FTO Positively Regulates Odontoblastic Differentiation via SMOC2 in Human Stem Cells from the Apical Papilla under Inflammatory Microenvironment
Abstract
:1. Introduction
2. Results
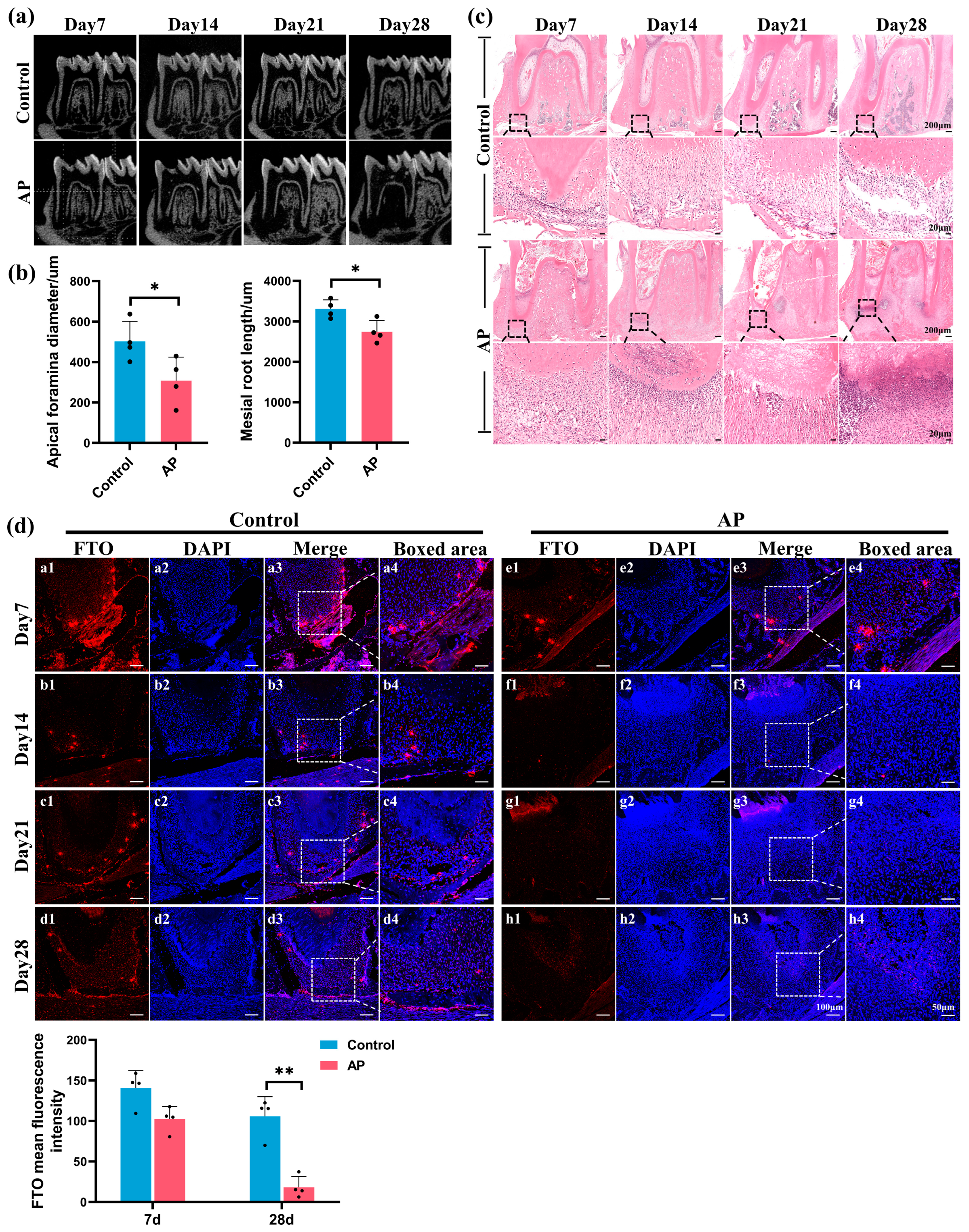
2.1. FTO Significantly Downregulated in Immature Teeth with AP
2.2. Characterization of hSCAPs and Establishment of In Vitro Model
2.3. Downregulation of FTO Expression and Odontoblastic Differentiation Ability in hSCAPs under LPS Stimulation
2.4. FTO Knockdown Impaired Odontoblastic Differentiation in hSCAPs
2.5. FTO Overexpression Rescued LPS-Induced Suppression of Odontoblastic Differentiation in hSCAPs
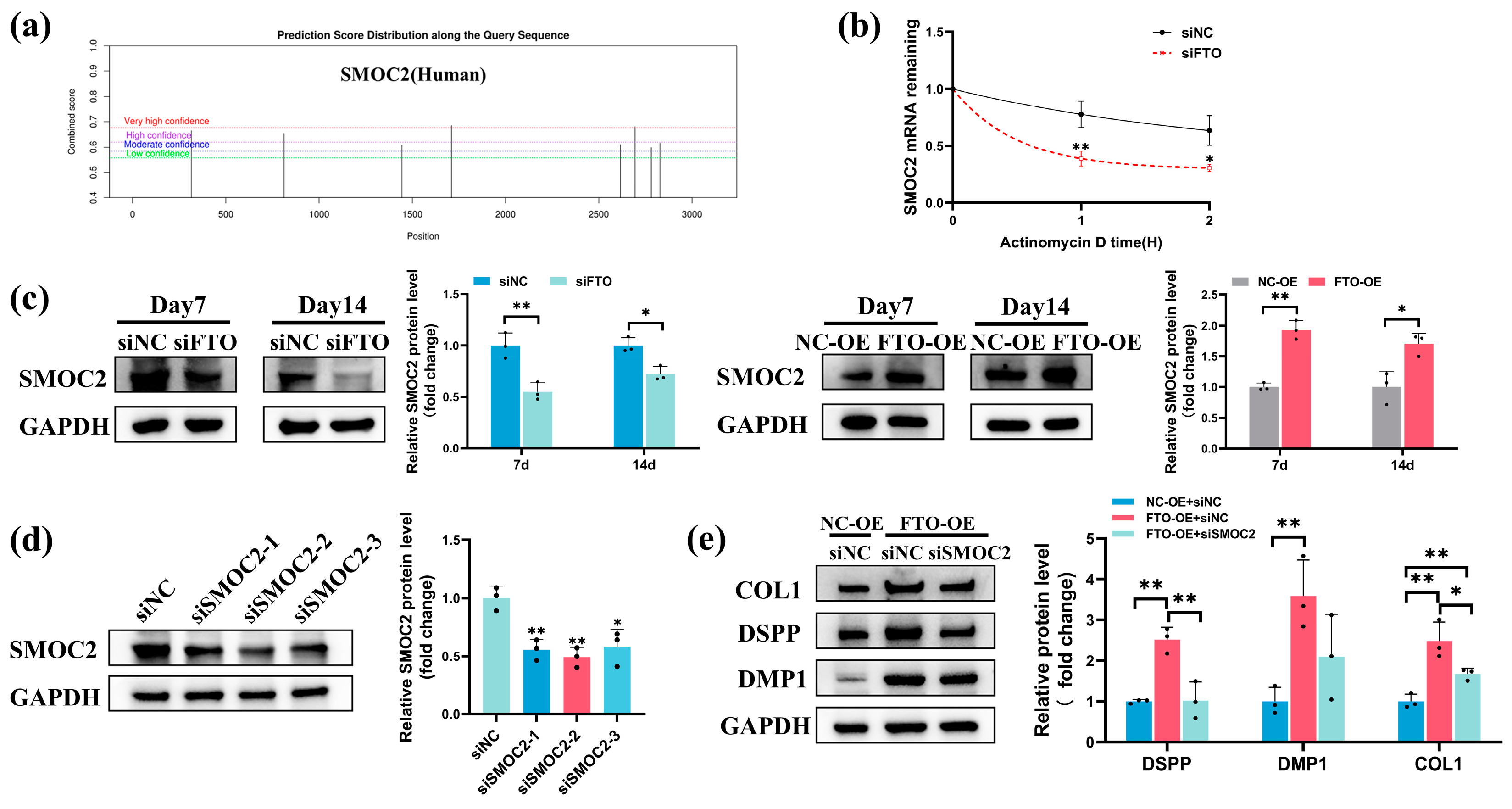
2.6. FTO Modulated SMOC2 Expression during Odontoblastic Differentiation of hSCAPs
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Micro-Computed Tomography (micro-CT) Analysis
4.3. Histological Analysis
4.4. Cell Culture and Identification
4.5. Cell Viability and Migration
4.6. Alkaline Phosphatase (ALP) and Alizarin Red S (ARS) Staining
4.7. Cell Transfection and IF Staining
4.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.9. mRNA Stability Assay
4.10. Western Blotting
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwon, S.K.; Kyeong, M.; Adasooriya, D.; Cho, S.W.; Jung, I.Y. Histologic and Electron Microscopic Characterization of a Human Immature Permanent Premolar with Chronic Apical Abscess 16 Years after Regenerative Endodontic Procedures. J. Endod. 2023, 49, 1051–1057. [Google Scholar] [CrossRef]
- Nosrat, A.; Kolahdouzan, A.; Khatibi, A.H.; Verma, P.; Jamshidi, D.; Nevins, A.J.; Torabinejad, M. Clinical, Radiographic, and Histologic Outcome of Regenerative Endodontic Treatment in Human Teeth Using a Novel Collagen-hydroxyapatite Scaffold. J. Endod. 2019, 45, 136–143. [Google Scholar] [CrossRef]
- Digka, A.; Sakka, D.; Lyroudia, K. Histological assessment of human regenerative endodontic procedures (REP) of immature permanent teeth with necrotic pulp/apical periodontitis: A systematic review. Aust. Endod. J. 2020, 46, 140–153. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, Y.; Chen, Y.; Chen, S.; Lyu, H.; Cai, Z.; Huang, X. Radiographic, Histologic, and Biomechanical Evaluation of Combined Application of Platelet-rich Fibrin with Blood Clot in Regenerative Endodontics. J. Endod. 2017, 43, 2034–2040. [Google Scholar] [CrossRef]
- Wei, X.; Yang, M.; Yue, L.; Huang, D.; Zhou, X.; Wang, X.; Zhang, Q.; Qiu, L.; Huang, Z.; Wang, H.; et al. Expert consensus on regenerative endodontic procedures. Int. J. Oral Sci. 2022, 14, 411–423. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; He, J. Stem Cells from the Apical Papilla (SCAPs): Past, Present, Prospects, and Challenges. Biomedicines 2023, 11, 2047. [Google Scholar] [CrossRef]
- Zymovets, V.; Razghonova, Y.; Rakhimova, O.; Aripaka, K.; Manoharan, L.; Kelk, P.; Landström, M.; Romani Vestman, N. Combined Transcriptomic and Protein Array Cytokine Profiling of Human Stem Cells from Dental Apical Papilla Modulated by Oral Bacteria. Int. J. Mol. Sci. 2022, 23, 5098. [Google Scholar] [CrossRef]
- Lei, S.; Liu, X.M.; Liu, Y.; Bi, J.; Zhu, S.; Chen, X. Lipopolysaccharide Downregulates the Osteo-/Odontogenic Differentiation of Stem Cells From Apical Papilla by Inducing Autophagy. J. Endod. 2020, 46, 502–508. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F., Jr.; Loghin, S.; Lin, L.M. Pulp and apical tissue response to deep caries in immature teeth: A histologic and histobacteriologic study. J. Dent. 2017, 56, 19–32. [Google Scholar] [CrossRef]
- Bucchi, C.; Bucchi, A.; Martínez-Rodríguez, P. Biological properties of stem cells from the apical papilla exposed to lipopolysaccharides: An in vitro study. Arch. Oral Biol. 2024, 159, 105876. [Google Scholar] [CrossRef]
- Cai, W.; Ji, Y.; Han, L.; Zhang, J.; Ni, Y.; Cheng, Y.; Zhang, Y. METTL3-Dependent Glycolysis Regulates Dental Pulp Stem Cell Differentiation. J. Dent. Res. 2022, 101, 580–589. [Google Scholar] [CrossRef]
- Luo, H.; Liu, W.; Zhang, Y.; Yang, Y.; Jiang, X.; Wu, S.; Shao, L. METTL3-mediated m6A modification regulates cell cycle progression of dental pulp stem cells. Stem Cell Res. Ther. 2021, 12, 159. [Google Scholar] [CrossRef]
- Xie, F.; Zhu, X.; Liu, X.; Chen, H.; Wang, J. N6-methyladenosine (m6A) RNA methylation mediated by methyltransferase complex subunit WTAP regulates amelogenesis. J. Biol. Chem. 2022, 298, 102715. [Google Scholar] [CrossRef]
- Li, Y.; Su, R.; Deng, X.; Chen, Y.; Chen, J. FTO in cancer: Functions, molecular mechanisms, and therapeutic implications. Trends Cancer 2022, 8, 598–614. [Google Scholar] [CrossRef]
- Boissel, S.; Reish, O.; Proulx, K.; Kawagoe-Takaki, H.; Sedgwick, B.; Yeo, G.S.; Meyre, D.; Golzio, C.; Molinari, F.; Kadhom, N.; et al. Loss-of-function mutation in the dioxygenase-encoding FTO gene causes severe growth retardation and multiple malformations. Am. J. Hum. Genet. 2009, 85, 106–111. [Google Scholar] [CrossRef]
- Daoud, H.; Zhang, D.; McMurray, F.; Yu, A.; Luco, S.M.; Vanstone, J.; Jarinova, O.; Carson, N.; Wickens, J.; Shishodia, S.; et al. Identification of a pathogenic FTO mutation by next-generation sequencing in a newborn with growth retardation and developmental delay. J. Med. Genet. 2016, 53, 200–207. [Google Scholar] [CrossRef]
- Sachse, G.; Church, C.; Stewart, M.; Cater, H.; Teboul, L.; Cox, R.D.; Ashcroft, F.M. FTO demethylase activity is essential for normal bone growth and bone mineralization in mice. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1846, 843–850. [Google Scholar] [CrossRef]
- Zhang, Q.; Riddle, R.C.; Yang, Q.; Rosen, C.R.; Guttridge, D.C.; Dirckx, N.; Faugere, M.C.; Farber, C.R.; Clemens, T.L. The RNA demethylase FTO is required for maintenance of bone mass and functions to protect osteoblasts from genotoxic damage. Proc. Natl. Acad. Sci. USA 2019, 116, 17980–17989. [Google Scholar] [CrossRef]
- Zhao, T.; Tao, Z.; Zhang, G.; Zhu, J.; Du, M.; Hua, F.; He, H. Fat mass and obesity-associated protein (FTO) affects midpalatal suture bone remodeling during rapid maxillary expansion. Eur. J. Orthod. 2024, 46, cjae009. [Google Scholar] [CrossRef]
- Xu, M.; Li, B.; Huang, J.; Jia, R.; Guo, J. The N6-methyladenosine demethylase FTO is required for odontoblast differentiation in vitro and dentine formation in mice by promoting RUNX2 exon 5 inclusion through RBM4. Int. Endod. J. 2023, 56, 1534–1549. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, T.; Li, B.; Li, M.; Luo, P.; Zhang, C.; Chen, G.; Cao, Z.; Li, Y.; Du, M.; et al. FTO/RUNX2 signaling axis promotes cementoblast differentiation under normal and inflammatory condition. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119358. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, C.; Liu, Y.; Chen, Z.; Liu, W.; Yang, F.; Zeng, F.; Guo, Q. Demethylase FTO promotes mechanical stress induced osteogenic differentiation of BMSCs with up-regulation of HIF-1α. Mol. Biol. Rep. 2022, 49, 2777–2784. [Google Scholar] [CrossRef]
- Chen, L.S.; Zhang, M.; Chen, P.; Xiong, X.F.; Liu, P.Q.; Wang, H.B.; Wang, J.J.; Shen, J. The m6A demethylase FTO promotes the osteogenesis of mesenchymal stem cells by downregulating PPARG. Acta Pharmacol. Sin. 2022, 43, 1311–1323. [Google Scholar] [CrossRef]
- Son, H.E.; Min, H.Y.; Kim, E.J.; Jang, W.G. Fat Mass and Obesity-Associated (FTO) Stimulates Osteogenic Differentiation of C3H10T1/2 Cells by Inducing Mild Endoplasmic Reticulum Stress via a Positive Feedback Loop with p-AMPK. Mol. Cells. 2020, 43, 58–65. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Z.; Shi, Y.; Wang, S.; Ren, J.; Yu, Z.; Huang, D.; Yan, K.; He, Y.; Liu, X.; et al. FTO stabilizes MIS12 and counteracts senescence. Protein. Cell. 2022, 13, 954–960. [Google Scholar] [CrossRef]
- Cao, Y.; Zhuang, Y.; Chen, J.; Xu, W.; Shou, Y.; Huang, X.; Shu, Q.; Li, X. Dynamic effects of Fto in regulating the proliferation and differentiation of adult neural stem cells of mice. Hum. Mol. Genet. 2020, 29, 727–735. [Google Scholar] [CrossRef]
- Su, T.; Zhu, Y.; Wang, X.; Zhu, Q.; Duan, X. Hereditary dentin defects with systemic diseases. Oral Dis. 2023, 29, 2376–2393. [Google Scholar] [CrossRef]
- Bloch-Zupan, A.; Jamet, X.; Etard, C.; Laugel, V.; Muller, J.; Geoffroy, V.; Strauss, J.P.; Pelletier, V.; Marion, V.; Poch, O.; et al. Homozygosity mapping and candidate prioritization identify mutations, missed by whole-exome sequencing, in SMOC2, causing major dental developmental defects. Am. J. Hum. Genet. 2011, 89, 773–781. [Google Scholar] [CrossRef]
- Alfawaz, S.; Fong, F.; Plagnol, V.; Wong, F.S.; Fearne, J.; Kelsell, D.P. Recessive oligodontia linked to a homozygous loss-of-function mutation in the SMOC2 gene. Arch. Oral Biol. 2013, 58, 462–466. [Google Scholar] [CrossRef]
- Ruan, W.; Duan, X. A new SMOC2 mutation within selective tooth agenesis, malformed teeth and dentin dysplasia. Clin. Genet. 2022, 102, 352–354. [Google Scholar] [CrossRef]
- Chen, D.; Li, X.; Lu, F.; Wang, Y.; Xiong, F.; Li, Q. Dentin dysplasia type I-A dental disease with genetic heterogeneity. Oral Dis. 2019, 25, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Morkmued, S.; Clauss, F.; Schuhbaur, B.; Fraulob, V.; Mathieu, E.; Hemmerlé, J.; Clevers, H.; Koo, B.K.; Dollé, P.; Bloch-Zupan, A.; et al. Deficiency of the SMOC2 matricellular protein impairs bone healing and produces age-dependent bone loss. Sci. Rep. 2020, 10, 14817. [Google Scholar] [CrossRef] [PubMed]
- Takahata, Y.; Hagino, H.; Kimura, A.; Urushizaki, M.; Kobayashi, S.; Wakamori, K.; Fujiwara, C.; Nakamura, E.; Yu, K.; Kiyonari, H.; et al. Smoc1 and Smoc2 regulate bone formation as downstream molecules of Runx2. Commun. Biol. 2021, 4, 1199. [Google Scholar] [CrossRef]
- Liu, D.; Li, R.; Xu, S.; Shi, M.; Kuang, Y.; Wang, J.; Shen, C.; Qiu, Q.; Liang, L.; Xiao, Y.; et al. SMOC2 promotes aggressive behavior of fibroblast-like synoviocytes in rheumatoid arthritis through transcriptional and post-transcriptional regulating MYO1C. Cell Death Dis. 2022, 13, 1035. [Google Scholar] [CrossRef]
- Almutairi, W.; Al-Dahman, Y.; Alnassar, F.; Albalawi, O. Intracanal calcification following regenerative endodontic treatment: A systematic review and meta-analysis. Clin. Oral Investig. 2022, 26, 3333–3342. [Google Scholar] [CrossRef]
- Jung, C.; Kim, S.; Sun, T.; Cho, Y.B.; Song, M. Pulp-dentin regeneration: Current approaches and challenges. J. Tissue Eng. 2019, 10, 2041731418819263. [Google Scholar] [CrossRef]
- Lu, J.; Lu, Y.; Lu, Z.; Kahler, B. Clinical and radiographic outcomes of regenerative endodontic procedures for traumatized permanent necrotic teeth with apical periodontitis and external root resorption. Int. Endod. J. 2023, 56, 802–818. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, Y.; Lin, Y.; Si, S.; Guo, B.; Zhao, X.; Cui, L. Epitranscriptomic modifications in mesenchymal stem cell differentiation: Advances, mechanistic insights, and beyond. Cell Death Differ. 2024, 31, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, C.; Wang, N.; Li, S.; Liu, Q.; Sun, Z.; Wang, K.; Yu, S.C.; Yang, Q. NADP modulates RNA m6A methylation and adipogenesis via enhancing FTO activity. Nat. Chem. Biol. 2020, 16, 1394–1402. [Google Scholar] [CrossRef]
- Mathiyalagan, P.; Adamiak, M.; Mayourian, J.; Sassi, Y.; Liang, Y.; Agarwal, N.; Jha, D.; Zhang, S.; Kohlbrenner, E.; Chepurko, E.; et al. FTO-Dependent N6-Methyladenosine Regulates Cardiac Function During Remodeling and Repair. Circulation 2019, 139, 518–532. [Google Scholar] [CrossRef]
- Sheng, R.; Wang, Y.; Wu, Y.; Wang, J.; Zhang, S.; Li, Q.; Zhang, D.; Qi, X.; Xiao, Q.; Jiang, S.; et al. METTL3-Mediated m6 A mRNA Methylation Modulates Tooth Root Formation by Affecting NFIC Translation. J. Bone Miner. Res. 2021, 36, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Chai, J.; Liu, W.; Zhang, X.; Li, Y.; Zuo, H.; Yuan, G.; Zhang, H.; Liu, H.; Chen, Z. Role of the Demethylase AlkB Homolog H5 in the Promotion of Dentinogenesis. Front. Physiol. 2022, 13, 923185. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, T.W.; Henry, M.A.; Hargreaves, K.M.; Diogenes, A. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J. Endod. 2011, 37, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Wang, S.; Tang, H.; Li, D.; Zhou, F.; Xin, L.; He, Q.; Hu, S.; Zhang, T.; Chen, T.; et al. Dynamically Bioresponsive DNA Hydrogel Incorporated with Dual-Functional Stem Cells from Apical Papilla-Derived Exosomes Promotes Diabetic Bone Regeneration. ACS Appl. Mater. Interfaces 2022, 14, 16082–16099. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Liu, Y.; Yu, S.; Jiang, L.M.; Song, B.; Chen, X. Potential immunomodulatory effects of stem cells from the apical papilla on Treg conversion in tissue regeneration for regenerative endodontic treatment. Int. Endod. J. 2019, 52, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, D.B.; Oliveira, A.R.; Seabra, C.M.; Palma, P.J.; Ramos, C.; Figueiredo, M.H.; Santos, A.C.; Cardoso, A.L.; Peça, J.; Santos, J.M. Regeneration of pulp-dentin complex using human stem cells of the apical papilla: In vivo interaction with two bioactive materials. Clin. Oral Investig. 2021, 25, 5317–5329. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xiong, H.; Chen, K.; Huang, Y.; Huang, Y.; Yin, X. Long-term exposure to pro-inflammatory cytokines inhibits the osteogenic/dentinogenic differentiation of stem cells from the apical papilla. Int. Endod. J. 2016, 49, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, S.; George, A. Multifunctional ECM proteins in bone and teeth. Exp. Cell Res. 2014, 325, 148–154. [Google Scholar] [CrossRef]
- Wang, J.; Fu, Q.; Yang, J.; Liu, J.L.; Hou, S.M.; Huang, X.; Cao, J.S.; Liu, T.L.; Wang, K.Z. RNA N6-methyladenosine demethylase FTO promotes osteoporosis through demethylating Runx2 mRNA and inhibiting osteogenic differentiation. Aging 2021, 13, 21134–21141. [Google Scholar] [CrossRef]
- Shen, G.S.; Zhou, H.B.; Zhang, H.; Chen, B.; Liu, Z.P.; Yuan, Y.; Zhou, X.Z.; Xu, Y.J. The GDF11-FTO-PPARγ axis controls the shift of osteoporotic MSC fate to adipocyte and inhibits bone formation during osteoporosis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3644–3654. [Google Scholar] [CrossRef]
- Rosset, E.M.; Bradshaw, A.D. SPARC/osteonectin in mineralized tissue. Matrix Biol. 2016, 52, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, M.; Zhou, Z.; Zou, X.; Song, G.; Zhang, Q.; Zhou, H. SMOC2 promoted vascular smooth muscle cell proliferation, migration, and extracellular matrix degradation by activating BMP/TGF-β1 signaling pathway. J. Clin. Biochem. Nutr. 2023, 73, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, S.; Shin, Y.; Lee, H.S.; Jeon, M.; Kim, S.O.; Cho, S.W.; Ruparel, N.B.; Song, J.S. Comparative Gene Expression Analysis of the Coronal Pulp and Apical Pulp Complex in Human Immature Teeth. J. Endod. 2016, 42, 752–759. [Google Scholar] [CrossRef]
- Krivanek, J.; Soldatov, R.A.; Kastriti, M.E.; Chontorotzea, T.; Herdina, A.N.; Petersen, J.; Szarowska, B.; Landova, M.; Matejova, V.K.; Holla, L.I.; et al. Dental cell type atlas reveals stem and differentiated cell types in mouse and human teeth. Nat. Commun. 2020, 11, 4816. [Google Scholar] [CrossRef] [PubMed]




| SMOC2 Transcript Variant 1 mRNA | SMOC2 Transcript Variant 2 mRNA | |
|---|---|---|
| Prediction using RF classifier | 0.55 | 0.55 |
| Prediction using SVM classifier | 0.908 | 0.897 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Sun, Y.; Huang, W.; Zhang, F.; He, H.; He, Y.; Huang, F. FTO Positively Regulates Odontoblastic Differentiation via SMOC2 in Human Stem Cells from the Apical Papilla under Inflammatory Microenvironment. Int. J. Mol. Sci. 2024, 25, 4045. https://doi.org/10.3390/ijms25074045
Huang Q, Sun Y, Huang W, Zhang F, He H, He Y, Huang F. FTO Positively Regulates Odontoblastic Differentiation via SMOC2 in Human Stem Cells from the Apical Papilla under Inflammatory Microenvironment. International Journal of Molecular Sciences. 2024; 25(7):4045. https://doi.org/10.3390/ijms25074045
Chicago/Turabian StyleHuang, Qi, Yumei Sun, Wushuang Huang, Fuping Zhang, Hongwen He, Yifan He, and Fang Huang. 2024. "FTO Positively Regulates Odontoblastic Differentiation via SMOC2 in Human Stem Cells from the Apical Papilla under Inflammatory Microenvironment" International Journal of Molecular Sciences 25, no. 7: 4045. https://doi.org/10.3390/ijms25074045
APA StyleHuang, Q., Sun, Y., Huang, W., Zhang, F., He, H., He, Y., & Huang, F. (2024). FTO Positively Regulates Odontoblastic Differentiation via SMOC2 in Human Stem Cells from the Apical Papilla under Inflammatory Microenvironment. International Journal of Molecular Sciences, 25(7), 4045. https://doi.org/10.3390/ijms25074045






