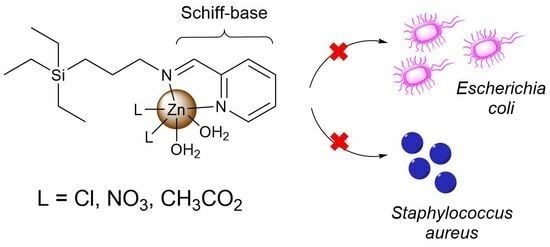
Zinc(II) Iminopyridine Complexes as Antibacterial Agents: A Structure-to-Activity Study
Abstract
1. Introduction
2. Results
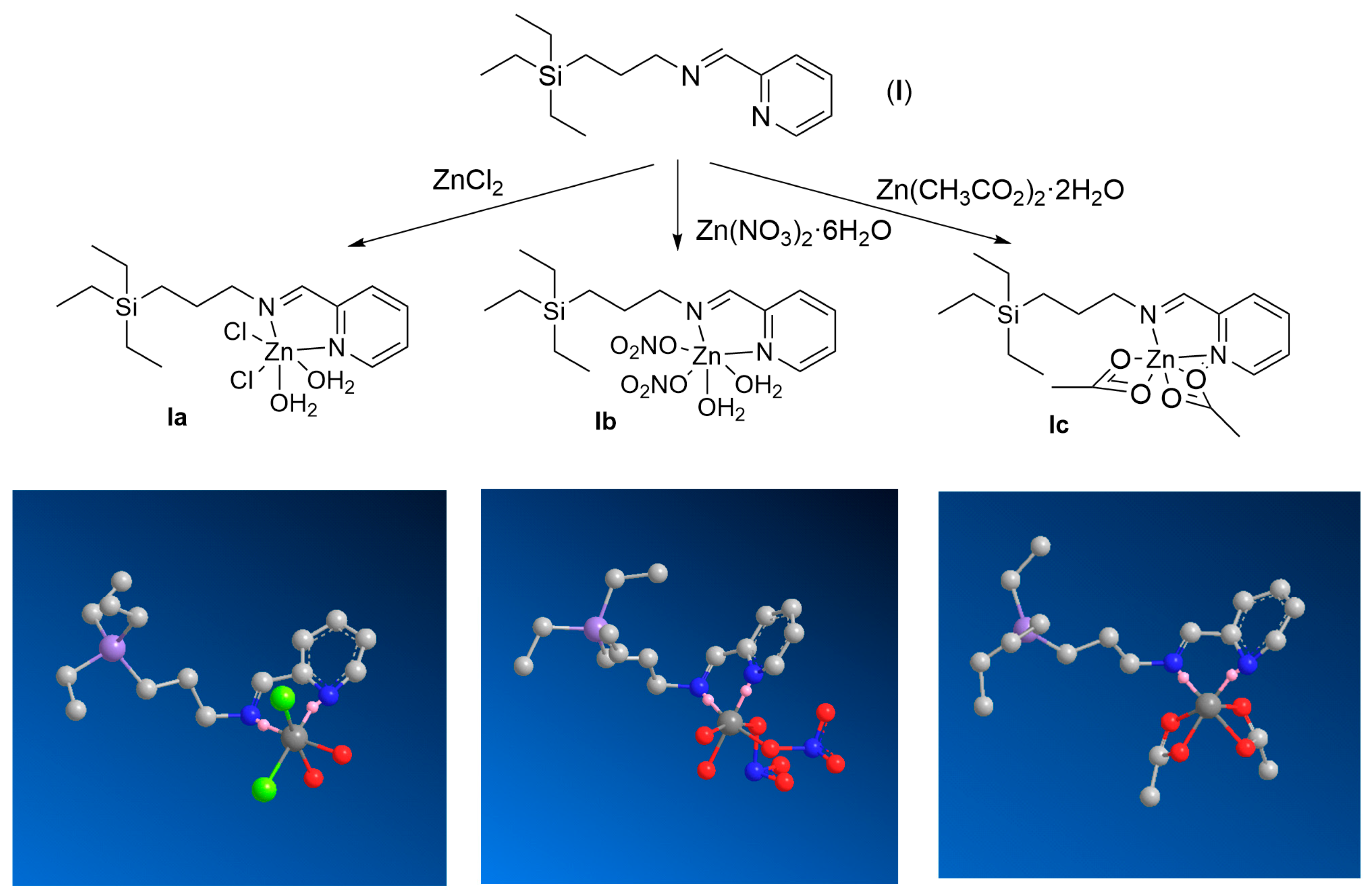
2.1. Synthesis of Zn(II) Iminopyridine Complexes
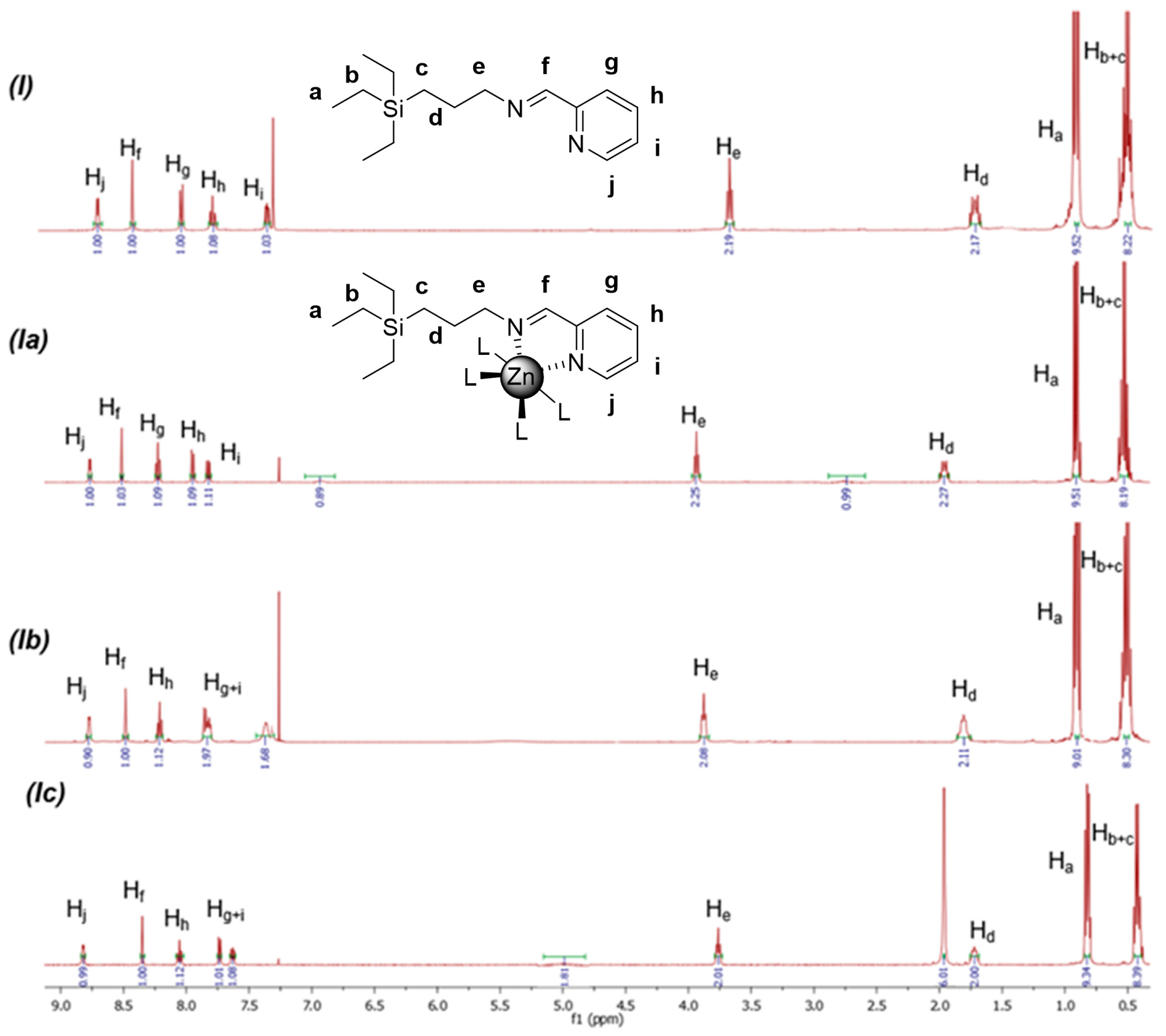
2.2. Structural Characterization of Zn(II) Complexes: Impact of the Metal Coordination and the Nature of the Inorganic Ligands
2.3. Antibacterial Activity
2.3.1. Biocidal Activity
2.3.2. Kinetic Studies
2.4. Evaluation of Topical Antibacterial Emulsion
3. Discussion
4. Materials and Methods
4.1. Synthesis and Characterization of Zn(II) Iminopyridine Complexes
- General synthetic protocol: A MeOH solution of the metal salt (1 eq., in 4 mL) was added over a MeOH of the ligand I (1 eq., in 4 mL). After 24 h stirring at r.t., the solvent was evaporated, and the product was isolated as dark brown oil with high yield.
4.1.1. G0[NCPh(o-N)ZnCl2·2H2O] (Ia)
- Ia was prepared according to the general protocol using the following reagents: ligand I (1.27 mmol) and ZnCl2 (1.27 mmol). Yield: 90%.
- 1H-NMR: δ 8.42 (N=CH); 8.81, 8.20, 7.84 (4H, CHpy); 3.95 (t, 2H, -CH2N); 1.98 (m, 2H, SiCH2CH2CH2N); 0.92 (m, 9H, SiCH2CH3); 0.54 (m, 8H, SiCH2CH3 & SiCH2CH2CH2N). 13C-NMR {1H}: δ 161.5 (N=CH); 146.5 (Cpy); 149.4, 142.1, 129.4, 128.2 (CHpy); 61.8 (SiCH2CH2CH2N); 24.7 (SiCH2CH2CH2N); 8.9 (SiCH2CH2CH2N); 7.3 (SiCH2CH3), 2.7 (SiCH2CH3). Elemental analysis (%): C15H30Cl2N2O2SiZn (434.8 g/mol) Calc.: C, 41.44%; H, 6.96%; N, 6.44%. Exp.: C, 41.77%; H, 6.40%; N, 6.55%. FT-IR: v(C=N): 1643.8 cm−1. ICP (Zn%): Calc. 15.0%, Exp. 19.1%.
4.1.2. G0[NCPh(o-N)Zn(NO3)2·2H2O] (Ib)
- Ib was prepared according to the general protocol using the following reagents: ligand I (0.487 mmol) and Zn(NO3)2·6H2O (0.487 mmol). Yield: 93%.
- 1H-NMR: δ 8.35 (N=CH); 8.79, 8.19, 7.80 (4H, CHpy); 3.88 (t, 2H, -CH2N); 1.81 (m, 2H, SiCH2CH2CH2N); 0.92 (m, 9H, SiCH2CH3); 0.54 (m, 8H, SiCH2CH3 & SiCH2CH2CH2N). 13C-NMR {1H}: δ 161.3 (N=CH); 146.1 (Cpy); 149.1, 141.1, 129.0, 127.4 (CHpy); 61.3 (SiCH2CH2CH2N); 24.0 (SiCH2CH2CH2N); 8.5 (SiCH2CH2CH2N); 7.1 (SiCH2CH3), 2.8 (SiCH2CH3). Elemental analysis (%): C15H30N4O8SiZn (487.9 g/mol). Calc.: C, 36.93%; H, 6.20%; N, 11.48%. Exp.: C, 36.51%; H, 6.11%; N, 10.96%. FT-IR: v(C=N): 1645.6 cm−1. ICP (Zn%): Calc. 13.4%, Exp. 14.1%.
4.1.3. G0[NCPh(o-N)Zn(OOCCH3)2] (Ic)
- Ic was prepared according to the general protocol using the following reagents: ligand I (0.497 mmol) and Zn(O2CCH3)2·2H2O (0.497 mmol). Yield: 98%.
- 1H-NMR: δ 8.34 (N=CH); 8.82, 8.05, 7.73, 7.63 (4H, CHpy); 3.76 (t, 2H, -CH2N); 1.91 (6H, CH3CO2); 1.73 (m, 2H, SiCH2CH2CH2N); 0.88 (m, 9H, SiCH2CH3); 0.40 (m, 8H, SiCH2CH3 & SiCH2CH2CH2N). 13C-NMR {1H}: δ 179.8 (CH3CO2); 159.6 (N=CH); 146.6 (Cpy); 149.9, 140.8, 128.4, 126.6 (CHpy); 61.8 (SiCH2CH2CH2N); 24.2 (SiCH2CH2CH2N); 22.5 (CH3CO2); 8.9 (SiCH2CH2CH2N); 7.2 (SiCH2CH3); 3.0 (SiCH2CH3). Elemental analysis (%): C19H32N2O4SiZn (445.9 g/mol). Calc.: C, 51.17%; H, 7.23%; N, 6.28%. Calc.(+H2O): C, 49.19%; H, 7.39%; N, 6.04%. Exp.: C, 49.17%; H, 7.27%; N, 5.92%. Calc.: FT-IR: v(C=N) & v(C=O): 1593.4 cm−1. ICP (Zn%): Calc. 14.1%, Exp. 20.0%.
4.2. Formulation in W/O Emulssion
4.3. Vertical Cell Diffusion Assay
4.4. Zeta Potential Evaluation
4.5. Bacterial Strains
4.6. In Vitro Antibacterial Activity Tests
4.7. Kinetic Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A.T. Metals to combat antimicrobial resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef]
- Lonergan, Z.R.; Skaar, E.P. Nutrient Zinc at the host-Pathogen interface. Trends Biochem. Sci. 2019, 44, 1041–1056. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liao, C.; Tjong, S.C. Recent advances in Zinc Oxide nanostructures with antimicrobial activities. Int. J. Mol. Sci. 2020, 21, 8836. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.H.; Scott, P. Chapter Seven-Antimicrobial Metallodrugs. In Inorganic and Organometallic Transition Metal Complexes with Biological Molecules and Living Cells; Lo, K.K.-W., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 205–243. [Google Scholar]
- Waters, J.E.; Stevens-Cullinane, L.; Siebenmann, L.; Hess, J. Recent advances in the development of metal complexes as antibacterial agents with metal-specific modes of action. Curr. Opin. Microbiol. 2023, 75, 102347. [Google Scholar] [CrossRef]
- Shekhar, S.; Khan, A.M.; Sharma, S.; Sharma, B.; Sarkar, A. Schiff base metallodrugs in antimicrobial and anticancer chemotherapy applications: A comprehensive review. Emergent Mater. 2022, 5, 279–293. [Google Scholar] [CrossRef]
- Malik, M.A.; Dar, O.A.; Gull, P.; Wani, M.Y.; Hashmi, A.A. Heterocyclic Schiff base transition metal complexes in antimicrobial and anticancer chemotherapy. Med. Chem. Commun. 2018, 9, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Sinicropi, M.S.; Ceramella, J.; Iacopetta, D.; Catalano, A.; Mariconda, A.; Rosano, C.; Saturnino, C.; El-Kashef, H.; Longo, P. Metal Complexes with Schiff Bases: Data Collection and Recent Studies on Biological Activities. Int. J. Mol. Sci. 2022, 23, 14840. [Google Scholar] [CrossRef]
- Mahmood, K.; Hashmi, W.; Ismail, H.; Mirza, B.; Twamley, B.; Akhter, Z.; Rozas, I.; Baker, R.J. Synthesis, DNA binding and antibacterial activity of metal(II) complexes of a benzimidazole Schiff base. Polyhedron 2019, 157, 326–334. [Google Scholar] [CrossRef]
- Mahmoud, W.H.; Deghadi, R.G.; Mohamed, G.G. Metal complexes of novel Schiff base derived from iron sandwiched organometallic and 4-nitro-1,2-phenylenediamine: Synthesis, characterization, DFT studies, antimicrobial activities and molecular docking. Appl. Organomet. Chem. 2018, 32, e4289. [Google Scholar] [CrossRef]
- İspir, E.; Kurtoğlu, M.; Toroğlu, S. The d10 metal chelates derived from Schiff base ligands having silane: Synthesis, characterization, and antimicrobial studies of Cadmium(II) and Zinc(II) complexes. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2006, 36, 627–631. [Google Scholar] [CrossRef]
- Llamazares, C.; Sanz Del Olmo, N.; Ortega, P.; Gómez, R.; Soliveri, J.; de la Mata, F.J.; García-Gallego, S.; Copa-Patiño, J.L. Antibacterial effect of carbosilane metallodendrimers in Planktonic cells of Gram-positive and Gram-negative bacteria and Staphylococcus aureus Biofilm. Biomolecules 2019, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Sanz del Olmo, N.; Carloni, R.; Ortega, P.; García-Gallego, S.; de la Mata, F.J. Chapter One-Metallodendrimers as a promising tool in the biomedical field: An overview. In Advances in Organometallic Chemistry; Pérez, P.J., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 74, pp. 1–52. [Google Scholar]
- Ortega, M.Á.; Guzmán Merino, A.; Fraile-Martínez, O.; Recio-Ruiz, J.; Pekarek, L.; GGuijarro, L.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J.; García-Gallego, S. Dendrimers and dendritic materials: From laboratory to medical practice in infectious diseases. Pharmaceutics 2020, 12, 874. [Google Scholar] [CrossRef] [PubMed]
- Maroto-Díaz, M.; Elie, B.T.; Gómez-Sal, P.; Pérez-Serrano, J.; Gómez, R.; Contel, M.; De La Mata, F.J. Synthesis and anticancer activity of carbosilane metallodendrimers based on arene ruthenium(II) complexes. Dalton Trans. 2016, 45, 7049–7066. [Google Scholar] [CrossRef] [PubMed]
- Tyula, Y.A.; Goudarziafshar, H.; Yousefi, S.; Dušek, M.; Eigner, V. Template synthesis, characterization and antibacterial activity of d10 (Zn2+, Cd2+, Hg2+) Schiff base complexes: A novel supramolecular Cd2+ complex with two 1D helical chains, and its Hirshfeld surface analysis. J. Mol. Struct. 2023, 1272, 134051. [Google Scholar] [CrossRef]
- Capitan, F.; Salinas, F.; Capitan-Vallvey, L.F. Study of the complexes formed with 2-pyrilidineaniline and copper(II), silver(I), zinc(II), cadmium(II) and mercury(II). An. Quim.) 1978, 74, 265–271. [Google Scholar]
- Deghadi, R.G.; Elsharkawy, A.E.; Ashmawy, A.M.; Mohamed, G.G. Antibacterial and anticorrosion behavior of bioactive complexes of selected transition metal ions with new 2-acetylpyridine Schiff base. Appl. Organomet. Chem. 2022, 36, e6579. [Google Scholar] [CrossRef]
- Mahmoud, W.H.; Mohamed, G.G.; El-Sayed, O.Y. Coordination compounds of some transition metal ions with new Schiff base ligand derived from dibenzoyl methane. Structural characterization, thermal behavior, molecular structure, antimicrobial, anticancer activity and molecular docking studies. Appl. Organomet. Chem. 2017, 32, e4051. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I. Infrared spectra of surface nitrates: Revision of the current opinions based on the case study of ceria. J. Catalysis. 2021, 394, 245–258. [Google Scholar]
- Edwards, D.A.; Hayward, R.N. Transition metal acetates. Can. J. Chem. 1968, 46, 3443–3446. [Google Scholar] [CrossRef]
- Kumar, U.; Thomas, J.; Thirupathi, N. Factors Dictating the Nuclearity/Aggregation and Acetate Coordination Modes of Lutidine-Coordinated Zinc(II)Acetate Complexes. Inorg. Chem. 2010, 49, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Dinku, D.; Demissie, T.B.; Beas, I.N.; Eswaramoorthy, R.; Abdi, B.; Desalegn, T. Antimicrobial activities and docking studies of new Schiff base ligand and its Cu (II), Zn (II) and Ni (II) Complexes: Synthesis and Characterization. Inorg. Chem. Commun. 2024, 160, 111903. [Google Scholar] [CrossRef]
- Tandon, S.S.; Chander, S.; Thompson, L.K. Ligating properties of tridentate Schiff base ligands, 2-[[(2-pyridinylmethyl)imino]methyl] phenol (HSALIMP) and 2-[[[2-(2-pyridinyl)ethyl]imino]methyl]phenol (HSALIEP) with zinc(II), cadmium(II), nickel(II) and manganese(III) ions. X-ray crystal structures of the [Zn(SALIEP)(NO3)]2 dimer, [Mn(SALIEP)2](ClO4), and [Zn(AMP)2(NO3)2]. Inorg. Chim. Acta 2000, 300–302, 683–692. [Google Scholar]
- Vicha, J.; Novotny, J.; Komorovsky, S.; Straka, M.; Kaupp, M.; Marek, R. Relativistic Heavy-Neighbor-Atom Effects on NMR Shifts: Concepts and Trends Across the Periodic Table. Chem. Rev. 2020, 120, 7065–7103. [Google Scholar] [CrossRef] [PubMed]
- Butera, V.; D’Anna, L.; Rubino, S.; Bonsignore, R.; Spinello, A.; Terenzi, A.; Barone, G. How the Metal Ion Affects the 1H NMR Chemical Shift Values of Schiff Base Metal Complexes: Rationalization by DFT Calculations. J. Phys. Chem. A. 2023, 127, 9283–9290. [Google Scholar] [CrossRef] [PubMed]
- Di Serio, M.; Carotenuto, G.; Cucciolito, M.E.; Lega, M.; Ruffo, F.; Tesser, R.; Trifuoggi, M. Shiff base complexes of zinc(II) as catalysts for biodiesel production. J. Mol. Cat. A 2012, 353–354, 106–110. [Google Scholar] [CrossRef]
- Benessere, V.; Cucciolito, M.E.; Esposito, R.; Lega, M.; Turco, R.; Ruffo, F.; Di Serio, M. A novel and robust homogeneous supported catalyst for biodiesel production. Fuel 2016, 171, 1–4. [Google Scholar] [CrossRef]
- Sharma, R.K.; Monga, Y. Silica encapsulated magnetic nanoparticles-supported Zn(II) nanocatalyst: A versatile integration of excellent reactivity and selectivity for the synthesis of azoxyarenes, combined with facile catalyst recovery and recyclability. Appl. Catal. A. 2013, 454, 1–10. [Google Scholar] [CrossRef]
- Lakshkar, R.R.; Yadav, S.; Thirumoorthi, R.; Ray, S.; Dash, C. Zinc-bis(imino)pyridine Complexes as Catalysts for Intermolecular Amidation of Benzylic C(sp3)−HBond. Chem. Select. 2024, 9, e202302804. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Naureen, B.; Miana, G.A.; Shahid, K.; Asghar, M.; Tanveer, S.; Sarwar, A. Iron (III) and zinc (II) monodentate Schiff base metal complexes: Synthesis, characterisation and biological activities. J. Mol. Structure. 2021, 1231, 129946. [Google Scholar] [CrossRef]
- Gómez-Casanova, N.; Torres-Cano, A.; Elias-Rodriguez, A.X.; Lozano, T.; Ortega, P.; Gómez, R.; Pérez-Serrano, J.; Copa-Patiño, J.L.; Heredero-Bermejo, I. Inhibition of Candida glabrata biofilm by combined effect of dendritic compounds and amphotericin. Pharmaceutics 2022, 14, 1604. [Google Scholar] [CrossRef] [PubMed]

| Compound | Zeta Potential, [mV] | S. aureus MIC [mg/L] | MBC [mg/L] | E. coli MIC [mg/L] | MBC [mg/L] |
|---|---|---|---|---|---|
| G0[NCPh(o-N)ZnCl2·2H2O] (Ia) | −0.33 ± 0.06 | 64 | 64 | 128 | 128 |
| G0[NCPh(o-N)Zn(NO3)2·2H2O] (Ib) | −6.28 ± 0.33 | 64 | 64 | 128 | 128 |
| G0[NCPh(o-N)Zn(O2CCH3)2] (Ic) | −8.43 ± 1.53 | 64 | 64 | 64–128 | 64–128 |
| G0[NCPh(o-N)CuCl2·H2O] a | 10.45 ± 1.25 | 64 | 64 | 256 | 256 |
| G0[NCPh(o-N)Cu(NO3)2·H2O] a | 14.79 ± 1.92 | 128 | 128 | 128 | 256 |
| G0[NCPh(o-N)Ru(Cp)(PTA)]Cl a | 18.70 ± 4.21 | 16 | 16 | 64 | 64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Mata Moratilla, S.; Casado Angulo, S.; Gómez-Casanova, N.; Copa-Patiño, J.L.; Heredero-Bermejo, I.; de la Mata, F.J.; García-Gallego, S. Zinc(II) Iminopyridine Complexes as Antibacterial Agents: A Structure-to-Activity Study. Int. J. Mol. Sci. 2024, 25, 4011. https://doi.org/10.3390/ijms25074011
de la Mata Moratilla S, Casado Angulo S, Gómez-Casanova N, Copa-Patiño JL, Heredero-Bermejo I, de la Mata FJ, García-Gallego S. Zinc(II) Iminopyridine Complexes as Antibacterial Agents: A Structure-to-Activity Study. International Journal of Molecular Sciences. 2024; 25(7):4011. https://doi.org/10.3390/ijms25074011
Chicago/Turabian Stylede la Mata Moratilla, Silvia, Sandra Casado Angulo, Natalia Gómez-Casanova, José Luis Copa-Patiño, Irene Heredero-Bermejo, Francisco Javier de la Mata, and Sandra García-Gallego. 2024. "Zinc(II) Iminopyridine Complexes as Antibacterial Agents: A Structure-to-Activity Study" International Journal of Molecular Sciences 25, no. 7: 4011. https://doi.org/10.3390/ijms25074011
APA Stylede la Mata Moratilla, S., Casado Angulo, S., Gómez-Casanova, N., Copa-Patiño, J. L., Heredero-Bermejo, I., de la Mata, F. J., & García-Gallego, S. (2024). Zinc(II) Iminopyridine Complexes as Antibacterial Agents: A Structure-to-Activity Study. International Journal of Molecular Sciences, 25(7), 4011. https://doi.org/10.3390/ijms25074011