Abstract
The improvement of in vitro embryo development is a gateway to enhance the output of assisted reproductive technologies. The Wnt and Hippo signaling pathways are crucial for the early development of bovine embryos. This study investigated the development of bovine embryos under the influence of a Hippo signaling agonist (LPA) and a Wnt signaling inhibitor (DKK1). In this current study, embryos produced in vitro were cultured in media supplemented with LPA and DKK1. We comprehensively analyzed the impact of LPA and DKK1 on various developmental parameters of the bovine embryo, such as blastocyst formation, differential cell counts, YAP fluorescence intensity and apoptosis rate. Furthermore, single-cell RNA sequencing (scRNA-seq) was employed to elucidate the in vitro embryonic development. Our results revealed that LPA and DKK1 improved the blastocyst developmental potential, total cells, trophectoderm (TE) cells and YAP fluorescence intensity and decreased the apoptosis rate of bovine embryos. A total of 1203 genes exhibited differential expression between the control and LPA/DKK1-treated (LD) groups, with 577 genes upregulated and 626 genes downregulated. KEGG pathway analysis revealed significant enrichment of differentially expressed genes (DEGs) associated with TGF-beta signaling, Wnt signaling, apoptosis, Hippo signaling and other critical developmental pathways. Our study shows the role of LPA and DKK1 in embryonic differentiation and embryo establishment of pregnancy. These findings should be helpful for further unraveling the precise contributions of the Hippo and Wnt pathways in bovine trophoblast formation, thus advancing our comprehension of early bovine embryo development.
1. Introduction
The development of mammalian embryos progresses from the zygotic stage to the blastocyst and includes processes such as embryo genome activation (EGA), the determination of cell lineages and the differentiation of cell fates [1]. The embryonic genome is activated at a certain point in embryonic development [2]. This development proceeds independently of the maternal genome, leading to the formation of three distinct cell lineages: the trophectoderm (TE), primitive endoderm (PE) and epiblast (EPI) [3]. The initial specific lineage differentiation occurs when the outer cells form the TE [4]. Subsequently, the inner cell mass (ICM) differentiates into PE and EPI cells, marking the second lineage-specific differentiation [5]. Finally, the TE gives rise to extraembryonic tissues, while the PE and EPI cells develop into the extraembryonic yolk sac and the actual embryo, respectively [6].
Wnt signaling is pivotal in orchestrating the developmental dynamics of the preimplantation embryo [7]. It is implicated in a myriad of developmental processes such as cellular differentiation [8,9] and proliferation [10], as well as lineage specification and the sustenance of pluripotency [11]. Furthermore, Wnt signaling is integral to axial elongation [12], the establishment of cell polarity and motility, and the regulation of epithelial–mesenchymal transition [13]. The endometrial secretory protein Dickkopf-1 (DKK1), a key mediator in maternal–embryonic crosstalk, inhibits the Wnt pathway by disrupting the formation of the Wnt ligand/Frizzled receptor/LRP5 or LRP6 complex, thereby facilitating embryonic differentiation [11,14,15].
The Hippo signaling pathway is highly conserved in mammals and is involved in follicle growth and follicular activation [16,17,18], and it has been shown to play a key role in embryonic development [19]. Recently, studies have elucidated the critical involvement of the Hippo-YAP pathway in the differentiation of the TE and ICM during preimplantation embryonic development [20]. The transcriptional coactivator YAP is implicated in embryonic development and organogenesis, which can also enhance cell proliferation and prevent apoptosis [21]. Lysophosphatidic acid (LPA) is a biologically active phospholipid that regulates a wide range of cellular effects through the activation of specific G protein-coupled receptors [22]. LPA-mediated signaling has been shown to play a key role in mouse embryo spacing and implantation time [23]. LPA is present in all mammalian cells and tissues, inducing cell proliferation, survival and migration [24,25,26].
The implementation of single-cell RNA sequencing (scRNA-seq) technology now facilitates the detailed characterization of single-cell transcriptomes across various developmental stages [27,28]. With the utilization of scRNA-seq technology, recent studies have elucidated the molecular features of gastrulation and organogenesis during the early stages of mouse development, as well as the cellular mechanisms in heart development [29,30]. Meanwhile, the effects of vitrification on sheep embryos have been analyzed at the single-embryo transcriptome level by scRNA-seq [31]. However, to our best knowledge, a comprehensive understanding of the signaling pathways and regulatory mechanism involved in early bovine embryo development at single-cell resolution is still lacking.
In this study, we analyzed the regulation of Hippo and Wnt signaling pathways by LPA and DKK1 supplemented in in vitro culture (IVC) medium, and how this affects the development of bovine embryos. The findings of this study will contribute to establishing an efficient approach to improve the developmental capacity of bovine embryos.
2. Results
2.1. Effect of LPA and DKK1 on the Development of Bovine Embryos
To examine how the Hippo and Wnt pathways are involved in embryo development, bovine oocytes were fertilized in vitro and then cultured to the blastocyst stage in the presence of LPA and DKK1. The cleavage rate and the blastocyst development rate of the LPA + DKK1 group (89.67 ± 4.53%, 47.11 ± 4.23%) were significantly higher than those of the LPA group (83.57 ± 3.60%, 40.04 ± 2.54%), the DKK1 group (81.97 ± 3.38%, 38.91 ± 3.10%) and the control group (74.18 ± 3.44%, 30.02 ± 3.30%; p < 0.05), as presented in Table 1.

Table 1.
Actions of LPA and DKK1 on the developmental ability of bovine embryos.
2.2. Effect of LPA and DKK1 on the YAP Fluorescence Intensity of Bovine Embryos
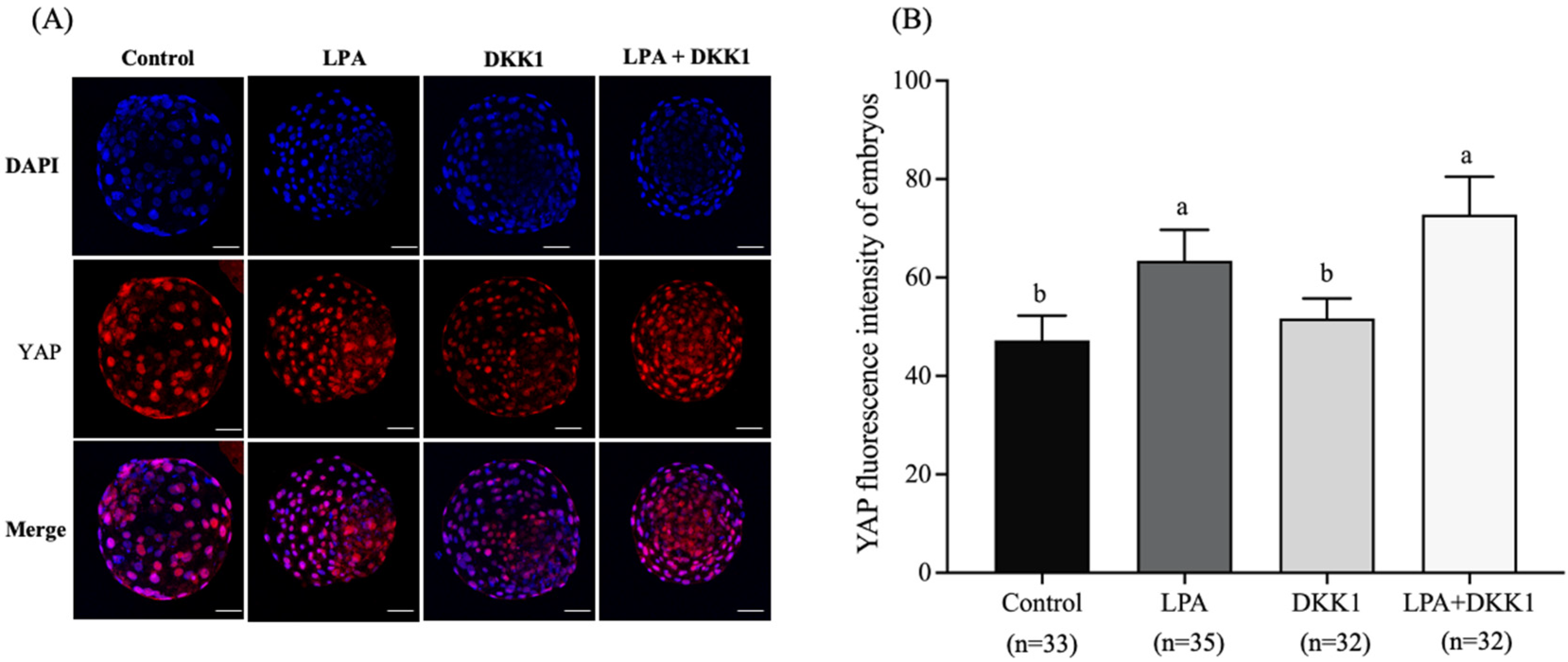
As shown in Figure 1, the YAP fluorescence intensity of the LPA group (63.41 ± 5.61) and the LPA + DKK1 group (72.81 ± 6.84) were significantly higher compared to the control group (47.21 ± 4.53) and the DKK1 group (51.66 ± 3.65, p < 0.05).
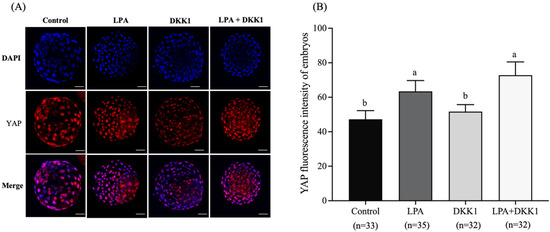
Figure 1.
Effect of LPA and DKK1 on the YAP fluorescence intensity of bovine embryos. (A) YAP immunofluorescence in the bovine embryos; (B) Effect of LPA and DKK1 on the YAP fluorescence intensity of bovine embryos. Scale bar = 50 μm. a, b Values with different superscripts indicate significant difference between groups (p < 0.05).
2.3. LPA and DKK1 Enhanced the Number of TE Cells of Bovine Embryos
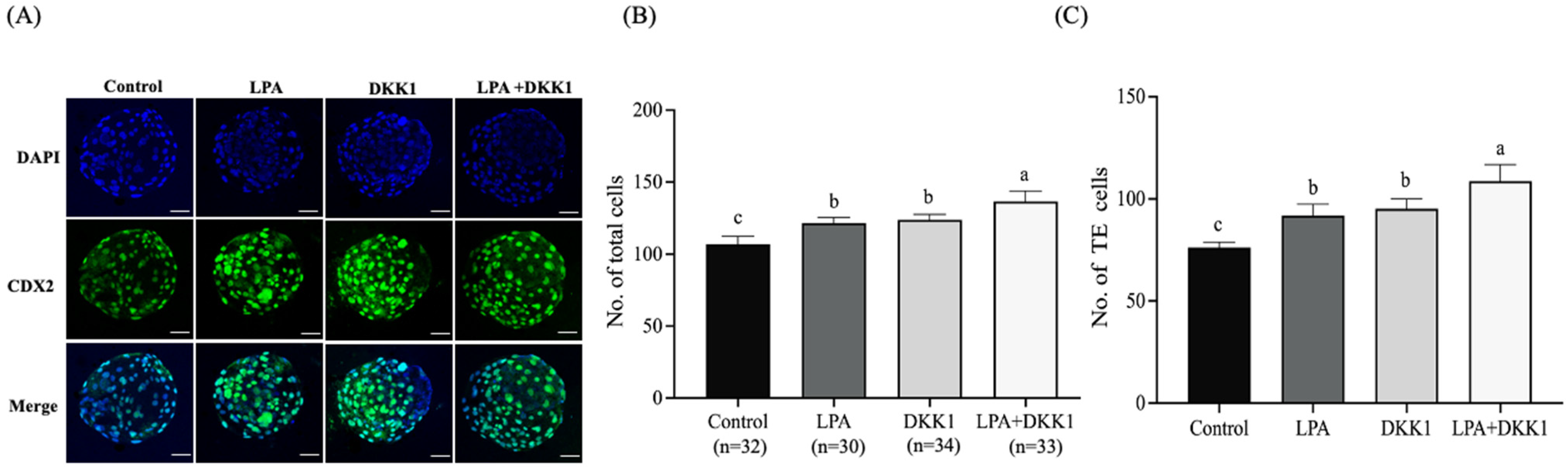
We evaluated the changes in the development and differentiation of embryonic TE cells with LPA and DKK1 treatments. As shown in Figure 2, the results showed that the total cell numbers and the TE cell numbers of the LPA group (121.63 ± 3.38, 91.82 ± 5.00), the DKK1 group (123.85 ± 3.40, 95.22 ± 4.28) and the LPA + DKK1 group (136.67 ± 6.52, 108.70 ± 7.27) were significantly higher compared to the control group (107.00 ± 4.82, 76.23 ± 2.31; p < 0.05).
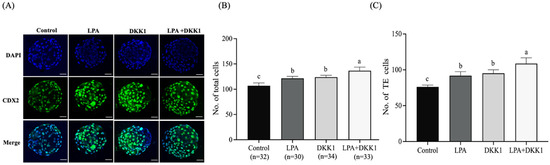
Figure 2.
LPA and DKK1 increase number of TE cells of bovine embryos. (A) Fluorogram of CDX2-stained bovine embryos; (B) total cell numbers of bovine embryos; (C) TE cell numbers of bovine embryos. Scale bar = 50 μm. a, b, c Values with different superscripts indicate significant difference between groups (p < 0.05).
2.4. LPA and DKK1 Suppressed the Apoptosis of Bovine Embryos
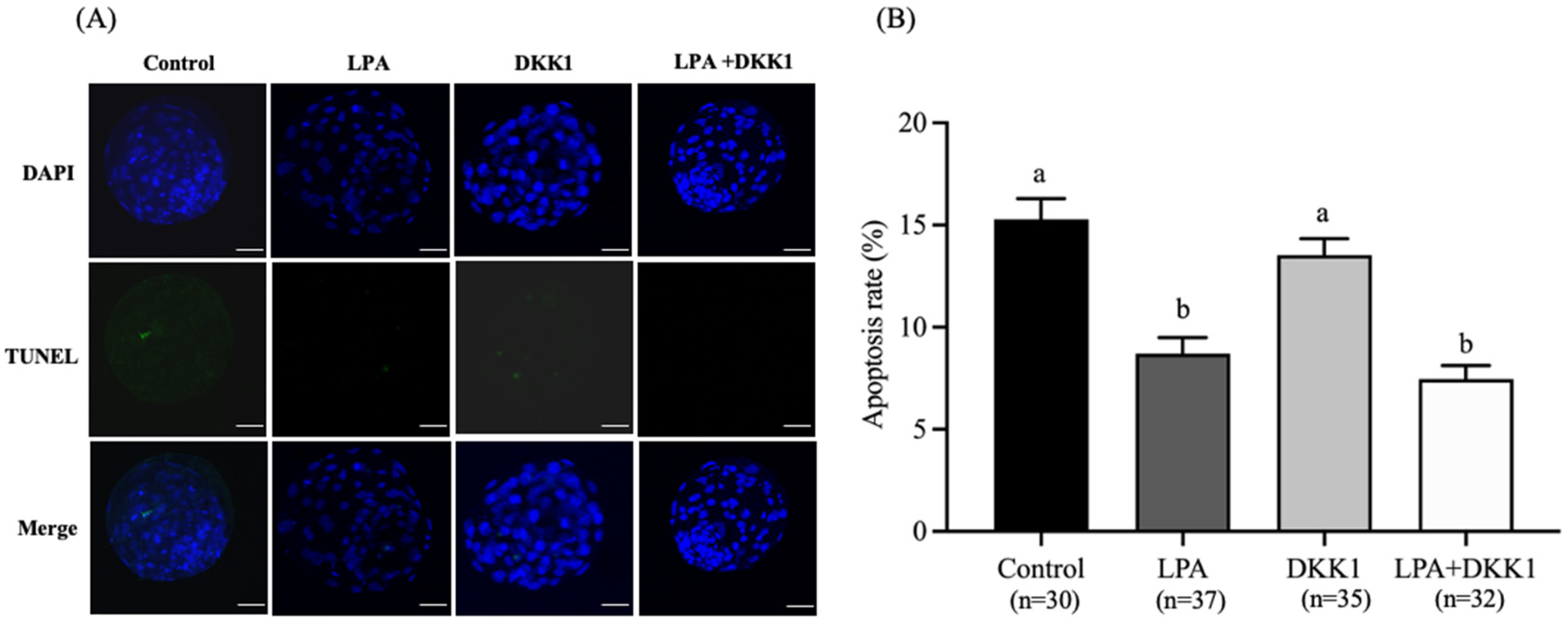
As shown in Figure 3, the apoptosis rates of blastocysts in the LPA group (8.63 ± 0.68%) and LPA + DKK1 group (7.45 ± 0.59%) were significantly lower compared to the control group (15.28 ± 0.91%) and the DKK1 group (13.53 ± 0.71%, p < 0.05).
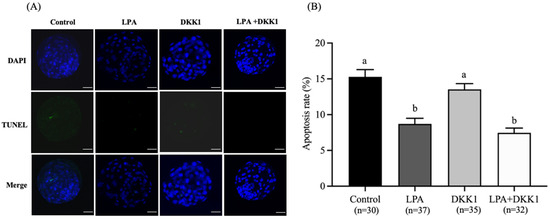
Figure 3.
Effect of LPA and DKK1 on the apoptosis of bovine embryos. (A) Fluorogram of TUNEL-stained embryos; (B) Effect of LPA and DKK1 on the apoptosis of bovine embryos. Scale bar = 50 μm. a, b Values with different superscripts indicate significant difference between groups (p < 0.05).
2.5. Transcriptomic Analysis of LPA- and DKK1-Treated Bovine Embryos
Using scRNA-seq for single embryos, the results of quality control are shown in Table 2; the expression level of each sample met the quality control requirements. We obtained approximately 52 million sequencing clean reads from duplicate samples of bovine blastocyst. The clean reads numbers of the control groups ranged from 44,891,384 to 62,536,622 and the clean reads rates were higher than 98.09%; the clean reads numbers of the LD groups varied from 40,869,626 to 54,470,954 and the clean reads rates were higher than 97.88%. The mapped ratios of the control group were higher than 97.51%, and the mapped ratios of the LD group were higher than 97.43%.

Table 2.
Summary of raw and clean reads of mRNA.
2.6. Screening of Differentially Expressed Genes (DEGs) in LPA- and DKK1-Treated Bovine Embryos
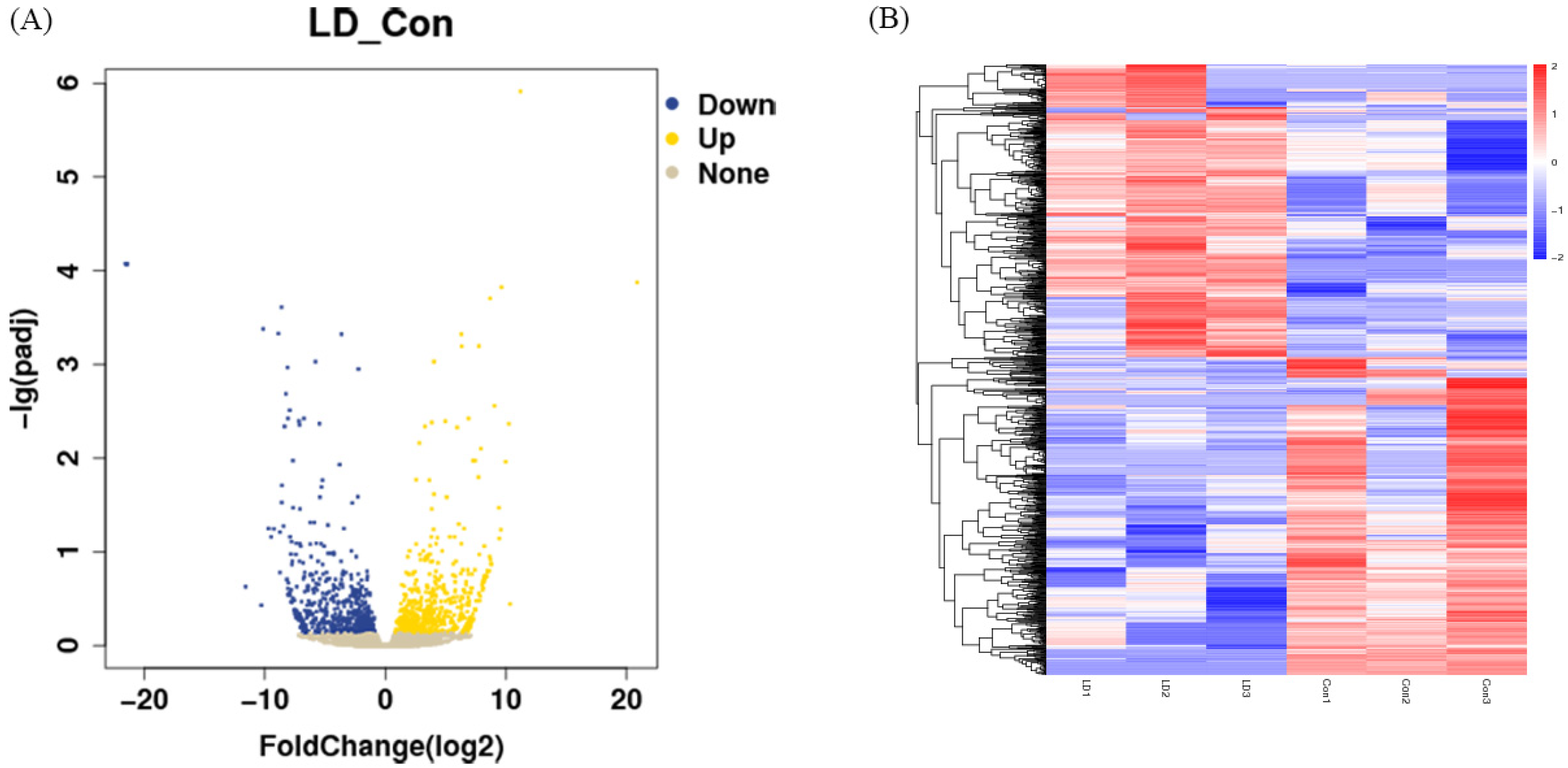
Differences in gene expression levels between the LD and control groups were depicted using volcano plots (Figure 4A). A total of 1203 DEGs were found between the LD and control groups, including 577 upregulated genes and 626 downregulated genes. Some of those DEGs are listed in Table 3, including apoptosis genes (BAD, FAS, BEX2), oxidative stress genes (GPX3, SLC25A27, MGST1), embryo development genes (ASTL, ABCB4, EED, TGFBR2, IGFBP7), endometrial-related genes (MX2, c15H11orf34, MUC1, COL5A3) and embryonic differentiation genes (FOS, GCM1, OVOL1, KRT7). The full list is provided in Supplementary Table S1. Cluster analysis of the DEGs was then performed according to the expression, and results showed that samples in the same group were well clustered together (Figure 4B).
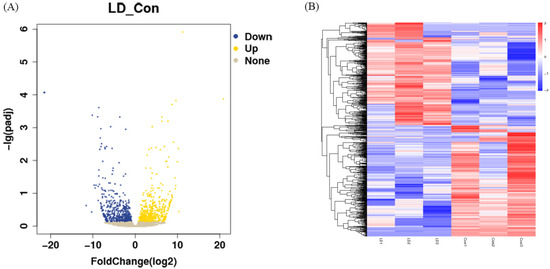
Figure 4.
Screening of differential expression of RNA. (A) Volcano plot of DEGs in LD and control groups. (B) Clustering analysis of differentially expressed genes. Red indicates high expression and blue indicates low expression.

Table 3.
Statistics of different expression of mRNA of LD and Control groups.
2.7. GO Enrichment Analysis of the DEGs
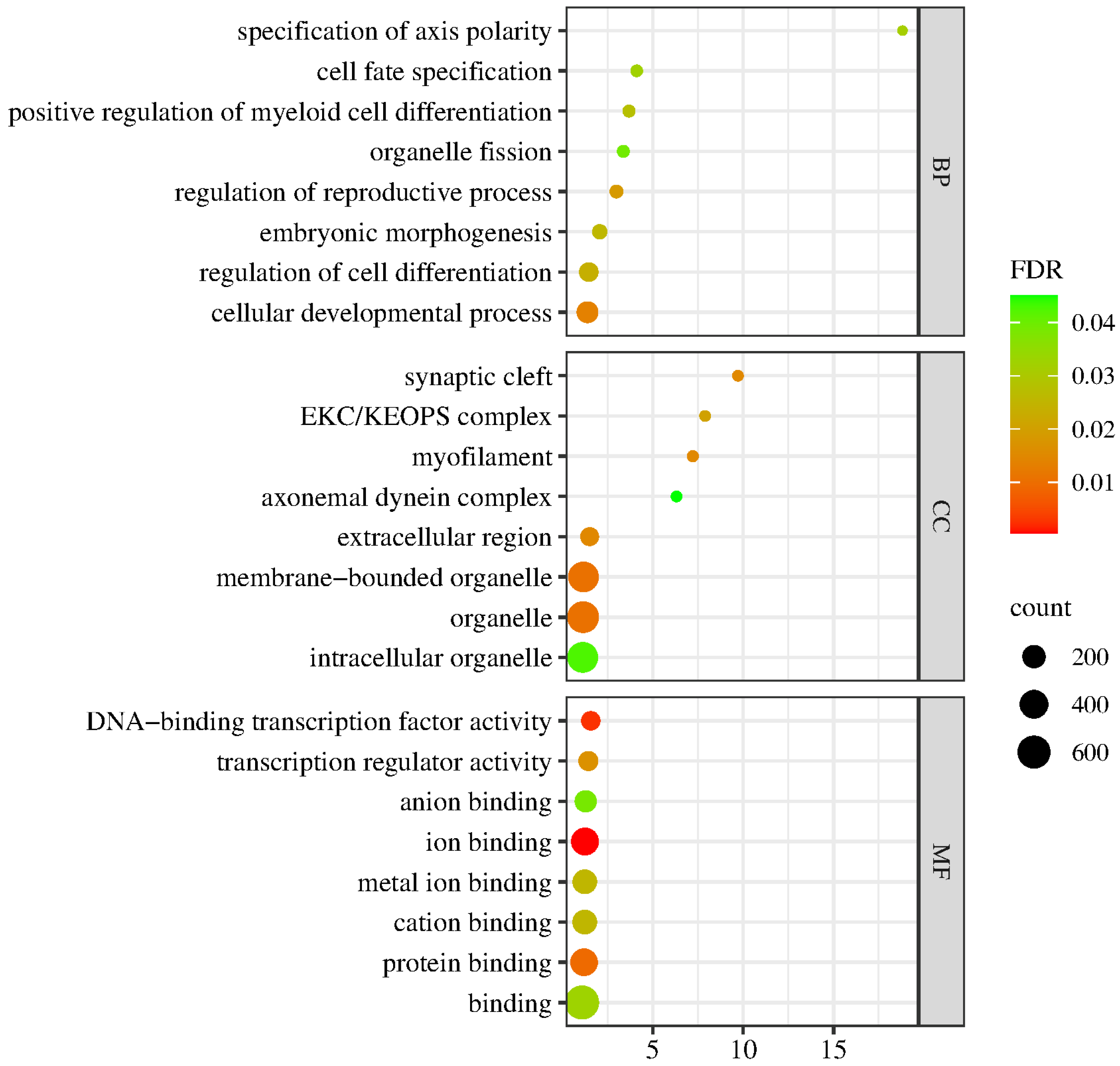
Differential expression analysis, functional annotation and functional enrichment analysis of DEGs were performed according to the expression level of the genes in the LD and control groups. As shown in Figure 5, the GO terms for genes associated with the DEGs between the LD and control groups were mainly involved in biological process (BP), cell component (CC) or molecular function (MF). The BP group mainly consisted of cell fate specification, regulation of cell differentiation and cellular development process; CC mainly consisted of synaptic cleft, myofilament, extracellular region and organelle; and MF mainly consisted of DNA-binding transcription factor activity, transcription regulator activity, ion binding and protein binding.
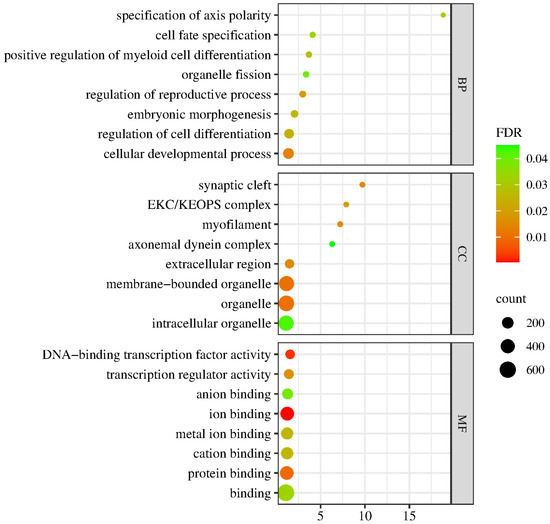
Figure 5.
GO enrichment analysis of the DEGs. The spot color represents the FDR, and the spot size represents the enriched gene number.
2.8. Significantly Regulated Biological Signaling Pathways in Response to LPA and DKK1 Treatment in Bovine Embryos
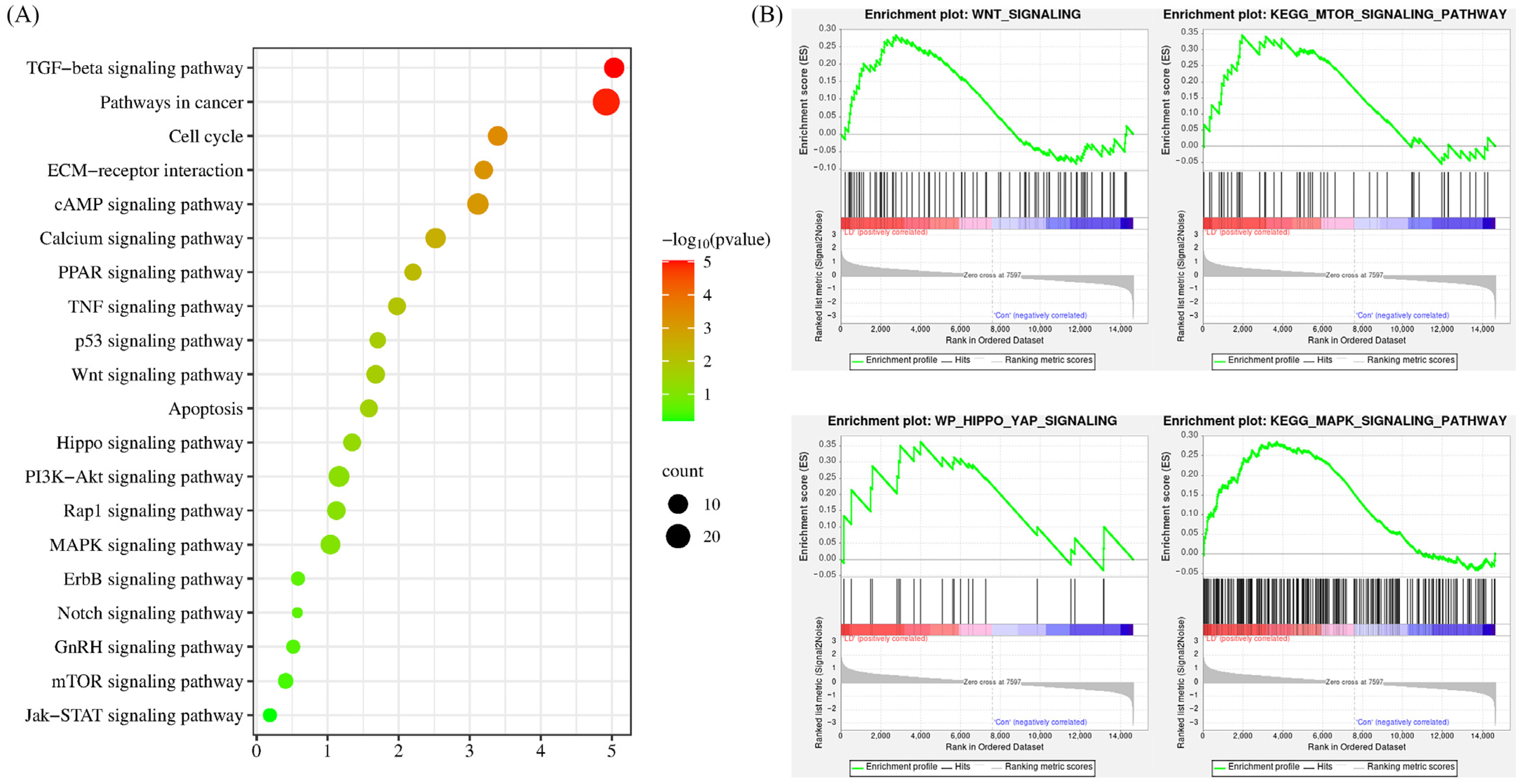
To enhance our comprehension of the signaling pathways regulated by LPA and DKK1 treatment, the identified DEGs were subjected to pathway analysis utilizing the KEGG. As shown in Figure 6A, the DEGs’ associated genes between the LD and control groups were mainly enriched with TGF-beta signaling pathway, ECM-receptor interaction, Wnt signaling pathway, Apoptosis, Hippo signaling pathway, MAPK signaling pathway and Notch signaling pathway. The overall changes in the gene set of the LD group analyzed by GSEA are shown in Figure 6B.
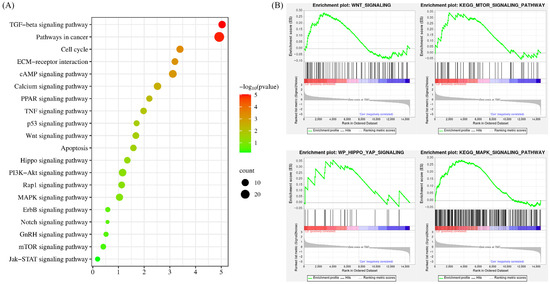
Figure 6.
KEGG pathway analysis of the DEGs. (A) Results from KEGG analysis; the color of each dot indicates the p value of the two sets of DEGs, and the size of the dot indicates the number of DEGs in each KEGG pathway. (B) The results from GSEA analysis.
3. Discussion
Culture environments typically control embryo cleavage, compaction, survival and development [32]. To elucidate the impact of IVC conditions on embryonic quality, the culture media were supplemented with the Hippo pathway agonist (LPA) and the Wnt pathway inhibitor (DKK1). Many research studies have endeavored to optimize culture systems to enhance blastocyst quality. Earlier studies have found that LPA treatment increases the abundance of transcripts of Connexin 43, GJC1 and CDH1, which would boost the cleavage rate and development of embryos [33]. For example, the addition of LPA into the medium can increase the cleavage rate and promote the development of mouse [34], porcine [35,36] and bovine [37,38] embryos. Consistent with these findings, our study observed that the application of LPA to the culture medium correlated with elevated cleavage and blastocyst development rates (Table 1). Our results found an improvement in the blastocyst rate following supplementation with DKK1 in the IVC medium. Similarly, previous research had reported a tendency for increased cleavage and blastocyst rates in bovine embryos treated with DKK1 [14,39]. Moreover, when LPA and DKK1 were used in combination, there was a notable enhancement in the developmental competence of bovine blastocysts. In essence, LPA and DKK1 exert a positive influence on early embryonic development, extending to the blastocyst stage, and thereby improving in vitro production (IVP) systems.
Yes-associated protein (YAP) is a signaling and transcriptional effector that is repressed by the Hippo pathway and induces pluripotency in embryonic stem cells [40]. YAP also plays a key role in regulating the differentiation of the TE and ICM lineages’ specification during porcine [41] and mouse [42] embryonic development. Studies have further elucidated YAP function, demonstrating that LPA activates YAP/TAZ and enhances YAP/TAZ signaling to promote cell proliferation and stemness maintenance [43,44]. Aligning with these findings, our experiments revealed a significant increase in YAP fluorescence intensity in embryos following LPA treatment, likely due to LPA-mediated inhibition of the Hippo pathway kinase LATS1/2, as previously reported [40,45]. Collectively, these data support the model that LPA acts through YAP/Hippo signaling to modulate cell fate decisions and embryonic patterning.
The transcription factor CDX2 plays a pivotal role in the specification of TE and the subsequent formation of blastocysts [46]. In our experiments, treatment of preimplantation embryos with LPA, DKK1 or both LPA and DKK1 resulted in an increase in total cell numbers, TE cell numbers and the ratio of TE cells to ICM cells compared to untreated control embryos. These findings aligned with previous work by Liu et al. which demonstrated that supplementation with LPA enhanced blastocyst differentiation, as evidenced by accelerated blastocyst outgrowth rates in vitro [47]. In the presence of LPA, embryos showed higher levels of CDX2 expression, which is regulated by the Hippo pathway [48]. In mouse [49] and bovine [50] embryos, nuclear CDX2 accumulation is dependent on YAP. Likewise, Zhang et al. [35] demonstrated that LPA supplementation in the culture medium significantly enhanced the total cell count and the number of TE cells in porcine blastocysts.
Furthermore, inhibition of the Wnt signaling pathway contributes to an increase in TE cell numbers and the total cell numbers of the blastocyst [51]. The likelihood is that DKK1 increases the expression of transcription factors that promote cell differentiation in the TE lineage [52]. Treatment of embryos with DKK1 from day 5 of development encourages the proliferation of the TE cell lineage in porcine [53], bovine [14,52,54,55] and other mammalian blastocysts. Aligning with these prior observations, our current results revealed that both TE cell lineage allocation and total cell numbers are increased following combined LPA and DKK1 treatment of bovine embryos (Table 2). Similarly, the expansion of the CDX2-positive TE cells in response to LPA and DKK1 (Figure 1) provides additional evidence supporting the interaction between Hippo and Wnt signaling during TE specification in the developing mammalian embryo. Further work is required to determine how LPA and DKK1 regulate the expression of genes involved in blastocyst differentiation.
Apoptosis is a process of programmed cell death that is involved in the homeostasis maintenance of many biological systems [56]. Recent studies have shown that LPA treatment increased the BCL2 and BCL2L1 expression and decreased the BAX and BAK expression of embryos [33,37]. Like earlier studies demonstrating that LPA supplementation decreases apoptotic cell numbers in porcine blastocysts [35,37], our current experiments revealed a significant decrease in the incidence of apoptosis in the LPA group and the LPA + DKK1 group, compared to the control and DKK1 groups. Collectively, these findings provide evidence that LPA and DKK1 act cooperatively to orchestrate gene regulatory networks governing cell fate decisions in the preimplantation embryo.
In the present investigation, scRNA-seq was utilized to perform a comprehensive transcriptomic analysis at the single-embryo level for both control group and LD group embryos. Several genes related to embryo differentiation (WNT3A, AMOT) and endometrium (MX2, c15H11orf34, MUC1) were found to be upregulated in the LD group compared to the control group. WNT3A is a classical canonical WNT ligand that plays an important role in embryonic developmental processes [54] and bovine TE development and differentiation [7]. It has been demonstrated that WNT3A regulates CDX2 through the regulation of the WNT-YAP/TAZ signaling pathway [57]. AMOT activates the Hippo signaling pathway during mouse preimplantation embryo development [58] and plays an important role in TE formation and function in bovine blastocyst development in vitro [50]. MX2 is an optimal gene for distinguishing pregnancy, which may trigger the migration of maternal neutrophils to the developing embryo [59]. MX2 gene expression is upregulated in early pregnancy and has an important role in regulating endometrial production, uterine recontouring and resistance to luteolysis activity [60]. c15H11orf34 is highly upregulated in endometrium of maternally pregnant bovines [61]. MUC1 is a cell surface glycoprotein which is expressed predominantly in the luminal epithelium of the mammalian endometrium and plays an important role in embryonic implantation and placentation [62,63]. The transcription of endometrial-related genes plays a critical role in the further development of the embryo and ultimately in the success of pregnancy [64].
The enrichment analysis of DEGs highlighted a significant overrepresentation of several signaling pathways, including Wnt, Notch, TGF-beta, mTOR, MAPK, ECM and Hippo. Notch has been detected in human, mouse and bovine preimplantation embryos [1,65]. The Notch pathway plays a key role in the first lineage specification and regulates TE-specific expression in mouse blastocysts [66,67], which explained the increased CDX2 expression in the LPA and DKK1 group. The TGF-beta signaling pathway is of notable importance in mediating cellular proliferation and differentiation, both of which are essential for uterine growth and the progression of fetal development [68]. Concurrently, the mTOR pathway promotes cell proliferation during the early stages of embryonic development [69]. Embryos deficient in mTOR signaling in mouse exhibit developmental failure around implantation, highlighting its essential role in mammalian embryogenesis [70,71]. It was found that pre-implantation inhibition of mTOR significantly reduced the ratio of developing embryos to blastocysts and impaired blastocyst quality as well as the proliferation and differentiation of TE cells [72]. Moreover, the MAPK signaling pathway is involved in cell proliferation, differentiation and apoptosis [73]. Additionally, MAPK plays an important role throughout preimplantation embryonic development [74] and is involved in embryonic and yolk sac angiogenesis during fetal placenta development [75]. Furthermore, the ECM signaling pathway is recognized as a key contributor to the development of the bovine placenta as well [76]. Alterations in the ECM interface that interact with stem cells are known to influence lineage commitment directly [77]. Collectively, these significantly enriched gene signaling pathways have been identified as integral to placental and embryo development. Since our work primarily focused on the early stages of embryonic development, more investigation is needed to determine the combined impact of the mTOR, MAPK and ECM signaling pathways during the preimplantation and placental development stages in bovine.
4. Materials and Methods
Animals were treated according to the requirements of the Institutional Animal Care and Use Committee of the Chinese Academy of Agricultural Sciences.
4.1. In Vitro Maturation of Oocytes
Bovine ovaries were collected from the slaughterhouse and transported to the laboratory within 2 h at 30–35 °C. Cumulus-oocyte complexes (COCs) were collected from 2–8 mm follicles by aspiration with an 18-gauge needle. COCs were washed and those with at least 3 layers of cumulus cells were chosen for the experiment. The IVM medium contained TCM199 (Gibco, Life Technology, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone; Gibco, Life Technology, Carlsbad, CA, USA), 1 µg/mL estradiol, 40 ng/mL insulin-like growth factor (IGF, Sigma-Aldrich, St. Louis, MO, USA), 10 µg/mL follicle-stimulating hormone (FSH, Sigma-Aldrich, St. Louis, MO, USA), 50 ng/mL epidermal growth factor (EGF, Sigma-Aldrich, St. Louis, MO, USA) and 10 µg/mL lutrinizing hormone (LH, Sigma-Aldrich, St. Louis, MO, USA).
4.2. In Vitro Fertilization (IVF) of Oocytes
The IVF procedure was that mature oocytes were washed twice or three times in Brackett and Oliphant (BO) [78] fertilization medium which contained 4 mg/mL fatty acid-free BSA (Sigma-Aldrich, St. Louis, MO, USA) and 10 μg/mL heparin (Sigma-Aldrich, St. Louis, MO, USA). Oocytes were transferred into a 90 μL drop of BO fertilization medium at a density of 20–25 COCs per group. Frozen semen was thawed in a water bath at 37 °C for 30 s, rinsed twice in 7 mL BO medium containing 4 mg/mL fatty acid-free BSA and 10 mmol/L caffeine (Sigma-Aldrich, St. Louis, MO, USA) by centrifugation at 1500 rpm for 5 min, and finally resuspended at a concentration of 1 × 106 spermatozoa per mL. For IVF, a 10 μL aliquot of the sperm suspension was added to each fertilization drop and the mixture was incubated for 16–18 h at 38.5 °C in humidified air containing 5% CO2.
4.3. IVC
After fertilization, cumulus cells and the sperm were stripped from the presumptive zygotes. The zygotes were washed twice and randomly cultured in different groups of 25 in droplets of 100 µL CR1aa medium supplemented with 6 mg/mL BSA at 38.5 °C under 5% CO2. The experiment was divided into four groups: (i) control group: culture in CR1aa medium; (ii) LPA group: CR1aa medium supplemented with 10−5 M LPA (Sigma-Aldrich, St. Louis, MO, USA) for 48 h at 38.5 °C under 5% CO2; (iii) DKK1 group: on day 5 after initiation of fertilization, CR1aa medium supplemented with 10% FBS and 100 ng/mL DKK1 (Sigma-Aldrich, St. Louis, MO, USA) for 48 h at 38.5 °C under 5% CO2; (iiii) LPA + DKK1 group: CR1aa medium supplemented with 10−5 M LPA and 100 ng/mL DKK1. Embryos were cultured at 38.5 °C under 5% CO2 until day 7 after initiation of fertilization. The cleavage rate was evaluated at day 2 and blastocyst rate was evaluated at day 7.
4.4. Immunofluorescence Staining of Embryo
Immunofluorescence was performed according to Yu et al. [79] with some modifications. The embryos were fixed in 4% paraformaldehyde for 1 h at 4 °C. Subsequently, the embryos were permeated with 0.5% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) at room temperature for 40 min. Then, the embryos were blocked with PBS containing 1% BSA at 4 °C overnight. The embryos were moved into the primary antibodies YAP (1:500, abcam, Cambridge, UK) and CDX2 (1:500, abcam, Cambridge, UK) diluted with 1% BSA at 4 °C overnight, and then washed three times with 0.5% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) for 10 min each. Embryos were incubated with the secondary antibodies Alexa Fluor 594 anti-mouse (1:1000, abcam, Cambridge, UK) and Alexa Fluor 488 anti-rabbit IgG (1:1000, Cell Signaling Technology, Danvers, MA, USA) at room temperature for 1 h in the dark. Finally, nuclei were stained with 5 mg/mL DAPI (Beyotime, Shanghai, China) for 10 min in the dark at room temperature. The fluorescent images were obtained by a confocal microscope (Leica, Wetzlar, Germany) and analyzed using Image J software version 1.8.0 (NIH, Bethesda, MD, USA). The total cell number of individual embryos was measured by DAPI (Beyotime, Shanghai, China) staining, and the number of putative TE cells was determined by CDX2 positive staining.
4.5. Detection of DNA Fragmentation by TUNEL
Apoptosis was analyzed using a TUNEL (terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling) assay kit (Solarbio, Beijing, China) following the manufacturer’s instructions. In brief, blastocysts were washed three times with PBS containing 0.1% PVA (PBS-PVA), fixed with 4% paraformaldehyde and permeabilized by incubation in 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) for 30 min at room temperature. Next, the embryos were washed three times with PBS-PVA and incubated with fluorescein-conjugated dUTP and the terminal deoxynucleotidyl transferase enzyme in the dark for 1 h at 37 °C. After incubation with 1 mg/mL Hoechst 33,342 for 5 min at 37 °C to label the nuclei, the samples were fixed on slides and examined by confocal microscope (Leica, Wetzlar, Germany). The total cell number of blastocysts (DAPI) and the number of apoptotic cells of blastocysts (TUNEL) were counted using ImageJ software version 1.8.0 (NIH, Bethesda, MD, USA). The apoptosis rate of blastocysts = the number of apoptotic cells of blastocysts/total cell number of blastocysts.
4.6. Sample Preparation, Library Construction and RNA Sequencing
The embryo zone pellucida was removed by 5 mg/mL pronase for 1–2 min. Each embryo was pooled and dissolved in lysis buffer. The protocol for scRNA-seq library preparation and sequencing was performed according to the method described by Gao et al. [80] with some modifications. A single cell was lysed to release RNAs, which were then reverse transcribed into first-strand cDNAs using six-base random primer random hexamers. The second strand cDNA was synthesized from dNTPs in the DNA polymerase I system, and then the double-stranded cDNA was purified by AMPure P beads. Finally, purified single-cell cDNAs were prepared for sequencing libraries according to Illumina’s TruSeq DNA sample preparation protocols. For the qualified cDNA libraries, sequencing was performed by the Illumina platform, and the PE150 sequencing strategy was run to obtain 150-bp double-ended sequencing reads.
4.7. Functional Enrichment Analysis
In the present study, gene ontology (GO) enrichment analysis was performed based on three terms including biological processes, cellular components and molecular functions (http://www.geneontology.org/), accessed on 20 October 2024). Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis distinguished significantly enriched biological pathways. Significance was set at a p-value of <0.05.
4.8. Statistical Analysis
All experiments were repeated at least three times, and all data were presented as mean ± standard error. All analyses were performed using SAS software version 9.2.0 (SAS Institute, Carrey, NC, USA) to compare the groups. Differences were regarded to be significant when p < 0.05.
5. Conclusions
In the context of our investigation, the addition of LPA and DKK1 was found to have remarkable results in improving early embryo quality. This improvement was observed to be intricately associated with its regulatory influence on the TE lineage, modulation of apoptosis and its interactive engagement with critical signaling pathways, including the Hippo, Wnt and MAPK pathways, during the in vitro development of bovine embryos.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25073912/s1.
Author Contributions
Methodology, H.Z., C.L., X.F. and J.C.; Formal analysis, W.D.; Investigation, M.S. and A.K.; Data curation, B.Y.; Writing—original draft, P.Z.; Visualization, S.-C.S.; Supervision, X.Z.; Funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the earmarked fund for the National Key R&D Program of China (2022YFE0100200), the National Natural Science Foundation of China (32161143032), the earmarked fund for CARS (CARS36), the National Germplasm Center of Domestic Animal Resources and the Agricultural Science and Technology Innovation Program (ASTIP-2016-IAS-06).
Institutional Review Board Statement
All animal processing conformed to guidelines developed by the Institutional Animal Care and Use Committee of the Chinese Academy of Agricultural Science (protocol code: IAS2023-4, date of approval: 8 February 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data supporting the conclusions in this article have been presented in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wei, Q.Q.; Zhong, L.; Zhang, S.P.; Mu, H.Y.; Xiang, J.Z.; Yue, L.; Dai, Y.P.; Han, J.Y. Bovine lineage specification revealed by single-cell gene expression analysis from zygote to blastocyst. Biol. Reprod. 2017, 97, 5–17. [Google Scholar] [CrossRef]
- Gassler, J.; Kobayashi, W.; Gáspár, I.; Ruangroengkulrith, S.; Mohanan, A.; Gómez Hernández, L.; Kravchenko, P.; Kümmecke, M.; Lalic, A.; Rifel, N.; et al. Zygotic genome activation by the totipotency pioneer factor Nr5a2. Science 2022, 378, 1305–1315. [Google Scholar] [CrossRef]
- Underhill, L.A.; Robins, J.C. Trophoblast development in the murine preimplantation embryo. Semin. Reprod. Med. 2016, 34, 57–62. [Google Scholar]
- Yao, C.; Zhang, W.; Shuai, L. The first cell fate decision in pre-implantation mouse embryos. Cell Regen. 2019, 8, 51–57. [Google Scholar] [CrossRef]
- Garg, V.; Yang, Y.; Nowotschin, S.; Setty, M.; Kuo, Y.Y.; Sharma, R.; Polyzos, A.; Salataj, E.; Murphy, D.; Jang, A.; et al. Single-cell analysis of bidirectional reprogramming between early embryonic states reveals mechanisms of differential lineage plasticities. bioRxiv 2023, 534648. [Google Scholar] [CrossRef]
- Perera, M.; Brickman, J.M. In vitro models of human hypoblast and mouse primitive endoderm. Curr. Opin. Genet. Dev. 2023, 83, 102115. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, B.; Haider, S.; Meinhardt, G.; Pollheimer, J.; Knöfler, M. WNT and NOTCH signaling in human trophoblast development and differentiation. Cell Mol. Life Sci. 2022, 79, 292. [Google Scholar] [CrossRef]
- Fan, R.; Kim, Y.S.; Wu, J.; Chen, R.; Zeuschner, D.; Mildner, K.; Adachi, K.; Wu, G.; Galatidou, S.; Li, J.; et al. Wnt/Beta-catenin/Esrrb signalling controls the tissue-scale reorganization and maintenance of the pluripotent lineage during murine embryonic diapause. Nat. Commun. 2020, 11, 5499. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, E.; Yang, M.; Lu, L. Overexpression of Wnt11 promotes chondrogenic differentiation of bone marrow-derived mesenchymal stem cells in synergism with TGF-β. Mol. Cell Biochem. 2014, 390, 123–131. [Google Scholar] [CrossRef]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef]
- Hayat, R.; Manzoor, M.; Hussain, A. Wnt signaling pathway: A comprehensive review. Cell Biol. Int. 2021, 46, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Zinovyeva, A.Y.; Yamamoto, Y.; Sawa, H.; Forrester, W.C. Complex network of Wnt signaling regulates neuronal migrations during Caenorhabditis elegans development. Genetics 2008, 179, 1357–1371. [Google Scholar] [CrossRef] [PubMed]
- Steinhart, Z.; Angers, S. Wnt signaling in development and tissue homeostasis. Development 2018, 146, dev146589. [Google Scholar] [CrossRef] [PubMed]
- Denicol, A.C.; Block, J.; Kelley, D.E.; Pohler, K.G.; Dobbs, K.B.; Mortensen, C.J.; Ortega, M.S.; Hansen, P.J. The WNT signaling antagonist Dickkopf-1 directs lineage commitment and promotes survival of the preimplantation embryo. FASEB J. 2014, 28, 3975–3986. [Google Scholar] [CrossRef] [PubMed]
- Minten, M.A.; Bilby, T.R.; Bruno, R.G.; Allen, C.C.; Madsen, C.A.; Wang, Z.; Sawyer, J.E.; Tibary, A.; Neibergs, H.L.; Geary, T.W.; et al. Effects of fertility on gene expression and function of the bovine endometrium. PLoS ONE 2013, 8, 69444. [Google Scholar] [CrossRef]
- Kawashima, I.; Kawamura, K. Regulation of follicle growth through hormonal factors and mechanical cues mediated by Hippo signaling pathway. Syst. Biol. Reprod. Med. 2018, 64, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Grosbois, J.; Demeestere, I. Dynamics of PI3K and Hippo signaling pathways during in vitro human follicle activation. Hum. Reprod. 2018, 33, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Masciangelo, R.; Hossay, C.; Chiti, M.C.; Manavella, D.D.; Amorim, C.A.; Donnez, J.; Dolmans, M.M. Role of the PI3K and Hippo pathways in follicle activation after grafting of human ovarian tissue. J. Assist. Reprod. Genet. 2020, 37, 101–108. [Google Scholar] [CrossRef]
- Hashimoto, M.; Sasaki, H. Epiblast formation by TEAD-YAP-dependent expression of pluripotency factors and competitive elimination of unspecified cells. Dev. Cell 2019, 50, 139–154. [Google Scholar] [CrossRef]
- Karasek, C.; Ashry, M.; Driscoll, C.S.; Knott, J.G. A tale of two cell-fates: Role of the Hippo signaling pathway and transcription factors in early lineage formation in mouse preimplantation embryos. Mol. Hum. Reprod. 2020, 26, 653–664. [Google Scholar] [CrossRef]
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007, 130, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Yung, Y.C.; Chen, A.; Chun, J. Lysophosphatidic acid signalling in development. Development 2015, 142, 1390–1395. [Google Scholar] [CrossRef]
- Hama, K.; Aoki, J.; Inoue, A.; Endo, T.; Amano, T.; Motoki, R.; Kanai, M.; Ye, X.; Chun, J.; Matsuki, N.; et al. Embryo Spacing and Implantation Timing Are Differentially Regulated by LPA3-Mediated Lysophosphatidic Acid Signaling in Mice1. Biol. Reprod. 2007, 77, 954–959. [Google Scholar] [CrossRef]
- Lin, M.E.; Herr, D.R.; Chun, J. Lysophosphatidic acid (LPA) receptors: Signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 2010, 91, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.T.; Yung, Y.C.; Herr, D.R.; Chun, J. Lysophosphatidic acid in vascular development and disease. IUBMB Life 2009, 61, 791–799. [Google Scholar] [CrossRef]
- Kim, D.; Li, H.Y.; Lee, J.H.; Oh, Y.S.; Jun, H.S. Lysophosphatidic acid increases mesangial cell proliferation in models of diabetic nephropathy via Rac1/MAPK/KLF5 signaling. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015, 161, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Zilionis, R.; Nainys, J.; Veres, A.; Savova, V.; Zemmour, D.; Klein, A.M.; Mazutis, L. Single-cell barcoding and sequencing using droplet microfluidics. Nat. Protoc. 2017, 12, 44–73. [Google Scholar] [CrossRef] [PubMed]
- Pijuan-Sala, B.; Griffiths, J.A.; Guibentif, C.; Hiscock, T.W.; Jawaid, W.; Calero-Nieto, F.J.; Mulas, C.; Ibarra-Soria, X.; Tyser, R.C.V.; Ho, D.L.L.; et al. A single-cell molecular map of mouse gastrulation and early organogenesis. Nature 2019, 566, 490–495. [Google Scholar] [CrossRef]
- Argelaguet, R.; Clark, S.J.; Mohammed, H.; Stapel, L.C.; Krueger, C.; Kapourani, C.A.; Imaz-Rosshandler, I.; Lohoff, T.; Xiang, Y.; Hanna, C.W.; et al. Multiomics profiling of mouse gastrulation at single-cell resolution. Nature 2019, 576, 487–491. [Google Scholar] [CrossRef]
- Ji, P.; Liu, Y.; Yan, L.; Jia, Y.; Zhao, M.; Lv, D.; Yao, Y.; Ma, W.; Yin, D.; Liu, F.; et al. Melatonin improves the vitrification of sheep morulae by modulating transcriptome. Front. Vet. Sci. 2023, 10, 1212047. [Google Scholar] [CrossRef] [PubMed]
- Im, G.S.; Lai, L.; Liu, Z.; Hao, Y.; Wax, D.; Bonk, A.; Prather, R.S. In vitro development of preimplantation porcine nuclear transfer embryos cultured in different media and gas atmospheres. Theriogenology 2004, 61, 1125–1135. [Google Scholar] [CrossRef]
- Shin, M.Y.; Lee, S.E.; Son, Y.J.; Park, Y.G.; Jeong, S.G.; Kim, E.Y.; Park, S.P. Lysophosphatidic acid accelerates development of porcine embryos by activating formation of the blastocoel. Mol. Reprod. Dev. 2018, 85, 62–71. [Google Scholar] [CrossRef]
- Jo, J.W.; Jee, B.C.; Suh, C.S.; Kim, S.H. Addition of lysophosphatidic acid to mouse oocyte maturation media can enhance fertilization and developmental competence. Hum. Reprod. 2014, 29, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Jiang, Y.; Lin, T.; Kang, J.W.; Lee, J.E.; Jin, D.I. Lysophosphatidic acid improves porcine oocyte maturation and embryo development in vitro. Mol. Reprod. Dev. 2015, 82, 66–77. [Google Scholar] [CrossRef]
- Zhu, X.; Li, L.; Gao, B.; Zhang, D.; Ren, Y.; Zheng, B.; Li, M.; Shi, D.; Huang, B. Early development of porcine parthenogenetic embryos and reduced expression of primed pluripotent marker genes under the effect of lysophosphatidic acid. Reprod. Domest. Anim. 2018, 53, 1191–1199. [Google Scholar] [CrossRef]
- Torres, A.C.; Boruszewska, D.; Batista, M.; Kowalczyk-Zieba, I.; Diniz, P.; Sinderewicz, E.; Saulnier-Blache, J.S.; Woclawek-Potocka, I.; Lopes-da-Costa, L. Lysophosphatidic acid signaling in late cleavage and blastocyst stage bovine embryos. Mediat. Inflamm. 2014, 2014, 678968. [Google Scholar] [CrossRef]
- Boruszewska, D.; Sinderewicz, E.; Kowalczyk-Zieba, I.; Grycmacher, K.; Woclawek-Potocka, I. Studies on lysophosphatidic acid action during in vitro preimplantation embryo development. Domest. Anim. Endocrinol. 2016, 54, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Shyam, S.; Goel, P.; Kumar, D.; Malpotra, S.; Singh, M.K.; Lathwal, S.S.; Chand, S.; Palta, P. Effect of Dickkopf-1 and colony stimulating factor-2 on the developmental competence, quality, gene expression and live birth rate of buffalo (Bubalus bubalis) embryos produced by hand-made cloning. Theriogenology 2020, 157, 254–262. [Google Scholar] [CrossRef]
- Qin, H.; Hejna, M.; Liu, Y.; Percharde, M.; Wossidlo, M.; Blouin, L.; Durruthy-Durruthy, J.; Wong, P.; Qi, Z.; Yu, J.; et al. YAP Induces Human Naive Pluripotency. Cell Rep. 2016, 14, 2301–2312. [Google Scholar] [CrossRef]
- Emura, N.; Saito, Y.; Miura, R.; Sawai, K. Effect of downregulating the Hippo pathway members YAP1 and LATS2 transcripts on early development and gene expression involved in differentiation in porcine embryos. Cell Reprogr. 2020, 22, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, N.; Inoue, K.I.; Adachi, K.; Kiyonari, H.; Ota, M.; Ralston, A.; Yabuta, N.; Hirahara, S.; Stephenson, R.O.; Ogonuki, N.; et al. The Hippo Signaling Pathway Components Lats and Yap Pattern Tead4 Activity to Distinguish Mouse Trophectoderm from Inner Cell Mass. Dev. Cell 2009, 16, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Van Sciver, N.; Ohashi, M.; Pauly, N.P.; Bristol, J.A.; Nelson, S.E.; Johannsen, E.C.; Kenney, S.C. Hippo signaling effectors YAP and TAZ induce Epstein-Barr Virus (EBV) lytic reactivation through TEADs in epithelial cells. PLoS Pathog. 2021, 17, e1009783. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, L.; Zhu, H.; Wu, Z.; Ding, X.; Hu, R.; Jiang, Y.; Tang, C.; Ding, S.; Guo, R. YAP Promotes Cell Proliferation and Stemness Maintenance of Porcine Muscle Stem Cells under High-Density Condition. Cells 2021, 10, 3069. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H.; et al. Regulation of the Hippo-YAP Pathway by G-Protein-Coupled Receptor Signaling. Cell 2012, 150, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Gentile, L.; Fuchikami, T.; Sutter, J.; Psathaki, K.; Esteves, T.C.; Araúzo-Bravo, M.J.; Ortmeier, C.; Verberk, G.; Abe, K.; et al. Initiation of trophectoderm lineage specification in mouse embryos is independent of Cdx2. Development 2010, 137, 4159–4169. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Armant, D.R. Lysophosphatidic acid regulates murine blastocyst development by transactivation of receptors for heparin- binding EGF-like growth factor. Exp. Cell Res. 2004, 296, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.; Okura, K.; Nagakura, S.; Ogawa, H. CDX2 downregulation in mouse mural trophectoderm during peri-implantation is heteronomous, dependent on the YAP-TEAD pathway and controlled by estrogen-induced factors. Reprod. Med. Biol. 2022, 21, e12446. [Google Scholar] [CrossRef] [PubMed]
- Liu-chittenden, Y.; Huang, B.; Shim, J.S.; Dev, G.; Chen, Q.; Lee, S.; Anders, R.A.; Liu, J.O.; Pan, D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012, 26, 1300–1305. [Google Scholar] [CrossRef]
- Negrón-Pérez, V.M.; Hansen, P.J. Role of yes-associated protein 1, angiomotin, and mitogen-activated kinase kinase 1/2 in development of the bovine blastocyst. Biol. Reprod. 2018, 98, 170–183. [Google Scholar] [CrossRef]
- Xie, H.; Tranguch, S.; Jia, X.; Zhang, H.; Das, S.K.; Dey, S.K.; Kuo, C.J.; Wang, H. Inactivation of nuclear Wnt-β-catenin signaling limits blastocyst competency for implantation. Development 2008, 135, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Amaral, T.F.; Gonella-Diaza, A.; Heredia, D.; Melo, G.D.; Estrada-Cortés, E.; Jensen, L.M.; Pohler, K.; Hansen, P.J. Actions of DKK1 on the preimplantation bovine embryo to affect pregnancy establishment, placental function, and postnatal phenotype†. Biol. Reprod. 2022, 107, 945–955. [Google Scholar] [CrossRef]
- Lim, K.T.; Gupta, M.K.; Lee, S.H.; Jung, Y.H.; Han, D.W.; Lee, H.T. Possible involvement of Wnt/β-catenin signaling pathway in hatching and trophectoderm differentiation of pig blastocysts. Theriogenology 2013, 79, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Denicol, A.C.; Dobbs, K.B.; McLean, K.M.; Carambula, S.F.; Loureiro, B.; Hansen, P.J. Canonical WNT signaling regulates development of bovine embryos to the blastocyst stage. Sci. Rep. 2013, 3, 1266. [Google Scholar] [CrossRef] [PubMed]
- Tribulo, P.; Bernal Ballesteros, B.H.; Ruiz, A.; Tríbulo, A.; Tríbulo, R.J.; Tríbulo, H.E.; Bo, G.A.; Hansen, P.J. Consequences of exposure of embryos produced in vitro in a serum-containing medium to dickkopf-related protein 1 and colony stimulating factor 2 on blastocyst yield, pregnancy rate, and birth weight. J. Anim. Sci. 2017, 95, 4407–4412. [Google Scholar] [CrossRef] [PubMed]
- Nabenishi, H.; Takagi, S.; Kamata, H.; Nishimoto, T.; Morita, T.; Ashizawa, K.; Tsuzuki, Y. The role of mitochondrial transition pores on bovine oocyte competence after heat stress, as determined by effects of cyclosporin A. Mol. Reprod. Dev. 2012, 79, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Han, X.; Zhou, Z.; Uyunbilig, B.; Huang, X.; Li, R.; Li, X. Wnt3a Activates the WNT-YAP/TAZ Pathway to Sustain CDX2 Expression in Bovine Trophoblast Stem Cells. DNA Cell Biol. 2019, 38, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Hirate, Y.; Hirahara, S.; Inoue, K.I.; Suzuki, A.; Alarcon, V.B.; Akimoto, K.; Hirai, T.; Hara, T.; Adachi, M.; Chida, K.; et al. Polarity-dependent distribution of angiomotin localizes hippo signaling in preimplantation embryos. Curr. Biol. 2013, 23, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Panda, B.S.K.; Mohapatra, S.K.; Chaudhary, D.; Alhussien, M.N.; Kapila, R.; Dang, A.K. Proteomics and transcriptomics study reveals the utility of ISGs as novel molecules for early pregnancy diagnosis in dairy cows. J. Reprod. Immunol. 2020, 140, 103148. [Google Scholar] [CrossRef]
- Casano, A.B.; Menchetti, L.; Trabalza-Marinucci, M.; Riva, F.; De Matteis, G.; Brecchia, G.; Inglesi, A.; Rossi, E.; Signorelli, F.; Barile, V.L.; et al. Gene expression of pregnancy-associated glycoproteins-1 (PAG-1), interferon-tau (IFNt) and interferon stimulated genes (ISGs) as diagnostic and prognostic markers of maternal-fetal cellular interaction in buffalo cows. Theriogenology 2023, 209, 89–97. [Google Scholar] [CrossRef]
- Adhikari, B.; Lee, C.N.; Khadka, V.S.; Deng, Y.; Fukumoto, G.; Thorne, M.; Caires, K.; Odani, J.; Mishra, B. RNA-Sequencing based analysis of bovine endometrium during the maternal recognition of pregnancy. BMC Genom. 2022, 23, 494. [Google Scholar] [CrossRef]
- Constantinou, P.E.; Morgado, M.; Carson, D.D. Transmembrane Mucin Expression and Function in Embryo Implantation and Placentation. Adv. Anat. Embryol. Cell Biol. 2015, 216, 51–68. [Google Scholar] [PubMed]
- Wang, X.; Zhu, B.; Xiong, S.; Sheng, X.; Qi, X.; Huang, Q.; Chen, C.; Guo, Y.; Ni, H. Expression and function of MUC1 in uterine tissues during early pregnancy in sheep after natural oestrous or artificially-induced oestrous. Theriogenology 2018, 108, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Sponchiado, M.; Gomes, N.S.; Fontes, P.K.; Martins, T.; Del Collado, M.; Pastore, A.A.; Pugliesi, G.; Nogueira, M.F.G.; Binelli, M. Pre-hatching embryo-dependent and -independent programming of endometrial function in cattle. PLoS ONE 2017, 12, e0175954. [Google Scholar] [CrossRef] [PubMed]
- Cormier, S.; Vandormael-Pournin, S.; Babinet, C.; Cohen-Tannoudji, M. Developmental expression of the Notch signaling pathway genes during mouse preimplantation development. Gene Expr. Patterns 2004, 4, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Rayon, T.; Menchero, S.; Nieto, A.; Xenopoulos, P.; Crespo, M.; Cockburn, K.; Canon, S.; Sasaki, H.; Hadjantonakis, A.K.; de la Pompa, J.L.; et al. Notch and hippo converge on Cdx2 to specify the trophectoderm lineage in the mouse blastocyst. Dev. Cell 2014, 30, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Menchero, S.; Rollan, I.; Lopez-Izquierdo, A.; Andreu, M.J.; de Aja, J.S.; Kang, M.J.; Adan, J.; Benedito, R.; Rayon, T.; Hadjantonakis, A.K.; et al. Transitions in cell potency during early mouse development are driven by Notch. Elife 2019, 8, e42930. [Google Scholar] [CrossRef] [PubMed]
- Ni, N.; Li, Q. TGFβ superfamily signaling and uterine decidualization. Reprod. Biol. Endocrinol. 2017, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Perez, C.A.; Moretti, L.; Lu, B. The mTOR signaling network: Insights from its role during embryonic development. Curr. Med. Chem. 2008, 15, 1192–1208. [Google Scholar] [CrossRef]
- Hentges, K.E.; Sirry, B.; Gingras, A.C.; Sarbassov, D.; Sonenberg, N.; Sabatini, D.; Peterson, A.S. FRAP/mTOR is required for proliferation and patterning during embryonic development in the mouse. Proc. Natl. Acad. Sci. USA 2001, 98, 13796–13801. [Google Scholar] [CrossRef]
- Murakami, M.; Ichisaka, T.; Maeda, M.; Oshiro, N.; Hara, K.; Edenhofer, F.; Kiyama, H.; Yonezawa, K.; Yamanaka, S. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol. Cell Biol. 2004, 24, 6710–6718. [Google Scholar] [CrossRef]
- Ma, C.; Li, Q.; Yang, Y.; Ge, L.; Cai, J.; Wang, J.; Zhu, M.; Xiong, Y.; Zhang, W.; Xie, J.; et al. mTOR hypoactivity leads to trophectoderm cell failure by enhancing lysosomal activation and disrupting the cytoskeleton in preimplantation embryo. Cell Biosci. 2023, 13, 219. [Google Scholar] [CrossRef] [PubMed]
- Radi, Z.A.; Marusak, R.A.; Morris, D.L. Species comparison of the role of p38 MAP kinase in the female reproductive system. J. Toxicol. Pathol. 2009, 22, 109–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bell, C.E.; Watson, A.J. p38 MAPK regulates cavitation and tight junction function in the mouse blastocyst. PLoS ONE 2013, 8, e59528. [Google Scholar] [CrossRef]
- Mudgett, J.S.; Ding, J.; Guh-Siesel, L.; Chartrain, N.A.; Yang, L.; Gopal, S.; Shen, M.M. Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis. Proc. Natl. Acad. Sci. USA 2000, 97, 10454–10459. [Google Scholar] [CrossRef] [PubMed]
- Jamioł, M.; Sozoniuk, M.; Wawrzykowski, J.; Kankofer, M. Effect of Sex Steroids and PGF2α on the Expression of Their Receptors and Decorin in Bovine Caruncular Epithelial Cells in Early-Mid Pregnancy. Molecules 2022, 27, 7420. [Google Scholar] [CrossRef]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- Brackett, B.G.; Oliphant, G. Capacitation of Rabbit Spermatozoa in vitro. Biol. Reprod. 1975, 12, 260–274. [Google Scholar] [CrossRef]
- Yu, B.; van Tol, H.T.A.; Oei, C.H.Y.; Stout, T.A.E.; Roelen, B.A.J. Lysophosphatidic Acid Accelerates Bovine In Vitro-Produced Blastocyst Formation through the Hippo/YAP Pathway. Int. J. Mol. Sci. 2021, 22, 5915. [Google Scholar] [CrossRef]
- Gao, S.; Yan, L.; Wang, R.; Li, J.; Yong, J.; Zhou, X.; Wei, Y.; Wu, X.; Wang, X.; Fan, X.; et al. Tracing the temporal-spatial transcriptome landscapes of the human fetal digestive tract using single-cell RNA-sequencing. Nat. Cell Biol. 2018, 20, 721–734. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).