Berberine Effects in Pre-Fibrotic Stages of Non-Alcoholic Fatty Liver Disease—Clinical and Pre-Clinical Overview and Systematic Review of the Literature
Abstract
1. Introduction
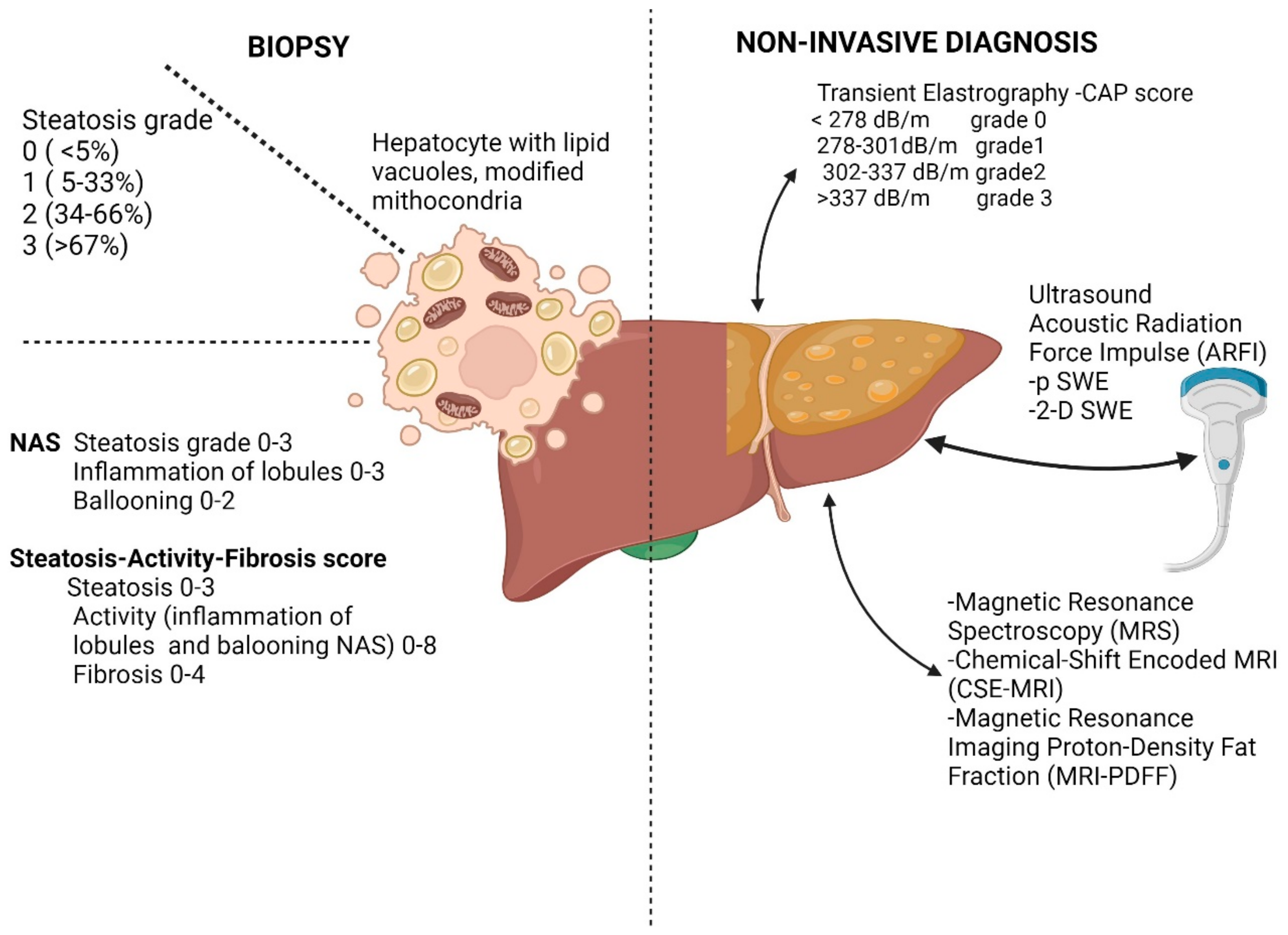
1.1. Diagnosis of Liver Steatosis
1.1.1. Pathology in Pre-Fibrotic Stages of NAFLD
1.1.2. Imagistic Studies in Pre-Fibrotic Stages in NAFLD
1.1.3. Serum Markers in NAFLD
1.2. Pathophysiology and Molecular Signaling in NAFLD
1.3. Actual Therapeutic Agents in NAFLD
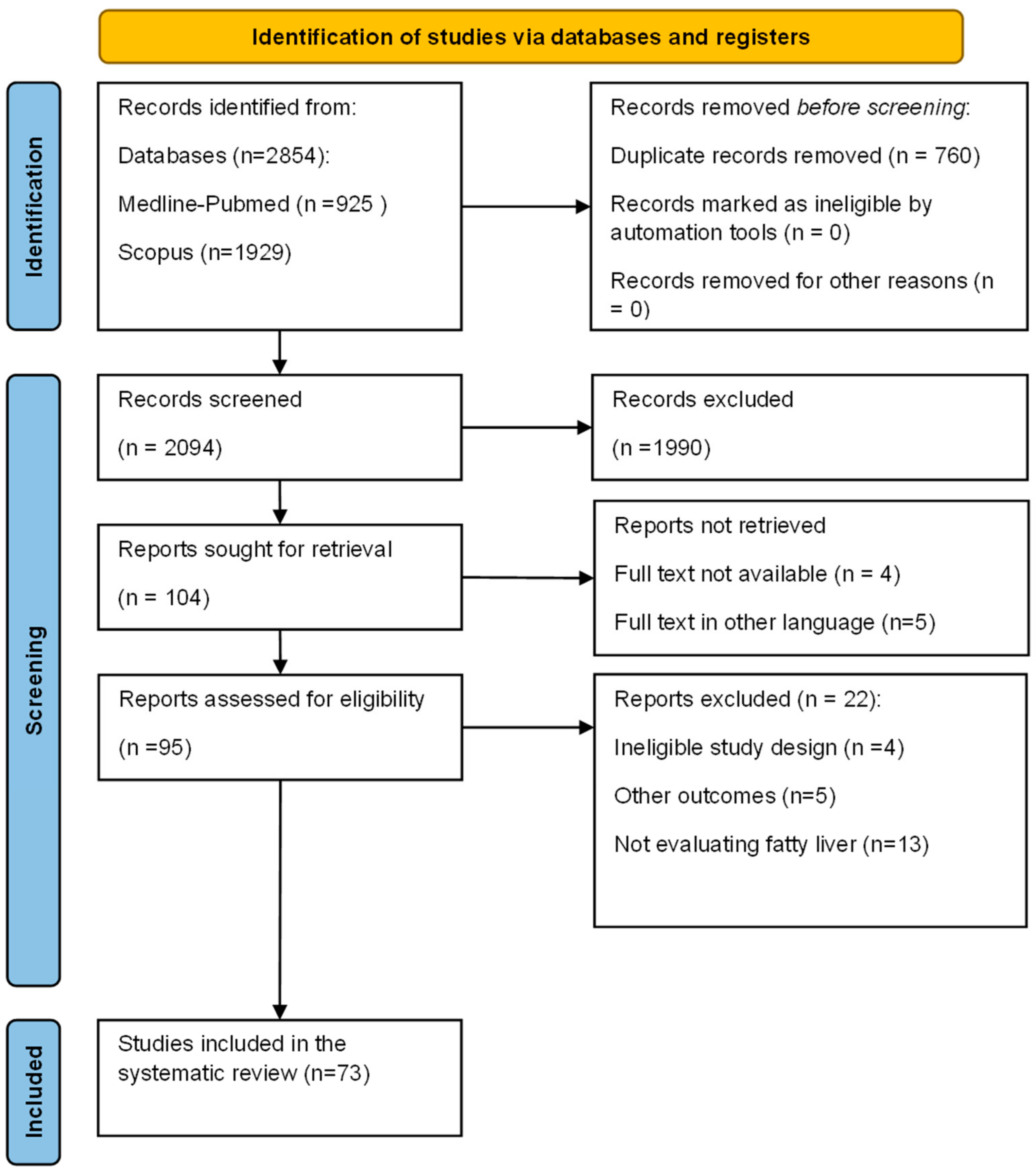
2. Methods
3. Results
3.1. Clinical Studies on BBR and NAFLD
3.2. Pre-Clinical Studies of Berberine Effects in Experimental Models
4. Discussion
4.1. Effects of Berberine in NAFLD Clinical Studies
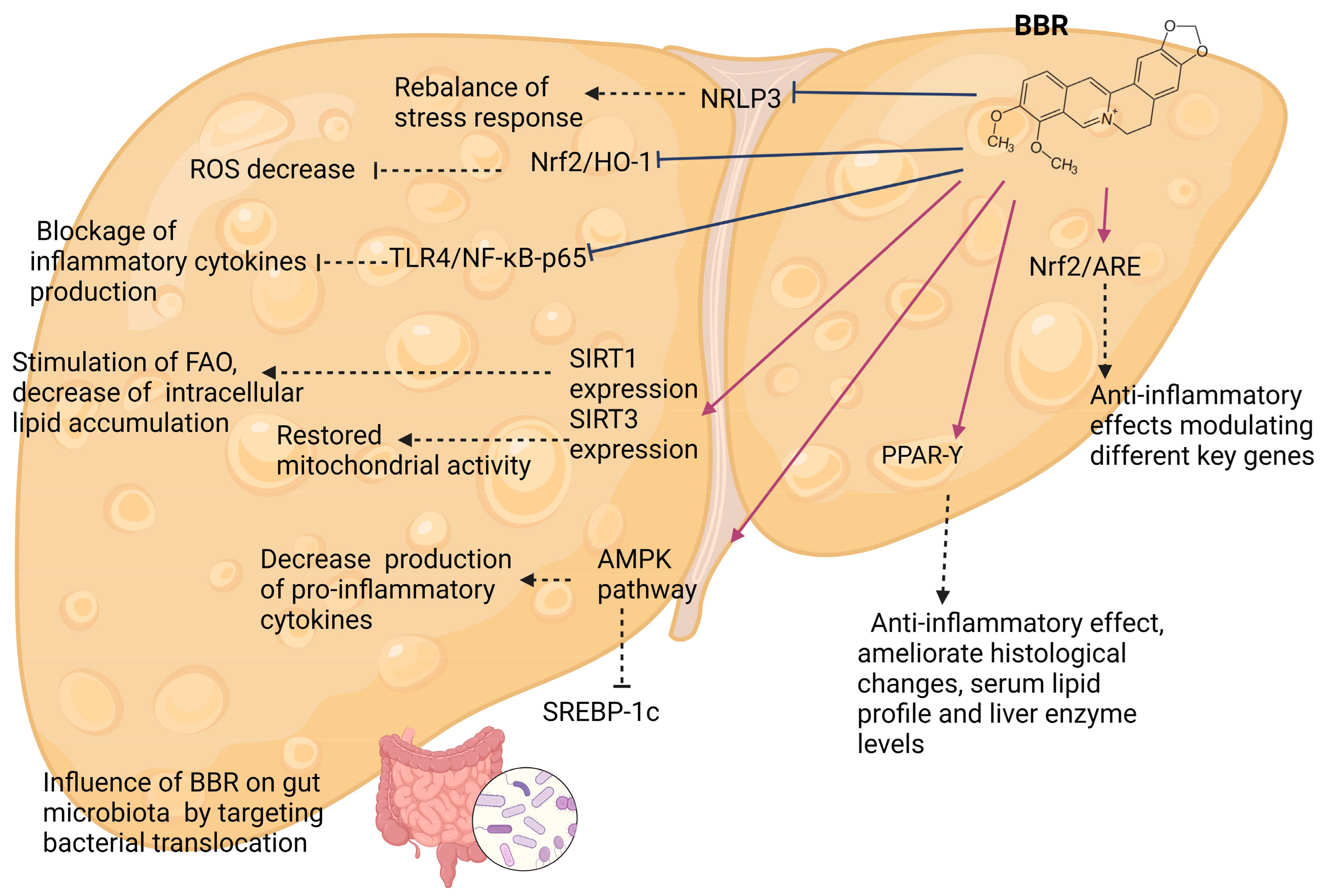
4.2. Effects of Berberine and Its Mechanisms—Experimental Models
4.2.1. AMPK-Kinase Pathways
4.2.2. Gut Microbiota
4.2.3. Sirtuins
4.2.4. PPAR-Υ
4.2.5. Inflammatory Pathways
4.2.6. Other Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, V.W.-S.; Ekstedt, M.; Wong, G.L.-H.; Hagström, H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol. 2023, 79, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Chaim, F.D.M.; Pascoal, L.B.; Chaim, F.H.M.; Palma, B.B.; Damázio, T.A.; da Costa, L.B.E.; Carvalho, R.; Cazzo, E.; Gestic, M.A.; Utrini, M.P.; et al. Histological grading evaluation of non-alcoholic fatty liver disease after bariatric surgery: A retrospective and longitudinal observational cohort study. Sci. Rep. 2020, 10, 8496. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. J. Hepatol. 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Chan, K.E.; Koh, T.J.L.; Tang, A.S.P.; Quek, J.; Yong, J.N.; Tay, P.; Tan, D.J.H.; Lim, W.H.; Lin, S.Y.; Huang, D.; et al. Global Prevalence and Clinical Characteristics of Metabolic-associated Fatty Liver Disease: A Meta-Analysis and Systematic Review of 10 739 607 Individuals. J. Clin. Endocrinol. Metab. 2022, 107, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Dao, A.D.; Nguyen, V.H.; Ito, T.; Cheung, R.; Nguyen, M.H. Prevalence, characteristics, and mortality outcomes of obese and nonobese MAFLD in the United States. Hepatol. Int. 2022, 17, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Ong, J.; Trimble, G.; AlQahtani, S.; Younossi, I.; Ahmed, A.; Racila, A.; Henry, L. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin. Gastroenterol. Hepatol. 2020, 19, 580–589.e5. [Google Scholar] [CrossRef]
- Vaz, K.; Clayton-Chubb, D.; Majeed, A.; Lubel, J.; Simmons, D.; Kemp, W.; Roberts, S.K. Current understanding and future perspectives on the impact of changing NAFLD to MAFLD on global epidemiology and clinical outcomes. Hepatol. Int. 2023, 17, 1082–1097. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef]
- Behl, T.; Singh, S.; Sharma, N.; Zahoor, I.; Albarrati, A.; Albratty, M.; Meraya, A.M.; Najmi, A.; Bungau, S. Expatiating the Pharmacological and Nanotechnological Aspects of the Alkaloidal Drug Berberine: Current and Future Trends. Molecules 2022, 27, 3705. [Google Scholar] [CrossRef] [PubMed]
- Bucurica, S.; Radu, F.I.; Bucurica, A.; Socol, C.; Prodan, I.; Tudor, I.; Sirbu, C.A.; Plesa, F.C.; Jinga, M. Risk of New-Onset Liver Injuries Due to COVID-19 in Preexisting Hepatic Conditions—Review of the Literature. Medicina 2022, 59, 62. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.-X.; Yang, P.; Tang, Q.; Cheng, P.; Liu, X.-B.; Zheng, Y.-J.; Liu, Y.-S.; Zeng, J.-G. Isoquinoline Alkaloids and Their Antiviral, Antibacterial, and Antifungal Activities and Structure-activity Relationship. Curr. Org. Chem. 2017, 21, 1920–1934. [Google Scholar] [CrossRef]
- Trowell, J.; Alukal, J.; Zhang, T.; Liu, L.; Maheshwari, A.; Yoo, H.Y.; Thuluvath, P.J. How Good Are Controlled Attenuation Parameter Scores from Fibroscan to Assess Steatosis, NASH, and Fibrosis? Dig. Dis. Sci. 2020, 66, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Harrison, S.A.; Abdelmalek, M.F.; Anstee, Q.M.; Bedossa, P.; Castera, L.; Dimick-Santos, L.; Friedman, S.L.; Greene, K.; Kleiner, D.E.; et al. Case definitions for inclusion and analysis of endpoints in clinical trials for nonalcoholic steatohepatitis through the lens of regulatory science. J. Hepatol. 2018, 67, 2001–2012. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.-S.; Chan, W.K.; Chitturi, S.; Chawla, Y.; Dan, Y.Y.; Duseja, A.; Fan, J.; Goh, K.-L.; Hamaguchi, M.; Hashimoto, E.; et al. Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 1: Definition, risk factors and assessment. J. Gastroenterol. Hepatol. 2018, 33, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Almanza, D.; Gharaee-Kermani, M.; Zhilin-Roth, A.; Rodriguez-Nieves, J.A.; Colaneri, C.; Riley, T.; Macoska, J.A. Nonalcoholic Fatty Liver Disease Demonstrates a Pre-fibrotic and Premalignant Molecular Signature. Dig. Dis. Sci. 2018, 64, 1257–1269. [Google Scholar] [CrossRef]
- Androutsakos, T.; Dimitriadis, K.; Revenas, K.; Vergadis, C.; Papadakis, D.-D.; Sakellariou, S.; Vallilas, C.; Hatzis, G. Liver Biopsy: To Do or Not to Do—A Single-Center Study. Dig. Dis. 2023, 41, 913–921. [Google Scholar] [CrossRef]
- Ratziu, V.; Charlotte, F.; Heurtier, A.; Gombert, S.; Giral, P.; Bruckert, E.; Grimaldi, A.; Capron, F.; Poynard, T. Sampling Variability of Liver Biopsy in Nonalcoholic Fatty Liver Disease. Gastroenterology 2005, 128, 1898–1906. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M. Nonalcoholic Fatty Liver Disease: Pathologic Patterns and Biopsy Evaluation in Clinical Research. Semin. Liver Dis. 2012, 32, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Nascimbeni, F.; Bedossa, P.; Fedchuk, L.; Pais, R.; Charlotte, F.; Lebray, P.; Poynard, T.; Ratziu, V. Clinical validation of the FLIP algorithm and the SAF score in patients with non-alcoholic fatty liver disease. J. Hepatol. 2019, 72, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, C.; Radu, F.I.; Jinga, M.; Nuță, P.; Bucurică, S.; Macadon, B.; Pătrășescu, M.; Popescu, A.; Balaban, V.; Voicu, L.; et al. From liver biopsy to non-invasive markers in evaluating fibrosis in chronic liver disease. Rom. J. Mil. Med. 2015, 118, 5–12. [Google Scholar] [CrossRef]
- van Werven, J.R.; Marsman, H.A.; Nederveen, A.J.; Smits, N.J.; ten Kate, F.J.; van Gulik, T.M.; Stoker, J. Assessment of Hepatic Steatosis in Patients Undergoing Liver Resection: Comparison of US, CT, T1-weighted Dual-Echo MR Imaging, and Point-resolved1H MR Spectroscopy. Radiology 2010, 256, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Monteiro, L.B.S. Ultrasound-based techniques for the diagnosis of liver steatosis. World J. Gastroenterol. 2019, 25, 6053–6062. [Google Scholar] [CrossRef] [PubMed]
- Ibacahe, C.; Correa-Burrows, P.; Burrows, R.; Barrera, G.; Kim, E.; Hirsch, S.; Jofré, B.; Blanco, E.; Gahagan, S.; Bunout, D. Accuracy of a Semi-Quantitative Ultrasound Method to Determine Liver Fat Infiltration in Early Adulthood. Diagnostics 2020, 10, 431. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The Severity of Ultrasonographic Findings in Nonalcoholic Fatty Liver Disease Reflects the Metabolic Syndrome and Visceral Fat Accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef]
- Ferraioli, G.; Wong, V.W.-S.; Castera, L.; Berzigotti, A.; Sporea, I.; Dietrich, C.F.; Choi, B.I.; Wilson, S.R.; Kudo, M.; Barr, R.G. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med. Biol. 2018, 44, 2419–2440. [Google Scholar] [CrossRef]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef]
- Balaban, D.; Popp, A.; Robu, G.; Bucurica, S.; Costache, R.; Nuta, P.; Radu, F.I.; Jinga, M. P323 Fatty liver assessment in inflammatory bowel disease patients using controlled attenuation parameter. J. Crohn’s Colitis 2017, 11 (Suppl. S1), S240–S241. [Google Scholar] [CrossRef]
- de Lédinghen, V.; Vergniol, J.; Capdepont, M.; Chermak, F.; Hiriart, J.-B.; Cassinotto, C.; Merrouche, W.; Foucher, J.; Brigitte, L.B. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: A prospective study of 5323 examinations. J. Hepatol. 2014, 60, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Reeder, S.B.; Sirlin, C.B.; Loomba, R. Noninvasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. J. Hepatol. 2018, 68, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; El-Abd, E.; Morsi, M.; El Safwany, M.M.; El-Sayed, M.Z. The effect of hepatic steatosis on 18F-FDG uptake in PET-CT examinations of cancer Egyptian patients. Eur. J. Hybrid Imaging 2023, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Keramida, G.; Peters, A.M. FDG PET/CT of the non-malignant liver in an increasingly obese world population. Clin. Physiol. Funct. Imaging 2020, 40, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Honka, M.-J.; Rebelos, E.; Malaspina, S.; Nuutila, P. Hepatic Positron Emission Tomography: Applications in Metabolism, Haemodynamics and Cancer. Metabolites 2022, 12, 321. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef]
- Zeng, Y.; He, H.; An, Z. Advance of Serum Biomarkers and Combined Diagnostic Panels in Nonalcoholic Fatty Liver Disease. Dis. Markers 2022, 2022, 1254014. [Google Scholar] [CrossRef]
- Tada, T.; Saibara, T.; Ono, M.; Takahashi, H.; Eguchi, Y.; Hyogo, H.; Kawanaka, M.; Kumada, T.; Toyoda, H.; Yasuda, S.; et al. Predictive value of cytokeratin-18 fragment levels for diagnosing steatohepatitis in patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2021, 33, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Jiang, S.-W.; Hu, A.-R.; Zhou, A.-W.; Hu, T.; Li, H.-S.; Fan, Y.; Lin, K. Non-invasive diagnosis of non-alcoholic fatty liver disease: Current status and future perspective. Heliyon 2024, 10, e27325. [Google Scholar] [CrossRef] [PubMed]
- Rios, R.S.; Zheng, K.I.; Targher, G.; Byrne, C.D.; Zheng, M.-H. Non-invasive fibrosis assessment in non-alcoholic fatty liver disease. Chin. Med. J. 2020, 133, 2743–2745. [Google Scholar] [CrossRef]
- Zhou, Y.-J.; Ye, F.-Z.; Li, Y.-Y.; Pan, X.-Y.; Chen, Y.-X.; Wu, X.-X.; Xiong, J.-J.; Liu, W.-Y.; Xu, S.-H.; Chen, Y.-P.; et al. Individualized risk prediction of significant fibrosis in non-alcoholic fatty liver disease using a novel nomogram. United Eur. Gastroenterol. J. 2019, 7, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Ganz, M.; Bukong, T.N.; Csak, T.; Saha, B.; Park, J.-K.; Ambade, A.; Kodys, K.; Szabo, G. Progression of non-alcoholic steatosis to steatohepatitis and fibrosis parallels cumulative accumulation of danger signals that promote inflammation and liver tumors in a high fat–cholesterol–sugar diet model in mice. J. Transl. Med. 2015, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Bucurica, S.; Lupanciuc, M.; Ionita-Radu, F.; Stefan, I.; Munteanu, A.E.; Anghel, D.; Jinga, M.; Gaman, E.L. Estrobolome and Hepatocellular Adenomas—Connecting the Dots of the Gut Microbial β-Glucuronidase Pathway as a Metabolic Link. Int. J. Mol. Sci. 2023, 24, 16034. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.P.; Cunningham, R.P.; Meers, G.M.; Johnson, S.A.; Wheeler, A.A.; Ganga, R.R.; Spencer, N.M.; Pitt, J.B.; Diaz-Arias, A.; Swi, A.I.A.; et al. Compromised hepatic mitochondrial fatty acid oxidation and reduced markers of mitochondrial turnover in human NAFLD. J. Hepatol. 2022, 76, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Beghini, M.; Wolf, P.; Pfleger, L.; Hackl, M.; Bastian, M.; Freudenthaler, A.; Harreiter, J.; Zeyda, M.; Baumgartner-Parzer, S.; et al. Leptin increases hepatic triglyceride export via a vagal mechanism in humans. Cell Metab. 2022, 34, 1719–1731.e5. [Google Scholar] [CrossRef] [PubMed]
- Li, X. FGFR and inhibitors. In Fibroblast Growth Factors; Elsevier: Amsterdam, The Netherlands, 2024; pp. 787–908. [Google Scholar] [CrossRef]
- Henriksson, E.; Andersen, B. FGF19 and FGF21 for the Treatment of NASH—Two Sides of the Same Coin? Differential and Overlapping Effects of FGF19 and FGF21 From Mice to Human. Front. Endocrinol. 2020, 11, 601349. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ge, H.; Baribault, H.; Gupte, J.; Weiszmann, J.; Lemon, B.; Gardner, J.; Fordstrom, P.; Tang, J.; Zhou, M.; et al. Dual actions of fibroblast growth factor 19 on lipid metabolism. J. Lipid Res. 2013, 54, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, S.; Liu, Y.; Wu, Y.; Zhang, D. Fibroblast Growth Factors for Nonalcoholic Fatty Liver Disease: Opportunities and Challenges. Int. J. Mol. Sci. 2023, 24, 4583. [Google Scholar] [CrossRef]
- Tezze, C.; Romanello, V.; Sandri, M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Pan, J.; Qu, N.; Lei, Y.; Han, J.; Zhang, J.; Han, D. The AMPK pathway in fatty liver disease. Front. Physiol. 2022, 13, 970292. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F. Role of acetylation in nonalcoholic fatty liver disease: A focus on SIRT1 and SIRT3. Explor. Med. 2020, 1, 248–258. [Google Scholar] [CrossRef]
- Tontonoz, P.; Spiegelman, B.M. Fat and Beyond: The Diverse Biology of PPARγ. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Liss, K.H.H.; Finck, B.N. PPARs and nonalcoholic fatty liver disease. Biochimie 2017, 136, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xue, W.; Wang, M.; Wu, Y.; Singh, M.; Zhu, Y.; Kumar, R.; Lin, S. MAFLD Criteria May Overlook a Subtype of Patient with Steatohepatitis and Significant Fibrosis. Diabetes Metab. Syndr. Obes. 2021, 14, 3417–3425. [Google Scholar] [CrossRef] [PubMed]
- Gawrieh, S.; Gawrieh, S.; Noureddin, M.; Noureddin, M.; Loo, N.; Loo, N.; Mohseni, R.; Mohseni, R.; Awasty, V.; Awasty, V.; et al. Saroglitazar, a PPAR-α/γ Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. J. Hepatol. 2021, 74, 1809–1824. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; Neuschwander-Tetri, B.A.; et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019, 394, 2012–2024. [Google Scholar] [CrossRef] [PubMed]
- Karim, G.; Bansal, M.B. Resmetirom: An Orally Administered, Small-molecule, Liver-directed, β-selective THR Agonist for the Treatment of Non-alcoholic Fatty Liver Disease and Non-alcoholic Steatohepatitis. Eur. Endocrinol. 2023, 19, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Ratziu, V.; Anstee, Q.M.; Noureddin, M.; Sanyal, A.J.; Schattenberg, J.M.; Bedossa, P.; Bashir, M.R.; Schneider, D.; Taub, R.; et al. Design of the phase 3 MAESTRO clinical program to evaluate resmetirom for the treatment of nonalcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2023, 59, 51–63. [Google Scholar] [CrossRef]
- Harrison, S.A.; Ruane, P.J.; Freilich, B.L.; Neff, G.; Patil, R.; Behling, C.A.; Hu, C.; Fong, E.; de Temple, B.; Tillman, E.J.; et al. Efruxifermin in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled, phase 2a trial. Nat. Med. 2021, 27, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.A.; Minnich, A.; Sanyal, A.J.; Loomba, R.; Du, S.; Schwarz, J.; Ehman, R.L.; Karsdal, M.; Leeming, D.J.; Cizza, G.; et al. Effect of pegbelfermin on NASH and fibrosis-related biomarkers and correlation with histological response in the FALCON 1 trial. JHEP Rep. 2023, 5, 100661. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Sanyal, A.; Harrison, S.A.; Wong, V.W.S.; Francque, S.; Goodman, Z.; Aithal, G.P.; Kowdley, K.V.; Seyedkazemi, S.; Fischer, L.; et al. Cenicriviroc Treatment for Adults with Nonalcoholic Steatohepatitis and Fibrosis: Final Analysis of the Phase 2b CENTAUR Study. Hepatology 2020, 72, 892–905. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Neuschwander-Tetri, B.A.; Wong, V.W.-S.; Abdelmalek, M.F.; Younossi, Z.M.; Yuan, J.; Pecoraro, M.L.; Seyedkazemi, S.; Fischer, L.; Bedossa, P.; et al. Cenicriviroc for the treatment of liver fibrosis in adults with nonalcoholic steatohepatitis: AURORA Phase 3 study design. Contemp. Clin. Trials 2019, 89, 105922. [Google Scholar] [CrossRef]
- Loomba, R.; Lawitz, E.; Mantry, P.S.; Jayakumar, S.; Caldwell, S.H.; Arnold, H.; Diehl, A.M.; Djedjos, C.S.; Han, L.; Myers, R.P.; et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. J. Hepatol. 2017, 67, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Gunn, N.; Neff, G.W.; Kohli, A.; Liu, L.; Flyer, A.; Goldkind, L.; Di Bisceglie, A.M. A phase 2, proof of concept, randomised controlled trial of berberine ursodeoxycholate in patients with presumed non-alcoholic steatohepatitis and type 2 diabetes. Nat. Commun. 2021, 12, 5503. [Google Scholar] [CrossRef]
- Yan, H.-M.; Xia, M.-F.; Wang, Y.; Chang, X.-X.; Yao, X.-Z.; Rao, S.-X.; Zeng, M.-S.; Tu, Y.-F.; Feng, R.; Jia, W.-P.; et al. Efficacy of Berberine in Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE 2015, 10, e0134172. [Google Scholar] [CrossRef]
- Ruiz-Herrera, V.V.; Navarro-Lara, S.A.; Andrade-Villanueva, J.F.; Alvarez-Zavala, M.; Sánchez-Reyes, K.; Toscano-Piña, M.; Méndez-Clemente, A.S.; Martínez-Ayala, P.; Valle-Rodríguez, A.; González-Hernández, L.A. Pilot study on the efficacy and safety of berberine in people with metabolic syndrome and human immunodeficiency virus infection. Int. J. STD AIDS 2023, 34, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Wang, Z.; Zhang, J.; Yan, H.; Bian, H.; Xia, M.; Lin, H.; Jiang, J.; Gao, X. Lipid profiling of the therapeutic effects of berberine in patients with nonalcoholic fatty liver disease. J. Transl. Med. 2016, 14, 266. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Zhang, P.; Liu, X.; Xue, L. Effect of berberine and bicyclol on Chinese patients with nonalcoholic fatty liver disease: A retrospective study. Postgrad. Med. 2022, 134, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Nejati, L.; Movahedi, A.; Salari, G.R.; Moeineddin, R.; Nejati, P. The Effect of Berberine on Lipid Profile, Liver Enzymes, and Fasting Blood Glucose in Patients with Non-alcoholic Fatty Liver Disease (NAFLD): A Randomized Controlled Trial. Med. J. Islam. Repub. Iran 2022, 36, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wu, W.; Chang, X.; Xia, M.; Ma, S.; Wang, L.; Gao, J. Gender differences in the efficacy of pioglitazone treatment in nonalcoholic fatty liver disease patients with abnormal glucose metabolism. Biol. Sex Differ. 2021, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Cossiga, V.; Lembo, V.; Guarino, M.; Tuccillo, C.; Morando, F.; Pontillo, G.; Fiorentino, A.; Caporaso, N.; Morisco, F. Berberis aristata, Elaeis guineensis and Coffea canephora Extracts Modulate the Insulin Receptor Expression and Improve Hepatic Steatosis in NAFLD Patients: A Pilot Clinical Trial. Nutrients 2019, 11, 3070. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xia, M.; Duan, Y.; Zhang, L.; Jiang, H.; Hu, X.; Yan, H.; Zhang, Y.; Gu, Y.; Shi, H.; et al. Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans. Cell Death Dis. 2019, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Kohli, K.; Mujtaba, A.; Malik, R.; Amin, S.; Alam, S.; Ali, A.; Barkat, A.; Ansari, M.J. Development of Natural Polysaccharide–Based Nanoparticles of Berberine to Enhance Oral Bioavailability: Formulation, Optimization, Ex Vivo, and In Vivo Assessment. Polymers 2021, 13, 3833. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Nakamura, N.; Akao, T.; Hattori, M. Pharmacokinetics of Berberine and Its Main Metabolites in Conventional and Pseudo Germ-Free Rats Determined by Liquid Chromatography/Ion Trap Mass Spectrometry. Drug Metab. Dispos. 2006, 34, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Majidzadeh, H.; Araj-Khodaei, M.; Ghaffari, M.; Torbati, M.; Dolatabadi, J.E.N.; Hamblin, M.R. Nano-based delivery systems for berberine: A modern anti-cancer herbal medicine. Colloids Surf. B Biointerfaces 2020, 194, 111188. [Google Scholar] [CrossRef]
- Jin, Q.; Cai, Y.; Li, S.; Liu, H.; Zhou, X.; Lu, C.; Gao, X.; Qian, J.; Zhang, J.; Ju, S.; et al. Edaravone-Encapsulated Agonistic Micelles Rescue Ischemic Brain Tissue by Tuning Blood-Brain Barrier Permeability. Theranostics 2017, 7, 884–898. [Google Scholar] [CrossRef]
- Azadi, R.; Mousavi, S.E.; Kazemi, N.M.; Yousefi-Manesh, H.; Rezayat, S.M.; Jaafari, M.R. Anti-inflammatory efficacy of Berberine Nanomicelle for improvement of cerebral ischemia: Formulation, characterization and evaluation in bilateral common carotid artery occlusion rat model. BMC Pharmacol. Toxicol. 2021, 22, 54. [Google Scholar] [CrossRef] [PubMed]
- Ghaffarzadegan, R.; Khoee, S.; Rezazadeh, S. Fabrication, characterization and optimization of berberine-loaded PLA nanoparticles using coaxial electrospray for sustained drug release. DARU J. Pharm. Sci. 2020, 28, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, A.; Khan, M.J.; Siddiqui, M.A. Nanocarrier Based Delivery of Berberine: A Critical Review on Pharmaceutical and Preclinical Characteristics of the Bioactive. Curr. Pharm. Biotechnol. 2023, 24, 1449–1464. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xiong, J.; Chen, G.; Zhang, Z.; Liu, Y.; Xu, J.; Xu, H. Comparing the Influences of Metformin and Berberine on the Intestinal Microbiota of Rats With Nonalcoholic Steatohepatitis. In Vivo 2023, 37, 2105–2127. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhu, W.; Zhou, J.; Shen, T. The combination of berberine and evodiamine ameliorates high-fat diet-induced non-alcoholic fatty liver disease associated with modulation of gut microbiota in rats. Braz. J. Med. Biol. Res. 2022, 55, e12096. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tai, Y.-L.; Zhao, D.; Zhang, Y.; Yan, J.; Kakiyama, G.; Wang, X.; Gurley, E.C.; Liu, J.; Liu, J.; et al. Berberine Prevents Disease Progression of Nonalcoholic Steatohepatitis through Modulating Multiple Pathways. Cells 2021, 10, 210. [Google Scholar] [CrossRef]
- Shu, X.; Li, M.; Cao, Y.; Li, C.; Zhou, W.; Ji, G.; Zhang, L. Berberine Alleviates Non-alcoholic Steatohepatitis Through Modulating Gut Microbiota Mediated Intestinal FXR Activation. Front. Pharmacol. 2021, 12, 750826. [Google Scholar] [CrossRef]
- Mehrdoost, S.; Yaghmaei, P.; Jafary, H.; Ebrahim-Habibi, A. The therapeutic effects of berberine plus sitagliptin in a rat model of fatty liver disease. Iran. J. Basic Med. Sci. 2021, 24, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-P.; Dou, Y.-X.; Huang, Z.-W.; Chen, H.-B.; Li, Y.-C.; Chen, J.-N.; Liu, Y.-H.; Huang, X.-Q.; Zeng, H.-F.; Yang, X.-B.; et al. Therapeutic effect of oxyberberine on obese non-alcoholic fatty liver disease rats. Phytomedicine 2021, 85, 153550. [Google Scholar] [CrossRef]
- Cossiga, V.; Lembo, V.; Nigro, C.; Mirra, P.; Miele, C.; D’argenio, V.; Leone, A.; Mazzone, G.; Veneruso, I.; Guido, M.; et al. The Combination of Berberine, Tocotrienols and Coffee Extracts Improves Metabolic Profile and Liver Steatosis by the Modulation of Gut Microbiota and Hepatic miR-122 and miR-34a Expression in Mice. Nutrients 2021, 13, 1281. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jia, Z.; Wang, B.; Zhang, B. Berberine inhibits liver damage in rats with non-alcoholic fatty liver disease by regulating TLR4/MyD88/NF-κB pathway. Turk. J. Gastroenterol. 2021, 31, 902–909. [Google Scholar] [CrossRef]
- Lu, Z.; Lu, F.; Wu, L.; He, B.; Chen, Z.; Yan, M. Berberine attenuates non-alcoholic steatohepatitis by regulating chemerin/CMKLR1 signalling pathway and Treg/Th17 ratio. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 394, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Alimujiang, M.; Hu, L.; Liu, F.; Bao, Y.; Yin, J. Berberine alleviates lipid metabolism disorders via inhibition of mitochondrial complex I in gut and liver. Int. J. Biol. Sci. 2021, 17, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, Y.; Xiao, L. Berberine ameliorates nonalcoholic fatty liver disease by decreasing the liver lipid content via reversing the abnormal expression of MTTP and LDLR. Exp. Ther. Med. 2021, 22, 1109. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; He, B.; Chen, Z.; Yan, M.; Wu, L. Anti-inflammatory activity of berberine in non-alcoholic fatty liver disease via the Angptl2 pathway. BMC Immunol. 2020, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, S.; Zheng, J.; Li, Y.; Li, P.; Hou, H. Berberine ameliorates intestinal mucosal barrier dysfunction in nonalcoholic fatty liver disease (NAFLD) rats. J. King Saud Univ.-Sci. 2020, 32, 2534–2539. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Y.; Tang, K.; Chen, R.; Liang, S.; Liang, Y.; Han, L.; Jin, L.; Liang, Z.; Chen, Y.; et al. Berberine Ameliorates High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in Rats via Activation of SIRT3/AMPK/ACC Pathway. Curr. Med. Sci. 2019, 39, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, X.-P.; Bai, J.-Y.; Xia, P.; Li, Y.; Lu, Y.; Li, X.-Y.; Gao, X. Berberine alleviates nonalcoholic fatty liver induced by a high-fat diet in mice by activating SIRT3. FASEB J. 2019, 33, 7289–7300. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tian, G.; Zhuang, Z.; Chen, J.; You, N.; Zhuo, L.; Liang, B.; Song, Y.; Zang, S.; Liu, J.; et al. Berberine prevents non-alcoholic steatohepatitis-derived hepatocellular carcinoma by inhibiting inflammation and angiogenesis in mice. Am. J. Transl. Res. 2019, 11, 2668–2682. [Google Scholar] [PubMed]
- Deng, Y.; Tang, K.; Chen, R.; Nie, H.; Liang, S.; Zhang, J.; Zhang, Y.; Yang, Q. Berberine attenuates hepatic oxidative stress in rats with non-alcoholic fatty liver disease via the Nrf2/ARE signalling pathway. Exp. Ther. Med. 2019, 17, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Wu, X.; Tong, P.; Yue, Y.; Gao, S.; Huang, D.; Huang, J. Inhibition of CCL19 benefits non-alcoholic fatty liver disease by inhibiting TLR4/NF-κB-p65 signaling. Mol. Med. Rep. 2018, 18, 4635–4642. [Google Scholar] [CrossRef]
- Feng, W.-W.; Kuang, S.-Y.; Tu, C.; Ma, Z.-J.; Pang, J.-Y.; Wang, Y.-H.; Zang, Q.-C.; Liu, T.-S.; Zhao, Y.-L.; Xiao, X.-H.; et al. Natural products berberine and curcumin exhibited better ameliorative effects on rats with non-alcohol fatty liver disease than lovastatin. Biomed. Pharmacother. 2018, 99, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cang, Z.; Sun, H.; Nie, X.; Wang, N.; Lu, Y. Berberine improves glucogenesis and lipid metabolism in nonalcoholic fatty liver disease. BMC Endocr. Disord. 2017, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ma, X.; Li, L.; Wang, L.; Chen, Y.; Liu, J.; Luo, Y.; Zhuang, Z.; Yang, W.; Zang, S.; et al. Berberine ameliorates non-alcoholic steatohepatitis in ApoE-/- mice. Exp. Ther. Med. 2017, 14, 4134–4140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, W.; Liu, L.; Wang, Y.; Mao, T.; Li, J. Effects of a combination of puerarin, baicalin and berberine on the expression of proliferator-activated receptor-γ and insulin receptor in a rat model of nonalcoholic fatty liver disease. Exp. Ther. Med. 2015, 11, 183–190. [Google Scholar] [CrossRef]
- Cao, Y.; Pan, Q.; Cai, W.; Shen, F.; Chen, G.-Y.; Xu, L.-M.; Fan, J.-G. Modulation of Gut Microbiota by Berberine Improves Steatohepatitis in High-Fat Diet-Fed BALB/C Mice. Arch. Iran. Med. 2016, 19, 197–203. [Google Scholar] [PubMed]
- Xue, M.; Zhang, L.; Yang, M.-X.; Zhang, W.; Li, X.-M.; Ou, Z.-M.; Li, Z.-P.; Liu, S.-H.; Li, X.-J.; Yang, S.-Y. Berberine-loaded solid lipid nanoparticles are concentrated in the liver and ameliorate hepatosteatosis in db/db mice. Int. J. Nanomed. 2015, 10, 5049–5057. [Google Scholar] [CrossRef] [PubMed]
- Ragab, S.M.M.; Elghaffar, S.K.A.; El-Metwally, T.H.; Badr, G.; Mahmoud, M.H.; Omar, H.M. Effect of a high fat, high sucrose diet on the promotion of non-alcoholic fatty liver disease in male rats: The ameliorative role of three natural compounds. Lipids Health Dis. 2015, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Heidarian, E.; Rafieian-Kopaei, M.; Khoshdel, A.; Bakhshesh, M. Metabolic effects of berberine on liver phosphatidate phosphohydrolase in rats fed on high lipogenic diet: An additional mechanism for the hypolipidemic effects of berberine. Asian Pac. J. Trop. Biomed. 2014, 4, S429–S435. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, J.S.; Duarte, F.V.; Gomes, A.P.; Varela, A.T.; Peixoto, F.M.; Rolo, A.P.; Palmeira, C.M. Berberine reverts hepatic mitochondrial dysfunction in high-fat fed rats: A possible role for SirT3 activation. Mitochondrion 2013, 13, 637–646. [Google Scholar] [CrossRef]
- Yang, Q.-H.; Hu, S.-P.; Zhang, Y.-P.; Xie, W.-N.; Li, N.; Ji, G.-Y.; Qiao, N.-L.; Lin, X.-F.; Chen, T.-Y.; Liu, H.-T. Effect of berberine on expressions of uncoupling protein-2 mRNA and protein in hepatic tissue of non-alcoholic fatty liver disease in rats. Chin. J. Integr. Med. 2011, 17, 205–211. [Google Scholar] [CrossRef]
- Xing, L.-J.; Zhang, L.; Liu, T.; Hua, Y.-Q.; Zheng, P.-Y.; Ji, G. Berberine reducing insulin resistance by up-regulating IRS-2 mRNA expression in nonalcoholic fatty liver disease (NAFLD) rat liver. Eur. J. Pharmacol. 2011, 668, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Yan, H.; Fei, J.; Jiang, M.; Zhu, H.; Lu, D.; Gao, X. Berberine reduces methylation of the MTTP promoter and alleviates fatty liver induced by a high-fat diet in rats. J. Lipid Res. 2010, 51, 2504–2515. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, X.; Li, R.; Cui, J.; Yu, H.; Ren, L.; Jiang, J.; Zhang, W.; Wang, L. Berberine-silybin salt achieves improved anti-nonalcoholic fatty liver disease effect through regulating lipid metabolism. J. Ethnopharmacol. 2024, 319, 117238. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Zhang, Y.; Lin, S.; Chen, Y.; Wang, Z.; Feng, H.; Fang, G.; Quan, S. Berberine Ameliorates Metabolic-Associated Fatty Liver Disease Mediated Metabolism Disorder and Redox Homeostasis by Upregulating Clock Genes: Clock and Bmal1 Expressions. Molecules 2023, 28, 1874. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-H.; Shen, H.-R.; Wang, L.-L.; Luo, Z.-G.; Zhang, J.-L.; Zhang, H.-J.; Gao, T.-L.; Han, Y.-X.; Jiang, J.-D. Berberine is a potential alternative for metformin with good regulatory effect on lipids in treating metabolic diseases. Biomed. Pharmacother. 2023, 163, 114754. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chai, X.; Li, J.; Li, C.; Wu, X.; Ye, X.; Ma, H.; Li, X. LCN2 contributes to the improvement of nonalcoholic steatohepatitis by 8-Cetylberberine. Life Sci. 2023, 321, 121595. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-M.; Fan, J.-H.; Xu, M.-M.; Xiong, M.-Y.; Wang, Q.-J.; Chai, X.; Li, X.-D.; Li, X.-G.; Ye, X.-L. Epiberberine regulates lipid synthesis through SHP (NR0B2) to improve non-alcoholic steatohepatitis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2023, 1869, 166639. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, R.; Li, Y.; Tan, S.; Jiang, J.; Liu, H.; Wei, X. Berberine alleviates non-alcoholic hepatic steatosis partially by promoting SIRT1 deacetylation of CPT1A in mice. Gastroenterol. Rep. 2022, 11, goad032. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, Z.; Xu, J.; Zhang, J.; Sun, R.; Zhou, J.; Lu, Y.; Gong, Z.; Huang, J.; Shen, X.; et al. Improving the ameliorative effects of berberine and curcumin combination via dextran-coated bilosomes on non-alcohol fatty liver disease in mice. J. Nanobiotechnology 2021, 19, 230. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cao, S.; Li, C.; Zhang, J.; Liu, C.; Qiu, F.; Kang, N. Berberrubine, a Main Metabolite of Berberine, Alleviates Non-Alcoholic Fatty Liver Disease via Modulating Glucose and Lipid Metabolism and Restoring Gut Microbiota. Front. Pharmacol. 2022, 13, 913378. [Google Scholar] [CrossRef]
- Li, H.; Liu, N.-N.; Li, J.-R.; Dong, B.; Wang, M.-X.; Tan, J.-L.; Wang, X.-K.; Jiang, J.; Lei, L.; Li, H.-Y.; et al. Combined Use of Bicyclol and Berberine Alleviates Mouse Nonalcoholic Fatty Liver Disease. Front. Pharmacol. 2022, 13, 843872. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Lu, S.; Shen, T.; Li, Y.; Chen, J. Methyltransferase SETD2 mediates hepatoprotection of berberine against steatosis. Ann. Transl. Med. 2022, 10, 552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wen, J.; Liu, D.; Qiu, Z.; Zhu, Q.; Li, R.; Zhang, Y. Demethylenetetrahydroberberine alleviates nonalcoholic fatty liver disease by inhibiting the NLRP3 inflammasome and oxidative stress in mice. Life Sci. 2021, 281, 119778. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-Y.; Shi, X.-Y.; Wu, Y.-R.; Zhang, Y.; Yao, Y.-H.; Qu, H.-L.; Zhang, W.; Guo, Y.-L.; Xu, R.-X.; Li, J.-J. Berberine attenuates atherosclerotic lesions and hepatic steatosis in ApoE-/- mice by down-regulating PCSK9 via ERK1/2 pathway. Ann. Transl. Med. 2021, 9, 1517. [Google Scholar] [CrossRef] [PubMed]
- Mai, W.; Xu, Y.; Xu, J.; Zhao, D.; Ye, L.; Yu, G.; Wang, Z.; Lu, Q.; Lin, J.; Yang, T.; et al. Berberine Inhibits Nod-Like Receptor Family Pyrin Domain Containing 3 Inflammasome Activation and Pyroptosis in Nonalcoholic Steatohepatitis via the ROS/TXNIP Axis. Front. Pharmacol. 2020, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Bian, H.; Wang, L.; Sun, X.; Xu, X.; Yan, H.; Xia, M.; Chang, X.; Lu, Y.; Li, Y.; et al. Berberine attenuates nonalcoholic hepatic steatosis through the AMPK-SREBP-1c-SCD1 pathway. Free Radic. Biol. Med. 2019, 141, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, Y.; Xia, M.; Xia, M.; Yan, H.; Yan, H.; Han, Y.; Han, Y.; Zhang, F.; Zhang, F.; et al. Berberine attenuates hepatic steatosis and enhances energy expenditure in mice by inducing autophagy and fibroblast growth factor 21. Br. J. Pharmacol. 2017, 175, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, Y. Berberine alleviates hepatic lipid accumulation by increasing ABCA1 through the protein kinase C δ pathway. Biochem. Biophys. Res. Commun. 2018, 498, 473–480. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, X.; Zhang, F.; Han, Y.; Chang, X.; Xu, X.; Li, Y.; Gao, X. Berberine ameliorates fatty acid-induced oxidative stress in human hepatoma cells. Sci. Rep. 2017, 7, 11340. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Lee, K.-Y.; Jung, S.-H.; Kim, H.S.; Shim, G.; Kim, M.-G.; Oh, Y.-K.; Oh, S.-H.; Jun, D.W.; Lee, B.-H. Activation of AMPK by berberine induces hepatic lipid accumulation by upregulation of fatty acid translocase CD36 in mice. Toxicol. Appl. Pharmacol. 2017, 316, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, B.; Meng, X.; Yao, S.; Jin, L.; Yang, J.; Wang, J.; Zhang, H.; Zhang, Z.; Cai, D.; et al. Berberine prevents progression from hepatic steatosis to steatohepatitis and fibrosis by reducing endoplasmic reticulum stress. Sci. Rep. 2016, 6, 20848. [Google Scholar] [CrossRef] [PubMed]
- Qiang, X.; Xu, L.; Zhang, M.; Zhang, P.; Wang, Y.; Wang, Y.; Zhao, Z.; Chen, H.; Liu, X.; Zhang, Y. Demethyleneberberine attenuates non-alcoholic fatty liver disease with activation of AMPK and inhibition of oxidative stress. Biochem. Biophys. Res. Commun. 2016, 472, 603–609. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Mei, D.; Sha, S.; Fan, S.; Wang, L.; Dong, M. ERK-dependent mTOR pathway is involved in berberine-induced autophagy in hepatic steatosis. J. Mol. Endocrinol. 2017, 59, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Woo, S.-L.; Guo, X.; Li, H.; Zheng, J.; Botchlett, R.; Liu, M.; Pei, Y.; Xu, H.; Cai, Y.; et al. Berberine Ameliorates Hepatic Steatosis and Suppresses Liver and Adipose Tissue Inflammation in Mice with Diet-induced Obesity. Sci. Rep. 2016, 6, 22612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chang, X.; Song, X.; Chen, C.; Chen, H.; Lu, Z.; Gao, X.; Lu, D. Berberine reverses abnormal expression of L-type pyruvate kinase by DNA demethylation and histone acetylation in the livers of the non-alcoholic fatty disease rat. Int. J. Clin. Exp. Med. 2015, 8, 7535–7543. [Google Scholar] [PubMed]
- Yuan, X.; Wang, J.; Tang, X.; Li, Y.; Xia, P.; Gao, X. Berberine ameliorates nonalcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles. J. Transl. Med. 2015, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, H.; Yeung, M.; Kowalski, S.; Krystal, G.; Elisia, I. Development of a novel human triculture model of non-alcoholic fatty liver disease and identification of berberine as ameliorating steatosis, oxidative stress and fibrosis. Front. Pharmacol. 2023, 14, 1234300. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.-Y.; Dai, Y.; Ren, X.-D.; Zheng, J.; Zhang, K.-B.; Chen, B.; Yan, J.; Xu, Z.-H. Berberine mitigates nonalcoholic hepatic steatosis by downregulating SIRT1-FoxO1-SREBP2 pathway for cholesterol synthesis. J. Integr. Med. 2021, 19, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, X.; Zhao, D.; Wang, X.; Gurley, E.C.; Liu, R.; Li, X.; Hylemon, P.B.; Chen, W.; Zhou, H. Berberine inhibits free fatty acid and LPS-induced inflammation via modulating ER stress response in macrophages and hepatocytes. PLoS ONE 2020, 15, e0232630. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Anand, S.K.; Singh, N.; Dwivedi, U.N.; Kakkar, P. Berbamine induced AMPK activation regulates mTOR/SREBP-1c axis and Nrf2/ARE pathway to allay lipid accumulation and oxidative stress in steatotic HepG2 cells. Eur. J. Pharmacol. 2020, 882, 173244. [Google Scholar] [CrossRef] [PubMed]
- Khorzoughi, R.B.; Namvarjah, F.; Teimouri, M.; Hosseini, H.; Meshkani, R. In-vitro Synergistic Effect of Metformin and Berberine on High Glucose-induced Lipogenesis. Iran. J. Pharm. Res. IJPR 2019, 18, 1921. [Google Scholar] [CrossRef]
- Rafiei, H.; Omidian, K.; Bandy, B. Dietary Polyphenols Protect Against Oleic Acid-Induced Steatosis in an in Vitro Model of NAFLD by Modulating Lipid Metabolism and Improving Mitochondrial Function. Nutrients 2019, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, H.; Omidian, K.; Bandy, B. Comparison of dietary polyphenols for protection against molecular mechanisms underlying nonalcoholic fatty liver disease in a cell model of steatosis. Mol. Nutr. Food Res. 2017, 61, 1600781. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Zhang, Z.X. Effect of berberine on a cellular model of non-alcoholic fatty liver disease. Int. J. Clin. Exp. Med. 2017, 10, 16360–16366. [Google Scholar]
- Brusq, J.-M.; Ancellin, N.; Grondin, P.; Guillard, R.; Martin, S.; Saintillan, Y.; Issandou, M. Inhibition of lipid synthesis through activation of AMP kinase: An additional mechanism for the hypolipidemic effects of berberine. J. Lipid Res. 2006, 47, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Samineni, R.; Chimakurthy, J.; Konidala, S. Emerging Role of Biopharmaceutical Classification and Biopharmaceutical Drug Disposition System in Dosage form Development: A Systematic Review. Turk. J. Pharm. Sci. 2022, 19, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Loomba, R.; Rinella, M.E.; Bugianesi, E.; Marchesini, G.; Neuschwander-Tetri, B.A.; Serfaty, L.; Negro, F.; Caldwell, S.H.; Ratziu, V.; et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2018, 68, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Ferré, P.; Foufelle, F. SREBP-1c Transcription Factor and Lipid Homeostasis: Clinical Perspective. Horm. Res. Paediatr. 2007, 68, 72–82. [Google Scholar] [CrossRef]
- Fang, J.; Yu, C.-H.; Li, X.-J.; Yao, J.-M.; Fang, Z.-Y.; Yoon, S.-H.; Yu, W.-Y. Gut dysbiosis in nonalcoholic fatty liver disease: Pathogenesis, diagnosis, and therapeutic implications. Front. Cell. Infect. Microbiol. 2022, 12, 997018. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wu, H.; Wei, J.; Miao, R.; Zhang, Y.; Tian, J. Research progress on the pharmacological effects of berberine targeting mitochondria. Front. Endocrinol. 2022, 13, 982145. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Alsahli, M.A.; Rahmani, A.H. Berberine: An Important Emphasis on Its Anticancer Effects through Modulation of Various Cell Signaling Pathways. Molecules 2022, 27, 5889. [Google Scholar] [CrossRef] [PubMed]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Qi, G.; Fan, R.; Ji, X.; Liu, Z.; Liu, X. EGCG ameliorates diet-induced metabolic syndrome associating with the circadian clock. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1575–1589. [Google Scholar] [CrossRef]
- Saran, A.R.; Dave, S.; Zarrinpar, A. Circadian Rhythms in the Pathogenesis and Treatment of Fatty Liver Disease. Gastroenterology 2020, 158, 1948–1966.e1. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ohta, M.; Desai, D.; Figueiredo, J.-L.; Whelan, M.C.; Sugano, T.; Yamabi, M.; Yano, W.; Faits, T.; Yabusaki, K.; et al. Angiopoietin Like Protein 2 (ANGPTL2) Promotes Adipose Tissue Macrophage and T lymphocyte Accumulation and Leads to Insulin Resistance. PLoS ONE 2015, 10, e0131176. [Google Scholar] [CrossRef]
- Chawla, H.; Bhosale, V.; Misra, R.; Sonkar, S.K.; Kohli, N.; Jamal, N.; Vimal, S.R.; Dangi, B.; Durgapal, K.; Singh, S.; et al. Lipocalin-2 levels increase in plasma of non-alcoholic fatty liver disease patients with metabolic syndrome. Int. J. Diabetes Dev. Ctries. 2022, 43, 105–112. [Google Scholar] [CrossRef]

| Authors | Clinical Study Type | Pharmaceutical Intervention and Dosage | Study Participants | Results and Key Impacts on NAFLD | NAFLD Assessments Methods |
|---|---|---|---|---|---|
| Harrison SA. et al., 2021 [68] | Randomized controlled, double-blind, placebo Phase 2 | Berberine ursodeoxycholate (BUDCA) (18 weeks) BUDCA 500 mg bid/BUDCA 1000 mg bid/ placebo. | 88 (27/30/32) | MRI-PDFF liver fat content was 2.4 times lower in the BUDCA higher dose group compared to placebo (4.8% vs. 2.0%) Secondary endpoints: Improvement in liver enzyme profile—mean ALT levels were 6.3 times lower than in the placebo group, and GGT levels were 15 times lower than in the placebo group. Regarding the serum lipide profile, BUDCA in higher doses shows better results in lowering LDL-c levels. | MRI-PDFF, Liver enzyme profile and serum lipid profile |
| Yan HM. et al., 2015 [69] | Randomized, parallel, multicenter, controlled, open-label clinical trial. | BBR 500 mg tid p.o 30 min before meal (16 weeks) Group A-lifestyle intervention (LSI)/Group B-LSI plus PGZ (15 mg q.d.) pioglitazone/Group C-LSI plus BBR | 155 (53/47/55) | 1H-MRS hepatic fat content was shown to improve better when the subject received lifestyle intervention and BBR rather than just dietary changes. (−17.4% vs. −12.1%, p < 0.008). Secondary endpoints: Improvement in liver enzyme profile as well as in serum lipide profile. | Hydrogen-1 MR spectroscopy (1H MRS) Liver enzyme profile and serum lipid profile |
| Ruiz-Herrera VV. et al., 2023 [70] | Randomized, double-blind, placebo-controlled clinical trial | BBR 500 mg tid (20 weeks) BBR tid/placebo (calcinate magnesia) 400 mg tid | 36 (19/17) | The BBR-treated group showed slight amelioration regarding lipid profile, but this was not statistically relevant. Secondary endpoints: Better control of glucose metabolism. Relevant improvements regarding Metabolic Syndrome features were found in the current study. | Liver enzymes profile and serum lipid profile. |
| Chang X. et al., 2016 [71] | Randomized, parallel-controlled, open-label, clinical trial | BBR 500 mg tid 30 min before meals (16 weeks) Group A-LSI/Group B-LSI plus BBR | 41 BBR/39 LSI 80 PTS | 1HMRS liver fat content decreased in the BBR-treated group compared with the LIS-only group. (13.6% vs. 20.3%, p = 0.021) Secondary endpoints: The lipid-lowering effect of BBR was reflected by various changes in lipid profile. | Hydrogen-1 MR spectroscopy (1H MRS) Liver enzyme profile and serum lipid profile |
| Geng Q. et al., 2022 [72] | Retrospective cohort | BBR 500 mg tid (16 weeks) BBR + Dietary modification and exercise/Bicyclol + Dietary modification and exercise /Dietary modification and exercise | 385 (112/145/128) | The Liver/spleen CT ratio was significantly improved in the BBR-treated group with a p-value < 0.0001. Liver steatosis was notably attenuated on liver biopsies after BBR treatment (4.44 vs. 5.28, p < 0.0001), as shown by an improved NAS score. Secondary endpoint: BBR treatment ameliorated liver function tests, as shown by a significant decrease in both AST and ALT levels (p < 0.0001, respectively, p < 0.001). No significant improvement in serum lipid profile was recorded in the BBR-treated group. | Liver/spleen ratio CT scans NAFLD activity score on liver biopsy (NAS score) Liver enzyme profile and serum lipid profile |
| Nejati L. et al., 2022 [73] | Open-label, double-blinded, randomized, controlled trial | 6.25 g daily p.o (7 weeks/45-day) | 48 (24/24) | BBR treatment did not improve liver function tests. | Ultrasonography Liver enzymes profile and serum lipid profile. |
| Yan H. et al. (2021) [74] | Randomized, parallel controlled, open-label clinical trial with three-arm. | BBR 500 mg tid (16 weeks) (1) LSI (2) LSI + PZG (3) LSI + BBR | 155 (85/70) | Liver fat content (LFC) was significantly decreased in BBR-treated women compared to lifestyle intervention alone (−11.88%, p = 0.02 BBR + LSI vs. LSI), and there were no significant outcomes in men. However, men responded better regarding LFC decrease in BBR + LSI vs. LSI + PZG (−11.29%, p = 0.07). BBR did not have a significant effect in reducing liver fat content compared to PZG or LSI groups (BBR + LSI vs. LSI, p = 0.124 and BBR + LSI vs. PGZ + LSI, p = 0.222). Secondary outcomes: Pioglitazone had superior effects on both glucose metabolism and liver fat content among women. | Ultrasonography. Hydrogen-1 MR spectroscopy (1H MRS). |
| Cossiga V. et al. (2019) [75] | Randomized controlled trial | Plant extracts consisting of: 500 mg BBR 30 mg tocotrienols 30 mg chlorogenic acid (duration not mentioned) (1) placebo (2) treated group | 49 (26/23) | Significant improvement in median CAP value after treatment compared to placebo (p < 0.01). Secondary outcomes: At the end of the study, the liver enzyme profile and serum lipid profile did not significantly change. Better control of glucose metabolism in the treated group. | Transient elastography—CAP value. Liver enzymes profile and serum lipid profile. |
| Wu L., 2019 [76] | Interventional prospective study /pre-clinical study | BBR 500 mg tid, p.o, 30 min before meals (4 weeks) | 10 | 18F-FDG PET/CT showed that 1 month of BBR treatment was associated with significantly enhanced recruitment and activity of brown adipose tissue (p < 0.05). Secondary endpoints: Mild improvement in basal metabolism after BBR treatment. No significant changes were found in the serum lipid profile. | 18F-FDG PET/CT and Micro 18F-FDG PET/CT imaging (for mice). Liver enzymes profile and serum lipid profile. |
| Authors | Experiment and Model Type | Pathway |
|---|---|---|
| Chen D. et al. (2023) [84] | Sprague–Dawley rats | Liver and spleen microbiota cultures. Genomic DNA analysis for gut microbiota. |
| Dai Y. et al. (2022) [85] | Sprague–Dawley rats | Genomic DNA analysis for gut microbiota. ELISA for inflammatory cytokines levels. |
| Wang Y. et al. (2021) [86] | Mixed background C57Bl/6J and 129S1/SvlmJ (B6/12) mice | Increased expression and analysis of multiple cellular pathways involved in NAFLD induction and progression. |
| Shu X. et al. (2021) [87] | Mice C57BL/6J | Upregulation of FXR pathway and FGF-15 levels. Genomic DNA analysis for gut microbiota. |
| Mehrdoost S. et al. (2021) [88] | Sprague–Dawley rats | Upregulation of Adiponectin receptor 2 (AdipoR2) levels and mitogen-activated protein kinase (MAPK) ERK expression and their influence on inflammation and progression of NAFLD. |
| Li QP. et al. (2021) [89] | Sprague–Dawley rats | Activation of AMPK pathway |
| Cossiga V. et al. (2021) [90] | C57BL/6J mice (in vivo) | Genomic DNA analysis for gut microbiota. |
| Wang L. et al. (2021) [91] | Sprague–Dawley rats | ELISA for inflammatory cytokine levels Downregulation of nuclear translocation of NF-κB via the TLR4/MyD88/NF-κB pathway. |
| Lu Z. et al. (2021) [92] | Wild-type (WT) Wistar rats | Downregulation of chemerin/CMKLR1 signaling pathway and Treg/TH17 ratio. |
| Yu M. et al. (2021) [93] | C57BL/6J | Upregulation of both mitochondrial activity and AMPK pathway, Genomic DNA analysis for gut microbiota |
| Chen P. et al. (2021) [94] | Sprague–Dawley rats | Downregulation of triglyceride transfer protein (MTTP), apolipoprotein B, and low-density lipoprotein receptor (LDLR) |
| Lu Z. et al. (2020) [95] | Sprague–Dawley rats | Upregulation of adipocyte macrophage-derived Angptl2 signaling pathway analysis. |
| Wang W. et al. (2020) [96] | Sprague–Dawley rats | Upregulation of NOD1, NOD2 pathway. NLRP3 inflammasome activity |
| Zhang YP. et al. (2019) [97] | Rats Sprague–Dawley | Activation of SIRT3/AMPK/ACC pathway. |
| Xu X. et al. (2019) [98] | C57BL/6J mice | Activation of SIRT3 pathway and its effects on lipid metabolism. Stimulation of mitochondrial β-oxidation levels. |
| Wu L. et al. (2019) [76] | C57BL/6J mice | Micro 18F-FDG PET/CT. Temperature and metabolic activity measurements. Fat adipose tissue analysis. |
| Luo Y. et al. (2019) [99] | C57BL/6J mice | Downregulation of p38MAPK/ERK-COX2 pathway. Analysis. |
| Deng Y. et al. (2019) [100] | Sprague–Dawley rats | Upregulation of Nrf2/ARE signaling pathway and genes involved in the inflammatory response. |
| Zhao J. et al. (2018) [101] | Sprague–Dawley Rats | Activation of AMPK pathway. |
| Feng WW. et al. (2018) [102] | Sprague–Dawley rats | Downregulation of sterol regulatory element-binding protein 1c (SREBP-1)c, pERK, TNF-α, and pJNK-genes involved in inflammatory response. |
| Zhao L. et al. (2017) [103] | Sprague–Dawley rats | Liver enzyme profile and serum lipid profile. Histological analysis. |
| Yang J. et al. (2017) [104] | C57BL/6J Apolipoprotein E-deficient (ApoE-/-) | Activation of C-X-C chemokine receptor type 4 (CXCR4)/CXCL12 signaling pathway. |
| Zhao W. et al. (2016) [105] | Sprague-Dawley rats | ELISA for detecting pro-inflammatory cytokine levels (TNF-α and IL-6). Upregulation of PPAR-γ and Insulin receptor (IR) activation. |
| Cao Y. et al. (2016) [106] | Mice BALB/c | Liver enzyme profile and lipid profile. Histological analysis. RT-PCR for key enzymes and proteins. |
| Xue M. et.al. (2015) [107] | db/db mice | Downregulation of lipogenic genes: fatty acid synthase (FAS), stearoyl-CoA desaturase (SCD1), and sterol regulatory element-binding protein 1c (SREBP1c). Upregulation of lipolytic gene carnitine palmitoyltransferase-1 (CPT1). |
| Ragab SM. et al. (2015) [108] | Wistar rats | Upregulation of peroxisome proliferator-activated receptor γ (PPARγ) in adipose tissue and liver. |
| Heidarian E. et al. (2014) [109] | Wistar rats | Downregulation of phosphatidate phosphohydrolase (PAP) activity. Antioxidants level assay. |
| Teodoro JS. et al., (2013) [110] | Sprague–Dawley-rats | Stimulation of mitochondrial activity and reactive oxygen species levels. Upregulation of SIRT3 activity level measurements. |
| Yang QH. et al. (2011) [111] | Sprague-Dawley rats | Downregulation of uncoupling protein-2 (UCP2) mechanism. |
| Xing LJ. et al. (2011) [112] | Wistar rats | Significant upregulation of insulin receptor substrate-2 (ISR-2) levels analysis. |
| Chang XX. et al. (2010) [113] | Sprague-Dawley rats /buffalo rat liver (BRL) | Downregulation of genes associated with lipid metabolism: CPT-1, MTTP, and LDLR. |
| Authors | Experiment and Model Type | Pathway |
|---|---|---|
| Ma X. et al. (2024) [114] | HepG2 cell culture/BALB/c mice | Upregulation of various genes involved in lipid metabolism. |
| Ye C. et al. (2023) [115] | C57BL/6J mice/HepG2 cell culture | ELISA for inflammatory cytokine levels. Downregulation of Clock and Bmall1 genes activity. |
| Guo HH. et al. (2023) [116] | ApoE(−/−) mice/Caco-2 cells | Genomic DNA analysis for gut microbiota. Downregulation of TLRs/NF-κB signaling pathway including TLR2, TLR4, and IKKβ. Upregulation of AMPK pathway, ELISA for inflammatory cytokine levels. |
| He H. et al. (2023) [117] | L02 and HepG2 cell lines C57BL/6J mice | Downregulation of lipocalin-2 (LCN2) activity. |
| Zhou LM. et al. (2023) [118] | C57BL/6 J wild-type (WT) mice and/HepG2 cell lines | Suppression of SREBP1/FASN pathway analysis. |
| Wang P. et al. (2022) [119] | HepG2 and AML12 cells line/C57BL/6J mice | Upregulation of SIRT1 and CPT1A activity. |
| Chen Y. et al. (2021) [120] | C57BL/6 J mice/Caco-2 human colon cancer cell line LO2 human normal liver cell line | Downregulation of NF-κB pathway activity. |
| Yang S. et al. (2022) [121] | Mice C57BL/6J HepG2 cell cultures | Liver enzyme profile and serum lipid profile. Genomic DNA analysis for gut microbiota. |
| Li H. et al. (2022) [122] | Mice C57BL/6J/HepG2 cells | Genomic DNA analysis for gut microbiota. |
| Dai L. et al. (2022) [123] | HepG2 cells culture/C57BL/6J mice | Downregulation of SETD2 pathway and histones activity (H3K36me3) |
| Zhang Y. et al. (2021) [124] | C57BL/6J mice/L02 cell culture | Pro-inflammatory cytokines levels analysis. Suppression of NRLP3 pathway and stress oxidative response. |
| Ma CY. et al. (2021) [125] | C57BL/6J mice, ApoE−/− mice/Hep G2 cells | Activation of MAPK/ERK1/2 signaling pathway. Downregulation of PCSK9 expression. |
| Mai W. et al. (2020) [126] | AML12 cell culture and/C57BLKS/J mice (in vivo and in vitro) | Suppression of Caspase-1 and Nod-like receptor family pyrin domain containing 3 (NLRP3) inflammasome activities analysis and ROS. |
| Zhu X. et al. (2019) [127] | C57BL/6 J and ob/ob mice/HepG2 and AML12 cells and six liver biopsies from NAFLD patients | Downregulation of AMPK-SREBP-1c-SCD1 pathways. |
| Sun Y. et al. (2018) [128] | C57BL/6J mice/hep G2 cell cultures | Upregulation of SIRT1 pathway/Autophagy/FGF21 activity. |
| Liang H. et al. (2018) [129] | C57BL/6J mice/Cell cultures | Downregulation of ATP-binding cassette transporter A1 (ABCA1) and protein kinase C δ pathway activities. |
| Sun Y. et al. (2017) [130] | C57BL/6J mice/Sprague Dawley rats/Human hepatoma cell lines Huh7 and HepG2 | Mn-SOD activity measurement and mitochondrial respiratory chain activity. Downregulation of Nrf2/HO-1 pathway activity. |
| Choi YJ. et al. (2017) [131] | Mice C57BL/6J/cell cultures HepG2 | Activation of AMPK, ERK-C/EBPβ pathways, and CD36 expression. |
| Zhang Z. et al. (2016) [132] | Mice C57BL/6J (db/db)/HepG2, FAO cell lines. AML 12 | Activation of ATF6/SREBP-1c pathway. |
| Qiang X. et al. (2016) [133] | Mice C57BLKS/J (db/db)/ICR mice | Activation of AMPK pathway. |
| He Q. et al. (2016) [134] | LO2 human cell line/C57BL/6 mice | Enhanced autophagy induced by activated ERK-dependent mTOR pathway. |
| Guo T. et al. (2016) [135] | Mice C57BL/6J/H4IIE cells (rat hepatoma cells) | Histological analysis. Western blot and RT-PCR for key enzymes and proteins. |
| Zhang Y. et al. (2015) [136] | Wistar rats/Cell cultures | Upregulation of L-pyruvate kinase (LK) activity. |
| Yuan X. et al. (2015) [137] | Sprague-Dawley rats/Huh7 Human hepatic cell line | Downregulation of various IncRNAs and mRNAs associated with NAFLD. |
| Authors | Experiment and Model Type | Pathway |
|---|---|---|
| Rafiei H. et al. (2023) [138] | Cell cultures HepG2,/LX-2 stellate cells, differentiated THP-1 cells | ELISA to quantify levels of inflammatory cytokines and chemokines. Lower levels of ROS. |
| Shan MY. et al. (2021) [139] | HepG2 cell culture | RT-PCR and Western blot for key enzymes. SIRT1-FoxO1-SREBP2 pathway downregulation. |
| Wang Y. et al. (2020) [140] | Macrophages (RAW264.7) and hepatocyte cell lines | Downregulation of endoplasmic reticulum stress response and ERK1/2 pathway. Suppression of PA/LPS-induced inflammation. |
| Sharma A. et al. (2020) [141] | Cell cultures HepG2 and Hepa 1–6 | Activation of AMPK/mTOR/SREBP-1c and AMPK/Nrf2 modulate lipid metabolism and inflammatory and oxidative stress. |
| Babaei Khorzoughi R. et al. (2019) [142] | HepG2 cell cultures | Downregulation of SREBP-1c and FAS expressions |
| Rafiei H. et al. (2019) [143] | HepG2 cell cultures | Upregulation of both mitochondrial and AMPK/SIRT1 pathway activity. |
| Rafiei H. et al. (2017) [144] | HepG2 cell line | Fluorescence analysis for reactive oxygen species analysis. Effects of different polyphenols against stress oxidative response. |
| Liu Y. (2017) [145] | HepG2 cell cultures | Downregulation of FXR/SREBP-1c/FAS pathway. |
| Brusq JM. et al. (2006) [146] | HepG2 cell cultures | Activation of AMPK pathway. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionita-Radu, F.; Patoni, C.; Nancoff, A.S.; Marin, F.-S.; Gaman, L.; Bucurica, A.; Socol, C.; Jinga, M.; Dutu, M.; Bucurica, S. Berberine Effects in Pre-Fibrotic Stages of Non-Alcoholic Fatty Liver Disease—Clinical and Pre-Clinical Overview and Systematic Review of the Literature. Int. J. Mol. Sci. 2024, 25, 4201. https://doi.org/10.3390/ijms25084201
Ionita-Radu F, Patoni C, Nancoff AS, Marin F-S, Gaman L, Bucurica A, Socol C, Jinga M, Dutu M, Bucurica S. Berberine Effects in Pre-Fibrotic Stages of Non-Alcoholic Fatty Liver Disease—Clinical and Pre-Clinical Overview and Systematic Review of the Literature. International Journal of Molecular Sciences. 2024; 25(8):4201. https://doi.org/10.3390/ijms25084201
Chicago/Turabian StyleIonita-Radu, Florentina, Cristina Patoni, Andreea Simona Nancoff, Flavius-Stefan Marin, Laura Gaman, Ana Bucurica, Calin Socol, Mariana Jinga, Madalina Dutu, and Sandica Bucurica. 2024. "Berberine Effects in Pre-Fibrotic Stages of Non-Alcoholic Fatty Liver Disease—Clinical and Pre-Clinical Overview and Systematic Review of the Literature" International Journal of Molecular Sciences 25, no. 8: 4201. https://doi.org/10.3390/ijms25084201
APA StyleIonita-Radu, F., Patoni, C., Nancoff, A. S., Marin, F.-S., Gaman, L., Bucurica, A., Socol, C., Jinga, M., Dutu, M., & Bucurica, S. (2024). Berberine Effects in Pre-Fibrotic Stages of Non-Alcoholic Fatty Liver Disease—Clinical and Pre-Clinical Overview and Systematic Review of the Literature. International Journal of Molecular Sciences, 25(8), 4201. https://doi.org/10.3390/ijms25084201








