MiR-21 Regulates Growth and Migration of Cervical Cancer Cells by RECK Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. miR-21 and RECK Gene Expression in HPV-Transformed Cervical Cancer Cells
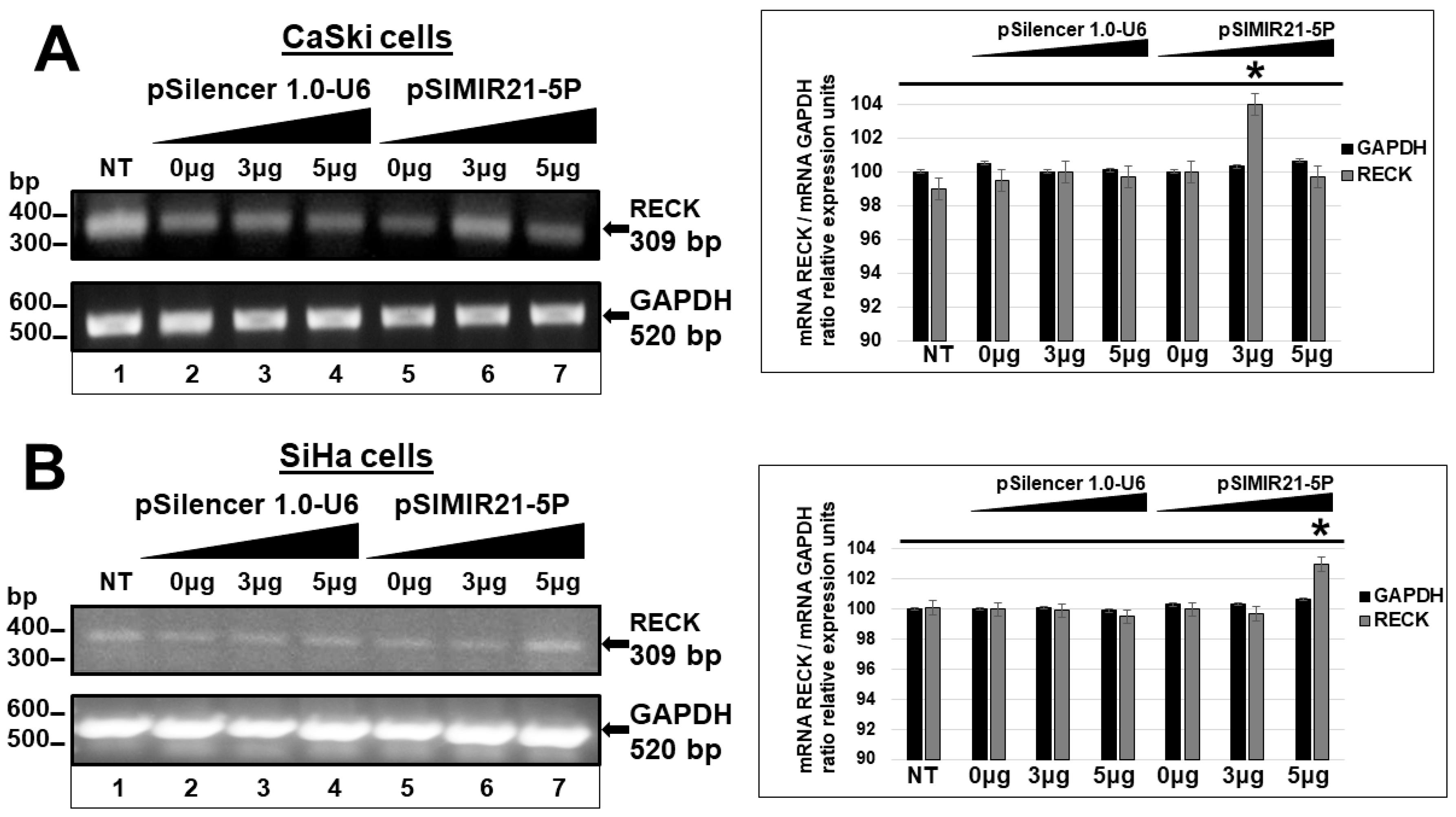
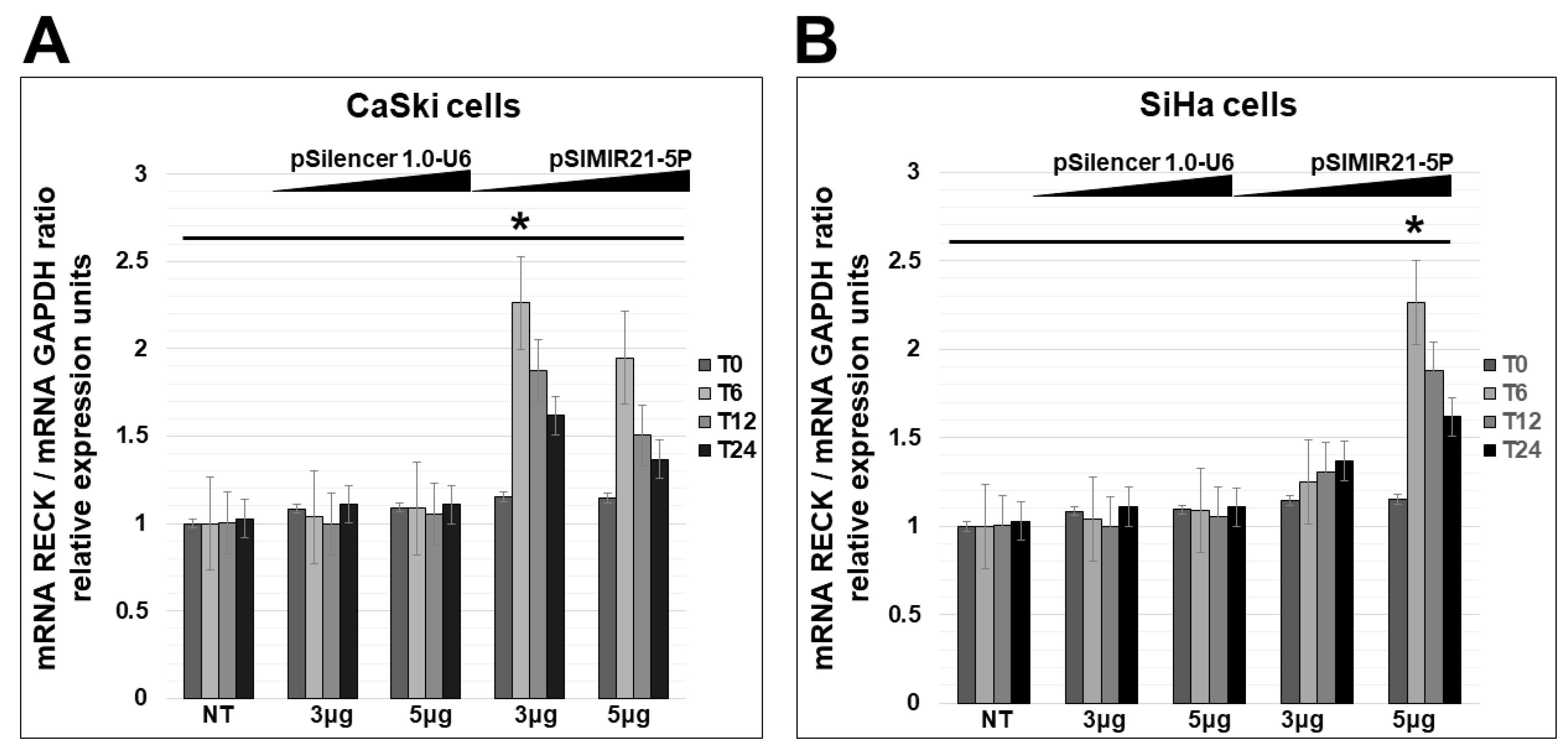
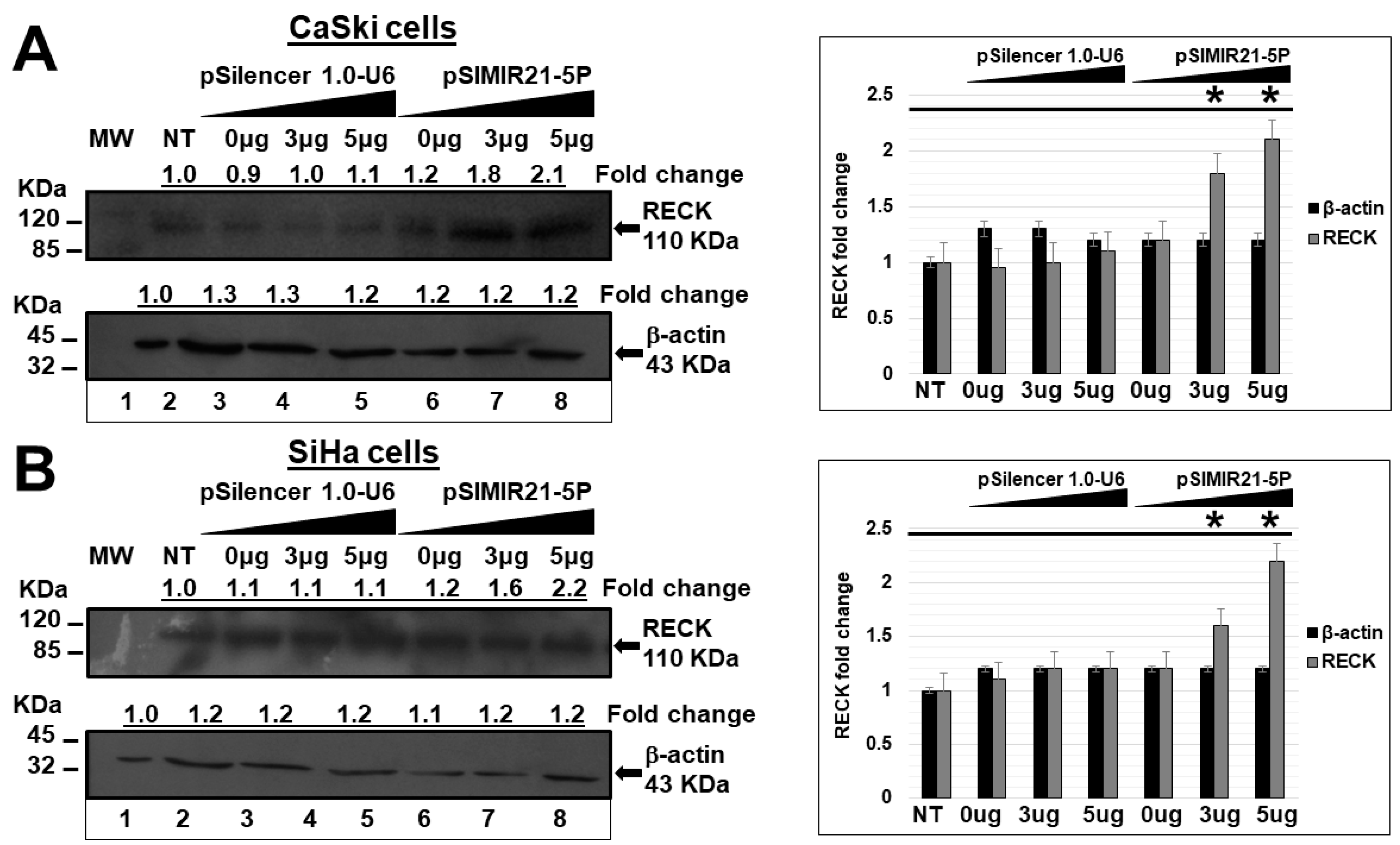
2.2. siRNA-Mediated Silencing of miR-21 Expression Has an Effect on RECK Expression
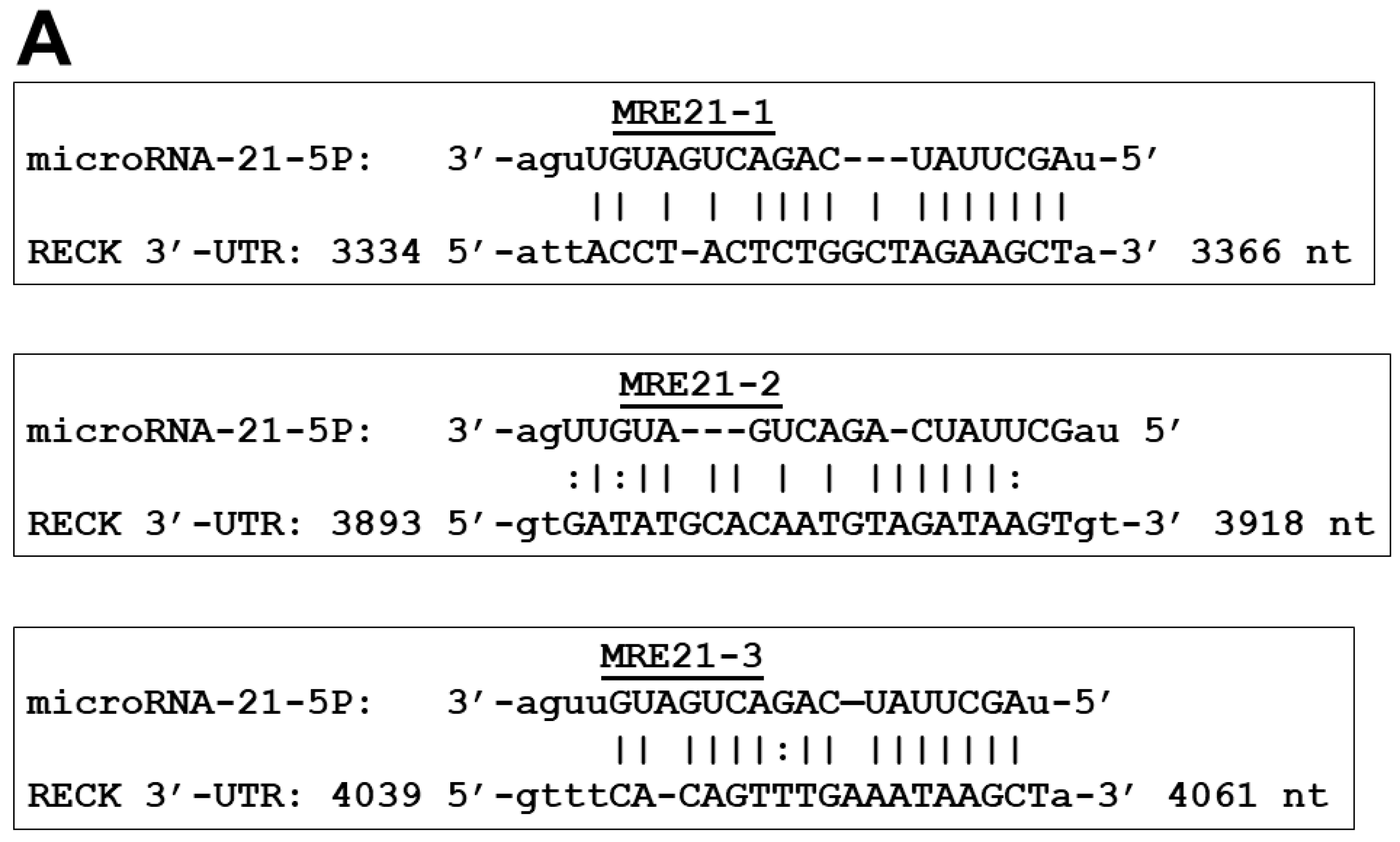
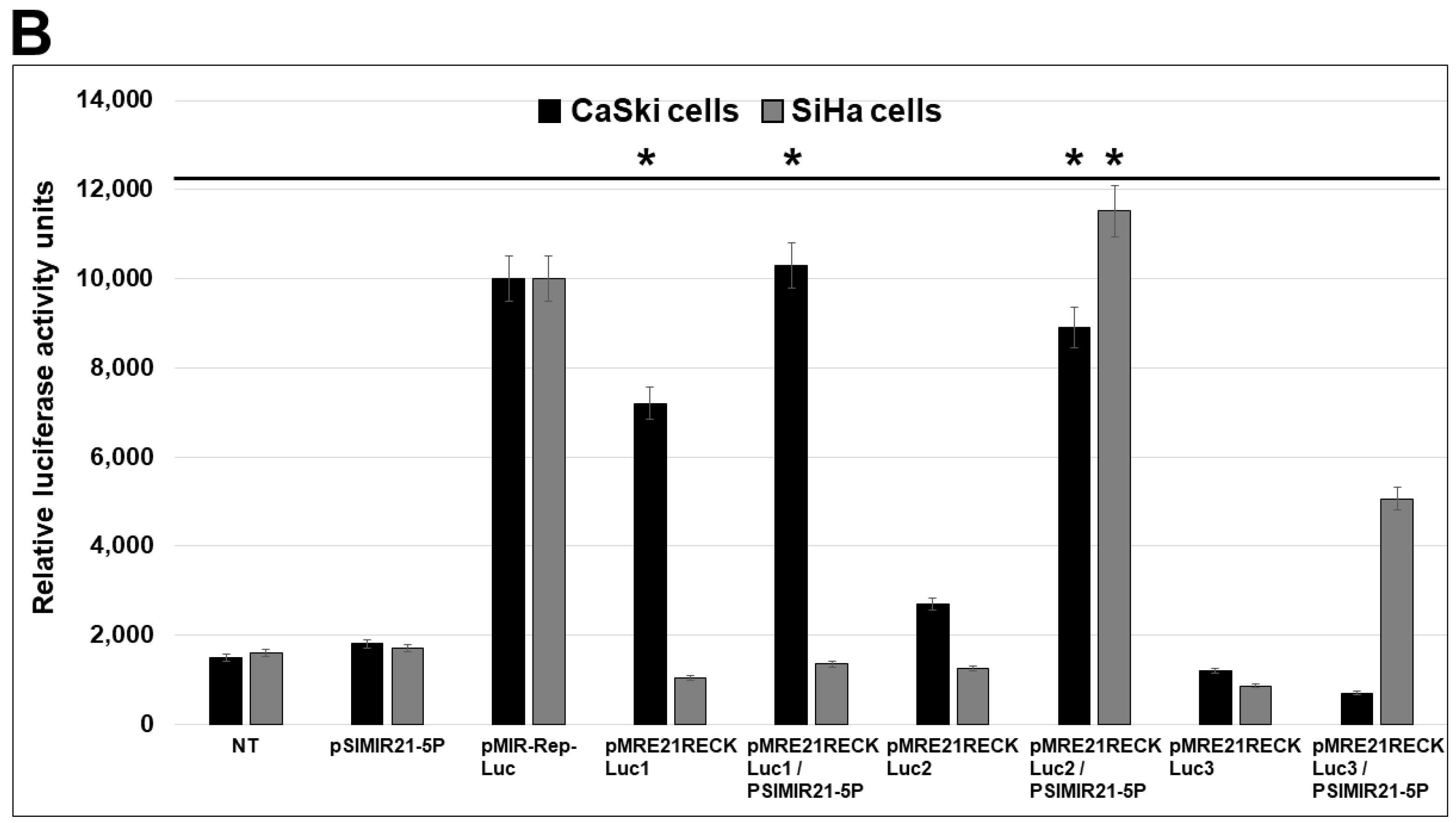
2.3. Specific MRE Recognition Sequences by miR-21 Are Critical for Regulation of RECK
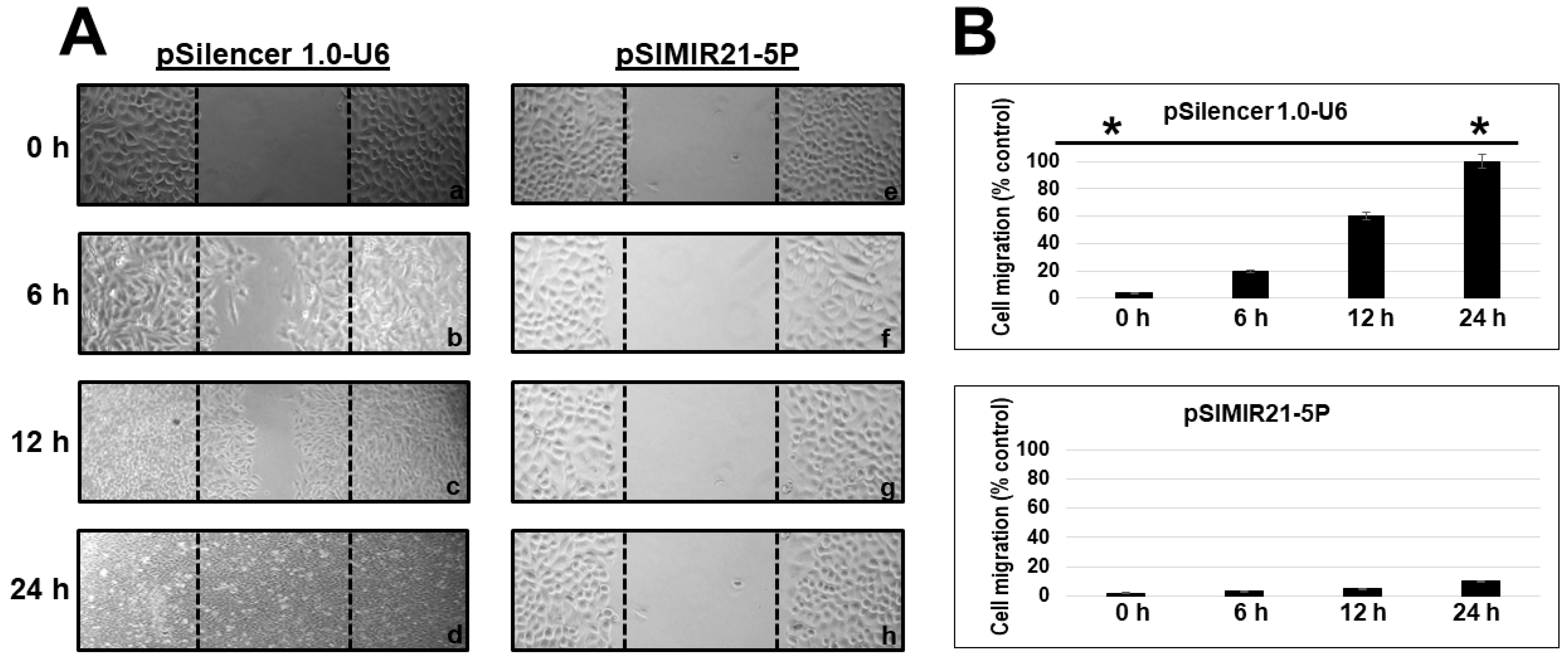
2.4. Silencing of miR-21 MicroRNA by siRNAs Induces Alterations in Tumor Cell Migration
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Conditions
4.2. Transfection Assays with siRNA Expression Plasmids to miR-21
4.3. Semiquantitative End-Point RT-PCR Analysis of RECK Gene
4.4. Quantitative Real-Time RT-PCR Analysis or RECK and miR-21 Genes
4.5. Western Blot Assays
4.6. Reporter Plasmids and Luciferase Activity Assays
4.7. Cellular Viability Assays
4.8. Cellular Migration Assays
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACAT1 | Acetyl-CoA acetyltransferase 1 |
| ATCC | American Type Culture Collection |
| BCA kit | (Bicinchoninic Acid) Protein Assay kit |
| BTG2 | Protein BTG2 also known as BTG family member 2 or NGF-inducible anti-proliferative protein PC3 or NGF-inducible protein TIS21 |
| CDH1 | Cadherin-1 or epithelial cadherin (E-cadherin) |
| CDK6 | Cyclin-dependent kinase 6 |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| DEPC | Diethylpyrocarbonate |
| DMEM | Dulbecco’s modified Eagle’s medium |
| EDTA | Ethylenediaminetetraacetic acid |
| FBS | Fetal bovine serum |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GAS5 | Growth arrest-specific 5 |
| GPI | Glycosylphosphatidylinositol |
| HIF1A | Hypoxia-inducible factor 1 alpha |
| HPV | Human papilloma virus |
| IDT | Integrated DNA Technologies |
| ITGB1 | Integrin beta-1, also known as CD29 |
| ITGB3 | Integrin beta-3, also known as CD61 |
| kDa | Kilo Dalton |
| MARCKS | Myristoylated alanine-rich C-kinase substrate |
| MMPs | Matrix metalloproteinases |
| MRE | MicroRNA response element |
| MTS | [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] |
| PDCD4 | Programmed cell death protein 4 |
| PEI | Polyethylenimine linear MW 25,000 transfection grade |
| PMSF | Phenylmethylsulfonyl fluoride |
| PTEN | Phosphatase and tensin homolog |
| RECK | Reversion-inducing-cysteine-rich protein with kazal motifs |
| RT-PCR | Reverse transcription polymerase chain reaction |
| RT-qPCR | Reverse transcription-quantitative polymerase chain reaction |
| SCC | Squamous cell carcinoma |
| siRNAs | Small interfering RNA, also known as short interfering RNA, or silencing RNA |
| TPM1 | Tropomyosin 1 |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Mackie, A.; Wentzensen, N. The IARC perspective on cervical cancer screening. Reply N. Engl. J. Med. 2022, 386, 607–608. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. Publisher Correction: The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 662, Erratum in Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Ismail, A.; Pappas-Gogos, G.; Boussios, S. HPV and cervical cancer: A review of epidemiology and screening uptake in the UK. Pathogens 2023, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- US National Institutes of Health. Database of Clinical Trials. Revision: v2.5.0. US Gov. Available online: http://clinicaltrials.gov/ct2/home (accessed on 31 March 2024).
- Mendonça, F.; Teles, A.M.; Nascimento, M.D.D.S.B.; Santos, A.P.A.D.; Lopes, F.F.; Paiva, A.; Brito, H.O.; Da Costa, R.G. Human papillomavirus modulates matrix metalloproteinases during carcinogenesis: Clinical significance and role of viral oncoproteins. In Vivo 2022, 36, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- Conlon, G.A.; Murray, G.I. Recent advances in understanding the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 2019, 247, 629–640. [Google Scholar] [CrossRef]
- Takahashi, C.; Sheng, Z.; Horan, T.P.; Kitayama, H.; Maki, M.; Hitomi, K.; Kitaura, Y.; Takai, S.; Sasahara, R.M.; Horimoto, A.; et al. Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. Proc. Natl. Acad. Sci. USA 1998, 95, 13221–13226. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.; Li, Y.; Zhang, H.; Sun, X.F. RECK, a novel matrix metalloproteinase regulator. Histol. Histopathol. 2008, 23, 1003–1010. [Google Scholar] [CrossRef]
- De Moraes, R.P.; Pimenta, R.; Mori, F.N.C.; Dos Santos, G.A.; Viana, N.I.; Guimarães, V.R.; de Camargo, J.A.; Leite, K.R.M.; Srougi, M.; Nahas, W.C.; et al. Tissue expression of MMP-9, TIMP-1, RECK, and miR338-3p in prostate gland: Can it predict cancer? Mol. Biol. Res. Commun. 2021, 10, 149–156. [Google Scholar] [CrossRef]
- Gorka, J.; Marona, P.; Kwapisz, O.; Rys, J.; Jura, J.; Miekus, K. The anti-inflammatory protein MCPIP1 inhibits the development of ccRCC by maintaining high levels of tumour suppressors. Eur. J. Pharmacol. 2020, 888, 173591. [Google Scholar] [CrossRef]
- Cardeal, L.B.; Boccardo, E.; Termini, L.; Rabachini, T.; Andreoli, M.A.; di Loreto, C.; Longatto-Filho, A.; Villa, L.L.; Maria-Engler, S.S. HPV16 oncoproteins induce MMPs/RECK-TIMP-2 imbalance in primary keratinocytes: Possible implications in cervical carcinogenesis. PLoS ONE 2012, 7, e33585. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Takahashi, R.; Kondo, S.; Mizoguchi, A.; Adachi, E.; Sasahara, R.M.; Nishimura, S.; Imamura, Y.; Kitayama, H.; Alexander, D.B.; et al. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell 2001, 107, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Yuki, K.; Dan, S.; Yamazaki, K.; Noda, M. Suppression of tumor metastasis by a RECK-activating small molecule. Sci. Rep. 2022, 12, 2319. [Google Scholar] [CrossRef]
- Sasahara, R.M.; Brochado, S.M.; Takahashi, C.; Oh, J.; Maria-Engler, S.S.; Granjeiro, J.M.; Noda, M.; Sogayar, M.C. Transcriptional control of the RECK metastasis/angiogenesis suppressor gene. Cancer Detect. Prev. 2002, 26, 435–443. [Google Scholar] [CrossRef]
- Xin, C.; Buhe, B.; Hongting, L.; Chuanmin, Y.; Xiwei, H.; Hong, Z.; Lulu, H.; Qian, D.; Renjie, W. MicroRNA-15a promotes neuroblastoma migration by targeting reversion-inducing cysteine-rich protein with Kazal motifs (RECK) and regulating matrix metalloproteinase-9 expression. FEBS J. 2013, 280, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Loayza-Puch, F.; Yoshida, Y.; Matsuzaki, T.; Takahashi, C.; Kitayama, H.; Noda, M. Hypoxia and RAS-signaling pathways converge on, and cooperatively downregulate, the RECK tumor-suppressor protein through microRNAs. Oncogene 2010, 29, 2638–2648. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Y.; Yang, L.; Jiang, R.; Li, W. MiR-25 promotes gastric cancer cells growth and motility by targeting RECK. Mol. Cell. Biochem. 2014, 385, 207–213. [Google Scholar] [CrossRef]
- Xie, J.; Tan, Z.H.; Tang, X.; Mo, M.S.; Liu, Y.P.; Gan, R.L.; Li, Y.; Zhang, L.; Li, G.Q. MiR-374b-5p suppresses RECK expression and promotes gastric cancer cell invasion and metastasis. World J. Gastroenterol. 2014, 20, 17439–17447. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Chiang, C.H.; Hung, W.C. STAT3 upregulates miR-92a to inhibit RECK expression and to promote invasiveness of lung cancer cells. Br. J. Cancer 2013, 109, 731–738. [Google Scholar] [CrossRef]
- Lei, L.; Huang, Y.; Gong, W. Inhibition of miR-92b suppresses nonsmall cell lung cancer cells growth and motility by targeting RECK. Mol. Cell Biochem. 2014, 387, 171–176. [Google Scholar] [CrossRef]
- Guo, H.; Li, Q.; Li, W.; Zheng, T.; Zhao, S.; Liu, Z. MiR-96 downregulates RECK to promote growth and motility of non-small cell lung cancer cells. Mol. Cell. Biochem. 2014, 390, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, D.; Bao, C.; Zhang, Y.; Chen, D.; Zhao, F.; Ding, J.; Liang, L.; Wang, Q.; Liu, L.; et al. MicroRNA-135b, a HSF1 target, promotes tumor invasion and metastasis by regulating RECK and EVI5 in hepatocellular carcinoma. Oncotarget 2015, 6, 2421–2433. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, J. GAS5 Regulates RECK expression and inhibits invasion potential of HCC cells by sponging miR-135b. Biomed. Res. Int. 2019, 2019, 2973289. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Ueno, K.; Shahryari, V.; Deng, G.; Tanaka, Y.; Tabatabai, Z.L.; Hinoda, Y.; Dahiya, R. MicroRNA-182-5p promotes cell invasion and proliferation by down regulating FOXF2, RECK and MTSS1 genes in human prostate cancer. PLoS ONE 2013, 8, e55502. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Ueno, K.; Shahryari, V.; Tanaka, Y.; Tabatabai, Z.L.; Hinoda, Y.; Dahiya, R. Oncogenic miRNA-182-5p targets Smad4 and RECK in human bladder cancer. PLoS ONE 2012, 7, e51056. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.H.; Hou, M.F.; Hung, W.C. Up-regulation of miR-182 by β-catenin in breast cancer increases tumorigenicity and invasiveness by targeting the matrix metalloproteinase inhibitor RECK. Biochim. Biophys. Acta 2013, 1830, 3067–3076. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhu, X.; Chen, X.; Guan, J.; Li, H. MicroRNA-182 suppresses malignant melanoma proliferation by targeting RECK. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liang, H.; Chen, W.; Zhang, H.; Wang, N.; Wang, F.; Zhang, S.; Liu, Y.; Zhao, C.; Yan, X.; et al. microRNA-200b and microRNA-200c promote colorectal cancer cell proliferation via targeting the reversion-inducing cysteine-rich protein with Kazal motifs. RNA Biol. 2015, 12, 276–289. [Google Scholar] [CrossRef]
- Qin, J.; Luo, M. MicroRNA-221 promotes colorectal cancer cell invasion and metastasis by targeting RECK. FEBS Lett. 2014, 588, 99–104. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C.; Li, R.; Liu, J.; Wang, J.; Wang, T.; Wang, B. The BAP31/miR-181a-5p/RECK axis promotes angiogenesis in colorectal cancer via fibroblast activation. Front. Oncol. 2023, 13, 1056903. [Google Scholar] [CrossRef]
- Chen, R.; Sheng, L.; Zhang, H.J.; Ji, M.; Qian, W.Q. miR-15b-5p facilitates the tumorigenicity by targeting RECK and predicts tumour recurrence in prostate cancer. J. Cell Mol. Med. 2018, 22, 1855–1863. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, W.; Zhang, R.; Zuo, J. Alkannin restrains oral squamous carcinoma cell growth, migration and invasion by regulating microRNA-9/RECK axis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3153–3162. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.W.; Shi, Y.L.; Ma, Y.; Qu, X.H.; Pang, G.M.; Yang, L. MiR-590-5p regulates cell proliferation, apoptosis, migration and invasion in oral squamous cell carcinoma by targeting RECK. Histol. Histopathol. 2021, 36, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.P.; Meng, F.L.; Wang, L.Y. miR-544a stimulates endometrial carcinoma growth via targeted inhibition of reversion-inducing cysteine-rich protein with Kazal motifs. Mol. Cell. Probes 2020, 53, 101572. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Xu, C.H.; Li, Y.P.; Tang, B.; Xie, S.H.; Zeng, E.M. Down-regulated microRNA-30b-3p inhibits proliferation, invasion and migration of glioma cells via inactivation of the AKT signaling pathway by up-regulating RECK. Biosci. Rep. 2019, 39, BSR20182226. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.K.; Giri, R.; Kumar, D.; Sharma, R.; Valis, M.; Kuca, K.; Garg, N. The role of microRNA-21 in the onset and progression of cancer. Future Med. Chem. 2021, 13, 1885–1906. [Google Scholar] [CrossRef] [PubMed]
- Gabriely, G.; Wurdinger, T.; Kesari, S.; Esau, C.C.; Burchard, J.; Linsley, P.S.; Krichevsky, A.M. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol. Cell Biol. 2008, 28, 5369–5380. [Google Scholar] [CrossRef]
- Han, L.; Yue, X.; Zhou, X.; Lan, F.M.; You, G.; Zhang, W.; Zhang, K.L.; Zhang, C.Z.; Cheng, J.Q.; Yu, S.Z.; et al. MicroRNA-21 expression is regulated by β-catenin/STAT3 pathway and promotes glioma cell invasion by direct targeting RECK. CNS Neurosci. Ther. 2012, 18, 573–583. [Google Scholar] [CrossRef]
- Reinhold, A.K.; Krug, S.M.; Salvador, E.; Sauer, R.S.; Karl-Schöller, F.; Malcangio, M.; Sommer, C.; Rittner, H.L. MicroRNA-21-5p functions via RECK/MMP9 as a proalgesic regulator of the blood nerve barrier in nerve injury. Ann. NY Acad. Sci. 2022, 1515, 184–195. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, Y.; Liu, J.S.; Long, S.Z.; Liu, H.L.; Zhang, J.; Zhang, T. The role of miR-21/RECK in the inhibition of osteosarcoma by curcumin. Mol. Cell Probes. 2020, 51, 101534. [Google Scholar] [CrossRef]
- Lin, J.; Liu, Z.; Liao, S.; Li, E.; Wu, X.; Zeng, W. Elevation of long non-coding RNA GAS5 and knockdown of microRNA-21 up-regulate RECK expression to enhance esophageal squamous cell carcinoma cell radio-sensitivity after radiotherapy. Genomics 2020, 112, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, Y.; Jiang, T.; Gao, C.; Wang, D.; Wang, X.; Wei, Y.; Liu, T.; Zhu, L.; Wang, P.; et al. Up-regulation of TIMP-3 and RECK decrease the invasion and metastasis ability of colon cancer. Arab. J. Gastroenterol. 2019, 20, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Murai, N.; Yanaihara, N.; Saito, M.; Saito, M.; Urashima, M.; Murakami, Y.; Matsufuji, S.; Okamoto, A. MicroRNA-21 is a candidate driver gene for 17q23-25 amplification in ovarian clear cell carcinoma. BMC Cancer 2014, 14, 799. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Wang, H.; Li, H.; Shu, L.; Han, Z.; Li, S.; Cheng, H.; Dai, X. miR-21-5p inhibits the proliferation, migration, and invasion of glioma by targeting S100A10. J. Cancer 2023, 14, 1781–1793. [Google Scholar] [CrossRef] [PubMed]
- Tiong, T.Y.; Chan, M.L.; Wang, C.H.; Yadav, V.K.; Pikatan, N.W.; Fong, I.H.; Yeh, C.T.; Kuo, K.T.; Huang, W.C. Exosomal miR-21 determines lung-to-brain metastasis specificity through the DGKB/ERK axis within the tumor microenvironment. Life Sci. 2023, 329, 121945. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, H.; Sun, G.; Zhang, X.; Ye, H.; Wang, P. Role of miR-21 in the diagnosis of colorectal cancer: Meta-analysis and bioinformatics. Pathol. Res. Pract. 2023, 248, 154670. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.J.; Yin, Y.N.; Lin, J.; Li, W.Y.; Long, D.R.; Mei, L. MicroRNA-21 in gynecological cancers: From molecular pathogenesis to clinical significance. Pathol. Res. Pract. 2023, 248, 154630. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Liu, T.; Yao, Y. Implications of viral infections and oncogenesis in uterine cervical carcinoma etiology and pathogenesis. Front. Microbiol. 2023, 14, 1194431. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Zaragoza, O.; Deas, J.; Meneses-Acosta, A.; De la O-Gómez, F.; Fernández-Tilapa, G.; Gómez-Cerón, C.; Benítez-Boijseauneau, O.; Burguete-García, A.; Torres-Poveda, K.; Bermúdez-Morales, V.H.; et al. Relevance of miR-21 in regulation of tumor suppressor gene PTEN in human cervical cancer cells. BMC Cancer 2016, 16, 215. [Google Scholar] [CrossRef]
- Mondin, A.; Bertazza, L.; Barollo, S.; Pedron, M.C.; Manso, J.; Piva, I.; Basso, D.; Merante Boschin, I.; Iacobone, M.; Pezzani, R.; et al. Validation of miRNAs as diagnostic and prognostic biomarkers, and possible therapeutic targets in medullary thyroid cancers. Front. Endocrinol. 2023, 14, 1151583. [Google Scholar] [CrossRef]
- Brahmbhatt, H.D.; Gupta, R.; Gupta, A.; Rastogi, S.; Subramani, D.; Mobeen, A.; Batra, V.V.; Singh, A. Differential regulation of miR-21-5p delays wound healing of melanocyte-deprived vitiligo skin by modulating the expression of tumor-suppressors PDCD4 and Maspin. J. Cell Physiol. 2022, 237, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Xu, X.; Lv, L.; Dai, H.; Chen, J.; Chen, B. miR-21 Overexpression promotes esophageal squamous cell carcinoma invasion and migration by repressing tropomyosin 1. Gastroenterol. Res. Pract. 2020, 2020, 6478653. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Wu, S.; Li, L.; Li, D.; Zhao, J.; Zhang, H.; Wang, Y.; Zhong, X.; Zhang, Z.; Li, P.; et al. Epigenetic inactivation of ACAT1 promotes epithelial-mesenchymal transition of clear cell renal cell carcinoma. Genes Genom. 2022, 44, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, Y.; Mu, J.; Yang, D.; Gu, X.; Zhang, J. Exosomal miR-21-5p contributes to ovarian cancer progression by regulating CDK6. Hum. Cell. 2021, 34, 1185–1196. [Google Scholar] [CrossRef]
- Kakotkin, V.V.; Semina, E.V.; Zadorkina, T.G.; Agapov, M.A. Prevention strategies and early diagnosis of cervical cancer: Current state and prospects. Diagnostics 2023, 13, 610. [Google Scholar] [CrossRef]
- Sweef, O.; Zaabout, E.; Bakheet, A.; Halawa, M.; Gad, I.; Akela, M.; Tousson, E.; Abdelghany, A.; Furuta, S. Unraveling therapeutic opportunities and the diagnostic potential of microRNAs for human lung cancer. Pharmaceutics 2023, 15, 2061. [Google Scholar] [CrossRef] [PubMed]
- Hillyar, C.R.; Kanabar, S.S.; Pufal, K.R.; Saw Hee, J.L.; Lawson, A.W.; Mohamed, Y.; Jasim, D.; Reed, L.; Rallis, K.S.; Nibber, A. A systematic review and meta-analysis of miRNAs for the detection of cervical cancer. Epigenomics 2023, 15, 593–613. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, S.; Wang, L.; Wu, X.; Wang, X.; Ou, C.; Yang, J.; Song, L.; Zhou, S.; Wu, Q.; et al. Hierarchically tumor-activated nanoCRISPR-Cas13a facilitates efficient microRNA disruption for multi-pathway-mediated tumor suppression. Theranostics 2023, 13, 2774–2786. [Google Scholar] [CrossRef] [PubMed]
- Márquez-González, R.M.; Saucedo-Sariñana, A.M.; Barros-Núñez, P.; Gallegos-Arreola, M.P.; Juárez-Vázquez, C.I.; Pineda-Razo, T.D.; Marin-Contreras, M.E.; Flores-Martínez, S.E.; Rosales-Reynoso, M.A. RECK variants are associated with clinicopathological features and decreased susceptibility in mexican patients with colorectal cancer. Tohoku J. Exp. Med. 2022, 257, 163–169. [Google Scholar] [CrossRef]
- miRTarBase update 2022: An Informative Resource for Experimentally Validated miRNA–Target Interactions. Nucleic Acids Research. 2022. Available online: https://mirtarbase.cuhk.edu.cn/~miRTarBase/miRTarBase_2022/php/index.php (accessed on 22 April 2022).
- Herbster, S.; Paladino, A.; de Freitas, S.; Boccardo, E. Alterations in the expression and activity of extracellular matrix components in HPV-associated infections and diseases. Clinics 2018, 73 (Suppl. 1), e551s. [Google Scholar] [CrossRef]
- Morgan, E.L.; Scarth, J.A.; Patterson, M.R.; Wasson, C.W.; Hemingway, G.C.; Barba-Moreno, D.; Macdonald, A. E6-mediated activation of JNK drives EGFR signalling to promote proliferation and viral oncoprotein expression in cervical cancer. Cell Death Differ. 2021, 28, 1669–1687. [Google Scholar] [CrossRef] [PubMed]
- Discacciati, M.G.; Gimenes, F.; Pennacchi, P.C.; Faião-Flores, F.; Zeferino, L.C.; Derchain, S.M.; Teixeira, J.C.; Costa, M.C.; Zonta, M.; Termini, L.; et al. MMP-9/RECK Imbalance: A mechanism associated with high-grade cervical lesions and genital infection by human papillomavirus and chlamydia trachomatis. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Ye, M.; Zhang, W. E6/E7 oncoproteins of high-risk HPV-16 upregulate MT1-MMP, MMP-2 and MMP-9 and promote the migration of cervical cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 4981–4989. [Google Scholar] [PubMed]
- Herbster, S.; Trombetta-Lima, M.; de Souza-Santos, P.T.; Paladino, A.; Silveira, C.R.F.; Sogayar, M.C.; Villa, L.L.; Lepique, A.P.; Boccardo, E. Low RECK expression is part of the cervical carcinogenesis mechanisms. Cancers 2021, 13, 2217. [Google Scholar] [CrossRef] [PubMed]
- Rotman, J.; den Otter, L.A.S.; Bleeker, M.C.G.; Samuels, S.S.; Heeren, A.M.; Roemer, M.G.M.; Kenter, G.G.; Zijlmans, H.J.M.A.A.; van Trommel, N.E.; de Gruijl, T.D.; et al. PD-L1 and PD-L2 expression in cervical cancer: Regulation and biomarker potential. Front. Immunol. 2020, 11, 596825. [Google Scholar] [CrossRef]
- Dong, Z.R.; Chen, Z.Q.; Yang, X.Y.; Ding, Z.N.; Liu, K.X.; Yan, L.J.; Meng, G.X.; Yang, Y.F.; Yan, Y.C.; Yao, S.Y.; et al. RECK expression is associated with angiogenesis and immunogenic tumor microenvironment in hepatocellular carcinoma, and is a prognostic factor for better survival. J. Cancer 2021, 12, 3827–3840. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Gonzalez, D.; Escalona-Rivano, R.; Takahashi, C.; Brandan, E. Reduced RECK levels accelerate skeletal muscle differentiation, improve muscle regeneration, and decrease fibrosis. FASEB J. 2021, 35, e21503. [Google Scholar] [CrossRef]
- Del Mar Díaz-González, S.; Rodríguez-Aguilar, E.D.; Meneses-Acosta, A.; Valadez-Graham, V.; Deas, J.; Gómez-Cerón, C.; Tavira-Montalván, C.A.; Arizmendi-Heras, A.; Ramírez-Bello, J.; Zurita-Ortega, M.E.; et al. Transregulation of microRNA miR-21 promoter by AP-1 transcription factor in cervical cancer cells. Cancer Cell Int. 2019, 19, 214. [Google Scholar] [CrossRef]
- Laban, M.; Chen, X.; Guo, B. Unmasking the hypoxia landscape in cervical cancer: S100A2 and its implication for immunotherapy resistance. Reprod. Sci. 2024, 31, 96–98. [Google Scholar] [CrossRef]
- Jung, H.M.; Phillips, B.L.; Patel, R.S.; Cohen, D.M.; Jakymiw, A.; Kong, W.W.; Cheng, J.Q.; Chan, E.K. Keratinization-associated miR-7 and miR-21 regulate tumor suppressor reversion-inducing cysteine-rich protein with kazal motifs (RECK) in oral cancer. J. Biol. Chem. 2012, 287, 29261–29272. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Gao, C.; Chen, P.; Chen, J.; Liu, W.; Xiao, S.; Lu, H. miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab. Investig. 2008, 88, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.J.; Ren, G.; Liu, J.L.; Zhao, Z.A.; Yu, Y.S.; Su, R.W.; Ma, X.H.; Ni, H.; Lei, W.; Yang, Z.M. MicroRNA expression and regulation in mouse uterus during embryo implantation. J. Biol. Chem. 2008, 283, 23473–23484. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.F.; Wu, Z.P.; Chen, Y.; Zhu, Q.S.; Hamidi, S.; Navab, R. MicroRNA-21 (miR-21) regulates cellular proliferation, invasion, migration, and apoptosis by targeting PTEN, RECK and Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS ONE 2014, 9, e103698. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, E.; Wang, X.; Cong, X.; Chen, X. MicroRNA-21 is a unique signature associated with coronary plaque instability in humans by regulating matrix metalloproteinase-9 via reversion-inducing cysteine-rich protein with Kazal motifs. Exp. Mol. Pathol. 2014, 96, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, Y.; Varley, P.; Chang, Y.; He, X.X.; Huang, H.; Tang, D.; Lotze, M.T.; Lin, J.; Tsung, A. High-mobility group box 1 promotes hepatocellular carcinoma progression through miR-21-mediated matrix metalloproteinase activity. Cancer Res. 2015, 75, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, W.; Lv, Q.; Zhu, D. Overexpression of miR-21 promotes the proliferation and migration of cervical cancer cells via the inhibition of PTEN. Oncol. Rep. 2015, 33, 3108–3116. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Li, Y.; Choi, B.R.; Matas-Rico, E.; Troncoso, J.; Takahashi, C.; Sockanathan, S. GDE2-RECK controls ADAM10 α-secretase-mediated cleavage of amyloid precursor protein. Sci. Transl. Med. 2021, 13, eabe6178. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Liu, Y.; Geng, P.; Li, G.; Yang, Y.; Song, H. RECK inhibits cervical cancer cell migration and invasion by promoting p53 signaling pathway. J. Cell Biochem. 2018, 119, 3058–3066. [Google Scholar] [CrossRef]
- Peralta-Zaragoza, O.; De-la-O-Gómez, F.; Deas, J.; Fernández-Tilapa, G.; Fierros-Zárate, G.d.S.; Gómez-Cerón, C.; Burguete-García, A.; Torres-Poveda, K.; Bermúdez-Morales, V.H.; Rodríguez-Dorantes, M.; et al. Selective silencing of gene target expression by siRNA expression plasmids in human cervical cancer cells. Methods Mol. Biol. 2015, 1249, 153–171. [Google Scholar] [CrossRef]
- Integrated DNA Technologies IDT.; Integrated DNA Technologies, Inc. Melb. Aust. 2023. Available online: https://www.idtdna.com/pages/products/qpcr-and-pcr/gene-expression (accessed on 22 April 2022).
- Huang, G.L.; Zhang, X.H.; Guo, G.L.; Huang, K.T.; Yang, K.Y.; Shen, X.; You, J.; Hu, X.Q. Clinical significance of miR-21 expression in breast cancer: SYBR-Green I-based real-time RT-PCR study of invasive ductal carcinoma. Oncol. Rep. 2009, 21, 673–679. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef] [PubMed]
- TargetScan Release 7.1; Bioinformatics and Research Computing; Whitehead Institute for Biomedical Research: Cambridge, MA 02142, USA. 2022; Available online: https://www.targetscan.org/vert_71/ (accessed on 22 April 2022).
- Monfared, H.; Jahangard, Y.; Nikkhah, M.; Mirnajafi-Zadeh, J.; Mowla, S.J. Potential therapeutic effects of exosomes packed with a miR-21-sponge construct in a rat model of glioblastoma. Front. Oncol. 2019, 9, 782. [Google Scholar] [CrossRef] [PubMed]
- Camargo, J.A.; Viana, N.I.; Pimenta, R.; Guimarães, V.R.; Dos Santos, G.A.; Candido, P.; Ghazarian, V.; Romão, P.; Silva, I.A.; Birbrair, A.; et al. The effect of gene editing by CRISPR-Cas9 of miR-21 and the indirect target MMP9 in metastatic prostate cancer. Int. J. Mol. Sci. 2023, 24, 14847. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Martínez, S.Y.; Campos-Viguri, G.E.; Medina-García, S.E.; García-Flores, R.J.; Deas, J.; Gómez-Cerón, C.; Pedroza-Torres, A.; Bautista-Rodríguez, E.; Fernández-Tilapa, G.; Rodríguez-Dorantes, M.; et al. MiR-21 Regulates Growth and Migration of Cervical Cancer Cells by RECK Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 4086. https://doi.org/10.3390/ijms25074086
Aguilar-Martínez SY, Campos-Viguri GE, Medina-García SE, García-Flores RJ, Deas J, Gómez-Cerón C, Pedroza-Torres A, Bautista-Rodríguez E, Fernández-Tilapa G, Rodríguez-Dorantes M, et al. MiR-21 Regulates Growth and Migration of Cervical Cancer Cells by RECK Signaling Pathway. International Journal of Molecular Sciences. 2024; 25(7):4086. https://doi.org/10.3390/ijms25074086
Chicago/Turabian StyleAguilar-Martínez, Seidy Y., Gabriela E. Campos-Viguri, Selma E. Medina-García, Ricardo J. García-Flores, Jessica Deas, Claudia Gómez-Cerón, Abraham Pedroza-Torres, Elizabeth Bautista-Rodríguez, Gloria Fernández-Tilapa, Mauricio Rodríguez-Dorantes, and et al. 2024. "MiR-21 Regulates Growth and Migration of Cervical Cancer Cells by RECK Signaling Pathway" International Journal of Molecular Sciences 25, no. 7: 4086. https://doi.org/10.3390/ijms25074086
APA StyleAguilar-Martínez, S. Y., Campos-Viguri, G. E., Medina-García, S. E., García-Flores, R. J., Deas, J., Gómez-Cerón, C., Pedroza-Torres, A., Bautista-Rodríguez, E., Fernández-Tilapa, G., Rodríguez-Dorantes, M., Pérez-Plasencia, C., & Peralta-Zaragoza, O. (2024). MiR-21 Regulates Growth and Migration of Cervical Cancer Cells by RECK Signaling Pathway. International Journal of Molecular Sciences, 25(7), 4086. https://doi.org/10.3390/ijms25074086






