Lipoxygenases at the Intersection of Infection and Carcinogenesis
Abstract
1. Introduction
2. Overview of Molecular Evolution of Lipoxygenases
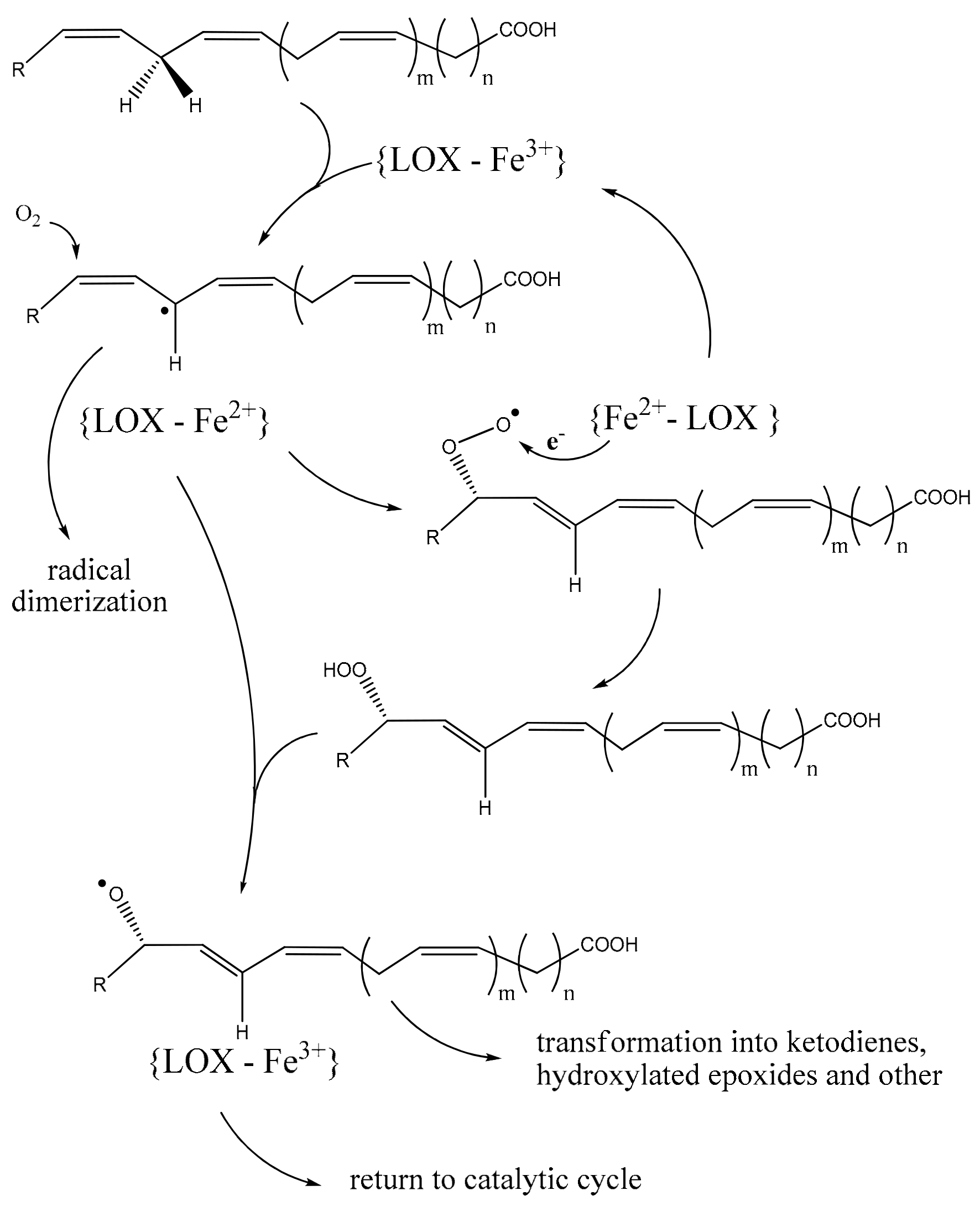
2.1. Structure-Functional Basis of PUFA Lipoxygenation
2.2. Plants and Fungi
2.3. Prokaryotes
2.4. Lipoxygenase (LoxA) in Pseudomonas aeruginosa
2.5. Bacterial Lipoxygenases, Cystic Fibrosis and Malignant Conditions
3. Inventory of Human and Mouse Lipoxygenase Isoforms
3.1. ALOX12
3.2. ALOX12B and ALOXE3
3.3. ALOX15
3.4. ALOX15B
3.5. ALOX12E
3.6. ALOX5 and ALOX5AP
3.7. ALOXes and Esterified PUFAs
4. Normal Function of Lipoxygenation in Human Metabolism and Immunity and Their Weaknesses
5. Pre-Malignant Conditions and Benign Outgrowth—Roles of Lipoxygenation
6. Lipoxygenases in Primary Carcinogenesis—Putative Intersections with Infections
- Catalyzing the activation of pro-carcinogens like aflatoxins.
- Disruption of interferon signaling, which ordinarily promotes the identification and elimination of cells with DNA damage, thereby pre-emptively targeting potential early-stage cancerous cells.
- Enhancing the invasiveness of existing cancerous cells by helping them evade immune surveillance.
- Boosting the resistance of cancer cells to specific anti-cancer drugs.
7. The Oncogenic Role of Human ALOX
8. The Anti-Tumorigenic Function of ALOXs
8.1. Hypoxia, Neoangiogenesis
8.2. P53, the ALOX Gene Family and Micro-RNAs
8.3. Lipoxygenases and Immune Suppression in Tumor Growth
8.4. Human ALOXes and Tumor-Associated Inflammation
- 15-HETE enhances T cell proliferation, particularly promoting Th1 cell activity.
- 12-HETE facilitates chemotaxis, although it is more specifically a chemoattractant for neutrophils, and the receptors for this activity are not yet identified.
- Lipoxin A4 (LXA4), through the BLT1 receptor, stimulates the production of cytokines.
- Leukotriene B4 (LTB4), also acting through the BLT1 receptor, encourages T cell homing.
- Cysteinyl leukotrienes D4 (CysLTD4) and E4 (CysLTE4), interacting with the CysLT1 receptor, are involved in inducing Th2 cell differentiation [157].
- Metabolites of ALOX5 and ALOX12 are elevated in human colon adenoma tissue and serve as therapeutic targets for colorectal cancer chemoprevention [220].
9. Inhibitors of ALOX in Anti-Cancer Therapies
10. Control of Lipoxygenation in Cancer Prevention and Treatment
11. Perspectives of ALOX15 Transgene, a Oncotherapeutic Booster
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Starkey, M.; Hickman, J.H.; Ma, L.; Zhang, N.; De Long, S.; Hinz, A.; Palacios, S.; Manoil, C.; Kirisits, M.J.; Starner, T.D.; et al. Pseudomonas aeruginosa Rugose Small-Colony Variants Have Adaptations That Likely Promote Persistence in the Cystic Fibrosis Lung. J. Bacteriol. 2009, 191, 3492–3503. [Google Scholar] [CrossRef]
- Dar, H.H.; Tyurina, Y.Y.; Mikulska-Ruminska, K.; Shrivastava, I.; Ting, H.-C.; Tyurin, V.A.; Krieger, J.; St Croix, C.M.; Watkins, S.; Bayir, E.; et al. Pseudomonas aeruginosa Utilizes Host Polyunsaturated Phosphatidylethanolamines to Trigger Theft-Ferroptosis in Bronchial Epithelium. J. Clin. Investig. 2018, 128, 4639–4653. [Google Scholar] [CrossRef] [PubMed]
- Aldrovandi, M.; Banthiya, S.; Meckelmann, S.; Zhou, Y.; Heydeck, D.; O’Donnell, V.B.; Kuhn, H. Specific Oxygenation of Plasma Membrane Phospholipids by Pseudomonas aeruginosa Lipoxygenase Induces Structural and Functional Alterations in Mammalian Cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Banthiya, S.; Pekárová, M.; Kuhn, H.; Heydeck, D. Secreted Lipoxygenase from Pseudomonas aeruginosa Exhibits Biomembrane Oxygenase Activity and Induces Hemolysis in Human Red Blood Cells. Arch. Biochem. Biophys. 2015, 584, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Norris, P.C. Eicosanoid Storm in Infection and Inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Watanabe, S.; Li, D.; Nakamura, T.; Juneja, L.R.; Takahashi, T.; Wichansawakun, S.; Wilczynska, A.; Jantan, I.; Sulaeman, A.; et al. Chapter 11—Effects of Polyunsaturated Fatty Acid–Rich Diets and Risk of Non-Communicable Diseases. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Singh, R.B., Watanabe, S., Isaza, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 165–185. ISBN 978-0-12-819815-5. [Google Scholar]
- Pohl, C.H.; Kock, J.L.F. Oxidized Fatty Acids as Inter-Kingdom Signaling Molecules. Molecules 2014, 19, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Haeggström, J.Z.; Funk, C.D. Lipoxygenase and Leukotriene Pathways: Biochemistry, Biology, and Roles in Disease. Chem. Rev. 2011, 111, 5866–5898. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.; Heydeck, D.; Hofheinz, K.; Roffeis, J.; O’Donnell, V.B.; Kuhn, H.; Walther, M. Molecular Enzymology of Lipoxygenases. Arch. Biochem. Biophys. 2010, 503, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arif, Y.; Miszczuk, E.; Bajguz, A.; Hayat, S. Specific Roles of Lipoxygenases in Development and Responses to Stress in Plants. Plants 2022, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, K.; Varakumar, P.; Reddy, R.; Basha, S.; Mehta, S.; Ampasala, A. Plant Lipoxygenases and Their Role in Plant Physiology. J. Plant Biol. 2020, 63, 83–95. [Google Scholar] [CrossRef]
- Mei, G.; Di Venere, A.; Nicolai, E.; Angelucci, C.B.; Ivanov, I.; Sabatucci, A.; Dainese, E.; Kuhn, H.; Maccarrone, M. Structural Properties of Plant and Mammalian Lipoxygenases. Temperature-Dependent Conformational Alterations and Membrane Binding Ability. Biochemistry 2008, 47, 9234–9242. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.; Banthiya, S.; van Leyen, K. Mammalian Lipoxygenases and Their Biological Relevance. Biochim. Biophys. Acta 2015, 1851, 308–330. [Google Scholar] [CrossRef]
- Garreta, A.; Val-Moraes, S.P.; García-Fernández, Q.; Busquets, M.; Juan, C.; Oliver, A.; Ortiz, A.; Gaffney, B.J.; Fita, I.; Manresa, À.; et al. Structure and Interaction with Phospholipids of a Prokaryotic Lipoxygenase from Pseudomonas aeruginosa. FASEB J. 2013, 27, 4811–4821. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A Programming Language for Software Integration and Development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Chrisnasari, R.; Hennebelle, M.; Vincken, J.-P.; van Berkel, W.J.H.; Ewing, T.A. Bacterial Lipoxygenases: Biochemical Characteristics, Molecular Structure and Potential Applications. Biotechnol. Adv. 2022, 61, 108046. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-E.; Lee, J.; An, J.-U.; Kim, T.-H.; Oh, C.-W.; Ko, Y.-J.; Krishnan, M.; Choi, J.; Yoon, D.-Y.; Kim, Y.; et al. Regioselectivity of an Arachidonate 9S-Lipoxygenase from Sphingopyxis macrogoltabida That Biosynthesizes 9S,15S- and 11S,17S-Dihydroxy Fatty Acids from C20 and C22 Polyunsaturated Fatty Acids. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2022, 1867, 159091. [Google Scholar] [CrossRef] [PubMed]
- Erba, F.; Mei, G.; Minicozzi, V.; Sabatucci, A.; Di Venere, A.; Maccarrone, M. Conformational Dynamics of Lipoxygenases and Their Interaction with Biological Membranes. Int. J. Mol. Sci. 2024, 25, 2241. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazy, H.; Abadi, S.; Martz, E.; Chay, O.; Mayrose, I.; Pupko, T.; Ben-Tal, N. ConSurf 2016: An Improved Methodology to Estimate and Visualize Evolutionary Conservation in Macromolecules. Nucleic Acids Res. 2016, 44, W344–W350. [Google Scholar] [CrossRef] [PubMed]
- Kobe, M.J.; Neau, D.B.; Mitchell, C.E.; Bartlett, S.G.; Newcomer, M.E. The Structure of Human 15-Lipoxygenase-2 with a Substrate Mimic. J. Biol. Chem. 2014, 289, 8562–8569. [Google Scholar] [CrossRef] [PubMed]
- Gillmor, S.A.; Villaseñor, A.; Fletterick, R.; Sigal, E.; Browner, M.F. The Structure of Mammalian 15-Lipoxygenase Reveals Similarity to the Lipases and the Determinants of Substrate Specificity. Nat. Struct. Biol. 1997, 4, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, N.C.; Gerstmeier, J.; Schexnaydre, E.E.; Börner, F.; Garscha, U.; Neau, D.B.; Werz, O.; Newcomer, M.E. Structural and Mechanistic Insights into 5-Lipoxygenase Inhibition by Natural Products. Nat. Chem. Biol. 2020, 16, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Banthiya, S.; Kalms, J.; Galemou Yoga, E.; Ivanov, I.; Carpena, X.; Hamberg, M.; Kuhn, H.; Scheerer, P. Structural and Functional Basis of Phospholipid Oxygenase Activity of Bacterial Lipoxygenase from Pseudomonas aeruginosa. Biochim. Biophys. Acta 2016, 1861, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.K.; West, S.I.; Hornostaj, A.R.; Lawson, D.M.; Fairhurst, S.A.; Sanchez, R.O.; Hough, P.; Robinson, B.H.; Casey, R. Probing a Novel Potato Lipoxygenase with Dual Positional Specificity Reveals Primary Determinants of Substrate Binding and Requirements for a Surface Hydrophobic Loop and Has Implications for the Role of Lipoxygenases in Tubers. Biochem. J. 2001, 353, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Vellosillo, T.; Aguilera, V.; Marcos, R.; Bartsch, M.; Vicente, J.; Cascón, T.; Hamberg, M.; Castresana, C. Defense Activated by 9-Lipoxygenase-Derived Oxylipins Requires Specific Mitochondrial Proteins. Plant Physiol. 2013, 161, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Vellosillo, T.; Martínez, M.; López, M.A.; Vicente, J.; Cascón, T.; Dolan, L.; Hamberg, M.; Castresana, C. Oxylipins Produced by the 9-Lipoxygenase Pathway in Arabidopsis Regulate Lateral Root Development and Defense Responses through a Specific Signaling Cascade. Plant Cell 2007, 19, 831–846. [Google Scholar] [CrossRef]
- Brodhun, F.; Feussner, I. Oxylipins in Fungi. FEBS J. 2011, 278, 1047–1063. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.; Saam, J.; Eibach, S.; Holzhütter, H.-G.; Ivanov, I.; Walther, M. Structural Biology of Mammalian Lipoxygenases: Enzymatic Consequences of Targeted Alterations of the Protein Structure. Biochem. Biophys. Res. Commun. 2005, 338, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Oliw, E.H. Iron and Manganese Lipoxygenases of Plant Pathogenic Fungi and Their Role in Biosynthesis of Jasmonates. Arch. Biochem. Biophys. 2022, 722, 109169. [Google Scholar] [CrossRef]
- Hashem, C.; Stolterfoht, H.; Rinnofner, C.; Steinberger, S.; Winkler, M.; Pichler, H. Secretion of Pseudomonas aeruginosa Lipoxygenase by Pichia Pastoris upon Glycerol Feed. Biotechnol. J. 2020, 15, e2000089. [Google Scholar] [CrossRef] [PubMed]
- Porta, H.; Rocha-Sosa, M. Lipoxygenase in Bacteria: A Horizontal Transfer Event? Microbiology 2001, 147, 3199–3200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hansen, J.; Garreta, A.; Benincasa, M.; Fusté, M.C.; Busquets, M.; Manresa, A. Bacterial Lipoxygenases, a New Subfamily of Enzymes? A Phylogenetic Approach. Appl. Microbiol. Biotechnol. 2013, 97, 4737–4747. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.; Feussner, I. Oxylipin Formation in Nostoc punctiforme (PCC73102). Phytochemistry 2007, 68, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Kurakin, G. Lipoxygenase in a Giant Sulfur Bacterium: An Evolutionary Solution for Size and Complexity? Biochem. Mosc. 2023, 88, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Kurakin, G.F.; Samoukina, A.M.; Potapova, N.A. Bacterial and Protozoan Lipoxygenases Could Be Involved in Cell-to-Cell Signaling and Immune Response Suppression. Biochem. Mosc. 2020, 85, 1048–1063. [Google Scholar] [CrossRef] [PubMed]
- Kurakin, G. Bacterial Lipoxygenases Are Associated with Host-Microbe Interactions and May Provide Cross-Kingdom Host Jumps. bioRxiv 2023. preprint. [Google Scholar] [CrossRef]
- Inglis, T.J.J.; Aravena-Roman, M.; Ching, S.; Croft, K.; Wuthiekanun, V.; Mee, B.J. Cellular Fatty Acid Profile Distinguishes Burkholderia pseudomallei from Avirulent Burkholderia thailandensis. J. Clin. Microbiol. 2003, 41, 4812–4814. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.-C.; Oh, D.-K. Lipoxygenases: Potential Starting Biocatalysts for the Synthesis of Signaling Compounds. Biotechnol. Adv. 2012, 30, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- An, J.-U.; Kim, B.-J.; Hong, S.-H.; Oh, D.-K. Characterization of an Omega-6 Linoleate Lipoxygenase from Burkholderia thailandensis and Its Application in the Production of 13-Hydroxyoctadecadienoic Acid. Appl. Microbiol. Biotechnol. 2015, 99, 5487–5497. [Google Scholar] [CrossRef]
- Neidig, M.L.; Wecksler, A.T.; Schenk, G.; Holman, T.R.; Solomon, E.I. Kinetic and Spectroscopic Studies of N694C Lipoxygenase: A Probe of the Substrate Activation Mechanism of a Nonheme Ferric Enzyme. J. Am. Chem. Soc. 2007, 129, 7531–7537. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, J.D.; Ogunsola, A.F.; Jameson, J.B.; Yasgar, A.; Flitter, B.A.; Freedman, C.J.; Melvin, J.A.; Nguyen, J.V.M.H.; Maloney, D.J.; Jadhav, A.; et al. Biochemical and Cellular Characterization and Inhibitor Discovery of Pseudomonas aeruginosa 15-Lipoxygenase. Biochemistry 2016, 55, 3329–3340. [Google Scholar] [CrossRef] [PubMed]
- Kühn, H.; Barnett, J.; Grunberger, D.; Baecker, P.; Chow, J.; Nguyen, B.; Bursztyn-Pettegrew, H.; Chan, H.; Sigal, E. Overexpression, Purification and Characterization of Human Recombinant 15-Lipoxygenase. Biochim. Biophys. Acta 1993, 1169, 80–89. [Google Scholar] [CrossRef]
- Wecksler, A.T.; Kenyon, V.; Deschamps, J.D.; Holman, T.R. Substrate Specificity Changes for Human Reticulocyte and Epithelial 15-Lipoxygenases Reveal Allosteric Product Regulation. Biochemistry 2008, 47, 7364–7375. [Google Scholar] [CrossRef] [PubMed]
- Wecksler, A.T.; Jacquot, C.; van der Donk, W.A.; Holman, T.R. Mechanistic Investigations of Human Reticulocyte 15- and Platelet 12-Lipoxygenases with Arachidonic Acid. Biochemistry 2009, 48, 6259–6267. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.F.; Browner, M.F.; Sloane, D.L.; Sigal, E. Defining the Arachidonic Acid Binding Site of Human 15-Lipoxygenase. Molecular Modeling and Mutagenesis. J. Biol. Chem. 1996, 271, 25412–25418. [Google Scholar] [CrossRef] [PubMed]
- Bender, G.; Schexnaydre, E.E.; Murphy, R.C.; Uhlson, C.; Newcomer, M.E. Membrane-Dependent Activities of Human 15-LOX-2 and Its Murine Counterpart: IMPLICATIONS FOR MURINE MODELS OF ATHEROSCLEROSIS. J. Biol. Chem. 2016, 291, 19413–19424. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Hoobler, E.K.; Perry, S.; Diaz, G.; Fox, B.; Holman, T.R. Kinetic and Structural Investigations into the Allosteric and pH Effect on the Substrate Specificity of Human Epithelial 15-Lipoxygenase-2. Biochemistry 2013, 52, 8026–8035. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, X.; Liu, S.; Feng, Y.; Rao, S.; Zhou, X.; Wang, M.; Du, G.; Chen, J. Enhanced Thermal Stability of Pseudomonas aeruginosa Lipoxygenase through Modification of Two Highly Flexible Regions. Appl. Microbiol. Biotechnol. 2014, 98, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, J.; Liu, S.; Zhang, D.; Xu, Z.; Wu, J.; Li, J.; Du, G.; Chen, J. Overproduction, Purification, and Characterization of Extracellular Lipoxygenase of Pseudomonas aeruginosa in Escherichia Coli. Appl. Microbiol. Biotechnol. 2013, 97, 5793–5800. [Google Scholar] [CrossRef] [PubMed]
- Smyrniotis, C.J.; Barbour, S.R.; Xia, Z.; Hixon, M.S.; Holman, T.R. ATP Allosterically Activates the Human 5-Lipoxygenase Molecular Mechanism of Arachidonic Acid and 5(S)-Hydroperoxy-6(E),8(Z),11(Z),14(Z)-Eicosatetraenoic Acid. Biochemistry 2014, 53, 4407–4419. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Kumar, R.B.; Balagunaseelan, N.; Hamberg, M.; Jegerschöld, C.; Rådmark, O.; Haeggström, J.Z.; Rinaldo-Matthis, A. Kinetic Investigation of Human 5-Lipoxygenase with Arachidonic Acid. Bioorg Med. Chem. Lett. 2016, 26, 3547–3551. [Google Scholar] [CrossRef] [PubMed]
- Ochi, K.; Yoshimoto, T.; Yamamoto, S.; Taniguchi, K.; Miyamoto, T. Arachidonate 5-Lipoxygenase of Guinea Pig Peritoneal Polymorphonuclear Leukocytes. Activation by Adenosine 5′-Triphosphate. J. Biol. Chem. 1983, 258, 5754–5758. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N.; Kaneko, S.; Yoshimoto, T.; Yamamoto, S. Purification of Arachidonate 5-Lipoxygenase from Porcine Leukocytes and Its Reactivity with Hydroperoxyeicosatetraenoic Acids. J. Biol. Chem. 1986, 261, 7982–7988. [Google Scholar] [CrossRef] [PubMed]
- Vance, R.E.; Hong, S.; Gronert, K.; Serhan, C.N.; Mekalanos, J.J. The Opportunistic Pathogen Pseudomonas aeruginosa Carries a Secretable Arachidonate 15-Lipoxygenase. Proc. Natl. Acad. Sci. USA 2004, 101, 2135–2139. [Google Scholar] [CrossRef]
- Williams, B.J.; Dehnbostel, J.; Blackwell, T.S. Pseudomonas aeruginosa: Host Defence in Lung Diseases. Respirology 2010, 15, 1037–1056. [Google Scholar] [CrossRef]
- Hauser, A.R. The Type III Secretion System of Pseudomonas aeruginosa: Infection by Injection. Nat. Rev. Microbiol. 2009, 7, 654–665. [Google Scholar] [CrossRef]
- Morello, E.; Pérez-Berezo, T.; Boisseau, C.; Baranek, T.; Guillon, A.; Bréa, D.; Lanotte, P.; Carpena, X.; Pietrancosta, N.; Hervé, V.; et al. Pseudomonas aeruginosa Lipoxygenase LoxA Contributes to Lung Infection by Altering the Host Immune Lipid Signaling. Front. Microbiol. 2019, 10, 1826. [Google Scholar] [CrossRef] [PubMed]
- Flitter, B.A.; Hvorecny, K.L.; Ono, E.; Eddens, T.; Yang, J.; Kwak, D.H.; Bahl, C.D.; Hampton, T.H.; Morisseau, C.; Hammock, B.D.; et al. Pseudomonas aeruginosa Sabotages the Generation of Host Proresolving Lipid Mediators. Proc. Natl. Acad. Sci. USA 2017, 114, 136–141. [Google Scholar] [CrossRef]
- Bleves, S.; Viarre, V.; Salacha, R.; Michel, G.P.F.; Filloux, A.; Voulhoux, R. Protein Secretion Systems in Pseudomonas aeruginosa: A Wealth of Pathogenic Weapons. Int. J. Med. Microbiol. 2010, 300, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Tamang, D.L.; Pirzai, W.; Priebe, G.P.; Traficante, D.C.; Pier, G.B.; Falck, J.R.; Morisseau, C.; Hammock, B.D.; McCormick, B.A.; Gronert, K.; et al. Hepoxilin A(3) Facilitates Neutrophilic Breach of Lipoxygenase-Expressing Airway Epithelial Barriers. J. Immunol. 2012, 189, 4960–4969. [Google Scholar] [CrossRef] [PubMed]
- Rafeeq, M.M.; Murad, H.A.S. Cystic Fibrosis: Current Therapeutic Targets and Future Approaches. J. Transl. Med. 2017, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Farinha, C.M.; Callebaut, I. Molecular Mechanisms of Cystic Fibrosis—How Mutations Lead to Misfunction and Guide Therapy. Biosci. Rep. 2022, 42, BSR20212006. [Google Scholar] [CrossRef] [PubMed]
- Farinha, C.M.; Canato, S. From the Endoplasmic Reticulum to the Plasma Membrane: Mechanisms of CFTR Folding and Trafficking. Cell Mol. Life Sci. 2017, 74, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, S.S.; Silva, I.A.L.; Amaral, M.D.; Farinha, C.M. Rare Trafficking CFTR Mutations Involve Distinct Cellular Retention Machineries and Require Different Rescuing Strategies. Int. J. Mol. Sci. 2021, 23, 24. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Z.; Csanády, L.; Gadsby, D.C.; Chen, J. Molecular Structure of the Human CFTR Ion Channel. Cell 2017, 169, 85–95.e8. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Lipoxins and Aspirin-Triggered 15-Epi-Lipoxin Biosynthesis: An Update and Role in Anti-Inflammation and pro-Resolution. Prostaglandins Other Lipid Mediat. 2002, 68–69, 433–455. [Google Scholar] [CrossRef] [PubMed]
- Bannenberg, G.L.; Aliberti, J.; Hong, S.; Sher, A.; Serhan, C. Exogenous Pathogen and Plant 15-Lipoxygenase Initiate Endogenous Lipoxin A4 Biosynthesis. J. Exp. Med. 2004, 199, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Hvorecny, K.L.; Dolben, E.; Moreau-Marquis, S.; Hampton, T.H.; Shabaneh, T.B.; Flitter, B.A.; Bahl, C.D.; Bomberger, J.M.; Levy, B.D.; Stanton, B.A.; et al. An Epoxide Hydrolase Secreted by Pseudomonas aeruginosa Decreases Mucociliary Transport and Hinders Bacterial Clearance from the Lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L150–L156. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.L. Polyunsaturated Fatty Acids: Conversion to Lipid Mediators, Roles in Inflammatory Diseases and Dietary Sources. Int. J. Mol. Sci. 2023, 24, 8838. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D.; Chen, X.-S.; Johnson, E.N.; Zhao, L. Lipoxygenase Genes and Their Targeted Disruption. Prostaglandins Other Lipid Mediat. 2002, 68–69, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Shornick, L.P.; Shannon, V.R.; Wilson, J.D.; Funk, C.D.; Pentland, A.P.; Holtzman, M.J. Epidermis Contains Platelet-Type 12-Lipoxygenase That Is Overexpressed in Germinal Layer Keratinocytes in Psoriasis. Am. J. Physiol. 1994, 266, C243–C253. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.N.; Brass, L.F.; Funk, C.D. Increased Platelet Sensitivity to ADP in Mice Lacking Platelet-Type 12-Lipoxygenase. Proc. Natl. Acad. Sci. USA 1998, 95, 3100–3105. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.N.; Nanney, L.B.; Virmani, J.; Lawson, J.A.; Funk, C.D. Basal Transepidermal Water Loss Is Increased in Platelet-Type 12-Lipoxygenase Deficient Mice. J. Investig. Dermatol. 1999, 112, 861–865. [Google Scholar] [CrossRef]
- Boeglin, W.E.; Kim, R.B.; Brash, A.R. A 12R-Lipoxygenase in Human Skin: Mechanistic Evidence, Molecular Cloning, and Expression. Proc. Natl. Acad. Sci. USA 1998, 95, 6744–6749. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; McDonnell, M.; Chen, X.S.; Lakkis, M.M.; Li, H.; Isaacs, S.N.; Elsea, S.H.; Patel, P.I.; Funk, C.D. Human 12(R)-Lipoxygenase and the Mouse Ortholog. Molecular Cloning, Expression, and Gene Chromosomal Assignment. J. Biol. Chem. 1998, 273, 33540–33547. [Google Scholar] [CrossRef] [PubMed]
- Coffa, G.; Brash, A.R. A Single Active Site Residue Directs Oxygenation Stereospecificity in Lipoxygenases: Stereocontrol Is Linked to the Position of Oxygenation. Proc. Natl. Acad. Sci. USA 2004, 101, 15579–15584. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yin, H.; Boeglin, W.E.; Elias, P.M.; Crumrine, D.; Beier, D.R.; Brash, A.R. Lipoxygenases Mediate the Effect of Essential Fatty Acid in Skin Barrier Formation: A Proposed Role in Releasing Omega-Hydroxyceramide for Construction of the Corneocyte Lipid Envelope. J. Biol. Chem. 2011, 286, 24046–24056. [Google Scholar] [CrossRef] [PubMed]
- Kinzig, A.; Heidt, M.; Fürstenberger, G.; Marks, F.; Krieg, P. cDNA Cloning, Genomic Structure, and Chromosomal Localization of a Novel Murine Epidermis-Type Lipoxygenase. Genomics 1999, 58, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Schneider, C.; Boeglin, W.E.; Marnett, L.J.; Brash, A.R. The Lipoxygenase Gene ALOXE3 Implicated in Skin Differentiation Encodes a Hydroperoxide Isomerase. Proc. Natl. Acad. Sci. USA 2003, 100, 9162–9167. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Schneider, C.; Boeglin, W.E.; Brash, A.R. Human and Mouse eLOX3 Have Distinct Substrate Specificities: Implications for Their Linkage with Lipoxygenases in Skin. Arch. Biochem. Biophys. 2006, 455, 188–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eckl, K.-M.; de Juanes, S.; Kurtenbach, J.; Nätebus, M.; Lugassy, J.; Oji, V.; Traupe, H.; Preil, M.-L.; Martínez, F.; Smolle, J.; et al. Molecular Analysis of 250 Patients with Autosomal Recessive Congenital Ichthyosis: Evidence for Mutation Hotspots in ALOXE3 and Allelic Heterogeneity in ALOX12B. J. Investig. Dermatol. 2009, 129, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Hotz, A.; Kopp, J.; Bourrat, E.; Oji, V.; Komlosi, K.; Giehl, K.; Bouadjar, B.; Bygum, A.; Tantcheva-Poor, I.; Hellström Pigg, M.; et al. Meta-Analysis of Mutations in ALOX12B or ALOXE3 Identified in a Large Cohort of 224 Patients. Genes 2021, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Benatzy, Y.; Palmer, M.A.; Brüne, B. Arachidonate 15-Lipoxygenase Type B: Regulation, Function, and Its Role in Pathophysiology. Front. Pharmacol. 2022, 13, 1042420. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.; Humeniuk, L.; Kozlov, N.; Roigas, S.; Adel, S.; Heydeck, D. The Evolutionary Hypothesis of Reaction Specificity of Mammalian ALOX15 Orthologs. Prog. Lipid Res. 2018, 72, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.; Kuhn, H.; Heydeck, D. Structural and Functional Biology of Arachidonic Acid 15-Lipoxygenase-1 (ALOX15). Gene 2015, 573, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M.A.; Safizadeh, B.; Eghtedari, A.R.; Ghorbanhosseini, S.S.; Rastegar, M.; Salimi, V.; Tavakoli-Yaraki, M. 15-Lipoxygenase and Its Metabolites in the Pathogenesis of Breast Cancer: A Double-Edged Sword. Lipids Health Dis. 2021, 20, 169. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Kakularam, K.R.; Reisch, F.; Rothe, M.; Stehling, S.; Heydeck, D.; Püschel, G.P.; Kuhn, H. Male Knock-in Mice Expressing an Arachidonic Acid Lipoxygenase 15B (Alox15B) with Humanized Reaction Specificity Are Prematurely Growth Arrested When Aging. Biomedicines 2022, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.; Jansen, C.; Roffeis, J.; Reddanna, P.; Forsell, P.; Claesson, H.-E.; Kuhn, H.; Walther, M. Applicability of the Triad Concept for the Positional Specificity of Mammalian Lipoxygenases. J. Biol. Chem. 2010, 285, 5369–5376. [Google Scholar] [CrossRef] [PubMed]
- Kakularam, K.R.; Canyelles-Niño, M.; Chen, X.; Lluch, J.M.; González-Lafont, À.; Kuhn, H. Functional Characterization of Mouse and Human Arachidonic Acid Lipoxygenase 15B (ALOX15B) Orthologs and of Their Mutants Exhibiting Humanized and Murinized Reaction Specificities. Int. J. Mol. Sci. 2023, 24, 10046. [Google Scholar] [CrossRef] [PubMed]
- Kinzig, A.; Fürstenberger, G.; Bürger, F.; Vogel, S.; Müller-Decker, K.; Mincheva, A.; Lichter, P.; Marks, F.; Krieg, P. Murine Epidermal Lipoxygenase (Aloxe) Encodes a 12-Lipoxygenase Isoform. FEBS Lett. 1997, 402, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Siebert, M.; Krieg, P.; Lehmann, W.D.; Marks, F.; Fürstenberger, G. Enzymic Characterization of Epidermis-Derived 12-Lipoxygenase Isoenzymes. Biochem. J. 2001, 355, 97–104. [Google Scholar] [CrossRef]
- Miyai, M.; Hamada, M.; Moriguchi, T.; Hiruma, J.; Kamitani-Kawamoto, A.; Watanabe, H.; Hara-Chikuma, M.; Takahashi, K.; Takahashi, S.; Kataoka, K. Transcription Factor MafB Coordinates Epidermal Keratinocyte Differentiation. J. Investig. Dermatol. 2016, 136, 1848–1857. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, C.; Lin, J.; Duan, P.; Long, J.; Zhu, H. ALOX5-5-HETE Promotes Gastric Cancer Growth and Alleviates Chemotherapy Toxicity via MEK/ERK Activation. Cancer Med. 2021, 10, 5246–5255. [Google Scholar] [CrossRef] [PubMed]
- Kummer, N.T.; Nowicki, T.S.; Azzi, J.P.; Reyes, I.; Iacob, C.; Xie, S.; Swati, I.; Darzynkiewicz, Z.; Gotlinger, K.H.; Suslina, N.; et al. Arachidonate 5 Lipoxygenase Expression in Papillary Thyroid Carcinoma Promotes Invasion via MMP-9 Induction. J. Cell Biochem. 2012, 113, 1998–2008. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.-X.; Ding, X.-L.; Wu, S.-B.; Zhang, H.-F.; Cao, W.; Qu, L.-S.; Zhang, H. Inhibition of 5-Lipoxygenase Triggers Apoptosis in Pancreatic Cancer Cells. Oncol. Rep. 2015, 33, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Selka, A.; Doiron, J.A.; Lyons, P.; Dastous, S.; Chiasson, A.; Cormier, M.; Turcotte, S.; Surette, M.E.; Touaibia, M. Discovery of a Novel 2,5-Dihydroxycinnamic Acid-Based 5-Lipoxygenase Inhibitor That Induces Apoptosis and May Impair Autophagic Flux in RCC4 Renal Cancer Cells. Eur. J. Med. Chem. 2019, 179, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xu, X.; Li, J.; Bai, M.; Zhu, W.; Liu, Y.; Liu, S.; Zhao, Z.; Li, T.; Jiang, N.; et al. ALOX5 Deficiency Contributes to Bladder Cancer Progression by Mediating Ferroptosis Escape. Cell Death Dis. 2023, 14, 800. [Google Scholar] [CrossRef] [PubMed]
- Rouzer, C.A.; Ford-Hutchinson, A.W.; Morton, H.E.; Gillard, J.W. MK886, a Potent and Specific Leukotriene Biosynthesis Inhibitor Blocks and Reverses the Membrane Association of 5-Lipoxygenase in Ionophore-Challenged Leukocytes. J. Biol. Chem. 1990, 265, 1436–1442. [Google Scholar] [CrossRef]
- Dixon, R.A.; Diehl, R.E.; Opas, E.; Rands, E.; Vickers, P.J.; Evans, J.F.; Gillard, J.W.; Miller, D.K. Requirement of a 5-Lipoxygenase-Activating Protein for Leukotriene Synthesis. Nature 1990, 343, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Byrum, R.S.; Goulet, J.L.; Griffiths, R.J.; Koller, B.H. Role of the 5-Lipoxygenase-Activating Protein (FLAP) in Murine Acute Inflammatory Responses. J. Exp. Med. 1997, 185, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Kilty, I.; Logan, A.; Vickers, P.J. Differential Characteristics of Human 15-Lipoxygenase Isozymes and a Novel Splice Variant of 15S-Lipoxygenase. Eur. J. Biochem. 1999, 266, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Green, A.R.; Barbour, S.; Horn, T.; Carlos, J.; Raskatov, J.A.; Holman, T.R. Strict Regiospecificity of Human Epithelial 15-Lipoxygenase-2 Delineates Its Transcellular Synthesis Potential. Biochemistry 2016, 55, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Nicolaou, K.C.; Webber, S.E.; Veale, C.A.; Dahlén, S.E.; Puustinen, T.J.; Samuelsson, B. Lipoxin A. Stereochemistry and Biosynthesis. J. Biol. Chem. 1986, 261, 16340–16345. [Google Scholar] [CrossRef] [PubMed]
- Ringholz, F.C.; Buchanan, P.J.; Clarke, D.T.; Millar, R.G.; McDermott, M.; Linnane, B.; Harvey, B.J.; McNally, P.; Urbach, V. Reduced 15-Lipoxygenase 2 and Lipoxin A4/Leukotriene B4 Ratio in Children with Cystic Fibrosis. Eur. Respir. J. 2014, 44, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Suraneni, M.V.; Moore, J.R.; Zhang, D.; Badeaux, M.; Macaluso, M.D.; DiGiovanni, J.; Kusewitt, D.; Tang, D.G. Tumor-Suppressive Functions of 15-Lipoxygenase-2 and RB1CC1 in Prostate Cancer. Cell Cycle 2014, 13, 1798–1810. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Srivastava, M.; Ahmad, N.; Sakamoto, K.; Bostwick, D.G.; Mukhtar, H. Lipoxygenase-5 Is Overexpressed in Prostate Adenocarcinoma. Cancer 2001, 91, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Horn, T.; Adel, S.; Schumann, R.; Sur, S.; Kakularam, K.R.; Polamarasetty, A.; Redanna, P.; Kuhn, H.; Heydeck, D. Evolutionary Aspects of Lipoxygenases and Genetic Diversity of Human Leukotriene Signaling. Prog. Lipid Res. 2015, 57, 13–39. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Yan, B.; Wang, C.; Zhang, L. The Role of 12/15-Lipoxygenase and Its Various Metabolites Generated from Multiple Polyunsaturated Fatty Acids as Substrates in Inflammatory Responses. Biomed. Res. Int. 2022, 2022, 4589191. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Spite, M.; Clària, J.; Serhan, C.N. Resolvins, Specialized Proresolving Lipid Mediators, and Their Potential Roles in Metabolic Diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Rådmark, O.; Werz, O.; Steinhilber, D.; Samuelsson, B. 5-Lipoxygenase, a Key Enzyme for Leukotriene Biosynthesis in Health and Disease. Biochim. Biophys. Acta 2015, 1851, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Ford-Hutchinson, A.W.; Bray, M.A.; Doig, M.V.; Shipley, M.E.; Smith, M.J. Leukotriene B, a Potent Chemokinetic and Aggregating Substance Released from Polymorphonuclear Leukocytes. Nature 1980, 286, 264–265. [Google Scholar] [CrossRef] [PubMed]
- Fürstenberger, G.; Krieg, P.; Müller-Decker, K.; Habenicht, A.J.R. What Are Cyclooxygenases and Lipoxygenases Doing in the Driver’s Seat of Carcinogenesis? Int. J. Cancer 2006, 119, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- El Kebir, D.; Filep, J.G. Modulation of Neutrophil Apoptosis and the Resolution of Inflammation through Β2 Integrins. Front. Immunol. 2013, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, R.G.; Brüne, B. Regulation and Functions of 15-Lipoxygenases in Human Macrophages. Front. Pharmacol. 2019, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, T.J.; Chan, C.-Y.; Ding, X.-Z.; Adrian, T.E. Lipoxygenase Inhibitors for the Treatment of Pancreatic Cancer. Expert. Rev. Anticancer. Ther. 2003, 3, 525–536. [Google Scholar] [CrossRef]
- Liu, Y.; Deguchi, Y.; Wei, D.; Liu, F.; Moussalli, M.J.; Deguchi, E.; Li, D.; Wang, H.; Valentin, L.A.; Colby, J.K.; et al. Rapid Acceleration of KRAS-Mutant Pancreatic Carcinogenesis via Remodeling of Tumor Immune Microenvironment by PPARδ. Nat. Commun. 2022, 13, 2665. [Google Scholar] [CrossRef] [PubMed]
- Suraneni, M.V.; Schneider-Broussard, R.; Moore, J.R.; Davis, T.C.; Maldonado, C.J.; Li, H.; Newman, R.A.; Kusewitt, D.; Hu, J.; Yang, P.; et al. Transgenic Expression of 15-Lipoxygenase 2 (15-LOX2) in Mouse Prostate Leads to Hyperplasia and Cell Senescence. Oncogene 2010, 29, 4261–4275. [Google Scholar] [CrossRef]
- Pelengaris, S.; Khan, M.; Evan, G.I. Suppression of Myc-Induced Apoptosis in Beta Cells Exposes Multiple Oncogenic Properties of Myc and Triggers Carcinogenic Progression. Cell 2002, 109, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Shureiqi, I.; Chen, D.; Day, R.S.; Zuo, X.; Hochman, F.L.; Ross, W.A.; Cole, R.A.; Moy, O.; Morris, J.S.; Xiao, L.; et al. Profiling Lipoxygenase Metabolism in Specific Steps of Colorectal Tumorigenesis. Cancer Prev. Res. 2010, 3, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.L.; Roberts, R.L.; Massion, P.P.; Olson, S.J.; Shyr, Y.; Shappell, S.B. 15-Lipoxygenase-2 Expression in Benign and Neoplastic Lung: An Immunohistochemical Study and Correlation with Tumor Grade and Proliferation. Hum. Pathol. 2004, 35, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Greene, E.R.; Huang, S.; Serhan, C.N.; Panigrahy, D. Regulation of Inflammation in Cancer by Eicosanoids. Prostaglandins Other Lipid Mediat. 2011, 96, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Chang, W.-C.; Chuang, J.-Y.; Chang, K.-Y.; Liou, J.-P.; Hsu, T.-I. The Complex Role of Eicosanoids in the Brain: Implications for Brain Tumor Development and Therapeutic Opportunities. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188957. [Google Scholar] [CrossRef] [PubMed]
- Hennig, R.; Kehl, T.; Noor, S.; Ding, X.-Z.; Rao, S.M.; Bergmann, F.; Fürstenberger, G.; Büchler, M.W.; Friess, H.; Krieg, P.; et al. 15-Lipoxygenase-1 Production Is Lost in Pancreatic Cancer and Overexpression of the Gene Inhibits Tumor Cell Growth. Neoplasia 2007, 9, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli-Yaraki, M.; Karami-Tehrani, F.; Salimi, V.; Sirati-Sabet, M. Induction of Apoptosis by Trichostatin A in Human Breast Cancer Cell Lines: Involvement of 15-Lox-1. Tumour Biol. 2013, 34, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.N.; Felipe da Costa, S.; Colquhoun, A. Eicosanoids and Cancer. Clinics 2018, 73, e530s. [Google Scholar] [CrossRef] [PubMed]
- Finetti, F.; Travelli, C.; Ercoli, J.; Colombo, G.; Buoso, E.; Trabalzini, L. Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity. Biology 2020, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Stearman, R.S.; Grady, M.C.; Nana-Sinkam, P.; Varella-Garcia, M.; Geraci, M.W. Genetic and Epigenetic Regulation of the Human Prostacyclin Synthase Promoter in Lung Cancer Cell Lines. Mol. Cancer Res. 2007, 5, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.F. The Clinical Evidence Linking Helicobacter Pylori to Gastric Cancer. Cell Mol. Gastroenterol. Hepatol. 2017, 3, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal Disease, Tooth Loss, and Cancer Risk. Epidemiol. Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Wu, L.; Leng, W.-D.; Fang, C.; Zhu, Y.-J.; Jin, Y.-H.; Zeng, X.-T. Periodontal Disease and Breast Cancer: A Meta-Analysis of 1,73,162 Participants. Front. Oncol. 2018, 8, 601. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium Nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Parhi, L.; Alon-Maimon, T.; Sol, A.; Nejman, D.; Shhadeh, A.; Fainsod-Levi, T.; Yajuk, O.; Isaacson, B.; Abed, J.; Maalouf, N.; et al. Breast Cancer Colonization by Fusobacterium Nucleatum Accelerates Tumor Growth and Metastatic Progression. Nat. Commun. 2020, 11, 3259. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Q.; Zhou, C.; Chen, K.; Chang, H.; Xiao, W.; Gao, Y. Colorectal Cancer, Radiotherapy and Gut Microbiota. Chin. J. Cancer Res. 2019, 31, 212–222. [Google Scholar] [CrossRef]
- Shureiqi, I.; Jiang, W.; Zuo, X.; Wu, Y.; Stimmel, J.B.; Leesnitzer, L.M.; Morris, J.S.; Fan, H.-Z.; Fischer, S.M.; Lippman, S.M. The 15-Lipoxygenase-1 Product 13-S-Hydroxyoctadecadienoic Acid down-Regulates PPAR-Delta to Induce Apoptosis in Colorectal Cancer Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 9968–9973. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Myers, C.E. Inhibition of Arachidonate 5-Lipoxygenase Triggers Massive Apoptosis in Human Prostate Cancer Cells. Proc. Natl. Acad. Sci. USA 1998, 95, 13182–13187. [Google Scholar] [CrossRef] [PubMed]
- Edderkaoui, M.; Hong, P.; Vaquero, E.C.; Lee, J.K.; Fischer, L.; Friess, H.; Buchler, M.W.; Lerch, M.M.; Pandol, S.J.; Gukovskaya, A.S. Extracellular Matrix Stimulates Reactive Oxygen Species Production and Increases Pancreatic Cancer Cell Survival through 5-Lipoxygenase and NADPH Oxidase. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G1137–G1147. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, T.; Murata, Y.; Takahashi, K.; Naito, T.; Ofuji, K.; Matsuda, H.; Ohtani, M.; Hiramatsu, K.; Imamura, Y.; Goi, T.; et al. Hepatocellular Carcinoma Progression Promoted by 5-Lipoxygenase Activity in CD163(+) Tumor-Associated Macrophages. Biomed. Pharmacother. 2023, 162, 114592. [Google Scholar] [CrossRef] [PubMed]
- Nathoo, N.; Prayson, R.A.; Bondar, J.; Vargo, L.; Arrigain, S.; Mascha, E.J.; Suh, J.H.; Barnett, G.H.; Golubic, M. Increased Expression of 5-Lipoxygenase in High-Grade Astrocytomas. Neurosurgery 2006, 58, 347–354; discussion 347–354. [Google Scholar] [CrossRef]
- Xu, X.-M.; Deng, J.-J.; Yuan, G.-J.; Yang, F.; Guo, H.-T.; Xiang, M.; Ge, W.; Wu, Y.-G. 5-Lipoxygenase Contributes to the Progression of Hepatocellular Carcinoma. Mol. Med. Rep. 2011, 4, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Watkins, G.; Douglas-Jones, A.; Mansel, R.E. Reduction of Isoforms of 15-Lipoxygenase (15-LOX)-1 and 15-LOX-2 in Human Breast Cancer. Prostaglandins Leukot. Essent. Fatty Acids 2006, 74, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Bhatia, B.; Maldonado, C.J.; Yang, P.; Newman, R.A.; Liu, J.; Chandra, D.; Traag, J.; Klein, R.D.; Fischer, S.M.; et al. Evidence That Arachidonate 15-Lipoxygenase 2 Is a Negative Cell Cycle Regulator in Normal Prostate Epithelial Cells. J. Biol. Chem. 2002, 277, 16189–16201. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, M.-T.; Chen, Y.; Yang, D.; Che, M.; Honn, K.V.; Akers, G.D.; Johnson, S.R.; Nie, D. Downregulation of Vascular Endothelial Growth Factor and Induction of Tumor Dormancy by 15-Lipoxygenase-2 in Prostate Cancer. Int. J. Cancer 2009, 124, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Wecksler, A.T.; Kenyon, V.; Garcia, N.K.; Deschamps, J.D.; van der Donk, W.A.; Holman, T.R. Kinetic and Structural Investigations of the Allosteric Site in Human Epithelial 15-Lipoxygenase-2. Biochemistry 2009, 48, 8721–8730. [Google Scholar] [CrossRef] [PubMed]
- Barooni, A.B.; Ghorbani, M.; Salimi, V.; Alimohammadi, A.; Khamseh, M.E.; Akbari, H.; Imani, M.; Nourbakhsh, M.; Sheikhi, A.; Shirian, F.I.; et al. Up-Regulation of 15-Lipoxygenase Enzymes and Products in Functional and Non-Functional Pituitary Adenomas. Lipids Health Dis. 2019, 18, 152. [Google Scholar] [CrossRef] [PubMed]
- Tunçer, S.; Tunçay Çağatay, S.; Keşküş, A.G.; Çolakoğlu, M.; Konu, Ö.; Banerjee, S. Interplay between 15-Lipoxygenase-1 and Metastasis-Associated Antigen 1 in the Metastatic Potential of Colorectal Cancer. Cell Prolif. 2016, 49, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Peng, Z.; Wu, Y.; Moussalli, M.J.; Yang, X.L.; Wang, Y.; Parker-Thornburg, J.; Morris, J.S.; Broaddus, R.R.; Fischer, S.M.; et al. Effects of Gut-Targeted 15-LOX-1 Transgene Expression on Colonic Tumorigenesis in Mice. J. Natl. Cancer Inst. 2012, 104, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, H.; Kamitani, H.; Calvo, B.F.; Foley, J.F.; Eling, T.E. Expression of 15-Lipoxygenase-1 in Human Colorectal Cancer. Cancer Res. 1999, 59, 360–366. [Google Scholar] [PubMed]
- Heslin, M.J.; Hawkins, A.; Boedefeld, W.; Arnoletti, J.P.; Frolov, A.; Soong, R.; Urist, M.M.; Bland, K.I. Tumor-Associated down-Regulation of 15-Lipoxygenase-1 Is Reversed by Celecoxib in Colorectal Cancer. Ann. Surg. 2005, 241, 941–946; discussion 946–947. [Google Scholar] [CrossRef]
- Yuri, M.; Sasahira, T.; Nakai, K.; Ishimaru, S.; Ohmori, H.; Kuniyasu, H. Reversal of Expression of 15-Lipoxygenase-1 to Cyclooxygenase-2 Is Associated with Development of Colonic Cancer. Histopathology 2007, 51, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Nixon, J.B.; Kim, K.S.; Lamb, P.W.; Bottone, F.G.; Eling, T.E. 15-Lipoxygenase-1 Has Anti-Tumorigenic Effects in Colorectal Cancer. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Barquissau, V.; Ghandour, R.A.; Ailhaud, G.; Klingenspor, M.; Langin, D.; Amri, E.-Z.; Pisani, D.F. Control of Adipogenesis by Oxylipins, GPCRs and PPARs. Biochimie 2017, 136, 3–11. [Google Scholar] [CrossRef]
- Scirpo, R.; Fiorotto, R.; Villani, A.; Amenduni, M.; Spirli, C.; Strazzabosco, M. Stimulation of Nuclear Receptor Peroxisome Proliferator-Activated Receptor-γ Limits NF-κB-Dependent Inflammation in Mouse Cystic Fibrosis Biliary Epithelium. Hepatology 2015, 62, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Fatty Acids, Eicosanoids and PPAR Gamma. Eur. J. Pharmacol. 2016, 785, 44–49. [Google Scholar] [CrossRef]
- Nicolaou, A.; Mauro, C.; Urquhart, P.; Marelli-Berg, F. Polyunsaturated Fatty Acid-Derived Lipid Mediators and T Cell Function. Front. Immunol. 2014, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Brinckmann, R.; Kühn, H. Regulation of 15-Lipoxygenase Expression by Cytokines. Adv. Exp. Med. Biol. 1997, 400B, 599–604. [Google Scholar] [PubMed]
- Liu, C.; Schain, F.; Han, H.; Xu, D.; Andersson-Sand, H.; Forsell, P.; Claesson, H.-E.; Björkholm, M.; Sjöberg, J. Epigenetic and Transcriptional Control of the 15-Lipoxygenase-1 Gene in a Hodgkin Lymphoma Cell Line. Exp. Cell Res. 2012, 318, 169–176. [Google Scholar] [CrossRef]
- Chen, X.; Ji, N.; Qin, N.; Tang, S.-A.; Wang, R.; Qiu, Y.; Duan, H.; Kong, D.; Jin, M. 1,6-O,O-Diacetylbritannilactone Inhibits Eotaxin-1 and ALOX15 Expression Through Inactivation of STAT6 in A549 Cells. Inflammation 2017, 40, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Nassar, G.M.; Morrow, J.D.; Roberts, L.J.; Lakkis, F.G.; Badr, K.F. Induction of 15-Lipoxygenase by Interleukin-13 in Human Blood Monocytes. J. Biol. Chem. 1994, 269, 27631–27634. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Cathcart, M.K. Induction of 15-Lipoxygenase Expression by IL-13 Requires Tyrosine Phosphorylation of Jak2 and Tyk2 in Human Monocytes. J. Biol. Chem. 1998, 273, 32023–32029. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.D.; Romano, M.; Chapman, H.A.; Reilly, J.J.; Drazen, J.; Serhan, C.N. Human Alveolar Macrophages Have 15-Lipoxygenase and Generate 15(S)-Hydroxy-5,8,11-Cis-13-Trans-Eicosatetraenoic Acid and Lipoxins. J. Clin. Investig. 1993, 92, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Wang, Y.; Ma, D.; Cheng, W.; Liu, J.; Yong, T.; Chen, H.; Wang, C. Immunotherapy: Reshape the Tumor Immune Microenvironment. Front. Immunol. 2022, 13, 844142. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Rao, G.N. Emerging Role of 12/15-Lipoxygenase (ALOX15) in Human Pathologies. Prog. Lipid Res. 2019, 73, 28–45. [Google Scholar] [CrossRef]
- Bodnarczuk, T.; Deskur, A.; Dolegowska, K.; Dolegowska, B.; Starzynska, T.; Blogowski, W. Hydroxyeicosatetraenoic Acids in Patients with Pancreatic Cancer: A Preliminary Report. Am. J. Cancer Res. 2018, 8, 1865–1872. [Google Scholar] [PubMed]
- Zhong, H.; Wang, R.; Kelavkar, U.; Wang, C.Y.; Simons, J. Enzyme 15-Lipoxygenase 1 Promotes Hypoxia-Inducible Factor 1α Turnover and Reduces Vascular Endothelial Growth Factor Expression: Implications for Angiogenesis. Cancer Med. 2014, 3, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Hennig, R.; Grippo, P.; Ding, X.-Z.; Rao, S.M.; Buchler, M.W.; Friess, H.; Talamonti, M.S.; Bell, R.H.; Adrian, T.E. 5-Lipoxygenase, a Marker for Early Pancreatic Intraepithelial Neoplastic Lesions. Cancer Res. 2005, 65, 6011–6016. [Google Scholar] [CrossRef] [PubMed]
- Hennig, R.; Ding, X.-Z.; Tong, W.-G.; Schneider, M.B.; Standop, J.; Friess, H.; Büchler, M.W.; Pour, P.M.; Adrian, T.E. 5-Lipoxygenase and Leukotriene B(4) Receptor Are Expressed in Human Pancreatic Cancers but Not in Pancreatic Ducts in Normal Tissue. Am. J. Pathol. 2002, 161, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.Z.; Wang, M.-T.; Nie, D. Regulations of Tumor Microenvironment by Prostaglandins. Cancers 2023, 15, 3090. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Pietrocola, F.; Guilbaud, E.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostini, M.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; et al. Apoptotic Cell Death in Disease-Current Understanding of the NCCD 2023. Cell Death Differ. 2023, 30, 1097–1154. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular Mechanisms and Health Implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Zhu, J.; Xiong, Y.; Zhang, Y.; Wen, J.; Cai, N.; Cheng, K.; Liang, H.; Zhang, W. The Molecular Mechanisms of Regulating Oxidative Stress-Induced Ferroptosis and Therapeutic Strategy in Tumors. Oxid. Med. Cell Longev. 2020, 2020, 8810785. [Google Scholar] [CrossRef]
- Liu, J.; Kuang, F.; Kroemer, G.; Klionsky, D.J.; Kang, R.; Tang, D. Autophagy-Dependent Ferroptosis: Machinery and Regulation. Cell Chem. Biol. 2020, 27, 420–435. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Liu, J.; Kang, R.; Klionsky, D.J.; Tang, D. Mitochondrial DNA Stress Triggers Autophagy-Dependent Ferroptotic Death. Autophagy 2021, 17, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the Ferroptosis Regulator Gpx4 Triggers Acute Renal Failure in Mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Shchepinov, M.S.; Pratt, D.A. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent. Sci. 2018, 4, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of Polyunsaturated Fatty Acids by Lipoxygenases Drives Ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef]
- Lee, H.; Zandkarimi, F.; Zhang, Y.; Meena, J.K.; Kim, J.; Zhuang, L.; Tyagi, S.; Ma, L.; Westbrook, T.F.; Steinberg, G.R.; et al. Energy-Stress-Mediated AMPK Activation Inhibits Ferroptosis. Nat. Cell Biol. 2020, 22, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zeng, G.; Xiong, B.; Zhu, X.; Guo, J.; Chen, D.; Zhang, S.; Luo, M.; Guo, L.; Cai, L. ALOX5 Promotes Autophagy-Dependent Ferroptosis by Activating the AMPK/mTOR Pathway in Melanoma. Biochem. Pharmacol. 2023, 212, 115554. [Google Scholar] [CrossRef] [PubMed]
- Anthonymuthu, T.S.; Kenny, E.M.; Shrivastava, I.; Tyurina, Y.Y.; Hier, Z.E.; Ting, H.-C.; Dar, H.H.; Tyurin, V.A.; Nesterova, A.; Amoscato, A.A.; et al. Empowerment of 15-Lipoxygenase Catalytic Competence in Selective Oxidation of Membrane ETE-PE to Ferroptotic Death Signals, HpETE-PE. J. Am. Chem. Soc. 2018, 140, 17835–17839. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Nam, M.; Son, H.Y.; Hyun, K.; Jang, S.Y.; Kim, J.W.; Kim, M.W.; Jung, Y.; Jang, E.; Yoon, S.-J.; et al. Polyunsaturated Fatty Acid Biosynthesis Pathway Determines Ferroptosis Sensitivity in Gastric Cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 32433–32442. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E.; Tyurina, Y.Y.; Zhao, J.; St Croix, C.M.; Dar, H.H.; Mao, G.; Tyurin, V.A.; Anthonymuthu, T.S.; Kapralov, A.A.; Amoscato, A.A.; et al. PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell 2017, 171, 628–641.e26. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Hu, M.; Wang, Z.; Ge, J.; Zhou, X.; Zhang, G.; Zheng, H. Ferroptosis-Related Genes in Lung Adenocarcinoma: Prognostic Signature and Immune, Drug Resistance, Mutation Analysis. Front. Genet. 2021, 12, 672904. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Bhat, A.A.; Goyal, A.; Singla, N.; Gupta, S.; Sharma, S.; Bhatt, S.; Dua, K. Exploring ACSL4/LPCAT3/ALOX15 and SLC7A11/GPX4/NFE2L2 as Potential Targets in Ferroptosis-Based Cancer Therapy. Future Med. Chem. 2023, 15, 1209–1212. [Google Scholar] [CrossRef]
- Shintoku, R.; Takigawa, Y.; Yamada, K.; Kubota, C.; Yoshimoto, Y.; Takeuchi, T.; Koshiishi, I.; Torii, S. Lipoxygenase-Mediated Generation of Lipid Peroxides Enhances Ferroptosis Induced by Erastin and RSL3. Cancer Sci. 2017, 108, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Anthonymuthu, T.S.; Tyurina, Y.Y.; Sun, W.-Y.; Mikulska-Ruminska, K.; Shrivastava, I.H.; Tyurin, V.A.; Cinemre, F.B.; Dar, H.H.; VanDemark, A.P.; Holman, T.R.; et al. Resolving the Paradox of Ferroptotic Cell Death: Ferrostatin-1 Binds to 15LOX/PEBP1 Complex, Suppresses Generation of Peroxidized ETE-PE, and Protects against Ferroptosis. Redox Biol. 2021, 38, 101744. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhu, Y.; Dong, Z.; Li, W.; Yang, N.; Wang, X.; Feng, L.; Liu, Z. Tumor-Killing Nanoreactors Fueled by Tumor Debris Can Enhance Radiofrequency Ablation Therapy and Boost Antitumor Immune Responses. Nat. Commun. 2021, 12, 4299. [Google Scholar] [CrossRef] [PubMed]
- Hernández Borrero, L.J.; El-Deiry, W.S. Tumor Suppressor P53: Biology, Signaling Pathways, and Therapeutic Targeting. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef] [PubMed]
- Barlev, N.A.; Sayan, B.S.; Candi, E.; Okorokov, A.L. The microRNA and P53 Families Join Forces against Cancer. Cell Death Differ. 2010, 17, 373–375. [Google Scholar] [CrossRef]
- Fedorova, O.; Petukhov, A.; Daks, A.; Shuvalov, O.; Leonova, T.; Vasileva, E.; Aksenov, N.; Melino, G.; Barlev, N.A. Orphan Receptor NR4A3 Is a Novel Target of P53 That Contributes to Apoptosis. Oncogene 2019, 38, 2108–2122. [Google Scholar] [CrossRef] [PubMed]
- Konopleva, M.; Martinelli, G.; Daver, N.; Papayannidis, C.; Wei, A.; Higgins, B.; Ott, M.; Mascarenhas, J.; Andreeff, M. MDM2 Inhibition: An Important Step Forward in Cancer Therapy. Leukemia 2020, 34, 2858–2874. [Google Scholar] [CrossRef]
- Rozenberg, J.M.; Zvereva, S.; Dalina, A.; Blatov, I.; Zubarev, I.; Luppov, D.; Bessmertnyi, A.; Romanishin, A.; Alsoulaiman, L.; Kumeiko, V.; et al. The P53 Family Member P73 in the Regulation of Cell Stress Response. Biol. Direct 2021, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Bulatov, E.; Sayarova, R.; Mingaleeva, R.; Miftakhova, R.; Gomzikova, M.; Ignatyev, Y.; Petukhov, A.; Davidovich, P.; Rizvanov, A.; Barlev, N.A. Isatin-Schiff Base-Copper (II) Complex Induces Cell Death in P53-Positive Tumors. Cell Death Discov. 2018, 4, 103. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, F.-E. Small-Molecule MDM2 Inhibitors in Clinical Trials for Cancer Therapy. Eur. J. Med. Chem. 2022, 236, 114334. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, P.; Aksenova, V.; Petrova, V.; Tentler, D.; Orlova, D.; Smirnov, S.; Gurzhiy, V.; Okorokov, A.L.; Garabadzhiu, A.; Melino, G.; et al. Discovery of Novel Isatin-Based P53 Inducers. ACS Med. Chem. Lett. 2015, 6, 856–860. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, W. P53 in Ferroptosis Regulation: The New Weapon for the Old Guardian. Cell Death Differ. 2022, 29, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hou, N.; Chen, B.; Kan, C.; Han, F.; Zhang, J.; Sun, X. Post-Translational Modifications of P53 in Ferroptosis: Novel Pharmacological Targets for Cancer Therapy. Front. Pharmacol. 2022, 13, 908772. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, B.; Ahmad, K.; Roos, J.; Lehmann, C.; Chiba, T.; Ulrich-Rückert, S.; Smeenk, L.; van Heeringen, S.; Maier, T.J.; Groner, B.; et al. 5-Lipoxygenase Is a Direct P53 Target Gene in Humans. Biochim. Biophys. Acta 2015, 1849, 1003–1016. [Google Scholar] [CrossRef]
- Catalano, A.; Caprari, P.; Soddu, S.; Procopio, A.; Romano, M. 5-Lipoxygenase Antagonizes Genotoxic Stress-Induced Apoptosis by Altering P53 Nuclear Trafficking. FASEB J. 2004, 18, 1740–1742. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Rodilossi, S.; Caprari, P.; Coppola, V.; Procopio, A. 5-Lipoxygenase Regulates Senescence-like Growth Arrest by Promoting ROS-Dependent P53 Activation. EMBO J. 2005, 24, 170–179. [Google Scholar] [CrossRef]
- Li, X.; Xiong, W.; Wang, Y.; Li, Y.; Cheng, X.; Liu, W. P53 Activates the Lipoxygenase Activity of ALOX15B via Inhibiting SLC7A11 to Induce Ferroptosis in Bladder Cancer Cells. Lab. Investig. 2023, 103, 100058. [Google Scholar] [CrossRef]
- Parfenyev, S.; Singh, A.; Fedorova, O.; Daks, A.; Kulshreshtha, R.; Barlev, N.A. Interplay between P53 and Non-Coding RNAs in the Regulation of EMT in Breast Cancer. Cell Death Dis. 2021, 12, 17. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, Y.; Jin, H.; Hou, B.; Li, H.; Li, X.; Liu, L.; Zhou, Y.; Li, Y.; Song, Y.S.; et al. The Suppression of Cervical Cancer Ferroptosis by Macrophages: The Attenuation of ALOX15 in Cancer Cells by Macrophages-Derived Exosomes. Acta Pharm. Sin. B 2023, 13, 2645–2662. [Google Scholar] [CrossRef] [PubMed]
- Le, M.T.N.; Teh, C.; Shyh-Chang, N.; Xie, H.; Zhou, B.; Korzh, V.; Lodish, H.F.; Lim, B. MicroRNA-125b Is a Novel Negative Regulator of P53. Genes. Dev. 2009, 23, 862–876. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-P.; Qiu, Z.-Z.; Li, X.-H.; Li, E.-Y. Propofol Induces Ferroptosis and Inhibits Malignant Phenotypes of Gastric Cancer Cells by Regulating miR-125b-5p/STAT3 Axis. World J. Gastrointest. Oncol. 2021, 13, 2114–2128. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Kon, N.; Chen, D.; Li, T.; Liu, T.; Jiang, L.; Song, S.; Tavana, O.; Gu, W. ALOX12 Is Required for P53-Mediated Tumour Suppression through a Distinct Ferroptosis Pathway. Nat. Cell Biol. 2019, 21, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, W.; Shi, X.; Chen, Y.; Yu, Y.; Du, B.; Tan, M.; Tong, L.; Wang, A.; Yin, X.; et al. S1PR1/S1PR3-YAP Signaling and S1P-ALOX15 Signaling Contribute to an Aggressive Behavior in Obesity-Lymphoma. J. Exp. Clin. Cancer Res. 2023, 42, 3. [Google Scholar] [CrossRef] [PubMed]
- Kwong, S.C.; Abd Jamil, A.H.; Rhodes, A.; Taib, N.A.; Chung, I. Fatty Acid Binding Protein 7 Mediates Linoleic Acid-Induced Cell Death in Triple Negative Breast Cancer Cells by Modulating 13-HODE. Biochimie 2020, 179, 23–31. [Google Scholar] [CrossRef]
- Gohara, A.; Eltaki, N.; Sabry, D.; Murtagh, D.; Jankun, J.; Selman, S.H.; Skrzypczak-Jankun, E. Human 5-, 12- and 15-Lipoxygenase-1 Coexist in Kidney but Show Opposite Trends and Their Balance Changes in Cancer. Oncol. Rep. 2012, 28, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Kelavkar, U.P.; Parwani, A.V.; Shappell, S.B.; Martin, W.D. Conditional Expression of Human 15-Lipoxygenase-1 in Mouse Prostate Induces Prostatic Intraepithelial Neoplasia: The FLiMP Mouse Model. Neoplasia 2006, 8, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.A. The Role of 15 Lipoxygenase 1 in Asthma Comes into Focus. J. Clin. Investig. 2022, 132, e155884. [Google Scholar] [CrossRef] [PubMed]
- Uderhardt, S.; Krönke, G. 12/15-Lipoxygenase during the Regulation of Inflammation, Immunity, and Self-Tolerance. J. Mol. Med. 2012, 90, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Heydeck, D.; Kakularam, K.R.; Labuz, D.; Machelska, H.; Rohwer, N.; Weylandt, K.; Kuhn, H. Transgenic Mice Overexpressing Human ALOX15 under the Control of the aP2 Promoter Are Partly Protected in the Complete Freund’s Adjuvant-Induced Paw Inflammation Model. Inflamm. Res. 2023, 72, 1649–1664. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; O’Donnell, V.B.; Balzar, S.; St Croix, C.M.; Trudeau, J.B.; Wenzel, S.E. 15-Lipoxygenase 1 Interacts with Phosphatidylethanolamine-Binding Protein to Regulate MAPK Signaling in Human Airway Epithelial Cells. Proc. Natl. Acad. Sci. USA 2011, 108, 14246–14251. [Google Scholar] [CrossRef]
- Rao, C.V.; Janakiram, N.B.; Mohammed, A. Lipoxygenase and Cyclooxygenase Pathways and Colorectal Cancer Prevention. Curr. Colorectal Cancer Rep. 2012, 8, 316–324. [Google Scholar] [CrossRef]
- Weisser, H.; Göbel, T.; Melissa Krishnathas, G.; Kreiß, M.; Angioni, C.; Sürün, D.; Thomas, D.; Schmid, T.; Häfner, A.-K.; Kahnt, A.S. Knock-out of 5-Lipoxygenase in Overexpressing Tumor Cells-Consequences on Gene Expression and Cellular Function. Cancer Gene Ther. 2023, 30, 108–123. [Google Scholar] [CrossRef]
- Janakiram, N.B.; Mohammed, A.; Bryant, T.; Ritchie, R.; Stratton, N.; Jackson, L.; Lightfoot, S.; Benbrook, D.M.; Asch, A.S.; Lang, M.L.; et al. Loss of Natural Killer T Cells Promotes Pancreatic Cancer in LSL-KrasG12D/+ Mice. Immunology 2017, 152, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Schmöcker, C.; Gottschall, H.; Rund, K.M.; Kutzner, L.; Nolte, F.; Ostermann, A.I.; Hartmann, D.; Schebb, N.H.; Weylandt, K.H. Oxylipin Patterns in Human Colon Adenomas. Prostaglandins Leukot. Essent. Fatty Acids 2021, 167, 102269. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, N.B.; Mohammed, A.; Rao, C.V. Role of Lipoxins, Resolvins, and Other Bioactive Lipids in Colon and Pancreatic Cancer. Cancer Metastasis Rev. 2011, 30, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Blogowski, W.; Dolegowska, K.; Deskur, A.; Dolegowska, B.; Starzynska, T. Lipoxins and Resolvins in Patients With Pancreatic Cancer: A Preliminary Report. Front. Oncol. 2021, 11, 757073. [Google Scholar] [CrossRef] [PubMed]
- Weigert, A.; Strack, E.; Snodgrass, R.G.; Brüne, B. mPGES-1 and ALOX5/-15 in Tumor-Associated Macrophages. Cancer Metastasis Rev. 2018, 37, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-C.; Shappell, S.B.; Liang, Z.; Song, S.; Menter, D.; Subbarayan, V.; Iyengar, S.; Tang, D.G.; Lippman, S.M. Reduced 15S-Lipoxygenase-2 Expression in Esophageal Cancer Specimens and Cells and Upregulation in Vitro by the Cyclooxygenase-2 Inhibitor, NS398. Neoplasia 2003, 5, 121–127. [Google Scholar] [CrossRef][Green Version]
- Hsi, L.C.; Wilson, L.C.; Eling, T.E. Opposing Effects of 15-Lipoxygenase-1 and -2 Metabolites on MAPK Signaling in Prostate. Alteration in Peroxisome Proliferator-Activated Receptor Gamma. J. Biol. Chem. 2002, 277, 40549–40556. [Google Scholar] [CrossRef] [PubMed]
- Orafaie, A.; Matin, M.M.; Sadeghian, H. The Importance of 15-Lipoxygenase Inhibitors in Cancer Treatment. Cancer Metastasis Rev. 2018, 37, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V.; Janakiram, N.B.; Madka, V.; Devarkonda, V.; Brewer, M.; Biddick, L.; Lightfoot, S.; Steele, V.E.; Mohammed, A. Simultaneous Targeting of 5-LOX-COX and EGFR Blocks Progression of Pancreatic Ductal Adenocarcinoma. Oncotarget 2015, 6, 33290–33305. [Google Scholar] [CrossRef]
- Tong, W.-G.; Ding, X.-Z.; Witt, R.C.; Adrian, T.E. Lipoxygenase Inhibitors Attenuate Growth of Human Pancreatic Cancer Xenografts and Induce Apoptosis through the Mitochondrial Pathway. Mol. Cancer Ther. 2002, 1, 929–935. [Google Scholar] [PubMed]
- Ding, X.Z.; Kuszynski, C.A.; El-Metwally, T.H.; Adrian, T.E. Lipoxygenase Inhibition Induced Apoptosis, Morphological Changes, and Carbonic Anhydrase Expression in Human Pancreatic Cancer Cells. Biochem. Biophys. Res. Commun. 1999, 266, 392–399. [Google Scholar] [CrossRef]
- Monga, J.; Subramani, D.; Bharathan, A.; Ghosh, J. Pharmacological and Genetic Targeting of 5-Lipoxygenase Interrupts c-Myc Oncogenic Signaling and Kills Enzalutamide-Resistant Prostate Cancer Cells via Apoptosis. Sci. Rep. 2020, 10, 6649. [Google Scholar] [CrossRef] [PubMed]
- Kothayer, H.; Rezq, S.; Abdelkhalek, A.S.; Romero, D.G.; Elbaramawi, S.S. Triple Targeting of Mutant EGFRL858R/T790M, COX-2, and 15-LOX: Design and Synthesis of Novel Quinazolinone Tethered Phenyl Urea Derivatives for Anti-Inflammatory and Anticancer Evaluation. J. Enzyme Inhib. Med. Chem. 2023, 38, 2199166. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-M.; Liu, S.-Q.; Zhu, K.-F.; Li, W.; Yang, Z.-J.; Yang, Q.; Zhu, Z.-C.; Chang, J. The ALOX5 Inhibitor Zileuton Regulates Tumor-Associated Macrophage M2 Polarization by JAK/STAT and Inhibits Pancreatic Cancer Invasion and Metastasis. Int. Immunopharmacol. 2023, 121, 110505. [Google Scholar] [CrossRef] [PubMed]
- Oguh-Olayinka, L.; Agarwal, V.; Ranatunge, D.; Campbell, A.; Laufer, S.; Cawkwell, L.; Lind, M.J. The Investigation of Lipoxygenases as Therapeutic Targets in Malignant Pleural Mesothelioma. Pathol. Oncol. Res. 2020, 26, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.Z.; Iversen, P.; Cluck, M.W.; Knezetic, J.A.; Adrian, T.E. Lipoxygenase Inhibitors Abolish Proliferation of Human Pancreatic Cancer Cells. Biochem. Biophys. Res. Commun. 1999, 261, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Koontongkaew, S.; Monthanapisut, P.; Saensuk, T. Inhibition of Arachidonic Acid Metabolism Decreases Tumor Cell Invasion and Matrix Metalloproteinase Expression. Prostaglandins Other Lipid Mediat. 2010, 93, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.; Ben-Shushan, D.; Peled, M.; Kamari, Y.; Isman, S.; Barshack, I.; Kuban, R.-J.; Kühn, H.; Harats, D.; Shaish, A. Specific Overexpression of 15-Lipoxygenase in Endothelial Cells Promotes Cancer Cell Death in an in vivo Lewis Lung Carcinoma Mouse Model. Adv. Med. Sci. 2020, 65, 111–119. [Google Scholar] [CrossRef]
- Lövey, J.; Nie, D.; Tóvári, J.; Kenessey, I.; Tímár, J.; Kandouz, M.; Honn, K.V. Radiosensitivity of Human Prostate Cancer Cells Can Be Modulated by Inhibition of 12-Lipoxygenase. Cancer Lett. 2013, 335, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Reedy, J.L.; Scroggins, B.T.; White, A.O.; Kwon, S.; Shankavaram, U.; López-Coral, A.; Chung, E.J.; Citrin, D.E. Senescence-Associated Tumor Growth Is Promoted by 12-Lipoxygenase. Aging 2022, 14, 1068–1086. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli Yaraki, M.; Karami Tehrani, F. Apoptosis Induced by 13-S-Hydroxyoctadecadienoic Acid in the Breast Cancer Cell Lines, MCF-7 and MDA-MB-231. Iran. J. Basic. Med. Sci. 2013, 16, 653–659. [Google Scholar] [PubMed]
- Doxorubicin Resistance in Breast Cancer Cells Is Mediated by Extracellular Matrix Proteins|BMC Cancer|Full Text. Available online: https://bmccancer.biomedcentral.com/articles/10.1186/s12885-017-3953-6 (accessed on 23 February 2024).
- Ho, C.F.-Y.; Bon, C.P.-E.; Ng, Y.-K.; Herr, D.R.; Wu, J.-S.; Lin, T.-N.; Ong, W.-Y. Expression of DHA-Metabolizing Enzyme Alox15 Is Regulated by Selective Histone Acetylation in Neuroblastoma Cells. Neurochem. Res. 2018, 43, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Brunner, E.; Giszas, B.; Iyer-Bierhoff, A.; Gerstmeier, J.; Börner, F.; Jordan, P.M.; Pace, S.; Meyer, K.P.L.; Hofstetter, R.K.; et al. Glucocorticoids Regulate Lipid Mediator Networks by Reciprocal Modulation of 15-Lipoxygenase Isoforms Affecting Inflammation Resolution. Proc. Natl. Acad. Sci. USA 2023, 120, e2302070120. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.D.C.; Ferreira, M.T.; Colquhoun, A. Influence of Lipoxygenase Inhibition on Glioblastoma Cell Biology. Int. J. Mol. Sci. 2020, 21, 8395. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A.; Colquhoun, A. Effect of Polyunsaturated Fatty Acids on Temozolomide Drug-Sensitive and Drug-Resistant Glioblastoma Cells. Biomedicines 2023, 11, 779. [Google Scholar] [CrossRef] [PubMed]
- Dragnev, K.H.; Dragnev, C.P.C.; Lubet, R.A. Major Hurdles to the Use of Tyrosine Kinase Inhibitors in Clinical Prevention/Interception Studies: Do Preclinical Studies with EGFR Inhibitors Suggest Approaches to Overcome Some of the Limitations. Front. Cell Dev. Biol. 2023, 11, 1170444. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Baek, S.J.; Bottone, F.G.; Sali, T.; Eling, T.E. Overexpression of 15-Lipoxygenase-1 Induces Growth Arrest through Phosphorylation of P53 in Human Colorectal Cancer Cells. Mol. Cancer Res. 2005, 3, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fang, B.; Yang, X.Q.; Wang, L.; Chen, D.; Krasnykh, V.; Carter, B.Z.; Morris, J.S.; Shureiqi, I. Therapeutic Molecular Targetingof 15-Lipoxygenase-1 in Colon Cancer. Mol. Ther. 2008, 16, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.G.; Bhatia, B.; Tang, S.; Schneider-Broussard, R. 15-Lipoxygenase 2 (15-LOX2) Is a Functional Tumor Suppressor That Regulates Human Prostate Epithelial Cell Differentiation, Senescence, and Growth (Size). Prostaglandins Other Lipid Mediat. 2007, 82, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, S.; Feng, Y.; Yang, Q.; Campbell, B.H.; Tang, X.; Campbell, W.B. Reduced Expression of 15-Lipoxygenase 2 in Human Head and Neck Carcinomas. Tumour Biol. 2006, 27, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Feng, Y.; Schultz, C.J.; Li, X.A.; Wu, H.; Wang, D. Synergistic Effect of 15-Lipoxygenase 2 and Radiation in Killing Head-and-Neck Cancer. Cancer Gene Ther. 2008, 15, 323–330. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kruglov, O.; Vats, K.; Soman, V.; Tyurin, V.A.; Tyurina, Y.Y.; Wang, J.; Williams, L.; Zhang, J.; Donahue Carey, C.; Jaklitsch, E.; et al. Melanoma-Associated Repair-like Schwann Cells Suppress Anti-Tumor T-Cells via 12/15-LOX/COX2-Associated Eicosanoid Production. Oncoimmunology 2023, 12, 2192098. [Google Scholar] [CrossRef] [PubMed]
- Juratli, T.A.; Schackert, G.; Krex, D. Current Status of Local Therapy in Malignant Gliomas—A Clinical Review of Three Selected Approaches. Pharmacol. Ther. 2013, 139, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Pacholska, A.; Wirth, T.; Samaranayake, H.; Pikkarainen, J.; Ahmad, F.; Ylä-Herttuala, S. Increased Invasion of Malignant Gliomas after 15-LO-1 and HSV-Tk/Ganciclovir Combination Gene Therapy. Cancer Gene Ther. 2012, 19, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Nishida, N.; Kudo, M. Radiofrequency Ablation of Liver Metastasis: Potential Impact on Immune Checkpoint Inhibitor Therapy. Eur. Radiol. 2019, 29, 5045–5051. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, L.; Yang, Z.; Zhao, D.; Deng, Z.; Xu, J.; Wu, Y.; Hao, Y.; Dong, Z.; Feng, L.; et al. Self-Fueling Ferroptosis-Inducing Microreactors Based on pH-Responsive Lipiodol Pickering Emulsions Enable Transarterial Ferro-Embolization Therapy. Natl. Sci. Rev. 2024, 11, nwad257. [Google Scholar] [CrossRef] [PubMed]









| Lipoxygenase | Optimum Temperature (°C) | Thermal Instability (°C) | pH | Vmax (µM/min) | Km (µM) | Kcat (s−1) | Kcat/Km (µM−1s−1) | References |
|---|---|---|---|---|---|---|---|---|
| Soybean ALOX15 | 35–40 | >60 | 9.0 | 15 b | 287 | 19 b | [12,42,43] | |
| Human ALOX15 | 22, 25–30 | >40 | 7.0, 7.5 | 1.03 a 4.9 b | 7.5 a 3 b | 5.3 7.8 | 2.0 a 2.5 b | [12,44,45] [46,47] |
| Human ALOX15B | 37 | >45 | 8.5 | 3.5 a | 0.74 0.14 | 0.1 a 0.013 b | [45,48,49] | |
| LoxA | 22–35, 25 | >45 °C, t1/2 = 10 min at 50 °C | 6.5, 7.5 | 0.226 b | 12 a 7 b | 181 28 | 16 a 3.8 b | [43,50,51] |
| Human ALOX5 | 21, 25 | >40 °C c | 8.0 | 2.56 a | 22.3 a | 0.06 | 0.054 a | [52,53,54,55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoah, A.-S.; Pestov, N.B.; Korneenko, T.V.; Prokhorenko, I.A.; Kurakin, G.F.; Barlev, N.A. Lipoxygenases at the Intersection of Infection and Carcinogenesis. Int. J. Mol. Sci. 2024, 25, 3961. https://doi.org/10.3390/ijms25073961
Amoah A-S, Pestov NB, Korneenko TV, Prokhorenko IA, Kurakin GF, Barlev NA. Lipoxygenases at the Intersection of Infection and Carcinogenesis. International Journal of Molecular Sciences. 2024; 25(7):3961. https://doi.org/10.3390/ijms25073961
Chicago/Turabian StyleAmoah, Abdul-Saleem, Nikolay B. Pestov, Tatyana V. Korneenko, Igor A. Prokhorenko, Georgy F. Kurakin, and Nickolai A. Barlev. 2024. "Lipoxygenases at the Intersection of Infection and Carcinogenesis" International Journal of Molecular Sciences 25, no. 7: 3961. https://doi.org/10.3390/ijms25073961
APA StyleAmoah, A.-S., Pestov, N. B., Korneenko, T. V., Prokhorenko, I. A., Kurakin, G. F., & Barlev, N. A. (2024). Lipoxygenases at the Intersection of Infection and Carcinogenesis. International Journal of Molecular Sciences, 25(7), 3961. https://doi.org/10.3390/ijms25073961





