Biomolecular Condensates Decipher Molecular Codes of Cell Fate: From Biophysical Fundamentals to Therapeutic Practices
Abstract
:1. Introduction
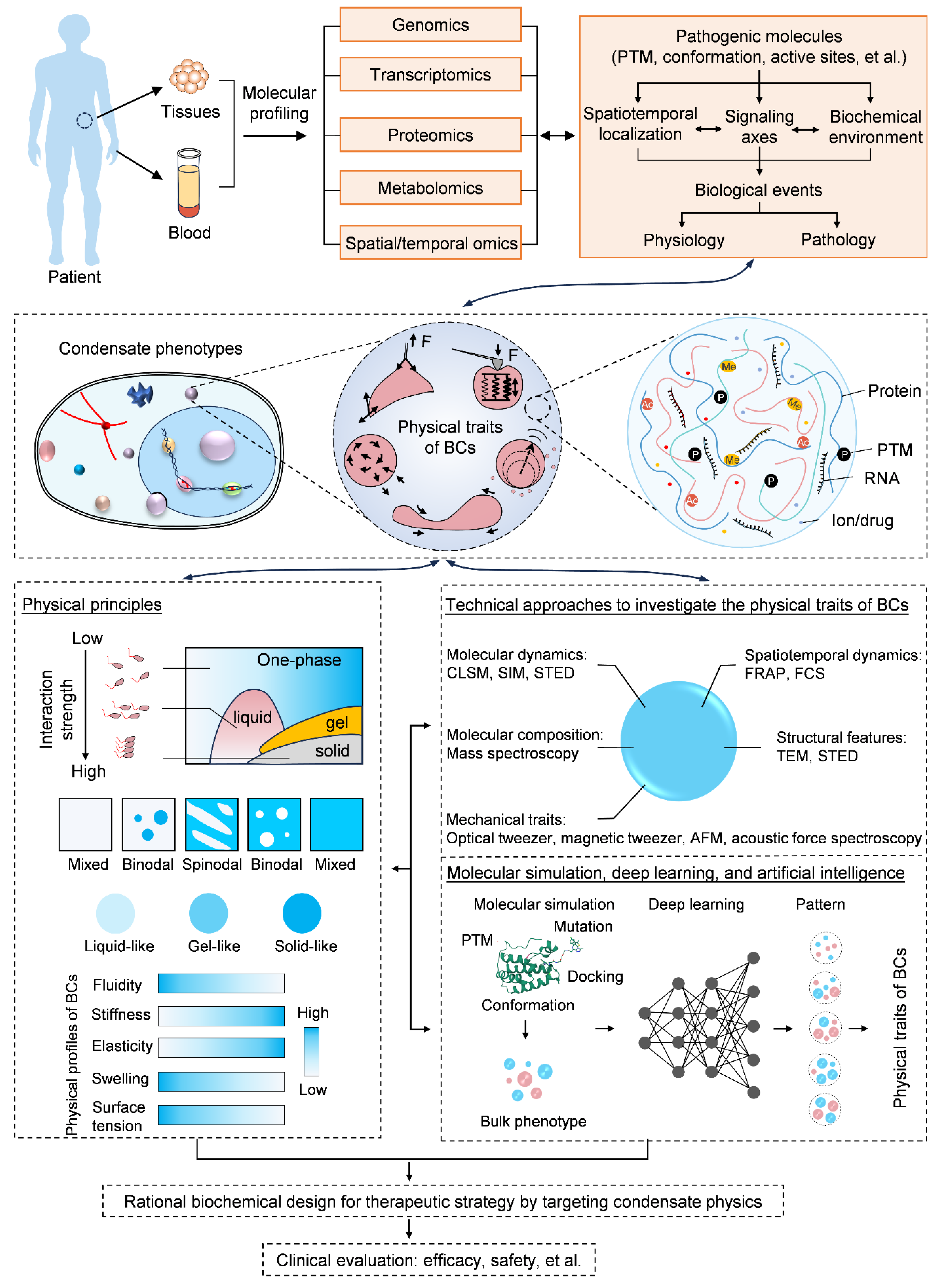
2. Physical Traits of BCs for Molecular Signaling
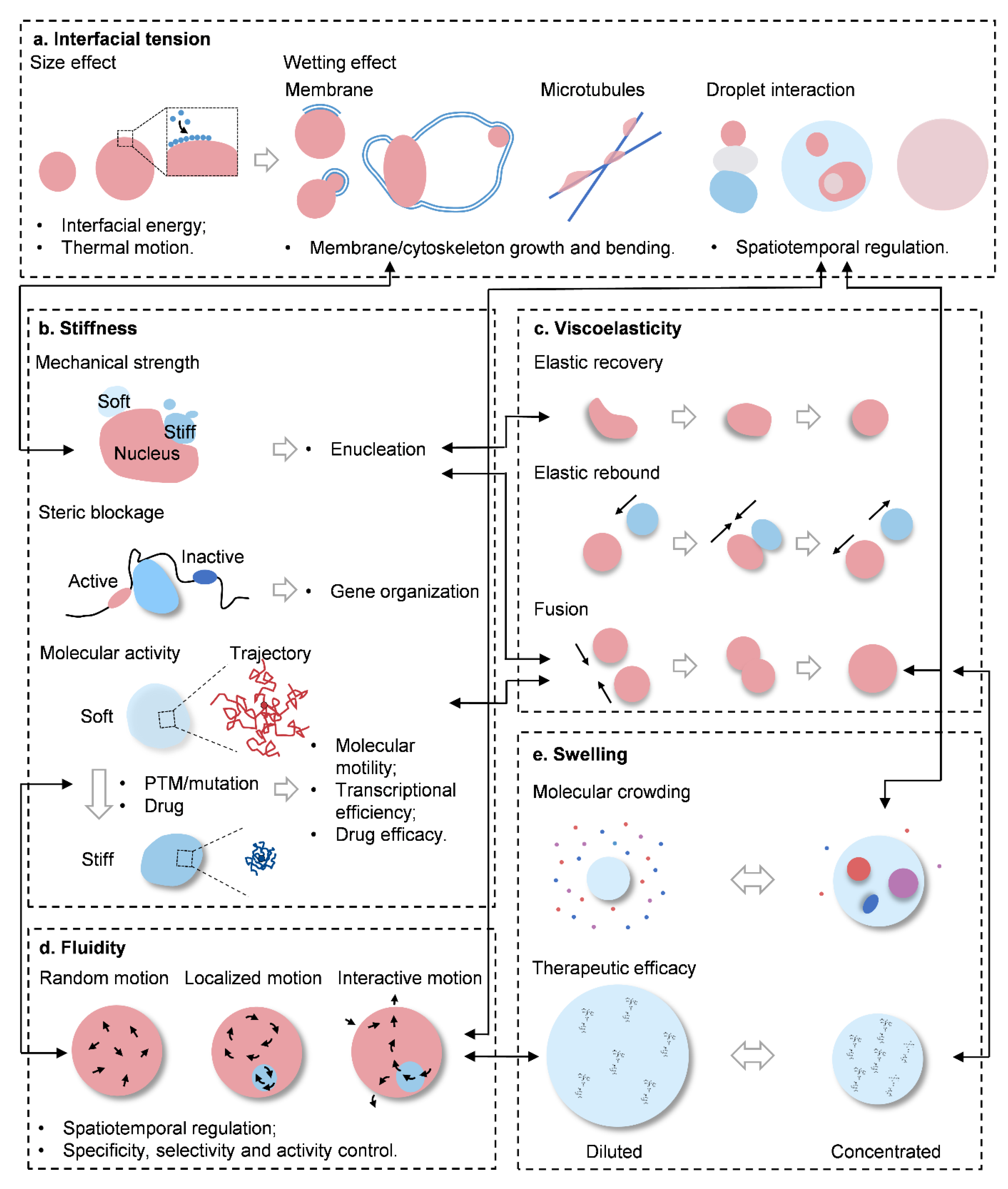
3. Interfacial Tension
3.1. Size Effects
3.2. Wetting
4. Stiffness
4.1. Mechanical Strength
4.2. Steric Blockage
4.3. Molecular Activity
5. Viscoelasticity
6. Fluidity
Swelling
7. The Interplay of Physical Traits
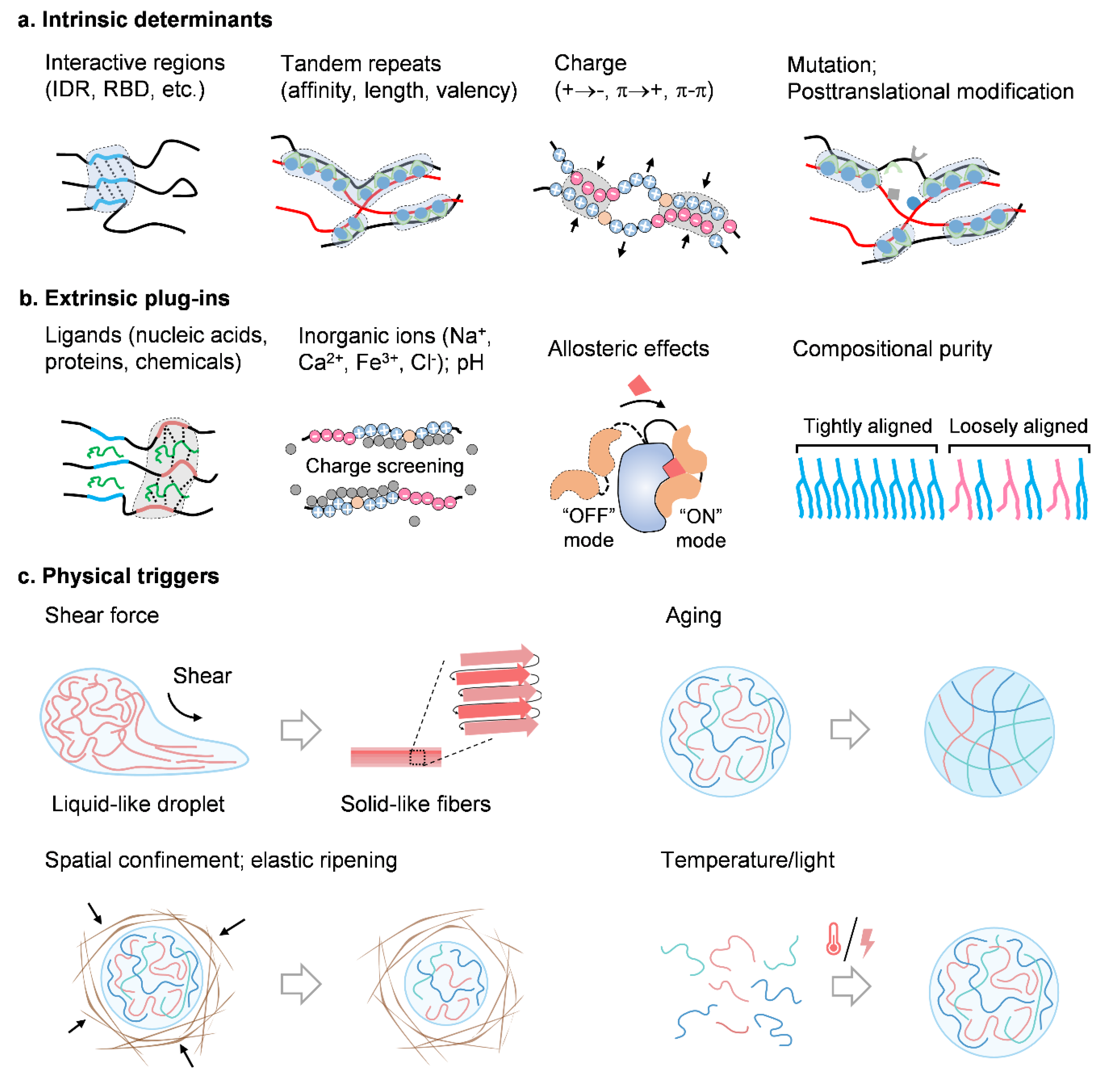
8. Determinants for Condensate Physics
8.1. Intrinsic Determinants
8.2. Extrinsic Plug-Ins
8.3. Physical Triggers
8.4. Shear Force
8.5. Aging
8.6. Spatial Confinement
8.7. Others
9. Clinical Significance of Condensate Physics
10. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Brangwynne, C.P.; Eckmann, C.R.; Courson, D.S.; Rybarska, A.; Hoege, C.; Gharakhani, J.; Jülicher, F.; Hyman, A.A. Germline P Granules Are Liquid Droplets That Localize by Controlled Dissolution/Condensation. Science 2009, 324, 1729–1732. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, F.G.; Quiroz, F.G.; Fiore, V.F.; Levorse, J.; Polak, L.; Wong, E.; Pasolli, H.A.; Fuchs, E. Liquid-liquid phase separation drives skin barrier formation. Science 2020, 367, eaax9554. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Ruggeri, F.S.; Vigolo, D.; Kamada, A.; Qamar, S.; Levin, A.; Iserman, C.; Alberti, S.; George-Hyslop, P.S.; Knowles, T.P.J. Biomolecular condensates undergo a generic shear-mediated liquid-to-solid transition. Nat. Nanotechnol. 2020, 15, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Strickfaden, H.; Strickfaden, H.; Tolsma, T.O.; Sharma, A.; Underhill, D.A.; Hansen, J.C.; Hendzel, M.J. Condensed Chromatin Behaves like a Solid on the Mesoscale In Vitro and in Living Cells. Cell 2020, 183, 1772–1784.e1713. [Google Scholar] [CrossRef] [PubMed]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Von Appen, A.; LaJoie, D.; Johnson, I.E.; Trnka, M.J.; Pick, S.M.; Burlingame, A.L.; Ullman, K.S.; Frost, A. LEM2 phase separation promotes ESCRT-mediated nuclear envelope reformation. Nature 2020, 582, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.A.; Boija, A.; Afeyan, L.K.; Hawken, S.W.; Fan, M.; Dall’Agnese, A.; Oksuz, O.; Henninger, J.E.; Shrinivas, K.; Sabari, B.R.; et al. Partitioning of cancer therapeutics in nuclear condensates. Science 2020, 368, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Peeples, W.; Rosen, M.K. Mechanistic dissection of increased enzymatic rate in a phase-separated compartment. Nat. Chem. Biol. 2021, 17, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Beutel, O.; Maraspini, R.; Pombo-García, K.; Martin-Lemaitre, C.; Honigmann, A. Phase Separation of Zonula Occludens Proteins Drives Formation of Tight Junctions. Cell 2019, 179, 923–936.e911. [Google Scholar] [CrossRef]
- Cai, D.; Feliciano, D.; Dong, P.; Flores, E.; Gruebele, M.; Porat-Shliom, N.; Sukenik, S.; Liu, Z.; Lippincott-Schwartz, J. Phase separation of YAP reorganizes genome topology for long-term YAP target gene expression. Nat. Cell Biol. 2019, 21, 1578–1589. [Google Scholar] [CrossRef]
- Bouchard, J.J.; Otero, J.H.; Scott, D.C.; Szulc, E.; Martin, E.W.; Sabri, N.; Granata, D.; Marzahn, M.R.; Lindorff-Larsen, K.; Salvatella, X.; et al. Cancer Mutations of the Tumor Suppressor SPOP Disrupt the Formation of Active, Phase-Separated Compartments. Mol. Cell 2018, 72, 19–36.e18. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.; Dugast-Darzacq, C.; Liu, Z.; Dong, P.; Dailey Gina, M.; Cattoglio, C.; Heckert, A.; Banala, S.; Lavis, L.; Darzacq, X.; et al. Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science 2018, 361, eaar2555. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.C.; Brangwynne, C.P. Getting RNA and Protein in Phase. Cell 2012, 149, 1188–1191. [Google Scholar] [CrossRef] [PubMed]
- Banani, S.F.; Rice, A.M.; Peeples, W.B.; Lin, Y.; Jain, S.; Parker, R.; Rosen, M.K. Compositional Control of Phase-Separated Cellular Bodies. Cell 2016, 166, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Alshareedah, I.; Moosa, M.M.; Raju, M.; Potoyan, D.A.; Banerjee, P.R. Phase transition of RNA−protein complexes into ordered hollow condensates. Proc. Natl. Acad. Sci. USA 2020, 117, 15650–15658. [Google Scholar] [CrossRef] [PubMed]
- Krypotou, E.; Townsend, G.E.; Gao, X.; Tachiyama, S.; Liu, J.; Pokorzynski, N.D.; Goodman, A.L.; Groisman, E. Bacteria require phase separation for fitness in the mammalian gut. Science 2023, 379, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Agudo-Canalejo, J.; Schultz, S.W.; Chino, H.; Migliano, S.M.; Saito, C.; Koyama-Honda, I.; Stenmark, H.; Brech, A.; May, A.I.; Mizushima, N.; et al. Wetting regulates autophagy of phase-separated compartments and the cytosol. Nature 2021, 591, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, B.; Kim, Y.; Shaevitz, J.W.; Petry, S.; Stone, H.A.; Brangwynne, C.P. Capillary forces generated by biomolecular condensates. Nature 2022, 609, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Chang, Y.-C.; Lee, D.S.W.; Berry, J.; Sanders, D.W.; Ronceray, P.; Wingreen, N.S.; Haataja, M.; Brangwynne, C.P. Liquid Nuclear Condensates Mechanically Sense and Restructure the Genome. Cell 2018, 175, 1481–1491.e1413. [Google Scholar] [CrossRef]
- Risso-Ballester, J.; Galloux, M.; Cao, J.; Le Goffic, R.; Hontonnou, F.; Jobart-Malfait, A.; Desquesnes, A.; Sake, S.M.; Haid, S.; Du, M.; et al. A condensate-hardening drug blocks RSV replication in vivo. Nature 2021, 595, 596–599. [Google Scholar] [CrossRef]
- Meng, L.; Lin, J. Elasticity generates indissoluble biomolecular condensates. bioRxiv 2022. [Google Scholar] [CrossRef]
- Bergeron-Sandoval, L.-P.; Kumar, S.; Heris, H.K.; Chang, C.L.A.; Cornell, C.E.; Keller, S.L.; François, P.; Hendricks, A.G.; Ehrlicher, A.J.; Pappu, R.V.; et al. Endocytic proteins with prion-like domains form viscoelastic condensates that enable membrane remodeling. Proc. Natl. Acad. Sci. USA 2021, 118, e2113789118. [Google Scholar] [CrossRef]
- Ghosh, A.; Kota, D.; Zhou, H.-X. Shear relaxation governs fusion dynamics of biomolecular condensates. Nat. Commun. 2021, 12, 5995. [Google Scholar] [CrossRef] [PubMed]
- Galvanetto, N.; Ivanović, M.T.; Chowdhury, A.; Sottini, A.; Nüesch, M.F.; Nettels, D.; Best, R.B.; Schuler, B. Extreme dynamics in a biomolecular condensate. Nature 2023, 619, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, A.; Alam, J.M.; Noshiro, D.; Hirata, E.; Fujioka, Y.; Suzuki, K.; Ohsumi, Y.; Noda, N.N. Liquidity Is a Critical Determinant for Selective Autophagy of Protein Condensates. Mol. Cell 2020, 77, 1163–1175.e1169. [Google Scholar] [CrossRef]
- Muzzopappa, F.; Hertzog, M.; Erdel, F. DNA length tunes the fluidity of DNA-based condensates. Biophys. J. 2021, 120, 1288–1300. [Google Scholar] [CrossRef]
- Watanabe, K.; Morishita, K.; Zhou, X.; Shiizaki, S.; Uchiyama, Y.; Koike, M.; Naguro, I.; Ichijo, H. Cells recognize osmotic stress through liquid–liquid phase separation lubricated with poly(ADP-ribose). Nat. Commun. 2021, 12, 1353. [Google Scholar] [CrossRef] [PubMed]
- Boyd-Shiwarski, C.R.; Shiwarski, D.J.; Griffiths, S.E.; Beacham, R.T.; Norrell, L.; Morrison, D.E.; Wang, J.; Mann, J.; Tennant, W.; Anderson, E.N.; et al. WNK kinases sense molecular crowding and rescue cell volume via phase separation. Cell 2022, 185, 4488–4506.e4420. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357, eaaf4382. [Google Scholar] [CrossRef]
- Brangwynne, C.P.; Mitchison, T.J.; Hyman, A.A. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 4334–4339. [Google Scholar] [CrossRef]
- Feric, M.; Vaidya, N.; Harmon, T.S.; Mitrea, D.M.; Zhu, L.; Richardson, T.M.; Kriwacki, R.W.; Pappu, R.V.; Brangwynne, C.P. Coexisting Liquid Phases Underlie Nucleolar Subcompartments. Cell 2016, 165, 1686–1697. [Google Scholar] [CrossRef]
- Case, L.B.; Zhang, X.; Ditlev, J.A.; Rosen, M.K. Stoichiometry controls activity of phase-separated clusters of actin signaling proteins. Science 2019, 363, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.P.T.; Zhang, W.L.; Seydoux, G. The conserved helicase ZNFX-1 memorializes silenced RNAs in perinuclear condensates. Nat. Cell Biol. 2022, 24, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Reber, S.; Jutzi, D.; Lindsay, H.; Devoy, A.; Mechtersheimer, J.; Levone, B.R.; Domanski, M.; Bentmann, E.; Dormann, D.; Mühlemann, O.; et al. The phase separation-dependent FUS interactome reveals nuclear and cytoplasmic function of liquid–liquid phase separation. Nucleic Acids Res. 2021, 49, 7713–7731. [Google Scholar] [CrossRef] [PubMed]
- Miné-Hattab, J.; Heltberg, M.; Villemeur, M.; Guedj, C.; Mora, T.; Walczak, A.M.; Dahan, M.; Taddei, A. Single molecule microscopy reveals key physical features of repair foci in living cells. eLife 2021, 10, e60577. [Google Scholar] [CrossRef] [PubMed]
- Pessina, F.; Giavazzi, F.; Yin, Y.; Gioia, U.; Vitelli, V.; Galbiati, A.; Barozzi, S.; Garre, M.; Oldani, A.; Flaus, A.; et al. Functional transcription promoters at DNA double-strand breaks mediate RNA-driven phase separation of damage-response factors. Nat. Cell Biol. 2019, 21, 1286–1299. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Gil, G.; Romero-Aristizabal, C.; Mateos, N.; Campelo, F.; de Llobet Cucalon, L.I.; Beato, M.; Lewenstein, M.; Garcia-Parajo, M.F.; Torreno-Pina, J.A. Stochastic particle unbinding modulates growth dynamics and size of transcription factor condensates in living cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2200667119. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.W.; Wingreen, N.S.; Brangwynne, C.P. Chromatin mechanics dictates subdiffusion and coarsening dynamics of embedded condensates. Nat. Phys. 2021, 17, 531–538. [Google Scholar] [CrossRef]
- Wu, X.; Ganzella, M.; Zhou, J.; Zhu, S.; Jahn, R.; Zhang, M. Vesicle Tethering on the Surface of Phase-Separated Active Zone Condensates. Mol. Cell 2021, 81, 13–24.e17. [Google Scholar] [CrossRef]
- Kusumaatmaja, H.; May, A.I.; Feeney, M.; McKenna, J.F.; Mizushima, N.; Frigerio, L.; Knorr, R.L. Wetting of phase-separated droplets on plant vacuole membranes leads to a competition between tonoplast budding and nanotube formation. Proc. Natl. Acad. Sci. USA 2021, 118, e2024109118. [Google Scholar] [CrossRef]
- Milovanovic, D.; Wu, Y.; Bian, X.; De Camilli, P. A liquid phase of synapsin and lipid vesicles. Science 2018, 361, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, Y.; Alam, J.M.; Noshiro, D.; Mouri, K.; Ando, T.; Okada, Y.; May, A.I.; Knorr, R.L.; Suzuki, K.; Ohsumi, Y.; et al. Phase separation organizes the site of autophagosome formation. Nature 2020, 578, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Astro, V.; de Curtis, I. Plasma membrane–associated platforms: Dynamic scaffolds that organize membrane-associated events. Sci. Signal. 2015, 8, re1. [Google Scholar] [CrossRef] [PubMed]
- Imasaki, T.; Kikkawa, S.; Niwa, S.; Saijo-Hamano, Y.; Shigematsu, H.; Aoyama, K.; Mitsuoka, K.; Shimizu, T.; Aoki, M.; Sakamoto, A.; et al. CAMSAP2 organizes a γ-tubulin-independent microtubule nucleation centre through phase separation. eLife 2022, 11, e77365. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.M.; Farcas, A.-M.; Kumar, A.; Ijavi, M.; Bill, R.T.; Stelling, J.; Dufresne, E.R.; Steinmetz, M.O.; Barral, Y. Multivalency ensures persistence of a +TIP body at specialized microtubule ends. Nat. Cell Biol. 2022, 25, 56–67. [Google Scholar] [CrossRef]
- Hernández-Vega, A.; Braun, M.; Scharrel, L.; Jahnel, M.; Wegmann, S.; Hyman, B.T.; Alberti, S.; Diez, S.; Hyman, A.A. Local Nucleation of Microtubule Bundles through Tubulin Concentration into a Condensed Tau Phase. Cell Rep. 2017, 20, 2304–2312. [Google Scholar] [CrossRef]
- Koppers, M.; Özkan, N.; Farías, G.G. Complex Interactions Between Membrane-Bound Organelles, Biomolecular Condensates and the Cytoskeleton. Front. Cell Dev. Biol. 2020, 8, 8733. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.; Chandrasekaran, A.; Wang, L.; Ladak, A.; Lafer, E.M.; Rangamani, P.; Stachowiak, J.C. Liquid-like VASP condensates drive actin polymerization and dynamic bundling. Nat. Phys. 2023, 19, 574–585. [Google Scholar] [CrossRef]
- Woodruff, J.B.; Ferreira Gomes, B.; Widlund, P.O.; Mahamid, J.; Honigmann, A.; Hyman, A.A. The Centrosome Is a Selective Condensate that Nucleates Microtubules by Concentrating Tubulin. Cell 2017, 169, 1066–1077.e1010. [Google Scholar] [CrossRef]
- Oshidari, R.; Huang, R.; Medghalchi, M.; Tse, E.Y.W.; Ashgriz, N.; Lee, H.O.; Wyatt, H.; Mekhail, K. DNA repair by Rad52 liquid droplets. Nat. Commun. 2020, 11, 695. [Google Scholar] [CrossRef]
- Song, X.; Yang, F.; Yang, T.; Wang, Y.; Ding, M.; Li, L.; Xu, P.; Liu, S.; Dai, M.; Chi, C.; et al. Phase separation of EB1 guides microtubule plus-end dynamics. Nat. Cell Biol. 2022, 25, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kalappurakkal, J.M.; Mayor, S.; Rosen, M.K. Phosphorylation of nephrin induces phase separated domains that move through actomyosin contraction. Mol. Biol. Cell 2019, 30, 2996–3012. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Raju, M.; Alshareedah, I.; Davis, R.B.; Potoyan, D.A.; Banerjee, P.R. Sequence-encoded and composition-dependent protein-RNA interactions control multiphasic condensate morphologies. Nat. Commun. 2021, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Wheeler, J.R.; Walters, R.W.; Agrawal, A.; Barsic, A.; Parker, R. ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell 2016, 164, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Mackowiak, S.D.; Niskanen, H.; Knezevic, D.; Asimi, V.; Grosswendt, S.; Geertsema, H.; Ali, S.; Jerković, I.; Ewers, H.; et al. Unblending of Transcriptional Condensates in Human Repeat Expansion Disease. Cell 2020, 181, 1062–1079.e1030. [Google Scholar] [CrossRef] [PubMed]
- Gall, J.G.; Bellini, M.; Wu, Z.A.; Murphy, C. Assembly of the Nuclear Transcription and Processing Machinery: Cajal Bodies (Coiled Bodies) and Transcriptosomes. Mol. Biol. Cell 1999, 10, 4385–4402. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, J.B.; Hyman, A.A.; Boke, E. Organization and Function of Non-dynamic Biomolecular Condensates. Trends Biochem. Sci. 2018, 43, 81–94. [Google Scholar] [CrossRef]
- Sanulli, S.; Trnka, M.J.; Dharmarajan, V.; Tibble, R.W.; Pascal, B.D.; Burlingame, A.L.; Griffin, P.R.; Gross, J.D.; Narlikar, G.J. HP1 reshapes nucleosome core to promote phase separation of heterochromatin. Nature 2019, 575, 390–394. [Google Scholar] [CrossRef]
- Li, W.; Hu, J.; Shi, B.; Palomba, F.; Digman, M.A.; Gratton, E.; Jiang, H. Biophysical properties of AKAP95 protein condensates regulate splicing and tumorigenesis. Nat. Cell Biol. 2020, 22, 960–972. [Google Scholar] [CrossRef]
- Jachowicz, J.W.; Strehle, M.; Banerjee, A.K.; Blanco, M.R.; Thai, J.; Guttman, M. Xist spatially amplifies SHARP/SPEN recruitment to balance chromosome-wide silencing and specificity to the X chromosome. Nat. Struct. Mol. Biol. 2022, 29, 239–249. [Google Scholar] [CrossRef]
- Gu, R.-X.; Baoukina, S.; Tieleman, D.P. Phase Separation in Atomistic Simulations of Model Membranes. J. Am. Chem. Soc. 2020, 142, 2844–2856. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, D.; Simons, K. Lipid Rafts As a Membrane-Organizing Principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef]
- Hug, C.B.; Grimaldi, A.G.; Kruse, K.; Vaquerizas, J.M. Chromatin Architecture Emerges during Zygotic Genome Activation Independent of Transcription. Cell 2017, 169, 216–228.e219. [Google Scholar] [CrossRef] [PubMed]
- Gamliel, A.; Meluzzi, D.; Oh, S.; Jiang, N.; Destici, E.; Rosenfeld, M.G.; Nair, S.J. Long-distance association of topological boundaries through nuclear condensates. Proc. Natl. Acad. Sci. USA 2022, 119, e2206216119. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Singh, N.; Kumar, R.; Patel, K.; Pandey, S.; Datta, D.; Mahato, J.; Panigrahi, R.; Navalkar, A.; Mehra, S.; et al. α-Synuclein aggregation nucleates through liquid–liquid phase separation. Nat. Chem. 2020, 12, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Sang, D.; Shu, T.; Pantoja, C.F.; Ibáñez de Opakua, A.; Zweckstetter, M.; Holt, L.J. Condensed-phase signaling can expand kinase specificity and respond to macromolecular crowding. Mol. Cell 2022, 82, 3693–3711.e3610. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Lee, H.O.; Jawerth, L.; Maharana, S.; Jahnel, M.; Hein, M.Y.; Stoynov, S.; Mahamid, J.; Saha, S.; Franzmann, T.M.; et al. A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 2015, 162, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Munder, M.C.; Midtvedt, D.; Franzmann, T.; Nüske, E.; Otto, O.; Herbig, M.; Ulbricht, E.; Müller, P.; Taubenberger, A.; Maharana, S.; et al. A pH-driven transition of the cytoplasm from a fluid- to a solid-like state promotes entry into dormancy. eLife 2016, 5, e09347. [Google Scholar] [CrossRef]
- Zhou, H.-X. Viscoelasticity of biomolecular condensates conforms to the Jeffreys model. J. Chem. Phys. 2021, 154, 041103. [Google Scholar] [CrossRef]
- Hubstenberger, A.; Noble, S.L.; Cameron, C.; Evans, T.C. Translation Repressors, an RNA Helicase, and Developmental Cues Control RNP Phase Transitions during Early Development. Dev. Cell 2013, 27, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Elbaum-Garfinkle, S.; Kim, Y.; Szczepaniak, K.; Chen Carlos, C.-H.; Eckmann Christian, R.; Myong, S.; Brangwynne Clifford, P. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl. Acad. Sci. USA 2015, 112, 7189–7194. [Google Scholar] [CrossRef] [PubMed]
- Sala, K.; Corbetta, A.; Minici, C.; Tonoli, D.; Murray, D.H.; Cammarota, E.; Ribolla, L.; Ramella, M.; Fesce, R.; Mazza, D.; et al. The ERC1 scaffold protein implicated in cell motility drives the assembly of a liquid phase. Sci. Rep. 2019, 9, 13530. [Google Scholar] [CrossRef] [PubMed]
- Huang William, Y.C.; Alvarez, S.; Kondo, Y.; Lee Young, K.; Chung Jean, K.; Lam Hiu Yue, M.; Biswas Kabir, H.; Kuriyan, J.; Groves Jay, T. A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science 2019, 363, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Trojanowski, J.; Frank, L.; Rademacher, A.; Mücke, N.; Grigaitis, P.; Rippe, K. Transcription activation is enhanced by multivalent interactions independent of phase separation. Mol. Cell 2022, 82, 1878–1893.e1810. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-C.; Fernandopulle, M.S.; Wang, G.; Choi, H.; Hao, L.; Drerup, C.M.; Patel, R.; Qamar, S.; Nixon-Abell, J.; Shen, Y.; et al. RNA Granules Hitchhike on Lysosomes for Long-Distance Transport, Using Annexin A11 as a Molecular Tether. Cell 2019, 179, 147–164.e120. [Google Scholar] [CrossRef] [PubMed]
- Quail, T.; Golfier, S.; Elsner, M.; Ishihara, K.; Murugesan, V.; Renger, R.; Jülicher, F.; Brugués, J. Force generation by protein–DNA co-condensation. Nat. Phys. 2021, 17, 1007–1012. [Google Scholar] [CrossRef]
- Kim Tae, H.; Tsang, B.; Vernon Robert, M.; Sonenberg, N.; Kay Lewis, E.; Forman-Kay Julie, D. Phospho-dependent phase separation of FMRP and CAPRIN1 recapitulates regulation of translation and deadenylation. Science 2019, 365, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Sabari Benjamin, R.; Dall’Agnese, A.; Boija, A.; Klein Isaac, A.; Coffey Eliot, L.; Shrinivas, K.; Abraham Brian, J.; Hannett Nancy, M.; Zamudio Alicia, V.; Manteiga John, C.; et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science 2018, 361, eaar3958. [Google Scholar] [CrossRef]
- Ahn, J.H.; Davis, E.S.; Daugird, T.A.; Zhao, S.; Quiroga, I.Y.; Uryu, H.; Li, J.; Storey, A.J.; Tsai, Y.-H.; Keeley, D.P.; et al. Phase separation drives aberrant chromatin looping and cancer development. Nature 2021, 595, 591–595. [Google Scholar] [CrossRef]
- Jawerth, L.; Fischer-Friedrich, E.; Saha, S.; Wang, J.; Franzmann, T.; Zhang, X.; Sachweh, J.; Ruer, M.; Ijavi, M.; Saha, S.; et al. Protein condensates as aging Maxwell fluids. Science 2020, 370, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.G.; Elnatan, D.; Keenen, M.M.; Trnka, M.J.; Johnston, J.B.; Burlingame, A.L.; Agard, D.A.; Redding, S.; Narlikar, G.J. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature 2017, 547, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Chen, Z.J. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science 2018, 361, 704–709. [Google Scholar] [CrossRef]
- Zhou, W.; Mohr, L.; Maciejowski, J.; Kranzusch, P.J. cGAS phase separation inhibits TREX1-mediated DNA degradation and enhances cytosolic DNA sensing. Mol. Cell 2021, 81, 739–755.e737. [Google Scholar] [CrossRef]
- Kagan, J.C.; Magupalli, V.G.; Wu, H. SMOCs: Supramolecular organizing centres that control innate immunity. Nat. Rev. Immunol. 2014, 14, 821–826. [Google Scholar] [CrossRef]
- Cho, W.-K.; Spille, J.-H.; Hecht, M.; Lee, C.; Li, C.; Grube, V.; Cisse Ibrahim, I. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science 2018, 361, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Dion, W.; Ballance, H.; Lee, J.; Pan, Y.; Irfan, S.; Edwards, C.; Sun, M.; Zhang, J.; Zhang, X.; Liu, S.; et al. Four-dimensional nuclear speckle phase separation dynamics regulate proteostasis. Sci. Adv. 2022, 8, eabl4150. [Google Scholar] [CrossRef] [PubMed]
- Jalihal, A.P.; Pitchiaya, S.; Xiao, L.; Bawa, P.; Jiang, X.; Bedi, K.; Parolia, A.; Cieslik, M.; Ljungman, M.; Chinnaiyan, A.M.; et al. Multivalent Proteins Rapidly and Reversibly Phase-Separate upon Osmotic Cell Volume Change. Mol. Cell 2020, 79, 978–990.e975. [Google Scholar] [CrossRef]
- Zhu, G.; Xie, J.; Kong, W.; Xie, J.; Li, Y.; Du, L.; Zheng, Q.; Sun, L.; Guan, M.; Li, H.; et al. Phase Separation of Disease-Associated SHP2 Mutants Underlies MAPK Hyperactivation. Cell 2020, 183, 490–502.e418. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; Du, Z.; Zhang, H. mTOR Regulates Phase Separation of PGL Granules to Modulate Their Autophagic Degradation. Cell 2018, 174, 1492–1506.e1422. [Google Scholar] [CrossRef]
- Yang, P.; Mathieu, C.; Kolaitis, R.-M.; Zhang, P.; Messing, J.; Yurtsever, U.; Yang, Z.; Wu, J.; Li, Y.; Pan, Q.; et al. G3BP1 Is a Tunable Switch that Triggers Phase Separation to Assemble Stress Granules. Cell 2020, 181, 325–345.e328. [Google Scholar] [CrossRef]
- Tsang, B.; Pritišanac, I.; Scherer, S.W.; Moses, A.M.; Forman-Kay, J.D. Phase Separation as a Missing Mechanism for Interpretation of Disease Mutations. Cell 2020, 183, 1742–1756. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Xu, L.; Boyko, S.; Surewicz, K.; Surewicz, W.K. Zinc promotes liquid–liquid phase separation of tau protein. J. Biol. Chem. 2020, 295, 5850–5856. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Choi, J.-M.; Holehouse, A.S.; Lee, H.O.; Zhang, X.; Jahnel, M.; Maharana, S.; Lemaitre, R.; Pozniakovsky, A.; Drechsel, D.; et al. A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 2018, 174, 688–699.e616. [Google Scholar] [CrossRef]
- Fisher, R.S.; Elbaum-Garfinkle, S. Tunable multiphase dynamics of arginine and lysine liquid condensates. Nat. Commun. 2020, 11, 4628. [Google Scholar] [CrossRef] [PubMed]
- Alshareedah, I.; Moosa, M.M.; Pham, M.; Potoyan, D.A.; Banerjee, P.R. Programmable viscoelasticity in protein-RNA condensates with disordered sticker-spacer polypeptides. Nat. Commun. 2021, 12, 6620. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, X.; Li, P.; Lin, Y. A brief guideline for studies of phase-separated biomolecular condensates. Nat. Chem. Biol. 2022, 18, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Courchaine, E.M.; Barentine, A.E.S.; Straube, K.; Lee, D.-R.; Bewersdorf, J.; Neugebauer, K.M. DMA-tudor interaction modules control the specificity of in vivo condensates. Cell 2021, 184, 3612–3625.e3617. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Takagi, M.; Kosako, H.; Hirano, T.; Yoshimura, S.H. Cell cycle-specific phase separation regulated by protein charge blockiness. Nat. Cell Biol. 2022, 24, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Jack, A.; Kim, Y.; Strom, A.R.; Lee, D.S.W.; Williams, B.; Schaub, J.M.; Kellogg, E.H.; Finkelstein, I.J.; Ferro, L.S.; Yildiz, A.; et al. Compartmentalization of telomeres through DNA-scaffolded phase separation. Dev. Cell 2022, 57, 277–290.e279. [Google Scholar] [CrossRef]
- Dzuricky, M.; Rogers, B.A.; Shahid, A.; Cremer, P.S.; Chilkoti, A. De novo engineering of intracellular condensates using artificial disordered proteins. Nat. Chem. 2020, 12, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.E.; Manteiga, J.C.; Henninger, J.E.; Sabari, B.R.; Dall’Agnese, A.; Hannett, N.M.; Spille, J.-H.; Afeyan, L.K.; Zamudio, A.V.; Shrinivas, K.; et al. Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature 2019, 572, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Guillén-Boixet, J.; Kopach, A.; Holehouse, A.S.; Wittmann, S.; Jahnel, M.; Schlüßler, R.; Kim, K.; Trussina, I.R.E.A.; Wang, J.; Mateju, D.; et al. RNA-Induced Conformational Switching and Clustering of G3BP Drive Stress Granule Assembly by Condensation. Cell 2020, 181, 346–361.e317. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xu, G.; Han, C.; Luan, P.-F.; Xing, Y.-H.; Nan, F.; Yang, L.-Z.; Huang, Y.; Yang, Z.-H.; Shan, L.; et al. lncRNA SLERT controls phase separation of FC/DFCs to facilitate Pol I transcription. Science 2021, 373, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Maharana, S.; Wang, J.; Papadopoulos, D.K.; Richter, D.; Pozniakovsky, A.; Poser, I.; Bickle, M.; Rizk, S.; Guillén-Boixet, J.; Franzmann, T.M.; et al. RNA buffers the phase separation behavior of prion-like RNA binding proteins. Science 2018, 360, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Riback, J.A.; Katanski, C.D.; Kear-Scott, J.L.; Pilipenko, E.V.; Rojek, A.E.; Sosnick, T.R.; Drummond, D. Stress-Triggered Phase Separation Is an Adaptive, Evolutionarily Tuned Response. Cell 2017, 168, 1028–1040.e1019. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Zhang, G.; Massett, M.; Cheng, J.; Guo, Z.; Wang, L.; Gao, Y.; Li, R.; Huang, X.; Li, P.; et al. Loci-specific phase separation of FET fusion oncoproteins promotes gene transcription. Nat. Commun. 2021, 12, 1491. [Google Scholar] [CrossRef]
- Iserman, C.; Roden, C.A.; Boerneke, M.A.; Sealfon, R.S.G.; McLaughlin, G.A.; Jungreis, I.; Fritch, E.J.; Hou, Y.J.; Ekena, J.; Weidmann, C.A.; et al. Genomic RNA Elements Drive Phase Separation of the SARS-CoV-2 Nucleocapsid. Mol. Cell 2020, 80, 1078–1091.e1076. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Song, D.; Jung, Y. Behavior control of membrane-less protein liquid condensates with metal ion-induced phase separation. Nat. Commun. 2020, 11, 5554. [Google Scholar] [CrossRef]
- Lyons, H.; Veettil, R.T.; Pradhan, P.; Fornero, C.; De La Cruz, N.; Ito, K.; Eppert, M.; Roeder, R.G.; Sabari, B.R. Functional partitioning of transcriptional regulators by patterned charge blocks. Cell 2023, 186, 327–345.e328. [Google Scholar] [CrossRef]
- Shih, P.-Y.; Fang, Y.-L.; Shankar, S.; Lee, S.-P.; Hu, H.-T.; Chen, H.; Wang, T.-F.; Hsia, K.-C.; Hsueh, Y.-P. Phase separation and zinc-induced transition modulate synaptic distribution and association of autism-linked CTTNBP2 and SHANK3. Nat. Commun. 2022, 13, 2664. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Chen, Y.; Chen, D.; Zhao, H.; Feng, Y.; Meng, Q.; Zhao, Y.; Zhang, H. Calcium transients on the ER surface trigger liquid-liquid phase separation of FIP200 to specify autophagosome initiation sites. Cell 2022, 185, 4082–4098.e4022. [Google Scholar] [CrossRef]
- Kim, A.C.; Lim, S.; Kim, Y.K. Metal Ion Effects on Aβ and Tau Aggregation. Int. J. Mol. Sci. 2018, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Gohrbandt, M.; Lipski, A.; Grimshaw, J.W.; Buttress, J.A.; Baig, Z.; Herkenhoff, B.; Walter, S.; Kurre, R.; Deckers-Hebestreit, G.; Strahl, H. Low membrane fluidity triggers lipid phase separation and protein segregation in living bacteria. EMBO J. 2022, 41, e109800. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Rutherford, S.T.; Silhavy, T.J.; Huang, K.C. Physical properties of the bacterial outer membrane. Nat. Rev. Microbiol. 2022, 20, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Galvagnion, C.; Buell, A.K.; Meisl, G.; Michaels, T.C.T.; Vendruscolo, M.; Knowles, T.P.J.; Dobson, C.M. Lipid vesicles trigger α-synuclein aggregation by stimulating primary nucleation. Nat. Chem. Biol. 2015, 11, 229–234. [Google Scholar] [CrossRef]
- Shi, B.; Li, W.; Song, Y.; Wang, Z.; Ju, R.; Ulman, A.; Hu, J.; Palomba, F.; Zhao, Y.; Le, J.P.; et al. UTX condensation underlies its tumour-suppressive activity. Nature 2021, 597, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Feng, Y.; Huang, W.; Tan, N.; Li, X.; Jie, M.; Feng, T.; Jiang, H.; Jiang, L. Liquid-Liquid Phase Separation in Cardiovascular Diseases. Cells 2022, 11, 3040. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Dormann, D. Liquid–Liquid Phase Separation in Disease. Annu. Rev. Genet. 2019, 53, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Boija, A.; Klein, I.A.; Young, R.A. Biomolecular Condensates and Cancer. Cancer Cell 2021, 39, 174–192. [Google Scholar] [CrossRef]
- Zhou, K.; Chen, Q.; Chen, J.; Liang, D.; Feng, D.; Liu, M.; Wang, Q.; Wang, R.; Ouyang, Q.; Quan, C.; et al. Spatiotemporal regulation of insulin signaling by liquid–liquid phase separation. Cell Discov. 2022, 8, 64. [Google Scholar] [CrossRef]
- Li, C.H.; Coffey, E.L.; Dall’Agnese, A.; Hannett, N.M.; Tang, X.; Henninger, J.E.; Platt, J.M.; Oksuz, O.; Zamudio, A.V.; Afeyan, L.K.; et al. MeCP2 links heterochromatin condensates and neurodevelopmental disease. Nature 2020, 586, 440–444. [Google Scholar] [CrossRef]
- Fasciani, A.; D’Annunzio, S.; Poli, V.; Fagnocchi, L.; Beyes, S.; Michelatti, D.; Corazza, F.; Antonelli, L.; Gregoretti, F.; Oliva, G.; et al. MLL4-associated condensates counterbalance Polycomb-mediated nuclear mechanical stress in Kabuki syndrome. Nat Genet 2020, 52, 1397–1411. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, J.; Zhang, M. Myosin VII, USH1C, and ANKS4B or USH1G Together Form Condensed Molecular Assembly via Liquid-Liquid Phase Separation. Cell Rep. 2019, 29, 974–986. [Google Scholar] [CrossRef]
- Mensah, M.A.; Niskanen, H.; Magalhaes, A.P.; Basu, S.; Kircher, M.; Sczakiel, H.L.; Reiter, A.M.V.; Elsner, J.; Meinecke, P.; Biskup, S.; et al. Aberrant phase separation and nucleolar dysfunction in rare genetic diseases. Nature 2023, 614, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Cunza, N.L.; Tan, L.X.; Thamban, T.; Germer, C.J.; Rathnasamy, G.; Toops, K.A.; Lakkaraju, A. Mitochondria-dependent phase separation of disease-relevant proteins drives pathological features of age-related macular degeneration. JCI Insight 2021, 6, e142254. [Google Scholar] [CrossRef] [PubMed]
- Horii, Y.; Matsuda, S.; Toyota, C.; Morinaga, T.; Nakaya, T.; Tsuchiya, S.; Ohmuraya, M.; Hironaka, T.; Yoshiki, R.; Kasai, K.; et al. VGLL3 is a mechanosensitive protein that promotes cardiac fibrosis through liquid–liquid phase separation. Nat. Commun. 2023, 14, 550. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; Saeedi, A.; Davtyan, A.; Fathi, M.; Sherman, M.B.; Safari, M.S.; Klindziuk, A.; Barton, M.C.; Varadarajan, N.; Kolomeisky, A.B.; et al. Mesoscopic protein-rich clusters host the nucleation of mutant p53 amyloid fibrils. Proc. Natl. Acad. Sci. USA 2021, 118, e2015618118. [Google Scholar] [CrossRef] [PubMed]
- Babinchak, W.M.; Dumm, B.K.; Venus, S.; Boyko, S.; Putnam, A.A.; Jankowsky, E.; Surewicz, W.K. Small molecules as potent biphasic modulators of protein liquid-liquid phase separation. Nat. Commun. 2020, 11, 5574. [Google Scholar] [CrossRef]
- Kilgore, H.R.; Mikhael, P.G.; Overholt, K.J.; Boija, A.; Hannett, N.M.; Van Dongen, C.; Lee, T.I.; Chang, Y.-T.; Barzilay, R.; Young, R.A. Distinct chemical environments in biomolecular condensates. Nat. Chem. Biol. 2023, 20, 291–301. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Q.; Wang, N.; Li, S.; Bian, W.; Sun, Z.; Wang, L.; Wang, L.; Liu, C.; Song, C.; et al. Oleic Acid Dissolves cGAS–DNA Phase Separation to Inhibit Immune Surveillance. Adv. Sci. 2023, 22, 06820. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, L.; Zou, Y.; Zhang, Y.; Zhang, M.; Xu, L.; Zheng, L.; He, W.; Yu, K.; Li, T.; et al. Disrupting the phase separation of KAT8–IRF1 diminishes PD-L1 expression and promotes antitumor immunity. Nat. Cancer 2023, 4, 382–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, B.; Wang, Y.; Jiang, R.; Liu, J.; Wei, Y.; Gao, X.; Zhu, Y.; Wang, X.; Sun, M.; et al. An extra-erythrocyte role of haemoglobin body in chondrocyte hypoxia adaption. Nature 2023, 622, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Słabicki, M.; Kozicka, Z.; Petzold, G.; Li, Y.-D.; Manojkumar, M.; Bunker, R.D.; Donovan, K.A.; Sievers, Q.L.; Koeppel, J.; Suchyta, D.; et al. The CDK inhibitor CR8 acts as a molecular glue degrader that depletes cyclin K. Nature 2020, 585, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Mayor-Ruiz, C.; Bauer, S.; Brand, M.; Kozicka, Z.; Siklos, M.; Imrichova, H.; Kaltheuner, I.H.; Hahn, E.; Seiler, K.; Koren, A.; et al. Rational discovery of molecular glue degraders via scalable chemical profiling. Nat. Chem. Biol. 2020, 16, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Dieter, S.M.; Siegl, C.; Codó, P.L.; Huerta, M.; Ostermann-Parucha, A.L.; Schulz, E.; Zowada, M.K.; Martin, S.; Laaber, K.; Nowrouzi, A.; et al. Degradation of CCNK/CDK12 is a druggable vulnerability of colorectal cancer. Cell Rep. 2021, 36, 109394. [Google Scholar] [CrossRef]
- Xie, J.; He, H.; Kong, W.; Li, Z.; Gao, Z.; Xie, D.; Sun, L.; Fan, X.; Jiang, X.; Zheng, Q.; et al. Targeting androgen receptor phase separation to overcome antiandrogen resistance. Nat. Chem. Biol. 2022, 18, 1341–1350. [Google Scholar] [CrossRef]



| Protein | Diseases | Location | Physical Traits | Description | Refs |
|---|---|---|---|---|---|
| IRS1 | Diabetes | Cytosol | Fluidity | IRS1 condensates mediate insulin signaling via recruiting PI3K, PDK1, PIP3 and PKB, whose formation is impaired in insulin resistant cells. | [121] |
| MeCP2 | Rett syndrome | Nucleus | Fluidity | MeCP2 condensates selectively concentrate heterochromatin cofactors rather than components of euchromatin. Mutations in MECP2 disrupt MeCP2 condensates, leading to Rett syndrome. | [122] |
| MLL4 | Kabuki syndrome | Nucleus | Interfacial tension | MLL4 condensates maintain the balance between transcriptional and PcG condensates; MLL4 LoF increased chromatin compaction and disrupted nuclear mechanics and architecture. | [123] |
| MYO7A, USH1C, ANKS4B, USH1G | Usher syndrome | Cytosol | Wetting | Densely packed MYO7A/USH1C/USH1G condensates stabilize tip-links in intestine microvilli and stereocilia. MYO7A mutations disrupt the binding of the motor to USH1 and impair condensates formation. | [124] |
| HOXD13, HMGB1 | Synpolydactyly | Nucleus | Interfacial tension | Alanine repeat expansions enhance the phase separation capacity of the HOXD13 IDR, and the IDR mutant unblend HOXD13 from transcriptional co-condensates, leading to disease phenotype. | [55,125] |
| SUMO-SIM | AD | Cytosol | Stiffness | Mechanical compression from molecular crowding shapes stiffness of condensates, therefore leading to phosphoregulatory network rewiring. | [67] |
| FUS | ALS | Nucleus | Fluidity, stiffness | FUS normally operate as liquid droplets, solid aggregation leads to ALS. | [68] |
| ApoE2, p62 | AMD | Cytosol | Universal | Mitochondrial injury drives phase separation of ApoE2 and p62 that nucleate drusen and regulate autophagy, respectively. | [126] |
| IB | RSV | Nucleus | Stiffness | A3E and cyclopamine inhibit RSV replication by hardening IB condensates. | [20] |
| VGLL3 | Cardiac fibrosis | Nucleus | Viscoelasticity | VGLL3 is incorporated into non-paraspeckle NONO condensates containing EWSR1 and suppresses miR-29b. | [127] |
| WNK | Stroke, hypertension, hyperkalemia | Cytosol | Swelling | WNK kinases sense molecular crowding and rescue cell volume via phase separation | [28] |
| MED1-BRD4, HP1⍺, SRSF2, FIB1, NPM1 | Cancer | Nucleus | Fluidity, swelling | Drug partitioning into nuclear condensates influences drug concentration and activity: swelling of MED1 condensates induces tamoxifen resistance; ER mutation alters drug affinity. | [7] |
| p53 | Cancer | Nucleus | Interfacial tension | p53 mediates the interplay of nuclear speckles and p21 for gene expression through DNA binding. p53 mutant R248Q condensates host and facilitate the nucleation of amyloid fibrils in cancer cells. | [128] |
| BRD4-MED1, TAF15, EWS, Sp1 | Cancer | Nucleus | Fluidity, wetting | Nuclear condensates create a dense phase with high concentration of transcriptional machinery, serving as interaction hubs for robust gene expression. | [12,79,86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Zhou, Y.; Wang, Z.; Peng, M.; Wei, X.; Xie, Y.; Wen, C.; Liu, J.; Ye, M. Biomolecular Condensates Decipher Molecular Codes of Cell Fate: From Biophysical Fundamentals to Therapeutic Practices. Int. J. Mol. Sci. 2024, 25, 4127. https://doi.org/10.3390/ijms25074127
Sun X, Zhou Y, Wang Z, Peng M, Wei X, Xie Y, Wen C, Liu J, Ye M. Biomolecular Condensates Decipher Molecular Codes of Cell Fate: From Biophysical Fundamentals to Therapeutic Practices. International Journal of Molecular Sciences. 2024; 25(7):4127. https://doi.org/10.3390/ijms25074127
Chicago/Turabian StyleSun, Xing, Yangyang Zhou, Zhiyan Wang, Menglan Peng, Xianhua Wei, Yifang Xie, Chengcai Wen, Jing Liu, and Mao Ye. 2024. "Biomolecular Condensates Decipher Molecular Codes of Cell Fate: From Biophysical Fundamentals to Therapeutic Practices" International Journal of Molecular Sciences 25, no. 7: 4127. https://doi.org/10.3390/ijms25074127





