Anti-Atherosclerotic Properties of Aronia melanocarpa Extracts Influenced by Their Chemical Composition Associated with the Ripening Stage of the Berries
Abstract
1. Introduction
2. Results and Discussion
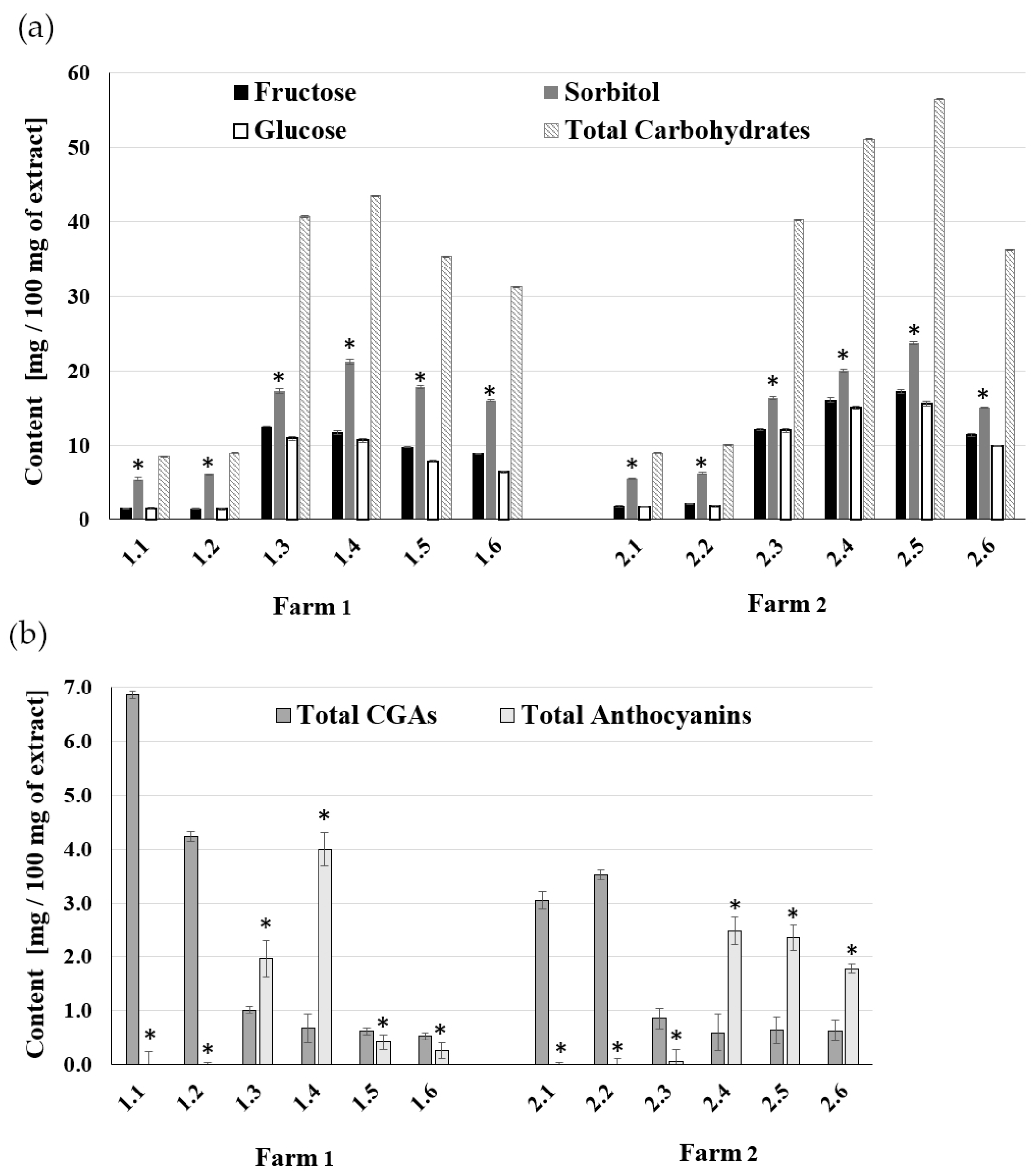
2.1. The Composition of A. melanocarpa Fruit Extracts
2.2. Anti-Atherosclerotic Activity in Human Endothelial Cells
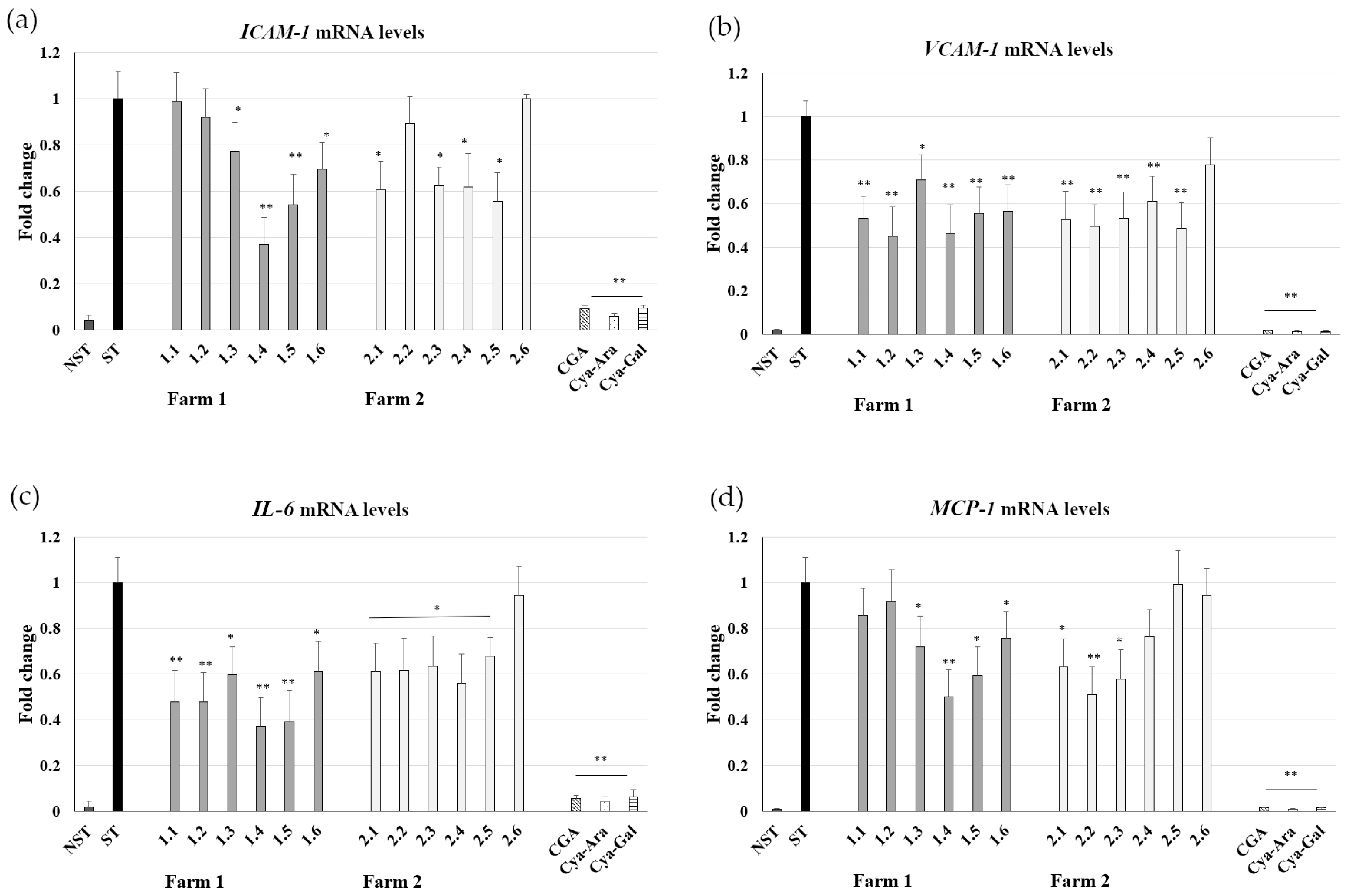
2.3. Influence on ICAM-1, VCAM-1, IL-6, and MCP-1 mRNA Expression
2.4. PCA Analysis
3. Materials and Methods
3.1. Plant Material and Extract Preparation
3.2. Content of Anthocyanins and Chlorogenic Acids in A. melanocarpa Extracts
3.3. Determination of Sorbitol and Sugars in Extracts of A. melanocarpa by HPLC-RI
3.4. Anti-Atherosclerotic Activity in Human Endothelial Cells
3.4.1. Materials
3.4.2. Cell Culture and Experimental Conditions
3.4.3. Cell Viability Assessment by MTT Assay
3.4.4. Measurement of ICAM-1 and VCAM-1 Expression in Human Umbilical Vein Endothelial Cells by Flow Cytometry
3.4.5. Determination of Gene Expression
3.5. PCA Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hardin, J.W. The Enigmatic Chokeberries (Aronia, Rosaceae). Bull. Torrey Bot. Club 1973, 100, 178–184. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Petruskevicius, A.; Viskelis, J.; Urbonaviciene, D.; Viskelis, P. Anthocyanin Accumulation in Berry Fruits and Their Antimicrobial and Antiviral Properties: An Overview. Horticulturae 2023, 9, 288. [Google Scholar] [CrossRef]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT Food Sci. Technol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Denev, P.; Kratchanova, M.; Petrova, I.; Klisurova, D.; Georgiev, Y.; Ognyanov, M.; Yanakieva, I. Black Chokeberry (Aronia melanocarpa (Michx.) Elliot) Fruits and Functional Drinks Differ Significantly in Their Chemical Composition and Antioxidant Activity. J. Chem. 2018, 2018, 9574587. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A Review on the Characteristic Components and Potential Health Effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Wojdyło, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Liu, X.; Chen, X.; Ding, C.; Dong, L.; Zhang, J.; Sun, S.; Ding, Q.; Khatoom, S.; et al. Chokeberry (Aronia melanocarpa) as a new functional food relationship with health: An overview. J. Future Foods 2021, 1, 168–178. [Google Scholar] [CrossRef]
- Blesso, C.N. Dietary Anthocyanins and Human Health. Nutrients 2019, 11, 2107. [Google Scholar] [CrossRef]
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M.M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Kay, C.; Mazza, G.; Holub, B.; Wang, J. Anthocyanin metabolites in human urine and serum. Br. J. Nutr. 2004, 91, 933–942. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on the safety and efficacy of niacin (nicotinamide) as a feed additive for all animal species based on a dossier submitted by EUROPE-ASIA Import Export GmbH. EFSA J. 2012, 10, 2789. [Google Scholar]
- Li, J.; Deng, Y.; Yuan, C.; Pan, L.; Chai, H.; Keller, W.J.; Kinghorn, A.D. Antioxidant and quinone reductase-inducing constituents of black chokeberry (Aronia melanocarpa) fruits. J. Agric. Food Chem. 2012, 60, 11551–11559. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Xiang, R.; Fu, C.; Qu, Z.; Liu, C. The Regulatory effect of chlorogenic acid on gut-brain function and its mechanism: A systematic review. Biomed. Pharmacother. 2022, 149, 112831. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Satti, N.K.; Sharma, P.; Sharma, V.K.; Suri, K.A.; Bani, S. Differential effects of chlorogenic acid on various immunological parameters relevant to rheumatoid arthritis. Phytother. Res. 2012, 26, 1156–1165. [Google Scholar] [CrossRef]
- Gao, X.H.; Zhang, S.D.; Wang, L.T.; Yu, L.; Zhao, X.L.; Ni, H.Y.; Wang, Y.Q.; Wang, J.D.; Shan, C.H.; Fu, Y.J. Anti-Inflammatory Effects of Neochlorogenic Acid Extract from Mulberry Leaf (Morus alba L.) Against LPS-Stimulated Inflammatory Response through Mediating the AMPK/Nrf2 Signaling Pathway in A549 Cells. Molecules 2020, 25, 1385. [Google Scholar] [CrossRef]
- Chrubasik, C.; Li, G.; Chrubasik, S. The clinical effectiveness of chokeberry: A systematic review. Phytother. Res. 2010, 24, 1107–1114. [Google Scholar] [CrossRef]
- Sidor, A.; Drożdżyńska, A.; Gramza-Michałowska, A. Black chokeberry (Aronia melanocarpa) and its products as potential health-promoting factors—An overview. Trends Food Sci. Technol. 2019, 89, 45–60. [Google Scholar] [CrossRef]
- Worsztynowicz, P.; Napierała, M.; Białas, W.; Grajek, W.; Olkowicz, M. Pancreatic α-amylase and lipase inhibitory activity of polyphenolic compounds present in the extract of black chokeberry (Aronia melanocarpa L.). Process. Biochem. 2014, 49, 1457–1463. [Google Scholar] [CrossRef]
- Skoczyńska, A.; Jedrychowska, I.; Poręba, R.; Affelska-Jercha, A.; Turczyn, B.; Wojakowska, A.; Andrzejak, R. Influence of chokeberry juice on arterial blood pressure and lipid parameters in men with mild hypercholesterolemia. Pharmacol. Rep. 2007, 59, 177–182. [Google Scholar]
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Tancheva, S.; Belcheva, A. Hypoglycemic and hypolipidemic effects of Aronia melanocarpa fruit juice in streptozotocin-induced diabetic rats. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Broncel, M.; Kozirog, M.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Chojnowska-Jezierska, J. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med. Sci. Monit. 2010, 16, Cr28–Cr34. [Google Scholar]
- Loo, B.-M.; Erlund, I.; Koli, R.; Puukka, P.; Hellström, J.; Wähälä, K.; Mattila, P.; Jula, A. Consumption of chokeberry (Aronia mitschurinii) products modestly lowered blood pressure and reduced low-grade inflammation in patients with mildly elevated blood pressure. Nutr. Res. 2016, 36, 1222–1230. [Google Scholar] [CrossRef]
- Parzonko, A.; Oświt, A.; Bazylko, A.; Naruszewicz, M. Anthocyans-rich Aronia melanocarpa extract possesses ability to protect endothelial progenitor cells against angiotensin II induced dysfunction. Phytomedicine 2015, 22, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef]
- Carlos, T.M.; Harlan, J.M. Leukocyte-endothelial adhesion molecules. Blood 1994, 84, 2068–2101. [Google Scholar] [CrossRef]
- Langer, H.F.; Chavakis, T. Leukocyte-endothelial interactions in inflammation. J. Cell. Mol. Med. 2009, 13, 1211–1220. [Google Scholar] [CrossRef]
- Blankenberg, S.; Barbaux, S.; Tiret, L. Adhesion molecules and atherosclerosis. Atherosclerosis 2003, 170, 191–203. [Google Scholar] [CrossRef]
- Manduteanu, I.; Simionescu, M. Inflammation in atherosclerosis: A cause or a result of vascular disorders? J. Cell. Mol. Med. 2012, 16, 1978–1990. [Google Scholar] [CrossRef]
- Kirichenko, T.V.; Sobenin, I.A.; Nikolic, D.; Rizzo, M.; Orekhov, A.N. Anti-cytokine therapy for prevention of atherosclerosis. Phytomedicine 2016, 23, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W.; Taheri, R.; Pei, R.; Kranz, S.; Yu, M.; Durocher, S.N.; Brand, M.H. Harvest date affects aronia juice polyphenols, sugars, and antioxidant activity, but not anthocyanin stability. Food Chem. 2015, 187, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Siudem, P.; Paradowska, K.; Gralec, M.; Kaźmierski, S.; Wawer, I. Aronia melanocarpa Fruits as a Rich Dietary Source of Chlorogenic Acids and Anthocyanins: 1H-NMR, HPLC-DAD, and Chemometric Studies. Molecules 2020, 25, 3234. [Google Scholar] [CrossRef]
- Nakashima, Y.; Raines, E.W.; Plump, A.S.; Breslow, J.L.; Ross, R. Upregulation of VCAM-1 and ICAM-1 at Atherosclerosis-Prone Sites on the Endothelium in the ApoE-Deficient Mouse. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 842–851. [Google Scholar] [CrossRef]
- Lampros, F.; Georgios, A.; Ioannis, S.V.; Alkistis, P.; Angeliki, M.; Maria, K.; Christos, V.; Dimitrios, T.; Dimitri, P.M.; Despina, P. Intercellular Adhesion Molecule (ICAM)-1 and Vascular Cell Adhesion Molecule (VCAM)-1 at the Early Stages of Atherosclerosis in a Rat Model. In Vivo 2012, 26, 243. [Google Scholar]
- Habas, K.; Shang, L. Alterations in intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in human endothelial cells. Tissue Cell 2018, 54, 139–143. [Google Scholar] [CrossRef]
- Chang, C.C.; Chu, C.F.; Wang, C.N.; Wu, H.T.; Bi, K.W.; Pang, J.H.; Huang, S.T. The anti-atherosclerotic effect of tanshinone IIA is associated with the inhibition of TNF-α-induced VCAM-1, ICAM-1 and CX3CL1 expression. Phytomedicine 2014, 21, 207–216. [Google Scholar] [CrossRef]
- Martin, D.; Taheri, R.; Brand, M.; Ii, A.; Sylvester, F.; Bolling, B. Anti-inflammatory activity of aronia berry extracts in murine splenocytes. J. Funct. Foods 2014, 8, 68–75. [Google Scholar] [CrossRef]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of Anthocyanins and Ellagitannins in Selected Foods Consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Cianciosi, D.; Quiles, J.L.; Mezzetti, B.; Xiao, J.; Giampieri, F.; Battino, M. The efficacy of berries against lipopolysaccharide-induced inflammation: A review. Trends Food Sci. Technol. 2021, 117, 74–91. [Google Scholar] [CrossRef]
- WHO Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 7 May 2021).
- Park, J.G.; Ryu, S.Y.; Jung, I.H.; Lee, Y.H.; Kang, K.J.; Lee, M.R.; Lee, M.N.; Sonn, S.K.; Lee, J.H.; Lee, H.; et al. Evaluation of VCAM-1 antibodies as therapeutic agent for atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 2013, 226, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Furuya, K.; Takeda, H.; Azhar, S.; McCarron, R.M.; Chen, Y.; Ruetzler, C.A.; Wolcott, K.M.; DeGraba, T.J.; Rothlein, R.; Hugli, T.E.; et al. Examination of several potential mechanisms for the negative outcome in a clinical stroke trial of enlimomab, a murine anti-human intercellular adhesion molecule-1 antibody: A bedside-to-bench study. Stroke 2001, 32, 2665–2674. [Google Scholar] [CrossRef] [PubMed]
- Garnacho, C.; Serrano, D.; Muro, S. A fibrinogen-derived peptide provides intercellular adhesion molecule-1-specific targeting and intraendothelial transport of polymer nanocarriers in human cell cultures and mice. J. Pharmacol. Exp. Ther. 2012, 340, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Thuan, D.T.B.; Phu, H.T.; Nguyen, T.H.D.; Hasan, H.; Halabi, S.; Abdelhady, S.; Nasrallah, G.K.; Eid, A.H.; Pintus, G. Herbal Medicine for Cardiovascular Diseases: Efficacy, Mechanisms, and Safety. Front. Pharmacol. 2020, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Taïlé, J.; Bringart, M.; Planesse, C.; Patché, J.; Rondeau, P.; Veeren, B.; Clerc, P.; Gauvin-Bialecki, A.; Bourane, S.; Meilhac, O.; et al. Antioxidant Polyphenols of Antirhea borbonica Medicinal Plant and Caffeic Acid Reduce Cerebrovascular, Inflammatory and Metabolic Disorders Aggravated by High-Fat Diet-Induced Obesity in a Mouse Model of Stroke. Antioxidants 2022, 11, 858. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Lewandowska, U. Inhibition of Pro-Inflammatory Cytokine Secretion by Polyphenol-Rich Extracts in Macrophages via NF-κB Pathway. Food Rev. Int. 2023, 39, 5459–5478. [Google Scholar] [CrossRef]
- Migoń, P. Wyjątkowe zdarzenia przyrodnicze na Dolnym Śląsku i ich skutki. Rozpr. Nauk. Inst. Geogr. Rozw. Reg. Uniw. Wrocławskiego 2010, 14, 21–25. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



| Date of Collection | Farm 1 | Farm 2 | Ripening/Color |
|---|---|---|---|
| 26 May | 1.1 | 2.1 | unripe/green |
| 26 June | 1.2 | 2.2 | |
| 24 July | 1.3 | 2.3 | ripe/dark red |
| 21 August | 1.4 | 2.4 | |
| 18 September | 1.5 | 2.5 | |
| 30 October | 1.6 | 2.6 | overripe/black |
| Compound | RT (min) | Calibration Curve | R2 | Linear Range (mg/mL) | LOD (mg/mL) | LOQ (mg/mL) |
|---|---|---|---|---|---|---|
| Sorbitol | 8.76 | A = 348,714c − 8658 | 1.000 | 0.50–12.0 | 0.06 | 0.18 |
| Fructose | 8.02 | A = 342,274c − 7039 | 0.999 | 0.30–10.0 | 0.08 | 0.25 |
| Glucose | 9.21 | A = 260,169c − 7766 | 0.999 | 0.30–10.0 | 0.09 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, A.; Bryk, D.; Paradowska, K.; Siudem, P.; Wawer, I.; Wrzosek, M. Anti-Atherosclerotic Properties of Aronia melanocarpa Extracts Influenced by Their Chemical Composition Associated with the Ripening Stage of the Berries. Int. J. Mol. Sci. 2024, 25, 4145. https://doi.org/10.3390/ijms25084145
Zielińska A, Bryk D, Paradowska K, Siudem P, Wawer I, Wrzosek M. Anti-Atherosclerotic Properties of Aronia melanocarpa Extracts Influenced by Their Chemical Composition Associated with the Ripening Stage of the Berries. International Journal of Molecular Sciences. 2024; 25(8):4145. https://doi.org/10.3390/ijms25084145
Chicago/Turabian StyleZielińska, Agnieszka, Dorota Bryk, Katarzyna Paradowska, Paweł Siudem, Iwona Wawer, and Małgorzata Wrzosek. 2024. "Anti-Atherosclerotic Properties of Aronia melanocarpa Extracts Influenced by Their Chemical Composition Associated with the Ripening Stage of the Berries" International Journal of Molecular Sciences 25, no. 8: 4145. https://doi.org/10.3390/ijms25084145
APA StyleZielińska, A., Bryk, D., Paradowska, K., Siudem, P., Wawer, I., & Wrzosek, M. (2024). Anti-Atherosclerotic Properties of Aronia melanocarpa Extracts Influenced by Their Chemical Composition Associated with the Ripening Stage of the Berries. International Journal of Molecular Sciences, 25(8), 4145. https://doi.org/10.3390/ijms25084145








