonics 2014, 54, 1125–1145. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.; Bolander, M.; Ryaby, J.P.; Hadjiargyrou, M. The Use of Low-Intensity Ultrasound to Accelerate the Healing of Fractures. J. Bone Jt. Surg. Am. 2001, 83, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chow, S.K.H.; Leung, K.S.; Cheung, W.H. Ultrasound as a Stimulus for Musculoskeletal Disorders. J. Orthop. Transl. 2017, 9, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Dalecki, D. Mechanical Bioeffects of Ultrasound. Annu. Rev. Biomed. Eng. 2004, 6, 229–248. [Google Scholar] [CrossRef]
- Bhan, K.; Patel, R.; Hasan, K.; Pimplé, M.; Sharma, S.; Nandwana, V.; Basta, M. Fracture Nonunions and Delayed Unions Treated With Low-Intensity Pulsed Ultrasound Therapy: A Clinical Series. Cureus 2021, 13, e17067. [Google Scholar] [CrossRef]
- Li, J.K.; Chang, W.H.; Lin, J.C.; Ruaan, R.C.; Liu, H.C.; Sun, J.S. Cytokine Release from Osteoblasts in Response to Ultrasound Stimulation. Biomaterials 2003, 24, 2379–2385. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.M.Z.; Qin, Y.X. Enhancement of Osteogenic Differentiation and Proliferation in Human Mesenchymal Stem Cells by a Modified Low Intensity Ultrasound Stimulation under Simulated Microgravity. PLoS ONE 2013, 8, e73914. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Yang, R.S.; Huang, T.H.; Lu, D.Y.; Chuang, W.J.; Huang, T.F.; Fu, W.M. Ultrasound Stimulates Cyclooxygenase-2 Expression and Increases Bone Formation through Integrin, Focal Adhesion Kinase, Phosphatidylinositol 3-Kinase, and Akt Pathway in Osteoblasts. Mol. Pharmacol. 2006, 69, 2047–2057. [Google Scholar] [CrossRef]
- Lai, C.H.; Chen, S.C.; Chiu, L.H.; Yang, C.B.; Tsai, Y.H.; Zuo, C.S.; Chang, W.H.S.; Lai, W.F. Effects of Low-Intensity Pulsed Ultrasound, Dexamethasone/TGF-β1 and/or BMP-2 on the Transcriptional Expression of Genes in Human Mesenchymal Stem Cells: Chondrogenic vs. Osteogenic Differentiation. Ultrasound Med. Biol. 2010, 36, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.; Lin, S.; Pounder, N.; Mikuni-Takagaki, Y. Mode & Mechanism of Low Intensity Pulsed Ultrasound (LIPUS) in Fracture Repair. Ultrasonics 2016, 70, 45–52. [Google Scholar] [CrossRef]
- Zhang, X.; Schwarz, E.M.; Young, D.A.; Puzas, J.E.; Rosier, R.N.; O’Keefe, R.J. Cyclooxygenase-2 Regulates Mesenchymal Cell Differentiation into the Osteoblast Lineage and Is Critically Involved in Bone Repair. J. Clin. Investig. 2002, 109, 1405–1415. [Google Scholar] [CrossRef]
- Tomas, M.; Čandrlić, M.; Juzbašić, M.; Ivanišević, Z.; Matijević, N.; Včev, A.; Cvijanović Peloza, O.; Matijević, M.; Perić Kačarević, Ž. Synthetic Injectable Biomaterials for Alveolar Bone Regeneration in Animal and Human Studies. Materials 2021, 14, 2858. [Google Scholar] [CrossRef]
- Migliorini, F.; Cuozzo, F.; Torsiello, E.; Spiezia, F.; Oliva, F.; Maffulli, N. Autologous Bone Grafting in Trauma and Orthopaedic Surgery: An Evidence-Based Narrative Review. J. Clin. Med. 2021, 10, 4347. [Google Scholar] [CrossRef]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of Bone Regeneration Using the Rat Critical Size Calvarial Defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef]
- de Freitas Silva, L.; de Carvalho Reis, E.N.R.; Barbara, T.A.; Bonardi, J.P.; Garcia, I.R.; de Carvalho, P.S.P.; Ponzoni, D. Assessment of Bone Repair in Critical-Size Defect in the Calvarium of Rats after the Implantation of Tricalcium Phosphate Beta (β-TCP). Acta Histochem. 2017, 119, 624–631. [Google Scholar] [CrossRef][Green Version]
- Kasuya, S.; Kato-Kogoe, N.; Omori, M.; Yamamoto, K.; Taguchi, S.; Fujita, H.; Imagawa, N.; Sunano, A.; Inoue, K.; Ito, Y.; et al. New Bone Formation Process Using Bio-Oss and Collagen Membrane for Rat Calvarial Bone Defect: Histological Observation. Implant. Dent. 2018, 27, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, M.; Wang, S.; Xiao, Z.; Xiong, Y.; Wang, G. Recent Advances of Osterix Transcription Factor in Osteoblast Differentiation and Bone Formation. Front. Cell Dev. Biol. 2020, 8, 601224. [Google Scholar] [CrossRef] [PubMed]
- Hojo, H.; Ohba, S.; He, X.; Lai, L.P.; McMahon, A.P. Sp7/Osterix Is Restricted to Bone-Forming Vertebrates Where It Acts as a Dlx Co-Factor in Osteoblast Specification. Dev. Cell 2016, 37, 238–253. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Probst, A.; Spiegel, H.U. Cellular Mechanisms of Bone Repair. J. Investig. Surg. 1997, 10, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.; Gupta, S.; Mohanty, S. Mesenchymal Stem Cells Modulate the Immune System in Developing Therapeutic Interventions. In Immune Response Activation and Immunomodulation; IntechOpen: London, UK, 2019. [Google Scholar]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P. V Current Concepts of Molecular Aspects of Bone Healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Kim, R.; Ham, H.J.; Park, S.I.; Lee, M.Y.; Kim, J.; Hwang, J.; Park, M.S.; Yoo, S.S.; Maeng, L.S.; et al. Focused Low-Intensity Pulsed Ultrasound Enhances Bone Regeneration in Rat Calvarial Bone Defect through Enhancement of Cell Proliferation. Ultrasound Med. Biol. 2015, 41, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Lavandier, B.; Gleizal, A.; Béra, J.C. Experimental Assessment of Calvarial Bone Defect Re-Ossification Stimulation Using Low-Intensity Pulsed Ultrasound. Ultrasound Med. Biol. 2009, 35, 585–594. [Google Scholar] [CrossRef]
- Hasuike, A.; Sato, S.; Udagawa, A.; Ando, K.; Arai, Y.; Ito, K. In Vivo Bone Regenerative Effect of Low-Intensity Pulsed Ultrasound in Rat Calvarial Defects. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, e12–e20. [Google Scholar] [CrossRef]
- Imafuji, T.; Shirakata, Y.; Shinohara, Y.; Nakamura, T.; Noguchi, K. Enhanced Bone Formation of Calvarial Bone Defects by Low-Intensity Pulsed Ultrasound and Recombinant Human Bone Morphogenetic Protein-9: A Preliminary Experimental Study in Rats. Clin. Oral Investig. 2021, 25, 5917–5927. [Google Scholar] [CrossRef]
- Gillman, C.E.; Jayasuriya, A.C. FDA-Approved Bone Grafts and Bone Graft Substitute Devices in Bone Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112466. [Google Scholar] [CrossRef] [PubMed]
- Jerbić Radetić, A.T.; Zoričić Cvek, S.; Tomas, M.; Erjavec, I.; Oguić, M.; Perić Kačarević, Ž.; Cvijanović Peloza, O. CSBD Healing in Rats after Application of Bovine Xenogeneic Biomaterial Enriched with Magnesium Alloy. Int. J. Mol. Sci. 2021, 22, 9089. [Google Scholar] [CrossRef]
- Cvijanović Peloza, O.; Jerbić Radetić, A.T.; Baričić, M.; Bukovac, L.; Zoričić Cvek, S. Dynamics of CSBD Healing after Implementation of Dentin and Xenogeneic Bone Biomaterial. Materials 2023, 16, 1600. [Google Scholar] [CrossRef]
- Forwood, M.R. Inducible Cyclo-Oxygenase (COX-2) Mediates the Induction of Bone Formation by Mechanical Loading in Vivo. J. Bone Miner. Res. 1996, 11, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.L.; Turner, C.H. Mechanotransduction and the Functional Response of Bone to Mechanical Strain. Calcif. Tissue Int. 1995, 57, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Kokubu, T.; Matsui, N.; Fujioka, H.; Tsunoda, M.; Mizuno, K. Low Intensity Pulsed Ultrasound Exposure Increases Prostaglandin E2 Production via the Induction of Cyclooxygenase-2 MRNA in Mouse Osteoblasts. Biochem. Biophys. Res. Commun. 1999, 256, 284–287. [Google Scholar] [CrossRef]
- Parfitt, A.M. Bone Histomorphometry: Standardization of Nomenclature, Symbols and Units (Summary of Proposed System). Bone 1988, 9, 67–69. [Google Scholar] [CrossRef]

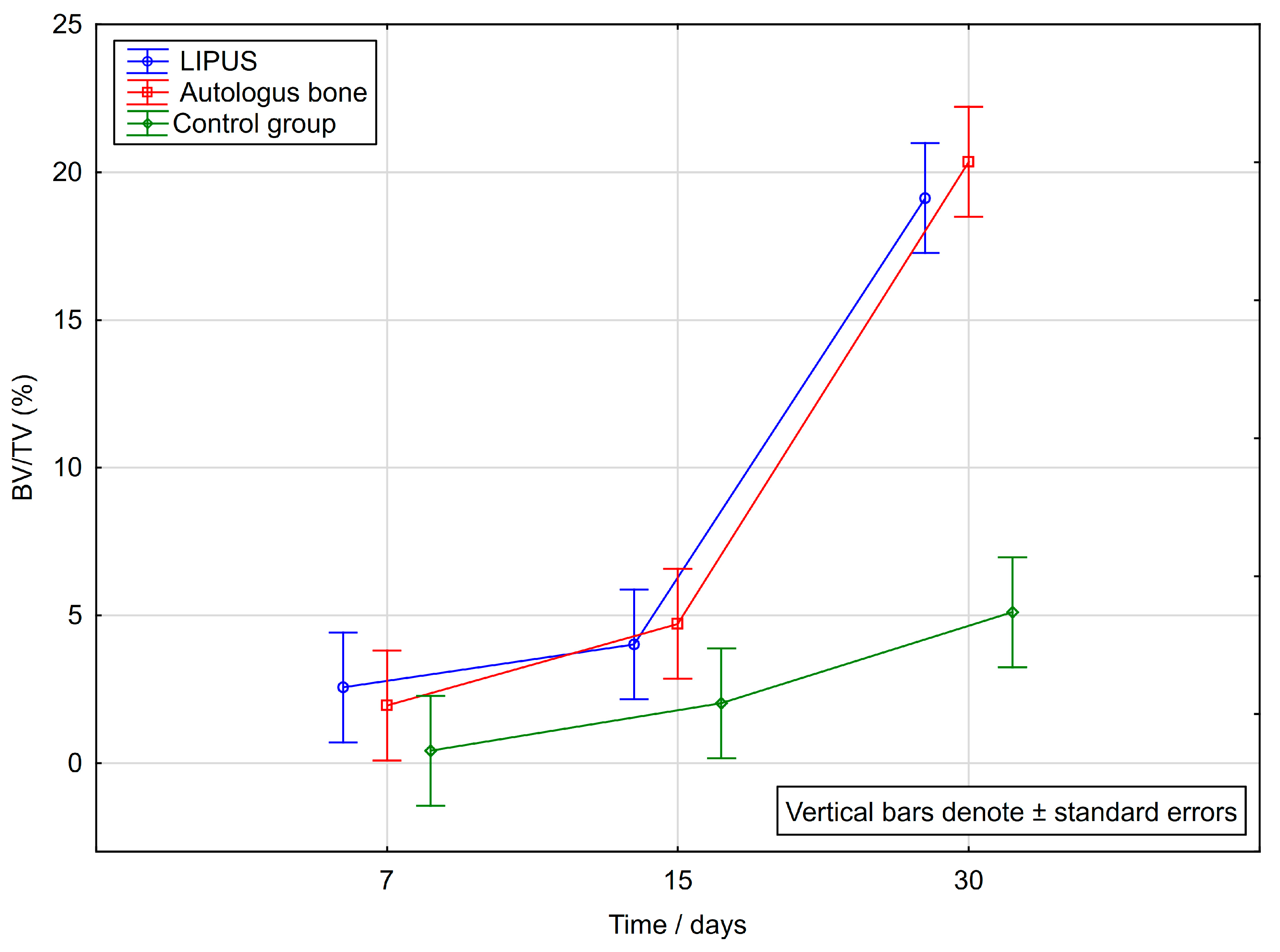

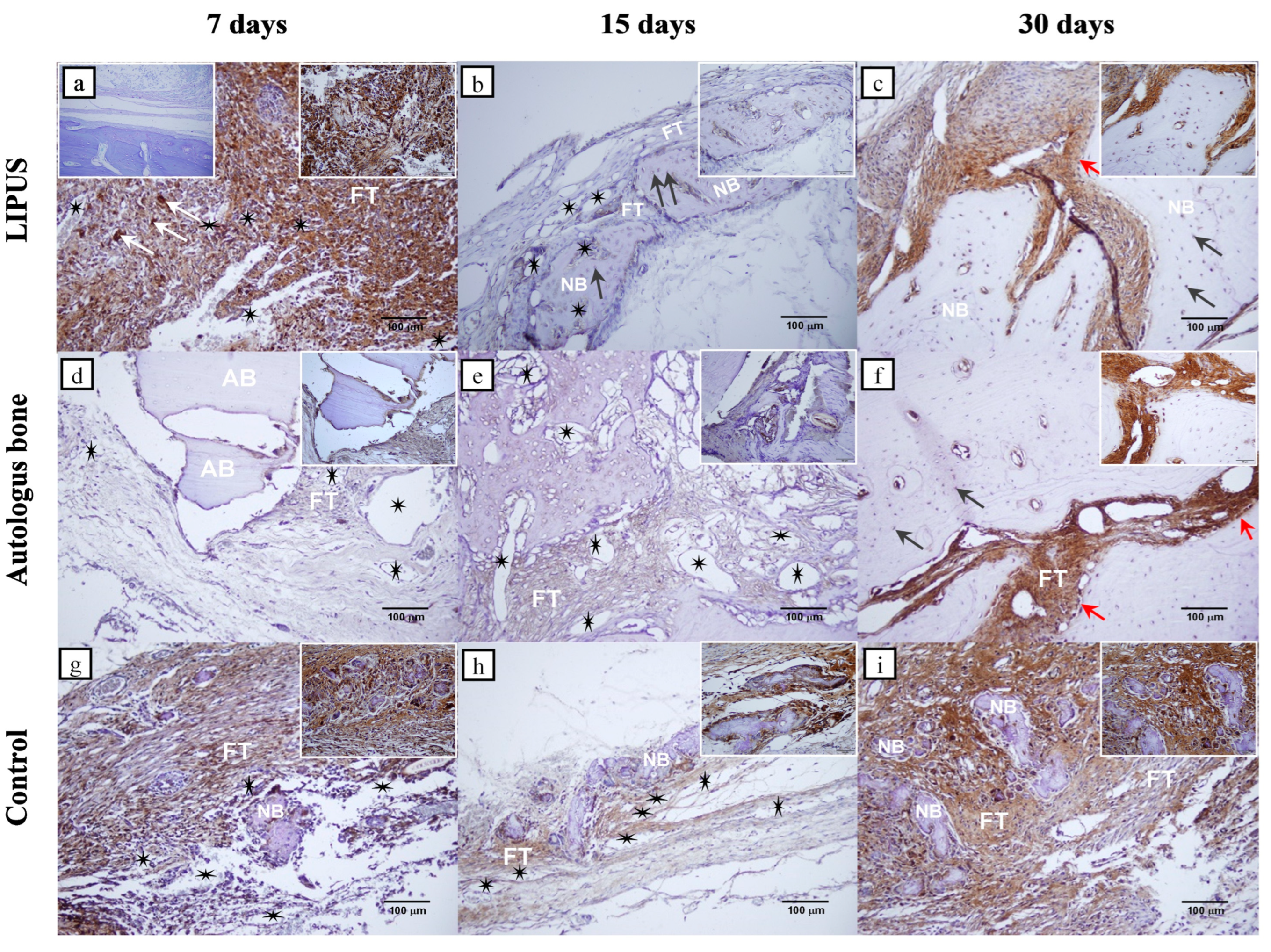

| Day | LIPUS Group | Autologous Bone Group | Control Group | Overall p | Pairwise Differences * |
|---|---|---|---|---|---|
| 7 | 2.56 ± 1.87 | 1.95 ± 1.32 | 0.42 ± 0.24 | 0.007 | cl |
| 15 | 4.02 ± 2.09 | 4.71 ± 4.65 | 2.03 ± 1.56 | 0.180 | / |
| 30 | 19.12 ± 8.53 | 20.35 ± 12.64 | 5.11 ± 3.71 | 0.002 | cl, ca |
| Day | LIPUS Group | Autologous Bone Group | Control Group | Overall p-Value * | Pairwise Differences ** | |
|---|---|---|---|---|---|---|
| COX-2 | 7 | 169.7 ± 1.6 | 55.5 ± 1.1 | 131.1 ± 1.6 | <0.001 | cl, ca, al |
| 15 | 92.7 ± 2.2 | 72.5 ± 2.2 | 64.3 ± 1.7 | <0.001 | cl, ca, al | |
| 30 | 81.4 ± 1.8 | 165.7 ± 1.1 | 111.0 ± 1.5 | <0.001 | cl, ca, al | |
| OSX | 7 | 131.9 ± 0.9 | 23.5 ± 0.9 | 102.2 ± 1.2 | <0.001 | cl, ca, al |
| 15 | 42.9 ± 0.9 | 64.7 ± 0.7 | 81.5 ± 1.8 | <0.001 | cl, ca, al | |
| 30 | 128.9 ± 1.2 | 177.1 ± 0.9 | 130.9 ± 0.5 | <0.001 | cl, ca, al |
| Group Number | Group | Number of Animals (N) | Time Points | Total |
|---|---|---|---|---|
| 1. | LIPUS | 5 | 3 (7, 15, 30 days) | 15 |
| 2. | Autologous bone | 5 | 3 (7, 15, 30 days) | 15 |
| 3. | Control | 5 | 3 (7, 15, 30 days) | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volarić, D.; Žauhar, G.; Chen, J.; Jerbić Radetić, A.T.; Omrčen, H.; Raič, A.; Pirović, R.; Cvijanović Peloza, O. The Effect of Low-Intensity Pulsed Ultrasound on Bone Regeneration and the Expression of Osterix and Cyclooxygenase-2 during Critical-Size Bone Defect Repair. Int. J. Mol. Sci. 2024, 25, 3882. https://doi.org/10.3390/ijms25073882
Volarić D, Žauhar G, Chen J, Jerbić Radetić AT, Omrčen H, Raič A, Pirović R, Cvijanović Peloza O. The Effect of Low-Intensity Pulsed Ultrasound on Bone Regeneration and the Expression of Osterix and Cyclooxygenase-2 during Critical-Size Bone Defect Repair. International Journal of Molecular Sciences. 2024; 25(7):3882. https://doi.org/10.3390/ijms25073882
Chicago/Turabian StyleVolarić, Darian, Gordana Žauhar, Jie Chen, Ana Terezija Jerbić Radetić, Hrvoje Omrčen, Antonio Raič, Roko Pirović, and Olga Cvijanović Peloza. 2024. "The Effect of Low-Intensity Pulsed Ultrasound on Bone Regeneration and the Expression of Osterix and Cyclooxygenase-2 during Critical-Size Bone Defect Repair" International Journal of Molecular Sciences 25, no. 7: 3882. https://doi.org/10.3390/ijms25073882
APA StyleVolarić, D., Žauhar, G., Chen, J., Jerbić Radetić, A. T., Omrčen, H., Raič, A., Pirović, R., & Cvijanović Peloza, O. (2024). The Effect of Low-Intensity Pulsed Ultrasound on Bone Regeneration and the Expression of Osterix and Cyclooxygenase-2 during Critical-Size Bone Defect Repair. International Journal of Molecular Sciences, 25(7), 3882. https://doi.org/10.3390/ijms25073882






