Long-Term Sodium Deficiency Reduces Sodium Excretion but Impairs Renal Function and Increases Stone Formation in Hyperoxaluric Calcium Oxalate Rats
Abstract
:1. Introduction
2. Results
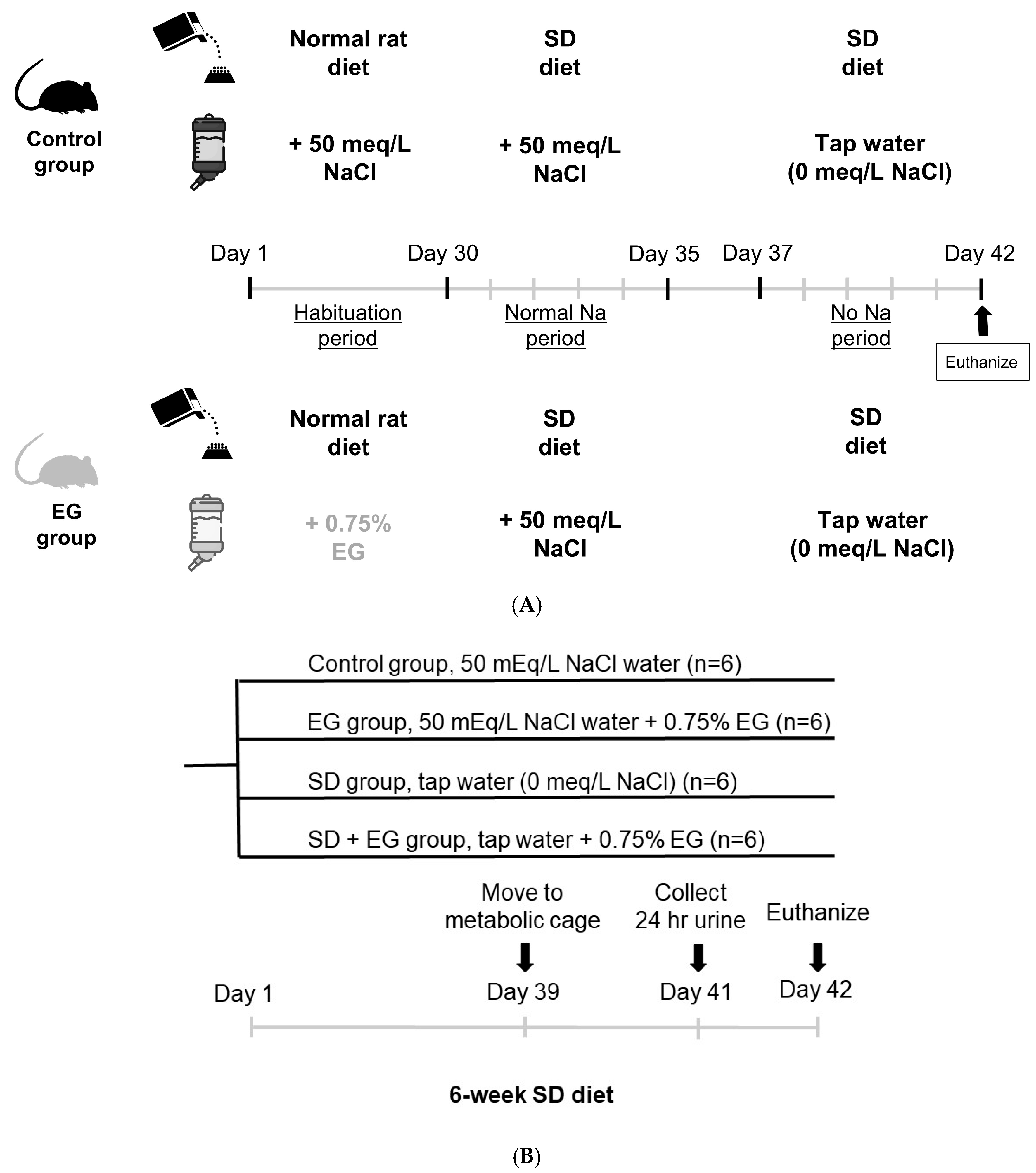
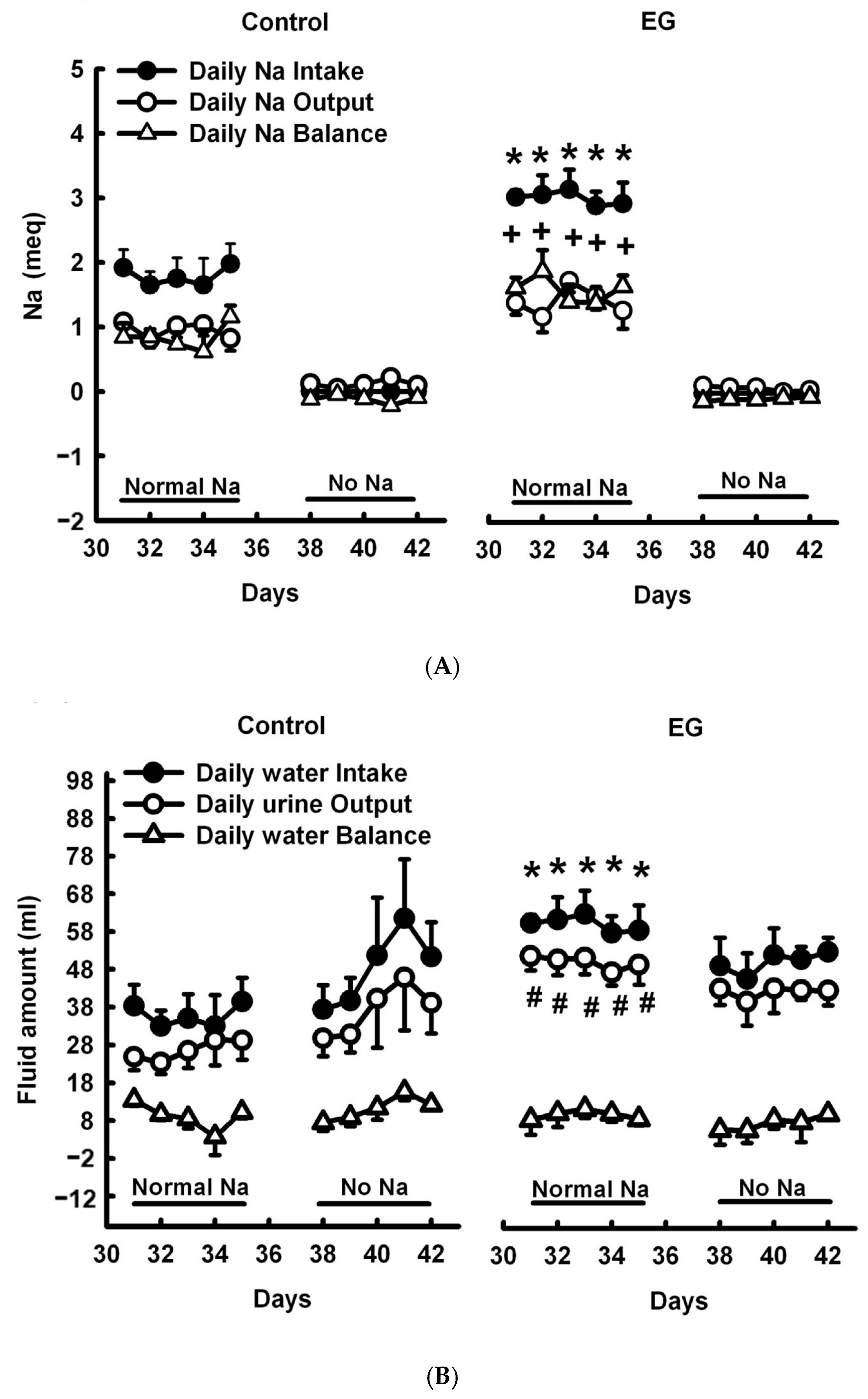
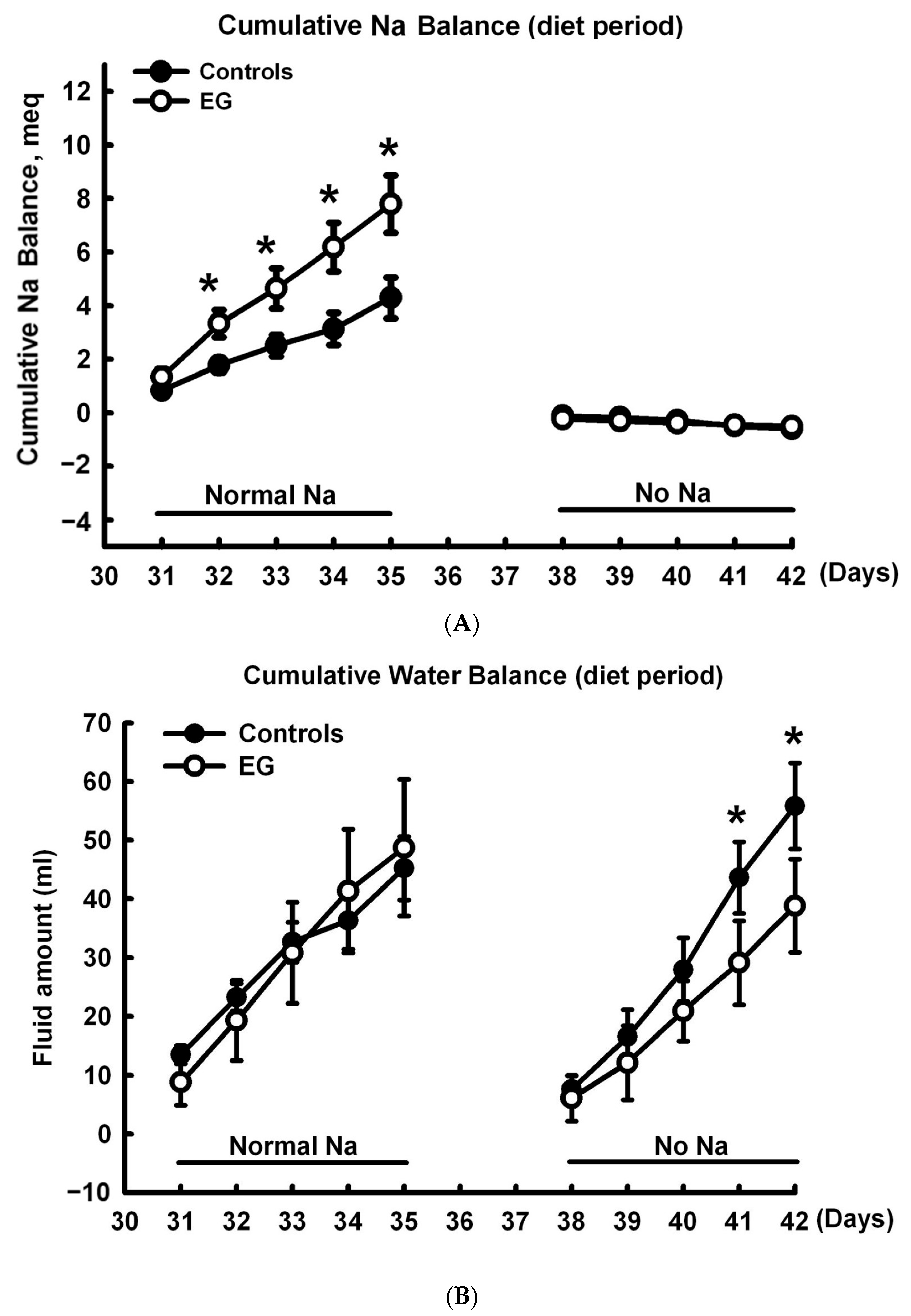
2.1. Short-Term Sodium and Water Balance Study
2.2. Effect of Long-Term SD Diet on Renal Function
2.3. Effect of Long-Term SD Diet on CaOx Stone Formation
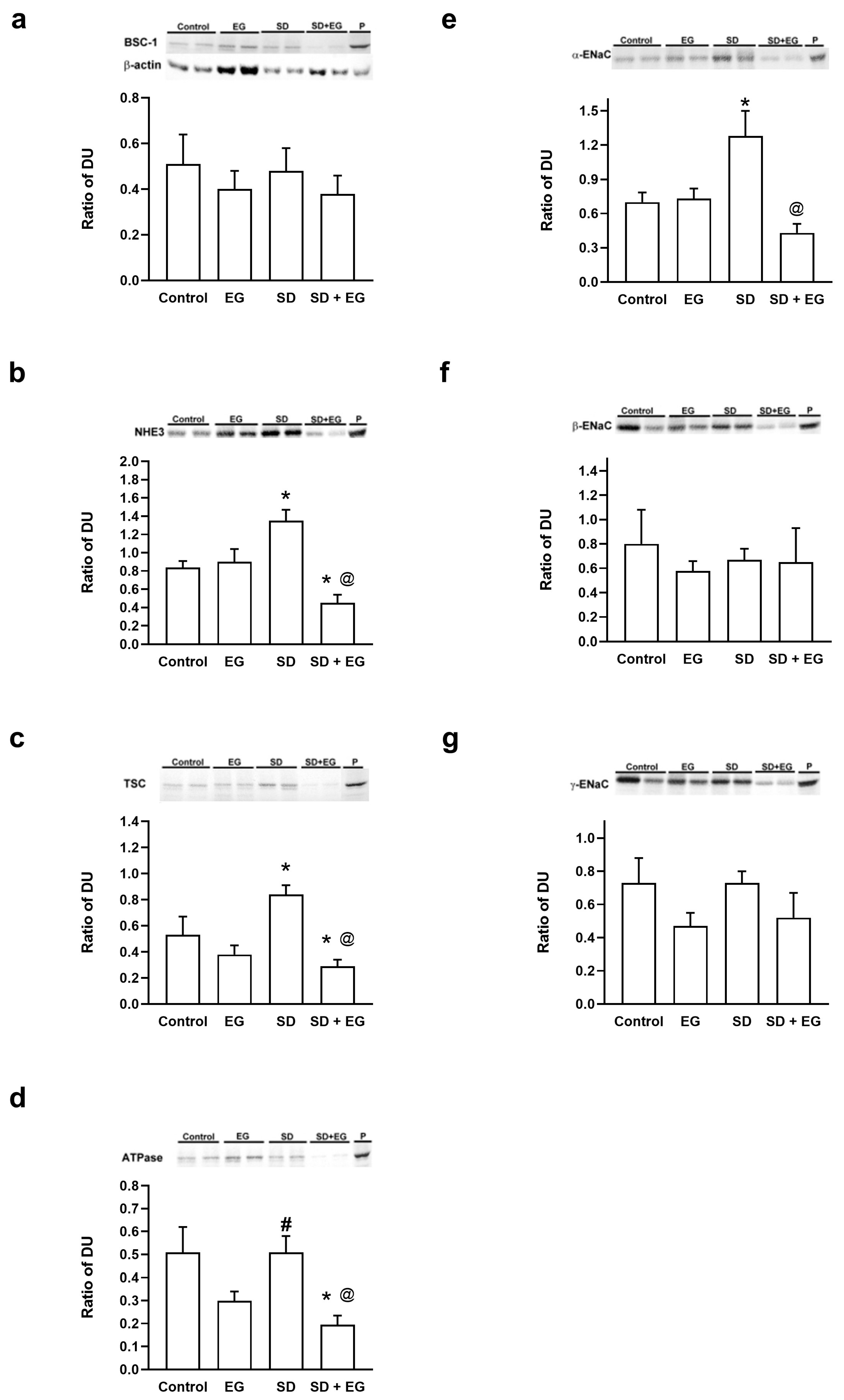
2.4. Changes in the Abundance of Major Renal Sodium Transporters
2.5. Changes in AQP Abundance
3. Discussion
4. Materials and Methods
Animals
- Urine and Plasma Chemistries
- Short-term Sodium Balance Study
- Effect of long-term SD diet feeding on CaOx crystal deposition
- Western blot analysis
- Statistical analyses
- Ethics approval
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Kell, A.L.; Grant, D.C.; Khan, S.R. Pathogenesis of calcium oxalate urinary stone disease: Species comparison of humans, dogs, and cats. Urolithiasis 2017, 45, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Littlejohns, T.J.; Neal, N.L.; Bradbury, K.E.; Heers, H.; Allen, N.E.; Turney, B.W. Fluid intake and dietary factors and the risk of incident kidney stones in UK Biobank: A population-based prospective cohort study. Eur. Urol. Focus 2020, 6, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Borghi, L.; Schianchi, T.; Meschi, T.; Guerra, A.; Allegri, F.; Maggiore, U.; Novarini, A. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N. Engl. J. Med. 2002, 346, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Hesse, A. Urinary Stones: Diagnosis, Treatment, and Prevention of Recurrence; Karger Medical and Scientific Publishers: Basel, Switzerland, 2009. [Google Scholar]
- Alexander, R.; Fuster, D.G.; Dimke, H. Mechanisms underlying calcium nephrolithiasis. Annu. Rev. Physiol. 2022, 84, 559–583. [Google Scholar] [CrossRef] [PubMed]
- Dahl, N.K.; Goldfarb, D.S. Nutritional prevention and treatment of urinary tract stones. In Nutritional Management of Renal Disease; Elsevier: Amsterdam, The Netherlands, 2022; pp. 685–697. [Google Scholar]
- Nouvenne, A.; Meschi, T.; Prati, B.; Guerra, A.; Allegri, F.; Vezzoli, G.; Soldati, L.; Gambaro, G.; Maggiore, U.; Borghi, L. Effects of a low-salt diet on idiopathic hypercalciuria in calcium-oxalate stone formers: A 3-mo randomized controlled trial. Am. J. Clin. Nutr. 2010, 91, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Himmerkus, N.; Plain, A.; Marques, R.D.; Sonntag, S.R.; Paliege, A.; Leipziger, J.; Bleich, M. AVP dynamically increases paracellular Na+ permeability and transcellular NaCl transport in the medullary thick ascending limb of Henle’s loop. Pflüg. Arch.-Eur. J. Physiol. 2017, 469, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Castrop, H.; Schnermann, J. Isoforms of renal Na-K-2Cl cotransporter NKCC2: Expression and functional significance. Am. J. Physiol.-Ren. Physiol. 2008, 295, F859–F866. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dong, C.; Xi, Y.-G.; Su, X. Thiazide-sensitive Na+–Cl− cotransporter: Genetic polymorphisms and human diseases. Acta Biochim. Biophys. Sin. 2015, 47, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Loffing, J.; Korbmacher, C. Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC). Pflüg. Arch. Eur. J. Physiol. 2009, 458, 111–135. [Google Scholar] [CrossRef]
- Hu, M.C.; Bobulescu, I.A.; Quinones, H.; Gisler, S.M.; Moe, O.W. Dopamine reduces cell surface Na+/H+ exchanger-3 protein by decreasing NHE3 exocytosis and cell membrane recycling. Am. J. Physiol. Ren. Physiol. 2017, 313, F1018–F1025. [Google Scholar] [CrossRef]
- Kitchen, P.; Salman, M.M.; Pickel, S.U.; Jennings, J.; Törnroth-Horsefield, S.; Conner, M.T.; Bill, R.M.; Conner, A.C. Water channel pore size determines exclusion properties but not solute selectivity. Sci. Rep. 2019, 9, 20369. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, B. Aquaporins in renal diseases. Int. J. Mol. Sci. 2019, 20, 366. [Google Scholar] [CrossRef] [PubMed]
- Urabe, Y.; Tanikawa, C.; Takahashi, A.; Okada, Y.; Morizono, T.; Tsunoda, T.; Kamatani, N.; Kohri, K.; Chayama, K.; Kubo, M.; et al. A genome-wide association study of nephrolithiasis in the Japanese population identifies novel susceptible Loci at 5q35.3, 7p14.3, and 13q14.1. PLoS Genet. 2012, 8, e1002541. [Google Scholar] [CrossRef] [PubMed]
- Renkema, K.Y.; Bindels, R.J.; Hoenderop, J.G. Role of the calcium-sensing receptor in reducing the risk for calcium stones. Clin. J. Am. Soc. Nephrol. 2011, 6, 2076–2082. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Glenton, P.; Byer, K. Modeling of hyperoxaluric calcium oxalate nephrolithiasis: Experimental induction of hyperoxaluria by hydroxy-L-proline. Kidney Int. 2006, 70, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Green, M.L.; Hatch, M.; Freel, R.W. Ethylene glycol induces hyperoxaluria without metabolic acidosis in rats. Am. J. Physiol.-Ren. Physiol. 2005, 289, F536–F543. [Google Scholar] [CrossRef] [PubMed]
- DiBona, G.F.; Sawin, L.L. Effect of arterial baroreceptor denervation on sodium balance. Hypertension 2002, 40, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Guron, G.; Nilsson, A.; DiBona, G.F.; Sundelin, B.; Nitescu, N.; Friberg, P. Renal adaptation to dietary sodium restriction and loading in rats treated neonatally with enalapril. Am. J. Physiol. 1997, 273, R1421–R1429. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; McKanna, J.A.; Akai, Y.; Jacobson, H.R.; Dubois, R.N.; Breyer, M.D. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J. Clin. Investig. 1994, 94, 2504–2510. [Google Scholar] [CrossRef]
- Huang, H.S.; Ma, M.C.; Chen, J.; Chen, C.F. Changes in the oxidant-antioxidant balance in the kidney of rats with nephrolithiasis induced by ethylene glycol. J. Urol. 2002, 167, 2584–2593. [Google Scholar] [CrossRef]
- Shao, W.; Seth, D.M.; Prieto, M.C.; Kobori, H.; Navar, L.G. Activation of the renin-angiotensin system by a low-salt diet does not augment intratubular angiotensinogen and angiotensin II in rats. Am. J. Physiol. Ren. Physiol. 2013, 304, F505–F514. [Google Scholar] [CrossRef]
- Seeger, H.; Kaelin, A.; Ferraro, P.M.; Weber, D.; Jaeger, P.; Ambuehl, P.; Robertson, W.G.; Unwin, R.; Wagner, C.A.; Mohebbi, N. Changes in urinary risk profile after short-term low sodium and low calcium diet in recurrent Swiss kidney stone formers. BMC Nephrol. 2017, 18, 349. [Google Scholar] [CrossRef] [PubMed]
- Masilamani, S.; Wang, X.; Kim, G.H.; Brooks, H.; Nielsen, J.; Nielsen, S.; Nakamura, K.; Stokes, J.B.; Knepper, M.A. Time course of renal Na-K-ATPase, NHE3, NKCC2, NCC, and ENaC abundance changes with dietary NaCl restriction. Am. J. Physiol. Ren. Physiol. 2002, 283, F648–F657. [Google Scholar] [CrossRef] [PubMed]
- Nwia, S.M.; Li, X.C.; Leite, A.P.O.; Hassan, R.; Zhuo, J.L. The Na+/H+ Exchanger 3 in the Intestines and the Proximal Tubule of the Kidney: Localization, Physiological Function, and Key Roles in Angiotensin II-Induced Hypertension. Front. Physiol. 2022, 13, 861659. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Cho, S.H.; Oh, B.S.; Yeum, C.H.; Choi, K.C.; Ahn, K.Y.; Lee, J. Diminished renal expression of aquaporin water channels in rats with experimental bilateral ureteral obstruction. J. Am. Soc. Nephrol. 2001, 12, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Fenton, R.A.; Poulsen, S.B.; de la Mora Chavez, S.; Soleimani, M.; Dominguez Rieg, J.A.; Rieg, T. Renal tubular NHE3 is required in the maintenance of water and sodium chloride homeostasis. Kidney Int. 2017, 92, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Fenton, R.A.; Poulsen, S.B.; de la Mora Chavez, S.; Soleimani, M.; Busslinger, M.; Dominguez Rieg, J.A.; Rieg, T. Caffeine-induced diuresis and natriuresis is independent of renal tubular NHE3. Am. J. Physiol. Ren. Physiol. 2015, 308, F1409–F1420. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Soleimani, M.; Zhu, D.; Rubera, I.; Tauc, M.; Zheng, X.; Zhang, J.; Chen, X.; Zhuo, J.L. Proximal Tubule-Specific Deletion of the NHE3 (Na+/H+ Exchanger 3) Promotes the Pressure-Natriuresis Response and Lowers Blood Pressure in Mice. Hypertension 2018, 72, 1328–1336. [Google Scholar] [CrossRef]
- Li, X.C.; Leite, A.P.O.; Zheng, X.; Zhao, C.; Chen, X.; Zhang, L.; Zhou, X.; Rubera, I.; Tauc, M.; Zhuo, J.L. Proximal Tubule-Specific Deletion of Angiotensin II Type 1a Receptors in the Kidney Attenuates Circulating and Intratubular Angiotensin II-Induced Hypertension in PT-Agtr1a−/− Mice. Hypertension 2021, 77, 1285–1298. [Google Scholar] [CrossRef]
- Afsar, B.; Kiremit, M.C.; Sag, A.A.; Tarim, K.; Acar, O.; Esen, T.; Solak, Y.; Covic, A.; Kanbay, M. The role of sodium intake in nephrolithiasis: Epidemiology, pathogenesis, and future directions. Eur. J. Intern. Med. 2016, 35, 16–19. [Google Scholar] [CrossRef]
- Huang, H.-S.; Ma, M.-C. High sodium-induced oxidative stress and poor anticrystallization defense aggravate calcium oxalate crystal formation in rat hyperoxaluric kidneys. PLoS ONE 2015, 10, e0134764. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-S.; Chen, J.; Chen, C.-F.; Ma, M.-C. Vitamin E attenuates crystal formation in rat kidneys: Roles of renal tubular cell death and crystallization inhibitors. Kidney Int. 2006, 70, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Kong, Z.; Mai, X.; Lan, Y.; Liu, Y.; Yang, Z.; Zhao, Z.; Deng, T.; Zeng, T.; Cai, C. Autophagy inhibition attenuates hyperoxaluria-induced renal tubular oxidative injury and calcium oxalate crystal depositions in the rat kidney. Redox Biol. 2018, 16, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Fong-Ngern, K.; Peerapen, P.; Sinchaikul, S.; Chen, S.-T.; Thongboonkerd, V. Large-scale identification of calcium oxalate monohydrate crystal-binding proteins on apical membrane of distal renal tubular epithelial cells. J. Proteome Res. 2011, 10, 4463–4477. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.S.; Chen, C.F.; Chien, C.T.; Chen, J. Possible biphasic changes of free radicals in ethylene glycol-induced nephrolithiasis in rats. BJU Int. 2000, 85, 1143–1149. [Google Scholar] [CrossRef]
- Tiselius, H.-G.; Ribeiro Nogueira Ferraz, R.; Pfeferman Heilberg, I. An approximate estimate of the ion-activity product of calcium oxalate in rat urine. Urol. Res. 2003, 31, 410–413. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Feng, D.; Fu, Y.; Wu, H.; Lu, J.; Bao, J. Calcium-sensing receptor promotes calcium oxalate crystal adhesion and renal injury in Wistar rats by promoting ROS production and subsequent regulation of PS ectropion, OPN, KIM-1, and ERK expression. Ren. Fail. 2021, 43, 465–476. [Google Scholar] [CrossRef]


| Groups | Control | EG | SD | SD + EG | |
|---|---|---|---|---|---|
| Parameters | |||||
| Water intake, mL/day | 25.6 ± 4.6 | 31.8 ± 3.3 | 29.6 ± 2.4 | 45.0 ± 4.9 * | |
| Urine amount, mL/day | 19.9 ± 1.5 | 27.1 ± 1.8 * | 16.2 ± 1.8 | 39.7 ± 1.4 *@ | |
| Urine protein, mg/day | 24.1 ± 4.2 | 55.1 ± 9.5 * | 21.3 ± 4.0 | 66.4 ± 9.4 *@ | |
| CCr, mL/min/kg | 2.7 ± 0.9 | 1.7 ± 0.3 | 3.8 ± 0.7 | 1.4 ± 0.4 @ | |
| Posm, mosmol/kg/H2O | 297.4 ± 4.6 | 337.0 ± 5.5 * | 292.3 ± 4.7 | 333.2 ± 6.0 *@ | |
| PNa, mmol/L | 150.3 ± 2.6 | 156.5 ± 1.6 | 147.4 ± 3.9 | 159.3 ± 1.7 | |
| PCr, mg/dL | 0.31 ± 0.01 | 0.57 ± 0.05 * | 0.31 ± 0.01 | 0.82 ± 0.15 * | |
| PUrea, mg/dL | 16.7 ± 2.9 | 40.0 ± 2.2 * | 19.4 ± 1.5 | 54.3 ± 6.6 *# | |
| Uosm, mosmol/kg/H2O | 1026.8 ± 188.0 | 626.8 ± 20.4 *@ | 1380.9 ± 132.5 *# | 702.8 ± 29.1 *@ | |
| U/Posm ratio | 2.9 ± 0.1 | 1.9 ± 0.0 * | 4.7 ± 0.4 * | 2.1 ± 0.1 *@ | |
| FENa (%) | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.1 ± 0.0 *# | 0.3 ± 0.1 *#@ | |
| UNa × Uv, μmol/min/kg | 1.0 ± 0.1 | 1.1 ± 0.2 | 0.3 ± 0.1 *# | 0.5 ± 0.0 *#@ | |
| TcH2O, μL/min/kg | 78.6 ± 10.4 | 52.1 ± 3.9 * | 108.3 ± 19.4 # | 77.6 ± 8.8 # | |
| Groups | Control | EG | SD | SD + EG | |
|---|---|---|---|---|---|
| Parameters | |||||
| BW, gm | 380.4 ± 4.2 | 397.2 ± 4.4 | 393.1 ± 2.5 | 396.7 ± 3.6 | |
| ΔBW, gm | +5.1 ± 1.0 | +7.2 ± 1.7 | +8.0 ± 2.8 | +5.0 ± 0.5 | |
| Food intake, g/day | 26.3 ± 3.5 | 18.5 ± 2.9 | 27.7 ± 3.4 | 16.2 ± 2.6 * | |
| Kidney weight, gm | 1.8 ± 0.1 | 2.6 ± 0.1 * | 1.8 ± 0.1 | 2.7 ± 0.1 * | |
| AP (CaOx)index | 4.8 ± 0.9 | 21.2 ± 6.1 * | 4.5 ± 1.0 | 24.9 ± 7.3 * | |
| Urine Ca/Cr | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.0 * | 0.8 ± 0.2 *#@ | |
| Urine oxalate, mg | 6.8 ± 2.2 | 68.3 ± 12.2 * | 4.4 ± 1.6 | 75.6 ± 23.3 * | |
| GAL, mU/mg | 3.4 ± 0.9 | 8.5 ± 2.1 * | 2.3 ± 0.2 | 38.9 ± 16.1 *# | |
| NAG, mU/mg | 4.0 ± 0.6 | 20.3 ± 5.8 * | 3.3 ± 0.2 | 43.9 ± 12.8 *# | |
| Urine MDA, μmole/g | 39.0 ± 8.3 | 402.8 ± 157.9 * | 53.0 ± 4.9 | 514.0 ± 208.5 * | |
| Urine TBARS, μmole/g | 156.6 ± 28.5 | 632.9 ± 229.4 * | 199.5 ± 16.7 | 883.9 ± 277.6 * | |
| Renal calculi (score) | 0 | II–III | 0 | III | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-C.; Liu, C.-J.; Lu, Z.-H.; Huang, H.-S. Long-Term Sodium Deficiency Reduces Sodium Excretion but Impairs Renal Function and Increases Stone Formation in Hyperoxaluric Calcium Oxalate Rats. Int. J. Mol. Sci. 2024, 25, 3942. https://doi.org/10.3390/ijms25073942
Huang Y-C, Liu C-J, Lu Z-H, Huang H-S. Long-Term Sodium Deficiency Reduces Sodium Excretion but Impairs Renal Function and Increases Stone Formation in Hyperoxaluric Calcium Oxalate Rats. International Journal of Molecular Sciences. 2024; 25(7):3942. https://doi.org/10.3390/ijms25073942
Chicago/Turabian StyleHuang, Yuan-Chi, Chan-Jung Liu, Ze-Hong Lu, and Ho-Shiang Huang. 2024. "Long-Term Sodium Deficiency Reduces Sodium Excretion but Impairs Renal Function and Increases Stone Formation in Hyperoxaluric Calcium Oxalate Rats" International Journal of Molecular Sciences 25, no. 7: 3942. https://doi.org/10.3390/ijms25073942





