Loading of Single Atoms of Iron, Cobalt, or Nickel to Enhance the Electrocatalytic Hydrogen Evolution Reaction of Two-Dimensional Titanium Carbide
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Characterization
2.2. Electrocatalytic Hydrogen Evolution Reaction Performance
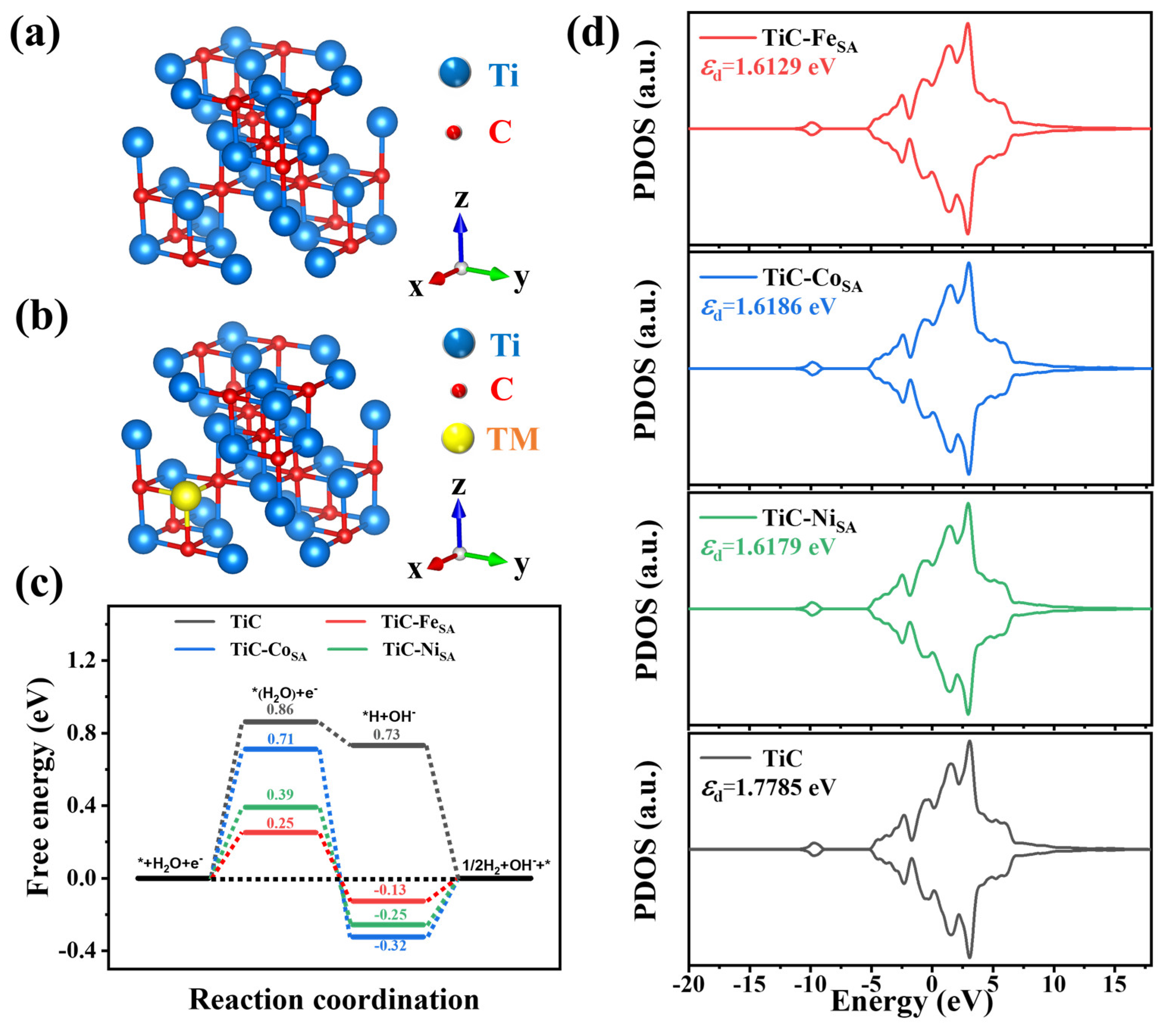
2.3. Density Functional Theory Calculations
3. Materials and Methods
3.1. Preparation of Ti2ZnC
3.2. Preparation of TiC
3.3. Preparation of TiC-FeSA, TiC-CoSA, and TiC-NiSA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the Oxygen Evolution Reaction: Recent Development and Future Perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Cui, Y.; Liu, N. The Path Towards Sustainable Energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.W.; Zhang, S.Q.; Chen, Q.; Zhang, C.; Wei, Y.; Jiang, H.N.; Lin, Y.X.; Zhao, M.T.; He, Q.Q.; Wang, X.G.; et al. Conversion of Intercalated MoO3 to Multi-Heteroatoms-Doped MoS2 with High Hydrogen Evolution Activity. Adv. Mater. 2020, 32, 2001167. [Google Scholar] [CrossRef] [PubMed]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An Overview of Hydrogen Production Technologies. Catalysis Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Zou, X.X.; Zhang, Y. Noble Metal-Free Hydrogen Evolution Catalysts for Water Splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and Fuels from Electrochemical Interfaces. Nat. Mater. 2017, 16, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, J.J.; Zou, X.L.; Hackenberg, K.; Zhou, W.; Chen, W.B.; Yuan, J.T.; Keyshar, K.; Gupta, G.; Mohite, A.; et al. Discovering Superior Basal Plane Active Two-Dimensional Catalysts for Hydrogen Evolution. Mater. Today 2019, 25, 28–34. [Google Scholar] [CrossRef]
- Liu, D.B.; Li, X.Y.; Chen, S.M.; Yang, H.; Wang, C.D.; Wu, C.Q.; Haleem, Y.A.; Duan, S.; Lu, J.L.; Ge, B.H.; et al. Atomically Dispersed Platinum Supported on Curved Carbon Supports for Efficient Electrocatalytic Hydrogen Evolution. Nat. Energy. 2019, 4, 512–518. [Google Scholar] [CrossRef]
- You, B.; Tang, M.T.; Tsai, C.; Abild-Pedersen, F.; Zheng, X.L.; Li, H. Enhancing Electrocatalytic Water Splitting by Strain Engineering. Adv. Mater. 2019, 31, 1807001. [Google Scholar] [CrossRef]
- Turner, J.; Sverdrup, G.; Mann, M.K.; Maness, P.C.; Kroposki, B.; Ghirardi, M.; Evans, R.J.; Blake, D. Renewable Hydrogen Production. Int. J. Energy Res. 2008, 32, 379–407. [Google Scholar] [CrossRef]
- Yang, F.; Xiong, T.Z.; Huang, P.; Zhou, S.H.; Tan, Q.R.; Yang, H.; Huang, Y.C.; Balogun, M.S. Nanostructured Transition Metal Compounds Coated 3d Porous Core-Shell Carbon Fiber as Monolith Water Splitting Electrocatalysts: A General Strategy. Chem. Eng. J. 2021, 423, 130279. [Google Scholar] [CrossRef]
- Ruqia, B.; Choi, S.I. Pt and Pt-Ni(OH)2 Electrodes for the Hydrogen Evolution Reaction in Alkaline Electrolytes and Their Nanoscaled Electrocatalysts. Chemsuschem 2018, 11, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Bhalothia, D.; Huang, T.H.; Chang, C.W.; Lin, T.H.; Wu, S.C.; Wang, K.W.; Chen, T.Y. High-Performance and Stable Hydrogen Evolution Reaction Achieved by Pt Trimer Decoration on Ultralow-Metal Loading Bimetallic Ptpd Nanocatalysts. ACS Appl. Energy Mater. 2020, 3, 11142–11152. [Google Scholar] [CrossRef]
- Ye, S.H.; Luo, F.Y.; Zhang, Q.L.; Zhang, P.Y.; Xu, T.T.; Wang, Q.; He, D.S.; Guo, L.C.; Zhang, Y.; He, C.X.; et al. Highly Stable Single Pt Atomic Sites Anchored on Aniline-Stacked Graphene for Hydrogen Evolution Reaction. Energy Environ. Sci. 2019, 12, 1000–1007. [Google Scholar] [CrossRef]
- Bhalothia, D.; Shuan, L.; Wu, Y.J.; Yan, C.; Wang, K.W.; Chen, T.Y. A Highly Mismatched NiO2 to Pd Hetero Structure as an Efficient Nanocatalyst for the Hydrogen Evolution Reaction. Sustain. Energy Fuels 2020, 4, 2541–2550. [Google Scholar] [CrossRef]
- Wang, A.Q.; Li, J.; Zhang, T. Heterogeneous Single-Atom Catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Fu, S.F.; Shi, Q.R.; Du, D.; Lin, Y.H. Single-Atom Electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 13944–13960. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Tong, Y.; Chen, P.Z.; Zhou, B.C.; Dong, X.P. Universal Strategy of Bimetal Heterostructures as Superior Bifunctional Catalysts for Electrochemical Water Splitting. ACS Sus. Chem. Eng. 2021, 9, 4206–4212. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wang, E.Q.; Cui, S.Q.; Yang, S.B.; Zou, X.L.; Gong, Y.J. Single-Atom Pt Anchored on Oxygen Vacancy of Monolayer Ti3C2Tx for Superior Hydrogen Evolution. Nano Lett. 2022, 22, 1398–1405. [Google Scholar] [CrossRef]
- Cui, Y.L.S.; Cao, Z.J.; Zhang, Y.Z.; Chen, H.; Gu, J.N.; Du, Z.G.; Shi, Y.Z.; Li, B.; Yang, S.B. Single-Atom Sites on MXenes for Energy Conversion and Storage. Small Sci. 2021, 1, 2100017. [Google Scholar] [CrossRef]
- Voiry, D.; Salehi, M.; Silva, R.; Fujita, T.; Chen, M.w.; Asefa, T.; Shenoy, V.B.; Eda, G.; Chhowalla, M. Conducting MoS2 Nanosheets as Catalysts for Hydrogen Evolution Reaction. Nano Lett. 2013, 13, 6222–6227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Fan, H.B.; Ignaszak, A.; Zhang, L.; Shao, S.Q.; Wilkinson, D.P.; Zhang, J.J. Compositing Doped-Carbon with Metals, Non-Metals, Metal Oxides, Metal Nitrides and Other Materials to Form Bifunctional Electrocatalysts to Enhance Metal-Air Battery Oxygen Reduction and Evolution Reactions. Chem. Eng. J. 2018, 348, 416–437. [Google Scholar] [CrossRef]
- Zhao, X.H.; Xue, Z.M.; Chen, W.J.; Wang, Y.Q.; Mu, T.C. Eutectic Synthesis of High-Entropy Metal Phosphides for Electrocatalytic Water Splitting. ChemSusChem 2020, 13, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.S.; Chen, J.P.; Jiang, P.P.; Leng, Y.; Jin, W. N-Doped Carbon-Coated Metal Sulfides/Phosphides Derived from Protic Salts for Oxygen Evolution Reaction. ChemCatChem 2019, 11, 1185–1191. [Google Scholar] [CrossRef]
- Kelly, T.G.; Hunt, S.T.; Esposito, D.V.; Chen, J.G. Monolayer Palladium Supported on Molybdenum and Tungsten Carbide Substrates as Low-Cost Hydrogen Evolution Reaction (HER) Electrocatalysts. Int. J. Hydrogen Energy 2013, 38, 5638–5644. [Google Scholar] [CrossRef]
- Kelly, T.G.; Chen, J.G. Metal Overlayer on Metal Carbide Substrate: Unique Bimetallic Properties for Catalysis and Electrocatalysis. Chem. Soc. Rev. 2012, 41, 8021–8034. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Rajbongshi, B.M.; Ramani, V.; Verma, A. Titanium Carbide: An Emerging Electrocatalyst for Fuel Cell and Electrolyser. Int. J. Hydrogen Energy 2021, 46, 12801–12821. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.H.; Shi, F.; Zhan, J.Y.; Tu, J.P.; Fan, H.J. Transition Metal Carbides and Nitrides in Energy Storage and Conversion. Sci. Adv. 2016, 3, 1500286. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.H.; Zhao, Y.; Zhou, G.M.; Lv, W.; Sun, P.J.; Kang, F.Y.; Li, B.H.; Yang, Q.H. An in-Plane Heterostructure of Graphene and Titanium Carbide for Efficient Polysulfide Confinement. Nano Energy 2017, 39, 291–296. [Google Scholar] [CrossRef]
- Rasaki, S.A.; Zhang, B.X.; Anbalgam, K.; Thomas, T.; Yang, M.H. Synthesis and Application of Nano-Structured Metal Nitrides and Carbides: A Review. Prog. Solid State Chem. 2018, 50, 1–15. [Google Scholar] [CrossRef]
- Chen, P.R.; Ye, J.S.; Wang, H.; Ouyang, L.Z.; Zhu, M. Recent Progress of Transition Metal Carbides/Nitrides for Electrocatalytic Water Splitting. J. Alloys Compd. 2021, 883, 160833. [Google Scholar] [CrossRef]
- Chen, W.F.; Muckerman, J.T.; Fujita, E. Recent Developments in Transition Metal Carbides and Nitrides as Hydrogen Evolution Electrocatalysts. Chem. Commun. 2013, 49, 8896–8909. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhao, X.H.; Xiong, Y.S.; Tang, Y.H.; Ma, X.T.; Tao, Q.; Sun, C.M.; Xu, W.L. A Review of Etching Methods of Mxene and Applications of MXene Conductive Hydrogels. Eur. Polym. J. 2022, 167, 111063. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lee, J.M. Recent Advances in Structural Engineering of MXene Electrocatalysts. J. Mater. Chem. A 2020, 8, 10604–10624. [Google Scholar] [CrossRef]
- Lu, J.; Persson, I.; Lind, H.; Palisaitis, J.; Li, M.; Li, Y.; Chen, K.; Zhou, J.; Du, S.; Chai, Z.; et al. Tin+1Cn MXenes with Fully Saturated and Thermally Stable Cl Terminations. Nanoscale Adv. 2019, 1, 3680–3685. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Li, R.X.; Jiang, C.L.; Qi, R.J.; Liu, M.Q.; Luo, C.H.; Lin, H.C.; Huang, R.; Peng, H. Facile Synthesis of Cobalt Modified 2D Titanium Carbide with Enhanced Hydrogen Evolution Performance in Alkaline Media. Int. J. Hydrogen Energy 2021, 46, 32536–32545. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, L.; Luo, B.; Lyu, M.; Wang, Z.L.; Huang, H.M.; Wang, S.C.; Du, A.J.; Wang, L.Z. Molten-Salt-Mediated Synthesis of an Atomic Nickel Co-Catalyst on TiO2 for Improved Photocatalytic H2 Evolution. Angew. Chem. Int. Ed. 2020, 59, 7230–7234. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, C.; Hu, R.M.; Du, Z.G.; Gu, J.N.; Cui, Y.L.S.; Chen, X.; Xu, W.J.; Cheng, Z.J.; Li, S.M.; et al. Selective Etching Quaternary MAX Phase toward Single Atom Copper Immobilized MXene (Ti3C2Clx) for Efficient Co2 Electroreduction to Methanol. ACS Nano 2021, 15, 4927–4936. [Google Scholar] [CrossRef]
- Song, H.R.; Du, R.; Wang, Y.W.; Zu, D.Y.; Zhou, R.; Cai, Y.; Wang, F.X.; Li, Z.; Shen, Y.M.; Li, C.P. Anchoring Single Atom Cobalt on Two-Dimensional Mxene for Activation of Peroxymonosulfate. Appl. Catal. B 2021, 286, 119898. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.B.; Chang, K.K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P.; et al. Element Replacement Approach by Reaction with Lewis Acidic Molten Salts to Synthesize Nanolaminated MAX Phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef] [PubMed]
- Halim, J.; Cook, K.M.; Naguib, M.; Eklund, P.; Gogotsi, Y.; Rosen, J.; Barsoum, M.W. X-Ray Photoelectron Spectroscopy of Select Multi-Layered Transition Metal Carbides (MXenes). Appl. Surf. Sci. 2016, 362, 406–417. [Google Scholar] [CrossRef]
- Han, M.K.; Yin, X.W.; Wu, H.; Hou, Z.X.; Song, C.Q.; Li, X.L.; Zhang, L.T.; Cheng, L.F. Ti3C2 Mxenes with Modified Surface for High-Performance Electromagnetic Absorption and Shielding in the X-Band. ACS Appl. Mat. Interfaces 2016, 8, 21011–21019. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Pei, Z.X.; Huang, Y.; Zhu, M.S.; Tang, Z.J.; Li, H.F.; Huang, Y.; Li, N.; Zhang, H.Y.; Zhi, C.Y. Mn3O4 Nanoparticles on Layer-Structured Ti3C2 MXene Towards the Oxygen Reduction Reaction and Zinc-Air Batteries. J. Mater. Chem. A 2017, 5, 20818–20823. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, Z.R.; Xiao, Y.Y.; Yin, Y.C.; Huang, W.M.; Huang, Z.C.; Zheng, Y.Z.; Mu, F.Y.; Huang, R.; Shi, G.Y.; et al. Electronic Metal-Support Interaction Modulates Single-Atom Platinum Catalysis for Hydrogen Evolution Reaction. Nat. Commun. 2021, 12, 3021. [Google Scholar] [CrossRef]
- Zhang, N.; Gan, S.; Wu, T.; Ma, W.; Han, D.; Niu, L. Growth Control of MoS2 Nanosheets on Carbon Cloth for Maximum Active Edges Exposed: An Excellent Hydrogen Evolution 3D Cathode. ACS Appl. Mat. Interfaces 2015, 7, 12193–12202. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.D.; Bai, L.; Li, M.; Guan, J.Q. Atomically Dispersed Cobalt- and Nitrogen-Codoped Graphene toward Bifunctional Catalysis of Oxygen Reduction and Hydrogen Evolution Reactions. ACS Sus. Chem. Eng. 2019, 7, 9249–9256. [Google Scholar] [CrossRef]
- Norskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Norskov, J.K. Trends in the Exchange Current for Hydrogen Evolution. J. Electrochem. Soc. 2005, 152, J23–J26. [Google Scholar] [CrossRef]
- Yu, J.; Li, J.; Xu, C.Y.; Li, Q.q.; Liu, Q.; Liu, J.Y.; Chen, R.R.; Zhu, J.H.; Wang, J. Modulating the D-Band Centers by Coordination Environment Regulation of Single-Atom Ni on Porous Carbon Fibers for Overall Water Splitting. Nano Energy 2022, 98, 107266. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Chang, K.C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N.M. Trends in Activity for the Water Electrolyser Reactions on 3d M(Ni,Co,Fe,Mn) Hydr(Oxy)Oxide Catalysts. Nat. Mater. 2012, 11, 550–557. [Google Scholar] [CrossRef]
- Danilovic, N.; Subbaraman, R.; Strmcnik, D.; Chang, K.C.; Paulikas, A.P.; Stamenkovic, V.R.; Markovic, N.M. Enhancing the Alkaline Hydrogen Evolution Reaction Activity through the Bifunctionality of Ni(OH)2/Metal Catalysts. Angew. Chem. Int. Ed. 2012, 51, 12495–12498. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.L.; Chen, C.; Guo, J.N.; Bi, R.; Chen, S.; Zhang, L.; Zhu, M. Pt Nanoclusters Anchored on Ordered Macroporous Nitrogen-Doped Carbon for Accelerated Water Dissociation toward Superior Alkaline Hydrogen Production. Chem. Eng. J. 2022, 436, 135186. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Mun, B.S.; Mayrhofer, K.J.J.; Ross, P.N.; Markovic, N.M.; Rossmeisl, J.; Greeley, J.; Norskov, J.K. Changing the Activity of Electrocatalysts for Oxygen Reduction by Tuning the Surface Electronic Structure. Angew. Chem. Int. Ed. 2006, 45, 2897–2901. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.; Norskov, J.K. Why Gold is the Noblest of All the Metals. Nature 1995, 376, 238–240. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab Initio Molecular-Dynamics Simulation of the Liquid-Metalamorphous-Semiconductor Transition in Germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, J.; Luo, B.; Tian, E.; Waterhouse, G.I.N. Central Metal and Ligand Effects on Oxygen Electrocatalysis over 3d Transition Metal Single-Atom Catalysts: A Theoretical Investigation. Chem. Eng. J. 2022, 427, 132038. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Norskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Deng, J.; Ren, P.; Deng, D.; Yu, L.; Yang, F.; Bao, X. Highly Active and Durable Non-Precious-Metal Catalysts Encapsulated in Carbon Nanotubes for Hydrogen Evolution Reaction. Energy Environ. Sci. 2014, 7, 1919–1923. [Google Scholar] [CrossRef]
- Liu, X.; Jiao, Y.; Zheng, Y.; Davey, K.; Qiao, S.-Z. A Computational Study on Pt and Ru Dimers Supported on Graphene for the Hydrogen Evolution Reaction: New Insight into the Alkaline Mechanism. J. Mater. Chem. A 2019, 7, 3648–3654. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Yu, J.; Liu, Q.; Liu, J.; Chen, R.; Zhu, J. Loading of Single Atoms of Iron, Cobalt, or Nickel to Enhance the Electrocatalytic Hydrogen Evolution Reaction of Two-Dimensional Titanium Carbide. Int. J. Mol. Sci. 2024, 25, 4034. https://doi.org/10.3390/ijms25074034
Wang K, Yu J, Liu Q, Liu J, Chen R, Zhu J. Loading of Single Atoms of Iron, Cobalt, or Nickel to Enhance the Electrocatalytic Hydrogen Evolution Reaction of Two-Dimensional Titanium Carbide. International Journal of Molecular Sciences. 2024; 25(7):4034. https://doi.org/10.3390/ijms25074034
Chicago/Turabian StyleWang, Kaijin, Jing Yu, Qi Liu, Jingyuan Liu, Rongrong Chen, and Jiahui Zhu. 2024. "Loading of Single Atoms of Iron, Cobalt, or Nickel to Enhance the Electrocatalytic Hydrogen Evolution Reaction of Two-Dimensional Titanium Carbide" International Journal of Molecular Sciences 25, no. 7: 4034. https://doi.org/10.3390/ijms25074034
APA StyleWang, K., Yu, J., Liu, Q., Liu, J., Chen, R., & Zhu, J. (2024). Loading of Single Atoms of Iron, Cobalt, or Nickel to Enhance the Electrocatalytic Hydrogen Evolution Reaction of Two-Dimensional Titanium Carbide. International Journal of Molecular Sciences, 25(7), 4034. https://doi.org/10.3390/ijms25074034







